Summary
Background
Although acute cardiac injury (ACI) is a known COVID-19 complication, whether ACI acquired during COVID-19 recovers is unknown. This study investigated the incidence of persistent ACI and identified clinical predictors of ACI recovery in hospitalized patients with COVID-19 2.5 months post-discharge.
Methods
This retrospective study consisted of 10,696 hospitalized COVID-19 patients from March 11, 2020 to June 3, 2021. Demographics, comorbidities, and laboratory tests were collected at ACI onset, hospital discharge, and 2.5 months post-discharge. ACI was defined as serum troponin-T (TNT) level >99th-percentile upper reference limit (0.014ng/mL) during hospitalization, and recovery was defined as TNT below this threshold 2.5 months post-discharge. Four models were used to predict ACI recovery status.
Results
There were 4,248 (39.7%) COVID-19 patients with ACI, with most (93%) developed ACI on or within a day after admission. In-hospital mortality odds ratio of ACI patients was 4.45 [95%CI: 3.92, 5.05, p<0.001] compared to non-ACI patients. Of the 2,880 ACI survivors, 1,114 (38.7%) returned to our hospitals 2.5 months on average post-discharge, of which only 302 (44.9%) out of 673 patients recovered from ACI. There were no significant differences in demographics, race, ethnicity, major commodities, and length of hospital stay between groups. Prediction of ACI recovery post-discharge using the top predictors (troponin, creatinine, lymphocyte, sodium, lactate dehydrogenase, lymphocytes and hematocrit) at discharge yielded 63.73%-75.73% accuracy.
Interpretation
Persistent cardiac injury is common among COVID-19 survivors. Readily available patient data accurately predict ACI recovery post-discharge. Early identification of at-risk patients could help prevent long-term cardiovascular complications.
Funding
None
Key Words: Machine learning, SARS-CoV-2, acute myocardial injury, heart failure
Research in context.
Evidence before this study
Acute cardiac injury (ACI) is a major complication of coronavirus disease 2019 (COVID-19). However, the incidence of persistent cardiac injury during and beyond hospitalization as well as the predictors of ACI recovery post hospital discharge are unknown.
Added value of this study
ACI incidence rate was 39.7% among hospitalized patients, with an in-hospital mortality odds ratio of 4.45. For those who survived and returned to our hospital system for follow-up care, 55.8% exhibited persistent cardiac injury 2.5 months (on average) after hospitalization from COVID-19. Troponin, creatinine, lymphocytes and sodium at discharge were the top predictors of ACI recovery at 2.5 months post discharge, yielding 63.73% to 75.73% prediction accuracy.
Implications of all the available evidence
Our findings suggest ACI is an important marker of future adverse outcomes in COVID-19. Awareness for cardiovascular complications is warranted when ACI is detected as cardiovascular prevention may be of lower priority given the urgency in treating SARS-CoV-2 infection. The ability to identify patients at-risk of persistent ACI early on could enable appropriate follow-up care to prevent long-term cardiac complications.
Alt-text: Unlabelled box
Introduction
Acute cardiac injury (ACI) is a major complication of coronavirus disease 2019 (COVID-19)1, 2, 3 and has been associated with elevated risk of critical illness and mortality.4,5 The virus responsible for COVID-19, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), could directly cause ACI1, 2, 3,6,7 because heart muscle has high density of angiotensin-converting enzyme 2 (ACE2) receptors through which SARS-CoV-2 virus enters cells.8 Systemic hypoxia, acute respiratory distress syndrome, hypercoagulation, hypotension, shock, sepsis, inflammation, cytokine storm, and other host-immune responses from COVID-19 complications could also contribute to ACI.1, 2, 3,6,7
Although ACI associated with COVID-19 has been documented,1, 2, 3,6, 7, 8, 9, 10, 11, 12 it is unknown whether COVID-19 ACI is transient or whether it has long-lasting effects, and what clinical variables are useful in predicting ACI recovery. Data on medium and long term ACI outcomes are generally lacking. There is direct evidence that SARS-CoV-2 causes persistent systemic infection and injury in the body in general for months.13 It is important to identify early on which COVID-19 patients will likely have persistent ACI such that appropriate follow-up care could be administered to prevent heart failure and other cardiovascular complications.
The goal of this study was thus to investigate whether ACI persisted among COVID-19 survivors and to identify predictors of ACI outcome at 2.5 months following hospital discharge. We analyzed demographic data, comorbidities, vital signs, and laboratory tests at ACI onset, hospital discharge, and 2.5 months post discharge of patients in the Montefiore Health System in the Bronx environs. Four predictive models were used to identify the best predictors hospital discharge that accurately predict ACI recovery status 2.5 months post hospital discharge from COVID-19.
Methods
Ethics
This study was approved by the Einstein-Montefiore Institutional Review Board (#2020-11389) with a waiver for informed consent due to the retrospective, observational nature of this study with de-identified patient data.
Data source
All data originated from the Montefiore Health System, one of the largest healthcare systems in New York City located in the Bronx, the lower Hudson Valley, and Westchester County. The Montefiore Health System serves a large, low-income, and racially and ethnically diverse population in the Bronx environs that was hit hard by COVID-19 early in the pandemic.14,15 Health data were searched and extracted as described previously.15,16 In short, de-identified data were made available for research by the Montefiore Einstein Center for Health Data Innovations after standardization to the Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM) version 6. OMOP CDM represents healthcare data from diverse sources, which are stored in standard vocabulary concepts,17 allowing for the systematic analysis of disparate observational databases, including data from the electronic medical record system, administrative claims, and disease classifications systems (e.g., ICD-10, SNOWMED, LOINC, etc.). ATLAS, a web-based tool developed by the Observational Health Data Sciences and Informatics (OHDSI) community that enables navigation of patient-level, observational data in the CDM format, was used to search vocabulary concepts and facilitate cohort building. Data were subsequently exported and queried as SQLite database files using the DB Browser for SQLite (version 3.12.0).
Study population
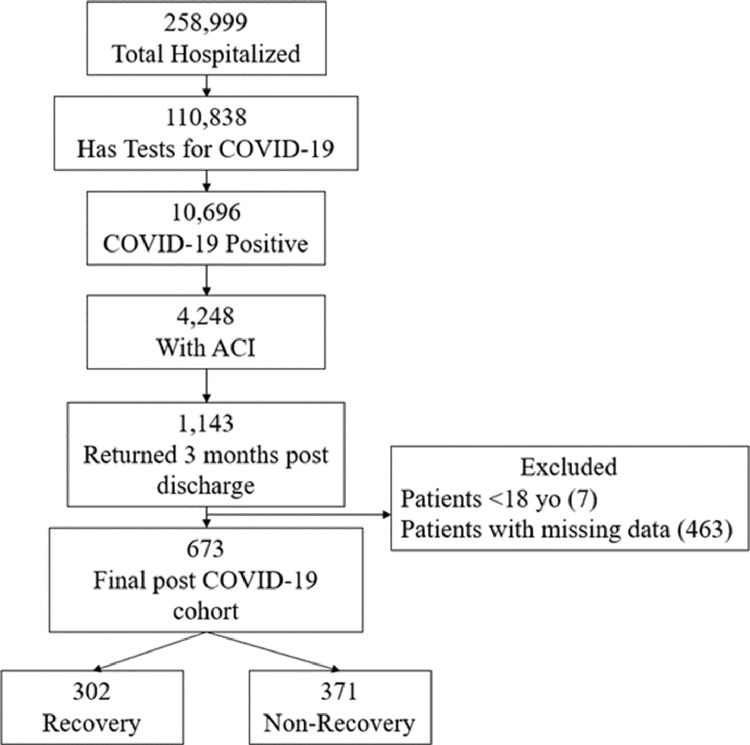
From March 11, 2020, to June 3, 2021, there were a total of 258,999 hospitalized patients in the Montefiore Health System (Figure 1). Of all hospitalized patients, 110,838 had tests for SARS-CoV-2 infection of which 10,696 had positive tests during or before hospitalization. Patients were considered to have ACI if they had a serum troponin T (TNT) level above the 99th percentile upper reference limit (0.014 ng/mL) any time during hospitalization.3,18, 19, 20 Recovery from ACI was defined as TNT dropping below this TNT threshold (for survivors only). Patients who were younger than 18 years old or without TNT data were excluded.
Figure 1.
Flowchart of hospitalized COVID-19 patients in the Montefiore Health System who recovered and who did not at 2.5 months post discharge (March 11, 2020 to June 3, 2021). ACI: acute cardiac injury.
Clinical variables
Demographics, chronic comorbidities, vital signs and laboratory tests were collected. Demographic data included age, gender, race and ethnicity. Race/ethnicity were based on patient self-identification and categorized as Hispanic, non-Hispanic Black, non-Hispanic White, Asian, other (comprising non-Hispanic patients indicating their race as multiple selected, American Indian or Alaska Native, or some other race), or unknown/declined. Chronic comorbidities included essential hypertension, asthma, chronic obstructive pulmonary disease (COPD), stroke, type-1 diabetes, type-2 diabetes, chronic kidney disease, coronary artery disease, heart failure, and liver disease. Vital signs including heart rate, temperature, respiratory rate, and oxygen saturation were collected at ACI onset, discharge, and 2.5 months after discharge. Laboratory values included alanine aminotransferase (ALT), aspartate aminotransferase (AST), brain natriuretic peptide (BNP), C-reactive protein (CRP), creatine kinase (CK), creatinine (Cr), D-dimer (DD), ferritin, hematocrit, potassium, sodium, lactate dehydrogenase (LDH), lymphocytes, troponin T (TNT), and white blood cell (WBC) count at ACI onset, discharge, and 2.5 months after discharge (mean: 71.5 days, median: 36 days, ranging from 14-380 days).
Predictive models
Predictive models were constructed to predict ACI recovery from ACI onset and at 2.5 months post discharge (primary outcomes) using Classification and Regression Trees (CART), Random Forest (RF), Neural Networks (NN) and Logistic Regression. BNP and ferritin were not used in our predictive models due to significant number of missing data (>80% missing). For the rest of the laboratory variables, missing data were <15% and were imputated using the median. The inputs to the predictive models included demographics, comorbidities, and clinical variables at ACI onset (or up to 2 days later) and at hospital discharge (up to 4 days prior).
The CART algorithm,21,22 is based on the Recursive Partitioning and Regression Trees (RPART), which works by repeatedly partitioning the data into multiple sub-spaces such that the outcomes in each final class are as homogeneous as possible. First a single predictor is found which best splits the training data into two subgroups. The dataset is separated, and then this process is applied separately to each subgroup, and so on recursively until there no improvement can be made. To evaluate the performance of the CART model, we used k-Fold Cross-Validation with k=10. Lastly, the CART tree was pruned using a tune length and cross validation of 10.
Breiman's RF algorithm23 aggregates the predictions made by multiple decision trees. Each tree in the forest is trained on a subset of our training set which called the boot strapped set or in-bag set. The RF model then analyzes each feature and reveals the importance of the feature in predicting the correct classification of the random forest machine learner.
NN24 uses a fully connected feed-forward neural network with one hidden layer consisting of three neurons implemented in the neural net package. Logistic function and ‘cross entropy’ loss function are utilized. For feature importance analysis and feature selection, Garson function in ‘NeuralNetTools’ library is used. To avoid overfitting, the top 9 features in the RF model is used.
As a benchmark, logistic regression analysis was also performed with the same top 9 features selected by RF. We first found the best subset out of the top 9 features from RF and inputted those variables into the model. We used the simplest feed-forward neural network model with no hidden layer, often referred to as the ‘perceptron’, and used a ‘logistic’ activation function and ‘cross entropy’ loss function, that is, the negative log likelihood function.
Statistical analysis
Statistical analysis was performed using R (https://www.R-project.org). Group comparisons (recovery vs non-recovery) of categorical variables were performed using the Chi-squared test. Group comparison of continuous variables in medians and interquartile ranges (IQR) were performed using the Wilcoxon test. All predictive models employed 80% training data and 20% testing with ten-fold cross validation (except logistic regression), implemented in R. In-hospital mortality odds ratio was adjusted for age, sex and significant comorbidities between ACI and non-ACI group. A p-value < 0.05 was used to indicate statistical significance unless otherwise specified.
Role of funding source
The authors declare no sources of funding and thus no funders had any influence in study design, data collection, data analyses, interpretation, or writing of the research.
Results
Of the 10,696 hospitalized patients with COVID-19, there were 4,248 (incidence rate of 39.7%) patients with ACI. Most (92.5%) ACI onset occurred on the day of or one day after hospital admission. The unadjusted in-hospital mortality rate was 32.2% for ACI COVID-19 patients compared to 13.8% of non-ACI COVID-19 patients and the adjusted odds ratio was 4.45 [95% CI: 3.92, 5.05, p<0.001]. Of the 2,880 ACI COVID-19 survivors, 1,114 (38.7%) returned to our hospital system 2.5 months (mean: 71.5 days, median: 36 days, ranging from 14 to 380 days) post discharge. After excluding patients < 18 yo and missing data, there were 673 patients in our final cohorts for 2.5 months follow up, of which only 302 (45%) recovered from ACI.
Table 1 summarizes the demographics and comorbidities stratified by recovery and non-recovery status. There was no significant difference in age, gender, race, ethnicity, comorbidities, and length of hospitalization between the two groups (p>0.05, Chi-squared test).
Table 1.
Demographics and comorbidities of patients who recovered vs not recovered from cardiac injury 2.5 months after hospital discharge from COVID-19. Group comparisons (recovery vs non-recovery) of categorical variables were performed using the Chi-squared test. Group comparison of continuous variables in medians and interquartile ranges (IQR) were performed using the Wilcoxon test. A p-value < 0.05 was considered statistically significant.
| Recovery(n=302) | Non-recovery(n=371) | p-value | |
|---|---|---|---|
| Demographics | |||
| Age, median (IQR) | 71 (59, 80) | 71 (61, 80) | 0.813 |
| Female sex, n (%) | 176 (58%) | 195 (53%) | 0.160 |
| Race, n (%) | 0.161 | ||
| White | 26 (9%) | 52 (14%) | |
| Black/African American | 132 (44%) | 135 (36%) | |
| Asian | 8 (3%) | 7 (2%) | |
| Other Pacific Islander | 0 | 1 (0.3%) | |
| Patient declined | 4 | 5 (1%) | |
| Other | 132 (44%) | 171 (46%) | |
| Ethnicity, n (%) | 0.732 | ||
| Hispanic | 127 (42%) | 162 (44%) | |
| Non-Hispanic | 175 (58%) | 209 (56%) | |
| Comorbidities, n (%) | |||
| Type-1 Diabetes | 17 (6%) | 11 (3%) | 0.127 |
| Type-2 Diabetes | 112 (37%) | 144 (39%) | 0.704 |
| Hypertension | 137 (45%) | 174 (47%) | 0.749 |
| Asthma | 26 (9%) | 30 (8%) | 0.917 |
| COPD | 13 (4%) | 30 (8%) | 0.066 |
| Coronary artery disease | 62 (21%) | 80 (22%) | 0.817 |
| Chronic kidney disease | 67 (22%) | 68 (18%) | 0.252 |
| Liver disease | 9 (3%) | 16 (4%) | 0.481 |
| Stroke | 30 (10%) | 43 (12%) | 0.574 |
| Heart failure | 91 (30%) | 114 (31%) | 0.934 |
| Length of hospital stay, median (IQR) | 8 (4, 16) | 8 (5, 13) | 0.289 |
Abbreviations: IQR, interquartile range. COPD, chronic obstructive pulmonary disease.
Table 2 summarizes the vital signs, laboratory values at ACI onset, hospital discharge, and 2.5 months post discharge stratified by the recovery and non-recovery cohorts.
Table 2.
Vital signs and lab values presented at ACI onset, at hospital discharge, and 2.5 months after hospital discharge from COVID-19 stratified by recovery and non-recovery from ACI. Group comparisons (recovery vs non-recovery) of categorical variables were performed using the Chi-squared test. Group comparison of continuous variables in medians and interquartile ranges (IQR) were performed using the Wilcoxon test. A p-value < 0.05 was considered statistically significant. Abbreviations: ACI, acute cardiac injury, eGFR, estimated glomerular filtration rate, IQR, interquartile range. Troponin, brain natriuretic peptide and creatine kinase are cardiac variables and are listed first.
| At ACI onset |
At hospital discharge |
At 2.5 months post discharge |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Recovery (n=492) | Non-recovery (n=622) | p-value | Recovery (n=302) | Non-recovery (n=371) | p-value | Recovery (n=302) | Non-recovery (n=371) | p-value | |
| Laboratory values, median, (IQR) | |||||||||
| Troponin T ng/mL | 0.03 (0.02, 0.06) | 0.06 (0.03, 0.16) | <0.001 | 0.02 (0.02, 0.05) | 0.06 (0.03, 0.15) | <0.001 | 0.1 (0.1, 0.1) | 0.07 (0.03, 0.15) | <0.001 |
| Brain natriuretic peptide, pg/mL | 228 (63, 804) | 916 (201, 4191) | <0.001 | 67 (18, 264) | 604 (173, 3001) | 0.004 | 60 (25, 175) | 775 (178, 2599) | <0.001 |
| Creatine kinase, U/L | 136 (72, 338) | 125 (72, 263) | 0.372 | 103 (47, 214) | 107 (59, 244) | 0.225 | 71 (38, 139) | 93 (50, 179) | <0.001 |
| Alanine aminotransferase, U/L | 23 (15, 41) | 20 (14, 31) | 0.050 | 24 (15, 41) | 21 (13, 36) | 0.166 | 17 (11, 29) | 20 (13, 35) | 0.011 |
| Aspartate aminotransferase, U/L | 32 (21, 53) | 28 (20, 45) | 0.086 | 29 (20, 43) | 26 (20, 43) | 0.594 | 22 (20, 31) | 27 (20, 43) | <0.001 |
| C-reactive protein, mg/dL | 8 (2, 19) | 6 (2, 14) | 0.117 | 1.0 (0.8, 1.6) | 3.9 (1.2, 8.0) | 0.093 | 3.7 (0.6, 12.4) | 7.2 (2.4, 13.9) | 0.027 |
| Creatinine mg/dL | 1.2 (0.9, 2.1) | 1.8 (1.1, 4.6) | <0.001 | 3.2 (1, 6.7) | 1.4 (0.9, 4.2) | <0.001 | 1.1 (0.8, 1.6) | 1.9 (1.1, 4.4) | <0.001 |
| D-dimer, ug/mL | 1.6 (0.9, 3.2) | 2.0 (1.0, 3.6) | 0.072 | 1.5 (0.7, 2.8) | 1.6 (0.8, 3.0) | 0.218 | 1.3 (0.8, 2.2) | 2.7 (1.4, 5.9) | <0.001 |
| eGFR mL/min | 44 (12, 67) | 23 (8, 49) | <0.001 | 51 (16, 70) | 32 (8, 61) | <0.001 | 63 (24, 82) | 23 (8, 53) | <0.001 |
| Ferritin, ng/mL | 657 (236, 1229) | 561 (178, 1441) | 0.662 | 483 (222, 813) | 542 (172, 1046) | 0.759 | 491 (130, 1425) | 796 (333, 1872) | 0.119 |
| Hematocrit, vol % | 38 (33, 42) | 35 (30, 41) | 0.007 | 34 (29, 38) | 32 (28, 37) | 0.019 | 35 (30, 40) | 33 (28, 38) | 0.001 |
| Potassium, mEq/L | 4.3 (3.9, 4.8) | 4.4 (3.9, 4.9) | 0.625 | 4.2 (3.8, 4.6) | 4.2 (3.9, 4.7) | 0.726 | 4.2 (3.8, 4.7) | 4.2 (3.8, 4.9) | 0.644 |
| Lactate dehydrogenase, U/L | 351 (245, 499) | 312 (251, 422) | 0.061 | 296 (222, 427) | 308 (239, 404) | 0.333 | 318 (224, 381) | 336 (247, 500) | 0.143 |
| Lymphocytes, x109/L | 14 (8, 22) | 13 (9, 20) | 0.574 | 21 (14, 29) | 18 (12, 25) | 0.003 | 20 (12, 28) | 15 (9, 21) | <0.001 |
| Sodium, mEq/L | 138 (135, 141) | 137 (134, 141) | 0.690 | 138 (136, 141) | 138 (135, 141) | 0.301 | 138 (136, 141) | 138 (135, 141) | 0.206 |
| White blood cell count, x109/L | 8 (6, 11) | 8 (6, 11) | 0.254 | 7 (5, 9) | 7 (5, 10) | 0.211 | 8 (6, 10) | 8.8 (6.1, 12.2) | <0.001 |
| Vital signs, median (IQR) | |||||||||
| Heart Rate, beats/min | 97 (96, 99) | 97 (96, 99) | 0.706 | 98 (96, 99) | 98 (97, 99) | 0.659 | 98 (97, 99) | 98 (96, 99) | 0.046 |
| Temperature, F | 98.4 (98, 99.1) | 98.4 (98.0, 99.2) | 0.774 | 98.1 (97.9, 98.4) | 98.1 (97.8, 98.3) | 0.639 | 98.2 (97.9, 98.5) | 98.2 (97.9, 98.6) | 0.873 |
| Respiratory Rate, breath/min | 18 (18, 19) | 18 (18, 19) | 0.678 | 18 (18, 19) | 18 (18, 19) | 0.810 | 18 (18, 20) | 19 (18, 20) | 0.511 |
| Oxygen saturation, % | 98 (96, 99) | 98 (96, 99) | 0.505 | 98 (96, 99) | 98 (97, 99) | 0.659 | 98 (96, 99) | 98 (97, 99) | 0.093 |
At ACI onset: Compared to recovery patients, non-recovery patients displayed higher levels of creatinine, hematocrit, TNT, and BNP (p<0.05 for all, Wilcoxon test), but not CK, ALT, AST, CRP, DD, ferritin, potassium, LDH, lymph, sodium, WBC, heart rate, temperature, respiratory rate, and oxygen saturation (p>0.05 for all, Wilcoxon test).
At discharge: Compared to recovery patients, non-recovery patients displayed higher levels TNT and BNP (p<0.05 for both, Wilcoxon test). Non-recovery patients also had lower levels of creatinine, hematocrit, and lymphocyte counts (p<0.05 for all, Wilcoxon test), but not CK, ALT, AST, CRP, DD, ferritin, potassium, LDH, sodium, WBC, heart rate, temperature, respiratory rate, and oxygen saturation (p>0.05 for all, Wilcoxon test).
At 2.5 months post discharge: Compared to recovery patients, non-recovery patients displayed higher levels of TNT, BNP, CK, ALT, AST, CRP, creatinine, D-dimer, WBC (p<0.05 for all, Wilcoxon test) and lower heart rate, hematocrit, and lymph, (p<0.05 for all, Wilcoxon test). Ferritin, potassium, LDH, sodium, temperature, respiratory rate, and oxygen saturation were not statistically different between groups (p>0.05 for all, Wilcoxon test). Note that Cr was elevated in the non-recovery group compared to recovery group at 2.5 months post discharge but not at ACI diagnosis and discharge. Similarly, eGFR was lower in the non-recovery group compared to recovery group at 2.5 months post discharge but not at ACI diagnosis and discharge.
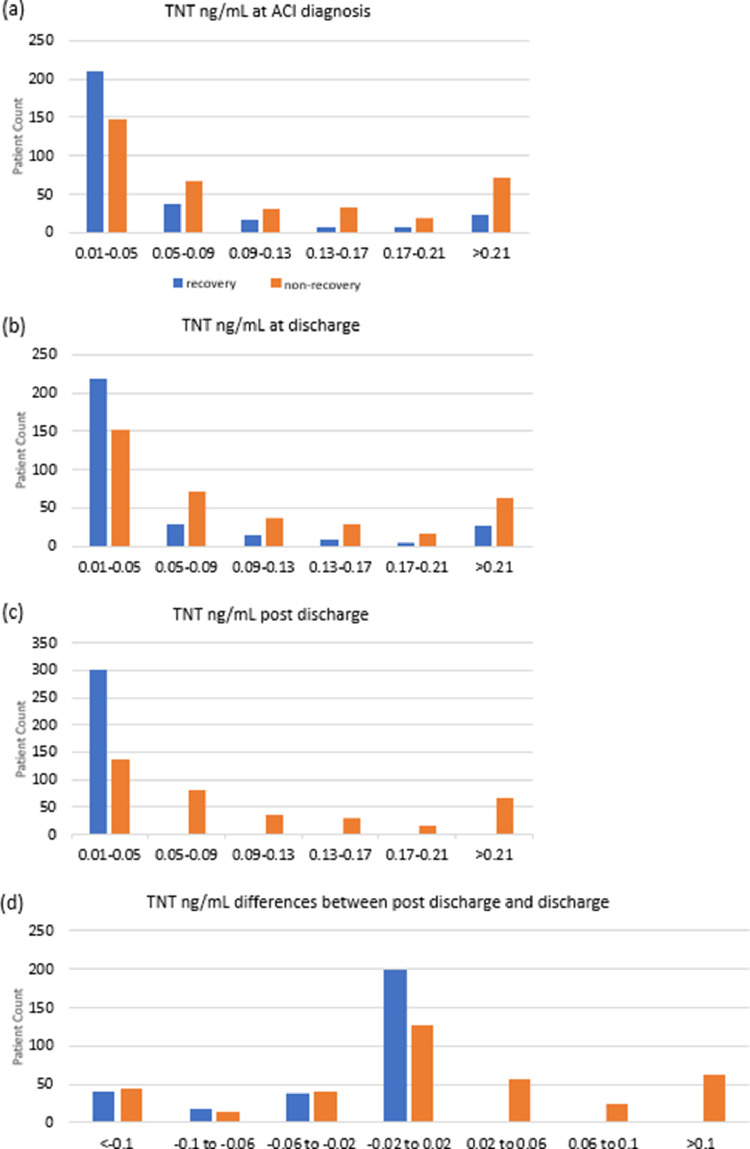
We further analyzed the TNT level in detail at ACI diagnosis, at hospital discharge, at 2.5 months post discharge, and changes in TNT between post discharge and hospital discharge stratified by patients who recovered from ACI and those who did not (Figure 2). At diagnosis, non-recovery group had more patients with higher TNT compared to the recovery group, except the lowest TNT level. At discharge, there were fewer patients at higher TNT levels overall, and the non-recovery and recovery group were more similar at each TNT bin. At 2.5 months post discharge, only the non-recovery group had significantly elevated TNT. The differences between 2.5 months post discharge and discharge showed that most non-recovery group increased in TNT level, whereas the recovery group showed either no worsening or reduced TNT level.
Figure 2.
Histograms of TNT level (a) at ACI diagnosis, (b) at discharge, (c) at 3 months post discharge, and (d) changes in TNT between 3 months post discharge and discharge separated by patients who recovered from ACI (blue) and those who did not (orange).
Predicting ACI recovery
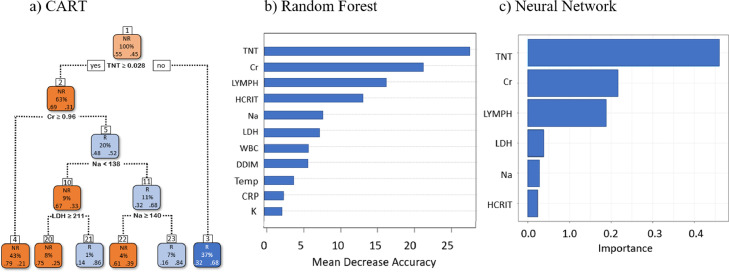
Figure 3a shows the CART prediction of ACI recovery using data at hospital discharge. The top predictors were TNT, Cr, Na, LDH during the index admission. The first split was TNT value ≥ 0.028 ng/mL. Patients were further split based on Cr ≥ 0.96 mg/dL, Na<138 mEq/L, LDH ≥ 211 U/L, and Na ≥ 140 mEq/L. The model yielded an accuracy of 70.66±2.37%, sensitivity of 65.63±7.93%, and specificity of 74.34±4.26% on an independent “test” dataset.
Figure 3.
Predictive models at discharge using CART, Random Forest and neural network at discharge. For CART, R: recovery, NR: non-recovery. In the CART panel, the % within the box indicates % of total patients that arrived at each leaf, and the fractions within the box panel indicate actual non-recovery (right) and recovery (left). HR: heart rate.
The RF model found the top predictors to be TNT, Cr, Lymph, Hcrit, Na, and LDH in order of importance (Figure 3b). This model had an accuracy of 72.07±2.79%, sensitivity of 66.27±4.76%, and specificity of 77.32±3.98% on an independent “test” dataset.
The neural network model ranked the top predictors to be TNT, Cr, Lymph, LDH, Na, Hcrit in order of importance (Figure 3c). This model yielded an accuracy of 75.73±3.61%, sensitivity of 67.96±3.28%, and specificity of 75.99±4.57% on an independent “test” dataset.
The logistic regression found the top predictors to be Cr and Lymph (in order of importance). This model yielded an accuracy of 63.73±3.30%, sensitivity of 52.09±6.61%, specificity of 72.72±6.47%.
Table 3a summarizes the top ACI predictors and their performance metrics using data at hospital discharge for all models. Overall, all models yielded remarkably similar top ACI predictors with TNT, creatinine, lymphocytes and sodium generally being the top predictors. The accuracy ranged from 63.73% to 75.73%. None of the comorbidities and vital signs were highly ranked. Potassium was also not highly ranked. Similarly, we also performed prediction using data at ACI onset (Table 3b). The top ACI predictors were less similar across different models, but common predictors nonetheless included D-dimer, creatinine, LDH, TNT, and CRP. The accuracy ranged from 61.19% to 74.79%, which were lower than of those using data at hospital discharge.
Table 3.
Top predictors of ACI recovery and performance metrics predicted using data at (A) hospital discharge and (B) ACI onset. DD: D-dimer, RR: respiratory rate, HCRIT: hematocrit, CKD: chronic kidney disease. Values are in mean ± standard deviation.
| (a) Prediction of recovery using data at discharge | ||||
|---|---|---|---|---|
| Models | Top predictors at discharge | Accuracy | Sensitivity | Specificity |
| CART | TNT, Cr, Na, LDH | 70.66% ± 2.37% | 65.63% ± 7.93% | 74.34% ± 4.26% |
| RF | TNT, Cr, Lymph, Hcrit, Na, LDH | 72.07% ± 2.79% | 66.27% ± 4.76% | 77.32% ± 3.98% |
| NN | TNT, Cr, Lymph, LDH, Na, Hcrit | 75.73% ± 3.61% | 67.96% ± 3.28% | 75.99% ± 4.57% |
| Logistic | Cr, Lymph | 63.73% ± 3.30% | 52.09% ± 6.61% | 72.72% ± 6.47 % |
| (b) Prediction of recovery using data at ACI onset | ||||
|---|---|---|---|---|
| Models | Top predictors at ACI onset | Accuracy | Sensitivity | Specificity |
| CART | DD, Cr, LDH | 69.04% ± 2.36% | 59.88% ± 5.24% | 76.19% ± 6.50% |
| RF | LDH, DD, TNT, CRP, Cr, Hcrit | 71.41% ± 4.18% | 61.73% ± 7.01% | 80.02% ± 4.63% |
| NN | TNT, LDH, Cr, DD, CRP | 74.79% ± 2.52% | 64.65% ± 5.85% | 74.51% ± 8.05% |
| Logistic | Cr, CRP | 61.19% ± 4.16% | 55.18% ± 8.21% | 66.70% ± 7.99% |
Temporal progression of top predictors
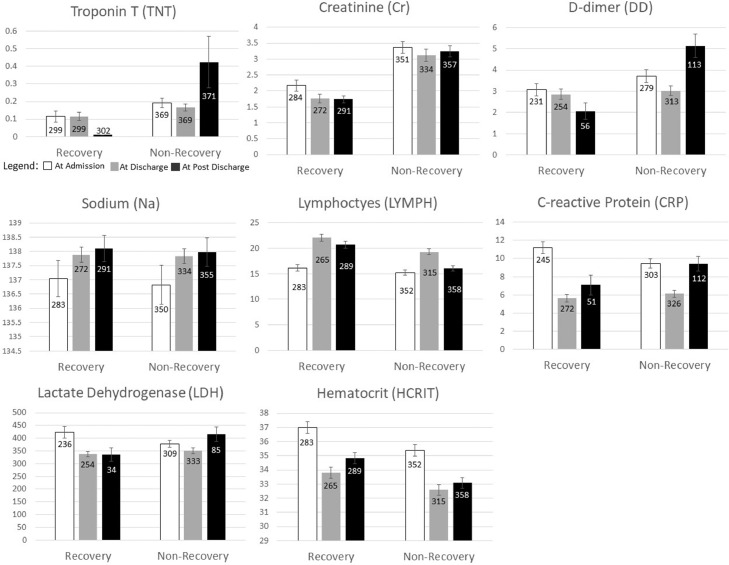
Figure 4 shows the time courses of the top predictors at ACI onset, hospital discharge, and 2.5 months post discharge separated by recovery and non-recovery status. In the recovery group, TNT, Cr, and D-dimer were generally low (less severe) at ACI onset and generally improved at discharge and 2.5 months post discharge, in marked contrast to the non-recovery group which showed these markers to be generally high at ACI onset and worsened or did not improve at discharge and 2.5 months post discharge. Sodium in both groups increased with time in both groups. Lymphocyte counts improved at later time points and were also higher (less severe) in the recovery group at discharge and 2.5 months after discharge, in contrast to the non-recovery group. CRP and LDH were significantly lowered with time in the recovery group although they were higher at ACI onset, in contrast to the non-recovery group which did not decrease with time.
Figure 4.
Bar charts of top predictors of ACI at ACI diagnosis, hospital discharge and 2.5 months post discharge. Sample sizes are shown for each group with error bars as standard errors. This figure highlights some top predictors in graphical forms with statistical tests for these variables provided in Table 1.
Discussion
This study investigated the incidence of persistent acute cardiac injury following COVID-19 and characterized the clinical variables that contributed to persistent cardiac injury in COVID-19 patients 2.5 months after hospital discharge. A significant number of COVID-19 patients developed ACI, typically on or within a day after admission. The risk of in-hospital mortality for ACI patients was 4.45 compared to non-ACI patients. Of the ACI survivors who returned to our hospital, only 44.2% fully recovered from ACI. There were no significant differences in demographics, race, ethnicity, major commodities, and length of hospital stay between groups. Prediction of ACI recovery at 2.5 months post-discharge using hospital discharge data were more accurate and more consistent across models than that using data at ACI diagnosis. Prediction of ACI recovery at 2.5 months post-discharge using the top predictors (troponin, creatinine, lymphocyte, sodium, lactate dehydrogenase, lymphocytes and hematocrit) at hospital discharge yielded 63.73% to 75.73% accuracy across four predictive models.
ACI incidence and onset: Our ACI incidence rate was 39.7% among hospitalized COVID-19 patients in the Montefiore Health System. Two meta-analysis reviews of COVID-19 data mostly from China reported ACI incidence of 16.1–23.8%10 and 21.2%.12 A study from the United States reported ACI incidence of 23%.11 The higher incidence rate in our cohort could be due to differences in race, ethnicity, socioeconomic status, among others. Our cohort in particular consisted of a large population of African American and Hispanic patients, and many were of lower socioeconomic status. Our adjusted mortality odds ratio was 4.45 among hospitalized COVID-19 patients in the Bronx environs, consistent with a previous study that found an adjusted OR to be 6.93 in COVID-19 patients with ACI.25 Although the incidence and mortality rates among COVID-19 patients with ACI are generally higher than the general COVID-19 population without ACI1, 2, 3,6,7 comparison studies controlling for race, ethnicity, among others are needed.
The majority of COVID-19 patients developed ACI very early on. There are no prior studies with which to compare. Heart muscle has a high density of ACE2 receptors through which SARS-CoV-2 virus enters cells 8. ACI could result from the primary effects of SARS-CoV-2. In contrast, other complications of COVID-19, such as acute kidney injury,26,27 and acute liver injury28 occur later in the clinical disease course, which could more likely arise from secondary effects of COVID-19 (i.e., systemic hypoxia, acute respiratory distress syndrome, hypotension, shock, sepsis, and host-immune responses) and/or therapeutic treatments of COVID-19.29,30 These secondary effects of COVID-19 could also contribute to ACI and its recovery.31, 32, 33
Laboratory variables: This study was enabled by the large number of patients (38.7%) in our catchment area returning to our health system for follow-up care. Of those who returned, only 44.2% of patients recovered from ACI, suggesting myocarditic process lingers at least 2.5 months on average among hospitalized COVID-19 survivors, although it is possible that this cohort is more likely to have post-acute sequela than the general COVID-19 survivors.
We found no differences in age, race, ethnicity, major comorbidities, and length of hospitalization with respect to ACI recovery status. With few exceptions (TNT, BNP, Cr, and Hcrit), most of the laboratory variables were also not statistically different between groups at diagnosis and at hospital discharge. However, many variables were markedly different between groups at 2.5 months post discharge, with the recovery cohort generally showing lower levels of many COVID-19 related laboratory values. Most of these variables have been associated with worse COVID-19 outcomes.34,35 Notably, the recovery group had lower TNT at ACI diagnosis and showed marked improvement in TNT at 2.5 months after discharge, in marked contrast to the non-recovery group. The differences between the two time points showed that the non-recovery group had increased TNT level, whereas the recovery group had either no worsening or reduced TNT level. Non-recovery patients had lower immunologic response but more cardiorenal involvement. It is not unexpected that chronic kidney disease and creatinine were lower in the recovery group because TNT is renally cleared, and thus the lack of ACI recovery is likely a marker of concomitant renal injury.36 These findings suggest early clinical variables can be used as predictors of ACI recovery status. The temporal progressions of laboratory variables further supported the notion that these laboratory variables could be predictive of ACI recovery.
Predictive models: It is challenging to interpret the large arrays of clinical data and thus we built predictive models to identify top predictors of ACI recovery. Using the discharge data, different predictive models of ACI recovery consistently identified the top predictors to be TNT, creatinine, lymphocyte, sodium, and LDH. Troponin T is known to be associated with worse cardiovascular outcomes and a predictor of newly developed heart failure, coronary heart disease, and general risk for worse cardiovascular outcomes.37, 38, 39 Note that elevated TNT is a marker of acute cardiac injury, not of chronic heart disease. Although it is used as a definition of ACI, TNT levels at earlier time points could be used to predict outcome at 2.5 months post discharge and thus the inclusion of TNT in the model is not circular. Creatinine, a marker of acute kidney injury, has been associated with severe COVID-19.40,41 Reduced lymphocyte count, a marker of altered immunological response, has been associated with COVID-19 disease severity,34,35 and thus it is not surprising that lymphocyte count is predictive of recovery status. Elevated or imbalance serum sodium has been associated with cardiac dysfunction, and thus it is not surprising that sodium level is predictive of recovery status. Elevated LDH (a marker for cell death) has been associated with COVID-19 disease severity. It is striking that none of the comorbidities and vital signs were among the highly ranked predictors. Potassium, a potential marker of cardiac dysfunction, was also not a top predictor of ACI recovery at 2.5 months post discharge. BNP was also not among the top predictors of ACI recovery. BNP (∼10% of our cohort had BNP data) was not routinely measured in our and most other hospitals and was measured only when clinically indicated. The missing data likely resulted in BNP not being highly ranked among the top predictors in the context of other variables.
Using the ACI onset data for prediction, the top predictors were similar but were less consistent across different models. Top predictors obtained using data at ACI onset and at discharge were also similar, but the ranking of top predictors differed slightly. The top predictors at ACI onset, consisted of cell death inflammatory response (LDH and DD), whereas the top predictors at discharge were of cardiac and kidney injury (TNT and creatinine). This is not unexpected as later changes (at discharge) were more likely associated with persistent cardiac injury whereas early changes (at ACI) were likely to be markers of COVID-19 severity. The overall prediction accuracy using the discharge data was higher than that of using data at ACI diagnosis. This is not unexpected because treatments during hospitalization could have modulated outcomes, including ACI recovery status. Taken together, these findings suggest that commonly available laboratory tests can be used to reliably predict ACI recovery at 2.5 months post discharge.
Consistency across predictive models provide assurance that these are likely reliable predictors. Moreover, machine learning models ranking predictors in the order of importance could have clinical value. The CART model also provided thresholds of the top clinical variable predictors that could be used to triage patients and make clinical decisions. We found more sophisticated machine learning methods generally performed better than logistic regression. Analysis of time courses showed that top predictors in the recovery cohort showed less severe disease than the non-recovery cohort at ACI onset and they generally improved at discharge and 2.5 months post discharge, supporting the notion that machine learning prediction of persistent cardiac injury. To our knowledge, there are no similar studies predicting ACI recovery in COVID-19 patients with which to compare.
Finally, we note that the longer-term outcome of COVID-19 patients with ACI and who are at risk of developing long-term cardiac injury sequela are unknown. Although there are no current clinical trials of cardiovascular disease drugs or proven medications to treat COVID-19 patients with ACI at this time, early identification of at-risk patients of developing persistent cardiac injury could enable more diligent follow-up care to minimize long-term cardiovascular complications, which could include preventative care and existing treatments. These findings could also inform clinical drug trial designs targeting cardiac injury sequela for COVID-19 patients.
Limitations
This study has several limitations. ACI defined by troponin is not 100% specific as there could be other causes of elevated troponin. We did not analyze other cardiovascular measurements such as EKGs, echocardiograms and MRI, nor analyze other cardiovascular complications such as heart failure, which would require individual chart reviews of a large patient cohort. We could not rule out that persistent abnormal troponin was not caused by poor renal clearance because reduced eGFR could result in elevated troponin.42 It is also possible that some patients returned at 2.5 months had a new event causing new ACI rather than residual injury associated with COVID-19. Our follow up was only 2.5 months post discharge and we did not follow patients who did not return to our health system. We used only a single method to inputate missing data because the relatively small percentage of missing data. Multiple imputation methods could be explored. It is also of interest to investigate functional status43,44 of patients with ACI at discharge. As with any retrospective study, there could be unintended patient selection bias and unaccounted confounds. Longer follow up and prospective studies are warranted. Future studies should investigate whether initiation of cardiovascular preventive therapies early on could mitigate future risks of cardiac complications in patients with COVID-19.
Conclusions
ACI is a common complication among hospitalized COVID-19 patients and many survivors exhibit persistent cardiac injury. Predictive models using a few readily available laboratory variables accurately predict ACI recovery status at 2.5 months post COVID-19. Our study has potential clinical implications because it suggests that ACI is an important marker of future adverse outcomes in COVID-19 and heightened awareness for cardiovascular complications is warranted when ACI is detected as cardiovascular prevention may have a lower priority in the context of treating SARS-CoV-2 infection. The ability to identify patients at-risk of persistent ACI early on would enable appropriate follow-up care to prevent long-term cardiac damage and other cardiovascular complications.
Declaration of interests
The authors declared no competing interests.
Acknowledgments
Contributors
J.Q. Lu – concept and design, collected data, analyzed data, created tables and figures, drafted paper
J.Y. Lu – concept and design, collected data, verified data, drafted paper
W. Wang, Y. Liu, and W. Zhu – concept and design, analyzed data
A. Buczek, R. Fleysher, W.S. Hoogenboom, W. Hou – concept and design, collected data, edited paper
C.J. Rodriguez – concept and design, edited paper
T.Q. Duong – concept and design, supervised, verified data, edited paper
All authors read and approved the final version of the manuscript.
Acknowledgements
None
Data sharing section
Data used is available from the corresponding author upon reasonable request.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo T, Fan Y, Chen M, et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippi G, Lavie CJ. Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–391. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bavishi C, Bonow RO, Trivedi V, Abbott JD, Messerli FH, Bhatt DL. Special Article - Acute myocardial injury in patients hospitalized with COVID-19 infection: A review. Prog Cardiovasc Dis. 2020;63(5):682–689. doi: 10.1016/j.pcad.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi S, Qin M, Cai Y, et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41(22):2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi S, Qin M, Shen B, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lala A, Johnson KW, Januzzi JL, et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J Am Coll Cardiol. 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasitlumkum N, Chokesuwattanaskul R, Thongprayoon C, Bathini T, Vallabhajosyula S, Cheungpasitporn W. Incidence of Myocardial Injury in COVID-19-Infected Patients: A Systematic Review and Meta-Analysis. Diseases. 2020;8(4):40. doi: 10.3390/diseases8040040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao YH, Zhao L, Yang XC, Wang P. Cardiovascular complications of SARS-CoV-2 infection (COVID-19): a systematic review and meta-analysis. Rev Cardiovasc Med. 2021;22(1):159–165. doi: 10.31083/j.rcm.2021.01.238. [DOI] [PubMed] [Google Scholar]
- 13.Stein SR, Ramelli SC, Grazioli A, et al. SARS-CoV-2 infection and persistence throughout the human body and brain. Nat Portfolio. 2021 doi: 10.1038/s41586-022-05542-y. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wadhera RK, Wadhera P, Gaba P, et al. Variation in COVID-19 Hospitalizations and Deaths Across New York City Boroughs. JAMA. 2020;323(21):2192–2195. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoogenboom WS, Pham A, Anand H, et al. Clinical characteristics of the first and second COVID-19 waves in the Bronx, New York: A retrospective cohort study. Lancet Reg Health Am. 2021;3 doi: 10.1016/j.lana.2021.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoogenboom WS, Fleysher R, Soby S, et al. Individuals with sickle cell disease and sickle cell trait demonstrate no increase in mortality or critical illness from COVID-19 - A fifteen hospital observational study in the Bronx, New York. Haematologica. 2021;106(11):3014–3016. doi: 10.3324/haematol.2021.279222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hripcsak G, Duke JD, Shah NH, et al. Observational Health Data Sciences and Informatics (OHDSI): Opportunities for Observational Researchers. Stud Health Technol Inform. 2015;216:574–578. [PMC free article] [PubMed] [Google Scholar]
- 18.Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361(9):858–867. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 19.Calvo-Fernandez A, Izquierdo A, Subirana I, et al. Markers of myocardial injury in the prediction of short-term COVID-19 prognosis. Rev Esp Cardiol (Engl Ed) 2021;74(7):576–583. doi: 10.1016/j.rec.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018) Circulation. 2018;138(20):e618–ee51. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 21.Saadati M, Bagheri A. Analysing First Birth Interval by A CART Survival Tree. Int J Fertil Steril. 2020;14(3):247–255. doi: 10.22074/ijfs.2020.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Therneau TM, Atkinson EJ. Mayo Foundation; Rochester, Minn: 1997. An Introduction to Recursive Partitioning Using the RPART Routines. [Google Scholar]
- 23.Livingston F. Implementation of Breiman's Random Forest Machine Learning Algorithm. ECE591Q Machine Learning Journal Paper. 2005 [Google Scholar]
- 24.FaF Günther, NeuralNet S. Training of Neural Networks. The R Journal. 2010;2:30–38. [Google Scholar]
- 25.Ni W, Yang X, Liu J, et al. Acute Myocardial Injury at Hospital Admission Is Associated With All-Cause Mortality in COVID-19. J Am Coll Cardiol. 2020;76(1):124–125. doi: 10.1016/j.jacc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu JY, Hou W, Duong TQ. Longitudinal prediction of hospital-acquired acute kidney injury in COVID-19: a two-center study. Infection. 2021 doi: 10.1007/s15010-021-01646-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu JY, Babatsikos I, Fisher MC, Hou W, Duong TQ. Longitudinal Clinical Profiles of Hospital vs. Community-Acquired Acute Kidney Injury in COVID-19. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.647023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu JY, Anand H, Frager SZ, Hou W, Duong TQ. Longitudinal progression of clinical variables associated with graded liver injury in COVID-19 patients. Hepatol Int. 2021 doi: 10.1007/s12072-021-10228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenz G, Moog P, Bachmann Q, P La Rosee, Schneider H, Schlegl M, et al. Title: Cytokine release syndrome is not usually caused by secondary hemophagocytic lymphohistiocytosis in a cohort of 19 critically ill COVID-19 patients. Sci Rep. 2020;10(1):18277. doi: 10.1038/s41598-020-75260-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo H, Sheng Y, Li W, et al. Coagulopathy as a Prodrome of Cytokine Storm in COVID-19-Infected Patients. Front Med (Lausanne) 2020;7 doi: 10.3389/fmed.2020.572989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oudit GY, Kassiri Z, Jiang C, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39(7):618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu JS, Ge P, Jiang C, et al. Deep-learning artificial intelligence analysis of clinical variables predicts mortality in COVID-19 patients. J Am Coll Emerg Physicians Open. 2020;1:1364–1373. doi: 10.1002/emp2.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen A, Zhao Z, Hou W, Singer AJ, Li H, Duong TQ. Time-to-Death Longitudinal Characterization of Clinical Variables and Longitudinal Prediction of Mortality in COVID-19 Patients: A Two-Center Study. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.661940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chesnaye NC, Szummer K, Barany P, et al. Association Between Renal Function and Troponin T Over Time in Stable Chronic Kidney Disease Patients. J Am Heart Assoc. 2019;8(21) doi: 10.1161/JAHA.119.013091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seliger SL, Hong SN, Christenson RH, et al. High-Sensitive Cardiac Troponin T as an Early Biochemical Signature for Clinical and Subclinical Heart Failure: MESA (Multi-Ethnic Study of Atherosclerosis) Circulation. 2017;135(16):1494–1505. doi: 10.1161/CIRCULATIONAHA.116.025505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen K, Fan W, Bertoni A, Budoff MJ, Defilippi C, Lombardo D, et al. N-terminal Pro B-type Natriuretic Peptide and High-sensitivity Cardiac Troponin as Markers for Heart Failure and Cardiovascular Disease Risks According to Glucose Status (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2020;125(8):1194–1201. doi: 10.1016/j.amjcard.2020.01.025. [DOI] [PubMed] [Google Scholar]
- 39.Daniels LB, Clopton P, deFilippi CR, et al. Serial measurement of N-terminal pro-B-type natriuretic peptide and cardiac troponin T for cardiovascular disease risk assessment in the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2015;170(6):1170–1183. doi: 10.1016/j.ahj.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelayo J, Lo KB, Bhargav R, et al. Clinical Characteristics and Outcomes of Community- and Hospital-Acquired Acute Kidney Injury with COVID-19 in a US Inner City Hospital System. Cardiorenal Med. 2020;10(4):223–231. doi: 10.1159/000509182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu JY, Buczek A, Fleysher R, et al. Outcomes of hospitalized patients with COVID-19 with acute kidney injury and acute cardiac injury frontier in cardiovascular medicine 2021, in press. [DOI] [PMC free article] [PubMed]
- 43.Musheyev B, Borg L, Janowicz R, et al. Functional status of mechanically ventilated COVID-19 survivors at ICU and hospital discharge. J Intensive Care. 2021;9(1):31. [DOI] [PMC free article] [PubMed]
- 44.Musheyev B, Janowicz R, Borg L, et al. Characterizing non-critically ill COVID-19 survivors with and without in-hospital rehabilitation. Sci Rep. 2021;11(1):21039. [DOI] [PMC free article] [PubMed]