Abstract
Background:
Cervical cancer is the fourth most common cancer among women. High parity has long been suspected with an increased risk of cervical cancer. Evidence from the existing epidemiological studies regarding the association between parity and cervical cancer is variable and inconsistent. Therefore, the objective of this systematic review and meta-analysis was to synthesize the best available evidence on the epidemiological association between parity and cervical cancer.
Methods:
Case–control studies reporting the association between parity and cervical cancer were systematically searched in databases like MEDLINE/PubMed, HINARI, Google scholar, Science direct, and Cochrane Libraries. All studies fulfilling the inclusion criteria and published between 2000 and 7 March 2020 were included in this meta-analysis. This study reported according to PRISMA guideline. Cochran’s Q-statistics and I2 tests were performed to assess heterogeneity among included studies. Egger’s regression analysis was performed to assess publication bias. A random-effect meta-analysis model was used to compute pooled odds ratio of the association between parity and cervical cancer.
Results:
A total of 6685 participants (3227 patients and 3458 controls) were incorporated in the 12 studies included in this meta-analysis. The meta-analysis revealed that women with high parity had 2.65 times higher odds of developing cervical cancer compared to their counterparts (odds ratio = 2.65, 95% confidence interval = 2.08–3.38).
Conclusion:
High parity is positively associated with cervical cancer. Strong epidemiological studies are recommended to further explore the mechanisms and role of parity in the causation of cervical cancer.
Keywords: case–control, cervical cancer, meta-analysis, parity, systematic review
Introduction
Cancer is a group of diseases characterized by the uncontrolled growth and spread of abnormal cells. 1 Cervical cancer is cancer that forms in tissues of the cervix, the organ connecting the uterus and the vagina. Cervical cancer is considered nearly completely preventable because of the generally slow progression of the disease and the availability of screening and the Human Papilloma Virus (HPV) vaccine.2,3 In 2018, about 570,000 women developed cervical cancer globally and 311,000 women died from it. 4 HPV is recognized as a necessary cause of cervical cancer.5 –10 However, HPV infection alone is not sufficient to cause cervical cancer and some cofactors modify the progression of the infections to cancer.5,9 Evidence suggests that women’s characteristics like age, number of live births or parity, number of pregnancies, age at first sexual intercourse, age at first pregnancy, history of sexually transmitted infections, having multiple sexual partners, and history of long-term oral contraceptives use play role in developing cervical cancer.11 –20
Previous studies reported a positive association between parity and cervical cancer.11,17,21 –24 Excess risk of cervical cancer among women with high parity is believed to be linked with a high rate of cervical abnormalities during pregnancy,25,26 a high detection rate of HPV among pregnant women,27,28 and some studies also suggest vaginal parity makes local changes to cervical cells due to traumas during birth. 11 Although several previous epidemiological studies documented parity as a risk factor for cervical cancer; the reported strength of association is variable and inconsistent. Therefore, this systematic review and meta-analysis aimed to estimate the pooled odds ratios (ORs) of the association between parity and cervical cancer. It will also highlight the strength of association between parity and cervical cancer which will, in turn, helps to ascertain risks of cervical cancer among women with high parity compared to those with low parity.
Methods
Formulation of the questions
The primary aim of this systematic review and meta-analysis is to determine the strength of association between parity and the risk of cervical cancer.
Search strategies
This review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline 29 (Additional File 1). To get potentially relevant studies, a comprehensive search was performed in the following databases: MEDLINE/Pub Med, HINARI, Google Scholar, Science Direct, and Cochrane Library. The following key terms in combination with Boolean operators were used: ((“parities” (All Fields) OR “parity” (MeSH Terms)) OR “parity” (All Fields)) AND ((“uterine cervical neoplasms” (MeSH Terms) OR ((“uterine” (All Fields) AND “cervical” (All Fields)) AND “neoplasms”(All Fields))) OR “uterine cervical neoplasms” (All Fields)). To ensure a comprehensive search of the literature, reference lists of included studies were scanned.
Inclusion criteria
Population: females of reproductive age and above at risk for cervical cancer.
Exposure of interest: parity.
Outcome of interest: cervical cancer.
Study designs: case–control studies examining the association between parity and cervical cancer were included in this review. Besides, the OR examining the association between parity and cervical cancer shall be given from the original studies to be considered for inclusion into the meta-analysis. Systematic reviews, cross-sectional, case-report, case-series, opinion reports, letters to the editor, short communications, and qualitative studies were excluded.
Setting: this systematic review and meta-analysis included all studies reporting the association between parity and cervical cancer regardless of their study areas.
Time frame: this review included all studies published from January 2000 to March 2020. An electronic database search was conducted from 6 February 2020 to 7 March 2020.
Publication condition: this review included articles published in peer-reviewed journals.
Language: only articles reported in English were considered.
Exclusion criteria
Studies with the following characteristics were excluded from this systematic review and meta-analysis:
Studies whose full text and data were inaccessible.
Studies which did not report the confounder adjusted OR of the association between parity and cervical cancer.
Studies conducted on precancerous lesion of cervix.
Qualitative studies, reviews, commentaries, editorials, letters, interventional studies, and other opinion papers.
Study selection
All identified articles through electronic databases were imported to EndNote X4 software. After removing duplicate articles, two authors (Y.T. and B.S.) independently screened all articles by their title, abstract, and full texts for their eligibility against the predetermined inclusion and exclusion criteria. Subsequently, identified articles were compiled together and discrepancies between the two authors were resolved through discussion to reach a consensus.
Risk of bias
We used Joanna Briggs Institute’s (JBI) Critical appraisal checklist for case–control studies. 30 The tool composed of 10 parameters: (1) Were the groups comparable other than the presence of disease in cases or absence of disease in controls? (2) Were cases and controls matched appropriately? (3) Were the same criteria used for the identification of cases and controls? (4) Was exposure measured in a standard, valid and reliable way? (5) Was exposure measured in the same way for cases and controls? (6) Were confounding factors identified? (7) Were strategies to deal with confounding factors stated? (8) Were outcomes assessed in a standard, valid and reliable way for cases and controls? (9) Was the exposure period of interest long enough to be meaningful? (10) Was appropriate statistical analysis used? Two authors (Y.T. and B.S.) evaluated the risk of bias of the full text considered to be included in the meta-analysis. Any disagreement between two authors was resolved through discussion. The overall risk of bias was then scored according to the number of high risks of bias per study: low (⩽2), moderate,3,4 and high (⩾5) (Additional File 2).
Data extraction
Data were extracted on Microsoft Office Excel spreadsheet. The data extraction format is composed of the primary author’s name, year of publication, study period, country, study design, study setup, number of cases, number of controls, OR, and 95% confidence intervals (CIs) for the association between parity and cervical cancer. Two authors (Y.T. and B.S.) independently extracted the information. Any discrepancies were resolved through discussion.
Statistical analysis
Extracted data were imported into STATA version 14 software (StataCorp LP.2015, College Station, TX, USA) to perform all statistical analyses. First, ORs were obtained from data reported in the original studies. Then, confounder adjusted ORs were pooled using generic inverse variance method by converting adjusted OR on logarithmic scale and back calculating standard error (SE) based on the 95% CIs. Heterogeneity between studies was assessed using Cochran’s Q-statistics and I 2 test. In this meta-analysis, the test statistics indicated the presence of significant heterogeneity (I 2 = 78.4%, p < 0.001). For this reason, the ORs were pooled using random-effect meta-analysis techniques (DerSimonian and Liard method), which accounts for the variation between studies. The pooled ORs along with their 95% CIs were presented using a forest plot. Subgroup analyses were conducted by countries of original studies, years of publication, confounders adjusted in multivariate analysis, and definitions of high parity on each original study. Univariate meta-regression analyses were also conducted to identify possible sources of heterogeneity. Variables considered in meta-regression were years of publication, study setups (hospital vs cancer registry), age of participants, factors adjusted as confounding variables, and definition of parity used in each original study. We also conducted sensitivity analysis using random-effect model to assess the effect single study on the pooled estimate. Publication bias of the meta-analysis was assessed using Egger’s test statistics, and there was no statistically significant publication bias (p-value = 0.2).
Operational definitions
Cervical cancer: in this study, authors included studies that diagnosed cervical cancer through the histological confirmation of cancer.
Parity: parity is defined as the number of times that a woman has given birth to a fetus with a gestational age of 24 weeks or more, regardless of whether the child was born alive or was stillborn.
Results
Description of study selection
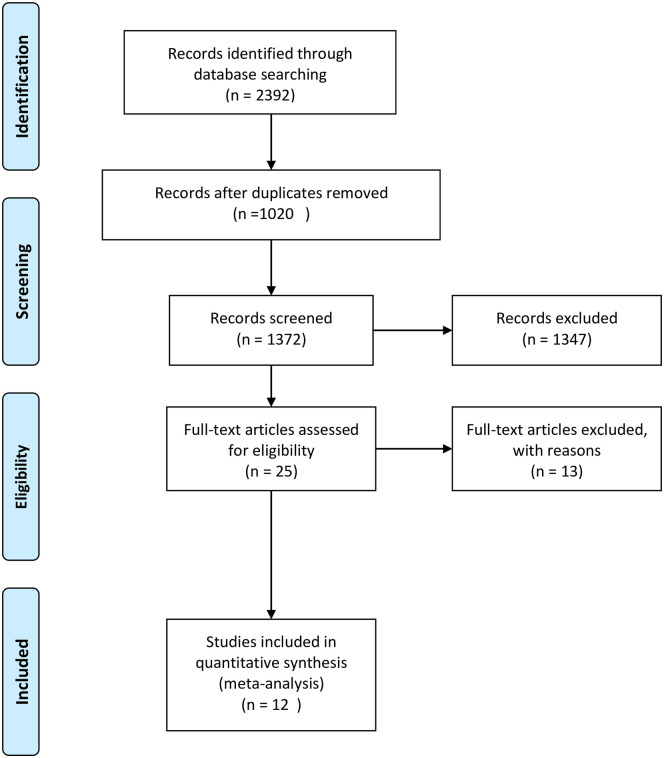
A total of 2392 studies were identified through all databases described above. Of these, 1020 duplicate studies were removed. After screening by their title and abstract, 1345 studies were excluded. Then, 27 studies were assessed for eligibility based on predefined eligibility criteria and risk of bias assessment. Further, 15 studies were excluded31 –45 due to inaccessibility of full text, full articles reported in languages other than English, and the outcome of interest is not reported separately (Additional File 3). Finally, 12 studies were included in this meta-analysis.13,17,46 –55 None of the primary studies included in the current review are type specific for high parity and its relation to cervical cancer (Figure 1).
Figure 1.
PRISMA flowchart of the article selection process for systematic review and meta-analysis of the association between cervical cancer and parity.
Characteristics of included studies
As described in Table 1, a total of 12 studies were included in this systematic review and meta-analysis. A total of 6685 participants (3227 patients and 3458 controls) were incorporated in the 12 articles included in the final meta-analysis. One study reported an association of parity with both adenocarcinoma and squamous cell carcinoma of the cervix; 56 hence, both results were included in the meta-analysis. All of the included articles were case–control studies. Of all studies included, one study was from China, one from Côte d’Ivoire, one study from Ethiopia, three studies from India, two studies from Indonesia, one article from Taiwan, one article from Thailand, one article from the United Kingdom, and one study from the United States. Regarding the year of publications, the earliest article included in this meta-analysis was published in 2003,50,51 and the latest was published in 2019.47,53 As described in Table 2, among the 12 studies that examined the association between parity and cervical cancer, 10 study reported positive association between high parity and cervical cancer.13,17,46 –48,50,51,53 –55
Table 1.
List of studies included in the systematic review and meta-analysis of the association between parity and risk of cervical cancer, 2020.
| Primary author | Year of publication | Study period | Country | Study setups/source | Study design | Number of cases | Number of controls | Age range (years) |
|---|---|---|---|---|---|---|---|---|
| Cai et al. 48 | 2008 | 2003–2005 | China | Hospital | Case–control | 110 | 110 | 22–72 |
| Adjorlolo-Johnson et al. 46 | 2010 | April 1997 to October 1999 | Côte d’Ivoire | Hospital | Case–control | 132 | 120 | 18–70 |
| Bezabih et al. 13 | 2015 | April 1 to 30 September 2010 | Ethiopia | Hospital | Case–control | 60 | 120 | Unreported |
| Franceschi et al. 50 | 2003 | June 1998 to May 1999 | India | Hospital | Case–control | 193 | 210 | Unreported |
| Sharma and Pattanshetty 54 | 2018 | Unreported | India | Hospital | Case–control | 91 | 182 | 20–80 |
| Thakur et al. 55 | 2015 | July 2008 to October 2009 | India | Hospital | Case–control | 226 | 226 | Unreported |
| Arfailasufandi et al. 47 | 2019 | October to December 2018 | Indonesia | Hospital | Case–control | 100 | 100 | Unreported |
| Putri et al. 53 | 2019 | March 2016 to August 2016 | Indonesia | Hospital | Case–control | 60 | 60 | 21–30 |
| Chen et al. 49 | 2005 | 1986–1992 | Taiwan | Hospital | Case–control | 45 | 54 | <36 |
| Natphopsuk et al. 52 | 2012 | February 2009 to August 2011 | Thailand | Hospital | Case–control | 177 | 177 | 27–81 |
| Green et al. 51 | 2003 | 1984–1989 | The United Kingdom | Cancer registry | Case–control | 180 | 923 | 20–44 |
| Green et al. 51 | 2003 | The United Kingdom | Cancer registry | Case–control | 180 | 923 | 20–44 | |
| Muñoz et al. 17 | 2002 | Unreported | Multicenter | Hospital | Case–control | 1673 | 253 | Unreported |
Table 2.
Primary studies with available adjusted odds ratios of the association between parity and cervical cancer.
| Primary author | Year of publication | Parity | AOR (95% CI) | Adjusted confounders |
|---|---|---|---|---|
| Adjorlolo-Johnson et al. 46 | 2010 | >2 | 5.1 (1.2–21.9) | a |
| Arfailasufandi et al. 47 | 2019 | ⩾3 | 3.94 (1.47–10.59) | Unreported |
| Bezabih et al. 13 | 2015 | 3 to 4 | 4.7 (0.8–27.2) | Unreported |
| Bezabih et al. 13 | 2015 | >4 | 12.4 (2.4–64.2) | |
| Cai et al. 48 | 2008 | 2 | 6.05 (0.93–38.59) | Unreported |
| Cai et al. 48 | 2008 | 3 | 9.06 (1.32–62.52) | |
| Cai et al. 48 | 2008 | >3 | 16.82 (18.1–150.95) | |
| Chen et al. 49 | 2005 | ⩾3 | 4.18 (0.71–24.69) | Unreported |
| Franceschi et al. 50 | 2003 | 0 | 0.5 (0.1–2.1) | b |
| Franceschi et al. 50 | 2003 | 3–4 | 2.6 (1.6–4.3) | |
| Franceschi et al. 50 | 2003 | 5–6 | 5.7 (3.0–11.1) | |
| Franceschi et al. 50 | 2003 | ⩾7 | 5.7 (2.4–13.3) | |
| Green et al. 51 | 2003 | 1 | 1.27 (0.69–2.34) | c |
| Green et al. 51 | 2003 | 2 | 1.14 (0.63–2.05) | |
| Green et al. 51 | 2003 | ⩾3 | 1.44 (0.76–2.73) | |
| Green et al. 51 | 2003 | 1 | 0.88 (0.55–1.4) | |
| Green et al. 51 | 2003 | 2 | 1.41 (0.92–2.17) | |
| Green et al. 51 | 2003 | ⩾3 | 1.86 (1.16–2.99) | |
| Natphopsuk et al. 52 | 2012 | ⩾3 | 1.63 (0.62–4.28) | Unreported |
| Putri et al. 53 | 2019 | ⩾3 | 2.89 (1.18–7.1) | Unreported |
| Sharma and Pattanshetty 54 | 2018 | 3–5 | 4.66 (2.04–10.66) | Unreported |
| Sharma and Pattanshetty 54 | 2018 | ⩾6 | 10.12 (4.33–23.87) | |
| Thakur et al. 55 | 2015 | ⩾3 | 1.7 (1.25–2.65) | Unreported |
| Muñoz et al. 17 | 2002 | 1–2 | 1.81 (1.31–2.52) | d |
| Muñoz et al. 17 | 2002 | 3–4 | 2.55 (1.95–3.34) | |
| Muñoz et al. 17 | 2002 | 5–6 | 2.83 (2.02–3.96) | |
| Muñoz et al. 17 | 2002 | ⩾7 | 3.82 (2.66–5.48) |
AOR: adjusted odds ratio; CI: confidence interval.
indicates age, low socioeconomic status, and lifetime number of sex partners.
indicates age and area of residence.
indicates age, recruitment center, age at first intercourse, duration of oral contraceptive use, level of education, number of negative screening results, smoking status and total number of sexual partners.
indicates study center, age, education, smoking status, age at first intercourse, number of sexual partners, oral contraceptive use, and history of Papanicolaou’s smears.
Association between parity and cervical cancer
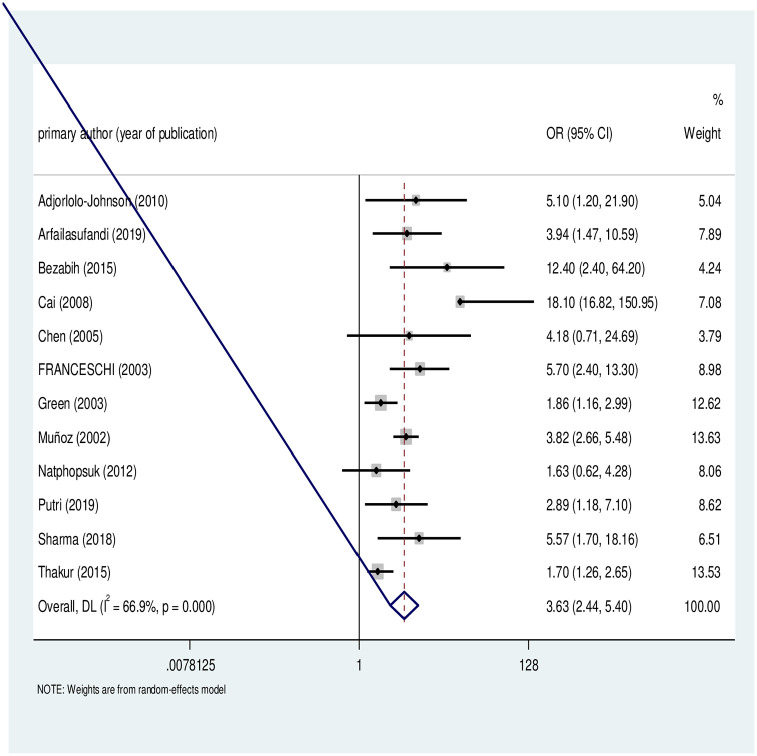
As describe in Figure 2, 12 case–control studies were included in this meta-analysis to determine the association between parity and cervical cancer. The studies exhibited significant heterogeneity (I 2 = 74.6, p < 0.001). Hence, a random-effect meta-analysis model was used to estimate the pooled OR. This meta-analysis revealed that parity is significantly associated with cervical cancer. The likelihood of developing cervical cancer was more than two times higher among women with high parity compared to their counterparts (OR = 2.65, 95% CI = 2.08–3.38).
Figure 2.
Forest plot of the individual and pooled odds ratios (OR) of association between cervical cancer and parity.
Sensitivity analysis for the association between parity and cervical cancer
To estimate the effect of individual study on the pooled estimate of the association between parity and cervical cancer, we performed sensitivity analysis using random-effect model. Based on the result, single study has no significant effect on the pooled estimate. The pooled estimated OR ranged between 2.50 (1.97–3.17) 54 and 2.77 (2.19–3.51) 51 after omitting each study (Figure 3).
Figure 3.
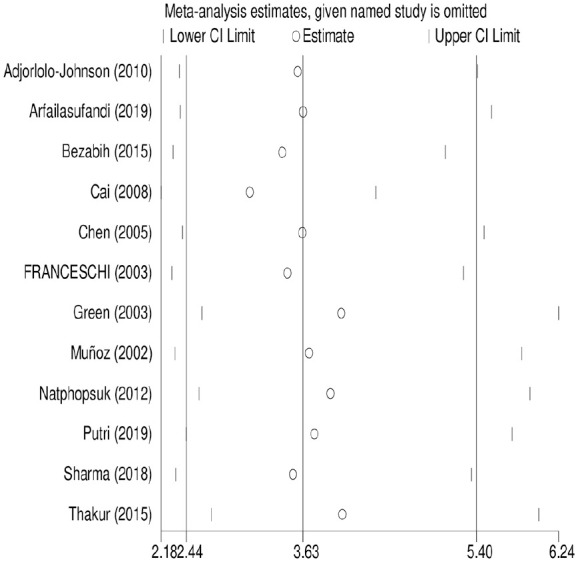
Sensitivity analysis for the pooled estimate of the association between parity and cervical cancer.
Exploration of heterogeneity and subgroup analysis
Meta-regression analysis was employed to assess potential sources of heterogeneity using factors like years of publication, study setups (hospital vs registry), age of participants, factors adjusted as confounding variables, and definition of parity used in each original study. Accordingly, study setup and factors adjusted for confounder in multivariate model were found to be significant sources of heterogeneity (Table 3). Furthermore, subgroup analysis was conducted by countries of primary studies, year of publications, study setup, and factors adjusted as confounder in multivariate models of original studies (Table 4).
Table 3.
Meta-regression of factors associated with the heterogeneity of the studies included in estimating the pooled effect of parity on cervical cancer.
| Variables | OR (95% CI) | p-value |
|---|---|---|
| Year of publication | 1.0 (1.001–1.1) | 0.04* |
| Age of participants | 0.84 (0.68–1.02) | 0.08 |
| Study setup | 0.39 (0.22–0.66) | 0.001* |
| Definition of parity | 1.02 (0.94–1.10) | 0.5 |
| Factors adjusted for confounder | 0.77 (0.60–0.98) | 0.03* |
OR: odds ratio; CI: confidence interval.
Significant at p < 0.05.
Table 4.
Subgroup analysis of the association between parity and cervical cancer.
| Variables | Subgroup | Number of studies | AOR (95% CI) | Heterogeneity across the studies | Heterogeneity between groups (p-value) | |
|---|---|---|---|---|---|---|
| I2 (%) | p-value | |||||
| Country | China | 1 | 12.6 (5.39–29.46) | 0 | <0.001 | <0.001 |
| Côte d’Ivoire | 1 | 5.1 (1.2–21.9) | 0 | <0.001 | ||
| Ethiopia | 1 | 7.9 (2.37–26.28) | 0 | 0.43 | ||
| India | 3 | 3.45 (1.95–6.12) | 79.4 | <0.001 | ||
| Indonesia | 2 | 3.32 (1.71–6.46) | 0 | 0.64 | ||
| Multicenter | 1 | 2.64 (1.99–3.5) | 67.8 | 0.025 | ||
| Taiwan | 1 | 4.18 (0.71–24.65) | 0 | – | ||
| Thailand | 1 | 1.63 (0.62–4.28) | 0 | – | ||
| The United Kingdom | 1 | 1.30 (1.05–1.62) | 5.8 | 0.37 | ||
| Year of publication | 2000–2010 | 6 | 2.39 (1.80–3.16) | 77.1 | <0.001 | 0.16 |
| 2011–2020 | 6 | 3.71 (2.12–6.51) | 68.4 | 0.002 | ||
| Study setups | Hospital | 11 | 3.45 (2.66–5.48) | 65.8 | 74.6 | <0.001 |
| Registry | 1 | 1.30 (1.04–1.62) | 5.8 | – | ||
| Factors adjusted in multivariate model | a | 1 | 5.1 (1.19–21.79) | 0 | – | <0.001 |
| b | 1 | 3.11 (1.47–6.58) | 72.8 | 0.012 | ||
| c | 1 | 1.3 (1.05–1.62) | 5.8 | 0.37 | ||
| d | 1 | 2.64 (1.99–3.5) | 67.8 | 0.025 | ||
| e | 8 | 4.67 (2.74–7.96) | 68.5 | <0.001 | ||
AOR: adjusted odds ratio; CI: confidence interval.
indicates age, low socioeconomic status, and lifetime number of sex partners.
indicates age and area of residence.
indicates age, recruitment center, age at first intercourse, duration of oral contraceptive use, level of education, number of negative screening results, smoking status, and total number of sexual partners.
indicates study center, age, education, smoking status, age at first intercourse, number of sexual partners, oral contraceptive use, and history of Papanicolaou’s smears.
Publication bias
In this meta-analysis, funnel plot and Egger’s test were used to assess presence of publication bias. However, the results show no publication bias at 95% confidence level (p-value = 0.1) (Figure 4).
Figure 4.
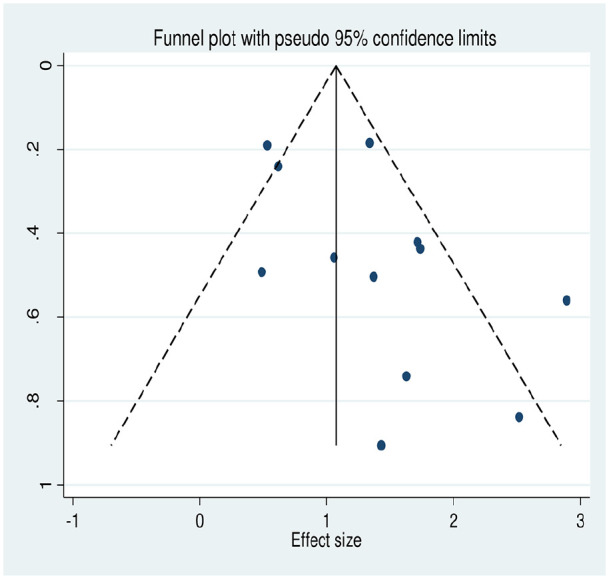
Funnel plot for the meta-analysis of the association between parity and cervical cancer.
Discussion
Cervical cancer is believed to be cancer emerging from infectious disease origin. 57 The HPV two types specifically, HPVs 16 and 18, explains approximately about 70% of cervical cancer cases. 58 Despite the fact HPV infection is the necessary cause in the etiology of cervical cancer, HPV infection alone is not a sufficient cause for the occurrence of the cases. 5 Several epidemiological studies investigated the role of different demographic, sexual, and reproductive factors in the progression of HPV infection into cervical carcinoma.11,17,39,59 –63 This systematic review and meta-analysis investigated the pooled OR of the association between multiple parity and cervical cancer.
In this meta-analysis, high parity is associated with a higher risk of cervical cancer. This finding is supported by the multicenter case–control study conducted by the International Agency for Research on Cancer (IARC). This multicenter study reported that nulliparous women were at lower risk of cervical cancer, whereas there were clear trends of increased risk of cervical cancer as the number of full-term pregnancies increased among parous women. 17 Several epidemiological studies also reported a positive association between parity and cervical cancer.39 –41,64,65
The previous studies reported an association between full-term pregnancy and cervical cancer. The possible explanations were concentrations of estrogen and progesterone level in blood are known to increase during pregnancy and reach the highest levels in the last weeks of gestation. These hormonal changes are perhaps responsible for the alterations in the junction between the squamous and columnar epithelium (transformation zone) occurring during pregnancy. Squamous metaplasia of the transformation zone also increases during pregnancy to reach a maximum during the third trimester. 66
Some other studies have also explained the association between multiple pregnancies and cervical cancer could be due to high detection of cervical abnormalities among pregnant women,25,26 probably due to migration of endocervix during pregnancy. 67 There are also assumptions that traumas to the uterine cervix during vaginal delivery might be a possible explanation for the positive association between cervical cancer and parity.22,68 Cesarean delivery was not associated with cervical cancer as vaginal delivery does, which might strengthen the speculation that traumas during the vaginal delivery might increase the risks. 17
A large cohort study conducted in Taiwan reported that high vaginal parity is not a sufficient cause by itself unless that women also HPV infected. They explained that if the woman is HPV infected and had high vaginal parity, the virus can easily integrate due to the birth traumas, and the risk of cervical cancer increases. However, if the woman is not HPV infected, vaginal parity does not make difference whether it is high or low because birth trauma can heal by itself. 69 Similarly, a multicenter case–control study by IARC reported that women with baseline HPV infection and multiple pregnancies had a higher risk of developing cervical cancer compared to women with a low number of pregnancies. 17
Even though several epidemiological studies examined the association between cervical cancer and different reproductive characteristics of women, the role of high parity and mechanisms in the causation of cervical cancer is unclear. There are several hypotheses regarding the effect of parity in the development of cervical cancer. A few studies suggest that vaginal parity could cause trauma to the cervix which could be responsible for cervical cancer developments and some other studies justified the role of parity by explaining hormonal changes during pregnancy might be responsible for the changes in cervical cells. There are also studies speculating high parity might be associated with a longer duration of oral contraceptive use 68 which might, in turn, leads to cervical cancer development. Despite there are debates regarding the mechanism and role of parity in the development of cervical cancer, there is plenty of strong evidence which supports the positive association between parity and cervical cancer.
Limitations
This systematic review and meta-analysis has several limitations and results should be interpreted considering the following points. First, this meta-analysis did not examine the effect of vaginal or cesarean parity separately. Also, this study did not explore separately the interaction between HPV infection and high parity on cervical cancer development. This meta-analysis included case–control studies which were published in the English language only and none of the included studies are cohort and exposure-specific primary studies. Finally, due to differences in definitions of high parity across studies, the pooled OR is not directly interpretable as a relative risk associated with a given number of births, but rather an indicator of the mean trend across studies which have examined high parity as a risk factor for cervical using different definitions.
Conclusion
This meta-analysis revealed that parity is positively associated with cervical cancer risks. Women with high parity had higher odds of developing cervical cancer compared to those with relatively low parity. Epidemiological studies with strong designs are recommended to examine the mechanisms and role of parity in the causation of cervical cancers.
Supplemental Material
Supplemental material, sj-docx-1-whe-10.1177_17455065221075904 for High parity is associated with increased risk of cervical cancer: Systematic review and meta-analysis of case–control studies by Yohannes Tekalegn, Biniyam Sahiledengle, Demelash Woldeyohannes, Daniel Atlaw, Sisay Degno, Fikreab Desta, Kebebe Bekele, Tesfaye Aseffa, Habtamu Gezahegn and Chala Kene in Women’s Health
Supplemental material, sj-docx-3-whe-10.1177_17455065221075904 for High parity is associated with increased risk of cervical cancer: Systematic review and meta-analysis of case–control studies by Yohannes Tekalegn, Biniyam Sahiledengle, Demelash Woldeyohannes, Daniel Atlaw, Sisay Degno, Fikreab Desta, Kebebe Bekele, Tesfaye Aseffa, Habtamu Gezahegn and Chala Kene in Women’s Health
Supplemental material, sj-xlsx-2-whe-10.1177_17455065221075904 for High parity is associated with increased risk of cervical cancer: Systematic review and meta-analysis of case–control studies by Yohannes Tekalegn, Biniyam Sahiledengle, Demelash Woldeyohannes, Daniel Atlaw, Sisay Degno, Fikreab Desta, Kebebe Bekele, Tesfaye Aseffa, Habtamu Gezahegn and Chala Kene in Women’s Health
Footnotes
Author contribution(s): Yohannes Tekalegn: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing.
Biniyam Sahiledengle: Conceptualization; Formal analysis; Methodology; Validation; Visualization; Writing—review & editing.
Demelash Woldeyohannes: Validation; Visualization; Writing—review & editing.
Daniel Atlaw: Validation; Visualization; Writing—review & editing.
Sisay Degno: Validation; Visualization; Writing—review & editing.
Fikreab Desta: Validation; Visualization; Writing—review & editing.
Kebebe Bekele: Validation; Visualization; Writing—review & editing.
Tesfaye Aseffa: Validation; Visualization; Writing—review & editing.
Habtamu Gezahegn: Validation; Visualization; Writing—review & editing.
Chala Kene: Validation; Visualization; Writing—review & editing.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Yohannes Tekalegn  https://orcid.org/0000-0001-6628-8180
https://orcid.org/0000-0001-6628-8180
Biniyam Sahiledengle  https://orcid.org/0000-0002-1114-4849
https://orcid.org/0000-0002-1114-4849
Daniel Atlaw  https://orcid.org/0000-0002-2968-4958
https://orcid.org/0000-0002-2968-4958
Chala Kene  https://orcid.org/0000-0003-1037-7496
https://orcid.org/0000-0003-1037-7496
Availability of data and materials: All relevant data are within the manuscript and its supporting information files.
Supplemental material: Supplemental material for this article is available online.
References
- 1. American Cancer Society. Cancer facts & figures 2018. Atlanta, GA: American Cancer Society, 2018. [Google Scholar]
- 2. American College of Obstetricians and Gynecologists, Committee on Adolescent Health Care, Immunization Expert Work Group. Committee opinion no. 641: human papillomavirus vaccination. Obstet Gynecol 2015; 126: e38–e43. [DOI] [PubMed] [Google Scholar]
- 3. Bernheim J, Bouche G, Jezdic S, et al. Cervical cancer: a guide for patients—information based on ESMO clinical practice guidelines. ESMO Anticancer Fund, 2012, https://www.esmo.org/content/download/19409/330414/file/Cervical-Cancer-Guide-for-Patients-RCT-ESMO.pdf
- 4. Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 2020; 8(2): e191–e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bosch FX, De Sanjosé S. Chapter 1: human papillomavirus and cervical cancer —burden and assessment of causality. J Natl Cancer Inst Monogr 2003; 31: 3–13. [DOI] [PubMed] [Google Scholar]
- 6. Waggoner SE. Cervical cancer. Lancet 2003; 361(9376): 2217–2225. [DOI] [PubMed] [Google Scholar]
- 7. Munoz N. Human papillomavirus and cancer: the epidemiological evidence. J Clin Virol 2000; 19(1–2): 1–5. [DOI] [PubMed] [Google Scholar]
- 8. Bosch FX, Lorincz A, Muñoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55(4): 244–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bosch FX, De Sanjosé S. The epidemiology of human papillomavirus infection and cervical cancer. Dis Markers 2007; 23(4): 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steben M, Duarte-Franco E. Human papillomavirus infection: epidemiology and pathophysiology. Gynecol Oncol 2007; 107(2Suppl. 1): S2–S5. [DOI] [PubMed] [Google Scholar]
- 11. Castellsagué X, Munoz N. Chapter 3: cofactors in human papillomavirus carcinogenesis—role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr 2003; 31: 20–28. [PubMed] [Google Scholar]
- 12. Castellsague X, Bosch FX, Munoz N. Environmental co-factors in HPV carcinogenesis. Virus Res 2002; 89(2): 191–199. [DOI] [PubMed] [Google Scholar]
- 13. Bezabih M, Tessema F, Sengi H, et al. Risk factors associated with invasive cervical carcinoma among women attending Jimma University Specialized Hospital, Southwest Ethiopia: a case control study. Ethiop J Health Sci 2015; 25(4): 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Teame H, Gebremariam L, Kahsay T, et al. Factors affecting utilization of cervical cancer screening services among women attending public hospitals in Tigray region, Ethiopia, 2018; case control study. PLoS One 2019; 14(3): e0213546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Getinet M, Gelaw B, Sisay A, et al. Prevalence and predictors of Pap smear cervical epithelial cell abnormality among HIV-positive and negative women attending gynecological examination in cervical cancer screening center at Debre Markos referral hospital, East Gojjam, Northwest Ethiopia. BMC Clin Pathol 2015; 15: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moreno V, Bosch FX, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case–control study. Lancet 2002; 359(9312): 1085–1092. [DOI] [PubMed] [Google Scholar]
- 17. Munoz N, Franceschi S, Bosetti C, et al. Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case–control study. Lancet 2002; 359(9312): 1093–1101. [DOI] [PubMed] [Google Scholar]
- 18. Muwonge R, Ngo Mbus L, Ngoma T, et al. Socio-demographic and reproductive determinants of cervical neoplasia in seven sub-Sahara African countries. Cancer Causes Control 2016; 27(12): 1437–1446. [DOI] [PubMed] [Google Scholar]
- 19. Vaccarella S, Herrero R, Dai M, et al. Reproductive factors, oral contraceptive use, and human papillomavirus infection: pooled analysis of the IARC HPV prevalence surveys. Cancer Epidemiol Biomarkers Prev 2006; 15(11): 2148–2153. [DOI] [PubMed] [Google Scholar]
- 20. Reproductive factors, oral contraceptive use, and human papillomavirus infection: pooled analysis of the IARC HPV prevalence surveys. Cancer Epidemiol Biomarkers Prev 2006; 15(11): 2148–2153. [DOI] [PubMed] [Google Scholar]
- 21. Hinkula M, Pukkala E, Kyyrönen P, et al. A population-based study on the risk of cervical cancer and cervical intraepithelial neoplasia among grand multiparous women in Finland. Br J Cancer 2004; 90(5): 1025–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brinton LA, Reeves WC, Brenes MM, et al. Parity as a risk factor for cervical cancer. Am J Epidemiol 1989; 130(3): 486–496. [DOI] [PubMed] [Google Scholar]
- 23. International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical carcinoma and reproductive factors: collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. Int J Cancer 2006; 119(5): 1108–1124. [DOI] [PubMed] [Google Scholar]
- 24. Abacjew-Chmyłk AO, Chmyłko Ł, Wydra DG, et al. Multiple multiparity is a negative prognostic factor for endometrial cancer in Poland. Ginekol Pol 2016; 87(3): 178–182. [DOI] [PubMed] [Google Scholar]
- 25. MacGregor JE, Teper S. Uterine cervical cytology and young women. Lancet 1978; l : 1029–1031. [DOI] [PubMed] [Google Scholar]
- 26. Orr JW, Shingleton HM. Cancer in pregnancy. Chicago, IL: Year Book Medical Publishers, Inc., 1983. [Google Scholar]
- 27. Garry R, Jones R. Relationship between cervical condylomata, pregnancy and subclinical papilloma virus infection. J Reprod Med 1985; 5: 393–399. [PubMed] [Google Scholar]
- 28. Schneider A, Hotz M, Gissmann L. Increased prevalence o f human papilloma viruses in the lower genital tract of pregnant women. Int J Cancer 1987; 40: 198–201. [DOI] [PubMed] [Google Scholar]
- 29. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015; 349: g7647. [DOI] [PubMed] [Google Scholar]
- 30. Moola S, Munn Z, Tufanaru C, et al. Chapter 7: systematic reviews of etiology and risk. JBI Manual for Evidence Synthesis, 2020, https://jbi-global-wiki.refined.site/space/MANUAL/3283910762/Chapter+7%3A+Systematic+reviews+of+etiology+and+risk
- 31. Atalah E, Urteaga C, Rebolledo A, et al. Diet, smoking and reproductive history as risk factors for cervical cancer. Rev Med Chil 2001; 129(6): 597–603. [PubMed] [Google Scholar]
- 32. Delgado-Enciso I, Martinez-Garza SG, Rojas-Martinez A, et al. [The effect of MTHFR polymorphisms, pregnancy and first intercourse on cervical cancer in a population from the Northeastern Mexico]. Rev Invest Clin 2006; 58(5): 462–469. [PubMed] [Google Scholar]
- 33. Aziz MF. Gynecological cancer in Indonesia. J Gynecol Oncol 2009; 20(1): 8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baskaran K, Kumar PK, Santha K, et al. Cofactors and their association with cancer of the uterine cervix in women infected with high-risk human papillomavirus in South India. Asian Pac J Cancer Prev 2019; 20(11): 3415–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bayo S, Bosch FX, De Sanjose S, et al. Risk factors of invasive cervical cancer in Mali. Int J Epidemiol 2002; 31(1): 202–209. [DOI] [PubMed] [Google Scholar]
- 36. Berraho M, Amarti-Riffi A, El-Mzibri M, et al. HPV and cofactors for invasive cervical cancer in Morocco: a multicentre case-control study. BMC Cancer 2017; 17(1): 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bhojani KR, Garg R. Cytopathological study of cervical smears and correlation of findings with risk factors. Int J Biol Med Res 2011; 2(3): 757–761. [Google Scholar]
- 38. Crauciuc E, Toma O, Crauciuc D, et al. The cervical cancer and its obstetrical antecedents. Analele Ştiinţ Ale Univ Alexandru Ioan Cuza Din Iașisectiunea II Genet Si Biol Mol 2011; 12(2): 17–24. [Google Scholar]
- 39. Luhn P, Walker J, Schiffman M, et al. The role of co-factors in the progression from human papillomavirus infection to cervical cancer. Gynecol Oncol 2013; 128(2): 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matos A, Moutinho J, Pinto D, et al. The influence of smoking and other cofactors on the time to onset to cervical cancer in a southern European population. Eur J Cancer Prev 2005; 14(5): 485–491. [DOI] [PubMed] [Google Scholar]
- 41. Paramita S, Soewarto S, Widodo MAA, et al. High parity and hormonal contraception use as risk factors for cervical cancer in East Kalimantan. Med J Indones 2010; 19: 268–272. [Google Scholar]
- 42. Pereira CRN, Rosa MLG, Vasconcelos Faria PC, et al. Human papillomavirus prevalence and predictors for cervical cancer among high-risk women from Rio de Janeiro, Brazil. Int J Gynecol Cancer 2007; 17(3): 651–660. [DOI] [PubMed] [Google Scholar]
- 43. Thulaseedharan JV, Malila N, Hakama M, et al. Effect of screening on the risk estimates of socio demographic factors on cervical cancer—a large cohort study from rural India. Asian Pac J Cancer Prev 2013; 14(1): 589–594. [DOI] [PubMed] [Google Scholar]
- 44. Nesrin R, Kilic D. Risk factors for cervical cancer: results from a hospital-based case-control study. Int J Hematol Oncol 2011; 28(4): 153–159. [Google Scholar]
- 45. Shields TS, Brinton LA, Burk RD, et al. A case-control study of risk factors for invasive cervical cancer among U.S. women exposed to oncogenic types of human papillomavirus. Cancer Epidemiol Biomarkers Prev 2004; 13(10): 1574–1582. [PubMed] [Google Scholar]
- 46. Adjorlolo-Johnson G, Unger ER, Boni-Ouattara E, et al. Assessing the relationship between HIV infection and cervical cancer in Cote d’Ivoire: a case-control study. BMC Infect Dis 2010; 10(1): 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arfailasufandi R, Mudigdo A, Sudiyanto A. The effect of obesity, oral contraceptive and passive smoking on the risk of cervical cancer. J Epidemiol Public Health 2019; 4(3): 189–197. [Google Scholar]
- 48. Cai HB, Ding XH, Zhou YF, et al. Risk factors for cervical cancer in China: a case-control study. Eur J Gynaecol Oncol 2008; 29(1): 72–75. [PubMed] [Google Scholar]
- 49. Chen T-C, Lee J-Y, Wang S-Y, et al. Relevant factors for cervical cancer among young women in Taiwan. Taiwan J Obstet Gynecol 2005; 44(2): 143–147. [Google Scholar]
- 50. Franceschi S, Rajkumar T, Vaccarella S, et al. Human papillomavirus and risk factors for cervical cancer in Chennai, India: a case-control study. Int J Cancer 2003; 107(1): 127–133. [DOI] [PubMed] [Google Scholar]
- 51. Green J, Berrington De, Gonzalez A, Sweetland S, et al. Risk factors for adenocarcinoma and squamous cell carcinoma of the cervix in women aged 20–44 years: the UK National Case-Control Study of Cervical Cancer. Br J Cancer 2003; 89(11): 2078–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Natphopsuk S, Settheetham-Ishida W, Sinawat S, et al. Risk factors for cervical cancer in northeastern Thailand: detailed analyses of sexual and smoking behavior. Asian Pac J Cancer Prev 2012; 13(11): 5489–5495. [DOI] [PubMed] [Google Scholar]
- 53. Putri AR, Khaerunnisa S, Yuliati I. Cervical cancer risk factors association in patients at the gynecologic-oncology clinic of Dr. Soetomo Hospital Surabaya. Indones J Cancer 2019; 13(4): 104–109. [Google Scholar]
- 54. Sharma P, Pattanshetty SM. A study on risk factors of cervical cancer among patients attending a tertiary care hospital: a case-control study. Clin Epidemiol Glob Health 2018; 6(2): 83–87. [Google Scholar]
- 55. Thakur A, Gupta B, Gupta A, et al. Risk factors for cancer cervix among rural women of a hilly state: a case-control study. Indian J Public Health 2015; 59(1): 45–48. [DOI] [PubMed] [Google Scholar]
- 56. Green J, Berrington De, Gonzalez A, Smith JS, et al. Human papillomavirus infection and use of oral contraceptives. Br J Cancer 2003; 88(11): 1713–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Viscidi RP, Shah KV. Chapter 156—papillomaviruses. In: Cohen J, Opal SM, Powderly WG. (eds) Infectious diseases. 3rd ed. London: Mosby, 2010, pp. 1565–1569, https://www.sciencedirect.com/science/article/pii/B9780323045797001568 [Google Scholar]
- 58. Castellsagué X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol 2008; 110(3Suppl. 2): S4–S7. [DOI] [PubMed] [Google Scholar]
- 59. Cox JT. The development of cervical cancer and its precursors: what is the role of human papillomavirus infection? Curr Opin Obstet Gynecol 2006; 18(Suppl. 1): s5–s13. [DOI] [PubMed] [Google Scholar]
- 60. Franco EL, Duarte-Franco E, Ferenczy A. Cervical cancer: epidemiology, prevention and the role of human papillomavirus infection. Cmaj 2001; 164(7): 1017–1025. [PMC free article] [PubMed] [Google Scholar]
- 61. Kyrgiou M, Mitra A, Moscicki A-B. Does the vaginal microbiota play a role in the development of cervical cancer? Transl Res 2017; 179: 168–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Melin A, Sparén P, Bergqvist A. The risk of cancer and the role of parity among women with endometriosis. Hum Reprod 2007; 22(11): 3021–3026. [DOI] [PubMed] [Google Scholar]
- 63. Misra JS, Das V, Srivastava AN, et al. Role of different etiological factors in progression of cervical intraepithelial neoplasia. Diagn Cytopathol 2006; 34(10): 682–685. [DOI] [PubMed] [Google Scholar]
- 64. Thulaseedharan JV, Malila N, Hakama M, et al. Socio demographic and reproductive risk factors for cervical cancer—a large prospective cohort study from rural India. Asian Pac J Cancer Prev 2012; 13(6): 2991–2995. [DOI] [PubMed] [Google Scholar]
- 65. Wilson HG, Marchbanks PA. #3 Parity, age at first birth, and risk of invasive cervical cancer: meta-analyses. Ann Epidemiol 2002; 12(7): 490–491. [Google Scholar]
- 66. Singer A. The uterine cervix from adolescence to the menopause. Br J Obstet Gynaecol 1975; 82(2): 81–99. [DOI] [PubMed] [Google Scholar]
- 67. Ostergard DR. The effect of pregnancy on the cervical squamocolumnar junction in patients with abnormal cervical cytology. Am J Obstet Gynecol 1979; 134(7): 759–760. [DOI] [PubMed] [Google Scholar]
- 68. Hildesheim A, Herrero R, Castle PE, et al. HPV co-factors related to the development of cervical cancer: results from a population-based study in Costa Rica. Br J Cancer 2001; 84(9): 1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liao SF, Lee WC, Chen HC, et al. Baseline human papillomavirus infection, high vaginal parity, and their interaction on cervical cancer risks after a follow-up of more than 10 years. Cancer Causes Control 2012; 23(5): 703–708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-whe-10.1177_17455065221075904 for High parity is associated with increased risk of cervical cancer: Systematic review and meta-analysis of case–control studies by Yohannes Tekalegn, Biniyam Sahiledengle, Demelash Woldeyohannes, Daniel Atlaw, Sisay Degno, Fikreab Desta, Kebebe Bekele, Tesfaye Aseffa, Habtamu Gezahegn and Chala Kene in Women’s Health
Supplemental material, sj-docx-3-whe-10.1177_17455065221075904 for High parity is associated with increased risk of cervical cancer: Systematic review and meta-analysis of case–control studies by Yohannes Tekalegn, Biniyam Sahiledengle, Demelash Woldeyohannes, Daniel Atlaw, Sisay Degno, Fikreab Desta, Kebebe Bekele, Tesfaye Aseffa, Habtamu Gezahegn and Chala Kene in Women’s Health
Supplemental material, sj-xlsx-2-whe-10.1177_17455065221075904 for High parity is associated with increased risk of cervical cancer: Systematic review and meta-analysis of case–control studies by Yohannes Tekalegn, Biniyam Sahiledengle, Demelash Woldeyohannes, Daniel Atlaw, Sisay Degno, Fikreab Desta, Kebebe Bekele, Tesfaye Aseffa, Habtamu Gezahegn and Chala Kene in Women’s Health