Abstract
Dietzia maris, an environmental actinomycete, has been implicated only once in human disease. We herein report the first D. maris isolate from a bone biopsy specimen in a patient hospitalized for a total hip prosthesis replacement. Cell wall fatty acid analysis and 16S ribosomal DNA gene sequencing were utilized to achieve its definite identification. This case report illustrates the usefulness of such methods for the accurate identification of actinomycetes.
The genus Dietzia includes two species of gram-positive, aerobic, mycolic acid-containing actinomycetes that lack aerial mycelium (9). Dietzia maris has been isolated from soil and from the skin and intestinal tract of a carp (8, 9), and Dietzia natronolimnaios was isolated from an east African soda lake (4). Previously classified as Rhodococcus maris (8), D. maris has subsequently been transferred to a new genus, Dietzia, proposed on the basis of the peculiar structure of polar lipids, the presence of short-chain mycolic acids, and 16S ribosomal DNA (rDNA) sequence-based phylogenetic evidence (9). Although 13 environmental isolates of D. maris have been reported (1–3, 7–11), only one report of a human infection with this unusual bacterial species has been published (2). We herein report on a case of total hip prosthesis infection due to D. maris.
Case report.
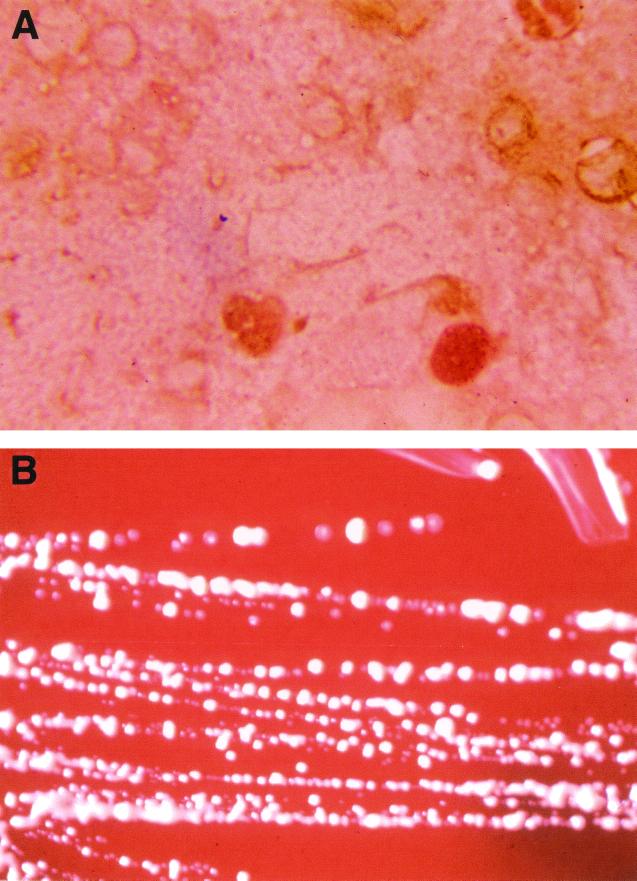
A 48-year-old man was admitted for a left total hip prosthesis replacement. His medical history included a traumatic hip fracture and total hip prosthesis in 1982 and two total hip prosthesis replacements for Staphylococcus epidermidis infection with unstable prosthesis. In 1998, a relapse of S. epidermidis infection led to total hip prosthesis ablation and the implantation of a vancomycin-impregnated cement spacer. Postoperative local care given to the patient included the cleansing of the wound with a physiological salt solution. Antibiotic treatment with vancomycin, rifampin, and ofloxacine was administrated, and another hip prosthesis was implanted in November 1999. At implantation, direct examination of a Gram-stained surgical bone biopsy specimen disclosed many leukocytes and gram-positive cocci that germinated into short rods (Fig. 1A). The specimen did not exhibit residual antibiotic activity as determined using a susceptible strain of Micrococcus luteus and of S. epidermidis as previously described (15). Culture of this specimen on 5% sheep blood agar (bioMérieux, Marcy l'Etoile, France) yielded rare, small, orange-pigmented colonies after 3 days of incubation at 37°C under aerobic conditions (Fig. 1B). The isolate was subcultured on 5% sheep blood agar and Mueller-Hinton agar (bioMérieux) within 72 h at 37°C under aerobic conditions or a 10% CO2 atmosphere and demonstrated short rods with coccoid forms that did not form mycelia. It did not stain by the Ziehl-Neelsen method. A test for catalase was positive. Biochemical characteristics determined by API Coryne strip (bioMérieux) were in accordance to those previously described (2, 5, 8, 9). Among the variable characters, the isolate lacked urease activity, hydrolyzed gelatin, and exhibited an alkaline phosphatase activity; it did not utilized glucose or xylose as a carbon source (Table 1). The isolate, identified as D. maris, was susceptible to amoxicillin (MIC of <4 μg/ml), imipenem (MIC of <4 μg/ml), gentamicin (MIC of 0.04 μg/ml), pristinamycin (MIC of <2 μg/ml), trimethoprim-sulfamethoxazole (MIC of 0.06 μg/ml), rifampin (MIC of 0.11 μg/ml), clindamycin (MIC of <2 μg/ml), and vancomycin (MIC of <4 μg/ml). Antibiotic susceptibility was performed by the disk diffusion method on Mueller-Hinton agar (bioMérieux) after 3 days of incubation at 37°C under aerobic conditions. All other microbiologic investigations, including those of a set of blood cultures, superficial specimen around the area of implantation, and spacer specimen, remained negative. The patient was successfully treated with teicoplanin for 4 months.
FIG. 1.
(A) Gram staining of a smear from a surgical biopsy specimen reveals gram-positive cocci which germinate into short rods. Magnification, ×1,000. (B) Growth of D. maris on 5% sheep blood agar after 3 days of incubation at 37°C under aerobic conditions, showing smooth and orange-pigmented colonies.
TABLE 1.
Phenotypic characteristics of an D. maris hip prosthesis infection isolate
| Characteristic or test | Results for isolate(s)
|
|
|---|---|---|
| Present work | Type strainsa | |
| Aerial hyphae | − | − |
| Color of colony | Orange | Orange |
| Acid-fast staining | − | − |
| Catalase production | + | + |
| Urease activity | − | Vb |
| Nitrate reductase activity | + | + |
| Hydrolysis of gelatin | − | V |
| Hydrolysis of esculin | − | − |
| Carbon substrate assimilation tests | ||
| Glucose | − | V |
| Xylose | − | V |
| Maltose | − | − |
| Lactose | − | − |
| Mannitol | − | − |
| Production of: | ||
| Alkaline phosphatase | + | V |
| α-Glucosidase | + | + |
| β-Galactosidase | − | − |
| Pyrazinamidase | + | + |
| G+C DNA content (mol%) | 71.70 ± 1.63c | 73.00 |
The cell wall fatty acid composition was determined by gas chromatography (13) on a culture grown for 48 h on Trypticase soy agar (bioMérieux). The number of peaks and retention times were exactly the same as those reported for the type strain DSM 43672 of D. maris and different from those for reference strains of other aerobic actinomycetes. The mean and standard deviation of guanosine plus cytosine (G+C) content were determined five times by using high-pressure liquid chromatography with a model 46200A system pump (Merck Clevenot, Nogent sur Marne, France). An aliquot of 5 μl of the hydrolysate was applied onto the Nucleosil 5C18 Lichrocart column (4 by 250 mm; Merck). Elution was carried out at room temperature with a mixture of 0.2 M NH4H2PO4 (pH 4.5) and acetonitrile (96:4 [vol/vol]). A flow rate of 1 ml/min was used, and the absorbance was monitored at 270 nm. The calibration curve was obtained from a mixture of four standard nucleotides (5 nmol/ml in distilled water; Sigma Chemical Co., St. Louis, Mo.). After chromatography, the relative concentration of each nucleotide was calculated on the basis of the peak area in the high-pressure liquid chromatography elution profile and was corrected as described previously (12). The mean and standard deviation of G+C content of five determinations were 71.70% ± 1.63%, a value similar to that previously reported (5).
DNA was later extracted from colonies in 100 μl of Tris-EDTA buffer (10 mM Tris, 1 mM EDTA, 0.1 M NaCl) at pH 8.0 and incubated for 1 h at 37°C. Digestion was supplemented by 40 μl of proteinase K solution (25 mg of proteinase K per ml) and 25 μl of 10% sodium dodecyl sulfate for 1 h at 55°C. A total of 200 μl of 4 M guanidine thiocyanate was added, left for 1 h at room temperature, and then heated at 100°C for 10 min with 50 μl of 0.5 M NaOH. Final extraction of nucleic acid was carried out by using a QIAmp kit (Qiagen, Hilden, Germany). PCR-mediated amplification of the 16S rDNA and sequence determinations were performed as previously described (6, 14). The 1,455-bp sequence was aligned and compared with all eubacterial 16S rDNA gene sequences available in the GenBank database by using the multisequence Advanced Blast National Center for Biotechnology Information comparison software. The highest 16S rDNA sequence similarity value of 98% was obtained for the D. maris 16S rDNA sequence (GenBank accession no. Y18883) and for the D. maris DSM 43672T 16S rDNA sequence (GenBank accession no. X79290).
In this case, a pathogenic role for D. maris, an environmental microorganism, was supported by its presence on direct examination of and its isolation in pure culture from a surgical biopsy specimen. Additionally, we did not isolate the organism from other clinical samples during the same period of time, ruling out laboratory contamination. D. maris has on one previous occasion been reported as responsible for bacteremia in an immunocompromised patient, and the infection was associated with the presence of a catheter (2). These two cases suggest that D. maris should be considered a potential nosocomial pathogen associated with foreign material. In addition, both D. maris and D. natronolimnaios have been recovered from salted environments (4, 8, 9). The fact that the surgical site had been washed using saline solutions may also have contributed to the growth of D. maris in our patient.
The API Coryne strip used for initial screening tests (enzymatic tests and carbohydrate fermentation reactions) allowed the identification of this D. maris strain as Rhodococcus species sensu lato. However, both cellular fatty acid analysis and molecular methods provided accurate identification of our isolate at the species level within two working days, and both methods could be alternatively proposed for the identification of D. maris. Accurate identification of D. maris, as well as other aerobic actinomycetes, cannot be achieved in a quick or straightforward manner by traditional phenotypic-assessment methods. A more widespread introduction of new methods, such as fatty acid analysis and, especially, 16S rDNA gene sequence analysis, to complement existing schemes is likely to help in their identification and should increase our knowledge regarding the clinical spectrum of D. maris human infections.
Acknowledgments
We acknowledge M. N. Mallet and M. J. Casagrande for their technical assistance and R. J. Birtles for reviewing the manuscript.
REFERENCES
- 1.Andersson M A, Mikkola R, Kroppenstedt R M, Rainey F A, Peltola J, Helin J, Sivonen K, Salkinoja-Salonen M S. The mitochondrial toxin produced by Streptomyces griseus strains isolated from an indoor environment is valinomycin. Appl Environ Microbiol. 1998;64:4767–4773. doi: 10.1128/aem.64.12.4767-4773.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bemer-Melchior P, Haloun A, Riegel P, Drugeon H B. Bacteremia due to Dietzia maris in an immunocompromised patient. Clin Infect Dis. 1998;29:1340–1341. doi: 10.1086/313490. [DOI] [PubMed] [Google Scholar]
- 3.Colquhoun J A, Mexson J, Goodfellow M, Ward A C, Horikoshi K, Bull A T. Novel rhodococci and other mycolate actinomycetes from the deep sea. Antonie Leeuwenhoek. 1998;74:27–40. doi: 10.1023/a:1001743625912. [DOI] [PubMed] [Google Scholar]
- 4.Duckworth A W, Grant S, Grant W D, Jones B E, Meijer D. Dietzia natrolimnaios sp. nov., a new member of the genus Dietzia isolated from an east African soda lake. Extremophiles. 1998;2:359–366. doi: 10.1007/s007920050079. [DOI] [PubMed] [Google Scholar]
- 5.Goodfellow M. Genus Rhodococcus. In: Williams S T, Sharpe M E, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 4. Baltimore, Md: The Williams & Wilkins Co.; 1989. pp. 2362–2370. [Google Scholar]
- 6.La Scola B, Birtles R J, Mallet M N, Raoult D. Massilia timonae gen. nov., sp. nov., isolated from blood of an immunocompromised patient with cerebellar lesions. J Clin Microbiol. 1998;36:2847–2852. doi: 10.1128/jcm.36.10.2847-2852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Misono H, Yonezawa J, Nagata S, Nagasaki S. Purification and characterization of a dimeric phenylalanine dehydrogenase from Rhodococcus maris K-18. J Bacteriol. 1989;171:30–36. doi: 10.1128/jb.171.1.30-36.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nesterenko O A, Nogina T M, Kasumova S A, Kvasnikow E I, Batrakov S G. Rhodococcus luteus nom. nov. and Rhodococcus maris nom. nov. Int J Syst Bacteriol. 1982;32:1–14. [Google Scholar]
- 9.Rainey F A, Klatte S, Kroppenstedt R M, Stackebrandt E. Dietzia, a new genus including Dietzia maris comb. nov., formerly Rhodococcus maris. Int J Syst Bacteriol. 1995;45:32–36. doi: 10.1099/00207713-45-1-32. [DOI] [PubMed] [Google Scholar]
- 10.Ruimy R, Riegel P, Boiron P, Monteil H, Christen R. Phylogeny of the genus Corynebacterium deduced from analyses of small-subunit ribosomal DNA sequences. Int J Syst Bacteriol. 1995;45:740–746. doi: 10.1099/00207713-45-4-740. [DOI] [PubMed] [Google Scholar]
- 11.Saadoun I, el-Migdadi F. Degradation of geosmin-like compounds by selected species of gram-positive bacteria. Lett Appl Microbiol. 1998;26:98–100. doi: 10.1046/j.1472-765x.1998.00280.x. [DOI] [PubMed] [Google Scholar]
- 12.Tamaoka J, Komagata K. Determination of DNA base composition by reversed-phase high-performance liquid chromatography. FEMS Microbiol Lett. 1984;25:125–128. [Google Scholar]
- 13.Van Graevenitz A, Osterhout G, Dick J. Grouping of some clinically relevant garm-positive rods by automated fatty acid analysis. Diagnostic implications. APMIS. 1991;99:147–154. doi: 10.1111/j.1699-0463.1991.tb05132.x. [DOI] [PubMed] [Google Scholar]
- 14.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zannier A, Drancourt M, Franceschi J P, Aubaniac J M, Raoult D. Intérêt d'une technique de lyse cellulaire par choc thermique et de la détermination de l'activité antibiotique des prélèvements dans l'isolement de bactéries responsables d'infections ostéo-articulaires. Pathol Biol. 1991;39:543–546. [PubMed] [Google Scholar]