Abstract
Background:
There is controversy about whether treatment of chronic lateral ankle instability (CLAI) with osteochondral lesions of the talus (OLT) can be performed concurrently.
Purpose:
To investigate the midterm results of arthroscopic treatment of CLAI combined with OLT in different surgical settings. It was hypothesized that the outcomes of treating both injuries at the same time would not be inferior to those of staged surgery.
Study Design:
Randomized controlled trial; Level of evidence, 1.
Methods:
Included were 103 patients with both CLAI and OLT who underwent arthroscopic microfracture surgery and an open, modified Broström-Gould procedure for ligament repair from January 2015 to December 2016. The patients were assigned randomly to a staged group (51 patients) and a single-stage group (52 patients). The staged group underwent arthroscopic debridement of the OLT and microfracture, then rehabilitation for 4 to 6 months before undergoing modified Broström-Gould ligament repair. The single-stage group underwent both procedures simultaneously. Clinical evaluations were performed on the day before surgery and at 12-month, 24-month, and final follow-up periods using the Karlsson-Peterson score, American Orthopaedic Foot & Ankle Society (AOFAS) score, and pain visual analog scale. The Karlsson-Peterson score at 24 months postoperatively was considered the primary outcome. The predefined noninferiority margin for the primary outcome was −5 points.
Results:
At the final follow-up, 50 patients in the single-stage group and 48 patients in the staged group completed the study. The median lesion size was 0.72 cm2 (interquartile range [IQR], 0.5-1.12 cm2) in the single-stage group and 0.84 cm2 (IQR, 0.7-1.05 cm2) in the staged group. At 12-month follow-up, the single-stage group had a significantly higher median Karlsson-Peterson score (79 [IQR, 70-85] vs 75 [IQR 65-80] for staged; P = .024) and median AOFAS score (85 [IQR, 76-89] vs 79.5 [IQR, 70-87] for staged; P = .045). At 24-month follow-up, the median difference in the Karlsson-Peterson score for single-stage versus staged surgery was 2 points (95% CI, −2 to 5 points), and the confidence interval was greater than the predefined value.
Conclusion:
At midterm follow-up, there was no clinical difference between single-stage versus staged surgery to treat CLAI with OLT. Single-stage surgery achieved better clinical outcomes than staged surgery at short-term follow-up.
Keywords: ankle, arthroscopy, chronic lateral ankle instability, osteochondral lesion
Ankle sprain is among the most common sports injuries. 8 In the United States, approximately 3 million people seek medical advice for ankle sprains every year. 33 Lateral ankle sprains are the most common type. A prospective cohort study has demonstrated that the incidence of chronic lateral ankle instability (CLAI) was 40% within 1 year after the initiating lateral ankle sprain. 7 Most patients fully recover from acute lateral ankle sprains after nonoperative treatment. However, approximately 20% of patients may develop CLAI because of mechanical or functional instability. 17 Patients with CLAI lose confidence in their ankle’s ability to support them. They fear walking on uneven ground and feel discomfort in the ankle joint at the beginning and end of walking. More seriously, CLAI may be accompanied by, or secondary to, related intra-articular lesions, particularly osteochondral lesions of the talus (OLT). 3 The incidence of OLT in patients with acute and chronic ankle instability ranges from 16% to 63%. 5,14,25,31 There is evidence that OLT rarely heals spontaneously. 24
The modified Broström procedure has been used widely in the repair of the anterior talofibular ligament (ATFL), and the bone marrow stimulation methods such as the microfracture technique for minor OLT have achieved encouraging results after midterm follow-up. 29 However, there is still some controversy about the timing of surgical intervention for patients with CLAI and OLT and whether CLAI and OLT should be managed concurrently. 9,16,38 The advantages of single-stage surgery lie in the relatively shorter recovery time, which saves the cost of the second operation and reduces the risk of complications caused by a second operation. The advantage of staging surgery is that it allows arthroscopic exploration and further management of the talus repair after microfracture surgery and, more importantly, it avoids the adverse effects that may arise from different rehabilitation programs for CLAI and OLT. A 3- to 6-week immobilization (2-3 weeks of casting followed by 3 weeks in a walking boot) was generally needed after ligament repair. 26,27 Early weightbearing and passive and active motion are recommended after osteochondral debridement and microfracture. 20,28 So far, there have been few studies about the timing of surgery for CLAI with OLT.
The purpose of the current study was to investigate the results of arthroscopic treatment of CLAI combined with OLT as a single-stage versus staged procedure. We hypothesized that the midterm clinical outcome of treating both injuries at the same time would not be inferior to staged surgery.
Methods
Patient Recruitment
After obtaining ethics committee approval and patient consent, we conducted this randomized clinical controlled study. The Strengthening the Reporting of Observational Studies in Epidemiology statement was followed for patient selection. From January 2015 to December 2016, a total of 122 patients with both CLAI and OLT underwent arthroscopic microfracture surgery and an open, modified Broström-Gould procedure for ligament repair by a senior sports medicine surgeon (M.W.) at our hospital. Of these patients, 103 constituted the study cohort. The inclusion criteria were patients with CLAI who met the International Ankle Consortium standards. 10 These patients reported a history of at least 1 significant ankle sprain, the initial sprain having occurred at least 12 months previously, and the most recent injury having occurred more than 3 months before study enrollment. All patients had a history of the previously injured ankle joint “giving way,” recurrent sprain, and/or “feelings of instability.”
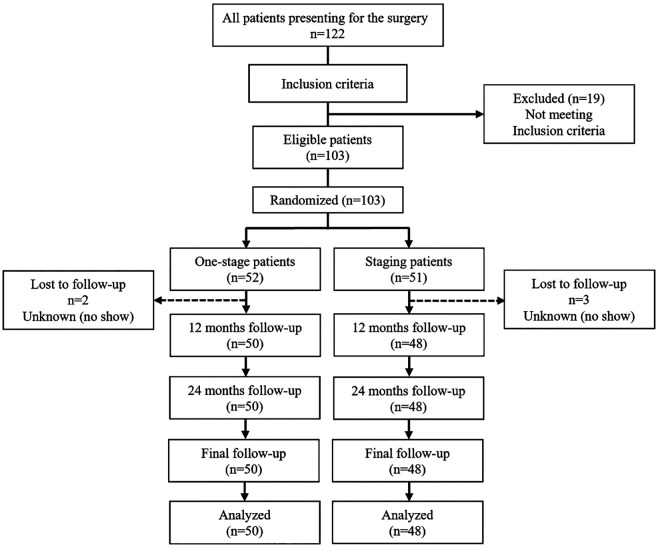
All patients were between 18 and 60 years old, had undergone a detailed history inquiry in the outpatient clinic, and had a positive result from an anterior drawer test. OLT was confirmed by radiograph, magnetic resonance imaging (MRI), and physical examination and had clear clinical symptoms that did not respond after 8 to 12 weeks of nonsurgical treatment (including immobilization, brace, and rehabilitation). In all cases, the lesion size was less than 150 mm2 (diameter <15 mm). 4,5 Findings from the foot alignment assessment were normal, without pes cavus deformity or flatfoot. Exclusion criteria were simultaneous osteochondral injury of the tibia (4 patients), OLT depth greater than 8 mm or requiring bone grafting or tissue-engineered transplantation (3 patients), concurrent tibiofibular syndesmosis ligament injury or other ligament injuries (8 patients), and bilateral lesions (4 patients). 1 Patients with only osteochondral lesion and complete and continuous articular cartilage were also excluded as unsuitable for microfracture techniques. Figure 1 shows the flowchart of patient enrollment in the study.
Figure 1.
The Strengthening the Reporting of Observational Studies in Epidemiology study diagram showing the patient enrollment process.
Included patients were grouped into the single-stage surgery group and the staged surgery group using a random number table. Enrollment and randomization of all patients were completed on December 15, 2016. In the single-stage surgery group, patients underwent arthroscopic debridement of the OLT and microfracture followed by a modified Broström-Gould ligament repair. In the staged surgery group, patients underwent arthroscopic debridement of the OLT and microfracture surgery, followed by functional rehabilitation training for 4 to 6 months, then modified Broström-Gould ligament repair.
OLT Evaluation
During arthroscopic surgery, osteochondral lesions were classified according to the Ferkel and Cheng system, 2 which consists of 6 stages ranging from A (smooth, intact, but soft or ballotable) to F (displaced fragment). The location of the lesion was recorded according to the 9-square-grid method (Figure 2). The widest point of the lesion in 2 planes was measured under direct arthroscopic vision using a custom-made probe with a scale (1.0 mm) (Smith & Nephew), and the lesion size was calculated. Measurements were performed independently by the senior sports medicine surgeon (M.W.) and another surgeon (Y.W.) and were rechecked if the difference exceeded 0.5 mm until consensus was reached. 36
Figure 2.
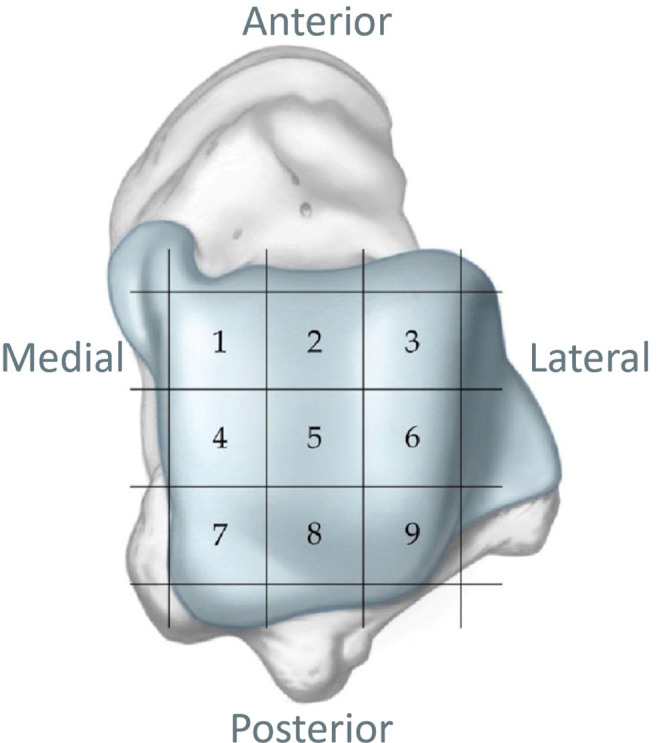
Nine-square-grid anatomic schematic of the talar dome used to define osteochondral lesion location.
MRI has been reported to have high sensitivity and specificity when diagnosing osteochondral lesions in the ankle joint. 6,22 All patients underwent MRI of the affected ankle preoperatively and at final follow up using a 1.5-T superconducting MRI scanner (uMR570; United-Imaging). The scanning sequence included T1- and T2-weighted imaging with turbo spin-echo fat suppression. No special coils were used. Lesions were evaluated and classified according to the Hepple system (stages 1 [articular cartilage injury] to 5 [subchondral cyst]). 13 A panel including the senior sports medicine surgeon and 2 senior radiologists not involved in this study came to a consensus for the classification of each patient.
Surgical Techniques
For OLT treatment, the patient was placed in a supine position, and the affected limb was disinfected routinely with iodine and alcohol. A 0.5-cm incision was made on the site medial to the tibialis anterior muscle and the site lateral to the extensor digitorum longus muscle at the ankle. The articular cavity was punctured for placement of the arthroscope, and then routine arthroscopy was performed. The conditions of other intra-articular injuries (soft tissue impact, osteophyte formation, lateral malleolus avulsion fracture, or loose body formation) were documented if present. A shaver and coblation wand were used to remove the hyperplastic synovium and grind the hyperplastic osteophyte. Exploration was performed to confirm the presence of an OLT. A shaver and curette were used to remove damaged cartilage of the talus and the unstable tissue around it. The actual area of the talus osteochondral removal was recorded, and then a microfracture awl was used to produce holes.
The modified Broström-Gould procedure was used to anatomically repair the lateral ankle ligaments. A curvilinear incision was made in the site anterior to the lateral malleolus. The incision was extended from the anterior distal lateral malleolus to the level of the peroneal tendon. The joint capsule interval was then recognized at the anterior border of the fibula, an ankle arthrotomy was performed, and the underlying ATFL remnant was identified and exposed. Two 2.3-mm suture anchors were used instead of transosseous suture. Then, the proximal extensor retinaculum was exposed and moved from the attachment to the distal fibula to make sure that the repair of ATFL could be further reinforced. When the ATFL was sutured, the ankle joint was kept in neutral dorsiflexion and slight eversion for a tension-free repair. Finally, range of motion (ROM) was checked once again, and an anterior drawer test and talar tilt were assessed to ensure sufficient ankle stability.
Postoperative Rehabilitation Program
The affected ankle was immobilized with a protective brace after surgery. On the first day after surgery (first day after initial surgery for the staged group), rehabilitation began with programs of different intensities. The patients in the staged surgery group received passive exercises of the ankle joint and muscle strength exercises, while those in the single-stage group received the same training but with half the intensity (see Appendix Table A1). All patients wore a brace for the first 4 weeks, then progressed to partial weightbearing for 6 weeks, with gradual removal of the brace at weeks 11 to 12. After that, patients in both groups began walking, balance, and mobility training. Patients in the single-stage group generally resumed daily activities 4 to 6 months after surgery. In the staged surgery group, at 4 to 6 months after the initial surgery, the patients underwent the modified Broström-Gould surgery. After ligament repair, patients in the staged group underwent the same rehabilitation protocol as prescribed for the single-stage group (phases 1-3 in Appendix Table A1).
Clinical Outcomes and Evaluation
We used the Karlsson-Peterson ankle function scale, 18 the American Orthopaedic Foot & Ankle Society (AOFAS) score (90-100 = excellent; 80-89 = good; 70-79 = fair; ≤69 = poor), 19 and a 10-point pain visual analog scale (VAS; 10 = most pain) to clinically evaluate patients on the day before surgery and at 12-month, 24-month, and final follow-up periods. The scores were assessed by 2 independent observers (J.S. and X.Y.). Surgery-related complications, including infection, surgical failure (eg, ligament repair failure or unhealed osteochondral lesion), postoperative ankle stiffness, lower limb nerve injury, and deep vein thrombosis, were assessed by 2 observers (M.W. and W.Q.) within the first 6 months postoperatively.
The Karlsson-Peterson score at 24 months postoperatively was considered the primary outcome measure. Secondary outcomes were the proportion of patients who achieved excellent and good AOFAS scores at 24 months postoperatively and the incidence of complications.
Statistical Methods
The calculation of sample size was based on a noninferiority design using the Karlsson-Peterson score at minimum 24-month follow-up. With means and standard deviations from a previous study, 3 inferiority was preset to −5 points. 21,32,35,39 If the limit of the 95% CI (for differences in median in Karlsson-Peterson score between the 2 groups) was greater than −5, then single-stage surgery could be considered as noninferior compared with the staged surgery. A power analysis was performed to calculate the minimum sample size required given a 95% CI limit at the 1-tailed significance level (P < .05). These calculations, performed using PASS software (NCSS), indicated that 50 patients were needed for each group.
Statistical analysis was performed using SPSS Statistics 22.0 (SPSS). The Kolmogorov-Smirnov test was used to assessed normality. Normally distributed data were expressed as means and ranges, and nonnormally distributed data were expressed as medians and interquartile ranges (IQRs). Chi-square tests were used to compare differences in categorical data (male-to-female ratio, Ferkel and Cheng classification, and Hepple classification), and independent-samples t tests were used to compare age, body mass index, and follow-up times. Two-way repeated-measures analysis of variance (ANOVA) was used to assess the within-patient effects of intervention and time. The Mann-Whitney U test was used to evaluate differences in the preoperative lesion size, AOFAS score, Karlsson-Peterson score, and VAS score between the 2 groups. The median difference between the Karlsson-Peterson and the AOFAS scores at each preoperative and postoperative follow-up time point in the 2 groups was assessed using the Hodges-Lehmann estimation to verify whether the results were consistent with our hypothesis. P < .05 indicated statistical significance. The content of the statistical analysis was reviewed independently by statistical experts.
Results
Patient Evaluation and Follow-up
In our study, 52 patients (34 men and 18 women) were included in the single-stage surgery group; the mean age was 40.9 years (range, 21-59 years). At the final follow-up, this group included 50 patients (33 men and 17 women). The staged surgery group consisted of 51 patients (32 men and 19 women) with a mean age of 40.6 years (range, 18-60 years). At the final follow-up, the group included 48 patients (31 men and 17 women). There was no significant difference in baseline characteristics between the 2 groups at the time of enrollment or the last follow-up (Table 1).
Table 1.
Baseline Characteristics of Patients Preoperatively and at Final Follow-up a
| Preoperative | Final Follow-up | |||||
|---|---|---|---|---|---|---|
| Single Stage (n = 52) |
Staged (n = 51) |
P | Single Stage (n = 50) |
Staged (n = 48) |
P | |
| Sex, male/female | 34/18 | 32/19 | .781 | 33/17 | 31/17 | .884 |
| Age, y | 40.9 (21-59) | 40.6 (18-60) | .878 | 41.0 (21-59) | 40.9 (18-60) | .822 |
| BMI, kg m-2 | 26.4 (20.1-34.4) | 26.7 (20.0-34.3) | .618 | 26.4 (20.1-34.4) | 26.6 (20.0-34.3) | .755 |
| Symptom duration, mo | 13 [11.5-36] | 12 [10-24] | .446 | 13 [12-36] | 12.5 [10-24] | .341 |
| Lesion size, cm2 | 0.72 [0.5 -1.16] | 0.84 [0.7 -1.05] | .320 | 0.72 [0.5 -1.12] | 0.84 [0.7 -1.05] | .186 |
| Follow-up time, mo | — | — | — | 57.7 (48-69) | 57.8 (48-69) | .942 |
a Data are reported as No., mean (range), or median [interquartile range]. BMI, body mass index. Dashes indicate not applicable.
There were no postoperative surgical complications (eg, wound nonhealing, infection, superficial peroneal nerve injury, or deep vein thrombosis) in any study patient.
In the single-stage surgery group, the average follow-up period was 57.7 months (48-69 months). In the staged surgery group, the average follow-up period was 57.8 months (48-69 months). By the time of the final follow-up, 2 patients (both in military service, 1 in each group) still presented with a suspiciously positive anterior drawer test result in the affected ankle joint, but this did not affect their daily activities. All patients underwent successful rehabilitation after the surgical procedures and resumed their daily work. Postoperative ankle stiffness was not reported.
Clinical Outcomes
The 2-way repeated-measures ANOVA showed that the different treatment methods (P = .128) and the cross-effect between the different treatment methods and postoperative time (P = .331) were not statistically significant influencing factors. Therefore, we focused on comparing the differences between the 2 groups at different follow-up time points.
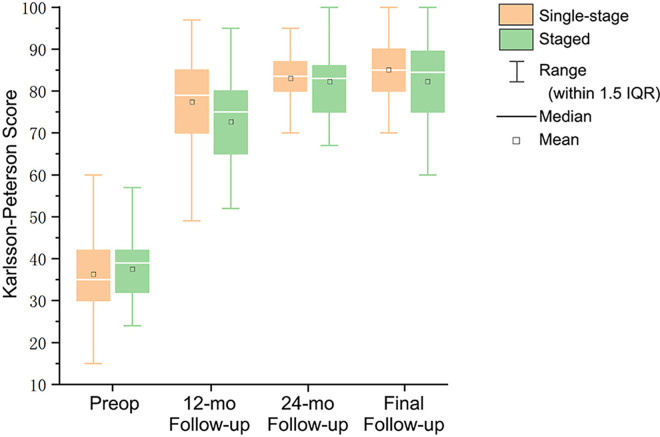
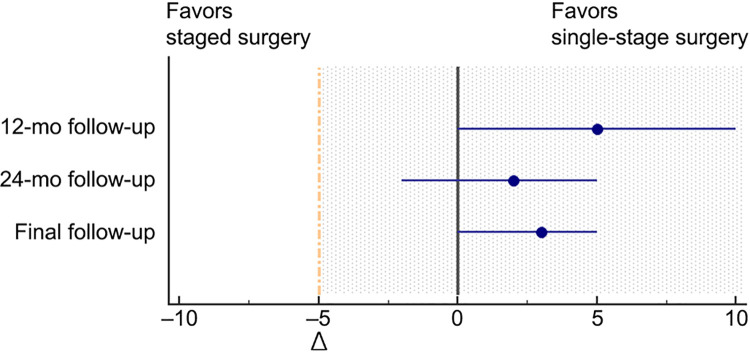
The median Karlsson-Peterson score increased from 35 (IQR, 30-42) before surgery to 85 (IQR, 80-90) at the last follow-up in the single-stage surgery group, while it increased from 39 (IQR, 32-42) before surgery to 84.5 (IQR, 75-89.5) in the staged surgery group. At 12 months after surgery, the score was 79 (IQR, 70-85) in the single-stage surgery group and 75 (IQR, 65-80) in the staged surgery, which was significantly different (P = .024). There was no significant difference between the 2 groups before surgery, 24 months after surgery, or the final follow-up (Figure 3). The median difference of Hodges-Lehmann estimation before surgery was 2 (95% CI, −2 to 5), and the median difference of each follow-up time after surgery was greater than the preset value (−5 points), which suggested that our study hypothesis was valid (Figure 4).
Figure 3.
Postoperative Karlsson-Peterson scores for the single-stage and staged groups. IQR, interquartile range; Preop, preoperative.
Figure 4.
Interpretation of the between-group difference in Karlsson-Peterson scores at different follow-up times. The blue lines represent the 95% CIs of the median difference (blue dots). Given that the confidence intervals for comparisons at 24-month follow-up are to the right of the noninferiority margin (Δ, −5 points) and also include zero, single-stage surgery was considered noninferior to staged surgery at 24-month follow-up. Dashed yellow line indicates noninferiority margin; shaded region indicates zone of noninferiority.
The median AOFAS and VAS scores preoperatively and at each follow-up point are shown in Table 2. At 12-month follow-up, there was a significant difference between the 2 groups (P = .045), but there was no significant difference at any other time point. According to the evaluation criteria, the rates of excellent and good AOFAS scores of the 2 groups were 82% (single-stage group) and 85% (staged group), which were not significantly different. There were no significant differences between the study groups in VAS score improvements at any time point.
Table 2.
AOFAS and VAS Scores at Each Follow-up Time a
| Single-Stage Group | Staged Group | P | |
|---|---|---|---|
| AOFAS score | |||
| Preoperative | 58 [49-69] | 59 [51-65.5] | .845 |
| 12-mo follow-up | 85 [76-89] | 79.5 [70-87] | .045 |
| 24-mo follow-up | 87 [76.5-90] | 85.5 [80-90] | .792 |
| Final follow-up | 90 [82-100] | 90 [84-98.5] | .621 |
| AOFAS grade at 24-mo follow-up, n | |||
| Excellent | 28 | 30 | .676 |
| Good | 13 | 11 | .758 |
| Fair | 8 | 6 | .647 |
| Poor | 1 | 1 | .977 |
| VAS pain score | |||
| Preoperative | 6 [5-7] | 6 [5-7] | .951 |
| 12-mo follow-up | 2 [1-4] | 1.5 [0-3] | .199 |
| 24-mo follow-up | 2 [1-3] | 1.5 [1-3] | .561 |
| Final follow-up | 1 [0-2] | 1 [0-2.5] | .595 |
a Data are reported as median [IQR] unless otherwise indicated. Bolded P value indicates statistically significant difference between groups (P < .05). AOFAS, American Orthopaedic Foot & Ankle Society; IQR, interquartile range; VAS, visual analog scale.
Arthroscopic Findings
Intraoperative arthroscopic findings according to the Ferkel and Cheng system are shown in Table 3. The cartilage of the talar surface was incomplete or discontinuous in all patients. The OLT location and other intra-articular lesions are shown in Table 4. In the single-stage group, the median lesion size was 0.72 cm2 (IQR, 0.5-1.12 cm2). In the staged surgery group, the median lesion size was 0.84 cm2 (IQR, 0.7-1.05 cm2). The classifications and lesion sizes were not significantly different between the 2 groups (P = .398, χ2 = 2.959;P = .186) (Table 4 and Table 1). All intra-articular diseases were managed with appropriate treatments, such as soft tissue resection, osteophyte grinding, and loose body removal.
Table 3.
Classification of OLT According to Ferkel and Cheng Classification a
| Grade b | Single-Stage Group (n = 50) | Staged Group (n = 48) | Total |
|---|---|---|---|
| A | 0 | 0 | 0 |
| B | 0 | 0 | 0 |
| C | 7 | 6 | 13 |
| D | 24 | 18 | 42 |
| E | 10 | 17 | 27 |
| F | 9 | 7 | 16 |
a Data are reported as number of patients. OLT, osteochondral lesions of the talus.
b Ferkel and Cheng classification grades: A, smooth, intact, but soft or ballotable; B, rough surface; C, fibrillation/fissuring; D, flap present or bone exposed; E, loose, undisplaced fragment; F, displaced fragment.
Table 4.
Intra-articular Lesions and Location of OLT a
| Single-Stage Group (n = 50) | Staged Group (n = 48) | |
|---|---|---|
| Intra-articular lesions | ||
| Soft tissue impingement | 35 | 34 |
| Osteophyte formation | 15 | 20 |
| Ossicles at lateral malleolus | 1 | 3 |
| Loose body formation | 3 | 7 |
| Osteochondral lesion area | ||
| Zone 4 | 32 | 28 |
| Zones 4 and 1 | 2 | 6 |
| Zones 4 and 6 | 1 | 3 |
| Zones 4 and 7 | 5 | 3 |
| Zones 4 and 2 | 0 | 1 |
| Zone 6 | 7 | 4 |
| Zone 7 | 2 | 2 |
| Zone 9 | 1 | 0 |
| Zones 3, 6, and 9 | 0 | 1 |
a Data are reported as number of patients. OLT, osteochondral lesions of the talus.
MRI Findings
The grading of articular lesions according to the Hepple classification system is shown in Table 5. There was no significant difference in the preoperative MRI grading between the single-stage and staged groups (χ2 = 3.828; P = .281). At the final follow-up, we obtained MRI scans of the patients within the previous month. Statistical results showed no significant difference between the 2 groups (χ2 = 0.675; P = .879).
Table 5.
Articular Lesions According to Hepple Classification Preoperatively and at Final Follow-up
| Preoperative | Final Follow-up | |||
|---|---|---|---|---|
| Grade a | Single Stage (n = 52) |
Staged (n = 51) |
Single Stage (n = 50) |
Staged (n = 48) |
| Normal | 0 | 0 | 2 | 1 |
| Stage 1 | 0 | 0 | 27 | 25 |
| Stage 2 (2a or 2b) | 6 | 2 | 18 | 20 |
| Stage 3 | 3 | 7 | 3 | 2 |
| Stage 4 | 10 | 8 | 0 | 0 |
| Stage 5 | 33 | 34 | 0 | 0 |
a Hepple classification grades: 1, articular cartilage injury; 2, cartilage injury with bony fracture and edema (2a, acute; 2b, chronic); 3, detached nondisplaced bony fragment with fluid rim beneath fragment; 4, displaced fragment and uncovered subchondral bone; and 5, subchondral cyst present.
Discussion
The most important finding of this study was that the clinical efficacy of arthroscopic treatment of OLT and modified Broström surgery to stabilize CLAI under open surgery was not inferior to that of staged surgery at the midterm follow-up. Both methods are safe and effective for the treatment of patients with CLAI and OLT. In fact, AOFAS and Karlsson-Peterson scores were higher in the patients undergoing single-stage surgery than in those undergoing staged surgery at the short-term (12-month) follow-up time point.
The ATFL and calcaneofibular ligament are the ligaments damaged most frequently in CLAI. If conservative treatment fails, surgery may be required to repair the ligament. Moreover, lateral ankle instability is often associated with other intra-articular lesions, among which OLT is considered an important predictor of poor prognosis after lateral ankle ligament reconstruction. 3 Ankle arthroscopy is usually performed during ligament repair or reconstruction to manage possible intra-articular injuries. 15 To treat OLT, arthroscopic microfracture as a bone marrow stimulation technique has also achieved good clinical results. 12,24,28
For patients with both CLAI and OLT, controversy remains regarding the application of single-stage versus staged surgery. The controversy mainly concerns the 2 different postoperative rehabilitation training programs and unfavorable factors such as an unclear surgical field caused by fluid extravasation during the open procedure after arthroscopic inspection. A retrospective study of 37 patients with an average follow-up period of 7.3 years confirmed that arthroscopy for OLT and open surgery for lateral ankle ligament repair can be performed safely and effectively at the same time. 9 However, no retrospective or prospective study was available to provide evidence of a difference between single-stage surgery and staged surgery during a middle- to long-term follow-up; filling this gap was our goal in this study.
An important finding of our study was that the median difference in the Karlsson-Peterson score (the primary outcome measure) between the single-stage surgery group and the staged surgery group was less than the prespecified value at all postoperative follow-up times, which validates our hypothesis that single-stage surgery for OLT with arthroscopy and open, modified Broström surgery to stabilize CLAI was not inferior to staged surgery at midterm follow-up. In this study of 98 patients, the main focus was OLT and CLAI; therefore, other lesions were considered confounding factors. A retrospective study including 64 patients found that inferior tibiofibular ligament and talar cartilage lesions and lateral malleolar avulsion fractures are risk factors that affect postoperative satisfaction. 3 To avoid these possible biases, we excluded patients with inferior tibiofibular syndesmosis injury during patient enrollment. Moreover, we carefully recorded intra-articular lesions in the patients and noted no significant difference between the 2 groups. No significant differences in Karlsson-Peterson or AOFAS scores were found before surgery, 24 months after surgery, or at the last follow-up between the single-stage surgery group and the staged surgery group. Furthermore, no significant difference in VAS scores was identified between the groups at any follow-up time point. At the final follow-up, the median AOFAS score in each group was 90, which is similar to the results reported by Gregush and Ferkel 9 and Jiang et al. 16
Different researchers have reported different rates of OLT associated with CLAI from 23.1% (15/65) to 63% (19/30). 3,25 In a word, surgeons are often tasked with proper management of OLT when performing lateral ankle ligament stabilization surgery. In recent years, some retrospective studies conducted on CLAI combined with OLT have obtained encouraging results. In studies treating these lesions simultaneously, postoperative AOFAS, VAS, Karlsson-Peterson, or Tegner scores were similar at the short- to midterm follow-up between patients with CLAI alone and those with CLAI combined with OLT. 3,15,16,21,37 Even though the latest consensus has indicated that surgical procedures can be performed on these 2 types of lesions simultaneously, the level of evidence is only 2C (weak recommendation, low level of evidence) because no prospective study with a large sample size has been performed. 30 Our study provides certain evidence to support such a recommendation from a prospective viewpoint. In the midterm follow-up results, AOFAS, Karlsson-Peterson, and VAS scores were similar between the single-stage and staged groups, and, at the final follow-up, the excellent and good rates of the 2 methods were 82% and 85%, respectively. Therefore, we believe that single-stage surgery can be a safe and effective choice for treatment of these 2 lesions.
Another important finding of our study is that, at 12 months after surgery, the AOFAS and Karlsson-Peterson scores of the patients in the single-stage surgery group were significantly higher than those in the staged surgery group, but no significant differences in VAS scores were identified. According to the rehabilitation training program that we provided to the patients, the patients in the single-stage surgery group had completed all postoperative rehabilitation training 6 months after surgery and had returned to work 12 months after surgery. However, in the staged surgery group, the time to the second procedure was around six months, and all postoperative rehabilitation was completed 12 months after surgery. The homogeneity of the enrolled patients convinces us that the main reason for this result was the choice of surgical timing. The occurrence of OLT has a significant negative impact on the overall results of surgery. 3 However, the timing of OLT management between the 2 groups of patients in this study was similar, which indirectly supports the results of previous studies and suggests that OLT may be the major source of pain in patients with CLAI and OLT.
The completely different postoperative rehabilitation plans between the 2 procedures are the main source of controversy. In postoperative rehabilitation for patients with CLAI, although the latest consensus suggests that ROM exercises and partial weightbearing (level of evidence:1C) should be carried out on the first postoperative day, 30 many orthopaedic physicians are accustomed to performing a long period of immobilization without weightbearing (wearing a cast for 3 weeks and then changing to orthotic walking boots for 3 weeks). 26,27 For patients with OLT undergoing microfracture surgery, ROM exercises should be performed in the early stage (usually the first week) to promote fibrocartilage healing, and weightbearing should be relatively delayed. 28,30 However, studies also show that early weightbearing and delayed weightbearing lead to similar healing outcomes after OLT repair. 20,34
In the current study, the rehabilitation program was based on consensus recommendations, with some modifications. Compared with other studies, 16,23 our rehabilitation training program was designed for earlier exercise and rehabilitation starting from the first day after surgery, including passive ROM exercises and isometric exercises. The difference between the 2 groups was the training intensity rather than the specific protocol. Rehabilitation training is an important factor in postoperative function. Our goal was to avoid bias caused by different postoperative rehabilitation programs. At the final follow-up, only 2 patients had a suspiciously positive anterior drawer test result (both patients were in military service and required additional field training and high-intensity physical strengthening). Moreover, no patients had mobility restrictions. This result is better than the results of the study by Jiang et al 16 (23.5%; 8/34 patients in their study), in which patients were permitted to remove splints or orthoses every day for continuous passive motion with machines in the second week after surgery, but the number of patients with OLT who had postoperative ROM limitations was still higher than those with CLAI alone. Therefore, we suggest more active rehabilitation training after microfracture surgery with concurrent lateral malleolar ligament stabilization surgery. Early reasonable functional rehabilitation will not affect the ligament stabilization effect.
Limitations
The present study, although carefully designed and thoroughly documented, has certain limitations. First, the lesion size of OLT may affect postoperative results. Previous studies have suggested that microfracture surgery is suitable for lesions with an area less than 150 mm2 (or a diameter less than 15 mm), 4,5 but the latest studies and a consensus have modified these parameters such that the ideal area of these lesions is less than 100 mm2 (or 107.4 mm2) or less than 10 mm (or 10.2 mm) in diameter. 11,29 However, since the patients enrolled in this study underwent surgery between January 2015 and December 2016, we used the guidelines in effect at the time. It should also be noted that the median lesion size in the 2 groups included in our study was relatively small. This suggests that our conclusions may not be applicable for larger lesions. More research may be needed to address this issue in the future. Second, we lack weightbearing radiographs due to equipment and manpower limitations of the medical imaging department. However, the detailed preoperative MRI assessment and intraoperative exploration classification results of enrolled patients enabled us to assess intra-articular lesions well and partially compensate for our limitations. Third, we lack more detailed subgroup analysis, and owing to the limited sample size, distinguishing medial and lateral lesions would result in a lack of sufficient data for our study. Finally, the Karlsson-Peterson function score contains many functional evaluations, so we do not have a separate rating scale for activity level, which was a deficiency of our study.
Conclusion
During midterm follow-up, no clinical difference was found between single-stage surgery and staged surgery for the arthroscopic treatment of OLT and open surgical repair of the lateral ankle ligament. Our results are consistent with previous results of OLT treatment. Single-stage surgery can achieve better clinical outcomes than staged surgery in the short term. For patients with combined CLAI and OLT with small defect areas, we recommend single-stage surgery.
APPENDIX
Table A1.
Postoperative Rehabilitation Program a
| Postoperative Timeline | Single-Stage Group (Microfracture and Ligament Repair) | Staged Group (Microfracture) b |
|---|---|---|
| Phase 1 (1 day to 4 weeks) |
Passive ankle flexion and extension: 1. Towel stretch: Use a towel to assist the ankle stretch, 20 times per day from postoperative day 1, and then gradually increased to 300 times per day by week 4 (Note: ankle joint was stretched in neutral position). 2. Stepping on the hard board: The patient’s foot is completely stepping on a hard board for isometric exercise of ankle joint muscle strength, about 10 times per day from postoperative day 1, and then gradually increased to 300 times per day by week 4. |
Passive ankle flexion and extension: 1. Towel stretch: Same exercise as the single-staged group, stretching about 20 times per day from postoperative day 1, gradually increasing to 600 times per day by week 4. 2. Stepping on the hard board: Same exercise as the single-staged group, exercising from postoperative day 1, about 20 times per day, gradually increasing to 600 times per day by week 4. |
| Phase 2 (5-12 weeks) |
1. Active joint ROM exercise: (a) Active
dorsiflexion and plantarflexion, eversion and inversion
(circular motion, letters A-Z movement); and (b) foot
treading roller exercise. Passive ROM exercise: same as
before. 2. Muscle strength exercise: lower limb muscle strength exercise. 3. Gait training: Start walking by the support of the crutches, and partial weightbearing on affected limbs. Weights started at 10 kg and increased gradually to 75% of body weight at 8 weeks and 100% at 9-10 weeks. Ankle brace was removed 11-12 weeks. |
|
| Phase 3 (13-24 weeks) |
1. Muscle strength training: on the basis of
phase 2 action training, heel-lifting training and
static squatting training were added. 2. Balance stability training, including balance pad assisted training. 3. Simple exercise training. |
|
a ROM, range of motion.
b After ligament repair, patients in the staged group repeated phases 1-3 as prescribed for the single-stage group.
Footnotes
Final revision submitted August 22, 2021; accepted October 7, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was funded by the National Natural Science Foundation of China (Nos. 82074244, 81473710), and the funds were used to pay for the postoperative magnetic resonance imaging examinations of patients with financial difficulties. The findings of this study do not necessarily reflect those of the National Natural Science Foundation of China. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Chinese People’s Liberation Army General Hospital (protocol No. S2020-185-01).
References
- 1. Angthong C, Yoshimura I, Kanazawa K, et al. Critical three-dimensional factors affecting outcome in osteochondral lesion of the talus. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1418–1426. [DOI] [PubMed] [Google Scholar]
- 2. Cheng JC, Ferkel RD. The role of arthroscopy in ankle and subtalar degenerative joint disease. Clin Orthop Relat Res. 1998;349:65–72. [DOI] [PubMed] [Google Scholar]
- 3. Choi WJ, Lee JW, Han SH, Kim BS, Lee SK. Chronic lateral ankle instability: the effect of intra-articular lesions on clinical outcome. Am J Sports Med. 2008;36(11):2167–2172. [DOI] [PubMed] [Google Scholar]
- 4. Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med. 2009;37(10):1974–1980. [DOI] [PubMed] [Google Scholar]
- 5. Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy. 2008;24(1):106–112. [DOI] [PubMed] [Google Scholar]
- 6. Deng E, Gao L, Shi W, et al. Both magnetic resonance imaging and computed tomography are reliable and valid in evaluating cystic osteochondral lesions of the talus. Orthop J Sports Med. 2020;8(9):2325967120946697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doherty C, Bleakley C, Hertel J, et al. Recovery from a first-time lateral ankle sprain and the predictors of chronic ankle instability: a prospective cohort analysis. Am J Sports Med. 2016;44(4):995–1003. [DOI] [PubMed] [Google Scholar]
- 8. Fong DT, Hong Y, Chan LK, Yung PS, Chan KM. A systematic review on ankle injury and ankle sprain in sports. Sports Med. 2007;37(1):73–94. [DOI] [PubMed] [Google Scholar]
- 9. Gregush RV, Ferkel RD. Treatment of the unstable ankle with an osteochondral lesion: results and long-term follow-up. Am J Sports Med. 2010;38(4):782–790. [DOI] [PubMed] [Google Scholar]
- 10. Gribble PA, Delahunt E, Bleakley C, et al. Selection criteria for patients with chronic ankle instability in controlled research: a position statement of the International Ankle Consortium. Br J Sports Med. 2014;48(13):1014–1018. [DOI] [PubMed] [Google Scholar]
- 11. Hannon CP, Bayer S, Murawski CD, et al. Debridement, curettage, and bone marrow stimulation: proceedings of the International Consensus Meeting on Cartilage Repair of the Ankle. Foot Ankle Int. 2018;39(1 suppl):16S–22S. [DOI] [PubMed] [Google Scholar]
- 12. Hannon CP, Smyth NA, Murawski CD, et al. Osteochondral lesions of the talus: aspects of current management. Bone Joint J. 2014;96(2):164–171. [DOI] [PubMed] [Google Scholar]
- 13. Hepple S, Winson IG, Glew D. Osteochondral lesions of the talus: a revised classification. Foot Ankle Int. 1999;20(12):789–793. [DOI] [PubMed] [Google Scholar]
- 14. Hintermann B, Boss A, Schäfer D. Arthroscopic findings in patients with chronic ankle instability. Am J Sports Med. 2002;30(3):402–409. [DOI] [PubMed] [Google Scholar]
- 15. Hua Y, Chen S, Li Y, Chen J, Li H. Combination of modified Broström procedure with ankle arthroscopy for chronic ankle instability accompanied by intra-articular symptoms. Arthroscopy. 2010;26(4):524–528. [DOI] [PubMed] [Google Scholar]
- 16. Jiang D, Ao YF, Jiao C, et al. Concurrent arthroscopic osteochondral lesion treatment and lateral ankle ligament repair has no substantial effect on the outcome of chronic lateral ankle instability. Knee Surg Sports Traumatol Arthrosc. 2018;26(10):3129–3134. [DOI] [PubMed] [Google Scholar]
- 17. Karlsson J, Lansinger O. Lateral instability of the ankle joint. Clin Orthop Relat Res. 1992;276:253–261. [PubMed] [Google Scholar]
- 18. Karlsson J, Peterson K. Evaluation of ankle joint function: the use of a scoring scale. Foot. 1991;1:15–19. [Google Scholar]
- 19. Kitaoka HB, Alexander IJ, Adelaar RS, et al. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349–353. [DOI] [PubMed] [Google Scholar]
- 20. Lee DH, Lee KB, Jung ST, et al. Comparison of early versus delayed weightbearing outcomes after microfracture for small to midsized osteochondral lesions of the talus. Am J Sports Med. 2012;40(9):2023–2028. [DOI] [PubMed] [Google Scholar]
- 21. Lee M, Kwon JW, Choi WJ, Lee JW. Comparison of outcomes for osteochondral lesions of the talus with and without chronic lateral ankle instability. Foot Ankle Int. 2015;36(9):1050–1057. [DOI] [PubMed] [Google Scholar]
- 22. Mintz DN, Tashjian GS, Connell DA, et al. Osteochondral lesions of the talus: a new magnetic resonance grading system with arthroscopic correlation. Arthroscopy. 2003;19(4):353–359. [DOI] [PubMed] [Google Scholar]
- 23. Miyamoto W, Takao M, Yamada K, Matsushita T. Accelerated versus traditional rehabilitation after anterior talofibular ligament reconstruction for chronic lateral instability of the ankle in athletes. Am J Sports Med. 2014;42(6):1441–1447. [DOI] [PubMed] [Google Scholar]
- 24. Murawski CD, Kennedy JG. Operative treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2013;95(11):1045–1054. [DOI] [PubMed] [Google Scholar]
- 25. Okuda R, Kinoshita M, Morikawa J, Yasuda T, Abe M. Arthroscopic findings in chronic lateral ankle instability: do focal chondral lesions influence the results of ligament reconstruction? Am J Sports Med. 2005;33(1):35–42. [DOI] [PubMed] [Google Scholar]
- 26. Pearce CJ, Tourné Y, Zellers J, et al. Rehabilitation after anatomical ankle ligament repair or reconstruction. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1130–1139. [DOI] [PubMed] [Google Scholar]
- 27. Petrera M, Dwyer T, Theodoropoulos JS, Ogilvie-Harris DJ. Short- to medium-term outcomes after a modified Broström repair for lateral ankle instability with immediate postoperative weightbearing. Am J Sports Med. 2014;42(7):1542–1548. [DOI] [PubMed] [Google Scholar]
- 28. Polat G, Erşen A, Erdil ME, et al. Long-term results of microfracture in the treatment of talus osteochondral lesions. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1299–1303. [DOI] [PubMed] [Google Scholar]
- 29. Ramponi L, Yasui Y, Murawski CD, et al. Lesion size is a predictor of clinical outcomes after bone marrow stimulation for osteochondral lesions of the talus: a systematic review. Am J Sports Med. 2017;45(7):1698–1705. [DOI] [PubMed] [Google Scholar]
- 30. Song Y, Li H, Sun C, et al. Clinical guidelines for the surgical management of chronic lateral ankle instability: a consensus reached by systematic review of the available data. Orthop J Sports Med. 2019;7(9):2325967119873852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sugimoto K, Takakura Y, Okahashi K, et al. Chondral injuries of the ankle with recurrent lateral instability: an arthroscopic study. J Bone Joint Surg Am. 2009;91(1):99–106. [DOI] [PubMed] [Google Scholar]
- 32. Ventura A, Terzaghi C, Legnani C, Borgo E. Treatment of post-traumatic osteochondral lesions of the talus: a four-step approach. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1245–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ, Jr. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92(13):2279–2284. [DOI] [PubMed] [Google Scholar]
- 34. Wei M, Wei Y, Liu Y. Effects of early weightbearing on microfracture treatment of osteochondral lesions of talus with subchondral bone defects. Curr Med Sci. 2019;39(1):88–93. [DOI] [PubMed] [Google Scholar]
- 35. Xu C, Li M, Wang C, Liu H. A comparison between arthroscopic and open surgery for treatment outcomes of chronic lateral ankle instability accompanied by osteochondral lesions of the talus. J Orthop Surg Res. 2020;15(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yasui Y, Hannon CP, Fraser EJ, et al. Lesion size measured on MRI does not accurately reflect arthroscopic measurement in talar osteochondral lesions. Orthop J Sports Med. 2019;7(2):2325967118825261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yasui Y, Murawski CD, Wollstein A, Kennedy JG. Reoperation rates following ankle ligament procedures performed with and without concomitant arthroscopic procedures. Knee Surg Sports Traumatol Arthrosc. 2017;25(6):1908–1915. [DOI] [PubMed] [Google Scholar]
- 38. Yasui Y, Takao M, Miyamoto W, Matsushita T. Simultaneous surgery for chronic lateral ankle instability accompanied by only subchondral bone lesion of talus. Arch Orthop Trauma Surg. 2014;134(6):821–827. [DOI] [PubMed] [Google Scholar]
- 39. Yeo ED, Lee KT, Sung IH, Lee SG, Lee YK. Comparison of all-inside arthroscopic and open techniques for the modified Broström procedure for ankle instability. Foot Ankle Int. 2016;37(10):1037–1045. [DOI] [PubMed] [Google Scholar]