Abstract
For cancer screening to be successful, it should primarily detect cancers with lethal potential or their precursors early, leading to therapy that reduces mortality and morbidity. Screening programmes have been successful for colon and cervical cancers, where subsequent surgical removal of precursor lesions has resulted in a reduction in cancer incidence and mortality. However, many types of cancer exhibit a range of heterogeneous behaviours and variable likelihoods of progression and death. Consequently, screening for some cancers may have minimal impact on mortality and may do more harm than good. Since the implementation of screening tests for certain cancers (for example, breast and prostate cancers), a spike in incidence of in situ and early-stage cancers has been observed, but a link to reduction in cancer-specific mortality has not been as clear. It is difficult to determine how many of these mortality reductions are due to screening and how many are due to improved treatments of tumours. In cancers with lower incidence but high mortality (for example, pancreatic cancer), screening has focused on high-risk populations, but challenges similar to those for general population screening remain, particularly with regard to finding lesions with difficult-to-characterize malignant potential (for example, intraductal papillary mucinous neoplasms). More sensitive screening methods are detecting smaller and smaller lesions, but this has not been accompanied by a comparable reduction in the incidence of invasive cancers. In this Opinion article, we focus on the contribution of screening in general and high-risk populations to overdiagnosis, the effects of overdiagnosis on patients and emerging strategies to reduce overdiagnosis of indolent cancers through an understanding of tumour heterogeneity, the biology of how cancers evolve and progress, the molecular and cellular features of early neoplasia and the dynamics of the interactions of early lesions with their surrounding tissue microenvironment.
The goal of cancer screening is to detect either a preneoplastic lesion or a cancer at an early stage where treatment will change the outcome and prolong survival. Two cancers for which screening has been shown to reduce cancer-specific mortality are colorectal cancer1 (for example, a UK trial that enrolled 170,000 patients and a US trial that enrolled 155,000 patients found that screening with flexible sigmoidoscopy reduced colorectal cancer mortality by 31% and 26%, respectively2,3) and cervical cancer (for example, a screening study in England in which 11,619 women were diagnosed with cervical cancer reported that screening resulted in a 70% reduction in cervical cancer deaths4). These reductions in mortality are largely due to the removal of preneoplastic lesions: colon polyps and cervical intraepithelial neoplasia. However, these major clinical and public health benefits come at a cost of detection of many lesions with little or no lethal potential or even risk of progression to invasive cancers. A review of US Medicare claims that, of 1.8 million colonoscopies performed, approximately 30% of the patients had polyps5. Most of these polyps will not develop into cancer, but they are surgically removed during colonoscopy. It is estimated that only approximately 5% of adenomas if not removed would progress to cancer. A polyp surveillance study in the USA reported that only 22% of 306 small polyps grew, 28% shrank (10% completely regressed) and 50% remained stable6. While colonoscopy is a relatively safe procedure, there are adverse events, including approximately 4 perforations and 8 instances of major bleeding per 10,000 colonoscopies7. However, on the basis of randomized controlled trials in both the USA and Europe, the United States Preventive Services Task Force (USPSTF) has concluded that, for asymptomatic adults aged 50–75 years, the benefits of screening outweigh the potential harms7. For other cancers, the benefits of screening, especially on the extent of mortality reduction, are less clear, and the harms of screening may outweigh the benefits. A major potential harm of screening is overdiagnosis.
Overdiagnosis is generally defined as the diagnosis of disease that would never cause symptoms or death during a given patient’s lifetime. Overdiagnosis is distinct from misdiagnosis. Overdiagnosis is primarily driven by screening asymptomatic patients. Screening can detect not only asymptomatic cancers that are destined to cause harm but also indolent or benign forms of the disease that will never harm the patient (FIG. 1). In fact, most available screening tests are better at detecting slow-growing tumours than detecting rapidly progressing ones, a phenomenon known as length-biased sampling (FIG. 2). Overdiagnosis should also not be confused with a false positive result: a positive test in an individual who does not have cancer. For example, a false positive occurs when a patient has a positive faecal immunochemical test but is found not to have cancer or polyps on a subsequent colonoscopy exam. By contrast, an overdiagnosed patient has a tumour that fulfils the histopathological criteria for cancer.
Fig. 1 |. Magnitude of the problem of overdiagnosis owing to screening.
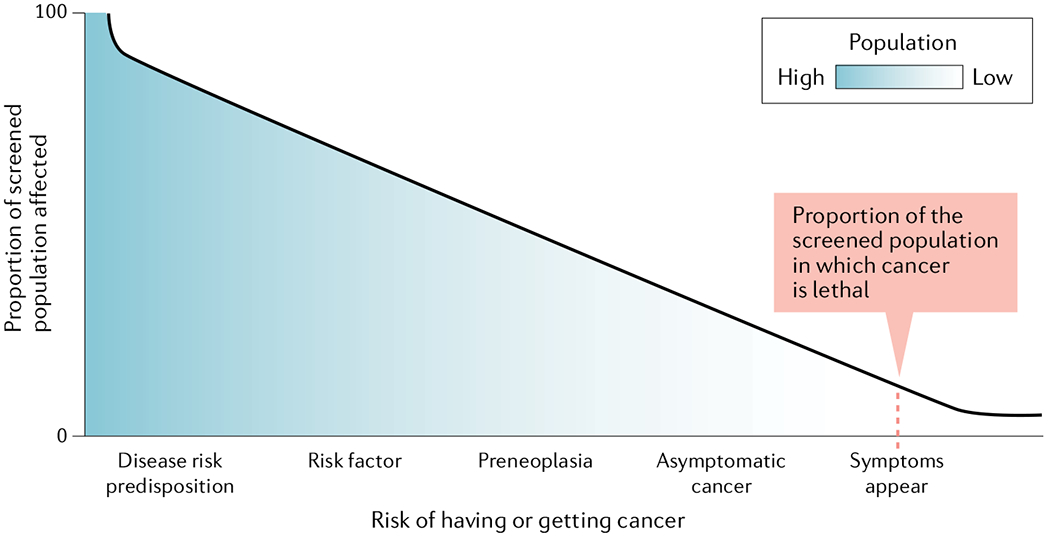
This schematic illustrates that only a small proportion of a screened population will have cancer that is lethal. However, screening for asymptomatic cancers, preneoplastic lesions or risk factors has the potential to label very large numbers of people as at risk , including those who were not destined to develop life-threatening disease.
Fig. 2 |. Slow versus rapid progressors — unpredictable tumour growth trajectory.
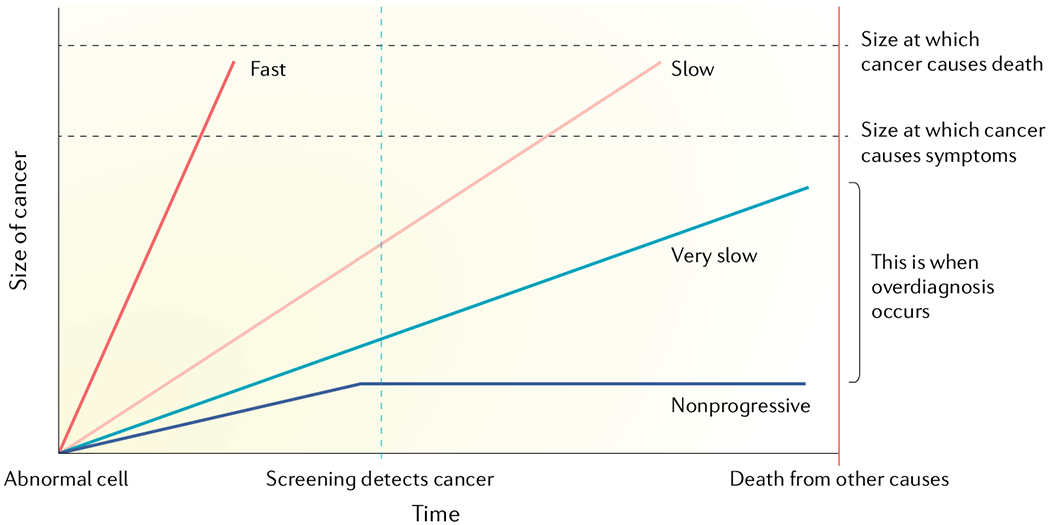
This schematic illustrates the large variability in growth rates and lethal potential of malignant cells. Overdiagnosis occurs when screen-detected cancers are either non-growing or so slow- growing that they would never cause medical problems before death from other causes. Therefore, the prerequisites of overdiagnosis are a reservoir of silent disease and a screening or detection activity that leads to detection of subclinical disease within the reservoir. Adapted with permission from REF.9, Oxford University Press.
USPSTF identified overdiagnosis as an important consideration for breast cancer (November 2009) when it recommended screening based on an individualized, informed decision rather than across the board8. Over the past 10 years, there has been an increasing awareness of the extent and clinical importance of overdiagnosis and related overtreatment and of the need to develop tests that better distinguish indolent or very-slow-growing cancers from aggressive ones9–13. Overdiagnosis occurs either when the detected cancer is never going to progress and cause symptoms or when the cancer will not progress rapidly enough to cause symptoms in a person with limited life expectancy. Using these criteria and the amalgamation of several studies, it can be estimated that approximately 25% of breast cancers detected by mammography, 50% of lung cancers detected by chest radiography and/or sputum examination, 13–25% of lung cancers detected by low-dose computed tomography (LDCT), and 50–60% of prostate cancers detected by prostate-specific antigen (PSA) are overdiagnosed8,9,14. Observational studies and population-based cancer statistics suggest that overdiagnosis also occurs for paediatric neuroblastoma, thyroid cancer, melanoma and kidney cancer9,14–16 (TABLE 1). Indeed, some degree of overdiagnosis may be the rule, rather than the exception, when commonly available screening tests are involved.
Table 1 |.
Overdiagnosed cancers
| Cancer type | Estimated amount of overdiagnosis | Screening modality |
|---|---|---|
| Breast | 25%9 | Mammography |
| Prostate | 50–60%9 | PSA |
| Lung | 13–25%14 | CT |
| Melanoma | Approximately 50–60%9,15 | Crude estimate based on population trend |
| Kidney | Twofold increase in incidence but no increase in deaths9 | Incidental detection on abdominal CT |
| Thyroid | Twofold increase in incidence but no increase in deaths9,16 | Incidental detection by imaging performed for other reasons including sinus symptoms and headaches or by palpitation of the neck |
CT, computed tomography; PSA, prostate-specific antigen.
In this Opinion article, we focus on several organ types for which a common screening test is available for the general population and there is supporting evidence of the magnitude of overdiagnosis through meta-analysis and/or randomized trials (for example, breast, prostate and lung cancers). We also discuss the use of screening in high-risk populations for cancers with relatively low incidence but high mortality, such as pancreatic cancer.
Factors influencing overdiagnosis
Challenges in estimating overdiagnosis.
Because most people who are diagnosed with a pre-malignant lesion or a preinvasive cancer are also treated, it is difficult to directly assess the unperturbed natural history of a given lesion and therefore to determine whether overdiagnosis has occurred in an individual patient. Thus, most inferences about overdiagnosis come from the study of populations, in which screening-associated disparities between tumour incidence and mortality are more obvious. A systematic review of methods to measure the extent of overdiagnosis grouped these studies into four categories17: (1) extended follow-up of well-designed randomized controlled trials, (2) imaging or pathological (including autopsy) studies, (3) statistical modelling studies and (4) ecological studies and cohort studies. The authors of that review identified strengths and weaknesses of each of these methodological approaches and concluded that, in the general population, well-conducted ecological and cohort studies in multiple settings are the most appropriate approach for quantifying and monitoring overdiagnosis in cancer screening programmes. However, the extent of overdiagnosis was found to vary widely even among studies that used similar methodological approaches. For example, 18 ecological and cohort studies reported widely different results for the proportion of breast cancers that were overdiagnosed, from as low as 5% to as high as 50%17. Even limiting these studies to those with moderate risk of bias and long-term follow-up, the extent of overdiagnosis varied from about 20% to 50% (for example, the extent of overdiagnosis was estimated to be 20% in the Norwegian Cancer Screening Program that studied 702,000 women18 and 45% in a Swedish study of approximately 750,000 women19). By contrast, the USPSTF concluded in their systematic review of breast cancer screening6 that three well-designed randomized controlled trials (two Canadian studies and one Swedish study that together enrolled approximately 130,000 women20) were the least biased estimates of overdiagnosis. In this case, the trials estimated 11–22% overdiagnosis in the screened population. This high degree of variation makes it difficult for clinicians to advise patients on the likelihood of their cancer being indolent or progressive and on which course of action to take — treatment or active surveillance.
Reservoir of indolent lesions.
The major contributory factor to overdiagnosis is slow-growing or indolent tumours that create a large reservoir of silent and non-lethal cancers that may be detected upon screening asymptomatic patients. Indeed, screening tests are more effective in detecting slow-growing tumours than ones that grow faster because of a longer preclinical asymptomatic period, and the number of indolent lesions in the reservoir may exceed the number of rapidly growing tumours with lethal potential. Autopsies of older men in the USA who died of causes other than prostate cancer showed that, among men aged 70–79 years, prostate cancer was found in 36% of white men and 51% of black men, which is an indication of the size of the reservoir of indolent cancer that could be detected by screening21,22. Developing more sensitive tests that can detect additional prostate cancers in this reservoir but do not distinguish indolent from aggressive cancer will likely do more harm than good by putting a larger number of patients through unnecessary intervention. Hence, the clinical dilemma of overdiagnosis exists — an increase in detection of cancer incidence without a comparable reduction in late-stage disease or mortality.
Extent and harms of overdiagnosis
Harms associated with overdiagnosis include the burden of unnecessary diagnostic procedures, surgeries, chemotherapy, adjuvant therapy, morbidities and in rare cases mortality associated with these treatments. Overdiagnosed individuals also often experience physical (for example, an unnecessary mastectomy or adverse effects of chemotherapy or radiation) and psychological harms (for example, stress and anxiety). There are also economic harms to both individual patients and health-care systems. Patients are often not educated about the harms and benefits of screening procedures, and it may also be difficult for clinicians to effectively communicate the concept of overdiagnosis to their patients.
Prostate cancer.
Prostate cancer is a heterogeneous disease with a wide spectrum of clinical behaviours ranging from prolonged latency during adult life to rapid spread and lethality23,24. The ideal screening method for prostate cancer, as with most cancers, would be one in which clinically relevant cancers that have substantial potential to cause morbidity or mortality are detected at a time when they are curable. Screening of asymptomatic men for prostate cancer with PSA is controversial primarily because of the very high rates of false positives and overdiagnosis25. The USPSTF analysis of multiple trials reported that two-thirds to three-quarters of men with elevated PSA (above 3–4 ng ml−1) do not have cancer detected by biopsy25. For example, the European Randomized Study of Screening for Prostate Cancer, which screened 61,404 men, reported that 76% of the prostate biopsies performed for an elevated PSA identified no cancer26. Of those with a positive biopsy, an estimated 20–50% represent overdiagnosis25 (for example, 50.4% in the European Randomized Study of Screening for Prostate Cancer, which enrolled 61,404 men26, and 20.7% in the US Prostate, Lung, Colorectal and Ovarian cancer Screening Trial (PLCO), which enrolled 148,000 men25). In addition, the false negative rate is approximately 15% (for example, in the Prostate Cancer Prevention Trial in the USA, 449 of the 2,950 men who never had PSA above 4 ng ml−1 were later diagnosed with prostate cancer27). Tumours with Gleason score 6 (Grade Group 1 tumour) (GS6/GG1) have a low capacity for metastasis and death28–30. It is estimated that approximately 20–30% of the 150,000 prostate cancers diagnosed each year in the USA are GS6/GG1. Even if 60–70% of these men select active surveillance, between 9,000 and 18,000 men with a Gleason score 6 will still be unnecessarily treated each year with radical surgery or radiotherapy.
In 2012, the USPSTF recommended against PSA screening, as the potential benefits did not outweigh the potential harms, including false positive results, complications from transrectal prostate biopsies and the harms of treatment (urinary incontinence and erectile dysfunction)31. In 2018, the USPSTF changed their recommendation: for men aged 55–69 years, the decision to undergo periodic PSA screening should be a personal one based on a discussion with their clinician about potential benefits and harms; they recommended against screening for men aged >70 years25. This change in recommendation was primarily based on additional evidence that screening reduces the risk of metastatic disease in some men who have sufficient remaining life expectancy to benefit. Public Health England’s 2016 guidance for prostate cancer risk concluded that the PSA test was not accurate enough to meet the requirements of a national screening programme (Public Health England prostate cancer risk management programme). This recommendation was based in large part on the conclusion that PSA detects slow-growing cancers that may never cause symptoms or shorten life, resulting in unnecessary treatments with side effects that can affect daily life. A recent meta-analysis of five randomized controlled trials concluded that PSA screening results in at best a small reduction in disease-specific mortality (less than 1 death avoided for 1,000 men screened over 10 years), but it has no effect on overall mortality32.
Although an increasing number of men with low-grade prostate cancer are electing to undergo more conservative management33,34, such as active surveillance, overdiagnosis is still common, and active surveillance can itself have serious side effects related to multiple prostate biopsies and can be costly over time to both individual patients and the health-care system. However, the increased awareness of overdiagnosis of prostate cancer has resulted in a decrease in radical prostatectomies for low-grade cancers33–35.
Breast cancer.
With the advent of screening mammography, the detection of ductal carcinoma in situ (DCIS) in the USA has increased from 10 per 100,000 (1975–1979) to 79 per 100,000 (2008–2012), but there has not been a commensurate decrease in invasive cancer36. If one assumes that half of the cases of DCIS progress to invasive carcinoma, this increase in DCIS detection should have resulted in a decrease of approximately 35 cases of invasive cancer per 100,000. However, rather than decreasing, the incidence of invasive cancer increased from 217 per 100,000 (1975–1979) to 281 per 100,000 (2008–2012)36, indicating that mammography detects a large number of DCIS cases that never progress to invasive cancer. Mammography also detects some cases of invasive stage 1 cancers that are not destined to progress, and their detection contributes to overdiagnosis. Statistical modelling and population trends suggest that much, or most, of the observed reduction in breast cancer mortality is attributable to improvements in treatment for stage 2 and stage 3 diseases37. Yet, recent data from Sweden found that women who participated in an organized mammography screening programme had a 60% reduction in the risk of dying from breast cancer 10 years after diagnosis and a 47% reduction in risk 20 years after diagnosis38. Interval cancers missed by screening mammography are usually more aggressive and faster growing and more likely to be diagnosed at an advanced stage39 and be oestrogen receptor (ER)-negative40–42 than screen-detected breast cancers. On the other end of the spectrum are invasive cancers that are indolent and will not progress (sometimes referred to as indolent lesions of epithelial origin (IDLE)43,44) and DCIS, which is considered noninvasive and unlikely to become invasive; these may account for at least half of the in situ and invasive cancers diagnosed today. These two extremes represent the conditions in which screening faces its biggest challenges.
Lung cancer.
The results from the US National Lung Screening Trial (NLST) of 53,453 high-risk current and former smokers found that screening by LDCT decreased lung cancer mortality by approximately 15–20%. However, 25% of the subjects in the LDCT arm of the NLST had abnormalities, and 95% of those lesions were determined to be false positives, and as many as 30% were overdiagnosed45,46. A recent analysis of the Danish Lung Cancer Screening Trial (2,050 subjects in the screened group and 2,050 subjects in the control group) estimated that 67% of the cancers detected by LDCT were due to overdiagnosis47. By contrast, the Italian Lung Screening Trial (1,613 subjects in the screened group and 1,593 subjects in the control group) found no evidence for overdiagnosis but did note nonsignificant reductions of 17% in overall mortality and 30% in lung cancer-specific mortality48. There are currently no organized lung screening programmes with LDCT either in North America or Europe49.
In the NLST45,46, most of the false positives were followed up only by additional imaging; however, 2.5% required an invasive procedure such as a needle biopsy. Lesions thought to be malignant on imaging often require additional diagnostic procedures resulting in increased radiation exposure, needle biopsy or other invasive procedures such as thoracotomy. Thoracotomy carries risk, especially in people with underlying cardiac or lung disease from years of tobacco smoking. Potentially serious complications can result from these procedures and delay appropriate treatment. A retrospective study of 174,702 patients in the USA who had an invasive diagnostic procedure (cytology, biopsy, bronchoscopy or surgery) to examine abnormalities found with lung cancer screening reported that 23% resulted in complications (19% after needle biopsies and 52% after surgery). In addition, the mean costs were approximately US$6,300 for minor complications and $57,000 for major complications50. Importantly, these patients did not have a diagnosis of lung cancer 1 year before or after the diagnostic procedure.
Pancreatic cancer.
Screening in the general population is not feasible for pancreatic ductal adenocarcinoma (PDAC) owing to its relatively low incidence and prevalence, but screening studies have been performed on asymptomatic individuals at high risk of PDAC, be it because of a strong family history or a predisposing germline mutation51,52. The benefits and risks of screening of high-risk individuals for PDAC have been highlighted in recently published studies, showing that while pancreatic cancer can be identified at early stages, other pancreatic lesions with variable malignant potential are also found51,53–56. For example, mucinous pancreatic cysts are commonly found in patients who participate in these high-risk screening protocols, and these mucinous cysts have the potential to become PDAC. In fact, these cysts present a broader problem for health-care providers beyond overdiagnosis owing to screening. Pancreatic cysts have increased in prevalence owing to increased use of cross-sectional abdominal imaging in the general population owing to reasons unrelated to the pancreas such as chronic abdominal pain, gastrointestinal discomfort or accidents57–59. Much may be gleaned from incidentalomas, despite the biology and clinical conundrums caused by incidental lesions perhaps being different than those related to screen-detected lesions. For example, a recent multi-institutional study found that 44% of pancreatectomies (141 out of 320) for pancreatic cysts were found to be either low-grade or intermediate-grade dysplasia, which is not considered to be life-threatening60. These results in the general population should give caution to the design and interpretation of screening protocols for individuals at high risk of pancreatic cancer: overdiagnosis as well as overtreatment may occur owing to incidental findings or discovery of indeterminate lesions within the pancreas or outside of it.
The finding of a pancreatic cyst can be worrisome for both physicians and patients owing to the fear of having PDAC61. The fundamental diagnostic uncertainty associated with whether pancreatic cysts are indolent or likely to progress to adenocarcinoma often triggers overtreatment, which would be surgical resection of the cyst and a portion of the pancreas. Although it is known that certain mucinous cysts, such as mucinous cystic neoplasms and intraductal papillary mucinous neoplasms (IPMNs), can progress to adenocarcinoma62,63, there are currently no diagnostic tests with sufficient specificity to identify the lesions that harbour cancer or are likely to undergo malignant transformation64. Work up of these potential precursors of cancer is invasive and can lead to major surgery with substantial postoperative morbidity and even a small risk of mortality. For example, current guidelines for management of IPMNs have relatively high sensitivity for detecting high-risk lesions65 but have low specificity, capturing a high proportion of nonprogressive lesions. Despite improved perioperative outcomes, pancreatic resections for pancreatic cysts have 2–4% operative mortality and 30–40% morbidity (these are estimations based on the amalgamation of several different studies)66–70. Furthermore, these surgeries are costly and require substantial recovery time, both of which can be potentially avoided through development of accurate diagnostic tests for low-risk and high-risk lesions.
The biology of overdiagnosis
There is an urgent need to improve the ability to identify overdiagnosis at the individual patient level and to accurately determine whether the cancer will progress. To date, discussions of overdiagnosis have largely been based on epidemiological findings rather than on the underlying biology. The identification of biological mechanism(s) that determine tumour aggressiveness could help identify biomarkers of aggressiveness and guide clinical decisions with respect to both extent and type of treatment and frequency of follow-up. The biological mechanisms that drive the development of aggressive tumours can be organized according to the ‘Hallmarks of Cancer’, as proposed by Hanahan and Weinberg71. What conceivably may distinguish indolent cancers from aggressive ones are the mechanisms that influence the activation of invasion and metastasis, as well as sustained proliferation. Indeed, recent work has revealed that gene expression signatures may predict indolent or aggressive biology (for example, for breast72 and prostate cancers73). Gene panels of aggressive cancers are enriched for genes related to proliferation and metastasis, and gene panels of indolent cancers are enriched for genes related to ageing and senescence73. The fundamental gap in knowledge is the underlying mechanisms that lead to these molecular signatures. Here, we do not attempt to review all aspects of the biological underpinnings of overdiagnosis for each tumour type but rather provide a few examples of different aspects: tumour evolution, tumour heterogeneity and the tumour microenvironment.
Tumour evolution.
The course of growth and development of a normal single cell (or a few cells) into a heterogeneous tumour mass is propelled by gene mutations or other molecular changes in the cell in concert with selection pressure from the microenvironment74. To develop a better understanding of tumour evolution, there is a need to understand the process of initiation and the causal intrinsic and extrinsic factors. To date, application of the principles of natural selection in breast cancer75, pancreatic cancer76, prostate cancer77 and lung cancer78 has revealed insights into the molecular events that make these diseases highly lethal. However, the selective pressures that lead to indolent lesions in these organs remain poorly understood. For example, pathologists have proposed a continuum of morphological features to describe the transition from DCIS to invasive breast cancer79, but comparative analysis of gene expression between DCIS and invasive cancer79–81 found no common or consistent genetic changes associated with this transition. Some protein differences were observed (for example, an increase in insulin-like growth factor binding protein 2 (IGFBP2)), but this is likely more a consequence of transition than a cause. Similarly, the step-wise model from pancreatic intraepithelial neoplasia transitioning to invasive carcinoma has also been challenged recently. The authors reported that a single neoplasm can give rise to multiple independent lesions that diverge spatially and genetically overtime82. These studies highlight the ways in which applications of evolutionary concepts can reveal new insights into these diseases and may help us better understand how to identify indolent and aggressive cancers using molecular methods.
Tumour heterogeneity.
Even for the same cancer type, tumours have distinct morphological and phenotypic profiles, including differences in gene expression, proliferation and metastatic potential. Intratumour heterogeneity is closely related to cancer progression, resistance to therapy and recurrence83. Deep sequencing and single cell sequencing have contributed greatly to our understanding of the extent of tumour genetic heterogeneity and are beginning to provide information that can be used to determine the likelihood a tumour will progress. For example, large-scale sequencing studies of both primary and metastatic cancers have shown that prostate cancer consists of different molecular subtypes24,84–87, with 74% falling into 7 categories: 59% with gene fusions (46% involving ERG, 8% involving ETS variant 1 (ETV1), 4% involving ETV4 and 1% involving friend leukaemia integration 1 transcription factor (FLI1)), 11% with speckle-type POZ protein (SPOP) mutations, 3% with FOXA1 (also known as HNF3α) mutations and 1% with isocitrate dehydrogenase 1 (IDH1) mutations24. The precise relationship between these molecular subgroups and the aggressiveness of the cancer is not currently known, but transmembrane protease serine 2–ERG (TMPRSS2–ERG) fusions appear to mediate invasion88. Other alterations occur in a non-mutually exclusive manner across many of the subtypes. Some alterations, such as PTEN loss, 8q24 gain and TP53 mutations, have been shown to be prognostic for poor outcome89–91.
One approach to develop biomarkers to distinguish indolent from aggressive cancers is to compare the genomic, transcriptomic, proteomic and/or immune profiles of what are thought to be indolent and aggressive cancers or more commonly to compare screen-detected (less aggressive) and interval or incidental cancers (more aggressive). This approach has been fruitful for prostate cancer24,84–87, and applications of omics approaches to breast cancers may also appear to help distinguish aggressive lesions from less aggressive or indolent ones. Specific findings from these studies have shown that there are molecular characteristics of tumours, such as germline DNA repair defects in prostate cancer that are associated with disease aggressiveness92 and TP53, protein phosphatase 1 regulatory subunit 3A (PPP1R3A) and histone-lysine N-methyltransferase 2B (KMT2B) mutations that occur more frequently in interval breast cancers than in screen-detected breast cancers93.
Tumour microenvironment.
There is evidence of the profound influence of stroma on growth and progression of tumours94. The main stromal components of the tumour microenvironment are angiogenic vascular cells, infiltrating immune cells and non-immune cells (for example, cancer-associated fibroblasts). The immune infiltrates of high-grade DCIS with a history of recurrence contain higher percentages of forkhead box P3 (FOXP3)+ cells, CD68+ and CD68+ proliferating cell nuclear antigen (PCNA)+ macrophages, human leukocyte antigen-DR isotype (HLA-DR)+ cells, CD4+ T cells, CD20+ B cells and total tumour-infiltrating lymphocytes than non-high-grade DCIS95. Tumours with similar molecular profiles may have very different growth trajectories dependent upon differences in stromal influence. For example, the lung microenvironment is thought to play a role in determining tumour progression96–99. Similarly, a hallmark feature of PDAC, including those that arise from mucinous cysts100, is an extensive desmoplastic reaction, and subtypes of PDAC may be defined based on stromal gene expression signatures101. An emerging area of interest related to the tumour microenvironment is the microbiome, which has been studied in the context of cancers of the lung, prostate, pancreas and other organs102. Further research on the influence of the microbiotic flora of an individual on cancer risk is expected to advance our understanding of the biology of overdiagnosis.
Research needs
One of the greatest needs is to develop biomarkers and/or imaging methods that can more accurately detect early-stage cancers or precancerous lesions and determine which are likely to progress and to determine how best to inform the patient. The biology and molecular pathways that lead to overdiagnosis are just beginning to be understood, and more research is needed to fully understand the mechanisms and to use this information to accurately determine whether a cancer is indolent or aggressive. Some of the resources needed to refine the characterization of overdiagnosis at the patient level are labour intensive, particularly those that require prospective, longitudinal collections of samples (BOX 1). The US National Cancer Institute (NCI) has developed two research consortia, the Early Detection Research Network (EDRN) and the Molecular and Cellular Characterization of Screen-Detected Lesions (MCL), whose missions include developing assays to better distinguish indolent from aggressive cancers (BOX 2). The two general research approaches in this area are to determine the molecular profiles of indolent versus aggressive cancers that can be used to develop biomarkers or panels of biomarkers and to understand the molecular and signalling pathways for cancer development that can be used to develop biomarkers or be targeted for preventive interventions. It is unclear which approach will be most productive in any given situation. Given the clinical importance of overdiagnosis and the level of research in this area, it seems likely that more accurate tests will be developed and deployed in the future. In the following section, we outline some of the needs, with attention to those that are tumour type-specific, However, some needs are common to multiple cancer types, including more accurately determining the extent of overdiagnosis in different patient populations, understanding tumour evolution, the role of the microenvironment and additional longitudinal studies.
Box 1 |. Resources needed to support research on cancer overdiagnosis.
Registries, such as Surveillance, Epidemiology, and End Results (SEER), National Health and Nutrition Examination Survey (NHANES), that can provide data on the incidence and outcomes of screen-detected, interval-detected and symptom-detected cancers.
Collections of clinically annotated normal, neoplastic and tumour tissues from both screen-detected and symptom-detected cancers that can be used to study the natural history of cancers. These specimens may come from longitudinal screening or surveillance programmes.
Specimens collected from cohorts and control arms of randomized screening and treatment trials with long-term follow-ups.
Precancerous and indolent lesions collected from surgical and autopsy specimens.
Specimens and imaging data from immunologically susceptible individuals to understand the selective forces shaping the evolution of cancer in its earliest stages.
Imaging and biological specimens obtained from animal models with strain-specific behaviours.
Box 2 |. Research focus of NCI’s MCL Consortium.
Examination of genomic and microenvironmental determinants that distinguish indolent tumours from aggressive cancers.
Evaluation of host and environmental factors that affect tumour development and progression, including the roles of the cells of origin, obesity, the microbiome, chronic infection, inflammation, immune response, ageing and DNA repair enzyme polymorphisms.
DNA sequencing and proteomic analysis of precursor lesions, circulating tumour cells and host niches to clarify the selection pressures that influence the phenotypic trajectory of a tumour.
Determination of molecular and genomic predictors of aggressive lesions using longitudinal data, as natural history studies must examine tumour dynamics over time and not at a single time point.
Collection of cross-sectional data (observing many subjects at the same point of time) with annotated samples from unique human cohorts, animal models with strain-specific behaviours and immunologically susceptible individuals to study the selective forces shaping the evolution of cancer in its earliest stages.
Collection of longitudinal data (for example, annotated biological specimens, annual imaging and medical records) from cohorts of patients who do not undergo treatment to understand tumour dynamics and trajectory.
Functional imaging and imaging of spatiotemporal modelling of tissues.
Modelling to merge various data sets and to consider the characteristics of tumour cells in a holistic way.
Construction of a Precancer Imaging Atlas to serve as a reference on indolent lesions.
MCL, Molecular and Cellular Characterization of Screen-Detected Lesions; NCI, National Cancer Institute.
Prostate cancer.
An important issue is whether a Gleason 6 cancer will progress to Gleason 7 or higher. A number of studies have shown that Gleason pattern 3 (GP3) cancers often harbour the same somatic DNA alterations as an adjacent Gleason pattern 4 (GP4) cancer, which may be indicative of either a common clonal origin or disease progression103–106. A single longitudinal study of the clonality of GS6/GG1 and higher-grade subsequent lesions in men on active surveillance supports a common clonal origin of GP3 and GP4 tumours in some cases, which suggests the potential for GP3 to progress to GP4 (REF.107). Improved molecular interrogation of prostate cancer needle biopsy samples is needed to determine whether samples with only GP3 represent adequate sampling of an indolent lesion or are part of a larger tumour already containing GP4 with more malignant potential. There is also a need for improvements in imaging technology, such that if a man is diagnosed with GS6/GG1 prostate cancer, clinicians can have sufficiently high confidence that the patient does not harbour any Gleason 7 disease. While imaging by itself may not be able to fully rule out high-grade cancer, magnetic resonance imaging (MRI)–ultrasound fusion-directed biopsies of suspicious lesions can lead to more accurate determinations of Gleason scores. This would make active surveillance more appealing for men with GS6/GG1 disease. However, further refinements in MRI will be necessary if it is to be accurate enough to detect high-grade cancer108.
Several tests have been reported to improve the detection of clinically important prostate cancer and to distinguish low-grade cancer from high-grade cancer. A meta-analysis of the diagnostic accuracy of the prostate health index (PHI)109 (a combination of total PSA (tPSA), free PSA (fPSA) and [−2]proPSA (p2PSA)) and a panel of four kallikreins (4 K-panel) reported that the PHI had a pooled sensitivity of 93% for high-grade prostate cancer (Gleason score 7 and above) at a specificity of 34% and that the 4 K-panel had a pooled sensitivity of 87% for high-grade prostate cancer at a specificity of 61%110. Combining PSA with prostate cancer antigen 3 (PCA3) and TMPRSS2-ERG urinary RNAs improves the specificity for aggressive prostate cancer (Gleason score 7 and above) to 39% compared with 18% for PSA alone while maintaining a sensitivity of 95%111. Furthermore, OncotypeDx Genomic Prostate Score (GPS), a 17-gene expression array, has been reported to be associated with increased risk of biopsy upgrading in men undergoing active surveillance and could be useful in managing these men112. Additional research is needed to determine whether these or other tests can result in a significant decrease in overdiagnosis.
Breast cancer.
To date, most attempts to discover biomarkers for aggressiveness have looked for molecular differences between noncancerous tissues and cancers with different degrees of aggressiveness. Many of the detected alterations are consistent with what is known about the biology of tumour development and progression113. Evidence suggests that breast cancer progression is influenced by signalling between cancer cells and non-malignant cells, such as macrophages, T lymphocytes and mast cells95,114, and profiling the microenvironment is another approach to develop assays to distinguish indolent from aggressive cancers.
Modelling can be applied to gene expression and sequence data to begin to answer questions about progression. One such exercise demonstrated a high probability that most cases of DCIS and accompanying or subsequent invasive carcinoma arise simultaneously from a common progenitor and evolve in parallel115. This would seem to contradict the concept of evolutionary selection pressure, which is assumed to be different between in situ versus invasive cancers, and an intrinsic cancer genomic instability and/or mutator phenotype. However, a better conceptual model might involve mutations (or other epigenetic and/or heritable changes) leading up to a point of in situ transformation and initiation followed by minimal ongoing instability. This has been shown in one context to be the result of shortening telomeres contributing to increased instability, followed by a re-stabilization of the telomere (survival of telomere-based crisis)116. Subsequent progression, latency, metastasis, plasticity and treatment sensitivity and so on may then be relatively fixed, with only a slow or rare additional selection of genetic changes117. In a recent single cell sequence approach, even the clonal heterogeneity of the invasive cancer was also seen in the DCIS118. If the malignant potential and rate of progression are relatively fixed at the time of initiation, several important questions remain. How is malignant progression programmed, or what biological properties at initiation lead to faster, slower or indolent progression rates? Can these properties be used to better stratify DCIS and localized invasive carcinomas? Finally, what are the likely biological properties, if they are not directly related to gene expression or mutation — is this the proof of concept for control of progression by the microenvironment, tumour and/or stroma cell metabolism or host factors including immune recognition?
Lung cancer.
As with other cancers, next-generation sequencing has been used to determine the mutational landscape of lung cancers119–122. While differences between cancer and normal lung tissues have been described123, the mutations that accurately distinguish aggressive from indolent cancers have not been determined. Dissecting the molecular pathways from preneoplasia to carcinoma in situ and a comparison of interval with screen-detected lung cancers at the genetic level will be critical for identifying biomarkers related to rapidly progressing and aggressive tumour phenotypes. As with other cancers, investigators are examining the role of the microenvironment in lung cancer progression94,124. There must also be a concerted effort to improve imaging analysis to better classify these tumours and an emphasis on molecular profiling to determine which computed tomography (CT)-detected lung nodules represent aggressive lung cancer. Current imaging methods have high sensitivity for lung cancer but low specificity and consequently very high false positive rates (approximately 95%)45,46. A number of investigators are exploring the use of radiomics to better classify LDCT images of lung nodules125,126. Radiomics is the process of converting standard of care digital medical images into quantitative image-based feature data that can be subsequently analysed using conventional biostatistics and machine learning methods. This will facilitate early treatment and thereby improve lung cancer outcomes. As over 70% of lung cancers in the USA occur in individuals who fall outside NLST criteria (persons aged 55–74 years who have a ≥30 packs a year smoking history and currently smoke or have quit in the past 15 years), there is also a need for research to develop molecular biomarkers to identify additional individuals who would benefit from LDCT screening127.
The recently announced SUMMIT study in the UK, a lung cancer screening project, plans to enrol 50,000 participants, half of which will be people who meet certain criteria based on whether they currently smoke or have smoked regularly in the past (equivalent to the current criteria for LDCT screening) and half who do not have a significant smoking history or will have never smoked. All participants will provide a blood sample annually for 2 years. The blood samples will be used to develop and evaluate a blood test for early lung cancer detection. Another aim is to determine the feasibility of implementing a lung cancer screening programme to help more at-risk people in the UK.
Pancreatic cysts.
Efforts have focused on using genetic-based, blood-based, microbiome-based and immune-based associations to develop biomarkers to help stratify indolent and aggressive mucinous cysts62,128,129. Studies are currently at the preclinical and translational stage64. Research is needed to determine how genetic drivers of pancreatic cyst formation interact with the surrounding tumour microenvironment to fuel malignant progression and to understand the interactions between the biology and physical attributes of cystic lesions of the pancreas. Although guidelines exist for the management of cystic lesions, they are increasingly being detected because of increased use and sensitivity of abdominal imaging modalities. It is challenging using current imaging techniques to determine which cysts are cancerous or are likely to become cancerous. Investigators are working to determine whether radiomic features on diagnostic imaging can be used to more accurately classify these lesions130,131. For effective management of the disease and to avoid putting patients through unnecessary resection, it will be useful if a correlation can be deciphered between imaging features and molecular predictors of malignancy.
Perspective
Screening tests can incur both benefits and harms. Benefits include the detection of an early-stage cancer or even a precursor lesion and the possibility of a better treatment outcome. Harms include overdiagnosis, negative side effects of unnecessary treatment and adverse events associated with the screening test itself and subsequent diagnostics, such as biopsies that can result in perforation or infection. Screening tests miss some aggressive life-threatening cancers that become symptomatic between scheduled screening tests. One possible approach to decrease the extent of overdiagnosis is to develop an initial screening test that detects fewer indolent cancers. For example, the PHI and 4K-panel tests are reported to be as sensitive as PSA for aggressive prostate cancer (Gleason 7 and above) but detect fewer low Gleason grade cancers. An alternate approach is to use a broad sensitive initial screen followed by a test that distinguishes indolent from aggressive cancers (FIG. 3). For example, LDCT for lung cancer, which is very sensitive, followed by a molecular test that would be very specific for aggressive cancer. Indeed, both approaches are being actively pursued.
Fig. 3 |. Molecular profiling to distinguish indolent from aggressive cancers.
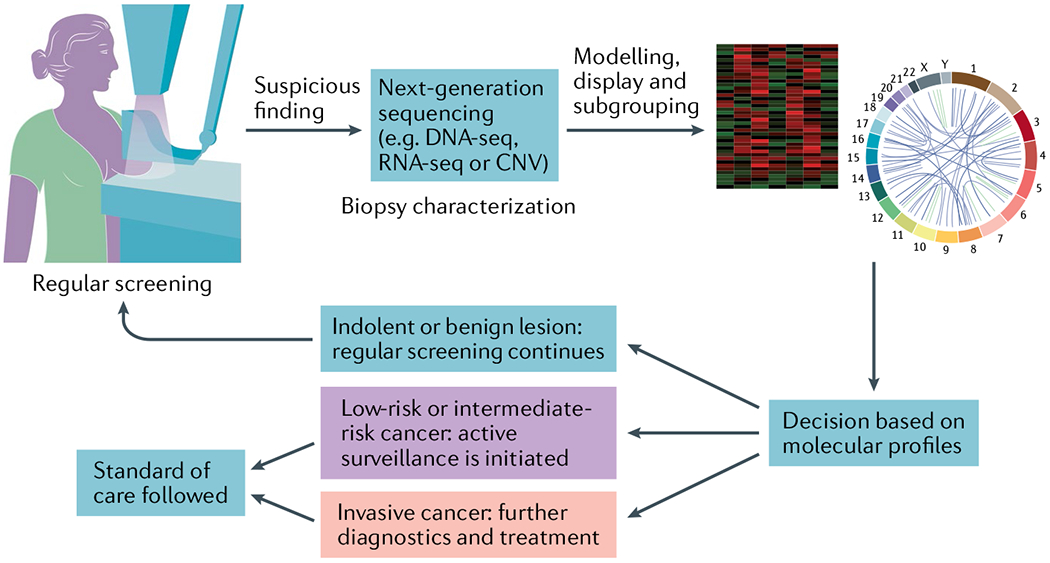
The schematic illustrates the use of validated molecular aberrations, including cellular and phenotypic changes, that could help develop decision criteria for clinical management. This hypothetical workflow assumes that there is a screening process in place, overdiagnosis occurs and validated molecular assays exist to stratify screen-detected lesions as low-risk , intermediate-risk or invasive cancer. The schematic is not intended to be the only possible workflow , and other clinical scenarios may require additional diagnostic work-ups at any stage of this workflow. CNV, copy number variation; seq, sequencing.
The estimates of overdiagnosis for specific types of cancer vary widely, depending on population, study design and statistical methods used, but whatever the frequency, overdiagnosis presents a potentially substantial harm to the patient and a clinical dilemma. How does one who is told that their cancer has a given chance of progressing to metastatic disease decide what to do? Do they undergo surgery, radiation, and/or chemotherapy or active surveillance? It is clear that tests, either imaging or molecular, that can accurately distinguish aggressive from indolent disease are needed, but there is also a need to improve decision sharing and decision aids. Although guidelines frequently recommend that the physician discuss the potential benefits and harms of screening with the patients, evidence indicates that more effort is needed to ensure that physicians spend more time discussing potential harms and benefits with their patients and that decision aids are effectively employed132–134.
Glossary
- Active surveillance
A treatment plan that involves closely watching a patient’s condition but not giving treatment unless there are changes in the test results that show the condition is worsening.
- Cohort studies
Research studies that compare a particular outcome (such as breast cancer) in groups of individuals who are alike in many ways but differ in certain characteristics (for example, women who are screened for breast cancer compared with those who are not).
- Decision aids
Evidence-based educational tools that facilitate shared decision making, improve knowledge of treatment options, may increase satisfaction with treatment choice and likely facilitate long-term quality of life. They include educational literature, videos and website interactive programmes.
- Ecological studies
Observational studies that focus on the comparison of groups rather than individuals. Data are analysed at the population or group level rather than at the individual level.
- Gleason pattern
In terms of microscopic appearance of prostatic carcinoma, there are a number of different recognizable patterns that range in number from 1 to 5, with pattern 1 most resembling normal glands and pattern 5 least resembling normal glands.
- Gleason score
Prostate cancer is often heterogeneous, with often more than one pattern being present in a given tumour nodule. Gleason score is the sum of the most common and second most common patterns (for example, 3 + 4 = 7) in prostatectomy specimens and the most common and highest pattern in needle biopsy samples. Gleason scores range from 2 to 10.
- Grade Group
With modern grading, it was found that almost all prostate cancers range from Gleason score 6 to 10. It is often assumed by many that a Gleason 6 out of 10 is quite aggressive, when in fact this is essentially the lowest grade one can have. Grade groups take this into account by referring to Gleason score 6 tumours as Grade Group 1 (GG1). It also forced a separation of Gleason 7 tumours (which could be either Gleason 3 + 4 = 7 or Gleason 4 + 3 = 7) into two groups because these are known to have a substantially different prognosis.
- Incidentalomas
Unanticipated findings that are not related to the original diagnostic inquiry.
- Interval cancers
Cancers missed during routine screening but diagnosed between scheduled screening tests.
- Overdiagnosis
A condition that fulfils standard diagnostic criteria but would not go on to cause symptoms or death. Cancer overdiagnosis occurs most frequently when a tumour is identified by a screening test but may also be detected as an incidentaloma on images of unrelated target organs.
- Reservoir of silent and non-lethal cancers
The existence of a substantial number of subclinical cancers that can be found through routine screening or imaging.
- Sigmoidoscopy
A procedure in which a flexible, narrow tube with a light and tiny camera on one end, called a sigmoidoscope or scope, is used to look inside a patient’s rectum and lower colon. During sigmoidoscopy, abnormal growths in the rectum and sigmoid colon can be removed for biopsy.
- SPOP
The SPOP gene encodes speckle-type POZ protein, which is thought to modulate the transcriptional repression activities of death-associated protein 6 (DAXX) and is part of an E3 ubiquitin ligase complex that is involved in controlling protein stability of the androgen receptor and some of its transcriptional co-activators.
- Thoracotomy
A surgical procedure in which a cut is made between the ribs to see and reach the lungs or other organs in the chest or thorax.
- TMPRSS2–ERG
Fusion of the genes ERG and transmembrane protease serine 2 (TMPRSS2) is the most frequent genomic alteration in prostate cancer. ERG is an oncogene that encodes a member of the family of ETS transcription factors. TMPRSS2 is an androgen-regulated gene that is preferentially expressed in the prostate.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Weinberg DS & Schoen RE In the clinic. Screening for colorectal cancer. Ann. Intern. Med 160, ITC5–1 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Atkin WS et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 375, 1624–1633 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Schoen RE et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N. Engl. J. Med 366, 2345–2357 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landy R et al. Impact of cervical screening on cervical cancer mortality: estimation using stage-specific results from a nested case-control study. Br. J. Cancer 115, 1140–1146 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper GS, Chak A & Koroukian S The polyp detection rate of colonoscopy: a national study of Medicare beneficiaries. Am. J. Med 118, 1413 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Pickhardt PJ et al. Assessment of volumetric growth rates of small colorectal polyps with CT colonography: a longitudinal study of natural history. Lancet Oncol. 14, 711–720 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin JS et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 315, 2576–2594 (2016). [DOI] [PubMed] [Google Scholar]
- 8.US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med 151 , 716–726 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Welch HG & Black WC Overdiagnosis in cancer. J. Natl Cancer Inst. 102, 605–613 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Esserman LJ et al. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol. 15, e234–e242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofmann B & Welch HG New diagnostic tests: more harm than good. BMJ 358, j3314 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Shieh Y et al. Population-based screening for cancer: hope and hype. Nat. Rev. Clin. Oncol 13, 550–565 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava S et al. Research needs for understanding the biology of overdiagnosis in cancer screening. J. Cell. Physiol 231, 1870–1875 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bach PB Reduced lung-cancer mortality with CT screening. N. Engl. J. Med 365, 2035–2038 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Welch HG, Woloshin S & Schwartz LM Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ 331, 481 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowenstein LM et al. Active surveillance for prostate and thyroid cancers: evolution in clinical paradigms and lessons learned. Nat. Rev. Clin. Oncol 16, 168–184 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter JL, Coletti RJ & Harris RP Quantifying and monitoring overdiagnosis in cancer screening: a systematic review of methods. BMJ 350, g7773 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falk RS et al. Overdiagnosis among women attending a population-based mammography screening program. Int. J. Cancer 133, 705–712 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zahl PH, Strand BH & Maehlen J Incidence of breast cancer in Norway and Sweden during introduction of nationwide screening: prospective cohort study. BMJ 328, 921–924 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marmot MG et al. The benefits and harms of breast cancer screening: an independent review. Br. J. Cancer 108, 2205–2240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter HB, Piantadosi S & Isaacs JT Clinical evidence for and implications of the multistep development of prostate cancer. J. Urol 143, 742–746 (1990). [DOI] [PubMed] [Google Scholar]
- 22.Etzioni R et al. Asymptomatic incidence and duration of prostate cancer. Am. J. Epidemiol 148, 775–785 (1998). [DOI] [PubMed] [Google Scholar]
- 23.Tolkach Y & Kristiansen G The heterogeneity of prostate cancer: a practical approach. Pathobiology 85, 108–116 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell 163, 1011–1025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. JAMA 319, 1901–1913 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Kilpelainen TP et al. False-positive screening results in the European randomized study of screening for prostate cancer. Eur. J. Cancer 47, 2698–2705 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Thompson IM et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N. Engl. J. Med 350, 2239–2246 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Ross HM et al. Do adenocarcinomas of the prostate with Gleason score (GS) </ = 6 have the potential to metastasize to lymph nodes? Am. J. Surg. Pathol 36, 1346–1352 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu JJ et al. Nationwide prevalence of lymph node metastases in Gleason score 3 + 3 = 6 prostate cancer. Pathology 46, 306–310 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassan O et al. Incidence of extraprostatic extension at radical prostatectomy with pure Gleason score 3+3=6 (grade group 1) cancer: implications for whether Gleason score 6 prostate cancer should be renamed “not cancer” and for selection criteria for active surveillance. J. Urol 199, 1482–1487 (2018). [DOI] [PubMed] [Google Scholar]
- 31.US Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med 157, 1–44 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Ilic D et al. Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ 362, k3519 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huland H & Graefen M Changing trends in surgical management of prostate cancer: the end of overtreatment? Eur. Urol 68, 175–178 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Cooperberg MR & Carroll PR Trends in management for patients with localized prostate cancer, 1990–2013. JAMA 314, 80–82 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Salmasi A et al. Radical prostatectomy then and now: surgical overtreatment of prostate cancer is declining from 2009 to 2016 at a tertiary referral center. Urol. Oncol 36, 401.e419–401.e425 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Welch HG et al. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N. Engl. J. Med 375, 1438–1447 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Narod SA, Iqbal J & Miller AB Why have breast cancer mortality rates declined? J. Cancer Policy 5, 8–17 (2015). [Google Scholar]
- 38.Tabar L et al. The incidence of fatal breast cancer measures the increased effectiveness of therapy in women participating in mammography screening. Cancer 125, 515–523 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otto SJ et al. Mammography screening and risk of breast cancer death: a population-based case-control study. Cancer Epidemiol. Biomarkers Prev 21, 66–73 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Nelson HD et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann. Intern. Med 151,727–737 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collett K et al. A basal epithelial phenotype is more frequent in interval breast cancers compared with screen detected tumors. Cancer Epidemiol. Biomarkers Prev 14, 1108–1112 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Porter PL et al. Breast tumor characteristics as predictors of mammographic detection: comparison of interval- and screen-detected cancers. J. Natl Cancer Inst 91, 2020–2028 (1999). [DOI] [PubMed] [Google Scholar]
- 43.Esserman L, Shieh Y & Thompson I Rethinking screening for breast cancer and prostate cancer. JAMA 302, 1685–1692 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Esserman LJ, Thompson IM Jr & Reid B Overdiagnosis and overtreatment in cancer: an opportunity for improvement. JAMA 310, 797–798 (2013). [DOI] [PubMed] [Google Scholar]
- 45.National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med 365, 395–409 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinsky PF Assessing the benefits and harms of low-dose computed tomography screening for lung cancer. Lung Cancer Manag. 3, 491–498 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heleno B, Siersma V & Brodersen J Estimation of overdiagnosis of lung cancer in low-dose computed tomography screening: a secondary analysis of the Danish Lung Cancer Screening Trial. JAMA Intern. Med 178, 1420–1422 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paci E et al. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax 72, 825–831 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Pinsky PF Lung cancer screening with low-dose CT: a world-wide view. Transl Lung Cancer Res. 7, 234–242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huo J et al. Complication rates and downstream medical costs associated with invasive diagnostic procedures for lung abnormalities in the community setting. JAMA Intern. Med 179, 324–332 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canto MI et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 142, 796–804 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Canto MI et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 62, 339–347 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verna EC et al. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin. Cancer Res 16, 5028–5037 (2010). [DOI] [PubMed] [Google Scholar]
- 54.Canto MI et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin. Gastroenterol. Hepatol 4, 766–781 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Vasen HF et al. Magnetic resonance imaging surveillance detects early-stage pancreatic cancer in carriers of a p16-Leiden mutation. Gastroenterology 140, 850–856 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Al-Sukhni W et al. Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J. Gastrointest. Surg 16, 771–783 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Nougaret S et al. Cystic pancreatic lesions: from increased diagnosis rate to new dilemmas. Diagn. Interv. Imaging 97, 1275–1285 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mendelson R Imaging for chronic abdominal pain in adults. Aust. Prescr 38, 49–54 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klibansky DA et al. The clinical relevance of the increasing incidence of intraductal papillary mucinous neoplasm. Clin. Gastroenterol. Hepatol 10, 555–558 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson GC et al. Are the current guidelines for the surgical management of intraductal papillary mucinous neoplasms of the pancreas adequate? A multi-institutional study. J. Am. Coll. Surg 224, 461–469 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Rahib L et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 74, 2913–2921 (2014). [DOI] [PubMed] [Google Scholar]
- 62.Matthaei H et al. Cystic precursors to invasive pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol 8, 141–150 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh M & Maitra A Precursor lesions of pancreatic cancer: molecular pathology and clinical implications. Pancreatology 7, 9–19 (2007). [DOI] [PubMed] [Google Scholar]
- 64.Fonseca AL et al. Intraductal papillary mucinous neoplasms of the pancreas: current understanding and future directions for stratification of malignancy risk. Pancreas 47, 272–279 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka M et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 17, 738–753 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Winter JM et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J. Gastrointest. Surg 10, 1199–1210 (2006). [DOI] [PubMed] [Google Scholar]
- 67.Valsangkar NP et al. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery 152, S4–S12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galanis C et al. Resected serous cystic neoplasms of the pancreas: a review of 158 patients with recommendations for treatment. J. Gastrointest. Surg 11,820–826 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Wilentz RE et al. Pathologic examination accurately predicts prognosis in mucinous cystic neoplasms of the pancreas. Am. J. Surg. Pathol 23, 1320–1327 (1999). [DOI] [PubMed] [Google Scholar]
- 70.Scheiman JM, Hwang JH & Moayyedi P American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 148, 824–848 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 72.Esserman LJ et al. Use of molecular tools to identify patients with indolent breast cancers with ultralow risk over 2 decades. JAMA Oncol. 3, 1503–1510 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Irshad S et al. A molecular signature predictive of indolent prostate cancer. Sci. Transl Med 5, 202ra 122 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vogelstein B & Kinzler KW The path to cancer — three strikes and you’re out. N. Engl. J. Med 373, 1895–1898 (2015). [DOI] [PubMed] [Google Scholar]
- 75.Polyak K Breast cancer: origins and evolution. J. Clin. Invest 117,3155–3163 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Makohon-Moore A & Iacobuzio-Donahue CA Pancreatic cancer biology and genetics from an evolutionary perspective. Nat. Rev. Cancer 16, 553–565 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gundem G et al. The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jamal-Hanjani M et al. Tracking the evolution of non-small-cell lung cancer. N. Engl. J. Med 376, 2109–2121(2017). [DOI] [PubMed] [Google Scholar]
- 79.Sgroi DC Preinvasive breast cancer. Anna. Rev. Pathol 5, 193–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Porter D et al. Molecular markers in ductal carcinoma in situ of the breast. Mol. Cancer Res 1,362–375 (2003). [PubMed] [Google Scholar]
- 81.Ma XJ et al. Gene expression profiles of human breast cancer progression. Proc. Natl Acad. Sci. USA 100, 5974–5979 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Makohon-Moore AP et al. Precancerous neoplastic cells can move through the pancreatic ductal system. Nature 561,201–205 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stanta G & Bonin S Overview on clinical relevance of intra-tumor heterogeneity. Front. Med 5, 85 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grasso CS et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 487, 239–243 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robinson D et al. Integrative clinical genomics of advanced prostate cancer. Cell 161, 1215–1228 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu YM et al. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell 173, 1770–1782 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Armenia J et al. The long tail of oncogenic drivers in prostate cancer. Nat. Genet 50, 645–651 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tomlins SA et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia 10, 177–188 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koh CM et al. MYC and prostate cancer. Genes Cancer 1,617–628 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guedes LB et al. Analytic, preanalytic, and clinical validation of p53 IHC for detection of TP53 missense mutation in prostate cancer. Clin. Cancer Res 23, 4693–4703 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jamaspishvili T et al. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol 15, 222–234 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pritchard CC et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med 375, 443–453 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li J et al. Molecular differences between screen-detected and interval breast cancers are largely explained by PAM50 subtypes. Clin. Cancer Res 23, 2584–2592 (2017). [DOI] [PubMed] [Google Scholar]
- 94.Lambrechts D et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med 24, 1277–1289 (2018). [DOI] [PubMed] [Google Scholar]
- 95.Campbell MJ et al. Characterizing the immune microenvironment in high-risk ductal carcinoma in situ of the breast. Breast Cancer Res. Treat 161, 17–28 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Borczuk AC et al. Lung adenocarcinoma global profiling identifies type II transforming growth factor-beta receptor as a repressor of invasiveness. Am. J. Respir. Crit. Care Med 172, 729–737 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Borczuk AC et al. Lung adenocarcinoma invasion in TGFbetaRII-deficient cells is mediated by CCL5/RANTES. Oncogene 27, 557–564 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fridman WH et al. The immune microenvironment: a major player in human cancers. Int. Arch. Allergy Immunol 164, 13–26(2014). [DOI] [PubMed] [Google Scholar]
- 99.Goc J et al. Tertiary lymphoid structures in human lung cancers, a new driver of antitumor immune responses. Oncoimmunology 3, e28976 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sarr MG et al. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann. Surg 231, 205–212 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moffitt RA et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat. Genet 47, 1168–1178 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schwabe RF & Jobin C The microbiome and cancer. Nat. Rev. Cancer 13, 800–812 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sowalsky AG et al. Clonal progression of prostate cancers from Gleason grade 3 to grade 4. Cancer Res. 73, 1050–1055 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kovtun IV et al. Lineage relationship of Gleason patterns in Gleason score 7 prostate cancer. Cancer Res. 73,3275–3284 (2013). [DOI] [PubMed] [Google Scholar]
- 105.Trock BJ et al. PTEN loss and chromosome 8 alterations in Gleason grade 3 prostate cancer cores predicts the presence of un-sampled grade 4 tumor: implications for active surveillance. Mod. Pathol 29, 764–771 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sowalsky AG et al. Gleason score 7 prostate cancers emerge through branched evolution of clonal Gleason pattern 3 and 4. Clin. Cancer Res 23, 3823–3833 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palapattu GS et al. Molecular profiling to determine clonality of serial magnetic resonance imaging/ultrasound fusion biopsies from men on active surveillance for low-risk prostate cancer. Clin. Cancer Res 23, 985–991 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Klotz L et al. Active Surveillance Magnetic Resonance Imaging Study (ASIST): results of a randomized multicenter prospective trial. Eur. Urol 75, 300–309 (2018). [DOI] [PubMed] [Google Scholar]
- 109.Catalona WJ et al. A multicenter study of [−2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range. J. Urol 185, 1650–1655 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Russo GI et al. A systematic review and meta-analysis of the diagnostic accuracy of prostate health index and 4-kallikrein panel score in predicting overall and high-grade prostate cancer. Clin. Genitourin. Cancer 15, 429–439 (2017). [DOI] [PubMed] [Google Scholar]
- 111.Sanda MG et al. Association between combined TMPRSS2:ERG and PCA3 RNA urinary testing and detection of aggressive prostate cancer. JAMA Oncol. 3, 1085–1093 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kornberg Z et al. Genomic prostate score, PI-RADSv2, and progression in men with prostate cancer on active surveillance. J. Urol 201,300–307 (2018). [DOI] [PubMed] [Google Scholar]
- 113.Russnes HG et al. Breast cancer molecular stratification: from intrinsic subtypes to integrative clusters. Am. J. Pathol 187, 2152–2162 (2017). [DOI] [PubMed] [Google Scholar]
- 114.Nagarajan D & McArdle SEB Immune landscape of breast cancers. Biomedicines 6, 20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sontag L & Axelrod DH Evaluation of pathways for progression of heterogeneous breast tumors. J. Theor. Biol 232, 179–189 (2005). [DOI] [PubMed] [Google Scholar]
- 116.Artandi SE Complex roles for telomeres and telomerase in breast carcinogenesis. Breast Cancer Res. 5, 37–41 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Risom T et al. Differentiation-state plasticity is a targetable resistance mechanism in basal-like breast cancer. Nat. Commun 9, 3815 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Casasent AK et al. Multiclonal invasion in breast tumors identified by topographic single cell sequencing. Cell 172, 205–217 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Govindan R et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 150, 1121–1134 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 489, 519–525 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pleasance ED et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 463, 184–190 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 511,543–550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Billatos E et al. The airway transcriptome as a biomarker for early lung cancer detection. Clin. Cancer Res 24, 2984–2992 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heinrich EL et al. The inflammatory tumor microenvironment, epithelial mesenchymal transition and lung carcinogenesis. Cancer Microenviron. 5, 5–18(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cherezov D et al. Delta radiomic features improve prediction for lung cancer incidence: a nested case-control analysis of the National Lung Screening Trial. Cancer Med. 7, 6340–6356 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wilson R & Devaraj A Radiomics of pulmonary nodules and lung cancer. Transl Lung Cancer Res 6, 86–91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pinsky PF & Berg CD Applying the National Lung Screening Trial eligibility criteria to the US population: what percent of the population and of incident lung cancer’s would be covered? J. Med. Screen 19, 154–156(2012). [DOI] [PubMed] [Google Scholar]
- 128.Singhi AD et al. Preoperative GNAS and KRAS testing in the diagnosis of pancreatic mucinous cysts. Clin. Cancer Res 20, 4381–4389 (2014). [DOI] [PubMed] [Google Scholar]
- 129.Zambirinis CP et al. Pancreatic cancer, inflammation, and microbiome. Cancer J 20,195–202 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Permuth JB et al. Combining radiomic features with a miRNA classifier may improve prediction of malignant pathology for pancreatic intraductal papillary mucinous neoplasms. Oncotarget 7, 85785–85797 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hanania AN et al. Quantitative imaging to evaluate malignant potential of IPMNs. Oncotarget 7, 85776–85784 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brenner AT et al. Evaluating shared decision making for lung cancer screening. JAMA Intern. Med 178, 1311–1316 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ivlev I et al. Prostate cancer screening patient decision aids: a systematic review and meta-analysis. Am. J. Prev. Med 55, 896–907 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Davies L et al. Defining, estimating, and communicating overdiagnosis in cancer screening. Ann. Intern. Med 169, 36–43 (2018). [DOI] [PubMed] [Google Scholar]


