Abstract
Myocarditis is an adverse event associated with coronavirus disease 2019 (COVID‐19) mRNA vaccination. A 50‐year‐old man presented with dyspnea and resting chest pain after receiving the second dose of the COVID‐19 mRNA vaccine and developed cardiogenic shock. Fulminant myocarditis was diagnosed by endomyocardial biopsy and treated with intravenous corticosteroids.
Keywords: COVID‐19 mRNA vaccination, fulminant myocarditis, steroid pulse therapy
Fulminant myocarditis is an adverse event associated with the COVID‐19 mRNA vaccination. Steroid pulse therapy may control the pathology of myocarditis caused by COVID‐19 mRNA vaccination.

1. INTRODUCTION
Vaccination is an essential component of the public health strategy to end the coronavirus 2019 (COVID‐19) pandemic; 1 however, various vaccine‐associated adverse events are emerging as an issue that cannot be overlooked. Although the most frequent adverse events following COVID‐19 mRNA vaccine administration include fever, fatigue, headache, myalgia, and injection‐site pain; several serious adverse events, including myocarditis and pericarditis, have also been reported, particularly in adolescents and young adults. 2 , 3 In a recent database in Israel, the estimated incidence of myocarditis within 21 days after the second dose of BNT162b2 mRNA vaccination was 0.21 per 100,000 persons among male recipients aged>50 years. 4 Although most cases had mild clinical courses, there are a few reports of fulminant myocarditis with cardiogenic shock. 3 Treatment of myocarditis is tailor‐made, depending on the case, and often includes NSAIDs, steroids, intravenous immunoglobulin, and colchicine. 5
2. CASE REPORT
A 50‐year‐old man presented with syncope and resting chest pain 10 days after receiving the second dose of COVID‐19 mRNA BNT162b2 vaccine and was admitted to a former hospital. On the day following admission, he was referred to our hospital due to hypotension and worsening chest pain. His vital signs included a blood pressure of 115/66 mmHg with inotropes, heart rate of 104 beats/min, and oxygen saturation of 94% with oxygen administration at a rate of 8 L/min.
His medical history was unremarkable, and he did not smoke or consume alcohol. The patient had no documented allergies or signs of a recent acute infection.
Differential diagnoses included pericarditis, myocarditis, acute myocardial infarction, Takotsubo cardiomyopathy, and sarcoidosis.
The reverse transcriptase‐polymerase chain reaction assay was negative for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) on nasopharyngeal swab, while antibodies against SARS‐CoV‐2 were present (IgG 280 AU/ml; negative<1). Electrocardiography showed sinus tachycardia (97 beats/min) and ST‐segment elevation in leads V1‐V4 with complete right bundle branch block (Figure 1). Laboratory blood tests revealed high levels of cardiac troponin‐I (14644 ng/L; negative<40) and creatine kinase‐MB (72 IU/L). The brain natriuretic peptide level was 248 pg/ml. Viral serologies, including Epstein–Barr virus, cytomegalovirus, adenovirus, respiratory syncytial virus, varicella zoster virus, measles morbillivirus, rubella virus, enterovirus, mumps orthorubulavirus, parvovirus, and coxsackie viruses, were all negative. A brief panel of autoantibodies was unremarkable. Chest radiography and chest computed tomography showed mild pulmonary edema but no pneumonia lesions. Echocardiography demonstrated a left ventricular ejection fraction (LVEF) of 35% with diffuse hypokinesis (Videos 1 and 2). On cardiac magnetic resonance imaging (MRI), late gadolinium enhancement imaging demonstrated linear mid‐myocardial enhancement of the septum walls at the base of the mid‐left ventricle, and T2‐weighted short‐axis inversion recovery imaging showed a high intensity of the global walls of the left ventricle (Figure 2). Coronary angiography revealed no obstructive coronary artery disease (Figure 3). An endomyocardial biopsy specimen showed multifocal cardiomyocyte damage with severe inflammation of lymphocytes and macrophages (Figure 4). The diagnosis of fulminant myocarditis was confirmed by histopathological examination.
FIGURE 1.
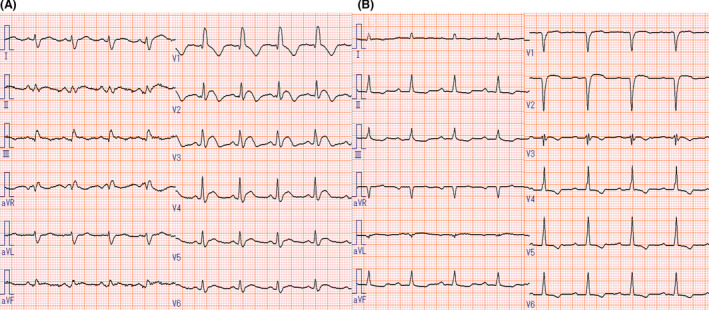
Electrocardiogram findings. Electrocardiogram at admission (A) shows ST‐segment elevation in leads V1‐V4 and complete right bundle branch block. The electrocardiogram at discharge (B) shows the ST‐segment resolution
FIGURE 2.
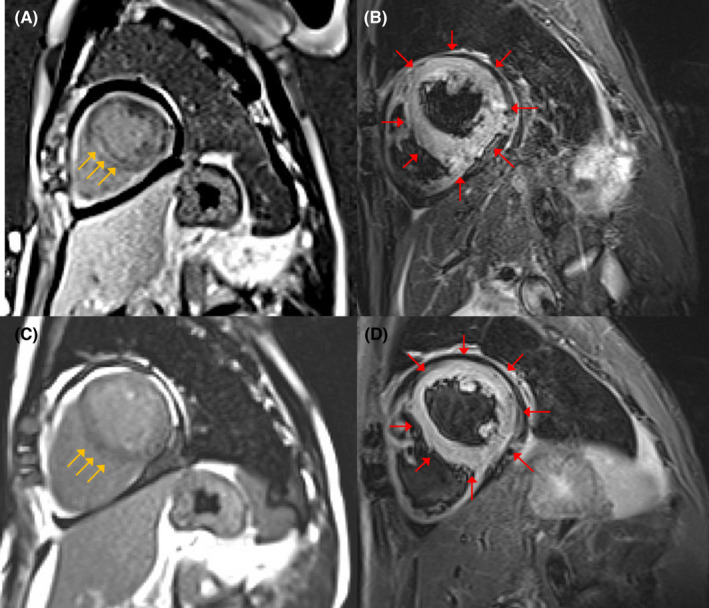
Cardiac magnetic resonance (CMR) findings. (A) CMR imaging shows linear mid‐myocardial late gadolinium enhancement (LGE) of the septum wall at the base of the mid‐ventricle (yellow arrows). (B) T2‐weighted short‐axis inversion recovery imaging shows a high intensity of the global walls of the left ventricle suggestive of myocardial edema (red arrows). CMR findings met the original Lake Louise criteria for the diagnosis of acute myocarditis. On CMR imaging after 5 weeks, LGE disappeared (C), and the high intensity of the global walls of the left ventricle was sustained (D)
FIGURE 3.
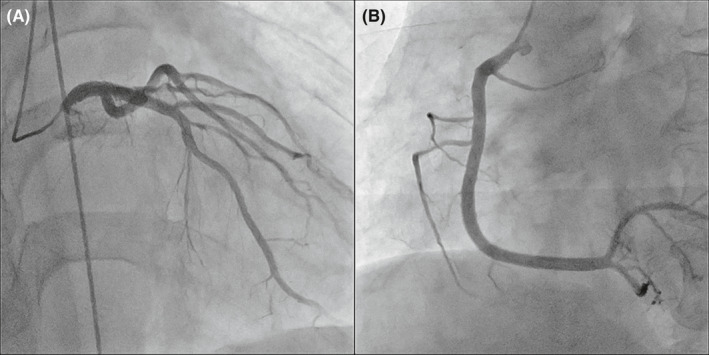
Coronary angiogram findings. (A) Coronary angiogram shows a normal left coronary artery and (B) right coronary artery
FIGURE 4.
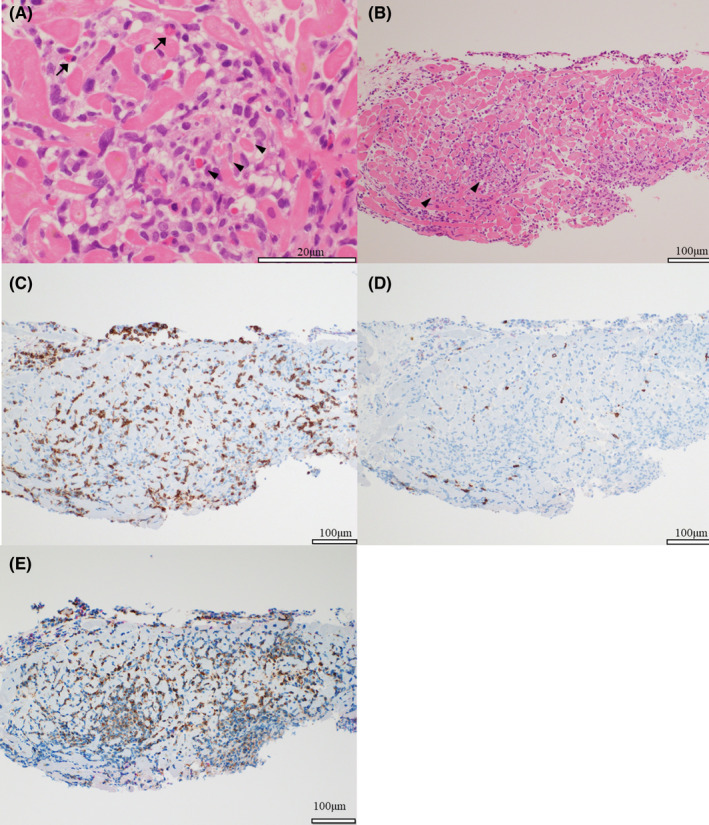
Histopathological Findings. Endomyocardial biopsy of the right ventricular septum was performed. (A, B) Hematoxylin–eosin staining of heart tissue specimens obtained by endomyocardial biopsy shows myocarditis with inflammatory cells and cardiomyocyte damage (arrowheads). A few eosinophils are observed (arrows). (C, D) Immunostaining shows more infiltration of T lymphocytes of CD3‐positive cells than those of CD20‐positive cells. (E) Immunostaining for CD68 shows the infiltration of several macrophages
The patient presented with cardiogenic shock and was transferred to the intensive care unit (Figure 5). He was treated with pulse steroid therapy (methylprednisolone, 1 g daily for 3 days) and required inotropes, even after pulse steroid therapy. On Day 6 post‐hospitalization, an advanced atrioventricular block was observed, and a temporary transvenous pacemaker was placed. After the first pulse steroid therapy, the cardiac troponin I level rapidly increased and peaked at 53292 ng/L. Subsequently, a second pulse steroid therapy was administered (methylprednisolone, 1 g daily for 3 days) following a daily dose of 40 mg of prednisolone, which was gradually reduced. On hospitalization Day 8, he did not require inotropes considering the fact that his blood pressure had improved. On hospitalization Day 9, the temporary transvenous pacemaker was removed. On hospitalization Day 18, the prednisolone treatment course was completed, and no atrioventricular block was detected. He eventually received guideline‐directed medical therapy for heart failure (bisoprolol, spironolactone, and sacubitril valsartan). On hospitalization Day 21, his cardiac troponin I level decreased to the normal range, and echocardiography showed a recovered LVEF of 60% with no regional wall motion abnormalities (Videos 3 and 4). The patient was discharged 22 days after admission.
FIGURE 5.
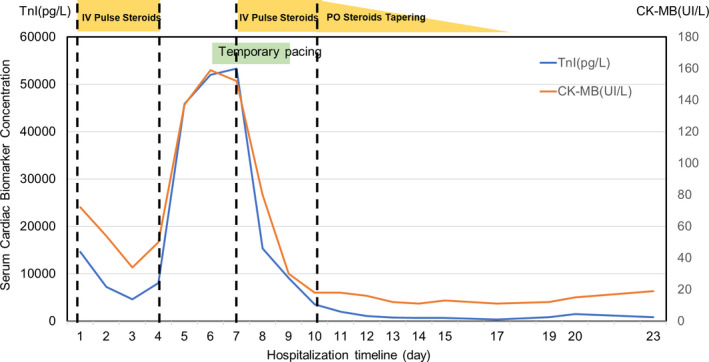
Troponin I (TnI) and creatine kinase‐MB (CK‐MB) levels. TnI and CK‐MB levels were suppressed by pulse steroid therapy: At their peak, an advanced atrioventricular block was observed. IV, intravenous injection; PO, per oral
At the 2‐week follow‐up, the patient presented with syncope and exacerbation of congestive heart failure. Electrocardiography revealed atrioventricular dissociation (62 beats/min), and an echocardiogram showed an LVEF of 60% with no regional wall motion abnormalities. The cardiac troponin I level slightly increased (223 ng/L), and the brain natriuretic peptide level was 1103 pg/ml. He was hospitalized because of heart failure, and diuretics were administered. He was discharged 1 week later.
3. DISCUSSION
The diagnosis of acute myocarditis in the present case was confirmed based on histological and MRI findings. Endomyocardial biopsy, which is the most definitive method, showed inflammatory infiltrates predominantly composed of lymphocytes and macrophages and cardiomyocyte damage accompanied by a small number of eosinophils, consistent with a previously reported case. 3 MRI findings are often used for the non‐invasive diagnosis of acute myocarditis. This case met the original Lake Louise criteria with T2 signal intensity and late gadolinium enhancement. 6 In other cases of myocarditis following mRNA vaccination, the usefulness of MRI for diagnosis has been reported. 7
Pulse steroid therapy was administered twice to suppress the inflammation. The troponin level, which was repeatedly confirmed, decreased after the first pulse steroid therapy. However, it rapidly increased with an advanced atrioventricular block. After the second pulse steroid therapy, the steroid dose was gradually decreased and was ultimately ceased during hospitalization. During the course, troponin levels reflected the severity of myocarditis. Although the treatment of myocarditis following mRNA vaccination is not yet well established, pulse steroid treatment may be considered for severe cases of cardiomyopathy.
Although a direct relationship could not be determined between myocarditis and the mRNA COVID‐19 vaccination in this case, blood examinations and endomyocardial biopsy failed to identify other causes of myocarditis. It is expected that the mechanism underlying mRNA COVID‐19 vaccination‐induced myocarditis will be clarified in the future.
4. CONCLUSIONS
This case demonstrated that myocarditis, which is a relatively mild and curable complication of the COVID‐19 mRNA vaccine, may follow a fulminant course. Further, our case revealed that steroid pulse therapy may be useful for controlling the pathology of myocarditis potentially resulting from the COVID‐19 mRNA vaccination.
CONFLICT OF INTEREST
All authors declare that they have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
AO contributed to patient care, conception of work, drafting of manuscript, interpretation of findings, performance of echocardiography, and acquisition of informed consent. YS contributed to patient care, conception of work, drafting of manuscript, and interpretation of findings. TM contributed to conception of work, drafting of manuscript, interpretation of findings, critical revision of the work, and editing of photographs and legends. MO and YK contributed to patient care, drafting of manuscript, and interpretation of findings. WT contributed to patient care, drafting of manuscript, interpretation of findings, and critical revision of the work. SU contributed to patient care, conception of work, drafting of manuscript, interpretation of findings, and performance of echocardiography. TO contributed to patient care, drafting of manuscript, interpretation of findings, and critical revision of the work. KN contributed to patient care, drafting of manuscript, interpretation of findings, and critical revision of the work. MD contributed to patient care, drafting of manuscript, and interpretation of findings. All authors read and approved the final version of the manuscript.
ETHICAL APPROVAL
None.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Supporting information
video S1
video S2
video S3
video S4
Supplementary Material
ACKNOWLEDGEMENT
The authors would like to thank Dr. Satoko Nakamura for her collaboration.
Oka A, Sudo Y, Miyoshi T, et al. Fulminant myocarditis after the second dose of COVID‐19 mRNA vaccination. Clin Case Rep. 2022;10:e05378. doi: 10.1002/ccr3.5378
Funding information
The authors report no financial support for the work presented in this case report
DATA AVAILABILITY STATEMENT
Data are available on request from authors.
REFERENCES
- 1. Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID‐19 vaccine strategies. Nat Rev Immunol. 2020;20:615‐632. doi: 10.1038/s41577-020-00434-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verma AK, Lavine KJ, Lin CY. Myocarditis after covid‐19 mRNA vaccination. N Engl J Med. 2021;385(14):1332‐1334. doi: 10.1056/NEJMc2109975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against covid‐19 in Israel. N Engl J Med. 2021;385:2140‐2149. doi: 10.1056/NEJMoa2109730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ammirati E, Frigerio M, Adler ED, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail. 2020;13:e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Friedrich MG, Sechtem U, Schulz‐Menger J, et al. Cardiovascular magnetic resonance in myocarditis: A JACC white paper. J Am Coll Cardiol. 2009;53:1475‐1487. doi: 10.1016/j.jacc.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel YR, Louis DW, Atalay M, Agarwal S, Shah NR. Cardiovascular magnetic resonance findings in young adult patients with acute myocarditis following mRNA COVID‐19 vaccination: a case series. J Cardiovasc Magn Reson. 2021;23:101. doi: 10.1186/s12968-021-00795-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
video S1
video S2
video S3
video S4
Supplementary Material
Data Availability Statement
Data are available on request from authors.


