SUMMARY
NMDA receptors (NMDARs) are a subtype of postsynaptic ionotropic glutamate receptors that function as molecular coincidence detectors, have critical roles in models of learning, and are associated with a variety of neurological and psychiatric disorders. To date, no auxiliary proteins that modify NMDARs have been identified. Here, we report the identification of NRAP-1, an auxiliary protein in C. elegans that modulates NMDAR function. NMDAR-mediated currents were eliminated in nrap-1 mutants, as was NMDA-dependent behavior. We show that reconstitution of NMDA-gated current in Xenopus oocytes, or C. elegans muscle cells, depends on NRAP-1 and that recombinant NRAP-1 can convert silent NMDARs to functional channels. Our data indicate that NRAP-1, secreted from presynaptic neurons, localizes to glutamatergic synapses where it associates with postsynaptic NMDARs to modify receptor gating. Thus, our studies reveal a novel mechanism for synaptic regulation via pre-synaptic control of NMDAR-mediated synaptic transmission.
Keywords: Glutamate, iGluR, NMDA receptor, auxiliary protein, receptor desensitization, synaptic plasticity, learning and memory, silent synapse
eTOC Blurb
Lei et al, have discovered NRAP-1, the first identified NMDAR auxiliary protein. NRAP-1, secreted from presynaptic neurons, modulates the properties of postsynaptic NMDA receptors. Trans-synaptic modulation of receptor function by NRAP-1 reveals a novel mechanism used to regulate synaptic strength.
INTRODUCTION
Most excitatory neurotransmission in the brain is mediated by the neurotransmitter glutamate, which depolarizes neurons by activating postsynaptic ionotropic glutamate receptors (iGluRs). AMPA, kainate and NMDA are agonists that preferentially activate different classes of iGluRs. Of these classes, NMDA receptors (NMDARs) have received intense interest because of their unique physiology and pharmacology (Paoletti et al., 2013). NMDARs function as molecular coincidence detectors, and have a central role in cellular models of learning and memory (Nicoll and Roche, 2013). Additionally, NMDAR dysfunction is implicated in a variety of neurological and psychiatric disorders (Paoletti et al., 2013; Volk et al., 2015; Zhou and Sheng, 2013).
iGluR subunits were first cloned by functional expression (Hollmann et al., 1989), and for many years these subunits were the only proteins known to contribute to the functional properites of iGluRs. This notion changed with the identification of TARPs (transmembrane AMPAR regulatory proteins) that modify the trafficking of AMPARs (Chen et al., 2000). Soon after, the first iGluR auxiliary protein shown to modify AMPAR function (SOL-1) was identified in C. elegans by screening for mutants with disrupted glutamatergic signaling (Zheng et al., 2004). TARPs were subsequently shown to also modify the gating of AMPARs in vertebrates (Tomita et al., 2005) and invertebrates (Walker et al., 2006b; Wang et al., 2008). It is now firmly established that several classes of auxiliary proteins modify AMPAR function (Farrow et al., 2015; Schwenk et al., 2009; Straub and Tomita, 2012; Zhang et al., 2014), and recent efforts have also identified auxiliary proteins that modify the function of kainate receptors (KARs) (Copits and Swanson, 2012; Howe, 2015; Tomita and Castillo, 2012; Zhang et al., 2009). Our genetic approach in C.elegans to discovering genes required for glutamatergic signaling has identified additional proteins that modify AMPAR function or localization, including SOL-2 (Wang et al., 2012) and CNI-1 (cornichon) (Brockie et al., 2013). We have now used a new genetic approach to identify NRAP-1, a secreted NMDAR auxiliary protein required for NMDAR-mediated current and behavior.
RESULTS
Identifying genes required for NMDAR function
To date, despite evidence supporting the notion that NMDARs interact with a large number of proteins (Frank et al., 2016), no auxiliary proteins for NMDARs have been described. With the goal of identifiying genes required for NMDAR function, we undertook a screen for genetic modifiers of a behavior dependent on NMDAR-mediated signaling in C. elegans.
The average time C. elegans spends moving in a forward direction before reversing (the forward duration) is increased in glr-1 AMPAR mutants and nmr-1 NMDAR mutants (Brockie et al., 2001b; Mellem et al., 2002). Mutations in egl-3, which encodes a proprotein convertase, can suppress glr-1 mutant defects (Kass et al., 2001; Mellem et al., 2002). Interestingly, the suppression of glr-1 by egl-3 is dependent on nmr-1. Thus, the average forward duration in nmr-1; glr-1; egl-3 triple mutants is significantly greater than glr-1; egl-3 double mutants (Figure 1A), and greater than in either glr-1 or nmr-1 single mutants (Brockie et al., 2001b; Mellem et al., 2002).
Figure 1. nrap-1 encodes a LDLa-domain protein expressed in presynaptic cells and required for NMDAR-dependent behavior.
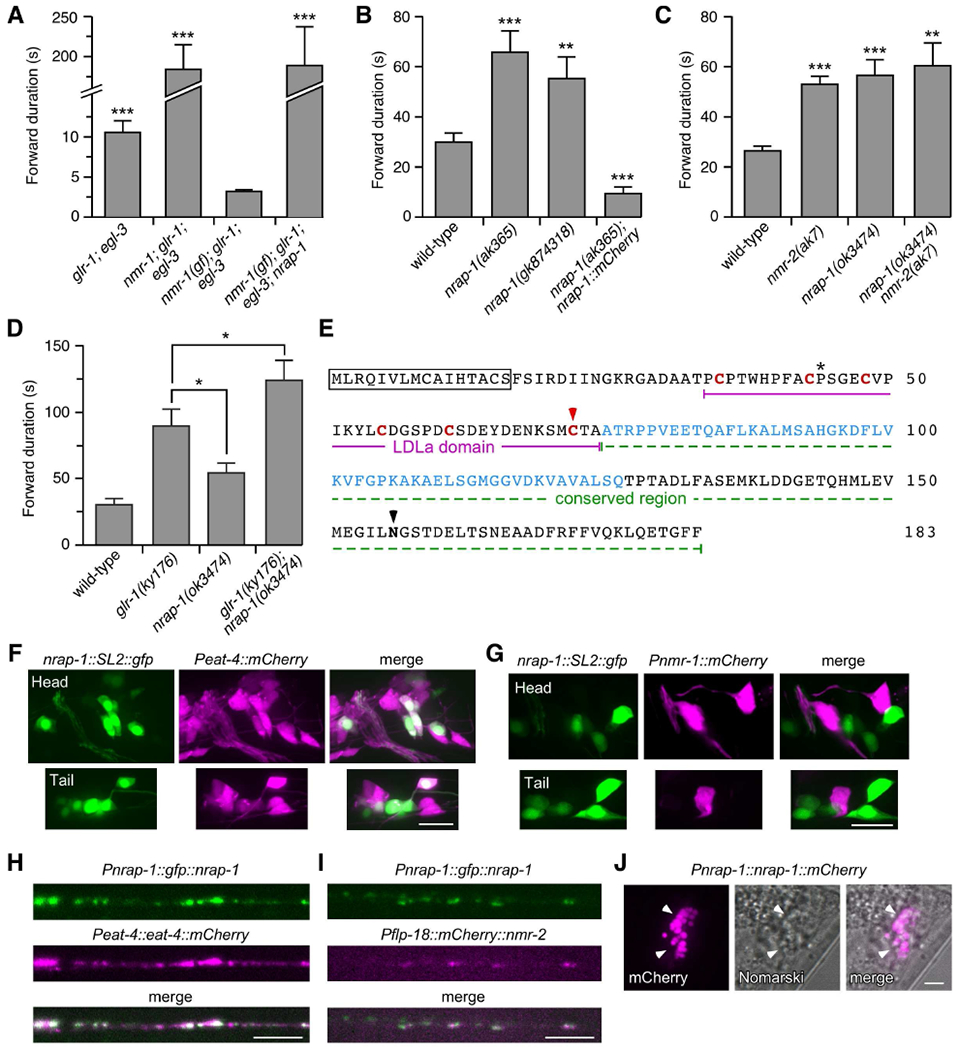
(A–D) Average forward duration (mean ± SEM). * p < 0.05, ** p < 0.01, *** p < 0.001 by Mann-Whitney U test (A, C and D) or Welch’s t-test (B). n=8 for all genotypes in (A). For genotypes in (B), wild-type, n=21; ak365, n=20; gk874318, n=20; ak365; Pnrap-1::nrap-1::mCherry, n=13. n=9 for all genotypes in (C). For genotypes in (D), wild-type, n=11; ky176, n=12; ok3474, n=7; ky176; ok3474, n=12. (E) NRAP-1 amino acid sequence. Signal peptide (boxed); conserved cysteins (red); residues mutated in nrap-1(gk874318) (astericks) and nrap-1(ak365) (red arrowhead); region deleted in nrap-1(ok3474) (blue); and predicted N-linked glycosylation (black arrowhead). (F and G) Images of anterior (head) and posterior (tail) neuronal cells bodies in transgenic worms that co-expressed Pnrap-1::nrap-1::SL2::gfp with either Peat-4::mCherry (F) or Pnmr-1::mCherry (G). GFP was tagged with a nuclear localization sequence. Scale bars, 10 μm. (H and I) Images of the VNC in transgenic worms that co-expressed Pnrap-1::gfp::nrap-1 with either Peat-4::eat-4::mCherry (H) or Pflp-18::mCherry::nmr-2 (I). Scale bars, 5 μm. (J) Images of a transgenic worm that expressed Pnrap-1::nrap-1::mCherry. Arrowheads indicate coelomocytes. Scale bar, 5 μm. See also Figures S1 and S2.
We discovered that transgenic nmr-1; glr-1; egl-3 triple mutants that expressed a gain-of-function (gf) variant of nmr-1 (Figure S1A) had exceedingly short forward durations, shorter even than that observed in glr-1; egl-3 double mutants (that expressed wild-type nmr-1) (Figure 1A). The dramatic locomotion defect of nmr-1(gf); glr-1; egl-3 transgenic mutants provided a highly sensitized background in which to screen for genes required for NMDAR function. Thus, we mutagenized transgenic nmr-1(gf); glr-1; egl-3 worms (short forward duration) and screened for mutants with a long forward duration consistent with a loss of NMDAR function. Using this strategy, we identified a mutant (nmr-1(gf); glr-1; egl-3; nrap-1) that was indistinguishable from the nmr-1; glr-1; egl-3 triple mutant (Figure 1A).
Whole-genome sequencing and genetic rescue experiments identified a mutation (ak365) in a gene that we named nrap-1 (NMDA Receptor Auxiliary Protein). Following genetic separation of the nrap-1(ak365) mutation from nmr-1(gf); glr-1; egl-3, we found that the average forward duration in nrap-1(ak365) single mutants was increased, consistent with loss of NMDAR function (Brockie et al., 2001b) (Figure 1B). We found the same change in forward movement in two independent nrap-1 mutant alleles, gk874318 (Figure 1B), and a large deletion allele, ok3474 (Figure 1C). Overexpression of NRAP-1::mCherry using its native promoter (Pnrap-1::nrap-1::mCherry) in transgenic nrap-1(ak365) mutants not only rescued the movement defect, but it also significantly decreased the forward duration compared to wild type, suggesting that NRAP-1 levels regulate the magnitude of NMDAR-mediated current.
We next used genetic analysis to address whether NRAP-1 function was limited to NMDAR-mediated signaling or whether it had additional effects. We found that the behavioral defects in nrap-1(ok3474) and nmr-2(ak7) (NMDAR) single mutants were similar, and that nrap-1 nmr-2 double mutants were indistinguishable from either single mutant (Figure 1C). In contrast, the average forward duration of glr-1(ky176) (AMPAR) single mutants was significantly different from nrap-1(ok3474) and an additive effect was observed in glr-1; nrap-1 double mutants (Figure 1D). Together, these data strongly support the hypothesis that nrap-1 function is specific for NMDAR-mediated synaptic signaling and behavior.
nrap-1 encodes a predicted glycosylated protein of 183 amino acids (a.a.) with only two recognizable domains: an N-terminal signal peptide and a 41 a.a. LDLa domain (Figure 1E). The sequence immediately downstream of the LDLa domain was found to be highly conserved in other LDLa-domain proteins (Figures 1E and S1B). The LDLa domain was first identified as peptide repeats in the LDR lipoprotein receptor. Six disulphide-bonded cysteines in LDLa domains provide a structure that complexes with a Ca2+ ion, and functions in ligand binding (Fass et al., 1997). LDLa domains have subsequently been identified in a large number of proteins with diverse functions, including AMPAR and KAR auxiliary proteins (Wang et al., 2012; Zhang et al., 2009), acetylcholine receptor-associated proteins (Gally et al., 2004), and one class of G-protein coupled receptors (Petrie et al., 2015). In nrap-1(ak365), a conserved cysteine is mutated to arginine and this mutation is predicted to disrupt the LDLa domain (Fass et al., 1997), and is thus likely a null. In nrap-1(gk874318) mutants a conserved proline in the LDLa domain is mutated to serine and nrap-1(ok3474) deletes 52 amino acids in the conserved region (Figure 1E). Using the BLAST algorithm, we found that NRAP-1 is highly conserved in protostomes, with homologous proteins in insects, beetles and molluscs (Figure S1B and S1C); however, we did not identify an obvious homologue in deuterostomes.
NRAP-1 colocalizes with postsynaptic NMDARs
To begin to address how NRAP-1 contributes to NMDAR-mediated signaling, we first sought to identify which cells express NRAP-1. We generated a bicistronic transcript expressing nuclear-localized green fluorescent protein (GFP) downstream of the nrap-1 genomic promoter and coding sequence (nrap-1::SL2::gfp). This transgene was co-expressed in transgenic worms with a second transgene that expressed soluble mCherry in either presynaptic glutamatergic neurons (Peat-4::mCherry) (Lee et al., 1999), or in postsynaptic neurons that express NMDARs (Pnmr-1::mCherry) (Brockie et al., 2001a). We observed nrap-1::SL2::gfp expression in a subset of the presynaptic neurons that expressed Peat-4::mCherry (Figure 1F). In contrast, we did not detect nrap-1 expression in postsynaptic neurons that express NMDARs, i.e., Pnmr-1::mCherry (Figure 1G). Thus, nrap-1 is expressed in glutamatergic neurons that provide synaptic input to NMDAR-expressing neurons.
To address whether NRAP-1 is found at presynaptic specializations in glutamatergic neurons, we co-expressed a functional full-length GFP::NRAP-1 chimera using the nrap-1 promoter (Pnrap-1::gfp::nrap-1) along with a functional EAT-4::mCherry chimera using the eat-4 promoter (Peat-4::eat-4::mCherry) (Figure S2). eat-4 encodes a worm homologue of vertebrate vesicular glutamate uptake proteins (Lee et al., 1999), which are localized to presynaptic release sites (Fremeau et al., 2004). We found extensive co-localization of EAT-4::mCherry and GFP::NRAP-1 along neuronal processes in the ventral nerve cord (VNC) (Pearson’s correlation coefficient (PCC): 0.52 ± 0.03, p < 0.01, n=7) (Figure 1H). We also found co-localization between GFP::NRAP-1 and a functional mCherry::NMR-2 fusion (Pflp-18::mCherry::nmr-2) (Figure S2) expressed in the AVA interneurons (one of 5 pairs of neurons that express NMDARs in C. elegans) (PCC: 0.33 ± 0.02, p < 0.01, n=13) (Figure 1I).
The primary amino acid sequence of NRAP-1 predicts a secreted protein. Support for this hypothesis comes from examination of worm coelomocytes. These macrophage-like scavanger cells non-specifically endocytose and degrade proteins in the pseudocoelomic fluid (Fares and Greenwald, 2001). NRAP-1 tagged at its C-terminus with mCherry can be identified in coelomocytes by mCherry fluorescence, indicating that the protein is secreted into the synaptic space from which it can eventually diffuse and be removed by the coelomocytes (Figure 1J). Together, these data indicate that while NRAP-1 is a secreted protein, it is found at highest concentration at glutamatergic synapses, suggesting that NRAP-1 interacts with proteins localized at synapses.
nrap-1 is required for NMDA-gated current
The majority of the glutamate-gated current in AVA interneurons is dependent on AMPARs containing the GLR-1 and GLR-2 receptor subunits, and receptor properties are modified by the auxiliary proteins SOL-1, SOL-2, STG-1/TARP and STG-2/TARP (Mellem et al., 2002; Wang et al., 2012; Wang et al., 2008; Zheng et al., 2004). In contrast, the NMDAR-mediated current is smaller in amplitude, slower to desensitize, outwardly rectifying and dependent on the NMR-1 and NMR-2 receptor subunits (Brockie et al., 2001b; Kano et al., 2008). Unlike AMPARs, NMDAR-mediated current is not modified by SOL-1, SOL-2, STG-1 or STG-2 (Walker et al., 2006a; Wang et al., 2012; Wang et al., 2008; Zheng et al., 2004).
Using standard patch-clamp techniques, we recorded in vivo glutamate-gated currents in the AVA neurons of dissected worms. The average peak amplitude of glutamate-gated current (at −60 mV) in nrap-1 mutants (624.3 ± 127.8 pA, n=3) was not diminished compared to wild type (634.8 ± 160.6 pA, n=4) (Figure 2A). In contrast, the slow, smaller amplitude and outwardly rectifying component, characteristic of NMDAR-mediated current, appeared to be eliminated in nrap-1 mutants. This is consistent with our behavioral analysis indicating that nrap-1 is specifically required for the NMDAR-mediated component of the glutamate-gated current, leaving the AMPAR-mediated component intact.
Figure 2. nrap-1 is specifically required for NMDA-gated current in vivo.
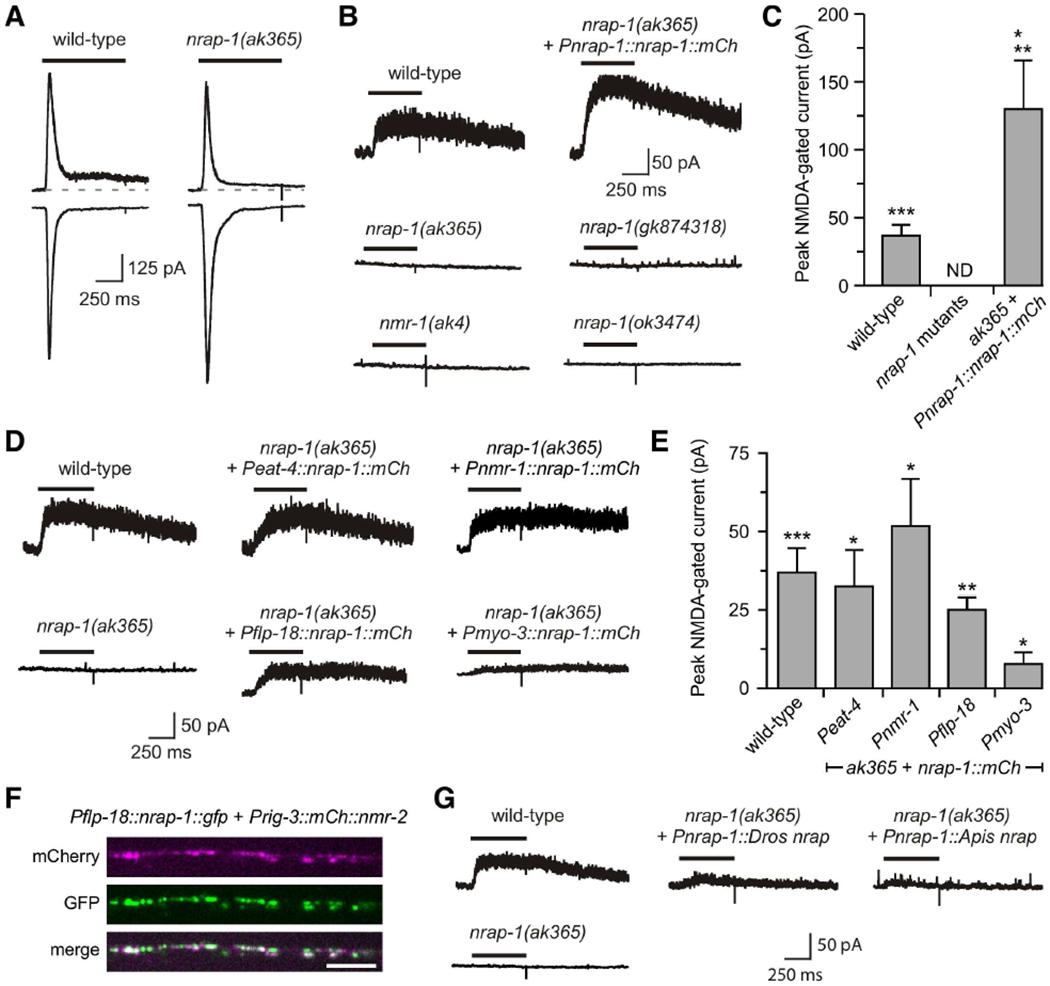
(A and B) Currents in AVA in response to glutamate (A) or NMDA (B). Dashed, gray line in (A) indicates baseline before glutamate application. (C) Average peak current (mean ± SEM). ** p < 0.01, significanlty different from wild type by Mann-Whitney U test. * p < 0.05, *** p < 0.001, significanlty different from nrap-1 mutants by one-sample, one-tailed Student’s t-test comparing the mean for each genotype with zero (i.e., no current). Wild-type, n=10; nrap-1 mutants, n=12; ak365 + Pnrap-1::nrap-1 ::mCh, n=4. ND, no current detected. (D) NMDA-gated currents in AVA. (E) Average peak current (mean ± SEM). Significantly different from nrap-1(ak365) mutants where no current was detected (data not shown), * p < 0.05, ** p < 0.01, *** p < 0.001 by one-sample, one-tailed Student’s t-test. Wild-type, n=10; Peat-4, Pnmr-1 and Pflp-18, n=4, and Pmyo-3, n=5. (F) Images of mCherry and GFP fluorescence in the VNC of a transgenic worm that expressed Pflp-18::nrap-1::gfp and Prig-3::mCherry::nmr-2 in AVA. Scale bar, 5 μm. (G) NMDA-gated currents in AVA in wild-type, mutant, and transgenic mutants. Wild-type average peak current shown in (C) and (E) was from pooled data. See also Figure S2.
To isolate the NMDAR-dependent component, we measured currents in response to application of NMDA, a NMDAR-specific agonist. In wild-type worms, we measured a slow-onset current that was eliminated in nmr-1(ak4) mutants (Figure 2B). This is consistent with previous results showing that NMDA-gated current is dependent on nmr-1 (Brockie et al., 2001b; Kano et al., 2008). We were unable to detect current in either the nrap-1(ak365), nrap-1(ok3474) or nrap-1(gk874318) mutants, indicating that NMDA-gated current is also dependent on nrap-1 (Figure 2B and 2C).
To confirm that mutations in nrap-1 were causative for the disrupted NMDAR-mediated current, we carried out genetic rescue experiments and found restoration of NMDA-gated currents in transgenic nrap-1(ak365) mutants that expressed a functional Pnrap-1::nrap-1::mCherry transgene (Figure 2B and 2C). Indeed, overexpression of NRAP-1 significantly increased NMDAR-mediated current compared to wild type, a result consistent with the behavioral rescue (Figure 1B). Together, our results show that nrap-1 is specifically required for the NMDAR-mediated component of the glutamate-gated current in vivo.
We next asked whether expression of NRAP-1 in specific subsets of neurons was sufficient to rescue NMDA-gated current in AVA. We found full rescue when NRAP-1 was expressed in presynaptic glutamatergic neurons using the eat-4 promoter (Figure 2D and 2E). This result was consistent with our studies showing that NRAP-1 was expressed in EAT-4-expressing neurons (Figure 1F).
Surprisingly, we also found full rescue of NMDA-gated current when the nmr-1 promoter was used to ectopically express NRAP-1 in postsynaptic interneuons, including AVA, that normally express NMDARs, and when expression was limited to AVA using the flp-18 promoter (Figure 2D and 2E). Imaging of functional fluorescently tagged NRAP-1 and NMR-2 (Figure S2) expressed in AVA revealed co-localization of the two proteins along the AVA processes (Figure 2F). Thus, NRAP-1 expressed cellularly either in trans or in cis co-localized with NMDARs, and rescued NMDA-gated currents in nrap-1 mutants.
We next asked whether NMDAR function was dependent on the cellular source of NRAP-1 secretion by ectopically expressing NRAP-1 in body wall muscle cells using the myo-3 promoter. Although small, we were able to measure NMDA-gated current in the AVA interneurons of transgenic nrap-1 mutants that expressed Pmyo-3::nrap-1::mCherry in muscle cells (Figure 2D and 2E). These results suggest that although NRAP-1 can diffuse from distant sites, NRAP-1 needs to be expressed in cells that are in close juxtaposition to NMDARs to be fully functional, i.e., near sites of synaptic contact.
As discussed earlier, we found homologues of NRAP-1 in organisms such as Drosophila melanogaster and Apis mellifera (Figure S1B and S1C). We next addressed whether these homologues were able to funcationally compensate for the loss of NRAP-1 by measuring currents in transgenic nrap-1 mutants that expressed either Drosophila or Apis NRAP using the nrap-1 promoter. We found partial rescue of the NMDA-gated current in transgenic nrap-1 mutants that expressed either Pnrap-1::Apis nrap (10.0 ± 1.5 pA, n=3) or Pnrap-1::Dro nrap (18.0 pA, n=1), indicating evolutionary conservation of function (Figure 2G).
NMDARs localize to synapses in nrap-1 mutants
To address whether the diminished NMDA-gated current in nrap-1 mutants was a consequence of a change in the number or surface expression of postsynaptic NMDARs, we generated transgenic strains that expressed a functional fusion protein (SEP::mCherry::NMR-2) (Figure S2) where NMR-2 was tagged at its N-terminal extracellular domain with a tandem array of two fluorophores: superecliptic phluorin (SEP), which is quenched at the acidic pH of intracellular vesicles, but visible when at the relatively alkaline pH of the extracellular space, and mCherry, which is not appreciably dependent on vesicular pH (Miesenbock et al., 1998). Punctate mCherry and SEP fluorescence was visible in transgenic wild-type worms and nrap-1 mutants that expressed SEP::mCherry::NMR-2 (Figure 3A), indicating that the localization of NMDARs was not appreciably dependent on NRAP-1. Furthermore, we observed no obvious difference in SEP fluorescence in nrap-1 mutants (0.84 ± 0.20, n=9; normalized to wild type, 1.0 ± 0.13, n=10), suggesting that the mutation in nrap-1 had no significant effect on NMDAR surface expression.
Figure 3. nrap-1 is not required for NMDAR localization or surface expression.
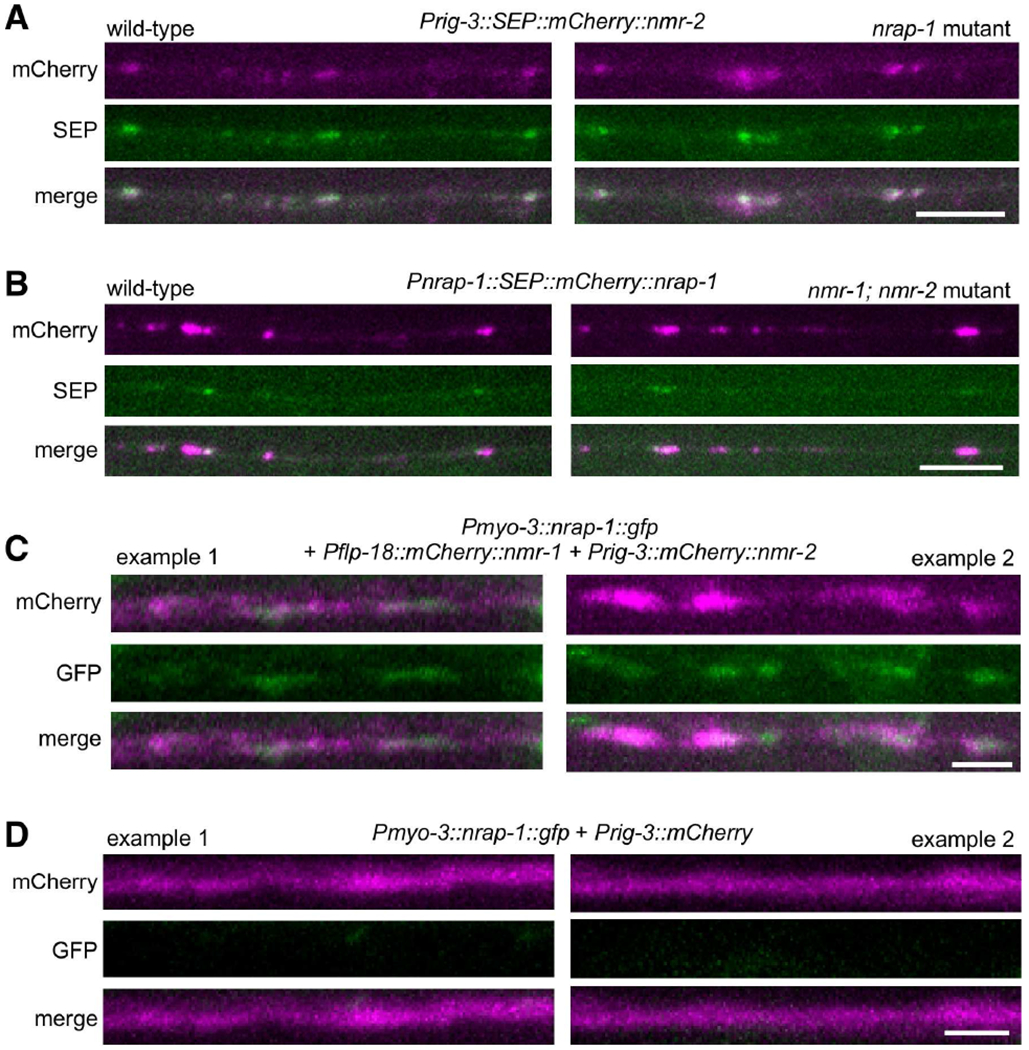
(A) Images of AVA processes in the VNC in a transgenic wild-type worm (left) or nrap-1 mutant (right) that expressed SEP::mCherry::nmr-2. Scale bar, 5 μm. (B) Images of neural processes in the VNC in a transgenic wild-type worm (left) or nmr-1; nmr-2 mutant (right) that expressed SEP::mCherry::nrap-1 in presynaptic neurons. Scale bar, 5 μm. (C and D) Images of the VNC in transgenic nmr-1; nmr-2 nrap-1 triple mutants that co-expressed Pmyo-3::nrap-1::gfp with either Pflp-18::mCherry::nmr-1 and Prig-3::mCherry::nmr-2 (C) or Prig-3::mCherry (D) (two examples from each strain). Scale bars, 2 μm. See also Figures S2 and S3.
We also addressed the converse question: Is the localization of NRAP-1 dependent on NMDARs? In transgenic strains that expressed functional SEP::mCherry::NRAP-1 (Figure S2), we observed punctate mCherry and SEP in transgenic wild-type worms and nmr-1; nmr-2 double mutants (Figure 3B), with no significant difference in SEP fluorescence in the mutants (1.39 ± 0.35, n=9; normalized to wild-type 1.0 ± 0.20, n=8), indicating that the localization of NRAP-1 was not wholly dependent on NMDARs. These experiments also provide additional evidence that NRAP-1 is extracellular and localized to synapses. However, because we do not have sufficient spatial resolution to distinguish presynaptic from postsynaptic NRAP-1, we cannot determine from these data whether NMDARs contribute to the stabilization of NRAP-1 at postsynaptic sites.
To address whether the localization of NRAP-1 was in part due to a direct interaction with NMDARs, we examined NRAP-1::GFP distribution in transgenic nmr-1; nrap-1 nmr-2 mutants that co-expressed Pmyo-3::nrap-1::gfp (ectopically expressed in muscle cells) with functional fluorescently tagged NMR-1 and NMR-2 (Brockie et al., 2001b) (Figure S2) expressed in the AVA neurons. We observed a punctate distribution of NRAP-1::GFP that co-localized with mCherry in the AVA processes (Figure 3C). In contrast, we did not observe a GFP signal along the neural processes in NMDAR mutants that expressed soluble mCherry in AVA (Figure 3D). Together, these data strongly suggest that secreted NRAP-1 physically interacts with postsynaptic NMDARs, but could also interact with additional synaptic molecules.
Our finding that NRAP-1 localizes to synapses, along with the electrophysiological and behavioral defects we observed in nrap-1 mutants, is consistent with defective NMDAR-mediated synaptic transmission. To address this more directly, we examined endogenous synaptic transmission. We measured miniature synaptic events in a genetic background that allowed us to isolate the glutamatergic component (see Method Details). When the NMDAR-mediated current was eliminated in these worms (nmr-1 mutants), we observed large, rapidly deactivating currents, consistent with AMPAR-mediated events, that were absent in nmr-1; glr-1 mutants (Figure S3A). The AMPAR-mediated synaptic events in nmr-1; nrap-1 mutants were indistinguishable from nmr-1 mutants (Figure S3A).
We next isolated the NMDAR-component by measuring endogenous activity in glr-1 (AMPAR) mutants and observed small, slowly deactivating currents that were consistent with the known kinetics of NMDAR-mediated miniature synaptic events (Figure S3B) (Prybylowski et al., 2002). In comparison, we observed no such miniature events if we also disrupted NMDARs (nmr-1; glr-1 mutants) or NRAP-1 function (glr-1; nrap-1 mutants) (Figure S3B). Furthermore, application of recombinant NRAP-1 was sufficient to restore NMDAR-mediated miniature synaptic events in glr-1; nrap-1 mutants (Figure S3B). These data further support our hypothesis that nrap-1 specifically modulates NMDAR function, and is required for NMDAR-mediated synaptic transmission.
NRAP-1 functions as a NMDAR auxiliary protein
Our finding that surface NMDARs are not appreciably altered in nrap-1 mutants suggests that the lack of NMDA-gated current in nrap-1 mutants is a consequence of defective NMDAR function. To test this hypothesis we turned to reconstitution experiments in Xenopus oocytes. We did not detect glutamate-gated currents in oocytes injected with cRNA encoding the NMR-1 and NMR-2 subunits. However, when we co-injected cRNAs for NMR-1, NMR-2 and NRAP-1, we recorded large, glutamate-gated or NMDA-gated currents (Figure 4A and 4B). We did not detect current from oocytes that expressed NRAP-1 alone, or in combination with either one of the NMDAR subunits (Figure 4A and 4B). These results are consistent with our genetic studies that tested the necessity of nmr-1, nmr-2 and nrap-1 for NMDAR-mediated current and behavior (Figures 1 and 2) (Brockie et al., 2001b; Kano et al., 2008), and suggest that NRAP-1 is a NMDAR auxiliary subunit.
Figure 4. NRAP-1 modifies the function of NMDARs.
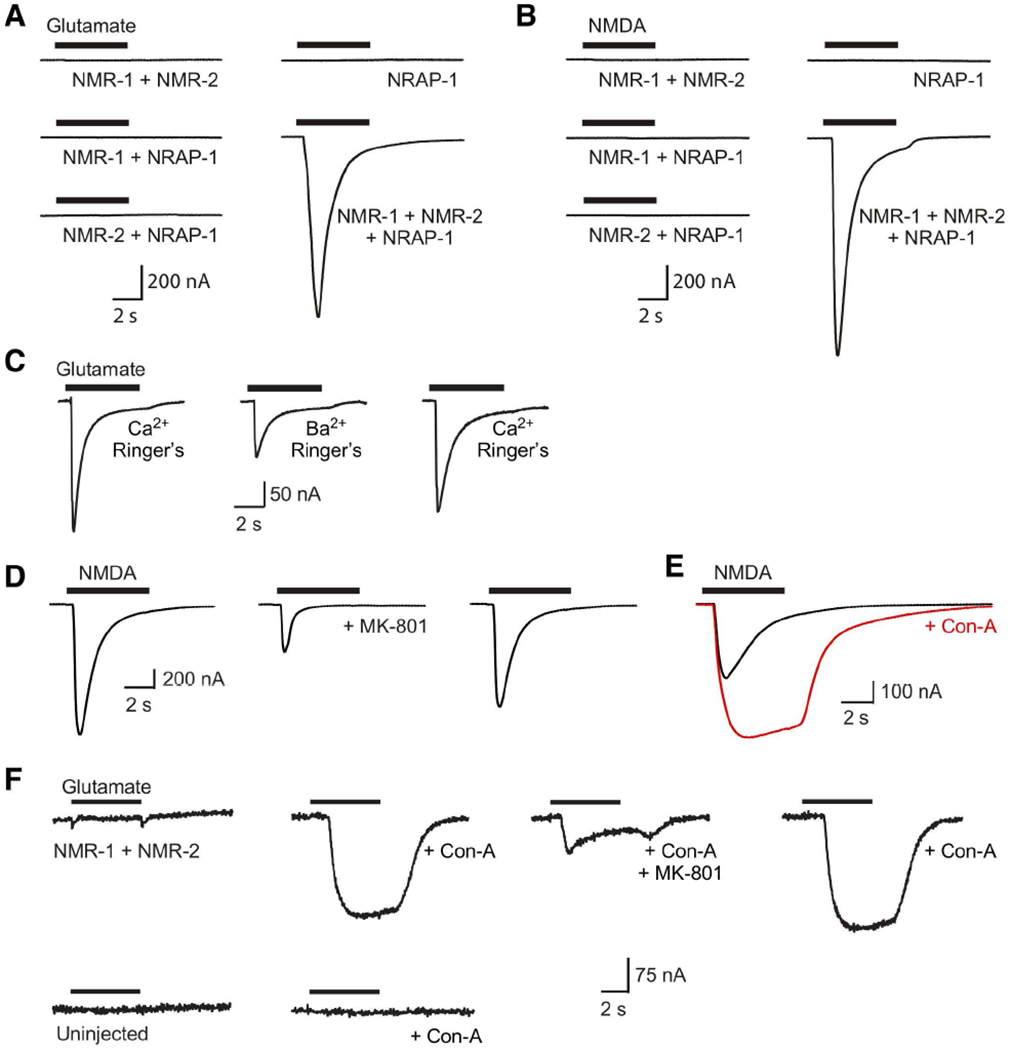
(A and B) Currents in response to 1 mM glutamate (A) or 1 mM NMDA (B) application to Xenopus oocytes that expressed various combinations of NMR-1, NMR-2 and NRAP-1 (Ca2+ Ringer’s solution). (C–E) Currents in response to NMDA or glutamate (200 μM) in oocytes that expressed NMR-1, NMR-2 and NRAP-1. (C) Current responses in oocytes bathed in either Ca2+ or Ba2+ containing Ringer’s solution. (D and E) Current responses before, during and after wash out of MK-801 (D); or before and during incubation in Con-A (E). (F) Current responses to 200 μM glutamate in an oocyte that expressed NMR-1 and NMR-2 (top). Current was not detected in uninjected, control oocytes (bottom).
Ca2+ ions are known to permeate NMDARs and this can lead to the activation of endogenous Ca2+-activated chloride currents that obscure the true NMDAR-mediated current in Xenopus oocyte reconstitution experiments. To isolate the NMDAR-mediated current, we replaced extracellular Ca2+ with Ba2+, an ion that can permeate NMDARs, but cannot activate Ca2+-activated chloride currents (Leonard and Kelso, 1990). We found that the replacement of extracellular Ca2+ with Ba2+ reduced the peak amplitude of NMDA-gated currents and slowed the apparent decay kinetics in oocytes that expressed NMR-1, NMR-2 and NRAP-1 (Figure 4C). This result indicates that C. elegans NMDARs are Ca2+ permeable, and is consistent with previous studies of recombinant vertebrate NMDARs expressed in Xenopus oocytes (Leonard and Kelso, 1990).
The anticonvulsant MK-801 is a non-competitive antagonist of vertebrate NMDARs that blocks the ion pore. We found that this drug was also a partial antagonist of C. elegans NMDARs (Figure 4D). Another drug known to influence iGluRs is the lectin Concanavalin-A (Con-A), which is best known for blocking desensitization of KARs and AMPARs (Partin et al., 1993; Thalhammer et al., 2002), but can also enhance NMDAR-mediated currents (Yue et al., 1995). Interestingly, we found that Con-A blocked receptor desensitization and enhanced NMDA-gated currents in oocytes that expressed NMR-1, NMR-2 and NRAP-1 (Figure 4E).
Con-A enhances receptor-mediated current by stabilizing the open state of the channel and preventing receptor desensitization; thus, it blocks the conformational change from an open, ligand-bound state to a ligand-bound, but closed and non-conductive state (Partin et al., 1993). To address whether NRAP-1 acts in part as a negative regulator of desensitization, we asked whether Con-A application can rescue NMDA-gated currents in oocytes in the absence of NRAP-1. While we failed to detect glutamate-gated currents in oocytes that co-expressed NMR-1 and NMR-2, following the acute application of Con-A, glutamate application elicited large currents that were partially and reversibly blocked by the NMDAR antagonist MK-801 (Figure 4F). In contrast, we did not detect glutamate-gated currents from uninjected oocytes either in the presence or absence of Con-A. Given the rapid time course of the effect and the known mechanism of action of Con-A, these results are most consistent with a model where Con-A shifts non-functional heteromeric NMR-1/NMR-2 NMDARs to a functional state by modifying channel properties, rather than other potential mechanisms such as new protein synthesis or membrane insertion of receptors.
Recombinant NRAP-1 restores NMDA-gated current in nrap-1 mutants
Our electrophysiological analysis together with the results of our imaging experiments suggest that NRAP-1 secreted from presynaptic glutamatergic neurons modulates NMDAR function. To test this hypothesis, we asked whether the acute application of recombinant NRAP-1 to nrap-1 mutants in vivo could switch non-functional NMDARs to a functional state. Recombinant NRAP-1 was generated by transient transfection of nrap-1 into mammalian HEK 293 cells, culturing the cells for several days and then collecting the cellular conditioned medium (CM) (see Method Details). As shown earlier, we were unable to detect NMDA-gated currents in nrap-1 mutants; however, we recorded large NMDA-gated currents in these mutants following the application of CM from NRAP-1 transfected HEK 293 cells (Figure 5A). In contrast, no current was detected after the addition of CM from non-transfected HEK 293 cells (control media; Figure 5A). In control experiments, recombinant NRAP-1 had no apparent effects on glutamate-gated currents mediated by AMPARs (Figure 5B; nmr-1 mutant, 462 pA; nmr-1 mutant plus recombinant NRAP-1, 444 pA). The rapid restoration of NMDA-gated currents by acute application of recombinant NRAP-1 demonstrates the essential role of NRAP-1 for NMDAR function, and further supports our hypothesis that NMDARs are present on the cell surface in nrap-1 mutants (Figure 3A).
Figure 5. Recombinant NRAP-1 restores current in nrap-1 mutants.
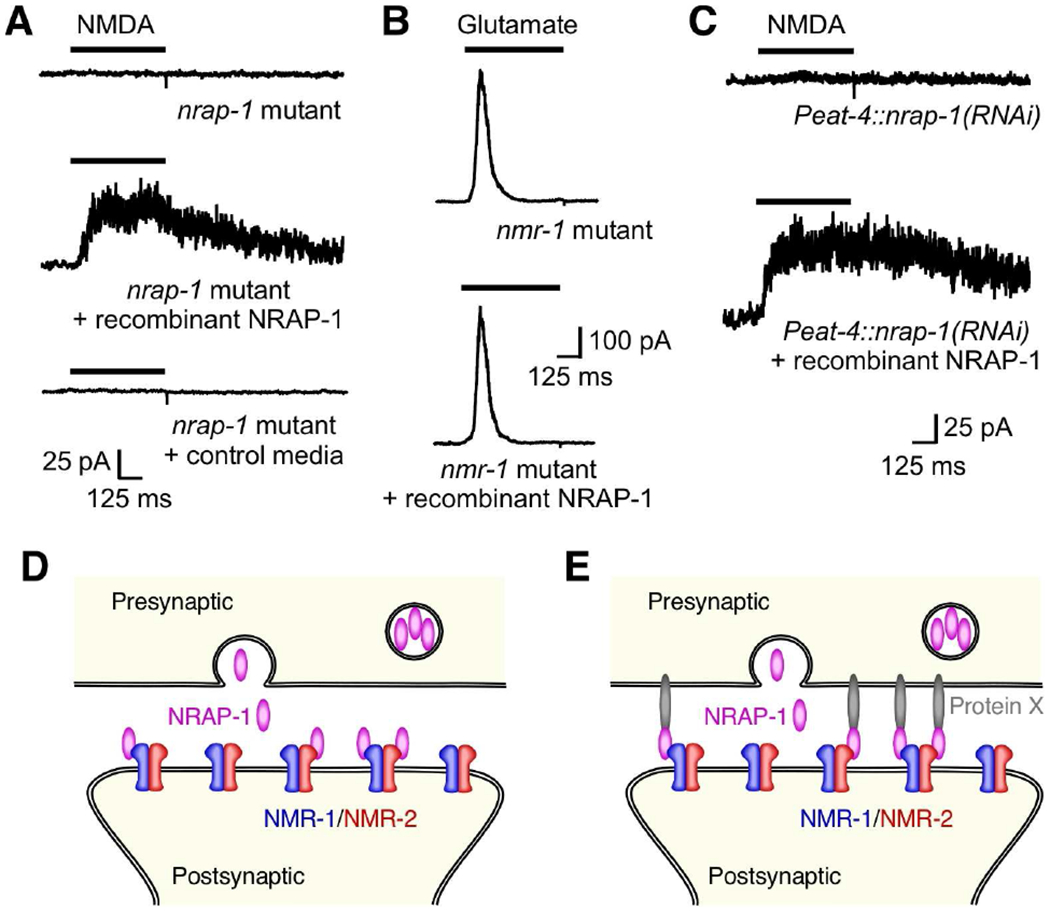
(A–C) Currents in AVA neurons in response to either NMDA or glutamate and in the presence or absence of recombinant NRAP-1, or control media from untransfected HEK 293 cells. In (C), recordings were from transgenic worms with selective knock down of NRAP-1 in presynaptic glutamatergic neurons. Neurons were held at +40 mV. (D and E) Model showing NRAP-1 secretion from presynaptic glutamatergic neurons followed by diffusion and binding to postsynaptic NMDARs (D), or NRAP-1 anchored to the presynaptic membrane via an unknown factor (Protein X) thereby stabilizing its association with postsynaptic NMDARs (E).
NRAP-1 knock down in presynaptic neurons reduces NMDA-gated current in postsynaptic cells
We found NRAP-1 expression in presynaptic, glutamatergic neurons (Figure 1F), but not in postsynaptic neurons that express NMDARs (Figure 1G). These findings, together with the ability of recombinant NRAP-1 to rescue NMDA-gated current in nrap-1 mutants, are consistent with a model where presynaptic release of secreted NRAP-1 modifies the function of postsynaptic NMDARs. However, our reconstitution experiments in Xenopus oocytes (Figure 4A and 4B) and promoter rescue experiments in vivo (Pnmr-1 and Pflp-18; Figure 2D and 2E) indicate that NRAP-1 can function cell autonomously in postsynaptic neurons. Although we did not detect expression of the nrap-1::SL2::gfp reporter construct in postsynaptic neurons, the formal possibility remains that these neurons might be the relevant source of NRAP-1 for NMDAR function.
To further test whether presynaptic release of NRAP-1 was required for NMDAR function, we used RNA inactivation (RNAi) to selectively knock down nrap-1 in presynaptic, glutamatergic neurons using the eat-4 promoter. We found that NMDA-gated current was dramatically reduced in postsynaptic AVA neurons of transgenic worms that expressed Peat-4::nrap-1(RNAi) (9.5 ± 1.3 pA, n=3) (Figure 5C). The small current that remained in these knock-down worms is consistent with both the known inefficiency of RNAi in providing complete knock down of gene function, and the cellular mosaicism associated with extrachromosomal delivery of the knock-down construct in transgenic worms.
The effects of RNAi were rescued following addition of recombinant NRAP-1 with the peak NMDA-gated current increasing approximately 10-fold following NRAP-1 application (Figure 5C). These results together with our cellular expression data are in strong support of a model in which NMDAR function depends on NRAP-1 secreted from presynaptic neurons. Additionally, these experiments confirm that in the absence of presynaptic NRAP-1, NMDARs are stably present at the cell surface.
Three additional lines of evidence support our hypothesis that NRAP-1 is secreted from presynaptic neurons and diffuses to postsynaptic NMDARs. First, small NMDA-gated currents can be recorded from transgenic nrap-1 mutants where the only source of NRAP-1 is from a transgene expressed in muscle cells (Figure 2D and 2E). Second, localization of GFP::NRAP-1 to the AVA processes, when ectopically expressed in muscle cells, was dependent on NMDAR expression (Figure 3C and 3D). Third, a functional NRAP-1::mCherry C-terminal fusion was observed in coelomocyte scavanger cells (Figure 1J). Thus, our data support a model where NRAP-1 secreted from presynaptic glutamatergic neurons diffuses to postsynaptic sites where it binds to and modulates the function of NMDARs (Figure 5D). Alternatively, additional mechanisms might act to stabilize NRAP-1 at synapses (Figure 5E). Both models are consistent with the diffusion of secreted NRAP-1, but the second model postulates that some fraction of secreted NRAP-1 is maintained at synapses by mechanisms distinct from an association with NMDARs. By examining the effects of recombinant NRAP-1 in wild-type and mutant worms, we sought to distinguish between these alternative models.
Recombinant NRAP-1 increases NMDA-gated current in wild-type worms
In vertebrates, surface NMDARs are relatively stable (Horak et al., 2014; Lavezzari et al., 2004; Won et al., 2016) and NMDA-gated currents do not appreciably run down during typical whole-cell, patch-clamp experiments (Harris and Pettit, 2007). To examine the stability of NMDA-evoked currents in our in vivo C. elegans preparation, we measured currents in the AVA neurons in response to repeated applications of NMDA. In wild-type worms we found little run down of current during a typical whole-cell, patch-clamp experiment where the bath volume was constantly perfused (bath exchange time typically less than 30 s) (Figure 6A). Notably, when we measured currents in nrap-1 mutants following acute application of recombinant NRAP-1, we found that the average peak current (115.0 ± 52.9 pA, n=3) was larger than that found in wild type (38.6 ± 6.2 pA, n=10). However, in contrast to wild-type worms, the NMDA-gated currents rapidly diminished in magnitude with wash out of recombinant NRAP-1 (Figure 6B).
Figure 6. Recombinant NRAP-1 reveals the presence of silent NMDARs.
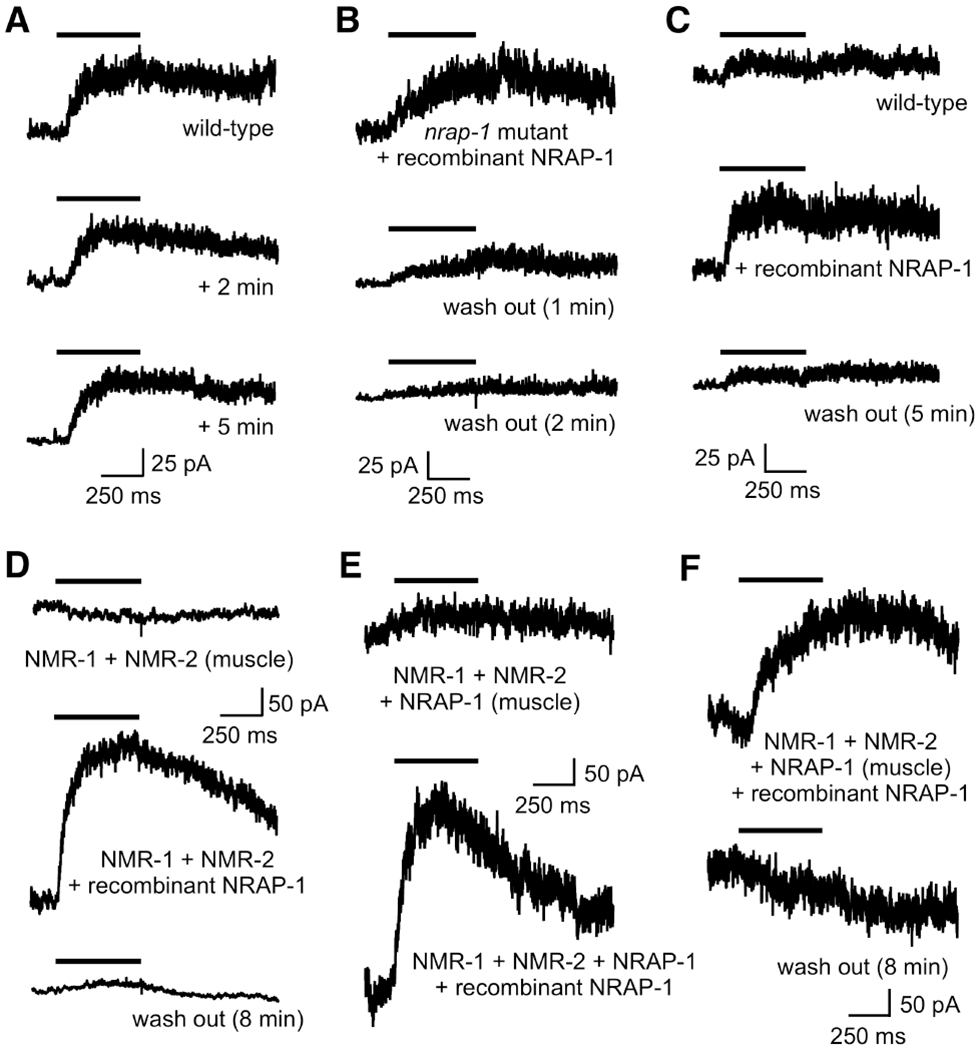
(A–C) Currents in AVA neurons in response to NMDA. Cells held at +40 mV. (A) The initial response in a wild-type worm, and that following 2 and 5 mins of bath solution exchange. (B) Current in a nrap-1 mutant in the presence of recombinant NRAP-1 and after 1 and 2 minutes of wash out. (C) Currents in a wild-type worm in the absence and presence of recombinant NRAP-1 and after 5 minutes of wash out. (D–F) Currents in response to NMDA in muscle cells of transgenic worms that ectopically express NMR-1 and NMR-2 (D) or NMR-1, NMR-2 and NRAP-1 (E and F) specifically in muscle cells. Shown are currents before (D and E) and after (D–F) recombinant NRAP-1 application, and after 8 minutes of wash out (D and F). See also Figure S4.
The differential effects of bath perfusion on NMDA-gated currents in wild-type worms compared to currents following application of recombinant NRAP-1 in nrap-1 mutants suggest that recombinant NRAP-1 is more easily displaced than endogenous NRAP-1. Additionally, the larger peak currents observed with addition of recombinant NRAP-1 compared to wild type suggest that a subset of NMDARs are functionally silent secondary to limiting amounts of NRAP-1.
To test this possibility, we compared NMDA-gated currents in wild-type worms before and after the application of recombinant NRAP-1, and following NRAP-1 wash out. We observed stable NMDA-gated currents that were dramatically increased with the addition of recombinant NRAP-1 (Figure 6C). Following wash out, the current magnitude returned to the baseline value recorded prior to the addition of NRAP-1.
Our finding that NMDAR-mediated current is increased following addition of recombinant NRAP-1 is consistent with the increased current measured in transgenic worms that overexpressed NRAP-1 (Figure 2B). Together, our results indicate that the number of functional NMDARs depends on NRAP-1 levels. Thus, in wild-type worms, a large pool of non-functional (silent) NMDARs is present on AVA, suggesting that secreted NRAP-1 might be used as a mechanism to modulate synaptic strength by regulating the number of functional NMDARs.
Reconstitution of NMDAR function in muscles cells
We next asked whether the effects of recombinant NRAP-1 depend on factors present in AVA or at AVA synapses. That is, could we use recombinant NRAP-1 to reconstitute NMDAR function in heterologous cells? In preliminary studies in Xenopus ooctyes, we were unable to reconstitute currents by the addition of recombinant NRAP-1 presumably because the vitelline membrane that surrounds oocytes limits access of NRAP-1 protein (Cristofori-Armstrong et al., 2015). Therefore, we attempted reconstitution of NMDAR function in C. elegans muscle cells.
We could not detect NMDA-gated currents in muscle cells in transgenic worms that expressed NMR-1 and NMR-2 under control of the myo-3 muscle-specific promoter (Figure 6D). However, when NRAP-1 was also ectopically expressed in muscle, we could elicit small NMDA-gated current (Figure 6E). This is consistent with our reconstitution studies in Xenopus oocytes, where NMDA-gated currents depended on co-expression of NMR-1, NMR-2 and NRAP-1 (Figure 4). With the addition of recombinant NRAP-1, we measured large NMDA-gated currents in transgenic worms that expressed either NMR-1 and NMR-2 (Figure 6D) or NMR-1, NMR-2 and NRAP-1 (Figure 6E and 6F) in muscle cells. These currents rapidly diminished during wash out of NRAP-1 from the bath (Figure 6D and 6F), indicating that this phenomenon was not specific to neuronally expressed NMDARs. However, in contrast to currents in AVA (Figure 6C), we found that the increase in current with addition of recombinant NRAP-1 was typically 20- to 30-fold, suggesting that NRAP-1 was either not well expressed in muscle, was processed differently, or was more easily washed out because of a lack of stabilizing factors.
Our demonstration that the dramatic effects of recombinant NRAP-1 on NMDA-gated currents can be reversed in minutes by wash out (bath exchange), suggests that the association between NRAP-1 and NMDARs is relatively unstable, and that other factors contribute to the stability of endogenous NMDAR-mediated currents. Immunoprecipitation data support the notion that NRAP-1 is not tightly associated with NMDARs. We expressed epitope tagged NMR-1, NMR-2 and NRAP-1 in Xenopus oocytes, i.e., the combination of proteins sufficient to reconstitute NMDA-gated currents (Figure 4), and were able to co-immunoprecipitate NMR-1 and NMR-2, but not NRAP-1 using an antibody directed against epitope tagged NMR-1 (Figure S4). This result indicates that heterologously expressed NRAP-1 does not tightly associate with NMDARs and suggests that additional proteins or processes contribute to NRAP-1 modulation of NMDAR function in vivo.
Defective gating of NMDARs in nrap-1 mutants
We made the unexpected discovery that many NMDARs in wild-type neurons are non-functional, and that they can be rapidly converted to functional receptors following addition of recombinant NRAP-1 (Figures 5 and 6). To address how NRAP-1 might contribute to the gating of NMDARs, we examined currents in response to rapid uncaging of glutamate. We did so to avoid potential confounds secondary to delivery of drug by pressure pefusion where exchange times are typically greater than 10 ms and might obscure the true kinetics of receptor activation. Therefore, we activated NMDARs by rapid photo-release of glutamate (< 1 ms exchange). Using our standard in vivo recording preparation, we included MNI-caged-L-glutamate (1 mM), which can be uncaged by UV light to release glutamate in a defined region (Sobczyk and Svoboda, 2007). We used a 1 ms pulse of UV light (405 nm) to illuminate a small circular area (8 μm in diameter) centered on the AVA processes in the nerve ring. To isolate the NMDAR-mediated current, we recorded currents from a genetic background that removed interfering ligand-gated currents (see Method Details).
With light uncaging, we were able to reproducibly elicit NMDAR-mediated currents and reduce noise by averaging multiple current records in glr-1 mutants (Figure 7A). In response to uncaging, we measured currents similar in time course to those we previously measured in response to the slower pressure perfusion of glutamate or NMDA (Figure 2). This was expected, since both the activation and deactivation kinetics of NMDARs are slow when compared, for example, to the rapid kinetics of AMPARs (Erreger et al., 2004). However, as with nmr-1; glr-1 mutants, even with averaging we were unable to detect glutamate-gated currents in glr-1; nrap-1 mutants (Figure 7A).
Figure 7. NRAP-1 is required for the gating of NMDARs.
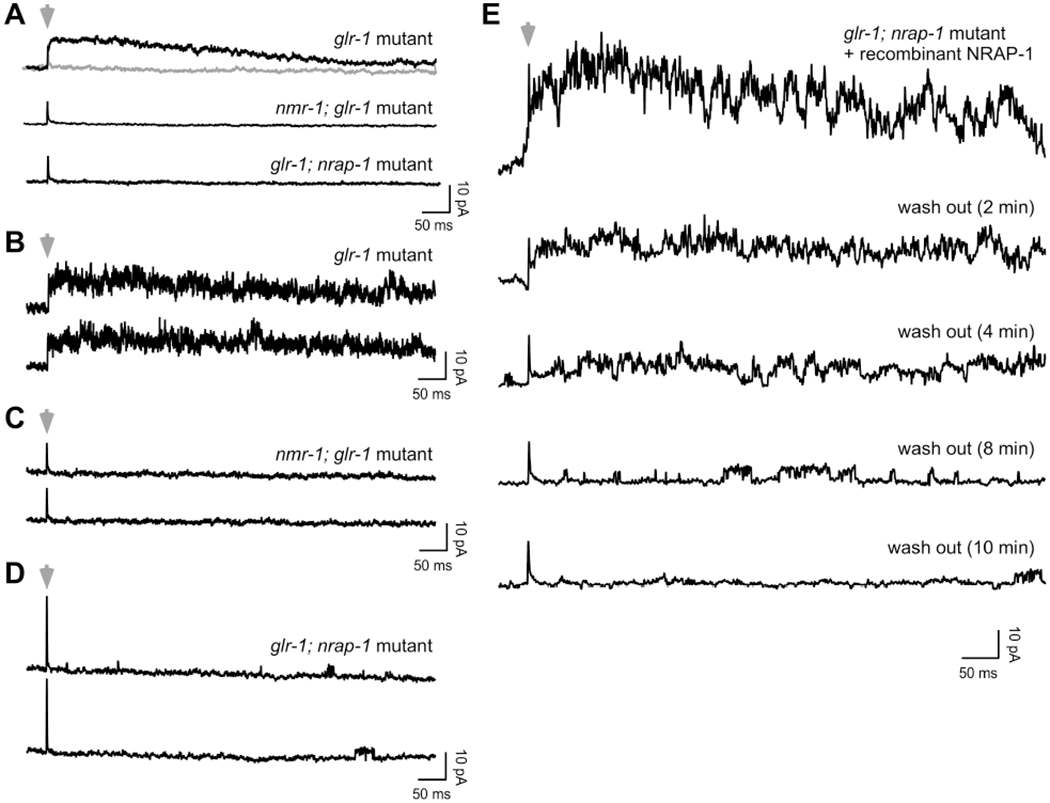
(A–E) Currents in response to rapid uncaging of glutamate. Cells were held at +40 mV. Gray arrowheads indicate a 1 ms pulse of UV light. Gray record shows the response to light in the absence of MNI-glutamate. (A) Shown are the averages of 10 uncaging events per genotype. (B–D) Individual current records (2 per genotype). (E) Individual current records in the presence of recombinant NRAP-1 and following 2, 4, 8 and 10 minutes of wash out.
We then examined individual current records for more subtle evidence of NMDAR activity. In glr-1 mutants, we observed large amplitude, noisy current traces, consistent with the parallel activity of independent channels (Figure 7B). In contrast, we did not detect current or single channel events in nmr-1; glr-1 mutants, indicating that these currents were mediated by NMDARs (Figure 7C). Notably, we observed infrequent, single channel events in glr-1; nrap-1 mutants following light activation in the presence of MNI-glutamate (Figure 7D), but not with light-only controls (data not shown). The channel activity was sporadic and the first appearance of channels opening typically occurred tens to hundreds of ms following glutamate uncaging.
To gain a better understanding of the relation between NRAP-1 and single channel activity, we added recombinant NRAP-1 to glr-1; nrap-1 mutants and used glutamate uncaging to elicit NMDAR-gated currents. We then measured the current during the wash out of NRAP-1 (Figure 7E). Using this strategy, we could clearly observe that the probability of channel events decreased with wash out of NRAP-1. Importantly, the currents during wash out were not simply scaled versions of the initial current. Instead, it appeared that the time-dependent probability of channel opening following uncaging decreased as NRAP-1 was washed out.
DISCUSSION
Presynaptic NRAP-1 mediates trans-synaptic regulation of NMDARs
We have described the identification and characterization of nrap-1, a gene that encodes a secreted, LDLa domain-containing protein required for NMDAR function and NMDAR-mediated synaptic transmission and control of behavior. NRAP-1 appears to be specifically required for NMDARs, as AMPAR-mediated current was not disrupted by the loss of nrap-1, and we observed additive behavioral defects in glr-1; nrap-1, but not nmr-2 nrap-1 double mutants. We demonstrated that NRAP-1 is expressed in presynaptic glutamatergic neurons yet co-localizes with and modulates the function of postsynaptic NMDARs. This suggests a model where NRAP-1 secreted from presynaptic neurons locally diffuses to and associates with NMDARs.
This model is further supported by our observation that NRAP-1 ectopically expressed in muscle cells not only co-localized with postsynaptic NMDARs, but was able to partially rescue NMDAR-mediated current in nrap-1 mutants. Two lines of evidence indicate that NRAP-1 diffusion is limited, however, and that NRAP-1 acts locally (synaptically) to modify NMDAR function. First, we did not detect NMDA-gated currents in muscle cells of transgenic worms that expressed NMR-1 and NMR-2 in muscle, and normal levels of NRAP-1 (from the endogenous nrap-1 gene), or even when NRAP-1 was overexpressed using its native promoter (data not shown). Second, we detected only very small currents in the AVA neurons of transgenic nrap-1 mutants that highly overexpressed NRAP-1 specifically in muscle cells (Figure 2).
The addition of recombinant NRAP-1 to the extracellular medium of nrap-1 mutants was sufficient to rapidly restore NMDA-gated current indicating that NRAP-1 modifies receptor function and is consistent with our hypothesis that NRAP-1 is not required for NMDAR surface expression. This observation, together with our reconstitution studies in Xenopus oocytes and C. elegans muscle cells, demonstrate that NRAP-1 functions as a NMDAR auxiliary protein that modulates receptor function. We also found that addition of the lectin Con-A increased current magnitude in oocytes that expressed NMR-1, NMR-2 and NRAP-1, and restored current in oocytes that expressed only NMR-1 and NMR-2. In vertebrates, Con-A is best known for modulating desensitization of kainate and AMPARs (Partin et al., 1993), but it can also enhance currents mediated by NMDARs (Yue et al., 1995). Because NRAP-1 and Con-A both impart function to C. elegans NMDARs, we hypothesize that NRAP-1 might in part modify the rate of NMDAR desensitization, or recovery from desensitzation. In this model, NMDARs are non-functional unless associated with NRAP-1.
We were unable to detect an effect of Con-A on either AMPAR- or NMDAR-mediated currents recorded from AVA interneurons in C. elegans (data not shown). The extent of protein glycosylation varies between different cell types (Vernon et al., 2017), and glycosylation of receptors in AVA might be insufficient for Con-A interaction. However, adding recombinant NRAP-1 rescued NMDA-gated currents in nrap-1 mutants, and was necessary to reconstitute NMDA-gated currents in muscle cells that ectopically expressed NMR-1 and NMR-2.
Application of recombinant NRAP-1 revealed the presence of silent, non-functional NMDARs
We found that addition of recombinant NRAP-1 to wild-type worms increased the magnitude of NMDA-gated current in AVA neurons. The increased current was dependent on the continued presence of recombinant NRAP-1. With exchange of the bath solution, the current rapidly approached the smaller peak magnitude measured prior to the addition of NRAP-1. These findings have several important implications. First, they suggest that a sizeable percentage of NMDARs are non-functional and might exist in a chronically desensitized state, but one that can be awakened by the addition of NRAP-1. Second, they indicate that the availability of NRAP-1 regulates the strength of NMDAR-mediated synaptic transmission. This notion is supported by behavioral and electrophysiological data from transgenic worms that overexpressed NRAP-1. Thus, NMDA-gated currents were significantly larger in these transgenic worms, resulting in a reduced average forward duration compared to wild-type worms (Figures 1 and 2).
NRAP-1 modifies NMDAR kinetics
We have provided multiple lines of evidence indicating that NMDARs are at synapses, but inactive, in nrap-1 mutants. These include analysis of endogenous synaptic events, reconstitution studies, the rapid effects of recombinant NRAP-1, imaging of synaptic NMDARs, and finally, glutamate uncaging experiments, which facilitated the analysis of NMDAR activation. In response to glutamate uncaging using a brief (1 ms) pulse of UV light, we observed characteristic activation of NMDAR-mediated currents. These currents were absent in nrap-1 mutants, but could be rescued by addition of exogenous NRAP-1. Subsequent wash out of NRAP-1 was particularly informative. The current elicited by uncaging progressively decreased in amplitude until only single channel events were detected. With additional wash out, the interval between single channel events increased resulting in an overall decrease in the number of events. These data provide a dramatic demonstration of the functional unsilencing of NMDARs by NRAP-1. Kinetic schemes for activation, desensitization, recovery from desensitization and deactivation are exceedingly complex (Erreger et al., 2004; Iacobucci and Popescu, 2017), thus, a complete and quantitative description of the concentration dependent effects of NRAP-1 on receptor function requires more detailed single channel analysis.
Trans-synaptic regulation of postsynaptic receptor function
Our results suggest a novel, trans-synaptic regulation of NMDARs that participates in the control of synaptic transmission. Our findings might also be relevant to current models of experience dependent plasticity. Thus, modifying either the secretion or synaptic stabilization of NRAP-1 might be mechanisms used to regulate the number of functional NMDARs, thereby modifying synaptic strength. With respect to secretion, it is of essential importance to identify the release machinery and whether patterns of synaptic activity differentially modify the probability of NRAP-1 secretion. With respect to stabilization, are molecules co-released that modify the stability of NRAP-1 at synapses? Do additional molecules contribute to the stability, localization or processing of synaptic NRAP-1, and how might these molecules and processes be regulated? One possible stabilization mechanism is the membrane anchoring of secreted proteins by glycosylphosphatidylinositol (GPI) linkage; however, NRAP-1 does not contain an obvious consensus sequence for GPI linkage. Regardless of the specific mechanisms, it is of significant interest to consider the impact of silent synaptic NMDARs on synaptic plasticity, and how receptors might be switched between silent and functional states. Perhaps inactive, extrasynaptic NMDARs can switch to functional receptors when modified by activity-dependent secretion of NRAP-1.
Auxiliary subunits modify function of all classes of iGluRs
Our identification of NRAP-1 as a NMDAR auxiliary protein revealed a new class of iGluR auxiliary protein that employs a novel cellular mechanism to modify synaptic transmission. It is notable that all iGluR auxiliary proteins identified to date, whether secreted (NRAP-1), or a multi- or single-pass transmembrane protein (Brockie et al., 2013; Farrow et al., 2015; Schwenk et al., 2009; Straub and Tomita, 2012; Wang et al., 2012; Zhang et al., 2014), modify the functional properties of iGluRs, in particular, the kinetics of receptor desensitization. Thus, these proteins can modify synaptic information processing by altering the band-pass characteristics of individual synapses.
This study firmly establishes the general principle that iGluR function, including NMDARs, is regulated by auxiliary proteins and highlights the value of genetic approaches to identify novel components required for synaptic function in vivo. Given the functional conservation of iGluR complexes documented to date, we can confidently predict that additional auxiliary proteins will be discovered. We anticipate that future studies will shed light on the orchestration of interactions between iGluR subunits and their auxiliary proteins at different synapses and cell types. With respect to NMDAR-mediated signaling, it will be of great interest to determine whether neuronal activity, or retrograde postsynaptic signals, modulate synaptic transmission by altering NRAP-1 expression, secretion or synaptic localization and stability.
Our identification of NRAP-1 might provide a valuable target for pharmacological intervention in neurological and psychiatric disorders. Because of the ancient origin of the LDLa domain and its widespread occurence in vertebrate proteins, we predict that functional homologues of NRAP-1 will be identified in vertebrates. Such was the case for SOL-1, an AMPAR auxiliary protein first identified using a genetic approach in C. elegans (Zheng et al., 2004), with related proteins subsequently identified in vertebrates (Straub and Tomita, 2012). Over a hundred human proteins contain LDLa domains, often in multiple repeats. Importantly, LDLa-containing proteins are found in the brain and in synaptic preparations, and have been implicated in neurological and psychiatric disorders (Nakajima et al., 2013; Vasli et al., 2016; Wasser et al., 2014; Xu et al., 2011).
STAR Methods
CONTACT FOR REAGENTS AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to, and will be fulfilled by, the Lead Contact, Andres V. Maricq (maricq@biology.utah.edu).
EXPERIMENTAL MODELS
C. elegans strains
Hermaphrodite nematodes, Caenorhabditis elegans (N2 Bristol strain, and mutant and transgenic strains derived from N2) were used in the study. All strains were raised at 20 °C under standard conditions. Germline transformation to generate transgenic strains was achieved as described (Brockie et al., 2001b). Transgenic worms were identified either by rescue of the lin-15(n765ts) mutant phenotype using a wild-type lin-15 transgene (pJM23), or by expression of a soluble fluorescent marker, e.g., Pegl-20::NLS::DsRed.
Transgenic arrays
Transgenic animals expressed the following transgenic arrays: akSi68, pCT48; akEx4537, pDM2416; akEx4572, pDM2431A + pDM1767 + pJM23; akEx4544, pDM2431A + pDM1196 + pJM23; akEx4687, pDM2392 + pDM1946 + pJM23; akEx4643, pDM2392 + pDM1807 + pJM23; akEx4647, pDM2500 + pJM23; akIs237, pDM2095 + pCT61; akEx4877, pDM2527 + pDM1490 + pDM2528 + pJM23; akEx4739, pDM2527 + pDM1494 + pJM23; akEx4539, pDM2396 + pDM1490; akIs151, pDM1580 + pWR43 + pJM23; akIs225, pDM1580 + pWR43 + pJM23; akEx4573, pDM2481 + pDM1436 + pJM23; akEx4579, pDM2482 + pDM1436 + pJM23; akEx5016, pDM2382 + pDM1436; akEx4674, pDM2480 + pDM1436 + pJM23; akEx4690, pDM2427 + pDM1436 + pJM23; akEx4672, pDM2502 + pDM1436 + pJM23; akEx4728, pDM2404 + pDM2398 + pCSW79 + pJM23; akEx4535, pDM2404 + pDM2398 + pDM2395 + pCSW79 + pJM23; akEx5033, pCT61 + Peat-4::nrap-1(sense RNA) + Peat-4::nrap-1(antisense RNA); akEx4500, pDM2392 + pDM1196.
METHOD DETAILS
Genetic screen
An F2 screen was used to isolate recessive mutations that suppressed the hyper-reversal behavior of transgenic nmr-1(ak4); glr-1(ky176); egl-3(gk238); akSi68 (Pnmr-1::nmr-1(N637Q; A674T)) worms. Approximately 200 transgenic worms (P0s) were mutagenized with 300 μM ENU at the L4 larval stage using standard techniques. F1 progeny of the mutagenized P0s, representing approximately 1300 haploid genomes, were clonally transferred (one F1 per plate) to NGM plates seeded with a lawn of the E. coli OP50 strain (food). F2 progeny of these worms were screened by simple visual inspection for those that no longer hyper-reversed (i.e., had a longer average forward duration than the starting strain). The nrap-1(ak365) mutation was identified by whole-genome sequencing and subsequent rescue of the mutant phenotype using a wild-type nrap-1 transgene expressed in transgenic nrap-1(ak365) mutants. The nrap-1(ak365) mutants were outcrossed with wild-type worms 5 times.
DNA plasmid constructs
The following plasmids were prepared using either a HiSpeed Plasmid Midi Kit (QIAGEN) or a PureLink HQ Mini Plasmid DNA Purification Kit (Invitrogen) and used to generate transgenic strains: pCT48, nmr-1(gf) allele: Pnmr-1::nmr-1(N637Q; A674T); pDM2416, Pnrap-1::nrap-1::mCherry; pDM2431A, Pnrap-1::nrap-1::SL2::NLS::gfp; pDM1767, Peat-4::mCherry; pDM1196, Pnmr-1::mCherry; pDM2392, Pnrap-1::gfp::nrap-1; pDM1946, Peat-4::eat-4(cDNA)::mCherry; pDM1807, Pflp-18::mCherry::nmr-2; pDM2500, Pnrap-1::SEP::mCherry::nrap-1(minigene); pDM2095, Pflp-18::SEP::mCherry::nmr-2; pCT61, Pegl-20::NLS::DsRed; pDM2527, Pmyo-3::nrap-1(cDNA)::gfp; pDM1494, Prig-3::mCherry; pDM2396, Pflp-18::nrap-1(cDNA)::gfp; pDM1490, Prig-3::mCherry::nmr-2; pDM2528, Pflp-18::mCherry:nmr-1; pDM1580, Pflp-18::cz-gfp; pWR43, Prig-3::nz-gfp; pDM2481, Peat-4::nrap-1(cDNA)::mCherry; pDM1436, Prig-3::gfp; pDM2482, Pnmr-1::nrap-1(cDNA)::mCherry; pDM2382, Pflp-18::nrap-1(cDNA)::mCherry; pDM2480; Pmyo-3::nrap-1(cDNA)::mCherry; pDM2427, Pnrap-1::Apis nrap; pDM2502, Pnrap-1::Dros nrap; pDM2404, Pmyo-3::nmr-1; pDM2398, Pmyo-3::nmr-2; pDM2395, Pmyo-3::nrap-1; pCSW79, Pmyo-3::gfp.
The nrap-1 promoter was 4.8 kb of genomic sequence immediately upstream of the ATG start codon. The nrap-1 minigene (derived from C. elegans transcript F58H1.7) included all 5 nrap-1 exons, but only intons 1 and 2 (introns 3 and 4 were deleted). N-terminal GFP::NRAP-1 and SEP::mCherry::NRAP-1, and C-terminal NRAP-1::GFP and NRAP-1::mCherry fusion proteins were generated by inserting the fluorophores in frame immediately after the predicted signal peptide (N-terminal fusions) or immediately before the predicted stop codon (C-terminal fusions). Fusion proteins were tested for function by rescue of either the behavioral or electrophysiology defects of the relevant mutant strain (Figure S2).
The nrap-1 cDNA was isolated by PCR amplification from clone yk1354b12, a gift from Y. Kohara. Analysis of the predicted NRAP-1 protein used the ExPASy suite of protein analysis programs (Gasteiger et al., 2003). The following plasmids were used to generate cRNA: pDM466, nmr-1 cDNA; pDM974, nmr-2 cDNA; pDM2388, nrap-1 cDNA; pDM2630, HA::nmr-1; pDM2632, Myc::nmr-2; and pDM2614, FLAG::nrap-1. All cDNAs used for expression in Xenopus oocytes were subcloned into the pSGEM expression vector. pDM2564, nrap-1 cDNA subcloned into pCDNA3.1, was used to transfect HEK 293 cells.
Microscopy
One-day old adult worms were mounted on 10% agarose pads and immobilized using approximately 1.5 μl of 30 mM muscimol. Confocal images were acquired using a Nikon Eclipse Ti microscope WaveFX-X1 spinning-disk confocal system (Quorum Technologies) and a Cascade II 1024 EMCCD camera (Photometrics).
Imaging analysis
The degree of co-localization between GFP::NRAP-1 and either EAT-4::mCherry or mCherry::NMR-2 in the ventral nerve chord (VNC) was assessed by first subtracting background fluorescence and then drawing a region of interest (ROI) in the VNC. The Coloc-2 analysis function in Fiji-ImageJ (https://imagej.net/Fiji) was then used to calculate Pearson’s correlation coefficient (PCC) between the two fluorescent signals within the ROI. Statistical significance between the PCC and that expected for no co-localization (a PCC value of 0) was determined using a one-sample, one-tailed Student’s t-test (McDonald and Dunn, 2013).
Quantification of SEP fluorescence in transgenic worms that expressed either SEP::mCherry::NRAP-1 or SEP::mCherry::NMR-2 was achieved by drawing a ROI along the VNC and measuring the ROI area and Integrated Density (IntDen) using ImageJ. A background measurement adjacent to the VNC was also made and the average fluorescence (corrected for background signal and ROI area) was determined: IntDen − (area x background).
Behavioral and electrophysiological studies
The average duration of forward movement (forward duration) was determined as previously described (Brockie et al., 2001b; Zheng et al., 2004), with the exception that a given worm’s movement was observed for 5 minutes. All behavioral assays were performed blind using young adult worms.
For electrophysiological recordings from AVA interneurons and muscle cells in vivo, drug delivery (3 mM glutamate or 1 mM NMDA) was by pressure application (Picospritzer II) and ligand-gated currents were measured using Patchmaster v2x73 (HEKA Electronik) and standard patch-clamp technology as previously described (Zheng et al., 2004). Cells were held at either +40 mV or −60 mV.
For rapid glutamate-uncaging (Sobczyk and Svoboda, 2007), we included MNI-caged-L-glutamate (1 mM) (ChiroBlock GMBH) in the preparation, and uncaged in a defined circular region using a 1 ms pulse of UV light (405 nm) that was 8 μm in diameter and targeted to AVA processes in the nerve.
To isolate current mediated by iGluRs in response to glutamate uncaging, and iGluR-mediated endogenous miniature synaptic events in AVA, all strains used in these experiments included the avr-14(ad1302) (GluCl channel subunit) and acr-16(ok789) (acetylcholine receptor subunit) mutations.
For expression in Xenopus oocytes, cRNAs were generated using mMessage mMachine (Invitrogen). Oocytes were injected with 5 ng of each cRNA using a Sutter NA-1 microinjector (Sutter Instruments Co.) and maintained in Modified Barth’s Saline for 4–8 days at 15 °C. Two-electrode voltage clamp recordings were performed as previously described (Walker et al., 2009). All recordings were made in a Ringer’s solution (NFR) consisting of NaCl (115 mM), CaCl2 (1.8 mM), KCl (2 mM) and HEPES (10 mM) with pH adjusted to 7.4 with NaOH; or a Barium Ringer’s where BaCl2 (1.8 mM) replaced CaCl2. Current electrodes were filled with KCl (3 M) and had resistances of approximately 0.5–1.5 MΩ. Voltage electrodes were filled with KCl (3 M) and had resistances of approximately 1-5 MΩ. Oocytes were held at −70 mV, and agonists or antagonists (NMDA, glutamate and MK-801) were applied by superfusion for 5 s. Concanavalin-A (2 mg/ml) (Sigma-Aldrich) was bath applied and allowed to incubate for 5–6 minutes to see potentiation of agonist-gated currents. Data were analyzed using IGOR Pro 7 (Wavemetrics).
Recombinant NRAP-1 and immunoprecipication
To generate recombinant NRAP-1, HEK 293 cells (80–90% confluent) were transfected with pDM2564 (nrap-1 in pCDNA3.1) DNA and Lipofectamine 2000 (Invitrogen) for 6 hrs in minimal Opti-MEM media. Complexes were removed before adding complete media and cells were allowed to grow for 6 days at 37 °C and 5% CO2 without changing the media. Conditioned media was then collected and spun at 1000 x g to pellet cells and debris before concentrating approximately 50–fold in Amicon Ultra-15 10K centrifugal filter (Millipore). Activity was seen with application of 50 ul of concentrated media. Concentrate was also run on LDS-PAGE gels and stained with Coomassie Brilliant Blue R-250. No band was discernible compared to non-transfected control media.
For immumoprecipitation experiments, isolated stage V to VI Xenopus laevis oocytes were injected with 10 ng of each cRNA and incubated for 6 days in Modified Barth’s media. Oocytes were collected in ice-cold IP buffer (150 mM NaCl, 25 mM Tris pH 7.5, 1% TX-100, 1 mM EDTA, 10% glycerol with cOmplete protease inhibitors (Sigma); 20 ul/oocyte) and homogenized with a microcentrifuge tube pestle. Lysates were spun at 1000 x g to pellet cell debris and carefully transferred to a fresh tube, with minimal transfer of yolk protein. 150 ul of total lysate was incubated with 2 ug of HA-probe antibody (sc-7392, Santa Cruz Biotechnology, Inc.) on ice for 2 hrs. 10 ul of Protein A-Agarose conjugated resin (sc-2001, SCBT) was washed and equilibrated with IP buffer before adding to immune complexes and agitated overnight at 4 °C. Samples were washed 3x with ice-cold IP buffer and protein was eluted by incubation with 2x Laemmli sample buffer and heating at 85 °C for 5 min. Samples were run on Bolt Bis-Tris 4-12% gels (Life Technologies) and transferred to nitrocellulose membranes. Blots were blocked overnight at 4 °C in Odyssey Blocking Buffer (LI-COR) and PBS plus 0.1% Tween-20. Identical blots were tested with either HA-probe (F-7, mouse monoclonal, sc-7392, SCBT) or c-Myc (9E11, mouse monoclonal, sc-47694, SCBT) and anti-FLAG antibody (rabbit polyclonal, F7425, Sigma). Blots were then treated with IRDye 680LT goat anti-rabbit and IRDye 800CW goat anti-mouse secondary antibodies and imaged with Odyssey Scanner (LI-COR).
QUANTIFICATION AND STATISTICAL ANALYSIS
Replication and randomization
Behavioral experiments (forward duration) were performed on young adult worms. The genotypes were randomized and the experiments were performed over multiple days to limit the effects of daily variation with all genotypes included each day. The behavioral assays reported in this study (performed by P.J.B.) were reproduced by N.L. (data not shown).
Electrophysiological recordings from AVA neurons and muscle cells in vivo were performed over multiple days with wild-type control animals included each day. Recordings were reproduced by measuring currents in multiple dissected animals per genotype (as stated in the figure legend).
Sample sizes used in this study were in accordance with previously published data. No data were excluded from the analysis.
Blinding
Blinding was achieved by assigning random numerical labels to each genotype. The number assigned to each genotype was revealed to the experimenter at the conclusion of the experiment.
Statistics
Statistical comparisons (software: GraphPad Prism 7) were performed using either a non-parametric Mann-Whitney U test (when sample sizes were small, i.e., n < 10), an unpaired (two-tailed) t-test with Welch’s correction, or a one-sample, one-tailed Student’s t-test as indicated in the figure legends and Results, and presented as mean ± standard error of the mean (SEM). Groups were considered statistically differenct from one another if p < 0.05. Samples sizes are listed in the figure legends and no statistical methods were used to predetermine sample size.
DATA AND SOFTWARE AVAILABILITY
Sequence data for the nrap-1 cDNA has been deposited in GenBank with the accession number MF282010.
Supplementary Material
Highlights.
NRAP-1 is a NMDA receptor auxiliary protein required for receptor function
NRAP-1 is secreted by presynaptic glutamatergic neurons
NRAP-1 co-localizes with postsynaptic NMDA receptors
In the absence of NRAP-1, NMDARs are on the cell surface, but non-functional.
ACKNOWLEDGMENTS
We thank members of the Maricq laboratory, Monica Vetter and Susumu Tomita (Yale University) for comments on the manuscript, Pierre Paoletti (Ecole Normale Superiéure) for early discussions about strategies to generate gain-of-function NMDARs, Colin Thacker for generating the akSi68 strain, Frédéric Hoerndli for help with imaging analysis, and Rui Wang for assistance with the genetic screen. We thank Y. Kohara (National Institute of Genetics, Japan) for the yk1354b12 clone. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). This research was supported by the National Institutes of Health Pioneer Award, and by support from the NARSAD foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Brockie PJ, Jensen M, Mellem JE, Jensen E, Yamasaki T, Wang R, Maxfield D, Thacker C, Hoerndli F, Dunn PJ, et al. (2013). Cornichons Control ER Export of AMPA Receptors to Regulate Synaptic Excitability. Neuron 80, 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockie PJ, Madsen DM, Zheng Y, Mellem J, and Maricq AV (2001a). Differential expression of glutamate receptor subunits in the nervous system of Caenorhabditis elegans and their regulation by the homeodomain protein UNC-42. J Neurosci 21, 1510–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockie PJ, Mellem JE, Hills T, Madsen DM, and Maricq AV (2001b). The C. elegans glutamate receptor subunit NMR-1 is required for slow NMDA-activated currents that regulate reversal frequency during locomotion. Neuron 31, 617–630. [DOI] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, and Nicoll RA (2000). Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 408, 936–943. [DOI] [PubMed] [Google Scholar]
- Copits BA, and Swanson GT (2012). Dancing partners at the synapse: auxiliary subunits that shape kainate receptor function. Nat Rev Neurosci 13, 675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofori-Armstrong B, Soh MS, Talwar S, Brown DL, Griffin JD, Dekan Z, Stow JL, King GF, Lynch JW, and Rash LD (2015). Xenopus borealis as an alternative source of oocytes for biophysical and pharmacological studies of neuronal ion channels. Sci Rep 5, 14763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Chen PE, Wyllie DJ, and Traynelis SF (2004). Glutamate receptor gating. Crit Rev Neurobiol 16, 187–224. [DOI] [PubMed] [Google Scholar]
- Fares H, and Greenwald I (2001). Genetic analysis of endocytosis in Caenorhabditis elegans: coelomocyte uptake defective mutants. Genetics 159, 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow P, Khodosevich K, Sapir Y, Schulmann A, Aslam M, Stern-Bach Y, Monyer H, and von Engelhardt J (2015). Auxiliary subunits of the CKAMP family differentially modulate AMPA receptor properties. eLife 4, e09693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass D, Blacklow S, Kim PS, and Berger JM (1997). Molecular basis of familial hypercholesterolaemia from structure of LDL receptor module. Nature 388, 691–693. [DOI] [PubMed] [Google Scholar]
- Frank RA, Komiyama NH, Ryan TJ, Zhu F, O’Dell TJ, and Grant SG (2016). NMDA receptors are selectively partitioned into complexes and supercomplexes during synapse maturation. Nature communications 7, 11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT Jr., Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, and Edwards RH (2004). Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science 304, 1815–1819. [DOI] [PubMed] [Google Scholar]
- Gally C, Eimer S, Richmond JE, and Bessereau JL (2004). A transmembrane protein required for acetylcholine receptor clustering in Caenorhabditis elegans. Nature 431, 578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, and Bairoch A (2003). ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 31, 3784–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AZ, and Pettit DL (2007). Extrasynaptic and synaptic NMDA receptors form stable and uniform pools in rat hippocampal slices. J Physiol 584, 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, O’Shea-Greenfield A, Rogers SW, and Heinemann S (1989). Cloning by functional expression of a member of the glutamate receptor family. Nature 342, 643–648. [DOI] [PubMed] [Google Scholar]
- Horak M, Petralia RS, Kaniakova M, and Sans N (2014). ER to synapse trafficking of NMDA receptors. Frontiers in cellular neuroscience 8, 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe JR (2015). Modulation of non-NMDA receptor gating by auxiliary subunits. J Physiol 593, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobucci GJ, and Popescu GK (2017). NMDA receptors: linking physiological output to biophysical operation. Nat Rev Neurosci 18, 236–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano T, Brockie PJ, Sassa T, Fujimoto H, Kawahara Y, Iino Y, Mellem JE, Madsen DM, Hosono R, and Maricq AV (2008). Memory in Caenorhabditis elegans is mediated by NMDA-type ionotropic glutamate receptors. Curr Biol 18, 1010–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass J, Jacob TC, Kim P, and Kaplan JM (2001). The EGL-3 proprotein convertase regulates mechanosensory responses of Caenorhabditis elegans. J Neurosci 21, 9265–9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, and Roche KW (2004). Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci 24, 6383–6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RY, Sawin ER, Chalfie M, Horvitz HR, and Avery L (1999). EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate cotransporter, is necessary for glutamatergic neurotransmission in Caenorhabditis elegans. J Neurosci 19, 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JP, and Kelso SR (1990). Apparent desensitization of NMDA responses in Xenopus oocytes involves calcium-dependent chloride current. Neuron 4, 53–60. [DOI] [PubMed] [Google Scholar]
- McDonald JH, and Dunn KW (2013). Statistical tests for measures of colocalization in biological microscopy. J Microsc 252, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellem JE, Brockie PJ, Zheng Y, Madsen DM, and Maricq AV (2002). Decoding of Polymodal Sensory Stimuli by Postsynaptic Glutamate Receptors in C. elegans. Neuron 36, 933–944. [DOI] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, and Rothman JE (1998). Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192–195. [DOI] [PubMed] [Google Scholar]
- Nakajima C, Kulik A, Frotscher M, Herz J, Schafer M, Bock HH, and May P (2013). Low density lipoprotein receptor-related protein 1 (LRP1) modulates N-methyl-D-aspartate (NMDA) receptor-dependent intracellular signaling and NMDA-induced regulation of postsynaptic protein complexes. J Biol Chem 288, 21909–21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, and Roche KW (2013). Long-term potentiation: peeling the onion. Neuropharmacology 74, 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, and Zhou Q (2013). NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14, 383–400. [DOI] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Winters CA, Mayer ML, and Buonanno A (1993). Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron 11, 1069–1082. [DOI] [PubMed] [Google Scholar]
- Petrie EJ, Lagaida S, Sethi A, Bathgate RA, and Gooley PR (2015). In a Class of Their Own - RXFP1 and RXFP2 are Unique Members of the LGR Family. Front Endocrinol (Lausanne) 6, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prybylowski K, Fu Z, Losi G, Hawkins LM, Luo J, Chang K, Wenthold RJ, and Vicini S (2002). Relationship between availability of NMDA receptor subunits and their expression at the synapse. J Neurosci 22, 8902–8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk J, Harmel N, Zolles G, Bildl W, Kulik A, Heimrich B, Chisaka O, Jonas P, Schulte U, Fakler B, et al. (2009). Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science 323, 1313–1319. [DOI] [PubMed] [Google Scholar]
- Sobczyk A, and Svoboda K (2007). Activity-dependent plasticity of the NMDA-receptor fractional Ca2+ current. Neuron 53, 17–24. [DOI] [PubMed] [Google Scholar]
- Straub C, and Tomita S (2012). The regulation of glutamate receptor trafficking and function by TARPs and other transmembrane auxiliary subunits. Curr Opin Neurobiol 22, 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalhammer A, Everts I, and Hollmann M (2002). Inhibition by lectins of glutamate receptor desensitization is determined by the lectin’s sugar specificity at kainate but not AMPA receptors. Molecular and cellular neurosciences 21, 521–533. [DOI] [PubMed] [Google Scholar]
- Tomita S, Adesnik H, Sekiguchi M, Zhang W, Wada K, Howe JR, Nicoll RA, and Bredt DS (2005). Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature 435, 1052–1058. [DOI] [PubMed] [Google Scholar]
- Tomita S, and Castillo PE (2012). Neto1 and Neto2: auxiliary subunits that determine key properties of native kainate receptors. J Physiol 590, 2217–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasli N, Ahmed I, Mittal K, Ohadi M, Mikhailov A, Rafiq MA, Bhatti A, Carter MT, Andrade DM, Ayub M, et al. (2016). Identification of a homozygous missense mutation in LRP2 and a hemizygous missense mutation in TSPYL2 in a family with mild intellectual disability. Psychiatr Genet 26, 66–73. [DOI] [PubMed] [Google Scholar]
- Vernon CG, Copits BA, Stolz JR, Guzman YF, and Swanson GT (2017). N-glycan content modulates kainate receptor functional properties. J Physiol 595, 5913–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk L, Chiu SL, Sharma K, and Huganir RL (2015). Glutamate Synapses in Human Cognitive Disorders. Annu Rev Neurosci. [DOI] [PubMed] [Google Scholar]
- Walker CS, Brockie PJ, Madsen DM, Francis MM, Zheng Y, Koduri S, Mellem JE, Strutz-Seebohm N, and Maricq AV (2006a). Reconstitution of invertebrate glutamate receptor function depends on stargazin-like proteins. Proc Natl Acad Sci U S A 103, 10781–10786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CS, Francis MM, Brockie PJ, Madsen DM, Zheng Y, and Maricq AV (2006b). Conserved SOL-1 proteins regulate ionotropic glutamate receptor desensitization. Proc Natl Acad Sci U S A 103, 10787–10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CS, Jensen S, Ellison M, Matta JA, Lee WY, Imperial JS, Duclos N, Brockie PJ, Madsen DM, Isaac JT, et al. (2009). A novel Conus snail polypeptide causes excitotoxicity by blocking desensitization of AMPA receptors. Curr Biol 19, 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Mellem JE, Jensen M, Brockie PJ, Walker CS, Hoerndli FJ, Hauth L, Madsen DM, and Maricq AV (2012). The SOL-2/Neto Auxiliary Protein Modulates the Function of AMPA-Subtype Ionotropic Glutamate Receptors. Neuron 75, 838–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Walker CS, Brockie PJ, Francis MM, Mellem JE, Madsen DM, and Maricq AV (2008). Evolutionary conserved role for TARPs in the gating of glutamate receptors and tuning of synaptic function. Neuron 59, 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser CR, Masiulis I, Durakoglugil MS, Lane-Donovan C, Xian X, Beffert U, Agarwala A, Hammer RE, and Herz J (2014). Differential splicing and glycosylation of Apoer2 alters synaptic plasticity and fear learning. Sci Signal 7, ra113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won S, Incontro S, Nicoll RA, and Roche KW (2016). PSD-95 stabilizes NMDA receptors by inducing the degradation of STEP61. Proc Natl Acad Sci U S A 113, E4736–4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Roos JL, Dexheimer P, Boone B, Plummer B, Levy S, Gogos JA, and Karayiorgou Karayiorgou M (2011). Exome sequencing supports a de novo mutational paradigm for schizophrenia. Nature genetics 43, 864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue KT, MacDonald JF, Pekhletski R, and Hampson DR (1995). Differential effects of lectins on recombinant glutamate receptors. Eur J Pharmacol 291, 229–235. [DOI] [PubMed] [Google Scholar]
- Zhang W, Devi SP, Tomita S, and Howe JR (2014). Auxiliary proteins promote modal gating of AMPA- and kainate-type glutamate receptors. Eur J Neurosci 39, 1138–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, St-Gelais F, Grabner CP, Trinidad JC, Sumioka A, Morimoto-Tomita M, Kim KS, Straub C, Burlingame AL, Howe JR, et al. (2009). A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron 61, 385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Mellem JE, Brockie PJ, Madsen DM, and Maricq AV (2004). SOL-1 is a CUB-domain protein required for GLR-1 glutamate receptor function in C. elegans. Nature 427, 451–457. [DOI] [PubMed] [Google Scholar]
- Zhou Q, and Sheng M (2013). NMDA receptors in nervous system diseases. Neuropharmacology 74, 69–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data for the nrap-1 cDNA has been deposited in GenBank with the accession number MF282010.


