Abstract
Background
Valproic acid (VPA) pharmacological mechanisms are related to the anti-inflammatory and anti-viral effects. VPA is a histone deacetylases inhibitor and serves a role in its immunomodulatory impacts. VPA has complex effects on immune cell’s mitochondrial metabolism. The SLC5A8 transporter of short fatty acids has an active role in regulating mitochondrial metabolism. The study aimed to investigate whether SLC5A8 expresses the sex-related difference and how SLC5A8 expression depends on gonadal hormones, VPA treatment, and NKCC1 expression in rat thymocytes.
Methods
Control groups and VPA-treated gonad-intact and gonadectomized Wistar male and female rats were investigated (n = 6 in a group). The VPA 300 mg/kg/day in drinking water was given for 4 weeks. The SLC5A8 (Slc5a8 gene) and NKCC1 (Slc12a2 gene) RNA expressions were determined by the RT-PCR method.
Results
The higher Slc5a8 expression was found in the gonad-intact males than respective females (p = 0.004). VPA treatment decreased the Slc5a8 expression in gonad-intact and castrated males (p = 0.02 and p = 0.03, respectively), and increased in gonad-intact female rats compared to their control (p = 0.03). No significant difference in the Slc5a8 expression between the ovariectomized female control and VPA-treated females was determined (p > 0.05). VPA treatment alters the correlation between Slc5a8 and Slc12a2 gene expression in thymocytes of gonad-intact rats.
Conclusion
VPA effect on the Slc5a8 expression in rat thymocytes is gender- and gonadal hormone-dependent.
Keywords: valproic acid, SLC5A8, thymocyte, sex-related difference
Introduction
VPA is one of the most frequently prescribed anti-epileptic drugs. 1 VPA is an inhibitor of histone deacetylases (HDACs). 2 Studies raise the possibility of the therapeutic anti-viral potential of VPA.3,4 The virus replication and survival depend on the produced mitochondrial energy; thus, the anti-viral therapy tactics may involve medicine preparations that change energetic mitochondrial functions and activate anti-inflammatory mechanisms. 5 As a simple fatty acid, VPA is a substrate for the fatty acid β -oxidation pathway. 6 VPA bioactivation directs the metabolite 4-ene-VPA into the mitochondria, forming the 2,4-diene-VPA-CoA ester by β-oxidation. 7 VPA metabolites induce the significant decrease of pyruvate‐driven oxidative phosphorylation in mitochondria by conflict with pyruvate transport. 6 Such VPA effect could interfere with the short fatty acid transporter SLC5A8 (Solute carrier family-5 member-8) activity. The SLC5A8, transporting short fatty acids as a substrate for the β-oxidation pathway in mitochondria, has a vital role in regulating cell metabolism.
SLC5A8 acts as electrogenic sodium (Na+) and chloride (Cl−)-dependent sodium-coupled transporter, transporting into the cell lactate, pyruvate, acetate, propionate, valerate, butyrate, and monocarboxylate drugs (dichloroacetate, nicotinate, salicylate, and 5-aminosalicylate) and ketone bodies.8–12 Research of adult male wild-type c/ebpδ+/– and c/ebpδ–/– mice shows that the SLC5A8 physiologic function operates an essential role in the short-chain fatty acids transport into the cell.13,14 If the transporter is not expressed, cells may not accumulate a substrate. Short fatty acids transported into immune cells through the SLC5A8 change the HDAC activity, exerting immunomodulatory effects: blockade of dendritic cell development, releasing cytokines and inducing T cell apoptosis, emphasizing the importance of SLC5A8 in the immune homeostasis, and suppression of inflammation.15,16 SLC5A8 triggers cell apoptosis by pyruvate-dependent repression of HDACs. 17 The silencing of the SLC5A8 gene is related to DNA methylation, and treating cancer cells with DNA-demethylating agents increases the SLC5A8 expression. 12 VPA could activate genes regulated by DNA methylation, and the VPA effect may be trigged to active DNA demethylation in cancer cells.18,19 The SLC5A8 activity in the male rat duodenum enterocytes depends on Na+-K+-2Cl− cotransporter (NKCC1) activity. 20 Changes in the NKCC1 activity might lead to mitochondrial function changes. In fibroblasts treated with NKCC1 inhibitor bumetanide, the increase in maximal respiration suggests enhanced substrate availability and mitochondrial oxidation. 21 VPA reduces NKCC1 RNA expression in males but not in female rat thymocytes. 22 Pro-inflammation-immune cells derive most of their energy from aerobic glycolysis rather than mitochondrial oxidation to generate more energy and support their intensified activity. 23 Sex differences in the immune response depending on the sexually dimorphic populations of mitochondria and gonadal hormones. 24
The present study aimed at the following questions: (1) whether SLC5A8 transporter expresses the sex-related difference in rat thymocytes? (2) does VPA treatment change the SLC5A8 expression in thymocytes? (3) how SLC5A8 expression depends on gonadal hormones, VPA treatment, and NKCC1 expression in thymocytes? The answers to these questions are significant in dealing with personalized VPA therapy purposes.
Methods
Animals and treatment
Permission of the study was obtained from the State Food and Veterinary Service of Lithuania to use experimental animals for research (2017-01-02 No. G2-53). The preclinical and clinical research of pharmaceuticals regulatory guidelines indicates the importance of evaluating sex differences, stressing that medicine development should provide adequate information about the drug effects in both genders. 25 The VPA treatment effect on the thymus was started in the 4-to-5-weeks old Wistar rats: gonad-intact and gonadectomized controls and VPA-treated groups of both genders (n = 6 each). The animals were housed in standard colony cages with free entrance to food in the state of constant temperature (21°C ± 1°C), light/dark cycle (12-h/12-h), and humidity. Rats got a commercial pellet diet ad libitum. The orchidectomy and ovariectomy were performed at 28 ± 2 days of age (in the peripubertal period). Male gonadectomy was performed by orchiectomy using the scrotal approach, and female ovariectomy was performed by midline laparotomy. The following preparations for anesthesia were used for gonadectomy: Sedator 1 mg/kg intramuscular injection (i.m.) (Eurovet Animal Health B.V., Bladel, the Netherlands), Bioketan 75 mg/kg i.m. (Vetoquinol Biowet, Gorzów, Poland), and Atipam 2 mg/kg i.m. as an antidote of Sedator (Eurovet Animal Health B.V.). The accommodation period after the castration was 1 week. After the accommodation, animals were treated for 28 days with VPA 300 mg/kg/day in the drinking water as reported. 22 The only source of drinking for treated groups was the VPA solution, and the fresh tap water for the control groups was provided. One ovariectomized VPA-treated female was eliminated from the study due to a fistula formed after the operation.
Thymus preparation
After treatment, the animals were euthanized in a 70% CO2 camera. The carotid arteries and the aorta were cut, and the animals exsanguinated to minimize the thymus contamination with red blood cells. After the euthanasia of the animals, their thymus was harvested, and the contaminating blood was removed by rinsing with Roswell Park Memorial Institute 1640 (RPMI-1640) medium (Biological Industries, Israel). The thymus surrounding connective tissue was removed, and the left lobe samples were stored in the RNAlaterRNA stabilization reagent (Qiagen, Germany) at −80°C until further RNA extraction and analysis.
Extraction of RNA from the thymus
The frozen tissue was ground in liquid nitrogen. According to the manufacturer’s instruction, the total RNA was extracted using the TRIzol Plus RNA Purification Kit (Life Technologies, USA). The RNA quality and concentration were assessed using a NanoDrop2000 spectrophotometer (Thermo Scientific, USA). The total RNA integrity was analyzed using the Agilent 2100 Bioanalyzer system (Agilent Technologies, CA) with an Agilent RNA 6000 Nano kit (Agilent Technologies, CA). RNA samples had the RNA integrity number (RIN) higher than 5. The samples of RNA were stored at −80°C until further analysis.
Determination of the Slc5a8 and Slc12a2 genes expression in thymocytes
RNA expression assays were performed for Slc5a8 (Rn01503812_m1), Slc12a2 (Rn00582505_m1), and Gapdh (Rn01775763_g1) genes. According to the manufacturer’s instruction, the reverse transcription was performed with High-Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (Applied Biosystems, USA) in 20 μL reaction volume containing 50 ng RNA using Biometra TAdvanced thermal cycler (Analytik Jena AG, Germany). The synthesized copy DNA (cDNA) was stored at 4°C until use or at −20°C for a longer time. Real-time polymerase chain reaction (PCR) was performed using an Applied Biosystems 7900 Fast Real-Time PCR System (Applied Biosystems, USA). The reactions were run in triplicate with 4 μL of cDNA template in a 20 μL reaction volume (10 μL of TaqMan Universal Master Mix II, no UNG (Applied Biosystems, USA), 1 μL of TaqMan Gene Expression Assay 20x (Applied Biosystems, USA), 5 μL of nuclease-free water (Invitrogen, USA) with the program running at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The amplification efficiency was about 100%. The amplicon length of TaqMan assays was 60 bp for the Slc5a8 gene, Slc12a2—67 bp, and Gapdh—174 bp.
Statistical analysis
The statistical analysis was performed using the Statistical Package for the Social Sciences, version 23.0 for Windows (IBM SPSS Statistics 23.0). The figures were performed using GraphPad Prism 7 and IBM SPSS Statistics 23.0 software. The normality assumption was conducted by the Shapiro–Wilk test. Differences between the two independent groups were evaluated using the nonparametric Mann–Whitney U test. To investigate the SLC5A8 (Slc5a8 gene) and NKCC1 (Slc12a2 gene) RNA expression changes in the VPA-treated and control groups, the threshold cycle (CT) value was normalized with the control Gapdh gene, and ΔCT value was obtained. For the gene expression study, the ΔΔCT (2−ΔΔCT) Livak method was used to calculate the expression level between the VPA-treated (test) and the control conditions of the target gene when compared to the reference gene. 26 The Spearman’s rank correlation coefficient (r) was used to assess relationships between the Slc5a8 and Slc12a2 genes (ΔCT values were used). R 2 linear was estimated by performing correlation plots. Differences at the value of p < 0.05 were considered significant.
Results
The VPA effect on Slc5a8 and Slc12a2 RNA expression in rat thymocytes
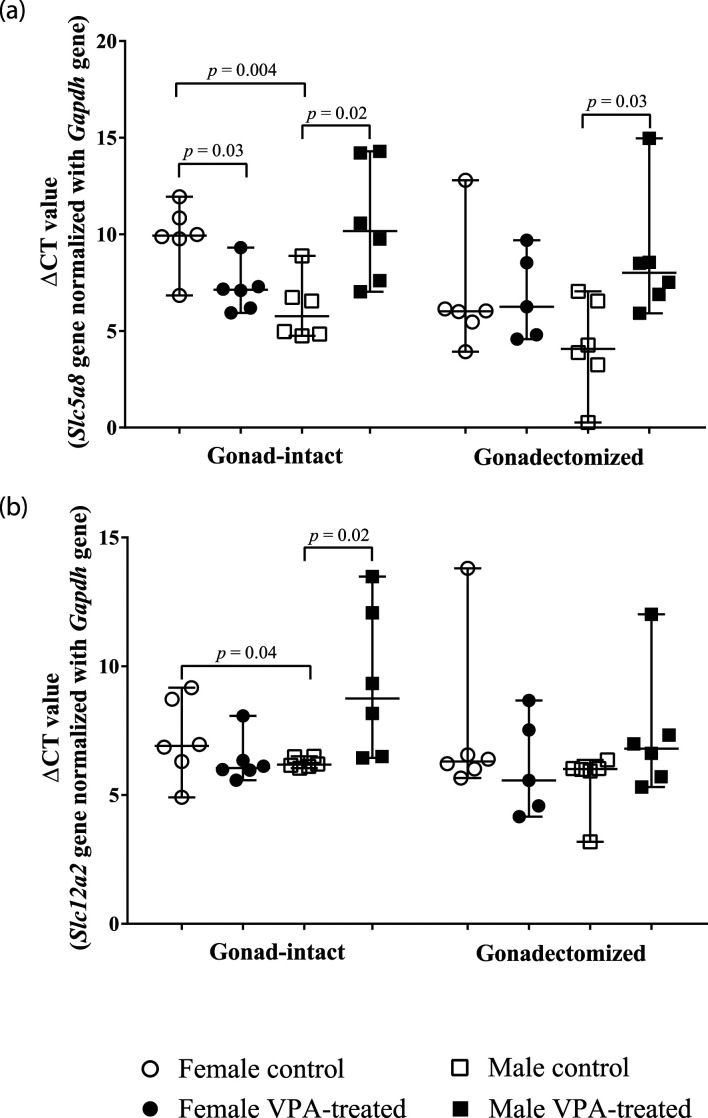
The Slc5a8 gene expression differences in gonad-intact and gonadectomized VPA-treated and control groups of both genders are considered in the ΔCT value (Table 1 and Figure 1(a)). A significantly 1.5-fold higher RNA expression was found in the gonad-intact male control than in the female control. The VPA treatment caused a statistically significantly decreased RNA gene expression in gonad-intact males and increased in gonad-intact female rats compared with their controls (Table 1 and Figure 1(a)).
Table 1.
RNA expression of SLC5A8 cotransporter in the thymus of study groups.
| Study group | CT mean | ΔCT | ΔΔCT | 2−ΔΔCT | |
|---|---|---|---|---|---|
| Gapdh | Slc5a8 | ||||
| Gonad-intact female | |||||
| Control | 23.530 | 33.406 | 9.876 | −2.708 | 6.534 |
| VPA-treated | 23.083 | 30.251 | 7.168 a | ||
| Gonad-intact male | |||||
| Control | 22.905 | 29.452 | 6.547 b | 4.034 | 0.061 |
| VPA-treated | 22.982 | 33.563 | 10.581 c | ||
| Gonadectomized female | |||||
| Control | 22.675 | 29.403 | 6.728 | 0.046 | 0.969 |
| VPA-treated | 24.831 | 31.604 | 6.774 | ||
| Gonadectomized male | |||||
| Control | 25.436 | 29.649 | 4.213 | 4.545 | 0.043 |
| VPA-treated | 22.319 | 31.077 | 8.758 d | ||
ap = 0.03, significant compared with the gonad-intact female control.
bp = 0.004, significant compared with the gonad-intact female control.
cp = 0.02, significant compared with the gonad-intact male control.
dp = 0.03, significant compared with the castrated male control.
Figure 1.
Slc5a8 (a) and Slc12a2 (b) genes expression in thymocytes of both gender gonad-intact and gonadectomized rats. Data after normalization with Galph gene. Delta threshold cycle (ΔCT) values were used for the graph (the horizontal bars represent the median; the short horizontal lines show the minimal and maximal values).
Comparing the thymocytes Slc5a8 gene expression of the gonad-intact of both sexes of rats with the expression in the respective gonadectomized animal groups, there is a clear increase trend: castration is associated with the increased gene expression in ovariectomized female (1.5-fold) and male (1.6-fold) groups; still, the difference is statistically insignificant. In castrated VPA-treated males, a statistically significant decrease of the Slc5a8 gene expression in thymocytes was found compared with their control rats. Data showed no significant effects of VPA treatment on the gene expression level of the ovariectomized females (Table 1 and Figure 1(a)). The relative Slc5a8 gene expression is shown in Table 1 and Supplemental material Figure 1(a).
The RNA expression of the Slc12a2 gene was found significantly higher in the gonad-intact males than the gonad-intact females. The comparison showed significantly increased Slc12a2 gene expression in gonad-intact VPA-treated females as compared with the control group. The Slc12a2 expression level in the gonad-intact VPA-treated males was significantly decreased as compared with the control group. Compared with the controls, there were no significant Slc12a2 gene expression changes in the gonadectomized female and male VPA-treated groups (Figure 1(b) and Table 2). The relative Slc12a2 gene expression is shown in Supplemental material Figure 1(b).
Table 2.
Delta threshold cycle (ΔCT) values correlation (r) between Slc5a8 and Slc12a2 (NKCC1) genes, R 2 linear in the study groups.
| Index | Gonad-intact female | Gonad-intact male | Gonadectomized female | Gonadectomized male | ||||
|---|---|---|---|---|---|---|---|---|
| Control | VPA-treated | Control | VPA-treated | Control | VPA-treated | Control | VPA-treated | |
| ΔCT Slc12a2 | 7.600 a | 6.344 | 6.229 | 9.332 b | 7.440 | 6.100 | 5.593 | 7.327 |
| Spearman correlation (r) | 0.941 c | 0 | 0.029 | 0.800 | 0.600 | 1 d | 0.700 | 0.800 |
| R 2 linear | 0.872 | 0.656 | 0.097 | 0.966 | 0.959 | 0.997 | 0.676 | 0.958 |
ap = 0.04, significant compared with gonad-intact male control.
bp = 0.02, significant compared with gonad-intact male control.
cp = 0.02, significant ΔCT values correlation between Slc5a8 and Slc12a2 genes in the study group.
dp = 0.02, significant ΔCT values correlation between Slc5a8 and Slc12a2 genes in the study group.
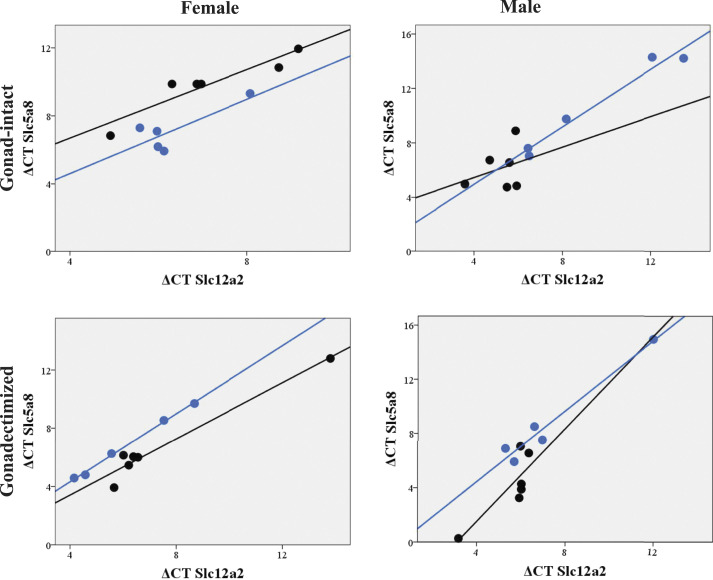
The VPA effect on the correlation between Slc5a8 and Slc12a2 RNA expression in rat thymocytes of study groups
The Slc12a2 gene expression, the correlation (r) between Slc5a8 and Slc12a2 RNA expression in thymocytes of gonad-intact, and gonadectomized VPA-treated and their control groups of both genders are shown in Figure 2 and Table 2.
Figure 2.
Correlation plots of the Slc5a8 and Slc12a2 (NKCC1) genes in the study rat groups. Black color represents control group, blue—VPA-treated group.
The gonad-intact females’ thymocytes showed a significantly strong correlation between Slc5a8 and Slc12a2 genes. The gonad-intact VPA-treated female thymocytes did not express this relationship. R 2 linear value in the VPA-treated female decreases in the VPA-treated female group compared with the control (Table 2).
Ovariectomized VPA-treated females showed a significant correlation between Slc5a8 and Slc12a2 genes. Study data did not establish a substantial relationship between Slc5a8 and Slc12a2 in thymocytes of tested male groups (Table 2 and Figure 2).
Discussion
Pharmacological impacts of VPA show the association with the therapeutic anti-viral7,27,28 and anti-inflammatory pathogenic mechanisms. 29 The immunomodulatory effect of VPA is related to the inhibition of class I and class II HDACs, which drives an increase in the acetylation of histones H2, H3, and H4, altering the gene expression related to immune cells activation.30–33 VPA represses the generation of pro-inflammatory factors such as NF-κB, IL-6, and TNF-α and prevents macrophages migration. 34 Our previous work reported that the 4 weeks of VPA treatment did not significantly impact thymus weight in the study gonad-intact and gonadectomized rat groups of both genders. 22 Others noted that the 8 weeks VPA treatment significantly reduced the lymph node and spleen weight and cellularity compared to control in MRL/lpr(−/−) mice females. 35 VPA suppresses T cell proliferation in vitro 33 and induces apoptosis of the lymphocyte. 36 Rat gonadectomy induces thymus hyperplasia by increased thymocyte proliferation. 37 In male rats, age-related thymus involution depends on testosterone. 38 Supplementation of testosterone in aged rhesus macaque males increases the number of naïve T cells by raising thymic output. 39
The present study of the VPA effect on rat thymocyte SLC5A8 transporter expression identified differences concerning gender, sex hormones, and NKCC1 expression potential VPA gender-related effects on cell function. Our study revealed that gonad-intact male rat thymocytes express a significantly higher SLC5A8 RNA level than gonad-intact females; the research shows an insignificant increase of SLC5A8 RNA expression in gonadectomized males and female rat controls, with no sex-related difference. The VPA treatment caused an opposite effect on the SLC5A8 RNA gene expression in female and male rats’ thymocytes. In gonad-intact females, the VPA treatment increased 2.7-fold of Slc5a8 gene expression while did not effect on ovariectomized female thymocytes. In VPA-treated gonad-intact and castrated males, the thymocyte Slc5a8 gene expression was decreased.
The SLC5A8 transporter function depends on NKCC1 cotransporter activity; its inhibition increases SLC5A8 activity and activates the mitochondrial function.20,21 The NKCC1 RNA expression in the gonad-intact male rat thymocytes is higher than in females. VPA downregulates NKCC1 expression in gonad-intact male rat thymocytes, and no VPA effect on the NKCC1 was found in castrated male, gonad-intact, and ovariectomized female rat thymocytes. 22 Our study shows the natural gender-related differences in the NKCC1 and SLC5A8 gene expression correlation in thymocytes. Such a biological gender-related relationship between SLC5A8 and NKCC1 gene expression differences disappeared in VPA-treated animals.
The SLC5A8 is a Na+-coupled and electrogenic transporter, able to concentrate short fatty acids into the cell: with a Na+ and substrate stoichiometry between 4:1 and 2:1. 40 The activity and expression of SLC5A8 and NKCC1 in the cell are related to the Na+ and Cl− ions transport into the cell; their intracellular and extracellular concentrations, which are sex-dependent, 41 and essential for immune-inflammatory, glycolytic processes. 42 A mice study shows that NKCC1 performs a novel role in acute lung inflammatory responses: a specific NKCC1 inhibition could benefit sepsis treatment. 43
By mitochondrial mechanisms, VPA inhibits HDACs activity, 31 inducing DNA demethylation and increase the SLC5A8 gene expression.12,18,19 Pro-inflammatory-immune cells derive most of their energy from aerobic glycolysis rather than mitochondrial oxidation to generate higher energy and maintain their enhanced activity. 23 The virus-activated T cells starting accelerated growth and proliferation need glucose uptake and glycolysis.44–46 VPA treatment decreases glucose blood concentration in animals and humans.47,48
VPA inhibits Th1 and Th17 cells and related pro-inflammatory cytokines. 49 VPA polarizes macrophages from M1 to M2 phenotype, which cannot induce naïve CD4+ T cells differentiation into a Th1 profile, favoring a Th2 phenotype 50 by mitochondria affected immune cell metabolism changing from the down TCA cycle to β-oxidation. 51 VPA does not affect the viability and the activation of CD8+ T lymphocytes exposed to viral peptides, 30 increased CD4+ T cell level with no change in CD8+ T cell level. 52 HDACs inhibition by VPA alters the gene expression related to cell differentiation and apoptosis.33,36 In females, aging does not lead to T cell dysfunction, and older females generate strong T cell immunity; men aging leads to T cell dysfunction. 53
VPA inhibits the aerobic glycolysis in tumor cells by reducing glucose uptake and decreasing lactate and ATP production via lowering the levels of E2F transcription factor 1; VPA affects E2F1 binding to the promoter of glycolytic genes GPI and PGK1. 54 Such VPA impact on glycolysis shows a new therapeutic strategy in researching the VPA effect on immune cells. The expression of SLC5A8 in T cells may likely affect the immune response and be related to the viral disease progression. It is known that the decreased SLC5A8 expression is the risk factor for Helicobacter pylori infection in children 55 ; the low SLC5A8 expression is related to papillomavirus-related cancerogenesis. 56 The limitation of the study is that the influence of sex hormones on thymocytes SLC5A8 expression has not been studied. Further studies are needed to determine the role of SLC5A8 transporter expression with sex-specific risk mechanisms for disease progression.
Conclusions
Gonad-intact male rat thymocytes express a higher SLC5A8 RNA level than gonad-intact females. The effect of VPA treatment on the SLC5A8 RNA gene expression in rat thymocytes depends on gender and gonadal hormones. The VPA treatment caused an oppositive effect on the SLC5A8 gene expression in gonad-intact female and male rats thymocytes. In gonadectomized rats, VPA decreases the Slc5a8 expression in castrated males, with no effect in ovariectomized female rat thymocytes.
Supplemental Material
Supplemental Material, sj-pdf-1-iji-10.1177_20587384211051954 for The effect of valproic acid on SLC5A8 expression in gonad-intact and gonadectomized rat thymocytes by Milda Juknevičienė, Ingrida Balnytė, Angelija Valančiūtė, Jūratė Stanevičiūtė, Kęstutis Sužiedėlis and Donatas Stakišaitis in International Journal of Immunopathology and Pharmacology
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present study was funded by the Research Fund of the Lithuanian University of Health Sciences [No.V-31; 2019-12-18].
Ethics approval: Ethical approval for this study was obtained from the STATE FOOD and VETERINARY SERVICE of LITHUANIA (2017-01-02 No. G2-53).
Animal welfare: The present study followed international, national, and/or institutional guidelines for human animal treatment and complied with relevant legislation.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Donatas Stakišaitis https://orcid.org/0000-0002-1547-7050
References
- 1.Perucca E. (2002) Pharmacological and therapeutic properties of valproate: a summary after 35 years of clinical experience. CNS Drugs 16: 695–714. [DOI] [PubMed] [Google Scholar]
- 2.Phiel CJ, Zhang F, Huang EY, et al. (2001) Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. Journal of Biological Chemistry 276: 36734–36741. [DOI] [PubMed] [Google Scholar]
- 3.Naasani I. (2020) Compare analysis, a bioinformatic approach to accelerate drug repurposing against Covid-19 and other emerging epidemics. SLAS DISCOVERY: Advancing the Science of Drug Discovery 26: 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreu S, Ripa I, Bello-Morales R, et al. (2020) Valproic acid and its amidic derivatives as new antivirals against alphaherpesviruses. Viruses 12: 1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunn AVW, Guy GW, Brysch W, et al. (2020) SARS-CoV-2 and mitochondrial health: implications of lifestyle and ageing. Immunity & Ageing 17: 33–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva MFB, Aires CCP, Luis PBM, et al. (2008) Valproic acid metabolism and its effects on mitochondrial fatty acid oxidation: a review. Journal of Inherited Metabolic Disease 31: 205–216. [DOI] [PubMed] [Google Scholar]
- 7.Ghodke-Puranik Y, Thorn CF, Lamba JK, et al. (2013) Valproic acid pathway: pharmacokinetics and pharmacodynamics. Pharmacogenetics and Genomics 23: 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gopal E, Fei Y-JJ, Miyauchi S, et al. (2005) Sodium-coupled and electrogenic transport of B-complex vitamin nicotinic acid by slc5a8, a member of the Na/glucose co-transporter gene family. Biochemical Journal 388: 309–316. Epub ahead of print 15 May 2005. DOI: 10.1042/BJ20041916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopal E, Fei Y-J, Sugawara M, et al. (2004) Expression of SLC5A8 in kidney and its role in Na+-coupled transport of lactate. Journal of Biological Chemistry 279: 44522–44532. [DOI] [PubMed] [Google Scholar]
- 10.Thangaraju M, Cresci G, Itagaki S, et al. (2008) Sodium-coupled transport of the short chain fatty acid butyrate by SLC5A8 and its relevance to colon cancer. Journal of Gastrointestinal Surgery 12: 1773–1782. [DOI] [PubMed] [Google Scholar]
- 11.Thangaraju M, Karunakaran SK, Itagaki S, et al. (2009) Transport by SLC5A8 with subsequent inhibition of histone deacetylase 1 (HDAC1) and HDAC3 underlies the antitumor activity of 3-bromopyruvate. Cancer 115: 4655–4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babu E, Ramachandran S, Coothankandaswamy V, et al. (2011) Role of SLC5A8, a plasma membrane transporter and a tumor suppressor, in the antitumor activity of dichloroacetate. Oncogene 30: 4026–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thangaraju M, Ananth S, Martin PM, et al. (2006) c/ebpδ Null mouse as a model for the double knock-out of slc5a8 and slc5a12 in kidney. Journal of Biological Chemistry 281: 26769–26773. [DOI] [PubMed] [Google Scholar]
- 14.Frank H, Gröger N, Diener M, et al. (2008) Lactaturia and loss of sodium-dependent lactate uptake in the colon of SLC5A8-deficient mice. Journal of Biological Chemistry 283: 24729–24737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh N, Thangaraju M, Prasad PD, et al. (2010) Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. Journal of Biological Chemistry 285: 27601–27608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmerman MA, Singh N, Martin PM, et al. (2012) Butyrate suppresses colonic inflammation through HDAC1-dependent fas upregulation and fas-mediated apoptosis of T cells. American Journal of Physiology-Gastrointestinal and Liver Physiology 302: G1405–G1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thangaraju M, Carswell KN, Prasad PD, et al. (2009) Colon cancer cells maintain low levels of pyruvate to avoid cell death caused by inhibition of HDAC1/HDAC3. Biochemical Journal 417: 379–389. [DOI] [PubMed] [Google Scholar]
- 18.Milutinovic S, D’Alessio AC, Detich N, et al. (2007) Valproate induces widespread epigenetic reprogramming which involves demethylation of specific genes. Carcinogenesis 28: 560–571. [DOI] [PubMed] [Google Scholar]
- 19.Veronezi GMB, Felisbino MB, Gatti MSV, et al. (2017) DNA methylation changes in valproic acid-treated HeLa cells as assessed by image analysis, immunofluorescence and vibrational microspectroscopy. PLoS One 12: e0170740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaji I, Iwanaga T, Watanabe M, et al. (2015) SCFA transport in rat duodenum. American Journal of Physiology-Gastrointestinal and Liver Physiology 308: G188–G197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omer S, Koumangoye R, Delpire E. (2020) A mutation in the Na-K-2Cl cotransporter-1 leads to changes in cellular metabolism. Journal of Cellular Physiology 235: 7239–7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juknevičienė M, Balnytė I, Valančiūtė A, et al. (2019) Valproic acid inhibits Na-K-2Cl cotransporter RNA expression in male but not in female rat thymocytes. Dose-Response 17: 1559325819852444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shyer JA, Flavell RA, Bailis W. (2020) Metabolic signaling in T cells. Cell Research 30: 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kloc M, Ghobrial RM, Kubiak JZ. (2020) The role of genetic sex and mitochondria in response to COVID-19 infection. International Archives of Allergy and Immunology 181: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.European Medicines Agency . 2011. ICH guideline S6 (R1) – preclinical safety evaluation of biotechnology-derived pharmaceuticals part I (parent guideline). Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002828.pdf (accessed 12 July 2018).
- 26.Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 27.Zhou P, Yang X-L, Wang X-G, et al. (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579: 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhavesh NS, Patra A. Virtual screening and molecular dynamics simulation suggest valproic acid co-A could bind to SARS-CoV2 RNA depended RNA polymerase. Epub ahead of print 26 March 2020. DOI: 10.20944/preprints202003.0393.v1. [DOI]
- 29.Hull EE, Montgomery MR, Leyva KJ. (2016) HDAC inhibitors as epigenetic regulators of the immune system: impacts on cancer therapy and inflammatory diseases. BioMed Research International 2016: 8797206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li G, Nowak M, Bauer S, et al. (2013) Levetiracetam but not valproate inhibits function of CD8+ T lymphocytes. Seizure 22: 462–466. [DOI] [PubMed] [Google Scholar]
- 31.Mihaylova MM, Vasquez DS, Ravnskjaer K, et al. (2011) Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell 145: 607–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett RL, Licht JD. (2018) Targeting epigenetics in cancer. Annual Review of Pharmacology and Toxicology 58: 187–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falkenberg KJ, Johnstone RW. (2014) Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nature Reviews Drug Discovery 13: 673–691. [DOI] [PubMed] [Google Scholar]
- 34.Ichiyama T, Okada K, Lipton JM, et al. (2000) Sodium valproate inhibits production of TNF-α and IL-6 and activation of NF-κB. Brain Research 857: 246–251. [DOI] [PubMed] [Google Scholar]
- 35.Dowdell KC, Pesnicak L, Hoffmann V, et al. (2009) Valproic acid (VPA), a histone deacetylase (HDAC) inhibitor, diminishes lymphoproliferation in the Fas-deficient MRL/lpr−/− murine model of autoimmune lymphoproliferative syndrome (ALPS). Experimental Hematology 37: 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan O, La Thangue NB. (2012) HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunology & Cell Biology 90: 85–94. [DOI] [PubMed] [Google Scholar]
- 37.Schneider T, Roman A, Basta-Kaim A, et al. (2008) Gender-specific behavioral and immunological alterations in an animal model of autism induced by prenatal exposure to valproic acid. Psychoneuroendocrinology 33: 728–740. [DOI] [PubMed] [Google Scholar]
- 38.Leposavic G, Perisic M. (2008) Age-associated remodeling of thymopoiesis: role for gonadal hormones and catecholamines. Neuroimmunomodulation 15: 290–322. [DOI] [PubMed] [Google Scholar]
- 39.Rais M, Wilson RM, Urbanski HF, et al. (2017) Androgen supplementation improves some but not all aspects of immune senescence in aged male macaques. GeroScience 39: 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyauchi S, Gopal E, Fei Y-J, et al. (2004) Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na+-coupled transporter for short-chain fatty acids. Journal of Biological Chemistry 279: 13293–13296. [DOI] [PubMed] [Google Scholar]
- 41.Grikiniene J, Volbekas V, Stakisaitis D. (2004) Gender differences of sodium metabolism and hyponatremia as an adverse drug effect. Medicina (Kaunas, Lithuania) 40: 935–942. [PubMed] [Google Scholar]
- 42.Amara S, Tiriveedhi V. (2017) Inflammatory role of high salt level in tumor microenvironment (review). International Journal of Oncology 50: 1477–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen MT, Pace AJ, Koller BH. (2007) Mice lacking NKCC1 are protected from development of bacteremia and hypothermic sepsis secondary to bacterial pneumonia. Journal of Experimental Medicine 204: 1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kidani Y, Elsaesser H, Hock MB, et al. (2013) Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nature Immunology 14: 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinclair LV, Rolf J, Emslie E, et al. (2013) Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nature Immunology 14: 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang R, Dillon CP, Shi LZ, et al. (2011) The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35: 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan S, Jena G. (2016) Valproic acid improves glucose homeostasis by increasing beta-Cell proliferation, function, and reducing its apoptosis through HDAC inhibition in Juvenile diabetic rat. Journal of Biochemical and Molecular Toxicology 30: 438–446. [DOI] [PubMed] [Google Scholar]
- 48.Rakitin A, Kõks S, Haldre S. (2015) Valproate modulates glucose metabolism in patients with epilepsy after first exposure. Epilepsia 56: e172–e175. [DOI] [PubMed] [Google Scholar]
- 49.Long J, Chang L, Shen Y, et al. (2015) Valproic acid ameliorates graft-versus-host disease by downregulating Th1 and Th17 Cells. The Journal of Immunology 195: 1849–1857. [DOI] [PubMed] [Google Scholar]
- 50.Wu C, Li A, Leng Y, et al. (2012) Histone deacetylase inhibition by sodium valproate regulates polarization of macrophage subsets. DNA and Cell Biology 31: 592–599. [DOI] [PubMed] [Google Scholar]
- 51.Angajala A, Lim S, Phillips JB, et al. (2018) Diverse roles of mitochondria in immune responses: novel insights into immuno-metabolism. Frontiers in Immunology 9: 1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee K, Vivithanaporn P, Siemieniuk RA, et al. (2010) Clinical outcomes and immune benefits of anti-epileptic drug therapy in HIV/AIDS. BMC Neurology 10: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi T, Wong P, Ellingson MK, et al. (2020) Sex differences in immune responses to SARS-CoV-2 that underlie disease outcomes. medRxiv 588: 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fang E, Wang J, Hong M, et al. (2019) Valproic acid suppresses Warburg effect and tumor progression in neuroblastoma. Biochemical and Biophysical Research Communications 508: 9–16. [DOI] [PubMed] [Google Scholar]
- 55.Orellana-Manzano A, O’Ryan MG, Lagomarcino AJ, et al. (2016) Helicobacter pylori infection is associated with decreased expression of SLC5A8, a cancer suppressor gene, in young children. Frontiers in Cellular and Infection Microbiology 6. Epub ahead of print 10 October 2016. DOI: 10.3389/fcimb.2016.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hernández-Juárez J, Vargas-Sierra O, Herrera LA, et al. (2019) Sodium-coupled monocarboxylate transporter is a target of epigenetic repression in cervical cancer. International journal of oncology 54: 1613–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-iji-10.1177_20587384211051954 for The effect of valproic acid on SLC5A8 expression in gonad-intact and gonadectomized rat thymocytes by Milda Juknevičienė, Ingrida Balnytė, Angelija Valančiūtė, Jūratė Stanevičiūtė, Kęstutis Sužiedėlis and Donatas Stakišaitis in International Journal of Immunopathology and Pharmacology