Abstract
Objectives
Lung cancer screening programmes offer an opportunity to address tobacco dependence in current smokers. The effectiveness of different approaches to smoking cessation in this context has not yet been established. We investigated if immediate smoking cessation support, including pharmacotherapy, offered as part of a lung cancer screening programme, increases quit rates compared to usual care (Very Brief Advice to quit and signposting to smoking cessation services).
Materials and methods
We conducted a single-blind randomised controlled trial of current smokers aged 55–75 years attending a Targeted Lung Health Check. On randomly allocated days smokers received either (1) immediate support from a trained smoking cessation counsellor with appropriate pharmacotherapy or (2) usual care. The primary outcome was self-reported quit rate at 3 months. We performed thematic analysis of participant interview responses.
Results
Of 412 people attending between January and March 2020, 115 (27.9%) were current smokers; 46% female, mean (SD) 62.4 (5.3) years. Follow-up data were available for 84 smokers. At 3 months, quit rates in the intervention group were higher 14/48 (29.2%) vs 4/36 (11%) (χ2 3.98, p=0.04). Participant interviews revealed four smoking-cessation related themes: (1) stress and anxiety, (2) impact of the COVID-19 pandemic, (3) CT scans influencing desire to quit and (4) individual beliefs about stopping smoking.
Conclusion
The provision of immediate smoking cessation support is associated with a substantial increase in quit rates at 3 months. Further research is needed to investigate longer-term outcomes and to refine future service delivery.
Trial registration number
Keywords: tobacco and the lung, lung cancer, imaging/CT MRI etc
Key messages.
What is already known on this topic?
Lung cancer screening clinics act as a teachable moment for smoking cessation and providing cessation support alongside screening clinics is reccomended.
What this study adds?
This study provides evidence that immediate cessation support plus pharmacotherapy support is an effective method of cessation support and can be delivered within a screening context.
How this study might affect research, practice or policy?
This study should be used to prompt larger, more definitive studies, to help guide the delivery of lung cancer screening services in the future.
Introduction
Tobacco smoking is the leading cause of preventable mortality and morbidity globally, killing an estimated 8.71 million (8.12–9.31) people (15.4% (14.6%–16.2%) of all deaths) in 2019 and causing substantial harm to the environment.1 2 In the UK, during 2018, 37% of respiratory deaths, and 26% of deaths from cancer were attributable to smoking, as well as 489 300 hospital admissions.3 Although UK smoking prevalence is falling and youth uptake rates are at a historic low,4 14.4% of adults are still current smokers.5
Lung cancer typically becomes symptomatic at a relatively late stage, often beyond the point where curative treatment is possible. This has led to the development of screening programmes for people with a smoking history using low-dose CT (LDCT), to identify disease at an earlier point.6 7 The UK Lung Cancer Screening trial (UKLS) is among studies that have demonstrated an increased quit rate in the intervention arm8–10 and attendance at lung cancer screening has been identified as a ‘teachable moment’ for smokers in relation to addressing their tobacco dependence.10–12 This is important because screening for lung cancer is a complex intervention, the value of which will depend on a range of potential impacts beyond the primary focus of reducing lung cancer mortality.
The provision of evidence-based smoking cessation interventions in the context of LDCT screening has been highlighted as an opportunity to increase quit rates within this population and increase the value of the LDCT intervention.13 Follow-up data from the NLST trial in the USA highlighted those smokers who attended yearly LDCT screens and successfully quit smoking had the largest reductions in mortality.14 Additionally, access to smoking cessation services in the UK, remains inadequate. For example, a recent British Lung Foundation survey found that a third of smokers with Chronic Obstructive Pulmonary Disease (COPD) reported not being offered any smoking cessation support.15 Although lung cancer screening interventions can promote smoking cessation, the most effective strategy remains unclear. A 2017 consensus review regarding smoking cessation during LDCT lung cancer screening noted: ‘we do not know if the addition of immediate smoking cessation interventions will produce a better outcome than a standard ’signposting to service’ model’.16
We, therefore, undertook the Quit Smoking Lung Health Intervention Trial (QuLIT) to compare the effectiveness of two approaches to smoking cessation support in smokers attending a lung health screening service; immediate smoking cessation support including pharmacotherapy, compared with usual care (UC) (Very Brief Advice (VBA) to quit and signposting to local services).
Materials and methods
Study design and randomisation
We conducted a single-blind, randomised controlled trial (randomised by day of attendance) comparing the effectiveness of two smoking cessation strategies based around targeted lung health checks (TLHC) undertaken primarily as a screening programme for lung cancer. On days randomly allocated as smoking intervention (SI) days, participants received immediate smoking cessation support including pharmacotherapy. On UC days, they were given VBA to quit and signposted to smoking cessation services.
Setting
The Healthy Lung Project (HLP) is a lung cancer screening pilot project delivered by the Royal Brompton Hospital, supported by RM Partners, West London Cancer Alliance with National Health Service (NHS) funding through the National Cancer Transformation Fund. The TLHC itself consists of two stages. An initial lung health check includes discussion with specialist nurses of participants’ current or historical smoking behaviour, medical history, familial cancer history and spirometry. If participants met the criteria according to the Prostate, Lung, Colorectal and Ovarian or Liverpool Lung Project screening risk models,17 18 they were invited to attend an LDCT scan.
Population and recruitment
Patients whose GP surgeries were within the London Borough of Hammersmith and Fulham London borough and had opted to attend the HLP programme were recruited. The process for referral to the HLP is as follows. Medical records were screened by the GP practice for eligibility criteria (age 55–75 recorded as ever smokers and consent for records to be shared) and this list was sent to the HLP team. Eligible participants were initially sent an invite letter to contact the HLP at the Royal Brompton Hospital to attend a TLHC. If no response had been received after 2 weeks, the HLP team sent a second invite letter. If there was still no response after a further 2 weeks, participants received a reminder phone call asking if they’d like to take up the offer of a TLHC.
Before any appointment was made, participants were asked several triage questions by an administrator; confirmation of GP practice, age, smoking history, if they had recent (<12 months) chest CT or were undergoing current treatment for cancer. During the period that the study was able to proceed (January–March 2020) a total of 3217 initial invite letters were mailed, 2122 second invite letters were mailed and a total of 1042 reminder calls were made, before the programme paused due to the COVID-19 pandemic.
Patient and public involvement
Patients and public were not involved in the design, conduct, reporting or dissemination plans of our research.
Interventions
Participants in QuLIT attended for TLHC between January and March 2020. During this time, half the days were allocated by random number generation as SI days and half as UC. Appointments to attend for the TLHC were made by an administrator who was unaware of which study arm days had been allocated to.
Smoking cessation
Participants in the SI arm attending the TLHC were seen immediately by the smoking cessation service, with immediate access to pharmacological options to support quit attempts and six sessions of one-to-one cessation support. The sessions were informed by the National Centre for Smoking Cessation and Training (NCSCT) and KickIT programmes.19 20 Each session would include a combination of motivational interviewing, behavioural support, and information on nicotine withdrawal alongside pharmacotherapy counselling and prescriptions. The prescriptions were provided free of charge. All sessions were conducted at the Royal Brompton Hospital, by a specialist research nurse and smoking cessation practitioner who was embedded into the HLP team.
Usual care
Those attending on UC days received VBA to quit (‘Stopping smoking is the most important thing that you can do to improve your health now and reduce the risk of health problems in the future.’) The VBA approach was as outlined by the NCSCT.21 Participants were also directed to the London Stop Smoking Portal https://london.stopsmokingportal.com/ which provides information to smokers about how to engage with local stop smoking services and a telephone Quitline service Nurses administering the TLHC appointments provided both signposting and VBA.
Follow-up was completed by telephone interview, 3 months after attending the TLHC. The interview was conducted by a researcher blind to study allocation and participants were asked not to reveal this until the primary endpoint had been ascertained. Interviews consisted of a short, predefined, scripted questionnaire exploring (1) self-reported 7-day point prevalence smoking abstinence (primary outcome), (2) the patient’s perception of treatments available and the value of any smoking cessation support offered (online supplemental appendix 1). The questionnaire was developed by NSH, a respiratory clinical academic and RM, a senior research nurse and smoking cessation practitioner. The questionnaire included open and closed questions, providing both quantitative and qualitative data exploring the effectiveness, and participants’ experience. If participants did not pick up on the first call attempt, they were called a second time, a total of three calls were made. If they did not pick up on the third attempt they were classed as lost to follow-up. Each call attempt was made at a different time of the day to increase the likelihood of response.
bmjresp-2021-001030supp001.pdf (83.9KB, pdf)
Statistical analysis
The primary outcome of the trial was the difference in self-reported point prevalence abstinence (PPA) from smoking at 3 months follow-up. The sample size was calculated using the findings of two trials; the EAGLES trial22 which found a 38% quit rate in the pharmacology arm and the UKLS Study,9 which found a 14% quit rate in the arm undergoing CT screening. Based on these rates, a superiority study (1:1 randomisation) with 90% power at a 5% significance level would require 136 participants (calculator at Sealed Envelope https://www.sealedenvelope.com/power/binary-superiority/). To improve the power of exploratory analyses comparing different subgroups (eg, those with or without airflow obstruction on spirometry), we had intended to recruit as many participants in the screening programme as possible (anticipating 500–1000), but enrolment in the TLHC programme was suspended during March 2020 because of the COVID-19 pandemic.
Smoking rates (7-day point prevalence) at 3 months were compared between study arms by χ2 test and a p<0.05 taken as statistically significant. Participants missing 3 months follow-up outcome data were excluded from the primary analysis, but as a sensitivity analysis, we repeated the analysis, assuming that all individuals lost to follow-up had continued smoking.
Interview conduct
Telephone interviews lasted between 5 and 25 min and took place 3 months after study enrolment. Due to the COVID-19 pandemic there was a 3-week interruption in study activities. As a result, 39 participants were interviewed later than planned, however, where this was the case, they were asked to answer questions based on what their smoking status had been at the 3-month point. Detailed notes were taken during each phone call and an anonymised questionnaire proforma saved for each patient on a password protected digital platform (Google Documents) accessible only to the thematic analysis (TA) coders to ensure confidentiality. Interviews were not recorded.
SCB, PW and KEJP conducted a TA based on the approach described by Braun and Clark.23 Initial codes were largely open, and these were then refined, using the context from initial readings. Preliminary themes were developed independently by SCB, PW and KEJP who then came together to discuss, refine, reorganise and agree on final themes. These were then reviewed with the co-authors, with discussion from the original data. Data were coded manually in Microsoft Excel. Quotations reported below are followed in brackets by the respondents’ gender, age group, study allocation, and smoking cessation outcome.
Results
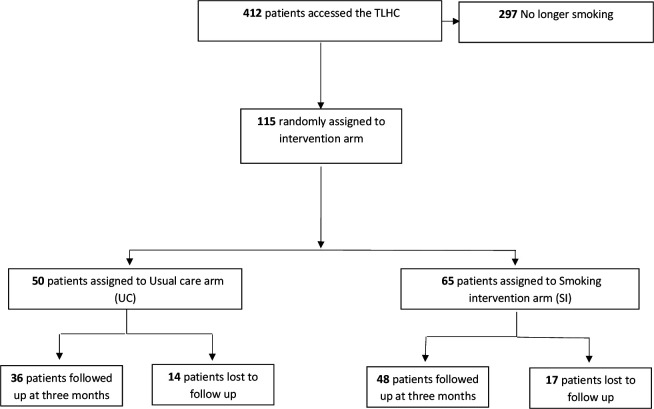
During the study period, 412 patients attended the TLHC. Of these, 115 (27%) were current smokers. Baseline demographic data are displayed in table 1. Figure 1 represents the flow of patients through the trial; 65 (57%) participants attended on SI days and 50 (43%) on days allocated to UC. The higher number of participants recruited to SI is due to the abrupt interruption of the TLHC programme caused by the COVID-19 pandemic. Of these, 48 (74%) and 36 (72%) were followed up in the SI and UC arms, respectively. Among those lost to follow-up (13/31) were uncontactable (invalid telephone numbers, telephone disconnected etc) and (18/31) did not answer on the final attempt.
Table 1.
Baseline data of participants
| Smoking cessation intervention (n=65) | Usual care (n=50) |
P value | |
| Age | 62.58±6.09 | 61.68±5.53 | 0.51 |
| Females | 30 (46%) | 26 (52%) | 0.43 |
| BMI | 26.41±5.04 | 25.65±5.39 | 0.63 |
| Average no cigarettes per day | 12.89±7.80 | 13.85±8.63 | 0.53 |
| Index of Multiple Deprivation (IMDD) | 0.34 | ||
| IMDD 3 | 24 (36.9%) | 18 (36%) | |
| IMDD 4 | 2 (3.1%) | 3 (6%) | |
| IMDD 5 | 2 (3.1%) | 8 (16%) | |
| IMDD 6 | 26 (40%) | 11 (22%) | |
| IMDD 7 | 5 (7.7%) | 1 (2%) | |
| IMDD 8 | 6 (9.2%) | 9 (18%) | |
| Baseline scan results | |||
| No evidence of nodules | 44 (68%) | 30 (60%) | 0.64 |
| Evidence of nodules | 4 (6%) | 2 (4%) | 0.63 |
| Did not meet threshold criteria for LDCT screening | 17 (26%) | 18 (36%) | 0.25 |
BMI, body mass index; LDCT, low-dose CT.
Figure 1.
Flow diagram to represent the flow of patients through the QuLIT trial. QuLIT, Quit Smoking Lung Health Intervention Trial; TLHC, Targeted Lung Health Check.
Among those followed up at 3 months 14 (29.2%) in the SI arm, and 4 (11%) in the UC arm reported that they had quit smoking, (χ2 3.98, p=0.04) (table 2). A sensitivity analysis, assuming that all participants that we had been unable to follow up were still smoking, produced a similar result (χ2 3.92, p=0.04). The effect of the intervention was more pronounced if analysis was limited to only those individuals who had undergone an LDCT SI arm 9/28 had quit vs 1/21 in the UC arm (χ2 5.53 p=0.01).
Table 2.
Effect of immediate smoking cessation intervention (SI) on smoking status at 3 months
| SI (n=48) | UC (n=36) | Pearson χ2 | P value | |
| Still smoking?* | Yes 34 (70.8%) | Yes 32 (89%) | 3.98 | 0.04 |
| No 14 (29.2%) | No 4 (11%) |
*Smoking abstinence measured via self-reported 7-day point prevalence.
UC, usual care.
Quit attempts and pharmacological methods used to support them are outlined in table 3. All participants in the SI group received at least one session of cessation counselling and only 3/36 participants in the UC arm had received counselling support via a local service. Of note, none of the UC participants who accessed counselling reported a successful quit, but all three reported a quit attempt. Of the participants who were still smoking at follow-up, 35.3% in the SI group had made a quit attempt vs 25% in the UC group. Although offered, most participants did not use any pharmacological aids to support quit attempts, while e-cigarette use was relatively low in this population.
Table 3.
Quit attempts, pharmacological and E-cigarette/vaping use by study arm
| Smoking cessation intervention (n=48) | Usual care (n=36) | |
| If quit smoking | (n=14) | (n=4) |
| What help you used to support your quit? | Varenicline 3 (21.4%) | Nothing 4 (100%) |
| NRT patches 1 (7.1%) | ||
| NRT inhaler 1 (7.1%) | ||
| Nothing 9 (64.4%) | ||
| If still smoking | (n=34) | (n=32) |
| Did you try to quit smoking? | Yes 12 (35.3%) | Yes 8 (25%) |
| No 22 (64.7%) | No 24 (75%) | |
| Attempted to quit unsuccessfully. | (n=12) | (n=8) |
| What help did you use to support attempts to quit? | Varenicline 2 (16.7%) | Varenicline 1 (12.5%) |
| NRT spray 1 (8.2%) | NRT spray 1 (12.5%) | |
| NRT gum 2 (16.7%) | NRT gum 2 (25%) | |
| NRT patches 2 (16.7%) | NRT patches 1 (12.5%) | |
| E-cigarettes/vape 2 (16.7%) | E-cigarettes/vape 2 (25%) | |
| Nothing 3 (25%) | Nothing 1 (12.5%) |
NRT, Nicotine Replacement Therapies.
Participant experience
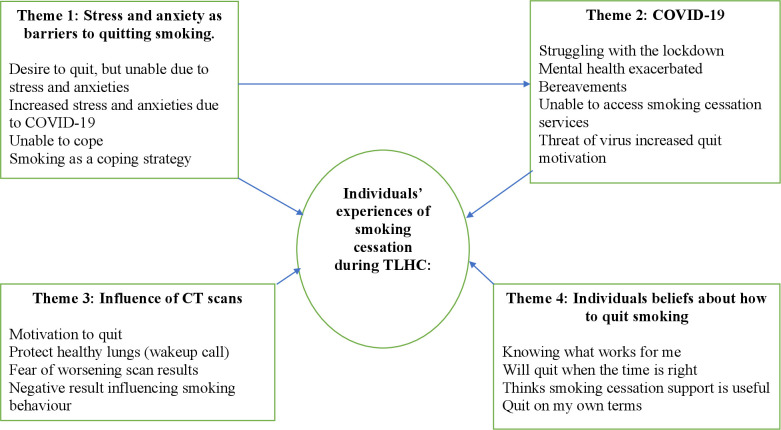
Experiences related to smoking cessation within the TLHC were shaped both by personal factors related to each individual and by the current context. Four themes were identified1 stress and anxiety as barriers to quitting smoking2; the COVID-19 pandemic and smoking3; CT scans influencing desire to quit4; Individual beliefs about stopping smoking (figure 2).
Figure 2.
Thematic map: individual experiences of smoking cessation during the targeted lung health check programme. A thematic map displaying the main four themes, sub themes and relationships. TLHC, Targeted Lung Health Check.
Stress and anxiety as barriers to quitting smoking
The most prominent theme throughout interviews was the impact that participants’ experience of stress or anxiety levels had on their ability to stop smoking. Frequently, despite reporting both the desire to quit and an understanding that it would be ‘the right thing to do’ (male, 60s, still smoking, SI arm), the reality of coping with mental health and well-being appeared to often modulate the perceived utility of the intervention.
It was ‘not the right time as I am in a stressful environment, but I would really like to quit soon’ (female, 60s, still smoking UC arm) and ‘I do think quitting is the right thing to do, I have just found this period very stressful’ (male, 60s, still smoking, SI arm) and ‘I really would like to quit, I have cut down, but I am struggling at the moment with my mental health’ (female, 50 s, still smoking, SI arm)
The COVID-19 pandemic was clearly a major contributing factor for most people and a clear source of stress and anxiety that made quitting smoking more difficult. ‘I think it was helpful at the time, but I have struggled over the COVID-19 with stress and anxieties’ (female, 50s, still smoking, SI arm).
Observations around mental health issues were typically ambiguous, making it unclear whether participants continued to smoke as a form of stress relief or because their anxieties made it difficult for them to commit to smoking cessation. ‘Found the Corona lockdown so hard I couldn’t give up’ (male, 60s, still smoking, UC arm). However, comments collected suggest that many participants view smoking as a comfort in challenging times.
‘It’s the only thing I’ve got’ (male, 50s, still smoking, UC arm) and ‘I have already given up drinking, so I don’t want to give up smoking as well, I need something!’
Impact of the COVID-19 pandemic
COVID-19 was often mentioned in relation to smoking cessation, and frequently identified as the cause of a failure to stop smoking, or of having relapsed to smoking despite finding the TLHC programme valuable.
At the beginning, I think It helped, but with COVID-19 things got harder. (female, 60s still smoking, UC arm), I thought the program was good, but I have just struggled over lockdown. (male, 60s, still smoking, SI arm)
While 44% of participants in the SI arm reported the smoking cessation support to have been beneficial, it was also apparent that many, particularly those who had not quit, believed it was only helpful within the context of their situation and if accessed at the correct time. The COVID-19 pandemic, including the prolonged period of social isolation has proved to be a challenge for many. 'Maybe if the virus hadn’t happened then I would have found it more helpful.’ (male, 60s, still smoking, SI arm)
Participants reported several barriers associated with attempting to quit smoking related to the pandemic. For example, challenges accessing smoking cessation services during lockdown were noted and therefore those who did not receive the SI and immediate counselling support were disadvantaged, regardless of desire to quit.
I have been wanting to quit for a while and tried to call the GP to go to a stopping smoking clinic, but they were not running due to COVID (female, 50s, still smoking, SI arm)
Others were more directly affected by COVID-19 with the death of family members which had negatively impacted quit attempts ‘I quit straight after I received my results and felt I was doing really well with it but then my mother died (two months ago) and this made me go back to smoking again.’ (female,70s, still smoking, SI arm) and ‘I did stop smoking for a few weeks and then my father caught COVID-19 and died. This caused me to go back to smoking.’(male, 50s, still smoking, SI arm)
However, a small number of participants felt the COVID-19-related social distancing measures had helped them to reduce or even cut out their smoking habit ‘I have cut down dramatically, by about 50% and I intend to fully quit by the end of the month. I have found that the current pandemic has spurred me on’ (male, 50s, still smoking, SI arm) and particularly those that associated smoking with being social. Also, ‘I don’t ever buy cigarettes but because of social isolation I have not had access to the social situations that I would usually smoke in’ (male, 50s, quit smoking, SI arm).
CT scans influencing desire to quit
Both participants who had stopped smoking, and those who had not, expressed a clear belief that the CT scan carried out as part of the TLHC was very useful in supporting smoking cessation. Participants who were still smoking, 54% stated that their views about quitting had changed since participating in the TLHC. Many, participants described the investigation as motivating because their scan was better than they thought ‘The lung screen has been a motivating factor in helping me quit. Observing my scan and realising that I did not have any lung cancer made me feel that it was worth making a change and relieved some of the stress that causes me to smoke in the first place.’ (male, 60s, quit smoking, SI group)
Seeing that I still had healthy lungs made me feel like I had something worth quitting for and preserving. (male, 60s, quit smoking, SI group).
Others reported being motivated by fear, which prompted them to make a change ‘When I got the result from my doctor and there was a shadow on my lung it really prompted me to want to make a change. It scared me.’ (male, 60s, quit smoking, SI group)
Among the 84 participants followed up at 3 months, 49/84 (58%) were eligible for an LDCT, being at high risk of lung cancer according to two validated risk models.17 24 Of these, 45/84 (53%) had no nodules requiring follow-up, 4/84 (5%) had nodules requiring follow-up. Additionally, 4/84 (5%) had emphysema (>15%) on their scan.
Despite some of the participants being undecided about whether the interventions had been helpful or not 19/84 (22%), many of these patients did mention the CT scan and believed it had been a powerful tool in making them change their mindset. ‘The program made me think more about quitting and I got in contact with my GP’s stopping smoking clinic’ (male, 70s, still smoking, UC arm)
For those who found the TLHC useful, it appeared to act as a prompt or wakeup call. It seemed people were already aware of the health risks, but that the screening and smoking cessation advice made these feel more real. Of note, for one participant, the scan caused the opposite effect and had prompted the decision to re-start smoking ‘when I found out how healthy my lungs were, and I had good test results I started to smoke again during lockdown.’ (male, 50s, still smoking, SI arm)
Individual beliefs about stopping smoking
Overall, there was a range of perceptions expressed, from those who thought the TLHC programme was very beneficial 37/84 (44%) to those who did not find it useful at all 15/84 (17%). There was also a strong feeling among those who had not yet managed to quit, that if they were to quit it was something that they needed to do by themselves, stating a preference for alternative approaches, or ‘knowing what works for me’ (male, 60s, still smoking, SI arm), for example, ‘I feel that I can do it on my own when the time is right’ (female, 50s, still smoking, UC arm);
I think I would be better to do it by myself. But then if I found I was unable to then I would seek help. (male, 70s, still smoking, SI arm).
A large proportion of participants showed a desire to stop smoking, and 38/84 (45.2%) reported having made a quit attempt since their attendance.
I have always known that smoking even a small amount is a silly idea, and now have committed to quit for good (female, 70s, quit smoking UC arm)
In contrast, out of the 66 participants who continued to smoke (25/66) 37% did not want to quit smoking and did not see their smoking as a problem. Many believed they did not smoke much or had cut down dramatically but there was a variety of ideas of what this meant.
I have always known smoking is bad for my health. I am occasionally smoking 1–2 cigarettes per week at the moment (female, 60s, still smoking, UC arm)
Didn’t want to quit and don’t want to now (male, 70s, still smoking, UC arm)
I have always known smoking is bad for me and I need to stop I just can't bite the bullet yet (male, 60s, still smoking, UC arm).
Not valuable at all: ‘I prefer to do things my own way, I quit before for over a year and can do it again (male, 60s, still smoking, SI arm).
Very significant: ‘Found the support very helpful (female, 60s still smoking, SI arm).
Discussion
The main finding of this study is that the immediate provision of smoking cessation support, including the offer of pharmacotherapy, to participants attending a TLHC, was associated with a substantially higher self-reported quit rate at 3 months, compared with a standard approach of VBA to quit and sign-posting participants to smoking cessation services.
Significance of findings
These results support the hypothesis that immediate smoking cessation support, including the provision of pharmacotherapy delivered during a TLHC, is an effective strategy for increasing quit rates in this high-risk population. The 3-month quit rate observed in our control arm (9%) was similar to that of 2-week self-reported quits observed in the UKLS trial (11%), 12-month CO verified quits in the Danish Lung Cancer Trial (DLCST) (12%), 2 years self- reported quits in the NELSON trial (16.6%) and 12-month CO verified quits in the ITALIA lung cancer screening trial (14.6%),9 10 25 26 suggesting that the data are generalisable. In our intervention arm, 29.2% reported that they had quit. The quit rate that we observed in the SI group was lower than with varenicline alone in the EAGLES trial, this may be attributed to the differences in sample population between the studies and the readiness to quit in the population sampled in the EAGLES trial. It is important to note that although pharmacotherapy was offered to participants in the SI group, many declined to take this up and interestingly, of those who did quit, most managed to do so without medication, despite being offered it. This may suggest that the immediate counselling alone acted as an additional prompt or motivational tool to support cessation.
Other studies have investigated smoking cessation in the context of lung cancer screening, though, the immediate, intense intervention is a novel aspect of our trial. In a pilot study, Marshall et al studied the impact of a smoking cessation intervention involving motivational interviewing with take home materials conducted on the day of screening, however, no follow-up support or pharmacotherapy was offered.27 A study in 92 patients attending lung cancer screening demonstrated higher quit rates in smokers randomised to receive telephone counselling compared withUC (17.4% vs 4.3% respectively).28 In this study, the telephone counselling was only initiated 1–2 days after the participants had received their screening results. By contrast, in the Alberta Lung Cancer Screening Study, no difference in 12-month quit rate was observed among 345 active smokers randomised to either telephone smoking cessation counselling or UC; 12.6% vs 14.0%, respectively.29 Of note in this study contact was initiated a median 16 days following randomisation, so this may have attenuated the immediate motivation associated with having just taken part in lung cancer screening. In people with a recent diagnosis of cancer (as distinct from a population going through screening for possible lung cancer), higher quit rates with telephone smoking cessation counselling compared with UC (34.5% vs 21.5%) have also been reported.30 There is some evidence to suggest that internet based materials are more likely to prompt quit attempts in this context than written ones31 and that intervention prior to screening may be more effective than post-screening.32
The impact of the coronavirus pandemic on our trial population’s ability to access services and its impact on mental health were main sub themes indicated in our tTA. Such experiences have been mirrored in individuals with chronic respiratory diseases across the UK.33 Of note our observed quit rate in the UC arm was similar to that seen in UKLS and DLCST, studies conducted before the pandemic, suggesting that the pandemic itself was not that impactful but rather that it was immediately available as a reason to support behavioural choices. Given the increased risk to smokers from COVID-19 (as well as other infections), there is a need to ensure that support to quit is incorporated into pandemic responses.3 34 35 An important theme highlighted in our qualitative analysis was the influence of having had a CT scan on smoking behaviour. Existing data demonstrate increased quit rates in people attending lung cancer screening compared with control groups9 25 26 and particularly in those who received an abnormal scan result.9 Kummer et al conducted semistructured interviews on current and ex-smokers who attended an LDCT scan in the UK, with many participants stating that they felt motivated to quit after receiving the CT scans or after having a discussion with a clinician at the appointment.36
The provision of immediate smoking cessation input for individuals attending TLHC, has multiple advantages; target populations for TLHC are typically older with a long smoking history, greater nicotine dependence and are more likely to live in socioeconomically deprived areas. These populations may often struggle to seek smoking cessation support from their primary care providers, thus provision of smoking support during TLHC may provide a vital resource for these less well-resourced populations, helping to address inequalities and deliver the agenda set out by The Royal College of Physicians to support smoking cessation by the NHS.3 Given the increased interest within this topic and the importance of proving cessation support for high-risk smokers, the National Cancer Institute in the US has funded the SCALE collaboration, which aims to improve knowledge, pool data from several RCTs provide guidance on the most effective cessation methods delivered during LCS.37
Strengths and limitations
Our results suggest that immediate smoking cessation intervention can increase quit rates in the context of lung cancer screening. However, it should be noted that although statistically significant, the sample size did not meet that required by the trial power calculation, increasing the risk of type 1 error. In addition to replicating these preliminary data, future research should also focus on exploring different ways services can provide access to smoking cessation support while accommodating individual patient choice, preference and capacity. This is likely to involve making a range of interventions available to suit different individuals, including face to face, online or hybrid approaches. For example, there is evidence that mobile phone-based interventions for smoking cessation are effective38 and may be relatively easy to implement. However, given issues of digital literacy and access as well as individual choice, many people will be unable or unwilling to access services in this manner. A variety of offers will need to be available to deliver a personalised approach to smoking cessation.
We did not biochemically verify smoking cessation, in order to keep study costs and participant burden low, as 3-month telephone follow-up of smokers was already part of the routine TLHC. Thus, our current findings should be interpreted with caution due to the use of self-reported data. Baseline measures of tobacco dependence and psychological status were not collected routinely in all study participants and given that these characteristics are known covariates for smoking cessation, this may have affected our results. The study arms were matched for number of cigarettes smoked daily. A larger cohort, as originally anticipated, would have allowed us to conduct subgroup analyses based on participant characteristics and CT scan outcomes, but this was not possible because of the COVID-19 pandemic. In addition, we do not have information on longer-term quit rates, although short-term quit rates often reflect longer term abstinence, as displayed in the UKLS trial which observed higher quit rates at 12 months (22% vs 11%).22 The longer-term impact on smoking behaviour cannot be inferred with certainty from the 3 months follow-up period covered by this study. The lost to follow-up observed in our cohort, is a limitation, though it is similar to that observed in other trials of smoking cessation within the context of screening when follow-up is conducted remotely.31 39
Conclusion
Our finding that immediate smoking cessation intervention appears to increase the impact of TLHCs should prompt definitive studies with longer follow-up to help guide the development and delivery of lung cancer screening services in the future. This will ensure delivery of the greatest health benefit to participants and thus maximise the value of lung health screening.
Acknowledgments
KEJP would like to acknowledge the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London for their support.
Footnotes
SCB and PW contributed equally.
Correction notice: The article has been corrected since it was published online. The co-authors' names have been amended: Emily Jade Bartlett to Emily Catharine Bartlett and Samual Kemp to Samuel V Kemp.
Contributors: NSH, ECB, AS, SVK, JA, JD, MC and KM designed the study, RM delivered the smoking cessation intervention. SCB and PW conducted follow up calls. SCB, PW and KEJP analysed qualitative data. SCB and PW produced the first draft to which all authors contributed. All authors have reviewed and approved the final version. NSH is the guarantor. KEJP was supported by the Imperial College Clinician Investigator Scholarship.
Funding: This work was supported by RM Partners, West London Cancer Alliance, hosted by The Royal Marsden NHS Foundation Trust.
Disclaimer: The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health
Competing interests: NSH is Chair of Action on Smoking and Health and Medical Director of The British Lung Foundation.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Anonymised research data will be shared with third parties via a request to the senior author (NSH).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by South Central – Oxford C Research Ethics Committee and the Health Research Authority (Ref:18/SC/0236). The requirement for individual consent was waived by the ethics committee, as obtaining this would itself have been an intervention and influenced outcomes in the control group.
References
- 1.Murray CJL, Aravkin AY, Zheng P, et al. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020;396:1223–49. 10.1016/S0140-6736(20)30752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zafeiridou M, Hopkinson NS, Voulvoulis N. Cigarette smoking: an assessment of tobacco's global environmental footprint across its entire supply chain. Environ Sci Technol 2018;52:8087–94. 10.1021/acs.est.8b01533 [DOI] [PubMed] [Google Scholar]
- 3.Royal College of Physicians . Hiding in plain sight: treating tobacco dependency in the NHS, 2018. Available: https://www.rcplondon.ac.uk/projects/outputs/hiding-plain-sight-treating-tobacco-dependency-nhs
- 4.Laverty AA, Filippidis FT, Taylor-Robinson D, et al. Smoking uptake in UK children: analysis of the UK millennium cohort study. Thorax 2019;74:607–10. 10.1136/thoraxjnl-2018-212254 [DOI] [PubMed] [Google Scholar]
- 5.Ash . Smoking statistics. Available: https://ash.org.uk/wp-content/uploads/2019/10/SmokingStatistics.pdf
- 6.National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020;382:503–13. 10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 8.Field JK, Duffy SW, Baldwin DR, et al. Uk lung cancer RCT pilot screening trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71:161–70. 10.1136/thoraxjnl-2015-207140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brain K, Carter B, Lifford KJ, et al. Impact of low-dose CT screening on smoking cessation among high-risk participants in the UK lung cancer screening trial. Thorax 2017;72:912–8. 10.1136/thoraxjnl-2016-209690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pistelli F, Aquilini F, Falaschi F, et al. Smoking Cessation in the ITALUNG Lung Cancer Screening: What Does “Teachable Moment” Mean? Nicotine Tobacco Research 2020;22:1484–91. 10.1093/ntr/ntz148 [DOI] [PubMed] [Google Scholar]
- 11.Iaccarino JM, Duran C, Slatore CG, et al. Combining smoking cessation interventions with LDCT lung cancer screening: a systematic review. Prev Med 2019;121:24–32. 10.1016/j.ypmed.2019.02.016 [DOI] [PubMed] [Google Scholar]
- 12.Tammemägi MC, Berg CD, Riley TL, et al. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst 2014;106:dju084. 10.1093/jnci/dju084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostroff JS, Copeland A, Borderud SP, et al. Readiness of lung cancer screening sites to deliver smoking cessation treatment: current practices, organizational priority, and perceived barriers. Nicotine Tob Res 2016;18:1067–75. 10.1093/ntr/ntv177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanner NT, Kanodra NM, Gebregziabher M, et al. The association between smoking abstinence and mortality in the National lung screening trial. Am J Respir Crit Care Med 2016;193:534–41. 10.1164/rccm.201507-1420OC [DOI] [PubMed] [Google Scholar]
- 15.Philip K, Gaduzo S, Rogers J, et al. Patient experience of COPD care: outcomes from the British lung Foundation patient Passport. BMJ Open Respir Res 2019;6:e000478. 10.1136/bmjresp-2019-000478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kathuria H, Detterbeck FC, Fathi JT, et al. Stakeholder research priorities for smoking cessation interventions within lung cancer screening programs. An official American thoracic Society research statement. Am J Respir Crit Care Med 2017;196:1202–12. 10.1164/rccm.201709-1858ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassidy A, Myles JP, van Tongeren M, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer 2008;98:270–6. 10.1038/sj.bjc.6604158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ten Haaf K, Jeon J, Tammemägi MC, et al. Risk prediction models for selection of lung cancer screening candidates: a retrospective validation study. PLoS Med 2017;14:e1002277. 10.1371/journal.pmed.1002277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.kick-it. healthcare professionals, 2018. Available: https://kick-it.org.uk/healthcare-professionals/
- 20.Training NCfSCa . NCSCT training, 2021. Available: https://elearning.ncsct.co.uk/
- 21.National Centre for Smoking Cessation and Training . Very brief advice training module, 2021. Available: www.ncsct.co.uk/vba [Accessed 11 Jan 2021].
- 22.Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (eagles): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016;387:2507–20. 10.1016/S0140-6736(16)30272-0 [DOI] [PubMed] [Google Scholar]
- 23.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 24.Ten Haaf K, Jeon J, Tammemägi MC, et al. Risk prediction models for selection of lung cancer screening candidates: a retrospective validation study. PLoS Med 2017;14:e1002277. 10.1371/journal.pmed.1002277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashraf H, Tønnesen P, Holst Pedersen J, et al. Effect of CT screening on smoking habits at 1-year follow-up in the Danish lung cancer screening trial (DLCST). Thorax 2009;64:388–92. 10.1136/thx.2008.102475 [DOI] [PubMed] [Google Scholar]
- 26.van der Aalst CM, van den Bergh KAM, Willemsen MC, et al. Lung cancer screening and smoking abstinence: 2 year follow-up data from the Dutch-Belgian randomised controlled lung cancer screening trial. Thorax 2010;65:600–5. 10.1136/thx.2009.133751 [DOI] [PubMed] [Google Scholar]
- 27.Marshall HM, Courtney DA, Passmore LH, et al. Brief tailored smoking cessation counseling in a lung cancer screening population is feasible: a pilot randomized controlled trial. Nicotine Tob Res 2016;18:1665–9. 10.1093/ntr/ntw010 [DOI] [PubMed] [Google Scholar]
- 28.Taylor KL, Hagerman CJ, Luta G, et al. Preliminary evaluation of a telephone-based smoking cessation intervention in the lung cancer screening setting: a randomized clinical trial. Lung Cancer 2017;108:242–6. 10.1016/j.lungcan.2017.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tremblay A, Taghizadeh N, Huang J, et al. A randomized controlled study of integrated smoking cessation in a lung cancer screening program. J Thorac Oncol 2019;14:1528–37. 10.1016/j.jtho.2019.04.024 [DOI] [PubMed] [Google Scholar]
- 30.Park ER, Perez GK, Regan S, et al. Effect of sustained smoking cessation counseling and provision of medication vs Shorter-term counseling and medication advice on smoking abstinence in patients recently diagnosed with cancer: a randomized clinical trial. JAMA 2020;324:1406–18. 10.1001/jama.2020.14581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark MM, Cox LS, Jett JR, et al. Effectiveness of smoking cessation self-help materials in a lung cancer screening population. Lung Cancer 2004;44:13–21. 10.1016/j.lungcan.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 32.Ferketich AK, Otterson GA, King M, et al. A pilot test of a combined tobacco dependence treatment and lung cancer screening program. Lung Cancer 2012;76:211–5. 10.1016/j.lungcan.2011.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Philip KEJ, Lonergan B, Cumella A, et al. COVID-19 related concerns of people with long-term respiratory conditions: a qualitative study. BMC Pulm Med 2020;20:319. 10.1186/s12890-020-01363-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hopkinson NS, Rossi N, El-Sayed Moustafa J, et al. Current smoking and COVID-19 risk: results from a population symptom APP in over 2.4 million people. Thorax 2021;76:714–22. 10.1136/thoraxjnl-2020-216422 [DOI] [PubMed] [Google Scholar]
- 35.Clift AK, von Ende A, Tan PS, et al. Smoking and COVID-19 outcomes: an observational and Mendelian randomisation study using the UK Biobank cohort. Thorax 2022;77:65–73. 10.1136/thoraxjnl-2021-217080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kummer S, Waller J, Ruparel M, et al. Mapping the spectrum of psychological and behavioural responses to low-dose CT lung cancer screening offered within a lung health check. Health Expect 2020;23:433–41. 10.1111/hex.13030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joseph AM, Rothman AJ, Almirall D, et al. Lung cancer screening and smoking cessation clinical trials. scale (smoking cessation within the context of lung cancer screening) collaboration. Am J Respir Crit Care Med 2018;197:172–82. 10.1164/rccm.201705-0909CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whittaker R, McRobbie H, Bullen C, et al. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev 2016;4:Cd006611. 10.1002/14651858.CD006611.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masiero M, Lucchiari C, Mazzocco K, et al. E-Cigarettes may support smokers with high smoking-related risk awareness to stop smoking in the short run: preliminary results by randomized controlled trial. Nicotine Tob Res 2019;21:119–26. 10.1093/ntr/nty047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2021-001030supp001.pdf (83.9KB, pdf)
Data Availability Statement
Anonymised research data will be shared with third parties via a request to the senior author (NSH).