Abstract
Vertebral hemangiomas are benign vascular tumors that are commonly asymptomatic. A low percentage might become aggressive; however, they are not known to be associated with scoliosis. We present a case of a third lumbar vertebral lesion coexisting with a moderate thoracolumbar scoliosis. The patient's initial presentation was back pain with bilateral lower limb radiculopathy and neurogenic claudication. Diagnosis was established using CT and MRI, which showed classical findings of an aggressive vertebral hemangioma. The patient underwent Partial hemangioma excision and scoliosis correction, with satisfactory outcome at 1 year follow up.
Introduction
Vertebral hemangiomas are benign vascular tumors or malformations occurring in the vertebral column. Prevalence of these tumors has been reported to be up to 10% in the population based on an autopsy survey. Among primary spine tumors, hemangioma represent around 2–3% [[1], [2], [3]]. Diagnosis is mainly by computed tomography (CT) and magnetic resonance imaging (MRI).
Hemangiomas can be classified based on radiological features to typical, atypical, and aggressive. The difference between typical and atypical hemangiomas mainly depends on the ratio of fatty to vascular components and associated edema. While they are labeled as aggressive hemangiomas when there are findings of extension beyond the vertebral body, cortical destruction, and erosion of the epidural and paravertebral spaces [4]. Another widely used clinical classification is the Enneking staging classification, which classifies the vertebral hemangioma into three types: Latent, active, and aggressive [5]. Latent (Enneking stage 1, S1): Mild bony destruction without symptoms. Active (Enneking stage 2, S2): bony destruction with pain. Aggressive (Enneking stage 3, S3): Epidural soft tissue extension with stenosis or neurological symptoms.
Most patients with hemangiomas are usually asymptomatic, however, 1–2% might experience symptoms such as back pain or neurological deficit [6,7]. Furthermore, most symptomatic lesions are either atypical or aggressive hemangiomas. Among different articles talking about hemangioma presentations, we found very few articles describing scoliosis coexisting with vertebral hemangiomas, and most were described in pediatric patients [8,9]. In this article, we will present an adult patient with a third lumbar (L3) aggressive vertebral hemangioma coexisting with thoracolumbar scoliosis.
Case presentation
A 60-year-old female, known case of hypertension, morbid obesity (Body mass index = 46 KG/MM2), presented with a 2-year history of chronic worsening low back pain, associated with bilateral lower limb radiculopathy, as well as neurogenic claudication. She denied any previous history of spine deformity and scoliosis. Physical examination showed morbid obesity, no obvious spine deformity, intact motor and sensory neurological examination, with normal reflexes in the lower limbs. Patient had a radiograph which showed an L3 lesion with left convex side scoliosis T11-L4 with a cobb angle of 45° (Fig. 1), associated with advanced degenerative changes. Long films radiographs showed balanced sagittal plane, with minimal left coronal malalignment (Convex malalignment)
Fig. 1.
(A&B) showing Anteroposterior (AP) + lateral standing scoliosis series
Fig. 1 (C&D) AP + lateral standing lumbar x-rays Images show the third lumbar (L3) lesion, associated with thoracolumbar scoliosis with a cobb angle of 45°.
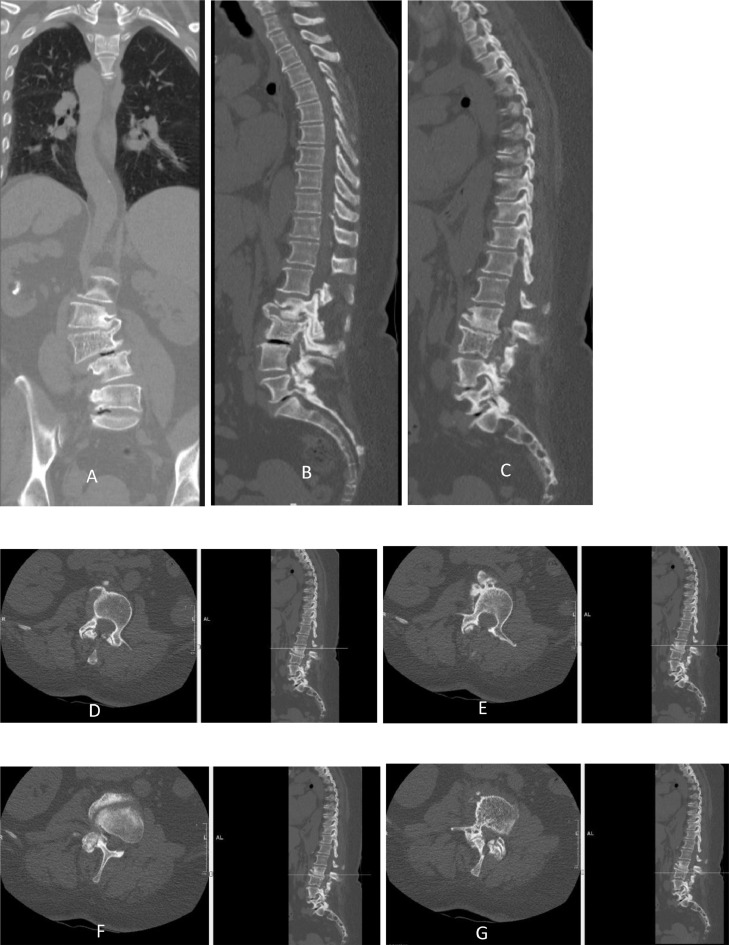
CT showed an L3 mass with the pathognomonic polka dotted appearance, cortical expansion, along with a soft tissue mass which goes with hemangiomas, it also shows advanced multilevel facet joints arthritis of the lumbar spine with multiple levels of vacuum phenomena at L5/S1 Disc & L3/4 Disc (Fig. 2). MRI also revealed a hyper intense lesion involving the entire vertebral body of L3 associated with epidural extension causing severe spinal stenosis. It also showed severe degenerative lumbar spinal stenosis at L3/4 and L4/5 levels, with moderate stenosis at L2/3 & L5/S1 levels (Fig. 3).
Fig. 2.
(A,B, &C): Coronal and sagittal lumbar spine Computed Tomography (CT)
Fig. 2 (D,E,F, &G): Axial lumbar spine CT, with its corresponding sagittal level
Fig. 2 shows CT images of the L3 lesion, with Fig. 2G showing the classical polka dot appearance. The extension beyond the vertebral body can also be seen. The facet arthritis can be appreciated in the axial Cuts.
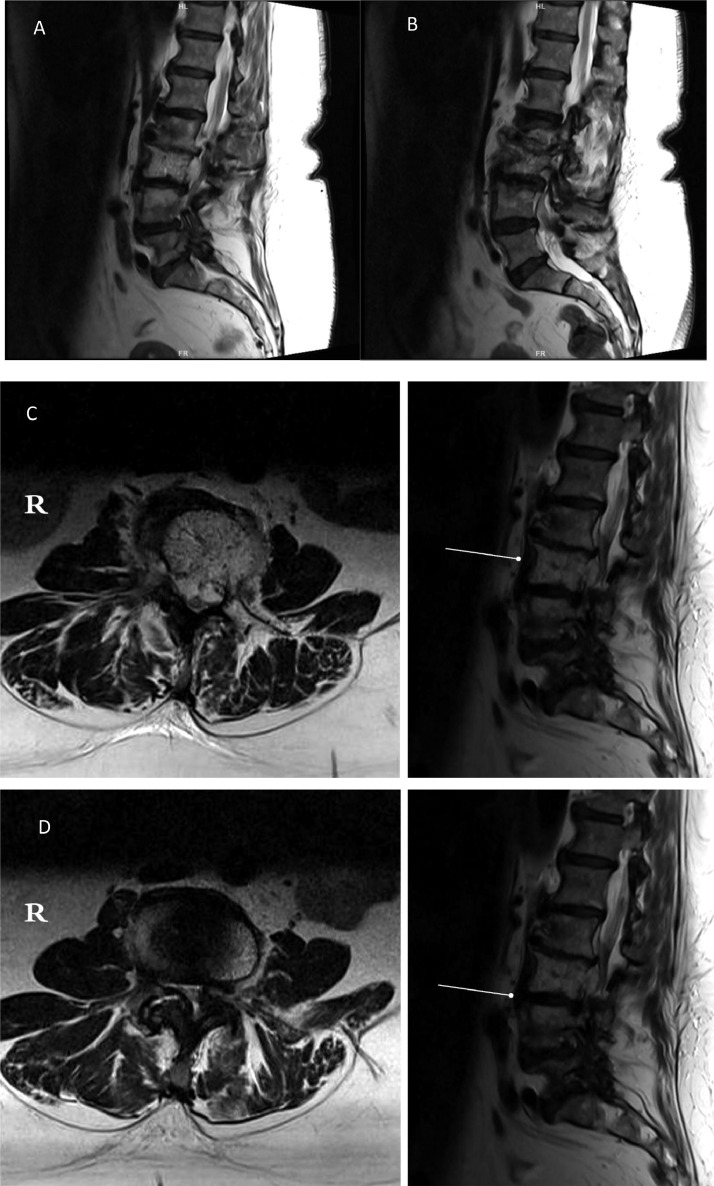
Fig. 3.
(A&B) Sagittal magnetic resonance imaging (MRI) cuts of the lumbar spine
Fig. 3 (C&D) Axial lumbar spine MRI with its corresponding sagittal levels
Fig. 3 MRI of the lumbar spine, showing the hemangioma extension to the epidural and paravertebral spaces.
Due to the classic radiological features of aggressive vertebral hemangioma, preoperative CT guided biopsy was not necessary. The patient underwent pre-operative angioembolization of the L3 segmental arteries, the embolization was not completed fully as one of the segmental vessels had branches connecting to the artery of Adamkiewicz.
Surgery was done 24 h following the embolization utilizing posterior approach to the thoracolumboscaral spine, screws from T10-S2AI (S2 Alar Iliac) were inserted, and decompressive laminectomies of the levels L2/3, L3/4 and L4/5 were performed with L5/S1 Bilateral Foraminotomies. Then, we performed partial tumor excision of the L3 hemangioma to ensure proper decompression of the dura. At this stage of the procedure we encountered 2 incidental durotomies, both of which were repaired primarily. Then, we performed an L3 vertebroplasty procedure as an adjunct to partial excision. We then proceeded with the scoliosis correction. The patient tolerated the procedure well, without symptoms of persistent CSF leakage. The procedure was done with Intraoperative neuromonitoring (IONM) without any signal changes throughout the procedure.
The estimated blood loss was roughly around 700 cc. The patient was able to mobilize on day 2 post-operatively, with no neurological deficit. She also required 2 units of packed red blood cells on day 3 post op due to low hemoglobin level. The patient was discharged on day 7, with an uneventful postoperative stay in general. Intra-operative biopsy results showed a multiple variably sized ectatic thick walled vascular spaces, with flattened endothelial lining, replacing the marrow spaces, and mixed with fatty tissue consistent with a hemangioma involving bone and soft tissue. At 1 year follow up, Patient had stable construct, no evidence of recurrence, and improvement of the back pain and neurological symptoms. (Fig. 4) Patient consent was taken.
Fig. 4.
(A&B) AP + lateral standing scoliosis series
Fig. 4 (C&D) AP + lateral standing lumbar spine x-rays
Fig. 4 shows Post-operative x-rays at 1 year follow up, showing the scoliosis correction and vertebroplasty at L3 with stable construct, and no evidence of recurrence.
Discussion
We present a case of Aggressive vertebral hemangioma of the lumbar spine with adult thoracolumbar scoliosis associated with multilevel lumbar spinal stenosis secondary to degenerative, deformity, and tumor aetiologies in the same setting, which added to the complexity of the management of this case. The patient's obesity added to the complexity as well. We couldn't accurately identify whether this is a causative relationship or just an incidental coexistence, the deformity was never noticed before, and all the patient's radiographic images were relatively new.
We were only able to find few articles associating scoliosis and hemangiomas and all were in the pediatric population, with no clear explanation of the relationship between the lesion and the deformity [8,9]. Other articles also in the pediatric and adolescent age group, have described association between scoliosis and different tumors (Osteoid osteomas, desmoid tumors, ganglioneuromas, and Kaposiform hemangioendothelioma) with different theories regarding the association [[10], [11], [12], [13]]. In the case of osteomas, mainly pain and paravertebral myositis are thought to be the cause of the deformity [10]. In the other three lesions, eccentric vertebral growth of the tumor, destruction of the vertebral side plates, or simply an incidental coexistence were theorized to be the association between the lesion and scoliosis [[11], [12], [13]].
In our patient, we could not establish whether there is an association or merely a coexistence. However, Since the lesion was symptomatic with compressive symptoms, we opted for the surgical management. The lesion was first confirmed to be an aggressive vertebral hemangioma with CT and MR imaging. CT is very useful in diagnosing hemangiomas, as it usually shows the pathognomonic polka dotted sign, which simply are hyperdense trabeculae surrounded by fatty stroma or vascular lacunae. Other features seen in aggressive hemangiomas include total vertebral involvement, extraosseous extension, and soft tissue mass [4]. While aggressive vertebral hemangiomas are more difficult to diagnose in MR due to the less fatty content, it is still a vital part of the diagnosis, and usually shows low signal intensity in T1 images, and high signal in T2 [4,7].
As for the management of such lesions, there are different modalities for management of symptomatic vertebral hemangiomas such as transarterial embolization, vertebroplasty, radiotherapy, and surgical decompression. Each of these modalities has been reported as a standalone or combined management with different reported success rates. Transarterial embolization alone, has been reported to be successful mainly in slowing or halting the hemangioma growth and alleviating the back pain, but has not demonstrated much benefit in compressive symptoms [14,15]. However, the use of pre-operative embolization, if done successfully, has shown to significantly decrease blood loss during surgery. A study by Robinson et al. showed that blood loss in the embolized treatment group (980 ± 683 mL) was lower than the non-embolized control group (1629 ± 946 mL) (16).
Percutaneous vertebroplasty was also beneficial in alleviating pain, but again did not improve compressive symptoms or obliterate the hemangioma [2]. Intraoperative vertebroplasty, however, has been reported to help in decreasing intra-operative blood loss and decrease the chances of recurrence [17]. Wang et al. reported treating 39 patients with aggressive vertebral hemangioma. Of which, 22 patients had decompression with intraoperative vertebroplasty as an adjunct. There was no recurrence and no major complications in the decompression with adjunct vertebroplasty group. While hemangiomas are known to be radiosensitive, the choice of radiotherapy as a sole modality remains controversial, as it may alleviate pain and neurological deficits. However, this happens over the course of weeks to a couple of months, with the risk of radionecrosis, pseudoarthrosis, skin complications, and malignancy [18,19]. Post-operative radiotherapy has been used as an adjuvant to surgery with decreased chances of recurrence [19].
Surgery with its different strategies (Subtotal resection, Gross total resection, or Enbloc resection) has proven to be effective in aggressive hemangiomas presenting with pain, neurological deficit, or both [20]. The patient presented in this case report had a mixture of symptoms which cannot be explained by the L3 aggressive vertebral hemangioma alone, our impression was that the lower back pain is due to the degenerative changes and the resulting scoliosis deformity, moreover, the neurogenic claudication is secondary to the lumbar spinal stenosis most noticeably at L3/4, L4/5 due to degenerative pathology and at the level of the body of L3 due to the Soft tissue extension of the tumor. At the L5/S1 level, bilateral foraminal stenosis with facets osteophytes was contributing to the bilateral L5 radiculopathy symptoms. For this reason, we felt that such a condition deserves a surgical intervention addressing all these pathologies during the same setting. Hence, such major surgery was required to address all of these contributing factors. Prior to the surgery, we elected to go with pre-operative embolization to decrease blood loss, unfortunately, the embolization was not completed fully due to the communication of the segmental vessels with the artery of Adamkiewicz.
As for the surgery, the L3 aggressive vertebral hemangioma was dealt with by performing partial resection to ensure proper decompression of the Spinal canal and using Intraoperative vertebroplasty as an adjunct treatment to prevent tumor recurrence. In addressing the deformity, choosing the Upper Instrumented vertebra (UIV) was based on the magnitude of the thoracolumbar scoliosis, as the curve extends between T11 - L4 levels, we elected to stop at T10 as the our UIV, the decision to extend the fusion down to the pelvis was based on many factors, first, the patient had severe stenosis requiring decompression at L4/5, As well as bilateral foraminotomies of the L5/S1 foramens, second, the patient had left convex coronal malalignment. Obaid etal, discussed in details treatment oriented guidelines for coronal malalignment in adult spine deformity, patients with Convex type coronal malalignment with degeneration and stiff lumbosacral junction needs fusion down to the pelvis with iliac screws, as stopping short of the pelvis will result in worsening of the coronal malalignment post operatively [21]. Moreover, the surgical team felt that stopping at L5 as the lower instrumented vertebra (LIV) will carry high risk of L5/S1 degeneration, adjacent level disease, L5 screws loosening & loss of sagittal alignment [[22], [23], [24]]. We did not proceed with post-operative radiotherapy to prevent wound complications, taking into consideration the patient's morbid obesity (BMI 46 KG/MM2), which is known to increase the risk of wound infection up to 5 folds, which could be due higher retraction forces and thick adipose tissue causing dead space, leading to less perfusion and higher necrosis rate [25,26]. The patient generally tolerated the surgery well, with improved symptoms, and at 1 year follow up, there was no evidence of recurrence or implant related complications.
Conclusion
An aggressive vertebral hemangioma, coexisting with degenerative scoliosis, needs surgical management. Pre-operative embolization will help in decreasing blood loss intra-op and with resection of the lesion. Intra-operative vertebroplasty also helps in decreasing blood loss, relieving pain, and reduces recurrence. Long term follow-up, with repeated imaging will be needed in this patient to monitor for recurrence.
Declaration of Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Patient informed consent
The authors declare that informed patient consent was taken from all the patients.
References
- 1.Pastushyn A.I., Slin'ko E.I., Mirzoyeva G.M. Vertebral hemangiomas: diagnosis, management, natural history and clinicopathological correlates in 86 patients. Surg Neurol. 1998;50(6):535–547. doi: 10.1016/s0090-3019(98)00007-x. [DOI] [PubMed] [Google Scholar]
- 2.Acosta F.L., Sanai N., Chi J.H., Dowd C.F., Chin C., Tihan T., Ames C.P. Comprehensive management of symptomatic and aggressive vertebral hemangiomas. Neurosurg Clin N Am. 2008;19(1):17–29. doi: 10.1016/j.nec.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Vasudeva V.S., Chi J.H., Groff M.W. Surgical treatment of aggressive vertebral hemangiomas. Neurosurg Focus. 2016;41(2) doi: 10.3171/2016.5.focus16169. [DOI] [PubMed] [Google Scholar]
- 4.Gaudino S., Martucci M., Colantonio R., Lozupone E., Visconti E., Leone A., Colosimo C. A systematic approach to vertebral hemangioma. Skeletal Radiol. 2014;44(1):25–36. doi: 10.1007/s00256-014-2035-y. [DOI] [PubMed] [Google Scholar]
- 5.Boriani S., Weinstein J.N., Biagini R. Primary bone tumors of the spine. Terminol Surg Stag Spine Phila Pa 1976. 1997;22:1036–1044. doi: 10.1097/00007632-199705010-00020. [DOI] [PubMed] [Google Scholar]
- 6.Chi J.H., Bydon A., Hsieh P., Witham T., Wolinsky J.P., Gokaslan Z.L. Epidemiology and demographics for primary vertebral tumors. Neurosurg Clin N Am. 2008;19:1–4. doi: 10.1016/j.nec.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Jiang L., Liu X.G., Yuan H.S., Yang S.M., Li J., Wei F., Liu Z.J. Diagnosis and treatment of vertebral hemangiomas with neurologic deficit: a report of 29 cases and literature review. Spine J. 2014;14(6):944–954. doi: 10.1016/j.spinee.2013.07.450. [DOI] [PubMed] [Google Scholar]
- 8.Ogura Y., Watanabe K., Hosogane N., Tsuji T., Ishii K., Nakamura M., Matsumoto M. Severe progressive scoliosis due to huge subcutaneous cavernous hemangioma: a case report. Scoliosis. 2011;6(1) doi: 10.1186/1748-7161-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asumu T.O., Williamson B., Hughes D.G. Symptomatic spinal hemangiomas in association with cutaneous hemangiomas. A case report. Spine Phila Pa 1976. 1996;21:1082–1084. doi: 10.1097/00007632-199605010-00018. [DOI] [PubMed] [Google Scholar]
- 10.Kawahara C., Tanaka Y., Kato H., Watanabe S., Kokubun S. Myolysis of the erector spinae muscles as the cause of scoliosis in osteoid osteoma of the spine. Spine. 2002;27(12) doi: 10.1097/00007632-200206150-00027. [DOI] [PubMed] [Google Scholar]
- 11.Shindle M.K., Khanna A.J., Mccarthy E.F., O'Neill P.J., Sponseller P.D. Desmoid tumor of the spinal canal causing scoliosis and paralysis. Spine. 2002;27(12) doi: 10.1097/00007632-200206150-00025. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y., Ren M., Yuan Z., Li K., Zhang Z., Zhang J., Yang Z. Thoracolumbar paravertebral giant ganglioneuroma and scoliosis: a case report and literature review. World J Surg Oncol. 2016;14(1) doi: 10.1186/s12957-016-0823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Y., Qiu G., Zhao H., Liang J., Shi X. Kaposiform hemangioendothelioma with adolescent thoracic scoliosis: a case report and review of literature. Eur Spine J. 2011;20(S2):309–313. doi: 10.1007/s00586-011-1731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayakumar P.N., Vasudev M.K. Srikanth SG: symptomatic vertebral haemangioma: endovascular treatment of 12 patients. Spinal Cord. 1997;35:624–628. doi: 10.1038/sj.sc.3100438. [DOI] [PubMed] [Google Scholar]
- 15.Smith T.P., Koci T., Mehringer C.M., Tsai F.Y., Fraser K.W., Dowd C.F., Higashida R.T., Halbach V.V., Hieshima G.B. Transarterial embolization of vertebral hemangioma. J Vasc Interv Radiol. 1993;4:681–685. doi: 10.1016/s1051-0443(93)71948-x. [DOI] [PubMed] [Google Scholar]
- 16.Robinson Y., Sheta R., Salci K., Willander J. Blood loss in surgery for aggressive vertebral haemangioma with and without embolisation. Asian Spine J. 2015;9(3):483. doi: 10.4184/asj.2015.9.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B., Han S.B., Jiang L., Liu X.G., Yang S.M., Meng N., Liu Z.J. Intraoperative vertebroplasty during surgical decompression and instrumentation for aggressive vertebral hemangiomas: a retrospective study of 39 patients and review of the literature. Spine J. 2018;18(7):1128–1135. doi: 10.1016/j.spinee.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Miszczyk L., Ficek K., Trela K., Spindel J. The efficacy of radiotherapy for vertebral hemangiomas. Neoplasma. 2001;48:82–84. [PubMed] [Google Scholar]
- 19.Piper K., Zou L., Li D., Underberg D., Towner J., Chowdhry A.K., Li Y.M. Surgical management and adjuvant therapy for patients with neurological deficits from vertebral hemangiomas. Spine. 2020;45(2) doi: 10.1097/brs.0000000000003181. [DOI] [PubMed] [Google Scholar]
- 20.Teferi N., Abukhiran I., Noeller J., Helland L.C., Bathla G., Ryan E.C., Hitchon P.W. Vertebral hemangiomas: diagnosis and management. A single center experience. Clin Neurol Neurosurg. 2020;190 doi: 10.1016/j.clineuro.2020.105745. [DOI] [PubMed] [Google Scholar]
- 21.Jiang L., Liu X.G., Yuan H.S., Yang S.M., Li J., Wei F., Liu Z.J. Diagnosis and treatment of vertebral hemangiomas with neurologic deficit: a report of 29 cases and literature review. Spine J. 2014;14(6):944–954. doi: 10.1016/j.spinee.2013.07.450. [DOI] [PubMed] [Google Scholar]
- 22.Cho K., Suk S., Park S., Kim J., Choi S., Yoon Y., Won M. Arthrodesis to L5 versus S1 in long instrumentation and fusion for degenerative lumbar scoliosis. Eur Spine J. 2009;18(4):531–537. doi: 10.1007/s00586-009-0883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhns C.A., Bridwell K.H., Lenke L.G., Amor C., Lehman R.A., Buchowski J.M., Christine B. Thoracolumbar deformity arthrodesis stopping at L5. Spine. 2007;32(24):2771–2776. doi: 10.1097/brs.0b013e31815a7ece. [DOI] [PubMed] [Google Scholar]
- 24.Edwards C.C., Bridwell K.H., Patel A., Rinella A.S., Berra A., Lenke L.G. Long adult deformity fusions to L5 and the sacrum a matched cohort analysis. Spine. 2004;29(18):1996–2005. doi: 10.1097/01.brs.0000138272.54896.33. [DOI] [PubMed] [Google Scholar]
- 25.Cao J., Kong L., Meng F., Zhang Y., Shen Y. Impact of obesity on lumbar spinal surgery outcomes. J Clin Neurosci. 2016;28:1–6. doi: 10.1016/j.jocn.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 26.Ojo O., Owolabi B., Oseni A., Kanu O., Bankole O. Surgical site infection in posterior spine surgery. Niger J Clin Pract. 2016;19(6):821. doi: 10.4103/1119-3077.183237. [DOI] [PubMed] [Google Scholar]