Abstract
Histoplasma capsulatum is an infrequent but serious cause of endocarditis. The definitive diagnosis requires culture, which may require a long incubation. We demonstrated the ability of the Histoplasma capsulatum AccuProbe to accurately identify this organism when applied directly on an excised valve that contained abundant yeast forms consistent with H. capsulatum.
Histoplasma capsulatum is a common cause of infection in the midwestern and southeastern United States. This fungus causes a broad array of clinical manifestations, and the diagnosis depends on a high index of suspicion. Furthermore, the presence of small budding yeast in tissue is suggestive but by no means diagnostic of this organism. The diagnosis of histoplasmosis is confirmed by isolation of H. capsulatum from body fluids or tissues. Unfortunately, H. capsulatum is a slow-growing dimorphic fungus that requires special media for recovery and may take up to 4 weeks to grow (range, 1 to 4 weeks) (8). If H. capsulatum is suspected in culture because of its characteristic tuberculate macroconidia, confirmatory testing is required to exclude the possibility of a contaminating environmental mold with similar conidia such as Sepedonium spp. Although mycelial-to-yeast-form conversion and exoantigen testing may be used to confirm a mold as H. capsulatum, today the Histoplasma capsulatum AccuProbe (GEN-PROBE, Inc., San Diego, Calif.), a DNA probe that recognizes a specific rRNA target sequence, is commonly used for more rapid and accurate results (5).
The occurrence of endocarditis secondary to H. capsulatum is rare. There are only a few cases reported in the literature (1, 2, 4). The characteristic histopathologic features of H. capsulatum endocarditis are clusters of yeast embedded in a fibrin mesh (3). In addition to the small yeast (2 to 5 μm in diameter) typical of H. capsulatum, rudimentary hyphal forms and enlarged yeast forms may be seen (7, 11). Although the histopathologic diagnosis of H. capsulatum endocarditis is strongly suggested by this morphology, confirmation by culture is necessary. However, the application of molecular methods directly to the excised tissue could potentially achieve an accurate diagnosis without the delays needed for culture.
A 58-year-old man from the midwestern United States, with a medical history of previous hypertension, was transferred to our institution for acute ascending aortic dissection. He underwent an emergent open-heart surgery with replacement of the ascending aorta and the aortic valve with a Hemashield and Carpentier-Edwards valve, respectively. His postoperative course was complicated by acute renal failure, rapid atrial fibrillation necessitating cardioversion, cardiac tamponade requiring a redosternotomy, and Pseudomonas aeruginosa mediastinitis with subsequent bacteremia necessitating mediastinal reexploration, abscess drainage, and prolonged intubation with subsequent tracheostomy. After a 2-month convalescence, he recovered well and was discharged to a rehabilitation facility on oral ciprofloxacin for lifelong suppression of the Pseudomonas infection. After a few months, he noted weight loss, fatigue, and anorexia. Six months later, he was admitted to a local hospital with worsening of these symptoms, in addition to high-grade fever and chills. Blood cultures grew P. aeruginosa that was resistant to ciprofloxacin. A transthoracic echocardiogram was performed that showed large vegetations on the prosthetic aortic valve and mild regurgitation. He was transferred to our institution, where he underwent an aortic valve replacement with a homograft for presumed Pseudomonas endocarditis. The histopathology of the excised valve, however, demonstrated large vegetations that consisted of a mixture of yeast forms and fibrin (Fig. 1). Although most of the yeast forms were small (2 to 5 μm in diameter), occasional large, globose forms were also seen. The morphology of the yeast was best demonstrated using the Grocott-Gomori methenamine silver stain (Fig. 2). These findings were thought to be most consistent with endocarditis caused by H. capsulatum. The portion of valve received for culture was macerated. Direct examination using Calcofluor white-KOH disclosed numerous small budding yeasts. Cultures for bacteria and fungi were performed. Two days later, the valvular tissue grew rare P. aeruginosa and a rare anaerobic nonsporeforming gram-positive bacillus, which was considered as a possible contaminant.
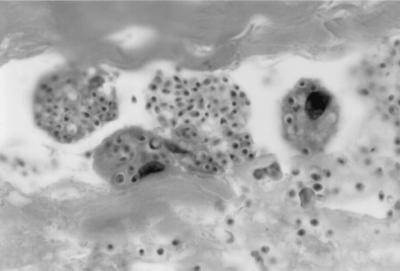
FIG. 1.
Small yeast (2 to 5 μm in diameter), as typically seen in histoplasmosis, are present in phagocytes in this vegetation. Hematoxylin and eosin strain. Magnification, ×1,000.
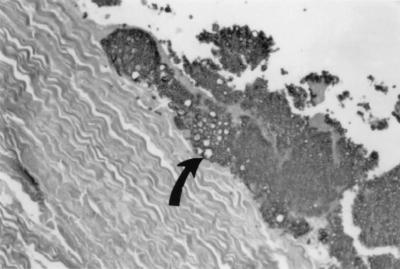
FIG. 2.
Enlarged, globose yeast forms (arrow), adherent to the cardiac valve, are best seen using a silver precipitation stain. Grocott-Gomori methenamine silver stain. Magnification, ×200.
We attempted to identify the fungus present directly from the excised valve, using the Histoplasma capsulatum AccuProbe, because of the abundance of the yeast seen and the suggestion that this probably represented H. capsulatum. Although the efficacy of this test has not yet been demonstrated on direct clinical specimens, the amount of fungus necessary to generate a positive result from culture is minimal (1 to 2 mm2), and this amount was judged to be present based on the histopathologic examination of the specimen. This test requires less than 1 h to perform, and the results are based on hybridization between a specific chemiluminescent DNA probe and rRNA sequence unique to H. capsulatum. The efficiency of hybridization is determined by light generation and was measured in a luminometer (Leader 450 i; GEN-PROBE, Inc.). Signals of ≥50,000 relative light units (RLU) are positive.
A 5-mm2 piece of the surgical specimen was sonicated for 20 min and then heated for 10 min at 95°C. With the exception of a longer sonication (5 min more than the usual time), the Histoplasma capsulatum AccuProbe procedure was performed as specified by the manufacturer. The signal produced for H. capsulatum in our specimen was 153,961 RLU, which was strongly positive. As a negative control, the same amount of tissue from the valve was tested using the Coccidiodes immitis AccuProbe and generated only 17,539 RLU, clearly negative. Of concern, red blood cells that can be present in a clinical specimen have been reported to yield false-positive probe results because of nonspecific chemiluminescence (10). This potential problem was ruled out in our experiment with the use of the Coccidiodes immitis AccuProbe as a negative control. One week later, the culture grew H. capsulatum, which was also confirmed with the Histoplasma capsulatum AccuProbe.
The valvular vegetations in patients with H. capsulatum endocarditis, like those produced by other fungi, are large, verrucous, and friable and contain abundant organisms. Our findings suggest that the Histoplasma capsulatum AccuProbe may be used for the rapid identification H. capsulatum in excised tissue if a sufficient number of organisms and histopathology suggestive of this organism are present. Since the sensitivity of the AccuProbe on a direct specimen has not been shown to be equivalent to that of culture methods (6, 9), additional studies are necessary to clarify the limits of detection (potential sample requirements, clinical relevance, etc).
REFERENCES
- 1.Alexander W J, Mowry R W, Gobbs C G, Dismukes W E. Prosthetic valve endocarditis caused by Histoplasma capsulatum. JAMA. 1979;242:1399–1400. [PubMed] [Google Scholar]
- 2.Blair T P, Waugh R A, Pollack M, Ashworth H E, Young N A, Anderson S E, Bem T P. Histoplasma capsulatum endocarditis. Am Heart J. 1980;99:783–788. doi: 10.1016/0002-8703(80)90630-4. [DOI] [PubMed] [Google Scholar]
- 3.Deepe G S. Histoplasma capsulatum. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 5th ed. Philadelphia, Pa: Churchill Livingstone; 2000. pp. 2718–2731. [Google Scholar]
- 4.Gaynes R P, Gardner P, Causey W. Prosthetic valve endocarditis caused by Histoplasma capsulatum. Arch Intern Med. 1981;141:1533–1537. [PubMed] [Google Scholar]
- 5.Hall G S, Pratt-Rippin K, Washington J A. Evaluation of a chemiluminescent probe assay for identification of Histoplasma capsulatum isolates. J Clin Microbiol. 1992;30:3003–3004. doi: 10.1128/jcm.30.11.3003-3004.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall G S. Probe technology for the clinical microbiology laboratory. Arch Pathol Lab Med. 1993;117:578–583. [PubMed] [Google Scholar]
- 7.Hutton J P, Durham J B, Miller D P, Everett E D. Hyphal forms of Histoplasma capsulatum. A common manifestation of intravascular infections. Arch Pathol Lab Med. 1985;109:330–332. [PubMed] [Google Scholar]
- 8.Koneman E W, Allen S D, Janda W M, Schreckenberger P C, Winn W C Jr, editors. Color atlas and textbook of diagnostic microbiology. 5th ed. Philadelphia, Pa: Lippincott; 1997. pp. 983–1069. [Google Scholar]
- 9.Sandin R L, Isada C M, Hall G S, Tomford J W, Rutherford I, Rogers A L, Washington J A. Aberrant Histoplasma capsulatum confirmation of identity by a chemiluminescence-labeled DNA probe. Diagn Microbiol Infect Dis. 1993;17:235–238. doi: 10.1016/0732-8893(93)90103-e. [DOI] [PubMed] [Google Scholar]
- 10.Stockman L, Clark K A, Hunt J M, Roberts G D. Evaluation of commercially available acridinium ester-labeled chemiluminescent DNA probes for culture identification of Blastomyces dermatitidis, Coccidiodes immitis, Cryptococcus neoformans, and Histoplasma capsulatum. J Clin Microbiol. 1993;31:845–850. doi: 10.1128/jcm.31.4.845-850.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svirbely J R, Ayers L W, Bueschlng W J. Filamentous Histoplasma capsulatum endocarditis involving mitral and aortic valve porcine bioprotheses. Arch Pathol Lab Med. 1985;109:273–276. [PubMed] [Google Scholar]