Abstract
Objectives
To assess the long-term safety and efficacy of the Janus kinase inhibitor upadacitinib versus adalimumab over 3 years in the ongoing long-term extension (LTE) of SELECT-COMPARE, a randomised controlled phase 3 trial of patients with active rheumatoid arthritis and inadequate response to methotrexate (MTX).
Methods
Patients on stable background MTX were randomised 2:2:1 to upadacitinib 15 mg, placebo or adalimumab 40 mg. Patients with an insufficient response were switched by week 26 from placebo to upadacitinib, upadacitinib to adalimumab or adalimumab to upadacitinib. Patients who completed the 48-week double-blind period could enter an LTE for up to 10 years. Safety and efficacy results were analysed here through 3 years. Treatment-emergent adverse events (AEs) were summarised based on exposure to upadacitinib and adalimumab. Efficacy was analysed by original randomised groups (non-responder imputation), as well as separately by treatment sequence (as observed).
Results
Rates of several AEs were generally comparable between upadacitinib and adalimumab, including AEs leading to discontinuation, serious infections and serious AEs, malignancies, major adverse cardiac events, venous thromboembolism and deaths. Consistent with earlier results, herpes zoster, lymphopaenia, hepatic disorder and CPK elevation were reported at higher rates with upadacitinib versus adalimumab. In terms of efficacy, upadacitinib continued to show numerically better clinical responses than adalimumab over 3 years across all endpoints, including low disease activity and remission.
Conclusion
The safety profile of UPA 15 mg was consistent with previous study-specific and integrated safety reports. Higher levels of clinical response continued to be observed with upadacitinib versus adalimumab through 3 years of treatment.
Keywords: adalimumab, therapeutics, tumor necrosis factor inhibitors, cardiovascular diseases, arthritis, rheumatoid
Key messages.
What is already known about this subject?
Safety and efficacy of upadacitinib have been evaluated in multiple rheumatoid arthritis (RA) populations in six global phase III trials, including SELECT-COMPARE, which demonstrated improvements in the signs and symptoms of RA in favour of upadacitinib 15 mg versus adalimumab through 72 weeks.
What does this study add?
Safety and efficacy data through 3 years, including treatment switch data are reported for a Janus kinase inhibitor versus a TNF inhibitor.
The safety profile of upadacitinib 15 mg observed through 3 years was generally similar to adalimumab for adverse events of special interest, including malignancies, major adverse cardiac events, venous thromboembolism and deaths.
Upadacitinib continued to show consistently better clinical responses compared with adalimumab through 3 years including rates of remission and low disease activity, physical function and pain severity; radiographic progression was inhibited to a similar extent.
How might this impact on clinical practice or further developments?
Based on its favourable benefit–risk profile for the treatment of patients with active RA, upadacitinib 15 mg continues to be a reasonable treatment choice after an inadequate response to methotrexate.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease associated with progressive cartilage and bone damage. Affecting approximately 0.5% of the adult population worldwide,1 RA can lead to functional impairment, loss of mobility and reduced quality of life. To counter these effects and improve long-term prognosis, the ‘treat to target’ strategy aims to optimise treatment until the goal of sustained remission or at least low disease activity (LDA) is met.2 3
In patients with an inadequate response or intolerance to methotrexate (MTX), alternative treatment options or combination therapies are usually helpful. While biological disease-modifying antirheumatic drugs (bDMARDs) have greatly improved the management of RA, upwards of 30%–40% of patients on TNF antagonists fail to achieve a satisfactory long-term clinical response,4 indicating a need for additional treatment options. Patient surveys also highlight the importance of oral medications, with oral delivery options strongly preferred over injection by patients with RA.5 6
Janus kinase (JAK) inhibitors, a relatively new class of orally administered small molecule inhibitors, have emerged as an effective alternative in RA patients with insufficient response or intolerance to conventional synthetic (cs) or bDMARDs. The safety and efficacy of the JAK inhibitor upadacitinib have been evaluated in a wide range of RA patients across six global randomised phase III trials.7–13 SELECT-COMPARE focuses on patients with active RA despite MTX treatment and uniquely includes a long-term comparator control arm with the TNF inhibitor adalimumab throughout the 10-year study. Notably, upadacitinib (15 mg orally once daily) plus MTX demonstrated statistically significant improvement in clinical and functional outcomes of RA when compared with adalimumab (40 mg subcutaneous injection every other week) plus MTX at week 12 of SELECT-COMPARE.13 Continued improvements in favour of upadacitinib were maintained through 72 weeks.14 Since RA is a chronic disease that requires long-term intervention, it is important to evaluate the long-term safety and efficacy of RA treatments. Here, we report the long-term safety, tolerability and efficacy of upadacitinib versus adalimumab, both in combination with MTX, over 3 years in the ongoing open-label, long-term extension (LTE) of SELECT-COMPARE.
Patients and methods
Patients
Study eligibility criteria and baseline demographics were described previously13 (summarised in online supplemental table 1). In brief, patients were at least 18 years of age with active RA (and meeting the 2010 ACR/EULAR classification criteria15) and were on MTX therapy for ≥3 months, attaining a stable dosage of 15–25 mg/week for ≥4 weeks prior to the first dose of study drug (or at least 10 mg/week MTX if intolerant of ≥12.5 mg/week). Patients also were required to have high-sensitivity C-reactive protein (CRP) ≥5 mg/L and evidence of erosive disease and/or seropositivity (as determined by ≥3 bone erosions on X-rays of hands and feet or ≥1 bone erosion and positive rheumatoid factor/anticyclic citrullinated peptide autoantibody). All patients were naive to targeted synthetic DMARDs and adalimumab. Patients were excluded if they had an inadequate response to prior bDMARD therapy.
rmdopen-2021-002012supp001.pdf (1.7MB, pdf)
Study design and treatment
SELECT-COMPARE included a 26-week, double-blind, placebo-controlled period, a 48-week, double-blind, active comparator-controlled period and an ongoing open-label LTE for an overall trial length of up to 10 years (online supplemental figure 1). Patients were randomised 2:2:1 to upadacitinib 15 mg once daily, placebo or adalimumab 40 mg every other week and continued to receive their background MTX dose. Patients not achieving sufficient clinical response were blindly rescued from placebo to upadacitinib, upadacitinib to adalimumab or adalimumab to upadacitinib within the first 26 weeks of the trial. Rescue occurred at week 14, 18 and 22 (for those who did not achieve at least 20% improvement in both tender joint counts (TJC) and swollen joint counts (SJC)) or week 26 (if LDA criteria were not met, defined by Clinical Disease Activity Index (CDAI) >10). All placebo patients not rescued previously were switched to upadacitinib at week 26. Initiation of, or change in, some background RA medications (including glucocorticoids, non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen) was allowed per investigator’s discretion starting at week 26; modification or initiation of csDMARDs was allowed beginning at week 48. Patients completing the 48-week period 1 could enter an ongoing LTE and continue to receive treatment with upadacitinib or adalimumab in a blinded manner until the last patient completed their week 48 visit.
Safety assessments
Safety assessments were based on available data up to week 156 for each patient. Treatment-emergent adverse events (TEAEs), including AEs of special interest (AESIs), per 100 patient-years (PY) were summarised up to 3 years based on exposure to upadacitinib or adalimumab. All patients were included in the safety analysis, with assignment of an AE based on drug exposure at the time of event. In addition, selected AESIs were summarised for the subset of patients receiving continuous upadacitinib or continuous adalimumab treatment. All safety data are reported as exposure-adjusted event rates.
Safety assessments were performed as previously described.13 16 Major adverse cardiac events (MACE) and venous thromboembolism (VTE) events were adjudicated by an independent Cardiovascular Adjudication Committee in a blinded manner. Kaplan-Meier curves were used to examine the time to occurrence of MACE and VTE events. Laboratory parameters were evaluated through week 156, including the proportion of patients meeting criteria for potentially clinically significant (grade 3 or 4) changes during the treatment period. The assessment of AE severity was made at the investigators’ discretion and in line with the study protocol. Transient AEs were determined based on the availability of an end date for that AE. A potential relationship between lymphocyte decreases and serious and opportunistic infectious events was also explored.
Efficacy measures
Efficacy assessments included the proportions of patients achieving LDA (defined by CDAI ≤10) or clinical remission (defined by CDAI ≤2.8),17 28-joint Disease Activity Score-CRP (DAS28(CRP)) ≤3.2 or <2.6,18 19 ACR/EULAR Boolean-based remission,20 ACR20/50/70 responses,21 and ACR components including Health Assessment Questionnaire-Disability Index (HAQ-DI) (rated on a 0–3 scale)22 and patient’s assessment of pain (scored on a 0–100 mm scale). Indicated assessments from period 1 (at weeks 4, 8, 12, 26 and 48)13 16 are summarised here; period 2 assessments were conducted at week 60 and every 12 weeks thereafter up to week 156.
Radiographic progression up to 2 years was assessed based on reading session 3, which included X-ray images from hands and feet from baseline and weeks 26, 48 and 96, as well as from premature discontinuation or unscheduled visits prior to week 96. Per study protocol, no X-ray assessments were performed at 3 years. Results from reading session 1 (images up to 6 months) and session 2 (up to 1-year reading) were previously reported.13 16 X-rays were evaluated by two independent readers who were blinded to treatment assignments and visit order and an adjudicator if needed. Assessments included mean change from baseline in modified Total Sharp Score (mTSS),23 24 erosion scores and joint space narrowing, as well as the proportion of patients who showed no radiographic progression (defined as change from baseline in mTSS ≤0).
Statistical analysis
Safety data were summarised by the following two groups, with assignment of AEs based on drug exposure at the time of event: any upadacitinib (including patients who started and remained on upadacitinib as well as the upadacitinib exposure from those rescued from placebo or adalimumab to upadacitinib) and any adalimumab (including patients who started and remained on adalimumab as well as the adalimumab exposure from those rescued from upadacitinib to adalimumab).
Efficacy data up to week 156 were analysed by originally assigned randomised treatment group (intention-to-treat analysis), as well as by treatment sequence, which includes: (1) placebo to upadacitinib, (2) continuous upadacitinib, (3) continuous adalimumab, (4) adalimumab to upadacitinib switchers and (5) upadacitinib to adalimumab switchers. For analysis of binary endpoints by randomised treatment group, non-responder imputation (NRI) was applied for rescue, premature discontinuation of study drug and missing data; treatment comparisons were made using the Cochran-Mantel-Haenszel test, adjusting for the stratification factor of prior bDMARD use. For analysis of continuous endpoints by randomised treatment group, last observation carried forward was applied to data observed after rescue, and missing data were not imputed. Analysis of continuous endpoints was conducted using the analysis of covariance model, where treatment was set as a fixed factor, and the stratification factor of prior bDMARD use and corresponding baseline value were covariates. As observed (AO) data by treatment sequence were reported without imputation for missing data. For radiographic endpoints, descriptive summaries were provided based on AO data. Patients for whom both baseline and week 96 X-ray readings were available are included in the present analysis.
Results
Patient disposition
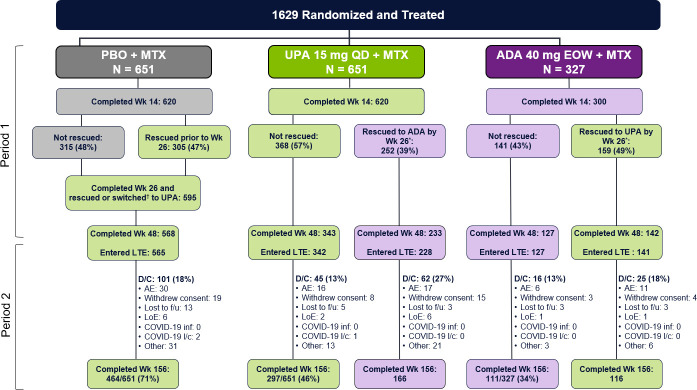
A total of 1629 patients were randomised and received at least one dose of study drug (651 to upadacitinib 15 mg, 651 to placebo and 327 to adalimumab 40 mg) (figure 1). Of the 651 patients randomised to upadacitinib, overall 252 (39%) were rescued to adalimumab between weeks 14 and 22 (if <20% improvement in TJC or SJC) or at week 26 (if CDAI >10), whereas a higher proportion of patients (159/327; 49%) randomised to adalimumab were rescued to upadacitinib. Over half of patients (342/651; 53%) randomised to upadacitinib completed period 1 on randomised therapy and entered the LTE at week 48 compared with 39% (127/327) of those randomised to adalimumab. Similarly, a higher proportion of patients randomised to upadacitinib completed 3 years of continuous treatment compared with those randomised to adalimumab (297/651 (46%) vs 111/327 (34%)). Of note, while the study was unblinded once the last patient in the study achieved the week 48 visit (end of period 1), over two-thirds of patients remained fully blinded at week 60, approximately one-third were still blinded at week 72, and the study was fully unblinded at week 156.
Figure 1.
Disposition of patients through week 156. Numbers of patients on study drug, with primary reason for discontinuation summarised during long-term extension through week 156. *Rescue occurred only before or at week 26; no rescue was allowed after week 26. †All placebo patients not previously rescued (at weeks 14, 18 or 22) were switched to upadacitinib at week 26. ADA, adalimumab; AE, adverse event; D/C, discontinued; EOW, every other week; f/u, follow-up; l/c, logistical restrictions; LOE, lack of efficacy; MTX, methotrexate; PBO, placebo; QD, once daily; UPA, upadacitinib; Wk, week.
Safety
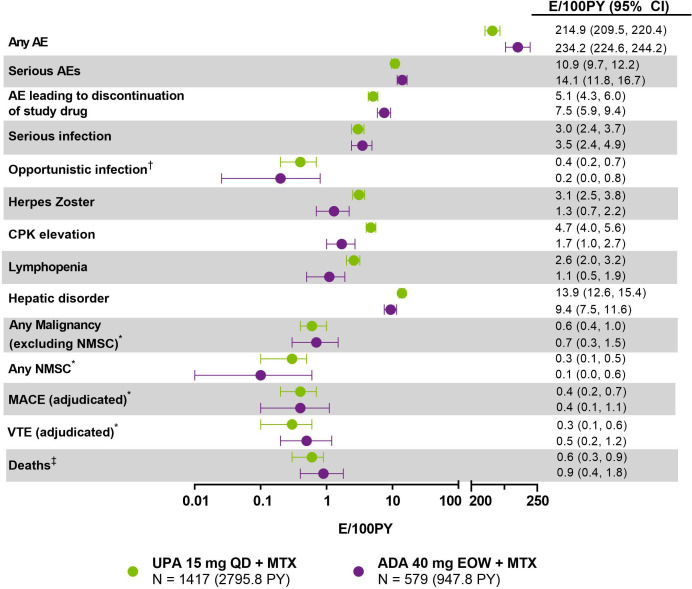
Through 3 years, the overall exposure to upadacitinib was approximately three-fold higher than the exposure to adalimumab (2796 PY and 948 PY, respectively). The overall rate of AEs was similar in patients receiving upadacitinib (214.9 E/100 PY) compared with adalimumab (234.2 E/100 PY) (figure 2). The most frequently reported TEAEs (>5 E/100 PY) in both groups included upper respiratory tract infection, urinary tract infection, nasopharyngitis, increased alanine aminotransferase (ALT) and bronchitis (online supplemental table 2). The exposure-adjusted event rates were numerically higher in patients randomised to adalimumab compared with upadacitinib for severe AEs (10.9 E/100 PY for upadacitinib and 14.1 E/100 PY for adalimumab), as well as AEs leading to discontinuation of study drug (5.1 E/100 PY for upadacitinib and 7.5 for adalimumab) (figure 2).
Figure 2.
Treatment-emergent adverse event summary through 156 weeks (E/100 PY (95% CI)). Data through 156 weeks include all patients receiving upadacitinib or adalimumab, including rescue groups, with assignment based on drug exposure at the time of event. The last week-156 visit was on 6 August 2020. Exposure-adjusted event rates (EAERs) are reported for all events. *Exposure-adjusted incidence rates (EAIRs) were the same as EAERs for malignancies, MACE and VTE. All events were considered AESIs except any AE, serious AEs, AE leading to discontinuation of study drug and deaths. †Opportunistic infections exclude herpes zoster, tuberculosis and oral candidiasis. Event rates for oral candidiasis and active tuberculosis were similar on upadacitinib and adalimumab (0.2 and 0.1 E/100 PY vs 0.3 and 0.2 E/100 PY, respectively). ‡Includes non-treatment emergent deaths. ADA, adalimumab; AE, adverse event; AESI, adverse event of special interest; CPK, creatine phosphokinase; EOW, every other week; MACE, major adverse cardiovascular event; MTX, methotrexate; NMSC, non-melanoma skin cancer; PY, patient-years; QD, once daily; UPA, upadacitinib; VTE, venous thromboembolism.
With the exception of herpes zoster (HZ), CPK elevation, hepatic disorder and lymphopaenia, which were reported at numerically higher rates with upadacitinib, AESI rates were generally comparable between upadacitinib and adalimumab (figure 2). Serious infections occurred at similar rates in patients on upadacitinib (3.0 E/100 PY) and adalimumab (3.5 E/100 PY), and the types of those infections were generally consistent with those expected in a study population comprised of patients with RA. HZ infection rates were higher on upadacitinib than adalimumab (3.1 and 1.3 E/100 PY, respectively) (figure 2). Most HZ infections were non-serious and involved one or two dermatomes (online supplemental material 1). No events were reported as having central nervous system or other non-cutaneous involvement; three events were reported as ophthalmic HZ (two on upadacitinib and one on adalimumab). Opportunistic infections (excluding tuberculosis, HZ and oral candidiasis) were reported in similar proportions of patients on either study therapy (0.4 E/100 PY on upadacitinib and 0.2 E/100 PY on adalimumab), with nonserious oesophageal candidiasis the most commonly reported (figure 2, online supplemental material 1).
The majority of CPK elevation events occurring on either upadacitinib or adalimumab were mild (68% and 50%, respectively) or moderate (25% and 44%), transient (77% and 81%), and, among the observations of increased CPK for which the presence or absence of symptoms were collected, 94% were asymptomatic; mostly transient muscle pain was reported for the remaining 6%. No case of rhabdomyolysis was observed. Most reports of hepatic disorder were based on ALT or aspartate aminotransferase (AST) elevations, with no Hy’s law case identified. The event rate for anaemia was the same for patients randomised to either upadacitinib or adalimumab (3.3 E/100 PY), and most cases were mild or moderate and transient. Through 3 years, one adjudicated GI perforation event was reported in a patient receiving upadacitinib (online supplemental material 1).
The event rates of malignancies, excluding nonmelanoma skin cancer (NMSC), were similar on upadacitinib and adalimumab through 156 weeks (0.6 and 0.7 E/100PY, respectively) (figure 2). There was no notable pattern in the types of malignancies that were observed. Malignancies in patients on upadacitinib included three malignant melanoma, three lung cancer and two breast cancer; malignancies occurring on adalimumab included three colon cancer and two lung cancer—all others on either treatment occurred in a single patient (detailed in online supplemental material 1). Nearly all malignancies took place in older patients (≥53 years of age). Malignancy (excluding NMSC) rates in the subgroup of patients receiving continuous treatment with upadacitinib or adalimumab, without rescue or switch, were also similar (0.8 and 1.1 E/100 PY, respectively) (table 1). Event rates for adjudicated MACE were equivalent on upadacitinib and adalimumab (0.4 E/100PY for each treatment) (figure 2). Adjudicated MACE in patients on upadacitinib included two non-fatal strokes, four non-fatal myocardial infarctions, four cardiovascular (CV) deaths; on adalimumab, three non-fatal strokes and one CV death were reported. Most MACE occurred in patients >50 years of age (upadacitinib: 8/10; adalimumab: 4/4). All patients with MACE had at least one CV risk factor; in fact, most had at least two CV risk factors, including hypertension, diabetes mellitus and smoking (online supplemental table 3). MACE was observed across the disease activity spectrum, with few patients in CDAI remission (upadacitinib: 20%; adalimumab: 0%) at the visit preceding MACE occurrence (40% and 50% of patients on upadacitinib and adalimumab were in CDAI LDA, respectively). Event rates for adjudicated VTE were similar on upadacitinib and adalimumab (0.3 and 0.5 E/100 PY, respectively) (figure 2). Adjudicated VTE events on upadacitinib included one venous thromboembolic death, three non-fatal deep vein thrombosis (DVT), three non-fatal pulmonary embolism (PE), two concurrent DVT and PE; on adalimumab, four PE and one DVT were reported. The majority of VTE events occurred in patients over 50 years of age (upadacitinib: 6/9; adalimumab: 5/5), and all had at least 1 CV or thrombosis risk factor (online supplemental table 4). Among patients who had AEs adjudicated as VTEs, most were in high or moderate disease activity (56% on upadacitinib and 60% on adalimumab; CDAI >10) at the visit preceding VTE occurrence, with few in CDAI remission (upadacitinib: 11%; adalimumab: 20%) (online supplemental figure 2). Overall, no clear pattern emerged in the timing of either MACE or VTE events during upadacitinib or adalimumab treatment (online supplemental figure 3). Event rates for adjudicated MACE and adjudicated VTE were also consistent in the subgroup of patients receiving continuous upadacitinib or adalimumab (table 1).
Table 1.
Exposure-adjusted event rates of malignancy (excluding NMSC), adjudicated MACE and VTE in patients receiving continuous upadacitinib or adalimumab
| Event | Continuous UPA 15 mg QD +MTX (n=398; PY=994.1) (95% CI) (events) |
Continuous ADA 40 mg EOW +MTX (n=168; PY=373.0) (95% CI) (events) |
| Malignancy (excluding NMSC)* |
0.8 (0.3 to 1.6) (8) | 1.1 (0.3 to 2.7) (4) |
| MACE (adjudicated)* | 0.1 (0.0 to 0.6) (1) | 0.5 (0.1 to 1.9) (2) |
| VTE (adjudicated)* | 0.2 (0 to 0.7) (2) | 0.8 (0.2 to 2.4) (3) |
*Exposure-adjusted incidence rates were the same as exposure-adjusted event rates for malignancy, MACE and VTE.
ADA, adalimumab; AE, adverse event; EOW, every other week; MACE, major adverse cardiovascular event; MTX, methotrexate; NMSC, non-melanoma skin cancer; PY, patient years; QD, once daily; UPA, upadacitinib; VTE, venous thromboembolism.
Twenty-five deaths (18 of which were treatment-emergent) were reported among patients who received upadacitinib (16 deaths) or adalimumab (9 deaths). The rate of deaths was generally similar on either upadacitinib or adalimumab (0.6 and 0.9 E/100 PY, respectively), and there was no detectable pattern observed in regard to the type of fatal events (online supplemental material 1).
The group mean values for haematology variables (haemoglobin, lymphocytes, neutrophils and platelets) were within normal range at baseline and at subsequent treatment visits. However, higher proportions of patients with grade 3/4 lymphocyte decreases and CPK elevation were reported on upadacitinib compared with adalimumab (table 2). There was no clear association between grade 4 lymphopaenia and the risk of infections, including serious and opportunistic infections (online supplemental table 5). The percentage of patients with a grade 3 increase in ALT/AST also occurred more frequently on upadacitinib than adalimumab.
Table 2.
Grade 3/4 laboratory abnormalities through week 156
| Parameter, % | UPA 15 mg QD +MTX |
ADA 40 mg EOW +MTX | |
| Haemoglobin (g/dL) | Grade 3 (decrease 2.1–2.9* or Hb ≥7.0 to <8.0) | 6.5 | 4.2 |
| Grade 4 (decrease ≥3* or Hb <7.0) | 2.1 | 2.1 | |
| Lymphocytes (x109/L) | Grade 3 (0.5 to <1.0) | 27.0 | 9.0 |
| Grade 4 (<0.5) | 2.9 | 0.5 | |
| Neutrophils (x109/L) | Grade 3 (0.5 to <1.0) | 1.3 | 0.5 |
| Grade 4 (<0.5) | 0.4 | 0.3 | |
| ALT (U/L) | Grade 3 (3.0–8.0 × ULN) | 5.4 | 2.1 |
| Grade 4 (>8.0 × ULN) | 0.6 | 0.7 | |
| AST (U/L) | Grade 3 (3.0–8.0 × ULN) | 3.3 | 1.4 |
| Grade 4 (>8.0 × ULN) | 0.4 | 0.9 | |
| CPK (U/L) | Grade 3 (>5.0 × ULN–10.0 × ULN) | 2.1 | 0.3 |
| Grade 4 (>10.0 × ULN) | 0.7 | 0.3 | |
| Creatinine (μMol/L) | Grade 3 (>3.0–6.0 × ULN) | 0.2 | 0.2 |
| Grade 4 (>6.0 × ULN) | 0.1 | 0 | |
Data are for patients with worsening in grade severity for laboratory parameters. Grading is based on Outcome Measures in Rheumatology (OMERACT) criteria, except for CPK and creatinine, where National Cancer Institute Common Terminology Criteria (NCI CTC) criteria were used.
*Decrease from baseline. Baseline is defined as the last observation on or before the date of the first dose of study drug in the corresponding treatment group.
ADA, adalimumab; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPK, creatine phosphokinase; EOW, every other week; MTX, methotrexate; QD, once daily; ULN, upper limit of normal; UPA, upadacitinib.
Efficacy
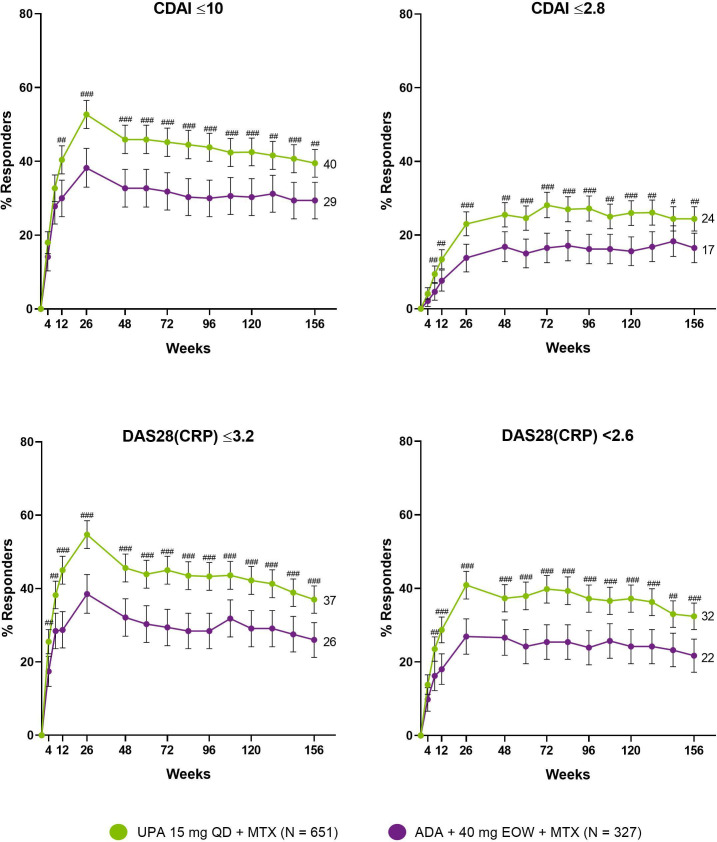
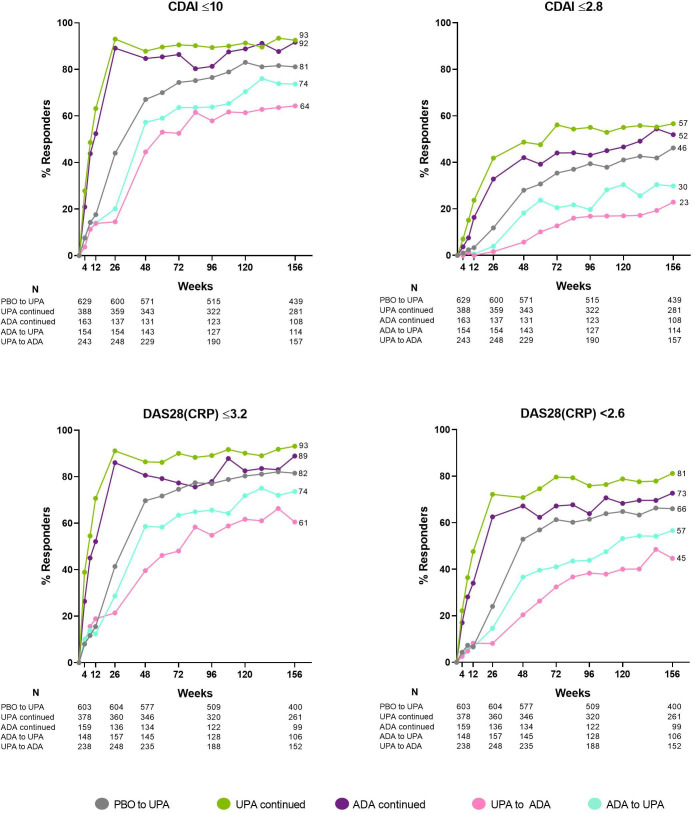
The proportion of patients attaining LDA and clinical remission by CDAI, as well as the proportion of patients reaching DAS28(CRP) ≤3.2/<2.6, was consistently higher in those randomised to upadacitinib compared with adalimumab over 3 years by NRI (figure 3). At week 156, CDAI LDA/remission was achieved by 40/24% randomised to upadacitinib and 29/17% randomised to adalimumab. Moreover, DAS28(CRP) ≤3.2/<2.6 was achieved by 37/32% of patients randomised to upadacitinib vs 26/22% of those randomised to adalimumab, and 20% vs 14% achieved the ACR/EULAR Boolean-based remission definition (nominal p=0.012). Upadacitinib also demonstrated improvements over time when data were analysed by treatment group sequence (AO) (figure 4). By this approach, numerically higher proportions of patients receiving continuous upadacitinib versus continuous adalimumab—without any treatment switching—achieved CDAI LDA and remission, as well as DAS28(CRP) ≤3.2/<2.6, over 3 years. By week 156, CDAI LDA/remission was achieved by 93/57% and 92/52% of patients receiving continuous upadacitinib or adalimumab, respectively (AO). Numerically higher responses for patients who switched from adalimumab to upadacitinib vs those who switched from upadacitinib to adalimumab were also observed (figure 4).
Figure 3.
Proportions of patients achieving CDAI LDA/remission and DAS28(CRP) ≤3.2/<2.6 through 156 Weeks (NRI). Treatment groups are by initial randomisation. #P<0.05, ##p<0.01, ###p<0.001 for upadacitinib plus MTX versus adalimumab plus MTX. All p values are nominal. NRI was used for patients who were rescued or prematurely discontinued study drug, as well as for missing data. Data points plotted here are shown in online supplemental table 6. ADA, adalimumab; CDAI, clinical disease activity index; DAS28(CRP), 28-joint Disease Activity Score based on C-reactive protein; EOW, every other week; MTX, methotrexate; NRI, non-responder imputation; PBO, placebo; QD, once daily; UPA, upadacitinib.
Figure 4.
Proportions of patients attaining disease activity states over 156 weeks (AO). Groups are by treatment sequence AO, without imputation for missing data. All patients in the placebo group who were not previously rescued were switched to upadacitinib at week 26. ADA, adalimumab; AO, as observed; CDAI, Clinical Disease Activity Index; DAS28(CRP), 28-joint Disease Activity Score based on C-reactive protein; EOW, every other week; MTX, methotrexate; QD, once daily; UPA, upadacitinib.
When evaluating the results by NRI, patients randomised to upadacitinib consistently achieved higher rates of ACR20, ACR50 and ACR70 responses compared with adalimumab (42/38/32% vs 30/27/21% at week 156) (online supplemental figure 4). Similarly, when analysed by treatment sequence group (AO), numerically higher ACR20/50/70 rates were observed in patients receiving continuous upadacitinib versus continuous adalimumab over 3 years (96/88/74% vs 94/82/66% at week 156) (online supplemental figure 5). In addition, numerically higher ACR responses were observed in patients who switched from adalimumab to upadacitinib than those who switched from upadacitinib to adalimumab. Patients randomised to upadacitinib also demonstrated greater numerical improvements from baseline in HAQ-DI at week 156 (−0.75 vs −0.60, nominal p<0.01) and a greater reduction in pain relative to adalimumab (−39.8 vs −31.2 at week 156, nominal p<0.001) (figure 5). Overall consistent results were observed for other ACR core components over 3 years, except SJC66, which was less prominent (online supplemental figure 6).
Figure 5.
Mean change from baseline in HAQ-DI and pain at week 156 (LOCF). Nominal ##p<0.01, ###p<0.001 for upadacitinib plus MTX versus adalimumab plus MTX. Treatment groups are by initial randomisation. Observations after rescue were replaced with the last observation prior to rescue, and analysis was based on ANCOVA model with treatment and prior bDMARD use as fixed factors and bL value as covariate. HAQ-DI is rated on a 0–3 scale; patient’s assessment of pain is scored on a 0–100 mm scale. ADA, adalimumab; bDMARD, biological disease modifying antirheumatic drug; bL, baseline; EOW, every other week; HAQ-DI, Health Assessment Questionnaire-Disability Index; LOCF, last observation carried forward; LS, least squares; MTX, methotrexate; QD, once daily; UPA, upadacitinib.
Through 2 years of treatment, similar proportions of patients administered continuous upadacitinib or continuous adalimumab demonstrated no radiographic progression (82.0% vs 75.2%, respectively, at 2 years) (figure 6A). The rate at which structural progression was inhibited was also comparable, as measured by a mean change from baseline in mTSS, erosion score and joint space narrowing (figure 6B; online supplemental figures 7 and 8).
Figure 6.
Radiographic outcomes at 6 months, 1 year and 2 years (AO). *Per study design, all patients in the placebo group who were not previously rescued were switched to upadacitinib at week 26. Data are reported AO. (A) Determination of no radiographic progression was assessed by a change from baseline in mTSS ≤0. (B) Least-squares mean change from baseline in mTSS. ADA, adalimumab; AO, as observed; bL, baseline; EOW, every other week; LS, least squares; MTX, methotrexate; PBO, placebo; QD, once daily; mTSS, modified Total Sharp Score; UPA, upadacitinib.
Discussion
The 3-year data from the SELECT-COMPARE trial provide the unique opportunity to assess the safety and efficacy of upadacitinib compared with the active comparator adalimumab. In contrast to other head-to-head RA studies evaluating JAK inhibitors where the comparator treatment arm ended after 1 year,25–27 SELECT-COMPARE is the only trial to our knowledge that maintains an active comparator through 3 years and longer. Given that RA is a chronic disease requiring continuing treatment, long-term safety considerations are of key clinical relevance.
The safety profile of upadacitinib 15 mg once a day observed through 3 years is consistent with prior evaluations of upadacitinib reported in SELECT-COMPARE and with the overall known safety profile of upadacitinib.10 13 16 There were no new safety findings. The rate of serious infections was similar for upadacitinib compared with adalimumab through 156 weeks. In line with the known increased risk of HZ on JAK inhibition,10 28–31 HZ rates were higher on upadacitinib. However, most HZ cases identified on upadacitinib were nonserious, involved one or two dermatomes, and did not result in discontinuation of treatment. Regarding geographical distribution, an integrated analysis of upadacitinib phase 3 RA data (including SELECT-COMPARE) reported that patients from Asia experience HZ more frequently.10 The event rate for malignancies (excluding NMSC) described here is consistent with that expected in RA populations,32–34 and results through 156 weeks were similar between upadacitinib and adalimumab. As would be expected, the vast majority of malignancies occurred in patients over 50 years of age. Event rates for MACE were also similar for both study drugs and consistent in the subgroup of patients receiving continuous upadacitinib or adalimumab without treatment switch. VTE rates reported here through week 156 were similar between upadacitinib and adalimumab (0.3 and 0.5 E/100 PY, respectively) and consistent with expected rates for patients with RA (rate of 0.3–0.8 E/100 PY).35 36 There was also no increase in VTE risk with duration of exposure to upadacitinib compared with week 48 results.16 All patients with MACE or thrombotic events had at least one risk factor in addition to their underlying RA. Of note, while MACE occurred in patients across the disease activity spectrum, few patients were in CDAI remission at the visit preceding the event (2/10 on upadacitinib and 0/4 on adalimumab). Given a recent cohort study suggesting that VTE risk may be associated with disease activity,37 we also assessed patient disease activity around the time of the VTE in affected patients. Similarly, more patients enrolled in SELECT-COMPARE showed higher disease activity at the visit prior to VTE occurrence, and few VTE events occurred among patients in CDAI remission (1/9 on upadacitinib and 1/5 on adalimumab). However, due to the limited number of events, no definitive conclusion can be drawn, and further research in a much larger population of patients at risk of the events are needed to answer this question. Other factors associated with VTE risk identified from an integrated analysis of upadacitinib 15 mg trial data included higher BMI, prior history of VTE, older age and NSAID or statin use.38
More recently, potential safety issues were reported in ORAL Surveillance, a randomised, postmarketing safety study for another JAK inhibitor.39 The study was prospective and randomised, with a primary endpoint comparing the incidence rate of malignancies and MACE in patients aged 50 and above with RA who had at least one CV risk factor.39 Although not powered for these endpoints, VTE and mortality were also assessed. This study showed a numerical difference in these events of special interest favouring the TNF inhibitor over the JAK inhibitor. SELECT-COMPARE enrolled an entirely different population that was much smaller, including only a subgroup of patients who would have qualified for ORAL Surveillance and was not powered to show these safety results. Notably, we observed similar rates of these events between upadacitinib and adalimumab among patients enrolled in SELECT-COMPARE.
To summarise safety findings, with the exception of known higher rates for HZ, CPK elevation, lymphopaenia and hepatic disorder on upadacitinib, safety over 3 years remained generally comparable between upadacitinib 15 mg once a day and adalimumab and consistent with findings from the integrated phase 3 safety analysis, which incorporated data from over 3500 patients worldwide with a combined exposure of 4000 PY.10 In addition, a post hoc analysis of SELECT-COMPARE using numbers needed to harm methodology identified comparable risks for serious infections, malignancies, MACE and VTEs among patients randomised to upadacitinib or adalimumab through 3 years.40 However, given that SELECT-COMPARE was not designed to show such safety differences, rates of malignancies, MACEs, VTEs and deaths need to be interpreted with caution.
In terms of efficacy, upadacitinib plus MTX continued to show better clinical responses compared with adalimumab plus MTX (nominal p<0.05) consistently through 3 years across all endpoints, including rates of remission and LDA, physical function and pain severity. Similar to previously described treatment switch data,16 numerically higher efficacy responses were consistently maintained for upadacitinib vs adalimumab over 3 years, regardless of whether patients were in the continuous upadacitinib or treatment switch group. Consistent with radiographic outcomes through 1 year,16 structural joint damage was inhibited to a similar extent on upadacitinib versus adalimumab over 2 years, as determined by mean change in mTSS score, erosion and joint space narrowing, as well as the proportion of patients with no radiographic progression.
Limitations of this study include that it was not designed or powered to detect differences in long-term safety events nor were adjustments possible for multiple comparisons to evaluate the long-term efficacy data. Thus, long-term comparative efficacy data should be interpreted with caution. In addition, the placebo treatment ended at week 26 for ethical reasons. Given the nature of LTEs, results based on AO data may be biased by those patients who remain in the study and who tolerate the drug and show response to drug treatment. To minimise this bias, a conservative NRI approach was also used for efficacy analysis. Regarding safety limitations, the reporting of uncommon AESIs may be biased against upadacitinib: the overall total PY of exposure was nearly threefold higher on upadacitinib versus adalimumab (2796 PY and 948 PY, respectively), providing more opportunities for rare AEs to present on upadacitinib. Nonetheless, despite these limitations, the 3-year data from this open-label LTE provide insights into the long-term benefit/risk of upadacitinib versus adalimumab in a controlled setting.
In summary, the safety profile of upadacitinib 15 mg once a day observed through 3 years was consistent with the results reported previously in SELECT-COMPARE13 16 and with the integrated phase 3 safety analysis.10 No new safety risks emerged. Upadacitinib continued to be effective in treating the signs and symptoms of RA over 3 years, with consistently higher proportions of patients achieving key clinical outcomes such as remission and LDA on upadacitinib vs adalimumab. Upadacitinib-treated patients also showed greater improvements in physical function and pain severity, key factors for patients given their strong influence on overall quality of life. In addition, radiographic progression through 2 years was low and similar between upadacitinib and adalimumab providing further reassurance that the disease activity was well controlled. Overall, the results from this LTE of SELECT-COMPARE continue to support a favourable benefit:risk profile for upadacitinib in the treatment of RA.
Acknowledgments
AbbVie and the authors thank the patients, trial sites and investigators who participated in this clinical trial. AbbVie was the trial sponsor, contributed to trial design, data collection, analysis and interpretation, and to writing, reviewing and approval of final version. No honoraria or payments were made for authorship. The authors thank Tim Shaw, Dr Anna Maniccia and Dr Heidi Camp of AbbVie for their support with the interpretation of the data. Medical writing support was provided by Matthew Eckwahl, PhD, of AbbVie.
Footnotes
Correction notice: This article has been changed since it was first published online. Under the Efficacy heading, the sentence 'Numerically higher responses for patients who switched from adalimumab to upadacitinib vs those who switched from adalimumab to upadacitinib were also observed' has been changed to 'Numerically higher responses for patients who switched from adalimumab to upadacitinib vs those who switched from upadacitinib to adalimumab were also observed'.
Contributors: RF, CGP, YL and I-HS contributed to study conception and design. RF, EM, LB, CGP, PD and JS participated in data acquisition. All authors participated in the analysis and interpretation of the data. All authors also contributed to the critical revision of the manuscript and approved the final version. RF is responsible for the overall content as the guarantor.
Funding: AbbVie was the trial sponsor, and the trial was designed by AbbVie, the authors and investigators. Clinical data were collected by the investigators, their teams and AbbVie. AbbVie was involved in data analysis, the interpretation of results and the preparation, review and approval of the final version of this report. All the authors had access to the data, reviewed and approved the final version, made the decision to submit the manuscript for publication, and attest to the accuracy and completeness of the data. The corresponding author had full access to all the data and the final responsibility to submit for publication. A medical writer, employed by AbbVie, assisted with preparing an initial draft under the direction of the authors.
Competing interests: RF: Research grants and consulting fees from AbbVie, Amgen, Astra-Zeneca, Biosplice, BMS, Flexion, Galvani, Genentech, Gilead, GSK, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi-Aventis, Teva, UCB, Viela, Vorso. EM: Research grants and consulting fees from AbbVie, Lilly, Pfizer, Roche, BMS, Sandoz, Amgen, AstraZeneca, GSK, Janssen, Novartis. LB: speaker, consulting fees and research support from Amgen, BMS, Janssen, Roche, UCB, AbbVie, Pfizer, Merck, Sanofi, Eli Lilly, Novartis, Teva, Gilead, Fresenius Kabi. CGP: Employee and shareholder: Spire Sciences; speaker: Amgen, Bristol-Myers Squibb; consultant: Aclaris, Centrexion, Daiichi-Sankyo, EMD Serono, Five Prime, Flexion Therapeutics, Genentech, Gilead, GlaxoSmithKline, Istresso, Eli Lilly, Myriad Genetics, Novartis, Roche, SetPoint, Sorrento, UCB. PD: Speaker fees from BMS, Sanofi, Eli Lilly, Celltrion. YT: speaker and/or received honoraria from Gilead, AbbVie, Boehringer Ingelheim, Eli Lilly, Mitsubishi-Tanabe, Chugai, Amgen, YL Biologics, Eisai, Astellas, Bristol-Myers, AstraZeneca; research grants from Asahi-Kasei, AbbVie, Chugai, Mitsubishi Tanabe, Eisai, Takeda, Corrona, Daiichi-Sankyo, Kowa, Boehringer Ingelheim. JS: Speaker, research grants and consulting fees from AbbVie, Sandoz, Pfizer, Roche, BMS, UCB, MSD, Accord, Janssen. NK, XB, YL, and I-HS: employees of AbbVie and may hold stock or options.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (eg, protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan and execution of a data sharing agreement. Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
The study was conducted as per the International Conference on Harmonisation guidelines, applicable regulations and the Declaration of Helsinki. Study-related documents were approved by the US Central Institutional Review Board (Quorum #31009) and other local institutional ethics committees and review boards.
References
- 1.Almutairi K, Nossent J, Preen D, et al. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int 2021;41:863–77. 10.1007/s00296-020-04731-0 [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. 10.1136/annrheumdis-2019-216655 [DOI] [PubMed] [Google Scholar]
- 3.Fraenkel L, Bathon JM, England BR, et al. 2021 American College of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res 2021;73:924–39. 10.1002/acr.24596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol 2017;13:707–18. 10.1038/nrrheum.2017.187 [DOI] [PubMed] [Google Scholar]
- 5.Louder AM, Singh A, Saverno K, et al. Patient preferences regarding rheumatoid arthritis therapies: a conjoint analysis. Am Health Drug Benefits 2016;9:84–93. [PMC free article] [PubMed] [Google Scholar]
- 6.Alten R, Krüger K, Rellecke J, et al. Examining patient preferences in the treatment of rheumatoid arthritis using a discrete-choice approach. Patient Prefer Adherence 2016;10:2217–28. 10.2147/PPA.S117774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018;391:2503–12. 10.1016/S0140-6736(18)31115-2 [DOI] [PubMed] [Google Scholar]
- 8.Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (select-beyond): a double-blind, randomised controlled phase 3 trial. Lancet 2018;391:2513–24. 10.1016/S0140-6736(18)31116-4 [DOI] [PubMed] [Google Scholar]
- 9.Smolen JS, Pangan AL, Emery P, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (select-monotherapy): a randomised, placebo-controlled, double-blind phase 3 study. Lancet 2019;393:2303–11. 10.1016/S0140-6736(19)30419-2 [DOI] [PubMed] [Google Scholar]
- 10.Cohen SB, van Vollenhoven RF, Winthrop KL, et al. Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the select phase III clinical programme. Ann Rheum Dis 2020. 10.1093/rheumatology/keaa111.204. [Epub ahead of print: 28 Oct 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubbert-Roth A, Enejosa J, Pangan AL, et al. Trial of Upadacitinib or abatacept in rheumatoid arthritis. N Engl J Med 2020;383:1511–21. 10.1056/NEJMoa2008250 [DOI] [PubMed] [Google Scholar]
- 12.van Vollenhoven R, Takeuchi T, Pangan AL, et al. Efficacy and safety of Upadacitinib monotherapy in Methotrexate-Naive patients with Moderately-to-Severely active rheumatoid arthritis (SELECT-EARLY): a multicenter, Multi-Country, randomized, double-blind, active Comparator-Controlled trial. Arthritis Rheumatol 2020;72:1607–20. 10.1002/art.41384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleischmann R, Pangan AL, Song I-H, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol 2019;71:1788–800. 10.1002/art.41032 [DOI] [PubMed] [Google Scholar]
- 14.Fleischmann R, Song IH, Enejosa J, et al. THU0201 long-term safety and effectiveness of upadacitinib or adalimumab in patients with rheumatoid arthritis: results at 72 weeks from the select-compare study. Ann Rheum Dis 2020;79:323–19. 10.1136/annrheumdis-2020-eular.1418 [DOI] [Google Scholar]
- 15.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 16.Fleischmann RM, Genovese MC, Enejosa JV, et al. Safety and effectiveness of upadacitinib or adalimumab plus methotrexate in patients with rheumatoid arthritis over 48 weeks with switch to alternate therapy in patients with insufficient response. Ann Rheum Dis 2019;78:1454–62. 10.1136/annrheumdis-2019-215764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aletaha D, Smolen J. The simplified disease activity index (SDAI) and the clinical disease activity index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 2005;23:S100–8. [PubMed] [Google Scholar]
- 18.Prevoo ML, van 't Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. 10.1002/art.1780380107 [DOI] [PubMed] [Google Scholar]
- 19.Wells G, Becker J-C, Teng J, et al. Validation of the 28-joint disease activity score (DAS28) and European League against rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 2009;68:954–60. 10.1136/ard.2007.084459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bykerk VP, Massarotti EM. The new ACR/EULAR remission criteria: rationale for developing new criteria for remission. Rheumatology 2012;51 Suppl 6:vi16–20. 10.1093/rheumatology/kes281 [DOI] [PubMed] [Google Scholar]
- 21.Felson DT, Anderson JJ. Methodological and statistical approaches to criteria development in rheumatic diseases. Baillieres Clin Rheumatol 1995;9:253–66. 10.1016/S0950-3579(05)80189-X [DOI] [PubMed] [Google Scholar]
- 22.Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol 1982;9:789–93. [PubMed] [Google Scholar]
- 23.van der Heijde D. How to read radiographs according to the Sharp/van Der Heijde method. J Rheumatol 1999;26:743–5. [PubMed] [Google Scholar]
- 24.van der Heijde DM, van Leeuwen MA, van Riel PL, et al. Biannual radiographic assessments of hands and feet in a three-year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum 1992;35:26–34. 10.1002/art.1780350105 [DOI] [PubMed] [Google Scholar]
- 25.Combe B, Kivitz A, Tanaka Y, et al. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: a phase III randomised clinical trial. Ann Rheum Dis 2021;80:848–58. 10.1136/annrheumdis-2020-219214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleischmann R, Mysler E, Hall S, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (oral strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 2017;390:457–68. 10.1016/S0140-6736(17)31618-5 [DOI] [PubMed] [Google Scholar]
- 27.Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med 2017;376:652–62. 10.1056/NEJMoa1608345 [DOI] [PubMed] [Google Scholar]
- 28.Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis 2017;76:1253–62. 10.1136/annrheumdis-2016-210457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee EB, Yamanaka H, Liu Y, et al. Efficacy and safety of tofacitinib for the treatment of rheumatoid arthritis in patients from the Asia-Pacific region: Post-hoc analyses of pooled clinical study data. Int J Rheum Dis 2019;22:1094–106. 10.1111/1756-185X.13516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaoka K, Tanaka Y, Kameda H, et al. The safety profile of Upadacitinib in patients with rheumatoid arthritis in Japan. Drug Saf 2021;44:711–22. 10.1007/s40264-021-01067-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smolen JS, Genovese MC, Takeuchi T, et al. Safety profile of Baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J Rheumatol 2019;46:7–18. 10.3899/jrheum.171361 [DOI] [PubMed] [Google Scholar]
- 32.Gross RL, Schwartzman-Morris JS, Krathen M, et al. A comparison of the malignancy incidence among patients with psoriatic arthritis and patients with rheumatoid arthritis in a large US cohort. Arthritis Rheumatol 2014;66:1472–81. 10.1002/art.38385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Askling J, Berglind N, Franzen S, et al. How comparable are rates of malignancies in patients with rheumatoid arthritis across the world? A comparison of cancer rates, and means to optimise their comparability, in five RA registries. Ann Rheum Dis 2016;75:1789–96. 10.1136/annrheumdis-2015-208105 [DOI] [PubMed] [Google Scholar]
- 34.Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum 2007;56:2886–95. 10.1002/art.22864 [DOI] [PubMed] [Google Scholar]
- 35.Holmqvist ME, Neovius M, Eriksson J, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis and association with disease duration and hospitalization. JAMA 2012;308:1350–6. 10.1001/2012.jama.11741 [DOI] [PubMed] [Google Scholar]
- 36.Scott IC, Hider SL, Scott DL. Thromboembolism with Janus kinase (JAK) inhibitors for rheumatoid arthritis: how real is the risk? Drug Saf 2018;41:645–53. 10.1007/s40264-018-0651-5 [DOI] [PubMed] [Google Scholar]
- 37.Molander V, Bower H, Frisell T, et al. Risk of venous thromboembolism in rheumatoid arthritis, and its association with disease activity: a nationwide cohort study from Sweden. Ann Rheum Dis 2021;80:169–75. 10.1136/annrheumdis-2020-218419 [DOI] [PubMed] [Google Scholar]
- 38.Choy E, Mcinnes I, Cush J. THU0195 incidence and risk of venous thromboembolic events among patients with rheumatoid arthritis enrolled in the upadacitinib select clinical trial program. Annals of the Rheumatic Diseases 2020;79:317–9. [Google Scholar]
- 39.Ytterberg S, Bhatt D, Mikuls T. Safety and efficacy of tofacitinib vs tnf inhibitors in ra patients aged 50 years or older with one or more cardiovascular risks: results from a phase 3b/4 randomized safety trial [abstract]. Arthritis Rheumatology 2021;73 https://acrabstracts.org/abstract/safety-and-efficacy-of-tofacitinib-vs-tnf-inhibitors-in-ra-patients-aged-50-years-or-older-with-one-or-more-cardiovascular-risks-results-from-a-phase-3b-4-randomized-safety-trial/ [Google Scholar]
- 40.Conaghan P, Cohen S, Burmester G, et al. Benefit-Risk analysis of Upadacitinib compared with adalimumab in the treatment of patients with moderate-to-severe rheumatoid arthritis. Rheumatol Ther 2021. 10.1007/s40744-021-00399-5. [Epub ahead of print: 23 11 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-002012supp001.pdf (1.7MB, pdf)
Data Availability Statement
Data are available on reasonable request. AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (eg, protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan and execution of a data sharing agreement. Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.