Significance Statement
Cisplatin is an effective chemotherapeutic agent for multiple solid tumors but its nephrotoxicity limits its clinical use. In this study in a mouse model, the researchers deliver an agonist peptide derived from renalase (RNLS), a secreted protein that enhances cell replication and decreases inflammation, specifically to the proximal tubule, the site of maximum renal damage induced by cisplatin. They provide evidence that this targeted delivery of the peptide (via encapsulation in mesoscale nanoparticles) protected against the development of cisplatin-mediated CKD, and that RNLS acts by inhibiting both regulated cell death and the proinflammatory state of CKD. These findings suggest that such an approach might provide a way to mitigate the nephrotoxicity of cisplatin and thus broaden its therapeutic efficacy in otherwise sensitive tumors.
Keywords: cisplatin, cisplatin nephrotoxicity, chronic renal disease, cytokines, macrophages, mitochondria, oxidative stress, proximal tubule, renal protection, cell death
Abstract
Background
Repeated administration of cisplatin causes CKD. In previous studies, we reported that the kidney-secreted survival protein renalase (RNLS) and an agonist peptide protected mice from cisplatin-induced AKI.
Methods
To investigate whether kidney-targeted delivery of RNLS might prevent cisplatin-induced CKD in a mouse model, we achieved specific delivery of a RNLS agonist peptide (RP81) to the renal proximal tubule by encapsulating the peptide in mesoscale nanoparticles (MNPs). We used genetic deletion of RNLS, single-cell RNA sequencing analysis, and Western blotting to determine efficacy and to explore underlying mechanisms. We also measured plasma RNLS in patients with advanced head and neck squamous cell carcinoma receiving their first dose of cisplatin chemotherapy.
Results
In mice with CKD induced by cisplatin, we observed an approximate 60% reduction of kidney RNLS; genetic deletion of RNLS was associated with significantly more severe cisplatin-induced CKD. In this severe model of cisplatin-induced CKD, systemic administration of MNP-encapsulated RP81 (RP81-MNP) significantly reduced CKD as assessed by plasma creatinine and histology. It also decreased inflammatory cytokines in plasma and inhibited regulated necrosis in kidney. Single-cell RNA sequencing analyses revealed that RP81-MNP preserved epithelial components of the nephron and the vasculature and suppressed inflammatory macrophages and myofibroblasts. In patients receiving their first dose of cisplatin chemotherapy, plasma RNLS levels trended lower at day 14 post-treatment.
Conclusions
Kidney-targeted delivery of RNLS agonist RP81-MNP protects against cisplatin-induced CKD by decreasing cell death and improving the viability of the renal proximal tubule. These findings suggest that such an approach might mitigate the development of CKD in patients receiving cisplatin cancer chemotherapy.
Over 37 million Americans have CKD, which is often progressive and increases the risk of heart disease, stroke, and death.1 Cisplatin is prominent among the toxins and drugs that damage the kidney. An observational cohort study of 233 human subjects who received 629 cycles of high-dose cisplatin (99±9 mg/m2) for treatment of head and neck cancer between 2005 and 2011 showed that 68% of patients developed AKI and cisplatin-associated severe AKI occurs in 20% of the patients and has a negative effect on long-term renal function and patient survival.2 As such, although cisplatin is an effective chemotherapeutic agent for multiple solid tumors, its nephrotoxicity limits its clinical use. We previously developed a mouse model that mimics cisplatin-induced CKD, which revealed failed repair as a prominent feature of the transition from acute to chronic injury.3 Activation of proteins involved in regulated necrosis and increased expression of kidney injury markers, cellular stress response regulators, and upstream activators of regulated necrosis all participate in this conversion from AKI to CKD.4 These studies also demonstrated that CKD is established before significant fibrosis occurred, and that unresolved injury and sustained activation of regulated necrosis pathways, rather than fibrosis, promotes the progression of cisplatin-induced AKI to CKD.
Renalase (RNLS) is a flavoprotein that displays cytoprotective and antiapoptotic properties as well as the ability to mitigate oxidative stress.5 RNLS minimizes injury in in vivo models of myocardial infarction,6 and AKI.7,8 We recently developed a sensitive and specific ELISA assay for human RNLS and applied it to measure serum RNLS in 267 patients with kidney disease. We discovered that a decrease in RNLS level is associated with lower GFR and higher creatinine.9 Therefore, we hypothesize that RNLS may play a role in maintaining kidney function and preventing progression of CKD.
We provide evidence that the first dose of cisplatin is associated with sustained (2 weeks) subclinical kidney injury. We use a mouse model of cisplatin-induced CKD to investigate and establish the therapeutic efficacy of a mesoscale nanoparticle (MNP)-encapsulated RP81 (RP81-MNP), a kidney-targeted RNLS peptide agonist. We employ genetic deletion on RNLS, single-cell RNA sequencing (scRNA-seq) analysis of kidney tissue, and Western blot analysis to explore the molecular mechanisms mediating RP81-MNP’s protective properties.
Methods
Chemicals and Reagents
Cisplatin was purchased from Sigma-Aldrich (P4394; St. Louis, MO). Renalase peptide RP81 (KIDVPWAGQYITSNPCIRFVSIDNKKRNIESSEIGP) was synthesized by CSBio (Menlo Park, CA). For kidney-specific delivery, the peptide was encapsulated into polymeric poly(lactic-co-glycolic acid)-polyethylene glycol (PLGA-PEG) MNPs, which prior work demonstrated to target the renal proximal tubular epithelial cells as evidenced by intravital microscopy and ex vivo imaging.10 Mice treated with MNPs exhibited no negative systemic consequences, immune reaction, liver impairment, or renal impairment.11 PLGA-PEG conjugation and nanoparticle formulation were performed as previously described.12 For particle formulation encapsulating RP81, an emulsion was prepared by adding 100 µg PBS-dissolved RP81 to 100 mg PLGA-PEG dissolved in 2 ml acetonitrile and bath sonicating for 1 minute. This mixture was added dropwise to 4 mL of purified water with 100 µl F-68 surfactant. Particles were characterized as described before for size and surface charge via dynamic and electrophoretic light scattering, respectively (Malvern). Peptide encapsulation was determined by acetonitrile-based particle extraction and quantification of liberated RP81 via a Micro-BCA quantification kit (Thermo). RNLS peptide-encapsulating MNPs (RP81-MNP) were 401.9±19.2 nm in diameter with a polydispersity index of 0.328±0.051. They encapsulated 1.47 µg of peptide per milligram MNP. MNPs without RP81 were used as negative control for RP81-MNP and were formulated in the same manner without emulsification before nanoprecipitation. Empty MNPs were 332.0±7.56 nm in diameter with a polydispersity index of 0.302±0.040. All parameters are within those previously determined to target the kidneys with high specificity.10 BSA (Sigma A2058) was used as a control for RP81. Antibodies used in this study are listed in detail in Supplemental Table 1.
Plasma RNLS, KIM-1, Creatinine, and GFR in Human Subjects Receiving the First Dose of Cisplatin
This study was approved by Yale Center for Clinical Investigation, HIC#2000020505. Study participants with advanced head and neck squamous cell carcinoma were recruited from October 2019 to October 2020 (Supplemental Table 2) and received pharmaceutical grade cisplatin at 100 mg/m2 on day 0. Blood samples was collected at days 0, 1, 2, and 14. Plasma RNLS levels were assayed as previously described.9 Plasma creatinine and GFR were measured by the Yale New Haven Hospital clinical laboratory. Plasma KIM-1 (kidney injury molecule-1) levels were determined using human KIM-1 ELISA assay kit from R&D Systems (Minneapolis, MN) according to the manufacturer’s instructions.
Murine Model of Cisplatin-Induced CKD
Wild-type (WT) mice C57BL/6J were purchased from Jackson Laboratory (Farmington, CT). Renalase global knockout (RNLS KO) mice were generated in house as described previously.13 Cisplatin was dissolved in sterile 0.9% sodium chloride at 0.875 mg/ml freshly before being given to the mice.
Five-month-old mice were administered two doses of cisplatin at 15 mg/kg subcutaneously, 2 weeks apart under brief isoflurane anesthesia. In some experiments, synthetic RNLS peptides RP81 were dissolved in sterile saline at 1 mg/ml and given to the mice by subcutaneous injection at 4 mg/kg three times a week for 4 weeks. RP81-MNPs were reconstituted in sterile saline at 20 mg/ml and injected into the mouse intravenously at 70 mg/kg once a week for 4 weeks. The mice were subsequently euthanized under general anesthesia. Blood and kidneys were collected, and plasma was prepared as described previously.4
Mice were maintained at Veterans Affairs Medical Center (VAMC), West Haven, CT. All experimental procedures were conducted according to the guidelines for animal care and use by the Institutional Animal Care and Use Committee of the VAMC.
Measurement of mRNA Levels in Mouse Kidney Tissues
RNA from mouse kidneys was extracted using RNeasy kit (QIAGEN) according to the manufacturer’s instructions. RNA was reverse transcribed into cDNA using QuantiTect Reverse Transcription Kit (QIAGEN) according to the manufacturer’s protocol using 1 µg RNA in a total of 20 µl reaction. Relative expression levels of various genes were assessed by quantitative PCR. The mRNA levels of RNLS, RIPK1, RIPK3, and GAPDH were assessed using the TaqMan Gene Expression real-time PCR assays (Applied Biosystems, Carlsbad, CA). The results were expressed as the threshold cycle (Ct). The relative quantification of the target transcripts normalized to the endogenous control 18s ribosomal RNA or β-actin was determined by the comparative Ct method (ΔCt) and the 2-ΔΔCt method was used to analyze the relative changes in gene expression between the tested cell lines according to the manufacturer’s protocol (User Bulletin No. 2, Applied Biosystems).
Western Blotting Analysis
Mouse kidney at different stages of CKD was homogenized in RIPA buffer supplemented with protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktails (Sigma). Kidney lysates were separated by SDS-PAGE and transferred to polyvinylidene difluoride membrane (Bio-Rad). Membranes were incubated with indicated antibodies according to the manufacturers’ instructions, followed by horseradish peroxidase–conjugated secondary antibodies (Cell Signaling Technology, Danvers, MA) and developed using a chemiluminescence detection system (PerkinElmer Life Sciences). GAPDH was used as loading control. Membranes were imaged and quantitated using ChemiDoc XRS+ System (Bio-Rad).
Renal Histology, Immunohistochemistry, and Immunofluorescence Staining
The kidney tissues were fixed in 10% neutral-buffered formalin overnight and embedded in paraffin. Then 5-μm thick sections were prepared for hematoxylin and eosin (H&E) staining for histologic assessment. Sections were examined under light microscopy for evaluation of tubular and glomerular changes. Histopathologic alteration or tubular damage was assessed by the degree of tubular dilatation, necrosis, apoptosis, and cast formation in the renal tubular cells. Immunohistochemical staining was performed for RNLS, CD68, CD3, and TUNEL as previously described.4,7,10 Immunofluorescence staining was performed in TKPTS cells grown on coverslips and treated with or without MNP or with RP81-MNP for 24 hours. Cells on coverslips were fixed in 4% paraformaldehyde at room temperature for 15 minutes followed by washing with PBS three times, 5 minutes each wash. Some of the cells on coverslips were covered with ice cold 100% methanol for 10 minutes at –20°C to permeabilize cells. Cells were blocked in 2.5% goat serum in PBS and 0.1% Tween at room temperature for 1 hour and incubated with rabbit anti-PEG at 4°C overnight followed by wash and incubation with Alexa Fluor 647–labeled goat anti-rabbit at room temperature for 1 hour. Coverslips were mounted with ProLong Gold Antifade Mountant with DAPI (Thermo Fisher Scientific).
TKPTS Cell Culture Model
Mouse proximal tubule TKPTS cell line was obtained from American Type Culture Collection (Manassas, VA) and maintained in DMEM:F12 medium (ATCC 30-2006) supplemented with 10% FBS (ATCC 30-2020) and 6 mg/L insulin (Sigma 19278). Cells were seeded and treated with either BSA or RP81 at 50 µg/ml for 24 hours, followed by addition of 25 µM cisplatin for the indicated time length. TKPTS cells were seeded in 96-well plates at 5 × 103 cells per well. After cells were attached to wells 6 hours later, culture medium was replaced with medium containing 50 µg/ml of BSA (as a control), or RNLS, or RP81, or MNP, or MNP containing RP81-MNP. Cells were then treated with or without 25 µM cisplatin for 48 hours. Cell viability was determined using the WST-1 reagent (Roche Diagnostics, Indianapolis, IN) as previously described.14 Relative cell viability was expressed by OD495 of cisplatin-treated/OD495 of mock.
Mouse Plasma Creatinine and KIM-1
Mouse plasma creatinine levels were measured by capillary electrophoresis at O’Brien Kidney Research Core, University of Texas Southwestern Medical Center. Plasma KIM-1 levels were measured using the mouse TIM-1/KIM-1/HAVCR Quantikine ELISA Kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
scRNA-seq
Kidneys were collected from each experimental mouse and weighed, then dissected into small pieces using a razor blade. The dissected tissue was incubated in 1.48 U/ml Liberase DL enzyme mixture (Roche) and 200 U/ml DNase I (Roche) at 37°C for 45 minutes on a rocker. Cell suspension was filtered through 40-μm filters then pelleted by centrifugation at 500 × g for 10 minutes. Cells were resuspended in ACK Lysing Buffer (Thermo Fisher) for 3 minutes at room temperature, followed by washing in DMEM 2% FBS. Cell debris was removed using the Miltenyi Debris Removal Solution. Live and dead cells were counted by Trypan Blue staining (Gibco) using a TC20 Cell Counter (Bio-Rad). Cells were diluted to a concentration of 106 cells/ml. We then proceeded to single cell library preparation.
scRNA-seq library preparation and sequencing were provided by the Yale Center for Genome Analysis. Briefly, single cells, reagents and a single Gel Bead containing barcoded oligonucleotides were encapsulated into nanoliter-sized Gel Bead in Emulsion using the GemCode Technology (10× Genomics). The cDNA libraries were constructed using the 10× Genomics Chromium Single Cell 3′ Library Kit. Qualitative analysis was performed using the Agilent Bioanalyzer High Sensitivity DNA assay. The final libraries from WT control (WT-CTRL), RNLS KO control (RNLSKO-CTRL), WT CKD (WT-CKD), and RNLS KO CKD (RNLSKO-CKD), as well as control- and RP81-MNP–treated kidneys, were sequenced on an Illumina HiSeq 4000 sequencer. Cell Ranger version 3.1.0 was used to process Chromium single-cell 3′ RNA sequencing output and align the read to the mouse reference transcriptome (mm10-3.0.0).
The downstream data analysis was performed using Seurat v4.1 R package. The Seurat integration strategy was performed to identify common cell types and enable comparative analyses between WT-CTRL, RNLSKO-CTRL, WT-CKD, and RNLSKO-CKD, as well as control- and RP81-MNP–treated kidneys.15,16 Briefly, WT-CTRL, RNLSKO-CTRL, WT-CKD, and RNLSKO-CKD datasets or control and RP81-MNP datasets were integrated using their identified anchors. A single integrated analysis was performed on all cells. In the quality control analysis, poor-quality cells with <200 (likely cell fragment) or >3500 (potentially cell doublet) unique expressed genes, cells with ≤650 total reads, and cells with ≥50% mitochondrial genes were excluded.17,18 Only genes expressed in five or more cells were used for further analysis. The data were then normalized using SCTransform, in which the confounding sources of variation including mitochondrial mapping percentage were removed.19 Principal component analysis was performed on the normalized/scaled data. The top 20 principal components were chosen for cell clustering because no marked changes were observed beyond 20 principal components. Uniform manifold approximation and projection (UMAP) was used to visualize the single cells on a two-dimensional space, and 20 neighboring points were used in local approximations of manifold structure to cluster the cells. Each cluster was screened for marker genes by differential expression analysis based on the nonparametric Wilcoxon rank sum test for all clusters with genes expressed in at least 25% of cells either inside or outside of a cluster. Clusters with poor-quality cells were further excluded in the downstream analyses. Based on the kidney cell and immune cell lineage-specific marker expression, 22 or 23 cell clusters were identified in each integrated dataset, respectively. The individual dataset was then split by their anchors from the integrated dataset for differential expression analyses. In each cluster, the differential gene expression between control- and RP81-MNP–treated kidneys was visualized in a volcano plot. We analyzed 86,944 cells (WT-CTRL, 9203; RNLSKO-CTRL, 14,048; WT-CKD, 9490; RNLSKO-CKD, 14,927; control, 16,733; and RP81-MNP, 22,543).
The raw data and processed data have been successfully deposited in Gene Expression Omnibus. GSE174014 is the reference Series. Within this Series, GSE174010 is associated with the datasets of control and RP81-MNP; and GSE174013 is associated with the datasets of WT-CTRL, WT-CKD, RNLSKO-CTRL, and RNLSKO-CKD. The details can be found below.
The following secure token has been created to allow review of record GSE174010 although it remains in private status:
Enter token qtyxceccplkdlix into the box.
The following secure token has been created to allow review of record GSE174013 although it remains in private status:
Enter token gvkzsgsqrdextul into the box.
The following secure token has been created to allow review of record GSE174014 although it remains in private status:
Enter token knajiugabtevdcr into the box.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism 7.01 (GraphPad Software, San Diego, CA). The Wilcoxon rank test and the Mann–Whitney test were used for paired and unpaired data, respectively. When appropriate, nonparametric repeated measures ANOVA (Friedman test) was used to evaluate statistical significance. When the Friedman test revealed statistical significance, Dunn’s test was used for pairwise comparisons. A Kaplan–Meier survival analysis was carried out, and a sample size calculation using an ANOVA for two groups indicates that a per-group sample size of 32 would permit detection of an effect size of 0.3 with 85% power. All data are mean±SEM, and values of P < 0.05 were accepted as a statistically significant difference.
Results
Evidence of Subclinical Injury in Human Subjects Following the First Cisplatin Dose
Repeated administration of cisplatin causes CKD, which limits its use in cancer treatment. The development of cisplatin-induced CKD can only be prevented by dose reduction or avoidance, which decreases cisplatin’s therapeutic effectiveness. A recent study reported time-dependent changes in urinary KIM-1 during an initial and subsequent cycles of cisplatin chemotherapy and found that KIM-1 levels failed to return to the initial baseline levels after subsequent cycles, consistent with unresolved kidney injury.20
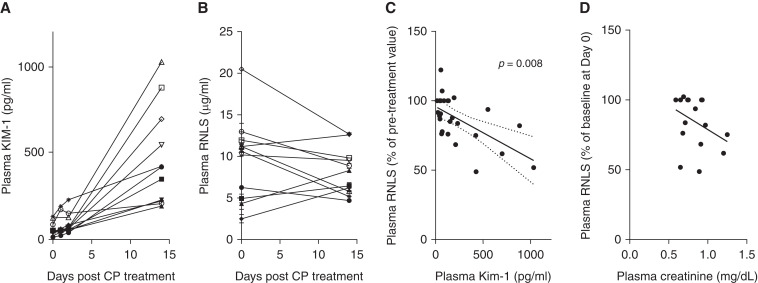
We previously observed that RNLS deficiency exacerbated cisplatin-mediated AKI,8 and asked, in the case of human subjects, whether the first dose of cisplatin was associated with a reduction in plasma RNLS and whether such changes correlated with plasma KIM-1 levels. We obtained pre and post (0, 1, 2, and 14 days) plasma samples from 11 patients who were receiving their first dose of cisplatin for cancer treatment. Plasma RNLS and KIM-1 levels were measured by ELISA. Renal function was evaluated using plasma creatinine and eGFR (Supplemental Table 2). At day 14 post the first dose of cisplatin, only one subject had an observable decrease in eGFR to 57 ml/min per 1.73m2 (Supplemental Table 2). By contrast, plasma KIM-1 levels increased significantly in all 11 patients (day 0 versus day 14: 60.62±11.84 pg/ml versus 474±86.35 pg/ml, P<0.001) confirming that a single dose of cisplatin is associated with unresolved, subclinical kidney injury (Figure 1A). In seven out of 11 subjects, plasma RNLS levels were reduced at day 14 post cisplatin treatment (Figure 1B), and in this subset, changes in plasma RNLS (from day 0 to day 1, 2, and 14) were significantly correlated with increased plasma KIM-1 (Figure 1C). A fall in plasma RNLS levels also trended toward elevated creatinine levels but did not reach statistical significance (Figure 1D). These data suggest that the first dose of cisplatin may be associated with subclinical renal injury that is unresolved up to 2 weeks later and may, in a subset of patients, be associated with a fall in plasma RNLS.
Figure 1.
Evidence of subclinical kidney injury in human subjects treated with cisplatin and correlation between changes in plasma RNLS and KIM-1 levels. (A) Plasma KIM-1 measured in 11 subjects at baseline, and at 1, 2, and 14 days post cisplatin treatment. (B) RNLS levels in patients prior to and 14 days post cisplatin treatment, n=11. (C) In the subset (n=7) of subjects with decreased plasma RNLS at day 14, correlation between change in RNLS levels from baseline and KIM-1 levels; (Pearson’s r=–0.6084; 95% confidence interval, –0.8028 to –0.2970 [dotted line on graph]; n=27 pairs, P=0.008). (D) Correlation between changes in plasma RNLS and creatinine levels (Pearson’s r=–0.3687; 95% confidence interval, 0.7215 to 0.1361; R squared=0.1359, n=17 pairs, P=0.15).
Stepwise Decrease in Kidney RNLS Expression in Cisplatin-Mediated CKD
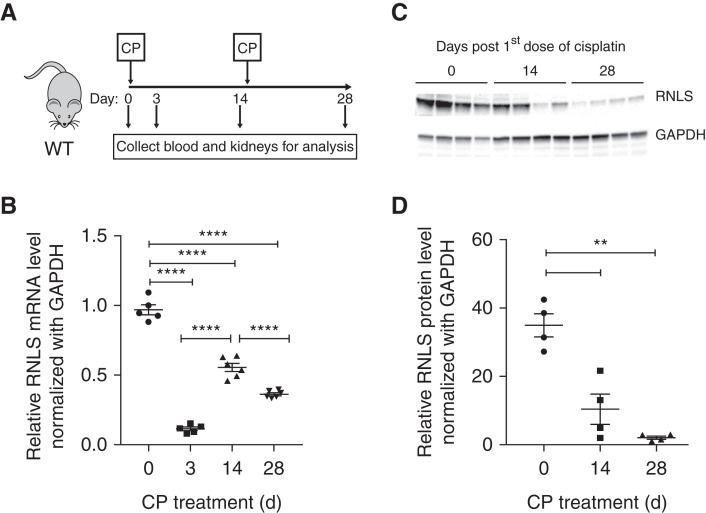
We previously showed that exogenous RNLS administration protects against AKI.8 To determine if RNLS expression changes during the development of CKD, we induced CKD in C57Bl/6J mice using two doses of cisplatin (Figure 2A). Mice were euthanized at days 3 and 14 after the first cisplatin dose, and also 14 days after the second dose (28 days after the first dose). At each time point, we measured plasma creatinine and collected kidney tissue to confirm AKI and CKD development (data not shown). As shown in Figure 2B, RNLS mRNA levels decreased by 88%±1.33% (n=5, P<0.001) 3 days post cisplatin treatment. Two weeks after the first cisplatin dose, although kidney function recovers as indicated by plasma creatinine level (data not shown), RNLS mRNA levels did not return to normal (55.64%±2.98% of baseline value, n=5, P<0.001). Moreover, 2 weeks after the second dose of cisplatin, RNLS mRNA levels were further reduced to 36.32%±1.04% of baseline value (n=6, P<0.001). Additional studies demonstrated that this reduction of RNLS mRNA was sustained for up to 6 weeks after the second dose of cisplatin (35.29%±1.62%, n=6, P<0.001). Similar to kidney RNLS mRNA expression, protein levels were reduced by 51.12%±2.38% (n=3, P<0.001) 2 weeks after the first dose of cisplatin and further by 57.44%±5.59% (n=3, P<0.001) 2 weeks after the second dose of cisplatin (Figure 2, C and D). We interpret these results to indicate that CKD development is associated with a stepwise reduction in RNLS expression at the level of both transcription and translation.
Figure 2.
Decreased kidney renalase expression in the cisplatin CKD mouse model. (A) Schematic representation of experimental design for mouse model of CKD. (B) Change in kidney RNLS mRNA levels during the course of CKD development measured by quantitative PCR (n=5, ****P<0.0001). (C) Change in kidney RNLS protein expression during CKD development measured by Western blot (n=3). (D) Quantification of immunoblot (values are the mean±SEM of three mice at each time point, **P<0.01). CP, cisplatin.
A Severe Model of Cisplatin-Mediated AKI and CKD Established by Genetic Deletion of RNLS
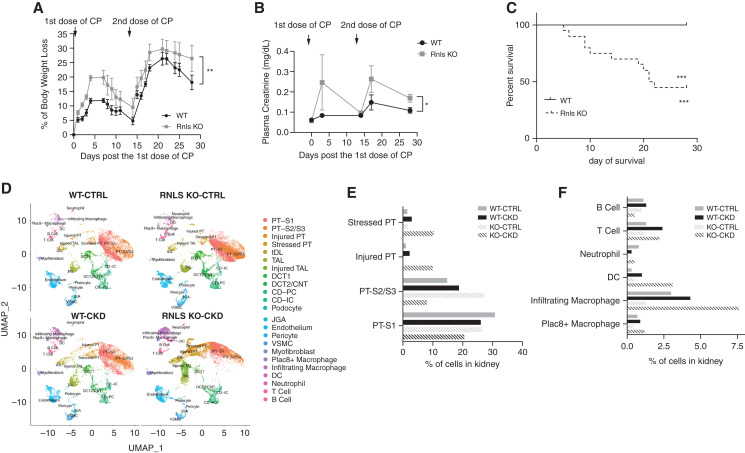
To determine whether this reduction of RNLS expression in CKD is central to the development of CKD, we compared the development of cisplatin-induced CKD in WT and RNLS KO mice. Body weight loss, a known side effect of cisplatin in mice,21 was significantly greater in RNLS KO mice compared with WT: 26.45%±4.60% versus 18.17%±2.51% (n=9, P<0.05) (Figure 3A). RNLS KO sustained more severe AKI (plasma creatinine: 0.247±0.136 mg/dl for KO versus 0.083±0.005 mg/dl for WT, n=6, P=0.29) and CKD (plasma creatinine: 0.17±0.02 mg/dl for KO versus 0.11±0.01 mg/dl for WT, n=6, P<0.05) (Figure 3B), and decreased survival at 4 weeks (55% for KO versus 100% for WT, n=20, P<0.005) (Figure 3C), These results provide strong evidence that RNLS deficiency promotes more severe cisplatin-induced AKI and CKD.
Figure 3.
Genetic deletion of RNLS associated with severe cisplatin-mediated AKI and CKD. RNLS KO mice and WT mice were given two intraperitoneal doses of cisplatin (15 mg/kg) administered two weeks apart. (A) Mouse body weight was measured five times a week for 4 weeks; the percentage of body weight loss was calculated as (body weight at day 0 − body weight)/(body weight at day 0)×100 and plotted, (**P<0.0005 at day 3, WT n=5, RNLS KO n=9). (B) Plasma creatinine level at baseline (day 0), 3 days post first CP dose (day 3), day 14 (before second dose), day 17 (3 days post second dose) and day 28 (at time of euthanization) (*P=0.029, n=6). (C) Survival of WT versus KO mice after cisplatin treatments. Data from (C) were analyzed using Kaplan–Meier curves and a log-rank test, ***P<0.0001. (D) UMAP plots from scRNA-seq, showing the renal cell populations in kidneys from the control and CP-induced CKD mice of WT and RNLS KO. (E and F) Percentage of PT and KIM-1 positive PT cells (E) and neutrophils, T cells, and macrophages (F) in kidneys of indicated mice. CP, cisplatin; PT, proximal tubule; tDL, thin descending limb; TAL, thick ascending limb; DCT, distal convoluted tubule; CNT, connecting tubule; CD, collecting duct; PC, principal cell; IC, intercalated cell; JGA, juxtaglomerular apparatus; VSMC, vascular smooth muscle cell; DC, dendritic cell.
To further evaluate the transcriptional changes in kidney that accompanied the development of cisplatin-induced CKD, we compared WT and RNLS KO control mice with those with cisplatin-induced CKD using scRNA-seq. Gene expression patterns were used to identify cell clusters (Supplemental Figures 1 and 2). UMAP plots are shown in Figure 3D and the results are quantified in Figure 3 E-F, Supplemental Table 3. The analysis also revealed a dramatic increase in injured proximal tubule (10.0% in KO versus 2.3% in WT), stressed proximal tubule (10.3% in KO versus 3.0% in WT), and injured thick ascending limb cells (8.0% in KO versus 1.0% in WT) in RNLS KO mice. The inflammatory response associated with cisplatin-induced CKD was more intense in RNLS KO compared with WT mice: 5.3-fold versus 1.4-fold increase in infiltrated macrophages, and a 5-fold increase of dendritic cells and neutrophils in RNLS KO but only a slight change in WT (Figure 3F).
These data strongly suggest that RNLS protects proximal tubular cells and reduces the inflammatory response associated with cisplatin-induced CKD.
Kidney-Targeted Renalase Agonist (RP81-MNP) Protects Severe Model of Cisplatin-Mediated CKD
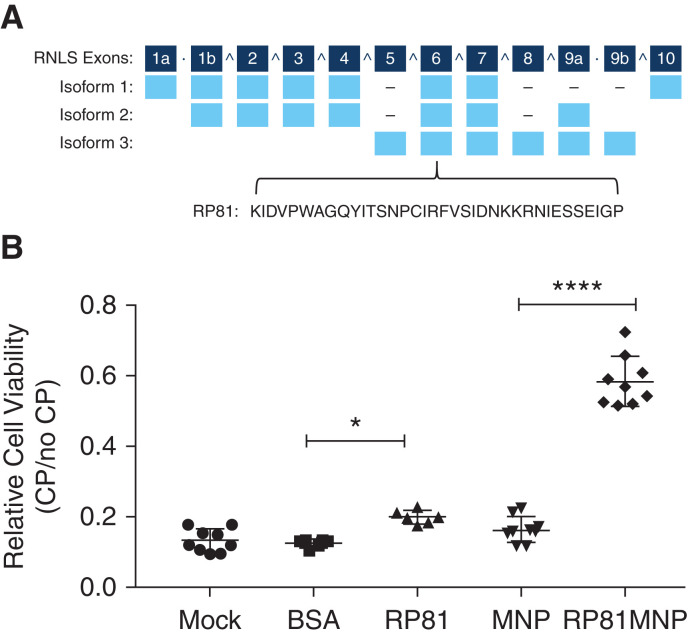
Because RNLS deficiency develops during cisplatin-induced CKD and the genetic deletion of RNLS results in more severe cisplatin-induced CKD, we hypothesized that a kidney-targeted RNLS agonist could rescue the severe phenotype. We previously identified the region of the RNLS protein that mediates binding to its cognate receptor PMCA4b and activates intracellular survival pathways.8 We previously demonstrated that a 20 amino acid RNLS peptide (RP220) derived from this region was as effective as recombinant RNLS in protecting HK-2 cells from toxic injury and preventing ischemic injury in WT mice.6 We tested several derivatives of RP220 and identified peptide RP81 (36 amino acids) as the most active in in vitro cisplatin cytotoxicity studies (Figure 4A). We sought to deliver RP81 to kidney tissue by conjugating it with kidney-specific MNPs.12
Figure 4.
RNLS agonist peptide RP81 protects in vitro against cisplatin-induced cytotoxicity. (A) Schematic drawing of RNLS gene showing exons (numbered), the three major isoforms, and sequences of peptides RP81 derived from exon 6, which is conserved in all splicing variants. (B) A representative WST-1 assay: mouse proximal tubular cells TKPTS were treated without (Mock) or with BSA, or RP81, or empty MNP, or RP81 encapsulated in MNP (RP81-MNP, n=9) for 24 hours, followed by 24-hour treatment with or without cisplatin. The relative cell viability is expressed as mean±SEM; n=9 for Mock, MNP, and RP81-MNP; n=3 for BSA; n=6 for RP81. *P<0.05; ****P<0.0001.
The TKPTS cell line exhibits light and ultramicroscopic proximal tubule morphology and is widely used to study renal proximal tubule cell transport, proximal tubule damage, cell death, and signaling pathways involved in the cytotoxicity of cisplatin.22 To determine whether the MNP accumulated in TKPTS cells, we performed immunofluorescence staining of cells treated with or without MNP for 24 hours followed by fixation alone or fixation and permeabilization using anti-PEG, a component of MNP, for visualizing MNP. As shown in Supplemental Figure 3, positive staining was detected only in permeabilized cells, indicating intracellular localization of MNP in TKPTS cells. We found that both RP81 and conjugated RP81 (RP81-MNP) reduced cisplatin-mediated injury in TKPTS cells and noted that RP81-MNP was significantly (2.9-fold) more effective than RP81 (Figure 4B).
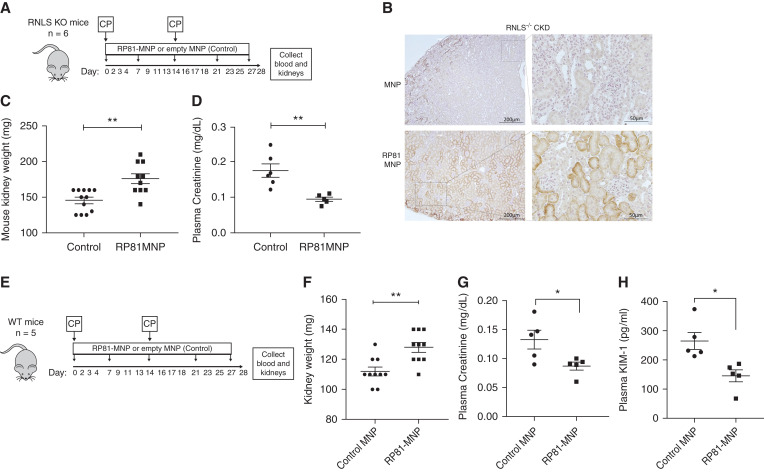
RNLS KO mice received two doses of cisplatin, 14 days apart along with either RP81-MNP or empty MNP once a week for 4 weeks (Figure 5A). We have previously shown that these MNPs targeted the proximal tubules and released payload in a controlled manner,11 and kidney delivery of RP81-MNP was documented by staining for the RNLS peptide in kidney sections using a peptide-specific monoclonal antibody (Figure 5B). Compared with MNP alone, RP81-MNP administration provided RNLS KO with significant protection against cisplatin-induced CKD as evidenced by preserved kidney weight (176±7.02 mg, n=10 versus 145.4±4.46 mg, n=10; P<0.05) (Figure 5C), and lower plasma creatinine levels (0.095±0.006 mg/dl for RP81-MNP, n=5 versus 0.177±0.019 mg/dl for MNP, n=6; P<0.005) (Figure 5D).
Figure 5.
Kidney-targeted delivery of RP81 (RP81-MNP) protects against cisplatin-induced CKD. RP81-MNP was tested in RNLS KO mice (A–D) and WT mice (E–H): (A,E) Schematic of treatment protocol; RP81 encapsulated in MNP (RP81-MNP) or empty MNP (control) was injected weekly and cisplatin (CP) was injected biweekly into RNLS KO mice (A) or WT mice (E); at the end of the experiment, blood was drawn for plasma and kidneys were collected. (B) Representative immunohistochemical staining for RP81 in RNLS KO kidneys from mice treated with MNP (top) or RP81-MNP (bottom) using rabbit monoclonal antibody m28 raised against RNLS 220–230 aa. Positive staining is represented by brown color in tubular epithelia. (C) RNLS KO mouse kidney weight: n=12 in control group, n=10 in RP81-MNP group, **P<0.005 by t test. (D) RNLS KO plasma creatinine: n=6 in control group, n=5 in RP81-MNP, **P<0.005 by t test. (F–H) WT kidney weight (n=10 in each group), plasma creatinine (n=5 in each group), and plasma KIM-1 (n=5 in each group) levels, *P<0.05; **P<0.005 by t test.
We also studied the effect of RP81 in protecting WT mice from cisplatin-induced CKD (Figure 5E). Both naked RP81 (Supplemental Figure 4) and RP81-MNP reduced the severity of CKD. Compared with those receiving MNP only, mice treated with RP81-MNP had higher kidney mass than the controls (128±3.266 mg versus 112±2.906 mg, n=10 each; P<0.005) (Figure 5F), lower plasma creatinine (0.0868±0.0069 mg/dl versus 0.1328±0.0161 mg/dl, n=5 each; P<0.05) (Figure 5G), and decreased plasma KIM-1 (145.2±20.68 pg/ml versus 264.5±29.38 pg/ml, n=5 each; P<0.05) (Figure 5H).
Taken together, these results indicate that the kidney-specific delivery of an RNLS agonist peptide was protective in cisplatin-induced CKD.
RP81-MNP Preserves Proximal Tubular Cell from Cisplatin-Induced Damage and Decreases Inflammation
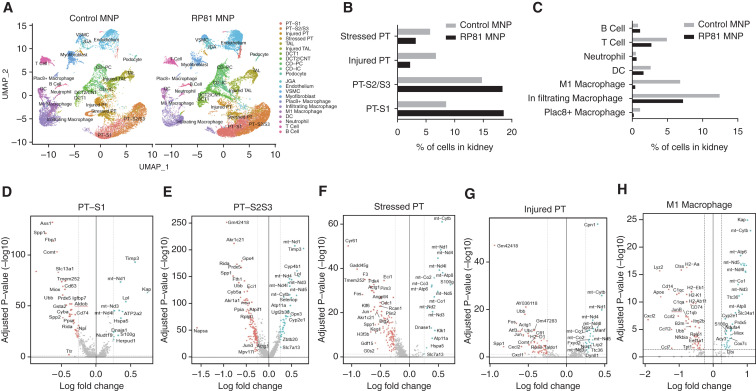
We used several methods to gain insight into the mechanisms underlying the protective action of RP81-MNP in cisplatin-induced CKD. First, scRNA-seq analysis was carried out to evaluate RP81-MNP effect on cisplatin-mediated transcriptional changes in the kidney. Unbiased clustering analysis of 43,412 and 49,439 cells isolated from MNP-treated and RP81-MNP–treated kidneys respectively revealed 18 clusters (Figure 6A, Supplemental Figures 5 and 6). Compared with control MNP treatment, RP81-MNP increased viable proximal tubular cells (PT-S1: 18.6% versus 8.5%; PT-S2/S3: 18.4% versus 14.8%), the distal convoluted tubule/connecting tubule (DCT/CNT, 6.0% versus 3.6%), and the collecting duct (CD-PC and CD-IC, 9.5% versus 5.2%). RP81-MNP also increased the percentage of viable endothelial cells (11.3% versus 7.5%). Conversely, it decreased the percentage of injured proximal tubule (2.2% versus 6.7%) and stressed proximal tubule cells (3.2% versus 5.7%) (Figure 6B and Supplemental Table 4), infiltrating T cells (2.7% versus 4.9%), infiltrating macrophages (7.2% versus 12.4%), inflamed M1 macrophages (0.4% versus 6.8%) (Figure 6C and Supplemental Table 4), and myofibroblasts (2.9% versus 5.8%).
Figure 6.
RP81-MNP decreases inflammatory cells and preserves proximal tubular cells. (A) UMAP plots show the renal cell populations in the kidneys of RNLS KO CKD mouse treated with empty MNP (control, on the left) or MNP loaded with RP81 (RP81-MNP, on the right). The plots are colored by orthogonally generated clusters labeled by manual cell type annotation. (B and C) Proportions of each cell type in control and RP81-MNP samples. (D–H) Volcano plots showing genes whose expression was significantly (Log2 fold change, P<0.05) deregulated by RP81-MNP treatment in kidney PT cells in S1 segment (D), S2/S3 segment (E), and stressed and injured PT (F and G, respectively), and in inflamed macrophages (H). Genes shown in blue on the left of volcano plots are downregulated by RP81-MNP and genes in red on the right of volcano plots are upregulated by RP81-MNP treatment. PT, proximal tubule; TAL, thick ascending limb; DCT, distal convoluted tubule; CNT, connecting tubule; CD, collecting duct; PC, principal cell; IC, intercalated cell; JGA: juxtaglomerular apparatus; VSMC, vascular smooth muscle cell; DC, dendritic cell.
In the renal proximal tubule, RP81-MNP downregulated genes encoding proteins involved in ferroptosis (Gpx4), autophagy (CD63), mitophagy (Ubb, Ubc, Cyba), stress (Ass1), and redox signaling (Prdx5). It reduced Toll-like receptor (TLR) signaling (secreted phosphoprotein 1 [Spp1]), reduced lysosome activation (CD63, Napsa), and modulated the effect of cisplatin in regulators of metabolic pathways (Comt, Fbp1, Miox). RP81-MNP upregulated mitochondrial genes that are involved in oxidative phosphorylation (Cytb, Nd1, Nd3, Nd4L, Nd5)23 in S3 proximal tubule cells (Figure 6, D–G), suggesting that RP81-MNP may stimulate mitochondrial biogenesis and ameliorate the well-described mitochondrial dysfunction observed in CKD. In inflamed macrophages, RP81-MNP downregulated chemokines (Cxcl2, Ccl12, Ccr12, Ccl7), proinflammatory cytokines (IL-1β, TNFα), complement/coagulation factors (C1qa, C1qb, C1qc), antigen process/presenting molecules (H2-Aa, K2-k1, Cd74), genes involved in TLR signaling (Cd14, Spp1), and oxidative stress gene Gadd45 (Figure 6H).
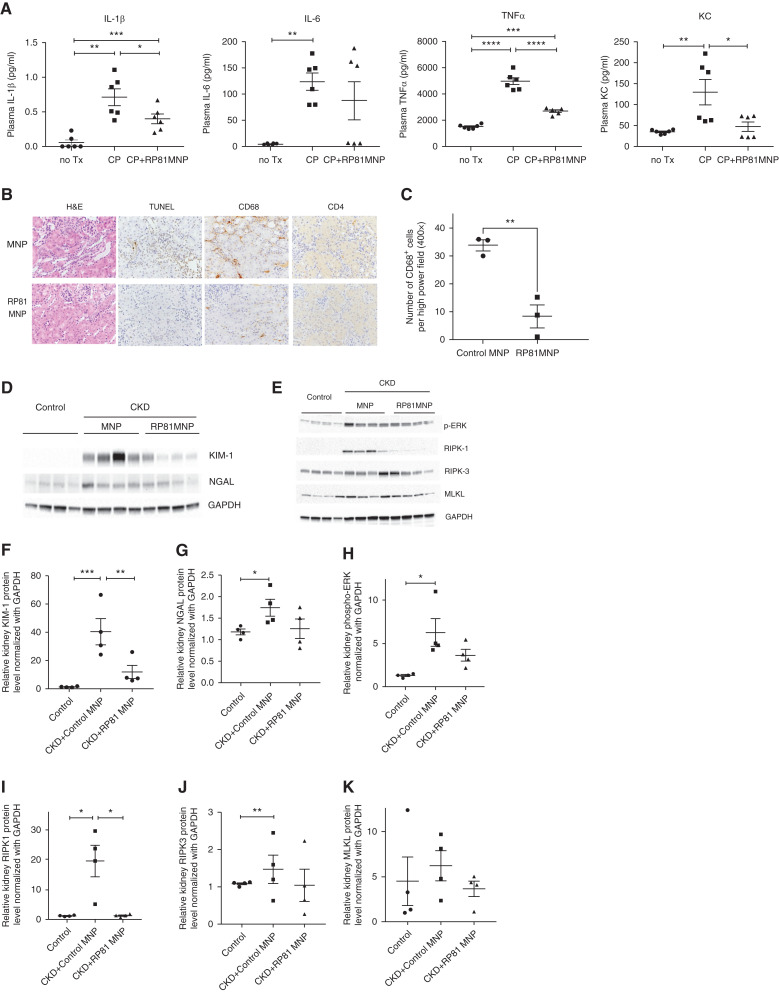
To evaluate the functional significance of the changes observed in gene expression, we measured proinflammatory cytokines in mouse plasma and evaluated the state of activation of proteins involved in regulated necrosis and inflammation. RNLS KO mice were treated without cisplatin or with two doses of cisplatin, 2 weeks apart, together with MNP or RP81-MNP. Plasma and kidneys were collected after 4 weeks of treatment for measurement of cytokines and protein, respectively. As shown in Figure 7A, elevated levels of IL-1β (0.7133±0.1195 pg/ml), IL-6 (124±16.5 pg/ml), TNFα (4959±265 pg/ml), and KC (130±30.3 pg/ml) were detected in the plasma of CKD mice when compared with control mice plasma: IL-1β (0.05667±0.03904 pg/ml), IL-6 (4.598±0.5562 pg/ml), TNFα (1513±65.12 pg/ml), and KC (34.95±2.07 pg/ml). RP81-MNP treatment significantly reduced plasma levels of IL-1β (0.4±0.06976 pg/ml), TNFα (2703±95.07 pg/ml), and KC (47.32±11.01 pg/ml) in CKD mice.
Figure 7.
RP81-MNP decreases inflammatory response and injury markers and regulated necrosis. (A) Plasma levels of cytokines IL-1β, IL-6, TNFα, and KC in RNLS KO control mice and CKD mice treated with empty MNP or RP81-MNP. (B) Representative images of H&E-, TUNEL-, CD68-, and CD4-stained kidney sections at a magnification 400× from CKD mice treated with MNP (control) or RP81-MNP. (C) Stained CD68- and CD4-positive cells at the cortical medullary junction in the interstitial space of kidneys from CKD mice treated with control or RP81-MNP. Five high power field (400×) per mouse, three mice from each group, were counted. Occasional defined clusters of CD68-positive cells were encountered in the control CKD RNLS KO mice but never in the RP81-MNP administered CKD RNLS KO mice. (D and E) Immunoblotting analysis of protein isolated from kidneys at day 28 after two doses of cisplatin and with or without RP81-MNP treatment. Four individual mice per treatment group were analyzed on the blots. (F–K) Quantifications of immunoblots shown in (D) and (E). Differences were analyzed for significance using one way ANOVA corrected for multiple comparisons. *P<0.05; **P<0.001, ***P<0.0005, ****P<0.0001.
Second, we evaluated the histologic and pathologic manifestations of the cisplatin-induced CKD and found that they were also diminished by RP81-MNP (Figure 7B). Nephrotoxicity in the cisplatin- and MNP-treated mice (control) was evidenced by tubular dilatation, tubular epithelial damage, intracellular cast formation, and nuclear irregularity (Figure 7B, upper left image); however, these changes were ameliorated by RP81-MNP treatment (Figure 7B, lower left image). In kidneys of mice treated with RP81-MNP, numbers of apoptotic cells, infiltrating macrophages (CD68 staining) and CD4 T cells were dramatically reduced (Figure 7, B and C).
Lastly, we used Western blot analysis to show that compared with MNP, RP81-MNP administration decreased kidney injury markers KIM-1 and NGAL, reduced ERK phosphorylation, and reduced activation of necroptosis molecules RIPK1, RIPK3, and MLKL (Figure 7, D-K). Single cell RNA sequencing revealed that RP81-MNP decreased NGAL expression in TAL, CD-PC, and neutrophil, KIM-1 in injured PT, and secreted phosphoprotein 1 (Spp1) in most cell types (Supplemental Figure 7).
The results obtained above indicate that RP81-MNP protects against cisplatin-induced CKD by preventing cell death, reducing the inflammatory response, and preserving renal proximal tubular function.
Discussion
Cisplatin is an effective chemotherapeutic agent for solid tumors but repeated doses lead to nephrotoxicity24 and prevention of CKD development in such treated patients is lacking. We previously reported that RNLS and an agonist peptide protected from cisplatin-induced AKI.8 In this study, we sought to uncover such a role for RNLS and its agonist peptide RP81 in cisplatin-induced CKD. RNLS is mainly expressed in the kidney and here we report that plasma RNLS is reduced in patients receiving cisplatin and correlates with elevated KIM-1 and creatinine. Its expression was also reduced in kidneys of mice during the course of cisplatin-induced CKD. In RNLS knockout mice, more severe CKD was induced after repeated doses of cisplatin as evidenced by increased plasma creatinine levels compared with WT mice. These data suggest a role of RNLS in prevention of CKD. RNLS levels in the kidney failed to be restored to normal 2 weeks after the first dose of cisplatin at a time when plasma creatinine was normalized. This suggests that the low level of RNLS at the time of exposure to the second dose may be linked to the development of CKD. Restoring RNLS levels may have reduced the potential of the second dose of cisplatin to cause the development of CKD. Furthermore, measurement of RNLS may be used as a susceptibility marker for development of CKD.
We chose to use a biologic active peptide instead of the recombinant protein to test for its therapeutic efficacy because such peptides are easy to synthesize and yield high lot-to-lot consistency. Furthermore, we have shown that such peptides have cytoprotective activity.5–7 RP81 protected cisplatin-induced cytotoxicity in vitro and in vivo. We utilized the technology of mesoparticles for kidney-specific delivery of RP81 in vivo because of the following: (1) cisplatin preferentially collects in the proximal tubules; (2) we speculated that such delivery of RP81 would be preferred in the background of cancer, as we have shown that dysregulated RNLS expression may be associated with tumor growth; and (3) there is a concern that RP81 might diminish the efficacy of cisplatin against cancer cells. We showed that we could deliver RNLS active peptide specifically to mouse kidney using MNPs and this RP81-MNP protected mice from cisplatin-induced CKD. This protective effect was greater than what was seen in naked RP81-administered WT mice.
RP81 delivery to the proximal tubule, the principal site of cisplatin-induced damage was the most prominent site of protection.2,3,24 Encapsulated RP81 peptide reduced proximal tubule damage, reduced oxygen stress, diminished mitochondrial damage, reduced inflammation, and restored expression of proximal tubule transport genes, all of which play a prominent role in cisplatin-induced AKI and CKD.25–28 From the scRNA-seq study, we found that RP81-MNP induced the mitochondrial genes Cytb, Nd1, Nd3, Nd4L, and Nd5. Cytb is a ubiquinol-cytochrome c reductase component of respiratory chain complex III, which plays a vital role in supporting the life of cells.25 Nd1, Nd3, Nd4L, and Nd5 encode subunits of NADH-ubiquinone oxidoreductase complex I, which is a key source of reactive oxygen species (ROS).26 The concentration and ratio of NAD+ and NADH, the redox states of the coenzyme Q, and many other factors control ROS production.27 The changes we observed in these genes both as a consequence of cisplatin-induced mitochondrial damage and the mitigation observed by RP81 suggest that RP81 increases the state of reduction of complex I. As ROS production and disposal plays a key role in cell signaling, apoptosis, and necrosis, the observation that RP81-MNP upregulated these reductase subunits suggests that RNLS agonist RP81 may ameliorate cisplatin-induced CKD, at least in part, by regulating mitochondrial ROS production and reducing oxidative stress.28
RP81-MNP treatment reduced the expression of Spp1 in all kidney cells activated after cisplatin as revealed by scRNA-seq analysis. Spp1 is involved in many physiologic and pathologic processes, including biomineralization, tissue remodeling, and inflammation29; it is a cytokine that plays a role in the recruitment of monocytes/macrophages and is a powerful mediator of the inflamed state.30,31
Cisplatin dramatically increased Spp1 expression and RP81-MNP treatment reduced it, suggesting that RNLS may suppress inflammation by inhibiting Spp1.
We observed that RP81-MNP not only reduced inflammation in kidney but also reduced proinflammatory cytokines such as IFNγ, IL-1β, IL-6, and TNFα in plasma. Because encapsulated RP81 is restricted to the kidney, these observations suggest that the origin of the circulating cytokines is derived from injured kidney and thus RP81 would be expected to reduce the systemic effects of CKD.
We identified proximal tubule cells that demonstrated diminished expression of transport proteins, as well as the reexpression of cell cycle–related genes and increased rates of DNA synthesis32,33 that occupy a space between proximal tubule and immune cells in the tSNE embedding, suggesting that these cells represent cisplatin-damaged tubular cells. These cells could behave like antigen-presenting cells that attract immune cells. Previously, we reported that repeated doses of cisplatin resulted in CKD by sustained activation of cell stress molecules and prolonged expression of genes that underlie regulated necrosis.4 Sustained activation of ERK and JNK after the second dose of cisplatin correlates with morphologic and functional indications of failed tissue repair, suggesting a role for these immediate early gene products in modulating injury and repair processes in cisplatin nephrotoxicity. This failure to resolve brief activation of the immediate early gene response seems to mark the conversion of AKI to CKD in cisplatin-induced CKD. In this study, we showed that treatment with RP81-MNP reduced ERK and JNK activation and reduced RIPK1 level and likely reduced the level of RIPK3 and MLKL, suggesting another molecular mechanism by which RP81-MNP protects the kidney from cisplatin-induced CKD. Unrelieved epithelial injury and the induction of regulated necrosis are key events in the development of cisplatin-induced CKD.2,3 Regulated necrosis can activate necroinflammation, a process characterized by the autoamplification loop between cell necrosis and inflammatory tissue response.32,34,35 In this study such enhanced necroptosis was inhibited by RP81 as was the reduction of the upstream regulators of necroptosis such as TNFα and TLR4, which are also important mediators in acute cisplatin nephrotoxicity.35,36 Cisplatin-induced injury causes a release of damage-associated molecular patterns that activate TLR4 to stimulate the production of various chemokines and cytokines, including TNFα, which further exacerbate cisplatin nephrotoxicity.32,33,37 Reeves and colleagues demonstrate that inhibiting TNFα and TLR4 protects against acute cisplatin nephrotoxicity, and reduces renal dysfunction, tubular injury, and inflammation after cisplatin treatment.34,37 Thus, RNLS may have direct and indirect effects on cell death pathways interrupting both the primary initiators of the process and the amplification of the process by continued cell death. Another process leading to cisplatin-induced cell death, ferroptosis, was also reduced by mesoparticle-delivered RP81 as increased Acsl4, a key driver of the process, was reduced by RP81. Ferroptosis plays a significant role in cisplatin-induced AKI and other models of CKD.38 The greater mortality not only may reflect the greater injury observed in the kidney but also may be a reflection of injury to other organs by enhancing the immunologic consequences of such injury.
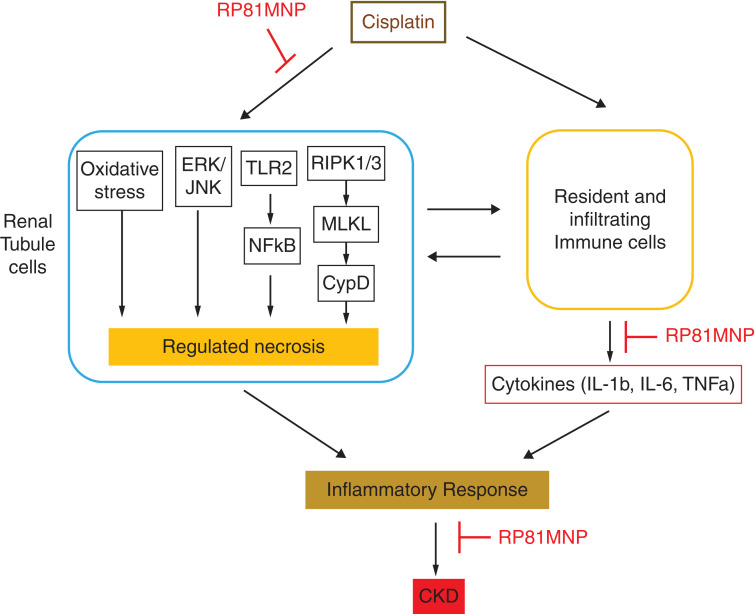
In summary, RNLS peptide RP81-MNP reduced tubular injury, oxidative stress, and inflammation in kidneys damaged by cisplatin, whereas RNLS deletion amplified these pathogenic pathways and led to more severe CKD (Figure 8). These findings suggest that RNLS agonists may be effective therapeutic agents to prevent CKD in patients treated with repeated doses of cisplatin.
Figure 8.
Proposed mechanism mediating the protective action of renalase against cisplatin-induced CKD. RNLS agonist RP81-MNP improved renal functions in cisplatin-induced CKD, possibly through inhibiting the regulated necrosis and the inflammatory response to cisplatin.
Disclosures
B. Burtness reports consultancy agreements with AstraZeneca, Cue, Debio, Genentech, IO Biotech, Kura, MacroGenics, Merck, Merck KGaA, Nektar, Nuvelution, Rakuten, and Vaccinex; reports research funding with Bristol Myers Squibb, Cue, Formation Biologics, Glaxo, and Merck; reports honoraria with AstraZeneca, Cue, Debio, Education Alliance, Genentech, i3, IO Biotech, Kura, MacroGenics, Merck, Merck KGaA, Nektar, Nuvelution, Oncology Education, PracticeUpdate, and Vaccinex; and reports other interests/relationships with Fanconi Anemia Research Fund, Stand Up to Cancer, American Society of Clinical Oncology, Eastern Cooperative Oncology Group-American College of Radiology Imaging Network, and the Society for Immunotherapy of Cancer. G Desir is a named inventor on several issued patents related to the discovery and therapeutic use of RNLS (licensed to Bessor Pharma); holds an equity position in Bessor Pharma and its subsidiary Personal Therapeutics; and reports scientific advisor or membership with Bessor Pharma and Personal Therapeutics. D. Heller reports consultancy agreements with Concarlo Holdings, Mediphage Bioceuticals, and Nanorobotics; reports ownership interest with Goldilocks Therapeutics, LipidSense, and Nirova BioSense; and reports scientific advisor or membership with Concarlo Holdings, Goldilocks Therapeutics, LipidSense, Mediphage Bioceuticals, Nanorobotics, and Nirova BioSense. R. Safirstein reports ownership interest with Apple, Apple XPO, Netflix, and Target; and reports scientific advisor or membership with RenalSense. R. Williams reports consultancy agreements with Goldilocks Therapeutics; reports ownership interest with Goldilocks Therapeutics; and reports scientific advisor or membership with Goldilocks Therapeutics. All remaining authors have nothing to disclose.
Funding
This work was supported in part by VAMC, Connecticut (to R. Safirstein and G.V. Desir) and by National Institutes of Health grants RC1DK086465, RC1DK086402, and R01DK081037 (to G.V. Desir) and grant K01DK120783 (to L. Xu).
Supplementary Material
Acknowledgments
Dr. Xiaojia Guo conceived and carried out some of the experiments and helped write the manuscript; Dr. Leyuan Xu analyzed the scRNA-seq data and helped write the manuscript; Dr. Heino Velazquez conceived and carried out some of the experiments and helped write the manuscript; Mr. Tian-min Chen carried out some of the experiments; Dr. Ryan M. Williams carried out some of the experiments and helped write the manuscript; Dr. Daniel A. Heller conceived some of the experiments and helped write the manuscript; Dr. Barbara Burtness conceived some of the experiments and helped write the manuscript; Dr. Robert Safirstein conceived some of the experiments and helped write the manuscript; and Dr. Gary V. Desir conceived some of the experiments and helped write the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Cisplatin-Induced Kidney Injury: Delivering the Goods,” on pages 255–256.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021040439/-/DCSupplemental.
Supplemental Figure 1. Dot plot of single cell RNA sequencing comparing WT versus KO kidneys.
Supplemental Figure 2.Heat map of gene expression levels in WT versus KO kidneys.
Supplemental Figure 3. Immunofluorescence staining of polyethylene glycol in TKPTS cells.
Supplemental Figure 4. RP81 protected WT mice from cisplatin-induced CKD.
Supplemental Figure 5. Dotplot of single cell RNA sequencing comparing kidneys from RNLS KO CKD mice treated with control vs. RP81MNP.
Supplemental Figure 6. Heat Map of gene expression levels in kidneys from RNLS KO CKD mice treated with control vs. RP81MNP.
Supplemental Figure 7A. Violin plots of NGAL, KIM1, and Spp1 in kidneys from WT and RNLS KO control and CKD mice.
Supplemental Figure 7B. Violin plots of NGAL, KIM1, and Spp1 in kidneys from RNLS KO CKD mice treated with RP81-MNP.
Supplemental Table 1. Description of Antibodies used.
Supplemental Table 2. Human subject characteristics, eGFR, serum creatinine, KIM-1, and RNLS levels.
Supplemental Table 3. Cell numbers in each cluster and % of cells in kidneys of WT vs. RNLS KO mice treated with or without 2 doses of cisplatin for 4 weeks.
Supplemental Table 4. Cell numbers in each cluster and % of cells in kidneys of RNLS KO mice treated with control or RP81 MNP.
References
- 1.Centers for Disease Control and Prevention : Chronic Kidney Disease in the United States, 2021, Atlanta, GA, US Department of Health and Human Services, Centers for Disease Control and Prevention, 2021 [Google Scholar]
- 2.Bhat ZY, Cadnapaphornchai P, Ginsburg K, Sivagnanam M, Chopra S, Treadway CK, et al. : Understanding the risk factors and long-term consequences of cisplatin-associated acute kidney injury: An observational cohort study. PLoS One 10: e0142225, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres R, Velazquez H, Chang JJ, Levene MJ, Moeckel G, Desir GV, et al. : Three-dimensional morphology by multiphoton microscopy with clearing in a model of cisplatin-induced CKD. J Am Soc Nephrol 27: 1102–1112, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landau SI, Guo X, Velazquez H, Torres R, Olson E, Garcia-Milian R, et al. : Regulated necrosis and failed repair in cisplatin-induced chronic kidney disease. Kidney Int 95: 797–814, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo X, Wang L, Velazquez H, Safirstein R, Desir GV: Renalase: Its role as a cytokine, and an update on its association with type 1 diabetes and ischemic stroke. Curr Opin Nephrol Hypertens 23: 513–518, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y, Xu J, Velazquez H, Wang P, Li G, Liu D, et al. : Renalase deficiency aggravates ischemic myocardial damage. Kidney Int 79: 853–860, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Lee HT, Kim JY, Kim M, Wang P, Tang L, Baroni S, et al. : Renalase protects against ischemic AKI. J Am Soc Nephrol 24: 445–455, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Velazquez H, Moeckel G, Chang J, Ham A, Lee HT, et al. : Renalase prevents AKI independent of amine oxidase activity. J Am Soc Nephrol 25: 1226–1235, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang J, Guo X, Rao V, Gromisch ES, Chung S, Kluger HM, et al. : Identification of two forms of human plasma renalase, and their association with all-cause mortality. Kidney Int Rep 5: 362–368, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams RM, Shah J, Ng BD, Minton DR, Gudas LJ, Park CY, et al. : Mesoscale nanoparticles selectively target the renal proximal tubule epithelium. Nano Lett 15: 2358–2364, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams RM, Shah J, Tian HS, Chen X, Geissmann F, Jaimes EA, et al. : Selective nanoparticle targeting of the renal tubules. Hypertension 71: 87–94, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han SJ, Williams RM, D’Agati V, Jaimes EA, Heller DA, Lee HT: Selective nanoparticle-mediated targeting of renal tubular Toll-like receptor 9 attenuates ischemic acute kidney injury. Kidney Int 98: 76–87, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, Li G, Wang P, Velazquez H, Yao X, Li Y, et al. : Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest 115: 1275–1280, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo X, Hollander L, MacPherson D, Wang L, Velazquez H, Chang J, et al. : Inhibition of renalase expression and signaling has antitumor activity in pancreatic cancer. Sci Rep 6: 22996, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R: Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol 36: 411–420, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang HM, Subramaniam M, Targ S, Nguyen M, Maliskova L, McCarthy E, et al. : Multiplexed droplet single-cell RNA-sequencing using natural genetic variation. Nat Biotechnol 36: 89–94, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M, et al. : Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360: 758–763, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudman-Melnick V, Adam M, Potter A, Chokshi SM, Ma Q, Drake KA, et al. : Single-cell profiling of AKI in a murine model reveals novel transcriptional signatures, profibrotic phenotype, and epithelial-to-stromal crosstalk. J Am Soc Nephrol 31: 2793–2814, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hafemeister C, Satija R: Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol 20: 296, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George B, Wen X, Mercke N, Gomez M, O’Bryant C, Bowles DW, et al. : Time-dependent changes in kidney injury biomarkers in patients receiving multiple cycles of cisplatin chemotherapy. Toxicol Rep 7: 571–576, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishiyama N, Okazaki S, Cabral H, Miyamoto M, Kato Y, Sugiyama Y, et al. : Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res 63: 8977–8983, 2003 [PubMed] [Google Scholar]
- 22.Hodeify R, Megyesi J, Tarcsafalvi A, Safirstein RL, Price PM: Protection of cisplatin cytotoxicity by an inactive cyclin-dependent kinase. Am J Physiol Renal Physiol 299: F112–F120, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szeto HH: Pharmacologic approaches to improve mitochondrial function in AKI and CKD. J Am Soc Nephrol 28: 2856–2865, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arany I, Safirstein RL: Cisplatin nephrotoxicity. Semin Nephrol 23: 460–464, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Blakely EL, Mitchell AL, Fisher N, Meunier B, Nijtmans LG, Schaefer AM, et al. : A mitochondrial cytochrome b mutation causing severe respiratory chain enzyme deficiency in humans and yeast. FEBS J 272: 3583–3592, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Larosa V, Remacle C: Insights into the respiratory chain and oxidative stress. Biosci Rep 38: BSR20171492, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robb EL, Hall AR, Prime TA, Eaton S, Szibor M, Viscomi C, et al. : Control of mitochondrial superoxide production by reverse electron transport at complex I. J Biol Chem 293: 9869–9879, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kausar S, Wang F, Cui H: The role of mitochondria in reactive oxygen species generation and its implications for neurodegenerative diseases. Cells 7: 274, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denhardt DT, Guo X: Osteopontin: A protein with diverse functions. FASEB J 7: 1475–1482, 1993 [PubMed] [Google Scholar]
- 30.Icer MA, Gezmen-Karadag M: The multiple functions and mechanisms of osteopontin. Clin Biochem 59: 17–24, 2018 [DOI] [PubMed] [Google Scholar]
- 31.Lamort AS, Giopanou I, Psallidas I, Stathopoulos GT: Osteopontin as a link between inflammation and cancer: The thorax in the spotlight. Cells 8: 815, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kers J, Leemans JC, Linkermann A: An overview of pathways of regulated necrosis in acute kidney injury. Semin Nephrol 36: 139–152, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB: Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2: 2490–2518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramesh G, Reeves WB: TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol 285: F610–F618, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Linkermann A, Stockwell BR, Krautwald S, Anders H-J: Regulated cell death and inflammation: An auto-amplification loop causes organ failure. Nat Rev Immunol 14: 759–767, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Linkermann A, Bräsen JH, Darding M, Jin MK, Sanz AB, Heller JO, et al. : Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A 110: 12024–12029, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB: TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J Am Soc Nephrol 19: 923–932, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belavgeni A, Meyer C, Stumpf J, Hugo C, Linkermann A: Ferroptosis and necroptosis in the kidney. Cell Chem Biol 27: 448–462, 2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.