Abstract
Aim:
The National Institute on Aging and the Alzheimer’s Association published new research criteria defining the Alzheimer’s continuum (AC) by the presence of positive amyloid-β biomarkers. Symptom severity of those on the AC is staged across six levels, including two preclinical stages (stages 1 and 2). AC stage 2 is defined by the presence of at least one of the following: (i) transitional cognitive decline; (ii) subjective cognitive decline; or (iii) neurobehavioural symptoms. In contrast, AC stage 1 is defined by the absence of symptoms.
Methods:
Initial empirical definitions for each symptom class were developed. These empirical criteria were then applied in a sample of 285 cognitively normal, amyloid-positive individuals from the Alzheimer’s Disease Neuroimaging Initiative for purposes of AC stage 1 and 2 classification.
Results:
In this sample, 56.10% of participants were asymptomatic and classified as AC stage 1. In contrast, 42.46% of individuals were positive for at least one symptom class: 22.11% for transitional cognitive decline, 20.35% for subjective cognitive decline, and 14.74% for neurobehavioural symptoms. AC stage was a predictor of cognitive/functional decline over 4 years of follow up in a longitudinal growth model (B = 0.33, P < 0.001).
Conclusions:
Results provide a methodology to operationalize the National Institute on Aging and the Alzheimer’s Association AC stage 1 and 2 criteria and include preliminary evidence of the validity of this approach. The methods outlined in this manuscript can be used to test hypotheses regarding prodromal Alzheimer’s disease, as well as implemented in clinical trial selection procedures.
Keywords: Alzheimer’s disease, preclinical, prodromal, staging, subjective cognitive decline, transitional cognitive decline
INTRODUCTION
The Alzheimer’s continuum
The 2018 National Institute on Aging and Alzheimer’s Association research framework introduced an Alzheimer’s continuum (AC). Under the framework, individuals are placed on the AC if they are positive for at least one biomarker suggestive of cerebral amyloid-β pathology. Individuals on the AC are then classified by severity of clinical symptoms across six stages. Stages 3–6 map onto current conceptualizations of mild cognitive impairment and mild, moderate, and severe dementia, with increasing levels of cognitive impairment and dependence in day-to-day tasks as the stage increases.1–3 Stages 1 and 2 are preclinical stages, which further refine the notion of a prodromal period of Alzheimer’s disease.4 These preclinical stages are not as well operationalized to date.
Consolidating across many different prior studies characterizing pre-dementia stages of Alzheimer’s disease,5 the differentiation between AC stage 1 and AC stage 2 considers three symptom classes: (i) transitional cognitive decline (TCD), marked by subtly reduced but unimpaired performance on objective cognitive tests; (ii) subjective cognitive decline (SCD), defined by self- or informant-reported reductions in cognitive abilities; and (iii) neurobehavioural symptoms (NBS), such as depression, anxiety, and apathy. In contrast, AC stage 1 applies when there is an absence of these symptoms. A nascent but growing body of research has emerged to better understand and define each of these symptom classes in preclinical samples.
Transitional cognitive decline
The concept of TCD arose in response to research that suggested that subtle cognitive changes not rising to the level of frank impairments can be detected as many as 18 years before the development of dementia.6 Currently, there are several promising methods for operationalizing TCD. For example, methods using data from serial assessments revealed that some individuals demonstrate small but statistically significant and clinically meaningful decreases in cognitive performance over time.7–9 Importantly, those who demonstrate such preclinical cognitive changes are at risk for future mild cognitive impairment or dementia diagnoses. In clinical trials and medical settings, however, an established testing history in participants is often lacking. In such situations, researchers have taken to applying demographically adjusted normative data to ascertain whether an individual demonstrates subtly lowered scores, compared to what might be expected, given the individual’s background.10–13 Thus, although there are several available methods to index TCD with preliminary validation, further research is necessary for a consensus to be reached on how best to operationalize TCD.
Subjective cognitive decline
For several years, self- or observer-reported cognitive concerns have served as a diagnostic criterion for mild cognitive impairment due to Alzheimer’s disease3; however, under the new research framework, the presence of SCD in isolation is enough to justify a stage 2 diagnosis.14 Subjective concerns expressed by participants and family members have been demonstrated to predict amyloid-β accumulation and are related to symptom progression over time among initially healthy individuals.15,16 Currently, there are guidelines from the Subjective Cognitive Decline Initiative Working Group on SCD in preclinical Alzheimer’s disease17; these outline important factors to consider when gathering data on SCD, such as age of onset, reporter identity (e.g. self vs caregiver vs provider), and domain of decline (e.g. memory, language). However, these guidelines fall short of providing a clear empirical definition of SCD and admit that ‘current knowledge is insufficient to comprehensively define the specific features of SCD in preclinical [Alzheimer’s disease]’.17 Given that there are well-validated psychometric measures of SCD, such as the Everyday Cognition Scale,18 it is be possible to take a similar approach to defining SCD as researchers have used to define TCD. This process would involve defining SCD positivity by a demographically adjusted score on the Everyday Cognition Scale at or above a chosen cut-off point for abnormality. Such an approach has yet to be tested scientifically, however.
Neurobehavioural symptoms
Neurobehavioural symptoms in Alzheimer’s disease include mental health symptoms and/or personality changes, such as depression, anxiety, and apathy.14 They are distinguished from their more prototypical manifestations in primary psychiatric disorders in that NBS are thought to represent phenotypes of the neurodegenerative process. Therefore, NBS in Alzheimer’s are typically de novo symptoms or exacerbations of existing symptoms not better accounted for by normal ageing or psychosocial factors.14 The presence of NBS among individuals with mild cognitive impairment is a well-documented risk factor for subsequent functional declines.19,20 Importantly, this work is beginning to be replicated in preclinical samples as well. For instance, Johansson et al. reported increased amyloid-β deposition and cognitive decline over time among those with NBS compared to those without such symptoms in a sample composed primarily of individuals in the preclinical stage.21 Given that past criteria for dementia and mild cognitive impairment due to Alzheimer’s either did not refer at all to NBS or mentioned them only obliquely,3,22 further study is needed to integrate NBS into the new diagnostic framework for the AC.
Integrating and validating symptom classes for staging preclinical Alzheimer’s
Prior studies have put forth AC staging criteria based solely on TCD or SCD.23–25 However, the three symptom classes (TCD, SCD, and NBS) have yet to incorporated into the more comprehensive operationalization system for staging AC stages 1 and 2 proffered by the framework by Jack et al.14 This goal is critical for the advancement of clinical trial research in the Alzheimer’s field for two reasons. First, as noted in recent US Food and Drug Administration guidance, ‘Treatment directed at [presymptomatic intervention] must begin before there are overt clinical symptoms’.26 Therefore, clear differentiation of AC stages 1 and 2 is necessary for accurate participant recruitment. Second, preclinical operationalization is needed to ascertain symptom progression in clinical trials—that is, to assess who converts from an asymptomatic (i.e. stage 1) to a symptomatic (i.e. stage 2) stage of disease.
Goals of the current study
In support of such clinical trial research, the first goal of the current manuscript was to develop an empirical method for defining AC stages 1 and 2 using all three symptom classes outlined by Jack et al.14 Data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) were used to achieve this purpose. This database was chosen because it includes a well-characterized sample with the relevant biomarker and clinical data necessary to define the early stages of the AC.
Once operationalized, the criteria need to be validated.14 Current models of the AC predict a more rapid clinical progression as individuals move closer to mild cognitive impairment from the preclinical stages.27 Thus, under the new Alzheimer’s disease research framework, individuals in stage 2 would be hypothesized to have more rapid declines in cognition or functioning over time than those in stage 1. The second goal of the current research was to test this hypothesis using follow-up data from ADNI, providing initial validation of the proposed AC staging procedures.
METHODS
Sample
The sample was drawn from publicly available data from ADNI and obtained from the online database at adni.loni.usc.edu in April 2020. ADNI is a public–private partnership led by Michael Weiner principal investigator (PI). Its goal is to test whether biological markers and clinical information can be combined to measure the progression of mild cognitive impairment and Alzheimer’s disease.
Because this project focused on examining individuals in the preclinical stages of cognitive decline, the sample was limited to individuals coded as cognitively unimpaired at baseline (n = 589). Next, participants were excluded if they had neuropsychological testing evidence of mild cognitive impairment at their baseline evaluation, per published Jak/Bondi criteria.11,28 Therefore, anyone rated clinically or empirically as having mild cognitive impairment or dementia was removed to ensure an unambiguously cognitively unimpaired group (n = 507). Finally, individuals were selected for AC staging if they were amyloid-positive based on an amyloid positron emission tomography scan. (Amyloid positivity is a requirement for inclusion on the AC under the National Institute on Aging and Alzheimer’s Association Research Framework.)14 Amyloid status of the sample was categorized in keeping with conventions established for ADNI.11,29,30 Specifically, a summary florbetapir cortical standard uptake value ratio, with the cerebellum as the reference region, was used; a cut-off of 1.11 was used to define amyloid positivity. There were 285 individuals in the final sample used for analyses (Fig. 1). The sample was 61.10% female, and the sample’s mean ± SD age was 72.32 ± 6.33 years and its mean education level was 16.79 ± 2.49 years. Participants self-reported ethnicity as non-Hispanic (94.70%), Hispanic/Latino (4.20%), and unknown (0.70%). The sample was primarily White (88.40%), with smaller percentages identifying as Black/African (7.40%), Asian (2.10%), Native American/Alaska Native (0.40%), more than one race (1.10%), and unknown (0.40%).
Figure 1.
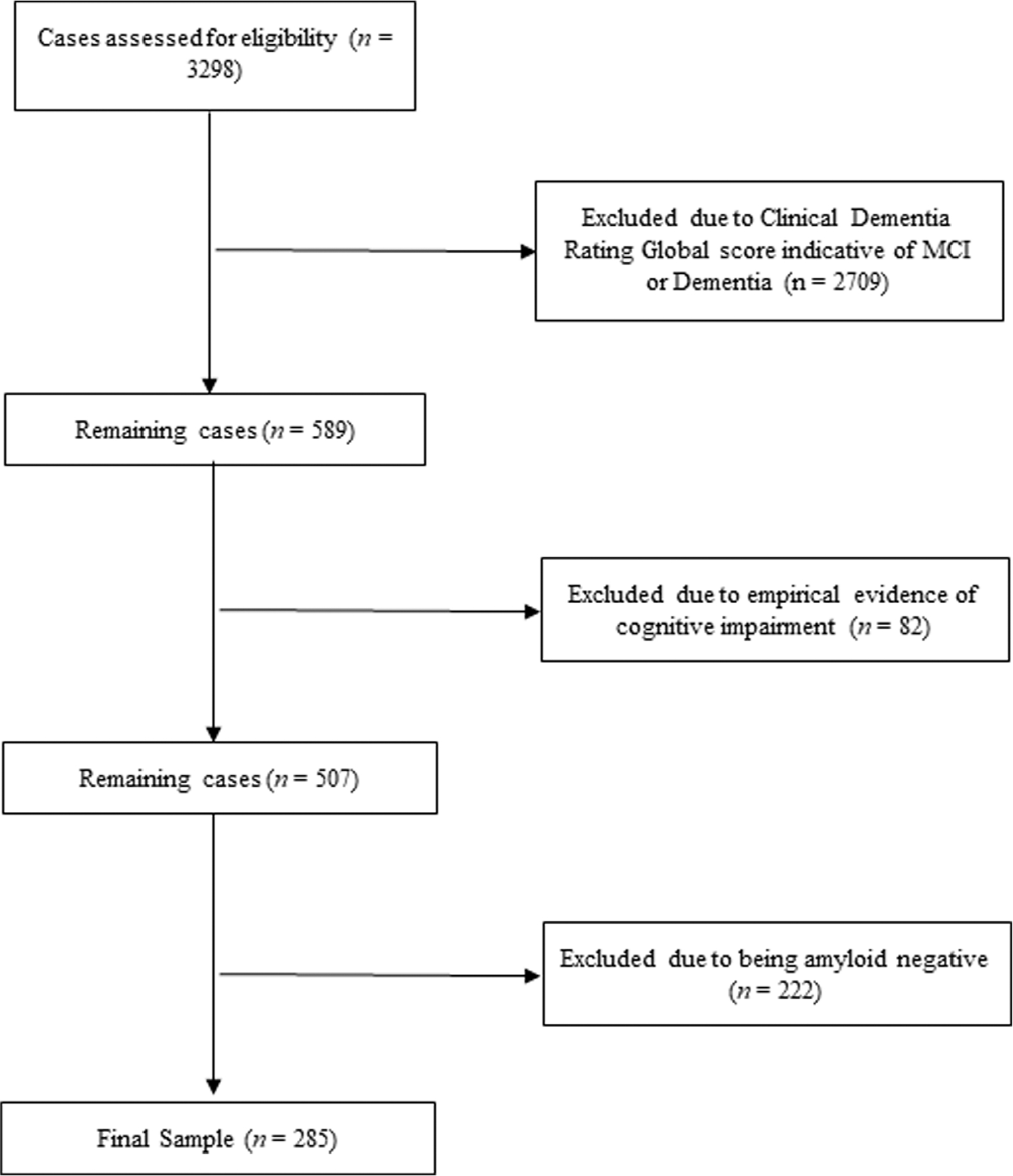
Flow chart for sample selection. MCI, mild cognitive impairment.
Operationalizing the three symptom classes for stages 1 and 2
The distinction between AC stages 1 and 2 is made on the basis of three symptom classes: TCD, SCD, and NBS.14 These symptom classes were operationalized as described below (also see Table 1).
Table 1.
Operational criteria for Alzheimer’s continuum (AC) stages 1 and 2†
| Symptom Class | AC Stage 1 | AC Stage 2 |
|---|---|---|
|
| ||
| Transitional cognitive decline (TCD) | Criteria for absence of TCD included: 1 No more than one low‡ score in a given cognitive domain§ 2 No more than one low process score¶ 3 May have one low process score or one low total test score but not both |
Positivity for TCD was defined by the presence of at least one of the following: 1 One low‡ cognitive test score in two different cognitive domains§ 2 Two low process scores¶ derived from the list learning measure 3 One low process score and one low total test score |
| Subjective cognitive decline | Everyday Cognition Scale demographically adjusted mean score ≤1 SD above the demographically adjusted mean | Positivity defined by self-reported symptoms on the Everyday Cognition Scale >1 SD above the demographically adjusted mean for participant or study partner report |
| Neurobehavioural symptoms (NBS) | Criteria for absence of NBS included: 1 No study partner-reported depression, anxiety, or apathy on the Neuropsychiatric Inventory 2 Self-reported score <5 on the Geriatric Depression Scale |
Positivity for NBS was defined by the presence of at least one of the following: 1 Study partner-reported depression, anxiety, or apathy on the Neuropsychiatric Inventory 2 Self-reported score ≥5 on the Geriatric Depression Scale |
AC stage 2 was defined by positivity on at least one symptom class, whereas AC stage 1 was defined by the absence of positivity on any symptom class.
Low scores were defined as those falling >1 SD below the demographically adjusted mean score for a robust normative group.
Cognitive domains included language, memory, and attention/executive functioning.
Process scores included indexes of learning slope, retroactive interference, and intrusion errors.
Transitional cognitive decline
To define TCD, procedures previously established in ADNI were followed.11,31 Specifically, data from a group of 365 robustly cognitively unimpaired individuals from ADNI were used to establish norms for neuropsychological measures. All individuals in the normative sample had at least 1 year of follow-up data available and were diagnosed as cognitively unimpaired across all available study visits. For each cognitive variable, regression-based Z-scores were calculated with adjustments for age, sex, and educational attainment to establish normative cut-offs (see Table S1 for results of regressions).
Of note, it was recognized that an alternative approach would be to limit the sample to cognitively normal, amyloid-negative individuals. Therefore, the regression analyses were repeated to determine if limiting the sample in such a manner would meaningfully change regression coefficients for creating demographically adjusted Z-scores. Regression coefficients did not significantly differ when the sample was limited to amyloid-negative individuals (Table S1).
Transition cognitive decline was defined by using criteria published Thomas et al.11,12 Individuals were coded as positive for TCD if they met one of the following criteria: (i) one low test score (i.e. > 1 SD below the mean of demographically adjusted normative data) in two different cognitive domains (e.g. language, memory, attention/executive functioning); (ii) two low process scores (i.e., learning slope, retroactive interference, intrusion errors) derived from the list learning measure; or (iii) one low process score and one low total test score. Learning slope consists of the Trial 5 score – Trial 1 scoreand indexes rate of learning over the five immediate recall trials. Retro-activeinterference consists of Trial 6 score – Trial 5 score and provides a measureof the impact of an intervening word list on recall of a previously learnedword list. Finally, intrusion errors are calculated as a sum of all non-listword answers provided during recall. This score indexes error pronenessduring recall.
Tests in ADNI include three domains of cognitive functioning. The first is language, which is measured by the 30-item Boston Naming Test or Multilingual Naming Test and animal fluency.32,33 The second is memory, as assessed by the Auditory-Verbal Learning Test34; process scores are also derived from this measure. The last domain is attention/executive function, assessed by Trail Making Tests A and B.35
Subjective cognitive decline
Subjective cognitive decline is indexed in ADNI based on the Everyday Cognition Scale, a questionnaire measuring subjective cognitive difficulties.18 This scale was completed by the participant and a study partner. Individuals were coded as positive for SCD if their reported daily cognitive difficulties were 1 SD greater than the demographically adjusted mean for either the participant or study partner report, again using normative data derived from the robustly normal ADNI sample (Table S1).
Neurobehavioural symptoms (NBS)
Neurobehavioral symptoms are indexed in two main ways in ADNI. First, study partners complete a structured clinical interview, the Neuropsychiatric Inventory,36 rated by a trained clinician. Variables of interest from this measure include depression, apathy, and anxiety, which commonly occur in Alzheimer’s disease and are the three neuropsychiatric symptoms mentioned in the framework by Jack et al.14,37 Second, participants completed a self-report measure of depressive symptoms, the shortform Geriatric Depression Scale.38 Individuals were categorized as having clinically significant self-reported depression if their summed score on the Geriatric Depression Scale was ≥5.39 Participants were labelled as having an NBS if they had any clinician-rated or participant-reported NBS, replicating previous procedures.40
Categorizing AC stages 1 and 2
Cognitively unimpaired, amyloid-positive participants were coded into AC stage 2 if they were positive for any one of the TCD, SCD, or NBS symptom classes. In contrast, if participants were negative for all three categories they were placed into AC stage 1.
Initial validation of early AC stages
Clinical outcome data for amyloid-positive participants were examined for up to 4 years of follow-up in ADNI. The Clinical Dementia Rating Sum of Boxes score (CDR-SB) was used as an outcome because it is one of the most commonly used measures of global functioning in Alzheimer’s disease research and has excellent psychometric properties.41,42
Latent growth models (LGMs) were conducted for CDR-SB in RStudio (RStudio, Boston, MA, USA),43 using the Lavaan package (https://cran.r-project.org/web/packages/lavaan/index.html).44 Figure 2 provides a visual of this model. Full information maximum likelihood was used to handle missing data45,46; this method is robust to violations of the normality assumption.47 Global model fit was examined using the χ2, comparative fit index (CFI), and the root mean square error of approximation (RMSEA). Good model fit is suggested by a lower (and ideally non-significant) χ2 value, CFI values closer to 1 (>0.90 for acceptable fit and >0.95 for good fit), and RMSEA values closer to zero (<0.08 for acceptable fit and <0.05 for good fit).48,49 Because measurements across time were expected to demonstrate residual correlations, modification indexes were examined after initial models were fit to determine whether any residual correlations needed to be added to the model.50
Figure 2.
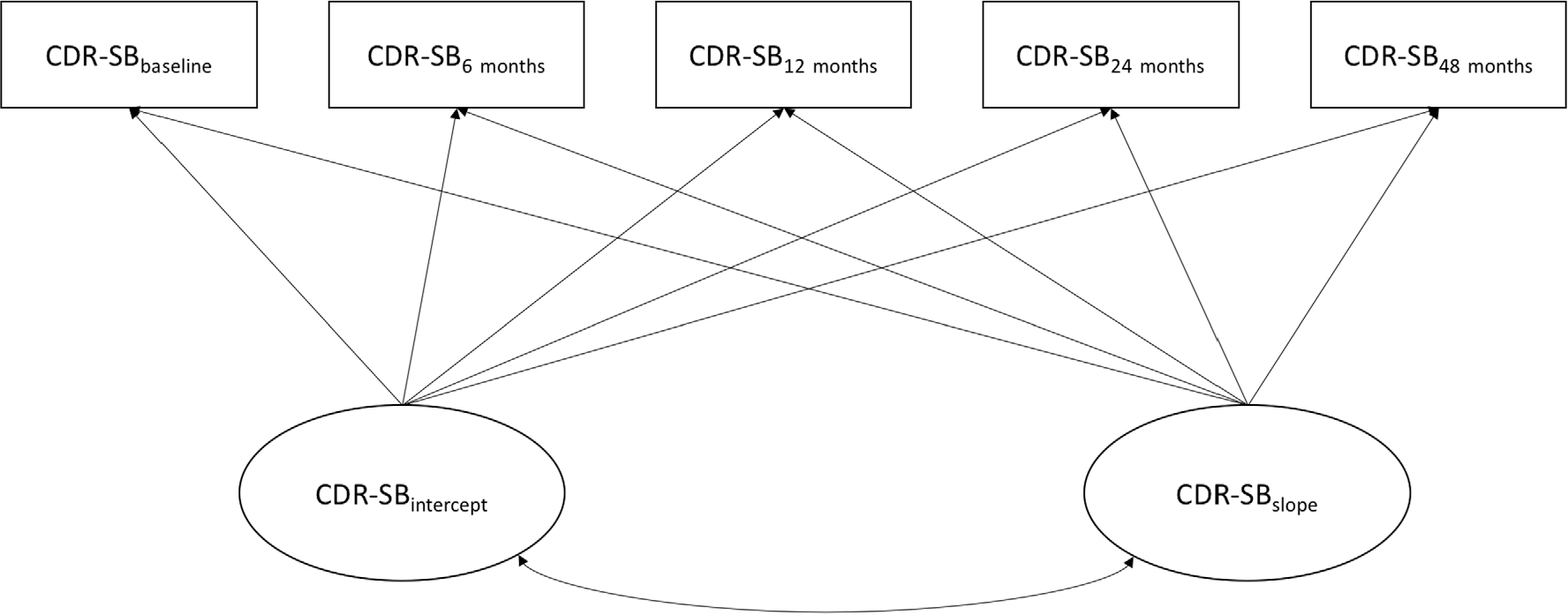
Latent growth model example for Clinical Dementia Rating Sum of Box scores (CDR-SB).
Once an adequate fitting LGM model for CDR-SB scores was established, analyses were conducted to examine whether AC stage would predict initial level (i.e. intercept) and change over time (i.e. slope) in cognitive/functional symptoms. Regression weights for AC stage as a predictor of CDR-SB intercept and slope were examined for statistical significance, and R2 was used as a measure of effect size. It was expected that AC stage would significantly predict change over time in CDR-SB scores, with individuals in AC stage 2 demonstrating more rapid declines in cognitive/functional status.
RESULTS
Transitional cognitive decline
Among the cognitively unimpaired, amyloid-positive individuals, 22.11% met the criteria for TCD. Subtle low memory scores were most common (18.20%), followed by subtle low scores in the language domain (17.50%) and executive domain (13.00%). Differences in rates of low scores across cognitive domains were not statistically significant (χ2(2) = 2.56, P = 0.278). The percentage of individuals with at least one low process score on the Audio-Verbal Learning Test was 28.10%.
Subjective cognitive decline
Among the cognitively unimpaired, amyloid-positive individuals, 20.35% met the criteria for SCD. The proportion of individuals with significantly elevated study partner-reported cognitive complaints (16.14%) was more than double the rate of self-reported cognitive complaints (7.72%) (χ2(1) = 14.74, P < 0.001).
Neurobehavioural symptoms
Among the cognitively unimpaired, amyloid-positive individuals, 14.70% were positive for NBS. Among these participants, depression was the most commonly endorsed manifestation on the Neuropsychiatric Inventory (9.10%), followed by anxiety (5.60%) and apathy (3.20%), (χ2(2) = 8.01, P = 0.018). The rate of positivity for self-reported depression (2.11%) was not significantly different from the rate of study partner-reported depression on the Neuropsychiatric Inventory (χ2(1) = 0.42, P = 0.517).
Operationalization of the early AC stages
Using the operationalization procedures, cognitively unimpaired, amyloid-positive individuals (n = 285) were classified into AC stages 1 and 2. Results of this process are presented in Figure 3. A higher proportion of asymptomatic individuals were classified as AC stage 1 (56.10%) compared with the proportion of symptomatic individuals (i.e. positive for at least one symptom) classified as AC stage 2 (42.47%) (χ2(1) = 5.41, P = 0.020). There was no significant difference in the rate of positivity across TCD (22.11%), SCD (20.35%), and NBS (14.70%) (χ2(2) = 4.28, P = 0.118).
Figure 3.
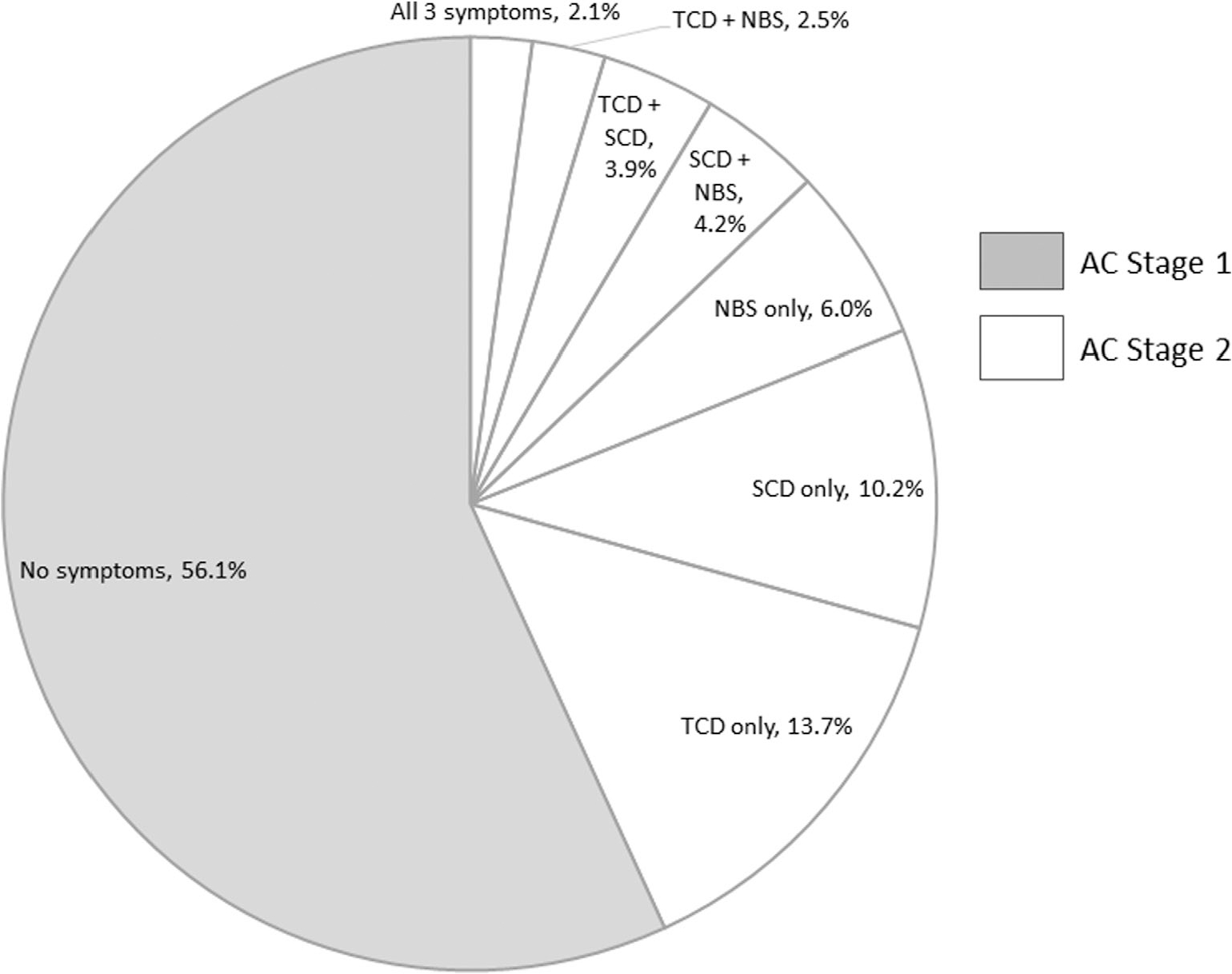
Pie chart demonstrating breakdown of symptom classes for early Alzheimer’s continuum (AC) staging. TCD, transitional cognitive decline; SCD, subjective cognitive decline; NBS, neurobehavioural symptoms.
Initial validation of the preclinical AC stages
Four years of outcome data for the cognitively unimpaired, amyloid-positive individuals were examined in ADNI. Six participants initially coded as AC stage 1 converted to mild cognitive impairment within 4 years; none converted to dementia. In contrast, among those initially coded as AC stage 2, one participant converted to dementia and ten converted to MCI. This group difference in the rate of conversion to a clinical state was not statistically significant (χ2(1) = 0.90, P = 0.342).
Descriptive statistics for CDR-SB scores over time are presented in Table 2. There was a small to moderate increase in scores over time (F2.81 = 3.46, P = 0.018, partial η2 = 0.04). LGMs were used to investigate individual differences in initial scores and change over time for this variable. The LGM for CDR-SB provided an acceptable but not excellent fit to the data initially (χ2(9) = 20.45, P = 0.015, CFI = 0.917, RMSEA = 0.067). After examination of modification indexes, residual correlations were added between the 6- and 24-month CDR-SB scores and the 12- and 24-month CDR-SB scores. This change yielded a substantial improvement in model fit (χ2(7) = 11.18, P = 0.131, CFI = 0.970, RMSEA = 0.046).
Table 2.
Sample sizes and descriptive statistics for Clinical Dementia Rating Sum of Box scores among individuals on the Alzheimer’s continuum over time
| Time point | Overall (n) | Overall, mean ± SD | AC Stage 1, mean ± SD | AC Stage 2, mean ± SD |
|---|---|---|---|---|
|
| ||||
| Baseline | 285 | 0.04 ± 0.13 | 0.03 ± 0.11 | 0.05 ± 0.15 |
| 6 months | 184 | 0.10 ± 0.25 | 0.08 ± 0.23 | 0.12 ± 0.27 |
| 12 months | 154 | 0.11 ± 0.31 | 0.06 ± 0.17 | 0.19 ± 0.44 |
| 24 months | 190 | 0.11 ± 0.28 | 0.05 ± 0.15 | 0.23 ± 0.39 |
| 48 months | 118 | 0.19 ± 0.49 | 0.14 ± 0.42 | 0.27 ± 0.57 |
When AC stage was added as a predictor to the model, fit remained good (χ2(10) = 12.88, P = 0.230, CFI = 0.981, RMSEA = 0.032). AC stage did not significantly predict CDR-SB intercept (β = 0.10, P = 0.218, R2 = 0.01), but it did significantly predict CDR-SB slope (β = 0.33, P < 0.001, R2 = 0.11). As hypothesized, those in AC stage 2 demonstrated more rapid increases in cognitive/functional problems over time with a moderate effect size.
DISCUSSION
This manuscript developed and provided preliminary validation of a method for classifying cognitively unimpaired, amyloid-positive individuals into AC stages 1 and 2 in ADNI, consistent with the National Institute on Aging and Alzheimer’s Association research framework.14
Transitional cognitive decline
Transitional cognitive decline was defined by using criteria based on work by Thomas et al.11,12 With this method, approximately 22.11% of the amyloid-positive sample was classified as positive for TCD; this is fairly similar to the 20% rate of classification reported by Thomas et al. in past research that used a larger ADNI sample, including both amyloid-positive and negative individuals.11 The Thomas et al. approach to defining TCD has now been validated in ADNI and is likely suitable for application to studies that wish to identify individuals with subtle cognitive declines that do not rise to the level of impairment. There are of course some limitations to the Thomas et al. approach worth noting, including its heavy reliance on process scores from the Auditory Verbal Learning task and its failure to index other cognitive domains, such as visuospatial skills and higher-level reasoning and problem-solving abilities. Furthermore, this method uses baseline data, and TCD could alternatively be defined by subtle declines across serial evaluations.9,14
Subjective cognitive decline
To our knowledge, this is the first study to classify positivity for SCD by using a demographically adjusted normative cut-off based on a psychometric instrument. This approach is likely preferable to simple yes/no assessments of SCD that have been employed in previous research,40,51 as psychometric instruments tend to capture multifaceted aspects of SCD,52 in addition to accounting for the influence of potentially confounding demographic factors on symptom presentations. Furthermore, using a normative approach may reduce false positives, as 71% of healthy older adults have reported at least some subjective cognitive symptoms.53 With the present method, the rate of positivity for SCD was a much more reasonable 20.35% among cognitively unimpaired, amyloid-positive individuals. In future research, the relative importance of different reporters to the AC staging process should be assessed. For instance, past research suggests that self-reports tend to be valid early in the disease process when impairment is low, whereas study partner reports may be more important in later stages when patient insight may be reduced.16
Neurobehavioural symptoms
Positivity for NBS was defined by the presence of anxiety, depression, or apathy symptoms reported by the participant or a study partner. With this approach, 14.70% of the cognitively unimpaired, amyloid-positive participants were positive for this symptom class. The prevalence of NBS in this group was unsurprisingly lower than the 35–75% prevalence rate of NBS reported in a systematic review of data for individuals with mild cognitive impairment.54 However, the rate of NBS was higher than that reported for mood and anxiety disorders of community-dwelling older adults, which range from about 6% to 12%, in epidemiological studies.55 That the rate of NBS for those on the early AC falls between the rates for older adults in the community and patients with mild cognitive impairment lends credence to the notion that neuropsychiatric changes can signal an early phase of neurodegeneration.
It must be noted that the present investigation included only depression, anxiety, and apathy, and more work is needed to determine the extent to which other neuropsychiatric symptoms (e.g. sleep disturbances, hallucinations) have relevance to the early AC staging process. Furthermore, it will be important to be more comprehensive in assessing NBS in future studies. For instance, the ADNI database includes a self-report measure of depression but no self-reports for apathy or anxiety. Finally, one must acknowledge the difficulty in ascertaining whether NBS are part of the neurodegenerative process or whether they have another cause. Detailed clinical interviews must be developed to rule out recurrence of longstanding mental health concerns, reactions to negative life events, side-effects of medications, and other potential explanations.56
AC stages 1 and 2
Stages 1 and 2 were defined by the absence or presence of TCD, SCD, and NBS. Based on objective criteria, 42.47% of individuals were classified into AC stage 2, while the remainder were classified as AC stage 1. This study is the first to provide prevalence rates of these early stages of the AC using all three symptom classes for classification. Results indicate that there is a high proportion of asymptomatic, amyloid-positive individuals. Importantly, symptomatic individuals (i.e. those classified as AC stage 2) demonstrated subtly faster rates of cognitive/functional decline, as measured by CDR-SB, over the course of 4 years of follow-up, consistent with prior research.11,16,21 These results provide support for separating preclinical individuals into asymptomatic and subtly symptomatic groups by using the methods outlined in this article. Of course, future research is necessary to replicate the present findings, particularly in samples with higher rates of subsequent clinical conversion.
Limitations
In addition, it is important to note that the ADNI sample suffers from some known disadvantages, such as a lack of ethnic diversity. This issue has been addressed in detail by other scholars,57 and work is ongoing to collect groups of participants from minority backgrounds to correct the imbalance. Similarly, participants had a university education on average, possibly limiting the generalizability of findings to individuals with less academic training. It was also beyond the scope of the current investigation to examine other outcomes in relation to the early AC stages, such as tau accumulation and neurodegeneration, which could be the focus of future research. Finally, it must be recognized that accumulating research suggests that efforts to change the course of Alzheimer’s disease may need to be initiated before the development of AC stages 1 and 2 and to focus on changing the trajectory of the disease before amyloid is widespread in the brain.58
Despite these limitations, this research represents an important step towards better understanding the early stages of the AC. This manuscript presented a specific, reproducible method for classifying individuals into AC stages 1 and 2 and demonstrated that group membership defined by this approach predicts rate of symptom progression over 4 years of follow-up. This method can be used to define the AC stages in future research aimed at testing hypotheses regarding prodromal Alzheimer’s disease. It can also be used for purposes of clinical trial selection when recruiting samples of individuals in preclinical stages.
Supplementary Material
Regressions coefficients for creating Z-scores, along with comparisons of regression weights between the entire normative sample and the sample restricted to amyloid negative participants.
ACKNOWLEDGMENTS
Funding for the preparation of this manuscript was provided by an Alzheimer’s Association Research Fellowship (2019-AARF-641693, PI Andrew Kiselica, PhD) and the 2019–2020 National Academy of Neuropsychology Clinical Research Grant (PI Andrew Kiselica, PhD).
Data collection and sharing for this project was funded by the ADNI (National Institutes of Health grant no. U01 AG024904) and DOD ADNI (Department of Defense award no W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EUROIMMUN; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research provides funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
The author thanks Dr Jared Benge for his feedback on initial drafts of the manuscript.
Alzheimer’s Association 2019-AARF-641693
National Academy of Neuropsychology 2019–2020 Clinical Research Grant
Footnotes
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Disclosure: None.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s website: http://onlinelibrary.wiley.com/doi//suppinfo.
REFERENCES
- 1.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 1997; 9: 173–176. [DOI] [PubMed] [Google Scholar]
- 2.Gauthier S, Patterson C, Chertkow H et al. Recommendations of the 4th Canadian Consensus Conference on the Diagnosis and Treatment of Dementia (CCCDTD4). Can Geriatrics J 2012; 15: 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert MS, DeKosky ST, Dickson D et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7: 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperling RA, Aisen PS, Beckett LA et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7: 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubois B, Hampel H, Feldman HH et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement 2016; 12: 292–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajan KB, Wilson RS, Weuve J, Barnes LL, Evans DA. Cognitive impairment 18 years before clinical diagnosis of Alzheimer disease dementia. Neurology 2015; 85: 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papp KV, Buckley R, Mormino E et al. Clinical meaningfulness of subtle cognitive decline on longitudinal testing in preclinical AD. Alzheimers Dement 2020; 16: 552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Small BJ, Bäckman L. Longitudinal trajectories of cognitive change in preclinical Alzheimer’s disease: a growth mixture modeling analysis. Cortex 2007; 43: 826–834. [DOI] [PubMed] [Google Scholar]
- 9.Kiselica AM, Kaser A, Webber T, Small B, Benge J. Development and preliminary validation of standardized regression-based change scores as measures of transitional cognitive decline. Arch Clin Neurospychol 2020; 35: 1168–1181. 10.1093/arclin/acaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiselica AM, Webber T, Benge J. Using multivariate base rates of low scores to understand early cognitive declines on the uniform data set 3.0 Neuropsychological Battery. Neuropsychology 2020; 34: 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas KR, Bangen KJ, Weigand AJ et al. Objective subtle cognitive difficulties predict future amyloid accumulation and neurodegeneration. Neurology 2020; 94: 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas KR, Edmonds EC, Eppig J, Salmon DP, Bondi MW. Alzheimer’s Dis Neuroimaging I. Using neuropsychological process scores to identify subtle cognitive decline and predict progression to mild cognitive impairment. J Alzheimers Dis 2018; 64: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW. Alzheimer’s Dis Neuroimaging I. Subjective cognitive complaints contribute to misdiagnosis of mild cognitive impairment. J Int Neuropsychol Soc 2014; 20: 836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jack CR, Bennett DA, Blennow K et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018; 14: 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amariglio RE, Buckley RF, Mormino EC et al. Amyloid-associated increases in longitudinal report of subjective cognitive complaints. Alzheimer’s Dementia 2018; 4: 444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan MM, Grill JD, Gillen DL, ADNI. Participant and study partner prediction and identification of cognitive impairment in preclinical Alzheimer’s disease: study partner vs. participant accuracy. Alzheimer’s Res Ther 2019; 11: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jessen F, Amariglio RE, Van Boxtel M et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 2014; 10: 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farias ST, Mungas D, Reed BR et al. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology 2008; 22: 531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dietlin S, Soto M, Kiyasova V et al. Neuropsychiatric symptoms and risk of progression to alzheimer’s disease among mild cognitive impairment subjects. J Alzheimers Dis 2019; 70: 25–34. [DOI] [PubMed] [Google Scholar]
- 20.Palmer K, Di Iulio F, Varsi AE et al. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J Alzheimers Dis 2010; 20: 175–183. [DOI] [PubMed] [Google Scholar]
- 21.Johansson M, Stomrud E, Lindberg O et al. Apathy and anxiety are early markers of Alzheimer’s disease. Neurobiol Aging 2020; 85: 74–82. [DOI] [PubMed] [Google Scholar]
- 22.McKhann GM, Knopman DS, Chertkow H et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jack CR, Knopman DS, Weigand SD et al. An operational approach to National Institute on Aging–Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol 2012; 71: 765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW. Subtle cognitive decline and biomarker staging in preclinical Alzheimer’s disease. J Alzheimers Dis 2015; 47: 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckerström M, Göthlin M, Rolstad S et al. Longitudinal evaluation of criteria for subjective cognitive decline and preclinical Alzheimer’s disease in a memory clinic sample. Alzheimer’s Dementia 2017; 8: 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Food and Drug Administration (US). Early Alzheimer’s Disease: Developing Drugs for Treatment, Guidance for Industry. Washington, DC: Department of Health and Human Services, 2018. [Google Scholar]
- 27.Aisen PS, Cummings J, Jack CR et al. On the path to 2025: understanding the Alzheimer’s disease continuum. Alzheimer’s Res Ther 2017; 9: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jak AJ, Preis SR, Beiser AS et al. Neuropsychological criteria for mild cognitive impairment and dementia risk in the Framingham Heart Study. J Int Neuropsychol Soc 2016; 22: 937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark CM, Pontecorvo MJ, Beach TG et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol 2012; 11: 669–678. [DOI] [PubMed] [Google Scholar]
- 30.Landau S, Thomas B, Thurfjell L et al. Amyloid PET imaging in Alzheimer’s disease: a comparison of three radiotracers. Eur J Nucl Med Mol Imaging 2014; 41: 1398–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edmonds EC, Delano-Wood L, Clark LR et al. Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimers Dement 2015; 11: 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gollan TH, Weissberger GH, Runnqvist E, Montoya RI, Cera CM. Self-ratings of spoken language dominance: a Multilingual Naming Test (MINT) and preliminary norms for young and aging Spanish–English bilinguals. Biling Lang Congn 2012; 15: 594–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jefferson AL, Wong S, Gracer TS, Ozonoff A, Green RC, Stern RA. Geriatric performance on an abbreviated version of the Boston Naming Test. Appl Neuropsychol 2007; 14: 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt M Rey Auditory Verbal Learning Test: A Handbook. Los Angeles, CA: Western Psychological Services, 1996. [Google Scholar]
- 35.Partington JE, Leiter RG. Partington pathways test. Psychol Serv Center J 1949; 1: 11–20. [Google Scholar]
- 36.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44: 2308–2314. [DOI] [PubMed] [Google Scholar]
- 37.Siafarikas N, Selbaek G, Fladby T, Benth JS, Auning E, Aarsland D. Frequency and subgroups of neuropsychiatric symptoms in mild cognitive impairment and different stages of dementia in Alzheimer’s disease. Int Psychogeriatr 2018; 30: 103–113. [DOI] [PubMed] [Google Scholar]
- 38.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol 1986; 5: 165–173. [Google Scholar]
- 39.Bijl D, van Marwijk HW, Adér HJ, Beekman AT, de Haan M. Test-characteristics of the GDS-15 in screening for major depression in elderly patients in general practice. Clin Gerontol 2006; 29: 1–9. [Google Scholar]
- 40.Kiselica AM, Kaser A, Benge JF. An initial empirical operationalization of the earliest stages of the Alzheimer’s continuum. Alzheimer’s Dis Assoc Disord 2021; 35: 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrison JK, Noel-Storr AH, Demeyere N, Reynish EL, Quinn TJ. Outcomes measures in a decade of dementia and mild cognitive impairment trials. Alzheimer’s Res Ther 2016; 8: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43: 2412–2414. [DOI] [PubMed] [Google Scholar]
- 43.Berlin KS, Parra GR, Williams NA. An introduction to latent variable mixture modeling (part 2): longitudinal latent class growth analysis and growth mixture models. J Pediatr Psychol 2014; 39: 188–203. [DOI] [PubMed] [Google Scholar]
- 44.Rosseel Y Lavaan: an R package for structural equation modeling and more. Version 0.5–12 (BETA). J Stat Softw 2012; 48: 1–36. [Google Scholar]
- 45.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods 2002; 7: 147–177. [PubMed] [Google Scholar]
- 46.Woodard JL. A quarter century of advances in the statistical analysis of longitudinal neuropsychological data. Neuropsychology 2017; 31: 1020–1035. [DOI] [PubMed] [Google Scholar]
- 47.Wang C, Su S, Weiss DJ. Robustness of parameter estimation to assumptions of normality in the multidimensional graded response model. Multivar Behav Res 2018; 53: 403–418. [DOI] [PubMed] [Google Scholar]
- 48.Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equation Model 1999; 6: 1–55. [Google Scholar]
- 49.Hooper D, Coughlan J, Mullen M. Structural equation modelling: guidelines for determining model fit. J Bus Res Methods 2008; 6: 53–60. [Google Scholar]
- 50.Kline RB. Principles and Practice of Structural Equation Modeling, 3rd edn. New York, NY: Guilford Press, 2011. [Google Scholar]
- 51.Lin M, Gong P, Yang T, Ye J, Albin RL, Dodge HH. Big data analytical approaches to the NACC Dataset: aiding preclinical trial enrichment. Alzheimer Dis Assoc Disord 2018; 32: 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langlois A-S, Belleville S Subjective cognitive complaint in healthy older adults: identification of major domains and relation to objective performance. Aging Neuropsychol Cognit 2014; 21: 257–282. [DOI] [PubMed] [Google Scholar]
- 53.Markova H, Andel R, Stepankova H et al. Subjective cognitive complaints in cognitively healthy older adults and their relationship to cognitive performance and depressive symptoms. J Alzheimers Dis 2017; 59: 871–881. [DOI] [PubMed] [Google Scholar]
- 54.Apostolova LG, Cummings JL. Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord 2008; 25: 115–126. [DOI] [PubMed] [Google Scholar]
- 55.Reynolds K, Pietrzak RH, El-Gabalawy R, Mackenzie CS, Sareen J. Prevalence of psychiatric disorders in US older adults: findings from a nationally representative survey. World Psychiatry 2015; 14: 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ismail Z, Smith EE, Geda Y et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement 2016; 12: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bachman DL, Stuckey M, Ebeling M et al. Establishment of a predominantly African-American cohort for the study of Alzheimer’s disease. Dement Geriatr Cogn Disord 2009; 27: 329–336. [DOI] [PubMed] [Google Scholar]
- 58.Uhlmann RE, Rother C, Rasmussen J et al. Acute targeting of pre-amyloid seeds in transgenic mice reduces Alzheimer-like pathology later in life. Nat Neurosci 2020; 16: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Regressions coefficients for creating Z-scores, along with comparisons of regression weights between the entire normative sample and the sample restricted to amyloid negative participants.


