Significance Statement
Plasma donor-derived cell-free DNA (cfDNA) measured as a percent of total cfDNA (dd-cfDNA[%]) has been proposed as a screening test for kidney transplant rejection. The prospective Trifecta study examined relationships between dd-cfDNA(%) measured at the time of indication biopsy and the genome-wide molecular findings in 300 biopsies from kidney transplant recipients assessed by microarrays. The dd-cfDNA(%) correlated with active rejection in the biopsy, and molecular scores predicted dd-cfDNA(%) ≥1.0% better than histologic scores. The top transcripts in the biopsy correlating with dd-cfDNA(%) were related to IFN-γ effects and natural killer cells. AKI and atrophy fibrosis were associated with mildly elevated dd-cfDNA(%), although some biopsies with high dd-cfDNA(%) revealed no rejection. These findings indicate that plasma dd-cfDNA levels are strongly related to the active molecular rejection processes in indication biopsies.
Keywords: cell-free DNA, rejection, transplantation, microarrays, genomics, biopsy, blood donors, phenotype
Abstract
Background
The relationship between the donor-derived cell-free DNA fraction (dd-cfDNA[%]) in plasma in kidney transplant recipients at time of indication biopsy and gene expression in the biopsied allograft has not been defined.
Methods
In the prospective, multicenter Trifecta study, we collected tissue from 300 biopsies from 289 kidney transplant recipients to compare genome-wide gene expression in biopsies with dd-cfDNA(%) in corresponding plasma samples drawn just before biopsy. Rejection was assessed with the microarray-based Molecular Microscope Diagnostic System using automatically assigned rejection archetypes and molecular report sign-outs, and histology assessments that followed Banff guidelines.
Results
The median time of biopsy post-transplantation was 455 days (5 days to 32 years), with a case mix similar to that of previous studies: 180 (60%) no rejection, 89 (30%) antibody-mediated rejection (ABMR), and 31 (10%) T cell–mediated rejection (TCMR) and mixed. In genome-wide mRNA measurements, all 20 top probe sets correlating with dd-cfDNA(%) were previously annotated for association with ABMR and all types of rejection, either natural killer (NK) cell–expressed (e.g., GNLY, CCL4, TRDC, and S1PR5) or IFN-γ–inducible (e.g., PLA1A, IDO1, CXCL11, and WARS). Among gene set and classifier scores, dd-cfDNA(%) correlated very strongly with ABMR and all types of rejection, reasonably strongly with active TCMR, and weakly with inactive TCMR, kidney injury, and atrophy fibrosis. Active ABMR, mixed, and active TCMR had the highest dd-cfDNA(%), whereas dd-cfDNA(%) was lower in late-stage ABMR and less-active TCMR. By multivariate random forests and logistic regression, molecular rejection variables predicted dd-cfDNA(%) better than histologic variables.
Conclusions
The dd-cfDNA(%) at time of indication biopsy strongly correlates with active molecular rejection and has the potential to reduce unnecessary biopsies.
Clinical Trial registration number:
Measurement of plasma donor-derived cell-free DNA (dd-cfDNA), expressed as percent of the total dd-cfDNA (dd-cfDNA[%]), was developed to screen for the presence of rejection in organ transplants,1–5 including in the lung,6–8 kidney,2,4,5,9–14 and heart.15,16 The relationship of dd-cfDNA(%) to histologic TCMR has been less consistent than for antibody-mediated rejection (ABMR).17 For example, in heart transplants the mean dd-cfDNA(%) levels measured by shotgun sequencing were five-fold higher in histologic ABMR than TCMR,15 and early post-transplant injury also elevated the dd-cfDNA(%). The dd-cfDNA(%) increased slightly in apparently stable kidney transplant patients over years post-transplant,18 possibly related to atrophy fibrosis.19
The relationship between dd-cfDNA(%) and the molecular phenotype in kidney transplants can be studied using automatic genome-wide microarray measurements in the Molecular Microscope Diagnostic System (MMDx). Microarrays measure gene expression with 99% reproducibility20 and interprets disease states by machine learning–derived classifiers and archetype scores. MMDx (molecular) diagnoses are assigned in one of two ways: automatic archetype assignments, which avoid all subjectivity, or by an expert report sign-out21,22 using rejection archetypes plus classifiers and gene sets. Molecular diagnoses shows strong agreement with histology, albeit with some discrepancies,21,22 in part due to the intrinsic variability in histology,23 for example, in TCMR.24,25 Some discrepancies reflect diagnostic conventions: for example, BK virus nephropathy often has molecular TCMR-like changes due to cognate T cell responses against virus or alloantigens,26 but the histologic convention is to not diagnose TCMR in BK virus nephropathy, despite typical lesions.26 All biopsy assessments—molecular or histologic—can be affected by sampling error, and have potential for error around boundaries of diagnostic classes.
We launched the Trifecta Study, an investigator-initiated, international prospective trial, to define the relationship between dd-cfDNA(%) at the time of indication biopsy and the genome-wide molecular findings in the biopsy, and with the diagnostic classification of the biopsy by molecular and histologic assessments. The present results are for the first 300 consecutive biopsies (94% for indications) processed by for molecular diagnoses with blood drawn for dd-cfDNA(%) just before the biopsy.
Methods
Study Population
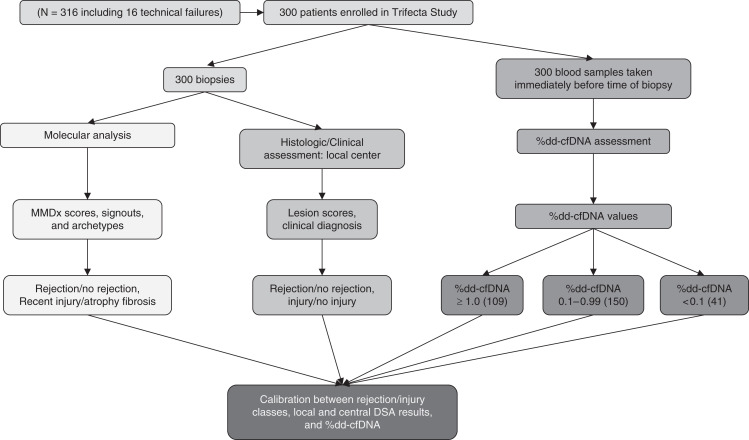
We analyzed 300 indication kidney transplant biopsies collected prospectively from 289 consenting patients. Eight biopsies with insufficient material for microarray processing and a further eight with unsuccessful dd-cfDNA(%) analyses or unavailable blood samples were excluded from 316 total biopsies to produce the n=300 cohort (Figure 1). Reaching 300 results triggered the present analysis, as per protocol.
Figure 1.
Study design flowchart. Workflow for collection and processing of the 300 biopsies for molecular biopsy assessment and concurrent blood samples processed for percent donor-derived cell-free DNA (%dd-cfDNA) in the Trifecta Study.
A list of centers is shown in Table 1. Demographics are shown in Table 2. Biopsies were contributed by established centers under local Institutional Review Board–approved protocols (ClinicalTrials.gov number NCT04239703).
Table 1.
Trifecta Study coauthors (n=300 biopsies): Institutions and investigators that have contributed biopsies and cfDNA data
| Investigators | Institution | Location |
|---|---|---|
| Beata Naumnik | Medical University in Bialystok | Białystok, Poland |
| Jonathan Bromberg | University of Maryland School of Medicine | Maryland, Baltimore |
| Matt Weir | ||
| Nadiesa Costa | ||
| Milagros Samaniego-Picota | Henry Ford Transplant Institute | Detroit, Michigan |
| Iman Francis | ||
| Anita Patel | ||
| Alicja Dębska-Ślizień | Medical University of Gdańsk | Gdańsk, Poland |
| Joanna Konopa | ||
| Andrzej Chamienia | ||
| Grzegorz Piecha | Medical University of Silesia | Katowice, Poland |
| Željka Veceric-Haler | University of Ljubljana | Ljubljana, Slovenia |
| Miha Arnol | ||
| Nika Kojc | ||
| Maciej Glyda | Wojewodzki Hospital | Poznan, Poland |
| Katarzyna Smykal-Jankowiak | ||
| Ondrej Viklicky | Institute for Clinical and Experimental Medicine | Prague, Czech Republic |
| Petra Hruba | ||
| Silvie Rajnochová Bloudíčkova | ||
| Janka Slatinská | ||
| Marius Miglinas | Centre of Nephrology, Vilnius University Hospital Santaros Klinikos | Vilnius, Lithuania |
| Marek Myślak | Pomeranian Medical University | Szczecin, Poland |
| Joanna Mazurkiewicz | ||
| Marta Gryczman | ||
| Leszek Domański | University Hospital n.2, Szczecin | |
| Rajendra Baliga | Tampa General Hospital | Tampa Bay, Florida |
| Agnieszka Perkowska-Ptasińska | Warsaw Medical University | Warsaw, Poland |
| Dominika Dęborska-Materkowska | ||
| Michał Ciszek | ||
| Magdalena Durlik | ||
| Ryszard Grenda | The Children’s Memorial Health Institute | |
| Mirosław Banasik | Medical University of Wrocław | Wrocław, Poland |
| Mladen Knotek | University Hospital Merkur | Zagreb, Croatia |
| Ksenija Vucur | ||
| Zeljka Jurekovic | ||
| Thomas Müller | University Hospital Zurich | Zurich, Switzerland |
| Thomas Schachtner | ||
| Andrew Malone | Washington University at St. Louis | St. Louis, MO, USA |
| Tarek Alhamad |
Table 2.
Demographics and clinical features of the Trifecta Study (n=300) biopsy cohort
| Biopsy Characteristics | |
|---|---|
| Days to biopsy post-transplant | |
| Mean | 1403 |
| Median (range) | 455 (5–11504) |
| Days to most recent follow-up after biopsy | |
| Mean | 177 |
| Median (range) | 169 (1–526) |
| Indication for biopsy (% of total) | |
| For cause | 278 (94%) |
| Surveillance | 18 (6%) |
| Missing | 4 (1%) |
| Patient demographics (n=300) | |
| Mean patient age (range) | 51 (19–80) |
| Age > 65 years (count) | 29 |
| Mean donor age (range) | 47 (6–81) |
| Patient sex | |
| Male (% of known) | 187 (63%) |
| Female (% of known) | 111 (37%) |
| Not available | 2 (1%) |
| Donor sex | |
| Male (% of known) | 150 (52%) |
| Female (% of known) | 137 (48%) |
| Not available | 13 (4%) |
| Patient ethnicity | |
| Black | 11 |
| Other | 288 |
| Not availablea | 1 |
| Donor type (% deceased donor transplants) | 224 (90%) |
| Status at last follow-up | |
| Functioning graft | 247 (90%) |
| Graft failure/return to dialysis | 23 (8%) |
| Patient death with functioning graft | 3 (1%) |
| Primary disease | |
| Diabetic nephropathy | 34 |
| Hypertension/ large vessel disease | 9 |
| Glomerulonephritis/ vasculitis | 107 |
| Interstitial nephritis/ pyelonephritis | 2 |
| Polycystic kidney disease | 0 |
| Others | 99 |
| Unknown etiology | 38 |
Some centers preferred not to identify ethnicity.
Sample and Data Collection
A portion of one core (mean length 3 mm)27 was immediately stabilized in RNALater and shipped to the Alberta Transplant Applied Genomics Centre (http://atagc.med.ualberta.ca) at ambient temperature for RNA extraction and processing as previously described.27 Gene expression was measured using Affymetrix PrimeView microarrays (n=49,495 probe sets) unless the biopsy was inadequate for analysis (e.g., too small or degraded: eight out of 308 in this dataset, and 4% in previous studies27). Of the 300 biopsies, 285 had available histologic diagnoses (nine missing, six with inadequate material for assessment). All molecular analyses and diagnoses were made without knowledge of the histology, donor-specific antibody (DSA), clinical data, or dd-cfDNA(%) results. Molecular reports were sent to the participating centers, usually within 2 working days of receiving the biopsy.
All blood samples for dd-cfDNA(%) testing were collected before the biopsies were performed to avoid any effect of the biopsy procedure on the dd-cfDNA(%) measurement.28 Blood samples were drawn in two 10 ml quantities in DNA Streck tubes using 20–21 gauge needles and shipped immediately per established protocols to Natera Inc. for analysis.
Histologic and clinical data were collected at each center and submitted to the study as available. All centers followed Banff 2019 guidelines for interpreting histology. The study was not permitted to request additional local tests that were not standard of care, and the centers did not agree to provide biopsies for central histology reading because it is not standard of care.
The Trifecta Study also includes centralized DSA measurements by One Lambda Inc., which are in progress and not reported here. A preliminary DSA analysis has been reported in abstract form29 and the full analysis will be described in a subsequent paper.
The MMDx
MMDx is a central biopsy diagnostic system that measures genome-wide mRNA expression and interprets the results using many previously derived and locked machine-learning–derived algorithms to assign molecular diagnoses, comparing new biopsies with results in a large reference set (n=1208). Automated output from ensembles of machine-learning algorithms (classifiers), both supervised and unsupervised,21,22 were used to make observer-independent assessments. Outputs include assessments of parenchymal injury and atrophy fibrosis. All molecular measurements were also assessed by an expert reader, who signed out the molecular MMDx report.
Principal Component Analysis and Archetypal Analysis
Principal component analysis (PCA) is a dimensionality reduction technique used for data visualization and interpretation. Archetypal analysis (AA) is a method related to clustering that represents each sample as a composite of a small number (n) of archetypes (clusters)—theoretical constructs representing diverse “types” of samples, each type with its own characteristic set of features. AA automatically assigns scores to each biopsy, describing its relationship to each archetype group. We named these groups on the basis of their associations with molecular and histologic findings: No rejection, TCMR1 (intense TCMR, sometimes mixed with ABMR30), TCMR2 (less active TCMR), early-stage ABMR (EABMR), fully developed ABMR (FABMR), and late-stage ABMR.
In this paper, PCA and AA are both constructed using the same 1208 (samples) × 7 (features) data matrix.30 Each of the seven input features is a score from a classifier trained and tested in the 1208 reference set. Two of the seven inputs are classifiers predicting histologic rejection—one for ABMR and one for TCMR. The other five are for predicting rejection-related histologic lesions: i>1, t>1, ptc>0, g>0, and cg>0. We used the PCA function in the R FactoMineR package31 and the stepArchetypes function in the archetype package.
Lists of Genes
Significant gene lists were determined by Spearman correlation coefficients (SCC) between the expression levels and the other variable of interest. The resulting transcript lists were ordered by correlation coefficient.
The dd-cfDNA(%) Assay
The dd-cfDNA(%) assays (Prospera) were performed on blood samples drawn at time of biopsy. The results were not reported to the center until many months postbiopsy, and never influenced the decision to biopsy or patient management. The Prospera assay uses massively multiplexed targeted PCR to identify 13,392 single nucleotide polymorphisms.9 For analytical purposes in this study, some analyses used the cutoff of dd-cfDNA(%) ≥1%. All dd-cfDNA(%) read as “<0.08%” (n=23) are analyzed as 0.08%.
Results
Case Mix in 300 Biopsies
Molecular diagnoses, rejection archetype groups, plus local standard-of-care histology, and DSA data are given in Table 3. Case mix was similar to the indication biopsies in the reference set.22 Archetypal assignments were No rejection 60%; TCMR 10% (TCMR1 3%; TCMR2 7%); and ABMR 30% (EABMR 10%, FABMR 13%, late-stage ABMR 6%). Molecular sign-outs were No rejection 58%; TCMR 7%; possible TCMR 2%; mixed 6%; ABMR 22%; and possible ABMR 4%. Histology assigned No rejection 43%; TCMR 10%; possible TCMR (borderline) 10%, ABMR 15%, possible ABMR 12%, and mixed 5%, with 5% inadequate for reporting.
Table 3.
Histologic diagnoses and DSA status in the Kidney 300 cohort (n, % of total)
| Molecular Diagnoses | Histology Diagnoses | |||
|---|---|---|---|---|
| n (% of 300) | % DSA Positive of Those Tested | n (% of 300) | % DSA Positive of Those Tested | |
| No rejection | 175 (58)a | 6 | 128 (43)a | 9 |
| Possible TCMR (pTCMR) | 6 (2) | 0 | 31 (10) | 10 |
| TCMR | 21 (7)a | 29 | 31 (10)a | 19 |
| Possible ABMR (pABMR) | 12 (4) | 50 | 35 (12) | 26 |
| ABMR | 67 (22)a | 33 | 45 (15)a | 44 |
| Mixed (ABMR+TCMR) | 19 (6)a | 50 | 15 (5)a | 40 |
| All ABMR (including Mixed) | 86 (29) | 36 | 60 (20) | 37 |
| Inadequate | — | — | 6 (2) | — |
| Missing | — | — | 9 (3) | — |
| Automated rejection archetypes | ||||
|---|---|---|---|---|
| n (% of 300) | % DSA positive of those tested | |||
| No rejection | 180 (60)a | 20 | ||
| TCMR1 (many mixed) | 9 (3)a | 50 | ||
| TCMR2 | 22 (7) | 29 | ||
| EABMR | 31 (10)a | 44 | ||
| FABMR | 40 (13)a | 58 | ||
| LABMR | 18 (6) | 38 | ||
LABMR, late-stage ABMR.
Groups with specific references in text.
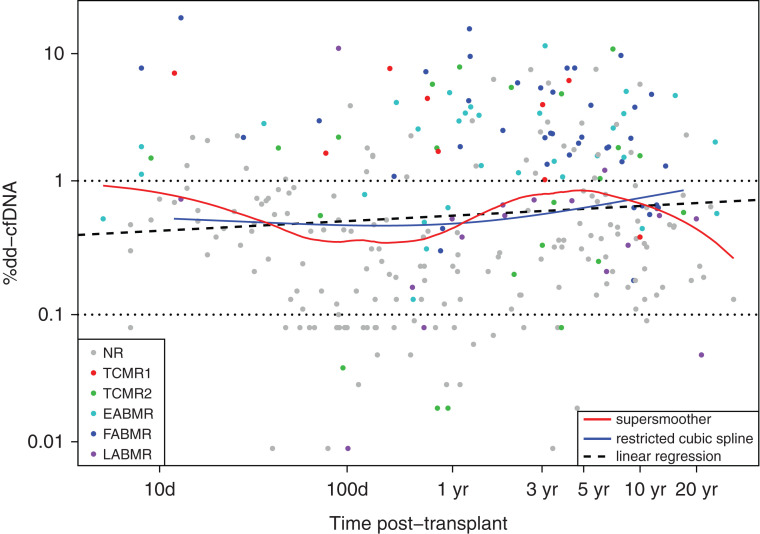
The dd-cfDNA(%) levels rose slightly with time of biopsy post-transplant (linear regression P=0.06). The “supersmoother” function shows these levels are also somewhat elevated in biopsies in the first weeks post-transplant (Figure 2). Very low levels of dd-cfDNA(%) were uncommon in early biopsies, presumably due to AKI resulting from recent donation-implantation.32 Most ABMR biopsies (blue) were late, whereas most early biopsies were No rejection (gray).
Figure 2.
Relationships between %dd-cfDNA, time of biopsy post-transplant, and molecular archetype groups in N=300 samples. Dots represent biopsies and corresponding paired blood sample %dd-cfDNA results, colored by archetype cluster assignments. Relationships between %dd-cfDNA and time post-transplant are represented by linear regression (thick dashed black line), restricted cubic spline (with three knots - blue line) and a supersmoother (red line). Overall, %dd-cfDNA increases slightly over time post-transplant (linear regression P=0.06). %dd-cfDNA ≥ 1 is used as the cutoff throughout this paper for positivity. NR – no rejection.
Top Transcripts Associated with dd-cfDNA(%)
Table 4 shows the top 20 transcripts correlated with dd-cfDNA(%); all were positively correlated. (Negative correlations were much weaker.) All of the top 20 (of 49,495 total probe sets) had previously been annotated as being highly associated with all types of rejection and with ABMR, particularly representing NK cell genes (e.g., GNLY, CCL4, TRDC, and S1PR5) and IFNG-inducible genes (e.g., PLA1A, IDO1, CXCL11, WARS). The top transcript was granulysin (GNLY), with a SCC of 0.56 (P=4E-26). Analysis of the top 20 unique genes correlated with dd-cfDNA(%) was similar (Supplemental Table 1): 19 had previously been annotated as expressed by NK cells (11) or IFNG-inducible (eight), and one had been annotated as inducible by injury.
Table 4.
Top probe sets positively correlated with dd-cfDNA(%) in the Trifecta Study (n=300) ordered by correlation coefficient
| Affy ID | Gene Symbol | Gene Name | Transcript Set Annotation | SCCa |
|---|---|---|---|---|
| 11751857_a_at | GNLYb | granulysin | ABMR-RAT, Rej-RAT | 0.56 |
| 11763715_a_at | GNLYb | granulysin | ABMR-RAT,Rej-RAT | 0.55 |
| 11746954_s_at | CCL4b | chemokine (C-C motif) ligand 4 | ABMR-RAT,Rej-RAT | 0.52 |
| 11761790_x_at | TRDCb | T cell receptor delta constant | ABMR-RAT,Rej-RAT | 0.51 |
| 11718982_s_at | CCL4b | chemokine (C-C motif) ligand 4 | ABMR-RAT,Rej-RAT | 0.51 |
| 11718983_x_at | CCL4b | chemokine (C-C motif) ligand 4 | ABMR-RAT,Rej-RAT | 0.51 |
| 11743168_at | IDO1c | indoleamine 2,3-dioxygenase 1 | ABMR-RAT,GRIT3,Rej-RAT | 0.51 |
| 11749245_a_at | CXCL11c | chemokine (C-X-C motif) ligand 11 | ABMR-RAT,GRIT3,Rej-RAT | 0.50 |
| 11753484_x_at | KLRD1b | killer cell lectin-like receptor subfamily D, member 1 | ABMR-RAT,Rej-RAT | 0.50 |
| 11727116_a_at | PLA1Ac | phospholipase A1 member A | ABMR-RAT, Rej-RAT | 0.50 |
| 11729649_at | PRF1b | perforin 1 (pore forming protein) | ABMR-RAT,QCAT,Rej-RAT | 0.50 |
| 11740452_x_at | KLRD1b | killer cell lectin-like receptor subfamily D, member 1 | ABMR-RAT,Rej-RAT | 0.50 |
| 11724900_a_at | GZMBb | granzyme B | ABMR-RAT,QCAT,Rej-RAT | 0.49 |
| 11726287_a_at | WARSc | tryptophanyl-tRNA synthetase | ABMR-RAT,GRIT3,Rej-RAT | 0.49 |
| 11733353_at | CRTAMb | cytotoxic and regulatory T-cell molecule | ABMR-RAT,RAT,Rej-RAT | 0.49 |
| 11756632_a_at | GNLYb | granulysin | ABMR-RAT,QCAT,Rej-RAT | 0.49 |
| 11744660_s_at | CCL4L1b | chemokine (C-C motif) ligand 4-like 1 | ABMR-RAT, Rej-RAT | 0.49 |
| 11732466_a_at | CXCL11c | chemokine (C-X-C motif) ligand 11 | ABMR-RAT,GRIT3,Rej-RAT | 0.49 |
| 11752664_a_at | S1PR5b | sphingosine-1-phosphate receptor 5 | ABMR-RAT,RAT | 0.49 |
| 11763756_x_at | GNLYb | granulysin | ABMR-RAT,QCAT,RAT | 0.49 |
Transcript sets are described on the home page at https://www.ualberta.ca/medicine/institutes-centres-groups/atagc/research/gene-lists.html. ABMR-RAT, antibody-mediated rejection-associated transcripts; GRIT, gamma-interferon-inducible transcripts, QCAT, Cytotoxic T cell-associated transcripts; Rej-RAT, rejection-associated transcripts.
All P values were <0.0001.
NK cell expressed.
IFNG-inducible.
Supplemental Figure 1 shows the correlation between dd-cfDNA(%) and the top dd-cfDNA(%)–associated gene, GNLY, with each biopsy colored by its archetypal cluster assignment, showing the strong association of EABMR and FABMR (cyan and blue symbols, respectively) with high GNLY expression and dd-cfDNA(%). Many archetypal TCMR1 biopsies (red) and some TCMR2 (green) also had high GNLY expression and dd-cfDNA(%).
Associations of Classifier and Transcript Set Scores with dd-cfDNA(%)
Table 5 analyzes the scores for previously annotated transcript sets and classifiers for their SCC with dd-cfDNA(%) in the 300 biopsies. The highest correlation was with the classifier trained on peritubular capillary inflammation, ptcProb (SCC 0.54). Other high correlations were related to scores for all-rejection or ABMR: the ABMRProb, gProb, ptcProb, and RejProb classifiers, and the ABMR-RAT transcript set. The correlations with scores for TCMR gene sets and classifiers were also high, although weaker than the associations with ABMR and all-rejection. Injury and atrophy-fibrosis gene set and classifier scores were weakly associated with dd-cfDNA(%), and the normal kidney transcript set score correlated negatively, reflecting injury-induced parenchymal dedifferentiation.
Table 5.
SCC of molecular rejection and injury transcript set and classifier scores with dd-cfDNA(%) in n=300 biopsies
| Variable | SCC | P Value | ||
|---|---|---|---|---|
| ABMR related | ABMR probability classifier | ABMRProba | 0.52a | <0.0001a |
| cg>0-probability classifier | cg>0Prob | 0.35 | <0.0001 | |
| g>0-probability classifier | g>0Proba | 0.50a | <0.0001a | |
| ptc>0 probability classifier | ptc>0Proba | 0.54a | <0.0001a | |
| DSA-probability classifier | DSAProb | 0.39 | <0.0001 | |
| ABMR transcripts | ABMR-RATa | 0.51a | <0.0001a | |
| All rejection | Rejection-probability classifier | RejProba | 0.52a | <0.0001a |
| IFNG-inducible transcripts | GRIT3 | 0.44 | <0.0001 | |
| TCMR related | Effector T cell transcripts | QCAT | 0.43 | <0.0001 |
| TCMR-probability classifier | TCMRProb | 0.22 | <0.0001 | |
| i>1-probability classifier | i>1Prob | 0.34 | <0.0001 | |
| t>1-probability classifier | t>1Prob | 0.26 | <0.0001 | |
| Macrophage related | Macrophage transcripts | QCMAT | 0.37 | <0.0001 |
| Alternative macrophage activation transcripts | AMAT1 | 0.39 | <0.0001 | |
| Recent injury | Recent injury-induced | IRRAT30 | 0.18 | 0.002 |
| Injury-induced day 3 | IRITD3 | 0.18 | 0.001 | |
| Parenchymal | Normal kidney transcripts | KT1 | −0.19 | 0.0001 |
| Atrophy fibrosis | ci>1-probability classifier | ci>1Prob | 0.16 | 0.005 |
| ct>1-probability classifier | ct>1Prob | 0.13 | 0.02 | |
Transcript sets are described on the home page at https://www.ualberta.ca/medicine/institutes-centres-groups/atagc/research/gene-lists.html.
Classifiers and gene sets with correlation >0.50.
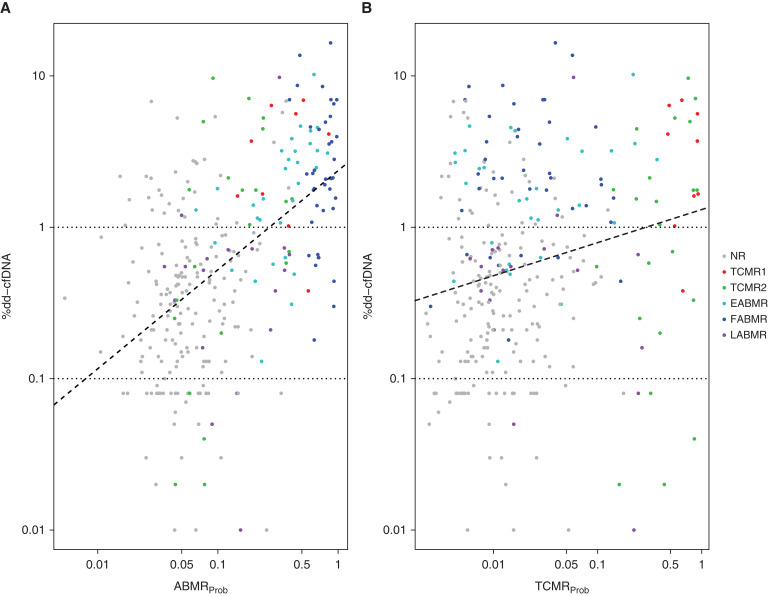
Figure 3 shows the relationship between dd-cfDNA(%) for each biopsy and the scores for the ABMR classifier (Figure 3A) or the TCMR classifier (Figure 3B). The regression lines showing best fit within the data demonstrated significant relationships with the ABMRProb and TCMRProb classifiers. The SCC of dd-cfDNA(%) with the ABMR classifier (0.52, P=6E-22) was higher than correlation with the TCMR classifier (0.22, P=9E-5), although this will be influenced by the greater abundance of ABMR in the case mix.
Figure 3.
Relationships between %dd-cfDNA, molecular archetype groups, and the ABMRProb and TCMRProb classifier scores in N=300 samples. Dots represent biopsies and corresponding paired blood sample %dd-cfDNA results, colored by archetype cluster assignments. Regression lines (dashed) show the relationship between the (A) ABMRProb and (B) TCMRProb classifier scores and %dd-cfDNA. Spearman correlations with dd-cfDNA were stronger for ABMRProb (0.52, P=6E-22) than TCMRProb (0.22, P=9E-5). NR, no rejection.
PCA of Rejection
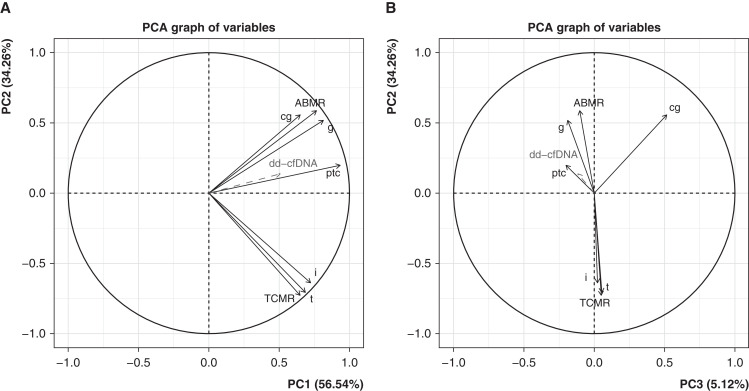
We used PCA to visualize the relationship between dd-cfDNA(%) and the seven rejection classifiers used to assign archetype scores21 (Figure 4). When we projected dd-cfDNA(%) scores into the PCA as a supplementary variable (not used as input), the dd-cfDNA(%) vector correlated positively with PC1 (all-rejection) and PC2 (ABMR, Figure 4A). In PC3, dd-cfDNA(%) performed like the active ABMR-related classifiers, reflecting association with early-stage or fully-developed ABMR rather than late-stage ABMR (Figure 4B). The dd-cfDNA(%) vector strongly resembled the peritubular capillaritis (ptc >1Prob) molecular classifier vector in all three dimensions.
Figure 4.
Rejection classifier PCA factor maps in N=300 samples, showing %dd-cfDNA as a supplementary variable. (A) PC2 versus PC1. (B) PC2 versus PC3. Input variables (classifier scores) are shown as solid black lines, and %dd-cfDNA in dashed grey as it is not included as input in the PCA. %dd-cfDNA behaves like a molecular rejection measurement, more closely aligned to ABMR than to TCMR.
dd-cfDNA(%) in Each Diagnostic Group
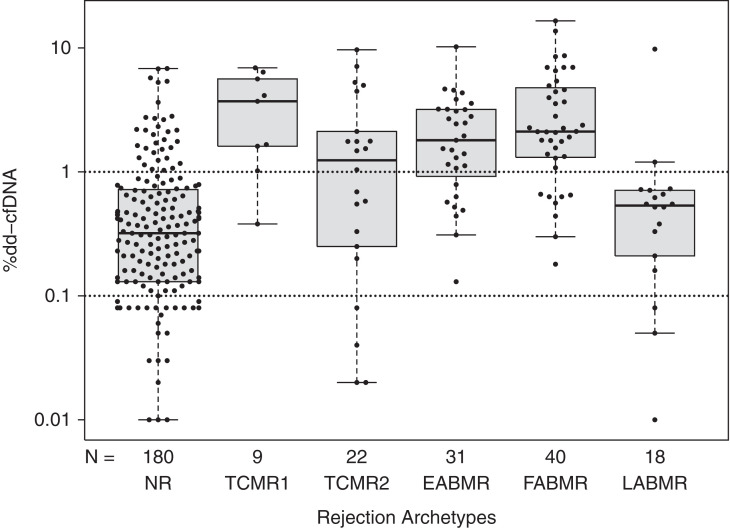
Among the archetype groups illustrated in Figure 5 the dd-cfDNA(%) was highest in FABMR, EABMR, TCMR1, and some TCMR2 biopsies. A small number of no rejection biopsies also had high values. Note that late-stage ABMR (which is often inactive30) often had low dd-cfDNA(%).
Figure 5.
Beeswarm/boxplots showing %dd-cfDNA measurements versus molecular rejection archetype groups in N=300 samples. Dots represent biopsies and corresponding paired blood sample %dd-cfDNA results.
Table 6 shows the mean and median dd-cfDNA(%) within each of the diagnostic categories: molecular sign-outs, automated rejection archetypes, and histology sign-outs. Because of the skewed distribution of the dd-cfDNA(%) data, medians are a more reliable measure of central tendency than means, thus we focus on medians.
Table 6.
Mean and median dd-cfDNA(%) levels in biopsies grouped by rejection diagnoses in n=300 biopsies
| Variables | dd-cfDNA(%) | n | |||
|---|---|---|---|---|---|
| Mean | Median | ||||
| Molecular sign-outs | No rejection | 0.62 | 0.33 | 175 | |
| TCMR related | TCMR (“pure” TCMR)a | 2.27 | 1.61 | 21 | |
| pTCMR | 0.55 | 0.27 | 6 | ||
| Mixeda | 2.84 | 1.56 | 19 | ||
| ABMR related | ABMRa | 3.12 | 2.11 | 67 | |
| pABMR | 0.85 | 0.57 | 12 | ||
| Automated rejection archetypes | No rejectiona | 0.70 | 0.32 | 180 | |
| TCMR related | TCMR1 (including mixed)a | 3.49 | 3.71 | 22 | |
| TCMR2a | 2.08 | 1.26 | 9 | ||
| ABMR related | EABMRa | 2.29 | 1.80 | 31 | |
| FABMRa | 3.54 | 2.12 | 40 | ||
| LABMR | 0.99 | 0.54 | 18 | ||
| Histology diagnostic sign-outs by the centers | No rejection | “AKI” (<6 weeks post-transplant)a | 0.90 | 0.68 | 14 |
| >6 weeks post-transplanta | 0.24 | 0.16 | 25 | ||
| Other | 0.72 | 0.36 | 89 | ||
| TCMR related | TCMR (“pure” TCMR)a | 1.53 | 0.88 | 31 | |
| pTCMR (borderline) | 0.78 | 0.26 | 31 | ||
| ABMR related | ABMRa | 2.16 | 1.57 | 45 | |
| pABMR | 2.78 | 1.14 | 35 | ||
| Mixed (ABMR+TCMR)a | 3.83 | 3.18 | 15 | ||
| Inadequate | 2.54 | 1.23 | 6 | ||
| Missing | 1.20 | 0.26 | 9 | ||
LABMR, late-stage ABMR.
The principal diagnostic categories.
Among molecular sign-out categories, median dd-cfDNA(%) was highest in ABMR, and mixed rejection, moderately high in TCMR, and lowest in No rejection.
Among rejection archetypes, median dd-cfDNA(%) was highest in TCMR1 (which includes some mixed) and lowest in No rejection.
In histology sign-outs, median dd-cfDNA(%) was highest in ABMR and mixed rejection biopsies; TCMR had moderate elevations. Biopsies with no histologic abnormalities, >6 weeks post-transplant (i.e., after recovery of AKI from transplantation, n=25) had the lowest median dd-cfDNA(%) (0.16), whereas biopsies with no histologic abnormalities <6 weeks post-transplant (n=14) had mildly elevated values (0.68, P=0.0003).
Note that “pure” TCMR identified by molecular sign-out diagnoses and by histology diagnoses had elevated mean and median dd-cfDNA(%) compared with No rejection biopsies.
Variable Importance in Predicting dd-cfDNA(%) ≥1
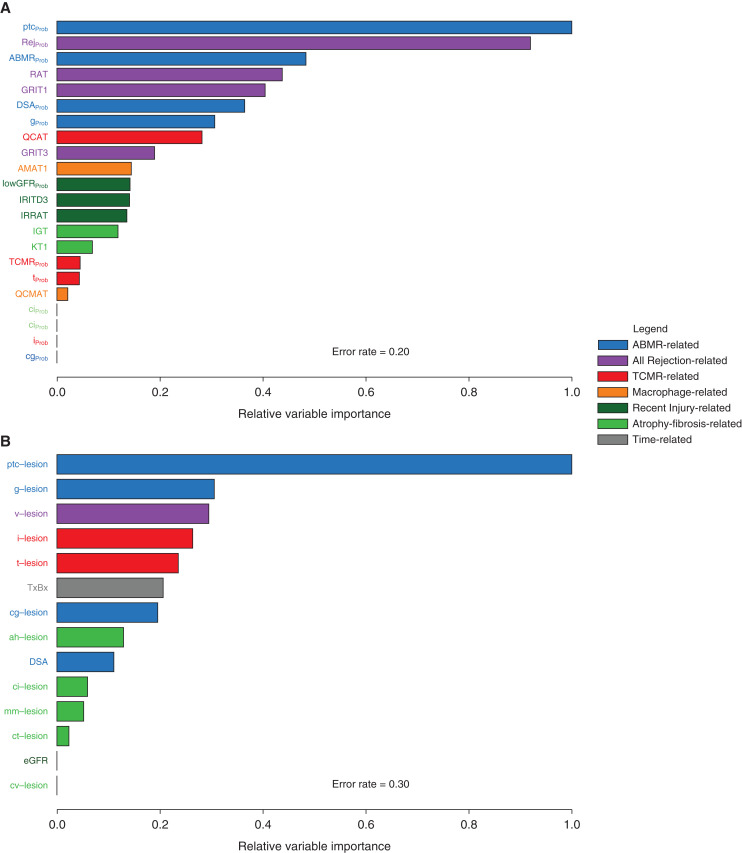
Figure 6 shows the relative variable importance for predicting dd-cfDNA(%) ≥1% using random forest analysis. In Figure 6A, the molecular classifiers and gene sets predicted dd-cfDNA(%) ≥1% with error rate of 0.20, with the top predictors being the molecular ptc-lesion classifier (ptcProb) and the all-rejection (RejProb) classifier. In general, ABMR-related (blue) and all rejection-related (purple) molecular features were the most important predictors of dd-cfDNA(%).
Figure 6.
Random forests demonstrating predictive ability of various molecular and histologic features of rejection for %dd-cfDNA ≥1.0 in N=300. (A) Relative variable importance using molecular predictors. (B) Relative variable importance using histologic predictors. In both figures, ABMR and overall rejection related variables are the most important predictors of high %dd-cfDNA.
In Figure 6B, the same analyses were repeated for histologic variables plus local DSA (error rate=0.30). The top variable was the histology ptc-lesion score. The overall prediction of dd-cfDNA(%) was more accurate with molecular variables than with histology variables and DSA (error rates: 0.20 versus 0.30, area under the receiver operating characteristic curves for the out-of-bag (bootstrapped) predicted values: 0.80 versus 0.71, P=0.003). DSA status was relatively unimportant compared with biopsy features, and excluding the local DSA status as a variable did not affect the predictions (data not shown).
Thus, the molecules associated with ptc lesions (as estimated by the ptcProb classifier), and to a lesser extent the ptc-lesions themselves, are the biopsy features in the biopsy with the strongest association with dd-cfDNA production.
Logistic regression models predicting dd-cfDNA(%) ≥1.0 were built using the top five variables from each random forest analysis. Models used histology variables alone, molecular variables alone, or all 10 combined. The combined model added significantly to the predictive value of histology alone (P=2×10−8, likelihood ratio test) but not to molecules alone (P=0.99). The same comparisons were run using linear regression to predict log10(dd-cfDNA[%]) as a continuous variable. Again, molecules added to histology alone (P=2×10−11), but histology did not add to molecules alone (P=0.95). Random forest models are not easily comparable with methods such as likelihood ratio tests, but the area under the receiver operating characteristic curves from their predicted values can be compared.
Discussion
This study prospectively recruited consenting patients undergoing kidney transplant indication biopsies (94% recorded as being for indications) to examine the relationship between dd-cfDNA(%) results taken just before the biopsy and gene expression in the biopsy. The 300 biopsies represent a cross-section of the international kidney transplant population undergoing indication biopsies from 5 days to 32 years post-transplant (median 453 days). The case mix was similar to previous indication biopsy studies, with approximately 60% of biopsies assigned molecular No rejection and 30% molecular ABMR by molecular sign-outs or automatic archetypes. Many of the elevated dd-cfDNA(%) values after 1 year had ABMR. All top 20 probe sets correlated with dd-cfDNA(%) were associated with all-rejection and/or with ABMR and expressed in NK cells or IFNG-inducible. The strongest correlation of multigene measurement scores (transcript sets or classifiers) with dd-cfDNA(%) were similar—strongest with ABMR and all-rejection scores, moderately with TCMR scores, and weakly with recent parenchymal injury, dedifferentiation, and atrophy-fibrosis scores. Using PCA, dd-cfDNA(%) performed as a variable associated with rejection, but specifically with active ABMR (EABMR and FABMR) rather than late-stage ABMR. By diagnostic categories, dd-cfDNA(%) was highest in both molecular and histologic ABMR, increased in TCMR biopsies, and mildly increased in AKI and atrophy fibrosis compared with histologically normal biopsies after 6 weeks post-transplant. The associations of dd-cfDNA(%) were strong with both molecular and histologic rejection, and were somewhat stronger with the molecular measurements, similar to the recent findings in an independent cohort.33 We conclude that dd-cfDNA(%) is strongly associated with active rejection, particularly ABMR and mixed but also the most active TCMR.
The top genes correlating with circulating dd-cfDNA(%) at the time of indication biopsies are predominantly those associated with active ABMR—genes expressed by NK cells or inducible by IFNG. These top transcripts are commonly expressed in active and fully developed ABMR in kidney and heart transplants.30,34 However, active ABMR is much more common than active TCMR in this population, and active pure TCMR also releases dd-cfDNA.
Our hypothesis is that the degree of dd-cfDNA(%) released by TCMR reflects the activation state of the effector T cells in those TCMR biopsies. TCMR varies in molecular activity, as illustrated by the TCMR1 and TCMR2 archetypes groups. TCMR1 often has ABMR features, but always has more intense TCMR activity (e.g., IFNG expression) than TCMR2.30 Some TCMR-like states recognized by MMDx (particularly TCMR2) or by histology are relatively inactive in molecular findings. Although associated ABMR contributes to the high dd-cfDNA(%) in some TCMR1 biopsies (e.g., TCMR1), it is likely that active pure TCMR also releases dd-cfDNA without ABMR activity, and TCMR biopsies associated with high dd-cfDNA(%) cannot be explained by associated ABMR activity. Our hypothesis is that some TCMR phenotypes release lower amounts of dd-cfDNA because of a lower activation state of the cognate T cells in these patients, due either to effects of immunosuppressive drugs or to adaptive changes in the T cells, such as exhaustion. Adaptive changes presumably explain why TCMR episodes become uncommon in transplant populations after 5–10 years.35 The potential relationship of dd-cfDNA(%) to cognate T cell activity within TCMR biopsies (as opposed to undetected ABMR activity) will be a subject of ongoing investigation in the Trifecta study.
The dd-cfDNA(%) test is of interest because of its potential to avoid unnecessary indication biopsies when dd-cfDNA(%) is low in patients with biopsy indications, although whether it actually does avoid unnecessary biopsies has not yet been established and was not examined in this study. (The use of dd-cfDNA[%] as a screening test in patients without biopsy indications is a separate issue and not addressed in this indication biopsy study.) The highest dd-cfDNA(%) values were typically associated with molecularly active rejection, and very low values were associated with pristine biopsies that did not have molecular or histologic rejection or other abnormalities. (Pristine biopsies were those that had recovered from the injury induced by the transplantation process, >6 weeks post-transplant, and without any signs of current rejection.) Although the dd-cfDNA test (Prospera) is typically only validated in patients meeting a set of criteria (e.g., >14 days post-transplant, no active cancer), a small number of biopsies in this study (approximately 10%) were taken from patients that would be excluded by these criteria. We designed the Trifecta study to find the associations in all indication biopsies with no selections, which permits us to draw conclusions from the largest possible dataset and can potentially inform future decisions for clinical use.
The explanation for the release of dd-cfDNA(%) by some kidneys with no molecular or histologic rejection could reflect parenchymal injury (acute injury or atrophy fibrosis), although low-level subthreshold ABMR should be considered.36 There is also a weak but significant association of dd-cfDNA(%) with AKI (e.g., in early transplants) and with atrophy fibrosis, and the source of the dd-cfDNA needs to be defined as potentially useful information about the renal parenchyma.
This study is limited in numbers of biopsies within individual rejection categories, which will be addressed in the ongoing extension of the study. One limitation of this study is that we did not include centralized reading of the biopsy histology, which would be of interest in addition to molecular readings and local histology readings.
In summary, Trifecta finds that dd-cfDNA(%) can be useful at the time of an indication biopsy—if dd-cfDNA(%) is very low, it means the probability of active rejection is low. In PCA, the orientation of dd-cfDNA(%) in blood was similar to the measurements in the biopsy itself, which is good evidence that plasma dd-cfDNA(%) is a useful addition to existing clinical information. In addition to guiding decisions at indication biopsy, and as part of routine screening, dd-cfDNA(%) could potentially be used to follow response-to-treatment, and avoid follow-up biopsies if the dd-cfDNA(%) signal normalizes. However, the potential benefits of these applications need to be proven.
Disclosures
A. Prewett reports current employment with, and an ownership interest in, Natera. K. Madill-Thomsen reports current employment with Transcriptome Sciences Inc., which is a University of Alberta Research Company; and reports receiving research funding from the Alberta Ministry of Advanced Education and Technology, the Canada Foundation for Innovation, Genome Canada, Industrial Research Assistance Program, the Mendez National Institute of Transplantation Foundation, the University of Alberta Hospital Foundation; partial support was also provided by funding from a licensing agreement with the One Lambda division of Thermo Fisher Scientific. P. Billings reports current employment with Natera Inc.; having consultancy agreements with Alveo Technologies, Baebies Inc., OmniSeq Inc., and Target BioPharma Inc.; reports having an ownership interest in 23andMe, Biological Dynamics, DNArx Inc., Fabric Genomics Inc., Iliad Bioscience Inc., LabCorp Inc., LungLifeAI Inc., MissionBio Inc., Natera Inc., OmniSeq Inc., PlumCare LLc; Precision Biosciences Inc., Roswell Biosciences, ThermoFisher Inc.; and reports being a scientific advisor or member of Alveo Technologies, Baebies Inc., Biological Dynamics, Fabric Genomics Inc., LungLifeAI Inc., MissionBio Inc., OmniSeq Inc., PlumCare LLc, and TargetBioPharma, Inc. The Trifecta study is an investigator-initiated study supported by a grant from Natera to Transcriptome Sciences Inc./Alberta Transplant Applied Genomics Centre. P. F. Halloran reports having shares in Transcriptome Sciences Inc., a University of Alberta research company with an interest in molecular diagnostics, and is a consultant to Natera; all Natera authors are employees and own equity at Natera, Inc.; reports having consultancy agreements with Astellas, and CSL Behring; reports having an ownership interest in giving lectures for Thermo Fisher Scientific; reports receiving research funding from Alberta Ministry of Advanced Education and Technology, Canada Foundation for Innovation, Genome Canada, Industrial Research Assistance Program the Mendez National Institute of Transplantation Foundation, and the University of Alberta Hospital Foundation, partial support was also provided by funding from a licensing agreement with the One Lambda division of Thermo Fisher Scientific; reports being a Canada Research Chair in Transplant Immunology; reports receiving honoraria from Astellas, Natera Inc., and One Lambda; reports being a scientific advisor or member as CEO, Transcriptome Sciences Inc., Editor-in-chief emeritus of the American Journal of Transplantation; and reports speakers bureau from Astellas, Natera Inc., and One Lambda. Z. Demko is employed by and owns stock and options to buy stock in Natera. All remaining authors have nothing to disclose.
Funding
The Microarray biopsy assessment project is supporte d in part by a licensing agreement with One Lambda/Thermo Fisher Scientific.
Supplementary Material
Acknowledgments
P. Halloran was the principal investigator, wrote and reviewed the manuscript, and was responsible for data interpretation and study design; K. Madill-Thomsen and J. Reeve wrote and reviewed the manuscript, and were responsible for data analysis and interpretation; P. Billings, Z. Demko, and A. Prewett were responsible for manuscript preparation, discussion of results, measurement of dd-cfDNA(%) (Prospera) and interpretation. Trifecta Investigators were responsible for biopsy collection and manuscript reviewing. We thank our valued clinicians in the Trifecta study group who partnered with us for this study by contributing biopsies and feedback. We also thank Dr. Martina Mackova and Mrs. Anna Hutton for biopsy processing for the Microarray biopsy assessment component of this study, and Natera Inc. for blood sample processing and Prospera results.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Comparing Plasma Donor-Derived Cell-free DNA to Indication Kidney Biopsy Tissue Gene Expression: Toward Understanding the Molecular Equivalents of Non-Invasive Tests,” on pages 256–258.
Data Sharing Statement
CEL files will be available on Gene Expression Omnibus upon publication.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021091191/-/DCSupplemental.
Supplemental Table 1. Top unique genes positively correlated with dd-cfDNA(%) in the Trifecta Study (n=300) ordered by correlation coefficient
Supplemental Figure 1. Relationships between dd-cfDNA(%), molecular archetype groups, and GNLY expression in n=300 samples. Dots represent biopsies and corresponding paired blood sample dd-cfDNA(%) results, colored by archetype cluster assignments. Regression lines (dashed) show the relationship between GNLY expression and dd-cfDNA(%).
References
- 1.Moinuddin I, Kumar D, Kamal L, King A, Kang L, Levy M, et al. : Calibration of donor-derived cell-free DNA criteria for rejection with molecular diagnoses of kidney transplant biopsies. Am J Transplant 20: 680, 2020 [Google Scholar]
- 2.Huang E, Sethi S, Peng A, Najjar R, Mirocha J, Haas M, et al. : Early clinical experience using donor-derived cell-free DNA to detect rejection in kidney transplant recipients. Am J Transplant 19: 1663–1670, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Dengu F: Next-generation sequencing methods to detect donor-derived cell-free DNA after transplantation. Transplant Rev (Orlando) 34: 100542, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Oellerich M, Shipkova M, Asendorf T, Walson PD, Schauerte V, Mettenmeyer N, et al. : Absolute quantification of donor-derived cell-free DNA as a marker of rejection and graft injury in kidney transplantation: Results from a prospective observational study. Am J Transplant 19: 3087–3099, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloom RD, Bromberg JS, Poggio ED, Bunnapradist S, Langone AJ, Sood P, et al. ; Circulating Donor-Derived Cell-Free DNA in Blood for Diagnosing Active Rejection in Kidney Transplant Recipients (DART) Study Investigators : Cell-free DNA and active rejection in kidney allografts. J Am Soc Nephrol 28: 2221–2232, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agbor-Enoh S, Wang Y, Tunc I, Jang MK, Davis A, De Vlaminck I, et al. : Donor-derived cell-free DNA predicts allograft failure and mortality after lung transplantation. EBioMedicine 40: 541–553, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharon E, Shi H, Kharbanda S, Koh W, Martin LR, Khush KK, et al. : Quantification of transplant-derived circulating cell-free DNA in absence of a donor genotype. PLOS Comput Biol 13: e1005629, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritsche J, Müller A, Hausmann M, Rogler G, Andreesen R, Kreutz M: Inverse regulation of the ADAM-family members, decysin and MADDAM/ADAM19 during monocyte differentiation. Immunology 110: 450–457, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altuğ Y, Liang N, Ram R, Ravi H, Ahmed E, Brevnov M, et al. : Analytical validation of a single-nucleotide polymorphism-based donor-derived cell-free DNA assay for detecting rejection in kidney transplant patients. Transplantation 103: 2657–2665, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moinuddin I, Kumar D, Halloran P, Kamal L, King A, Kimball P, et al. : Correlation of donor-derived cell-free DNA with histology and molecular diagnoses of kidney transplant biopsies. Am J Transplant 19: 521, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Sigdel TK, Archila FA, Constantin T, Prins SA, Liberto J, Damm I, et al. : Optimizing detection of kidney transplant injury by assessment of donor-derived cell-free DNA via massively multiplex PCR. J Clin Med 8: E19, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thongprayoon C, Vaitla P, Craici IM, Leeaphorn N, Hansrivijit P, Salim SA, et al. : The use of donor-derived cell-free DNA for assessment of allograft rejection and injury status. J Clin Med 9: E1480, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kataria A, Kumar D, Gupta G: Donor-derived cell-free DNA in solid-organ transplant diagnostics: Indications, limitations, and future directions. Transplantation 105: 1203–1211, 2021 [DOI] [PubMed] [Google Scholar]
- 14.Bloom RD, Augustine JJ: Beyond the biopsy: Monitoring immune status in kidney recipients. Clin J Am Soc Nephrol 16: 1413–1422, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agbor-Enoh S, Shah P, Tunc I, Hsu S, Russell S, Feller E, et al. ; GRAfT Investigators : Cell-free DNA to detect heart allograft acute rejection. Circulation 143: 1184–1197, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khush KK, Patel J, Pinney S, Kao A, Alharethi R, DePasquale E, et al. : Noninvasive detection of graft injury after heart transplant using donor-derived cell-free DNA: A prospective multicenter study. Am J Transplant 19: 2889–2899, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callemeyn J, Lerut E, de Loor H, Arijs I, Thaunat O, Koenig A, et al. : Transcriptional changes in kidney allografts with histology of antibody-mediated rejection without Anti-HLA donor-specific antibodies. J Am Soc Nephrol 31: 2168–2183, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malhotra R, Siew ED: Biomarkers for the early detection and prognosis of acute kidney injury. Clin J Am Soc Nephrol 12: 149–173, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venner JM, Famulski KS, Reeve J, Chang J, Halloran PF: Relationships among injury, fibrosis, and time in human kidney transplants. JCI Insight 1: e85323, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klebanov L, Yakovlev A: How high is the level of technical noise in microarray data? Biol Direct 2: 9, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeve J, Böhmig GA, Eskandary F, Einecke G, Gupta G, Madill-Thomsen K, et al. ; INTERCOMEX MMDx-Kidney Study Group : Generating automated kidney transplant biopsy reports combining molecular measurements with ensembles of machine learning classifiers. Am J Transplant 19: 2719–2731, 2019 [DOI] [PubMed] [Google Scholar]
- 22.Madill-Thomsen K, Perkowska-Ptasińska A, Böhmig GA, Eskandary F, Einecke G, Gupta G, et al. ; MMDx-Kidney Study Group : Discrepancy analysis comparing molecular and histology diagnoses in kidney transplant biopsies. Am J Transplant 20: 1341–1350, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Furness PN, Taub N, Assmann KJ, Banfi G, Cosyns JP, Dorman AM, et al. : International variation in histologic grading is large, and persistent feedback does not improve reproducibility. Am J Surg Pathol 27: 805–810, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Reeve J, Sellarés J, Mengel M, Sis B, Skene A, Hidalgo L, et al. : Molecular diagnosis of T cell-mediated rejection in human kidney transplant biopsies. Am J Transplant 13: 645–655, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Bachelet T, Couzi L, Lepreux S, Legeret M, Pariscoat G, Guidicelli G, et al. : Kidney intragraft donor-specific antibodies as determinant of antibody-mediated lesions and poor graft outcome. Am J Transplant 13: 2855–2864, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Halloran PF, Madill-Thomsen KS, Böhmig GA, Myslak M, Gupta G, Kumar D, et al. : A 2-fold approach to polyoma virus (BK) nephropathy in kidney transplants: Distinguishing direct virus effects from cognate T cell-mediated inflammation. Transplantation 105: 2374–2384, 2021 [DOI] [PubMed] [Google Scholar]
- 27.Halloran PF, Reeve J, Akalin E, Aubert O, Bohmig GA, Brennan D, et al. : Real time central assessment of kidney transplant indication biopsies by microarrays: The INTERCOMEX Study. Am J Transplant 17: 2851–2862, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Kyeso Y, Bhalla A, Smith AP, Jia Y, Alakhdhair S, Ogir SC, et al. : Donor-derived cell-free DNA kinetics post-kidney transplant biopsy. Transplant Direct 7: e703, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halloran PF, Demko Z, Prewett A, Reeve J, Billings P: The Trifecta Study: Calibrating circulating donor-derived cell-free DNA at the time of indication biopsies against the molecular phenotype of the biopsy reveals a prominent association with NK cell genes. Am J Transplant 21[Suppl 3]: 2021 [Google Scholar]
- 30.Reeve J, Böhmig GA, Eskandary F, Einecke G, Lefaucheur C, Loupy A, et al. ; MMDx-Kidney study group : Assessing rejection-related disease in kidney transplant biopsies based on archetypal analysis of molecular phenotypes. JCI Insight 2: e94197, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lê S, Josse J, Husson F: FactoMineR: AnRPackage for multivariate analysis. J Stat Softw 25: 18, 2008 [Google Scholar]
- 32.Famulski KS, de Freitas DG, Kreepala C, Chang J, Sellares J, Sis B, et al. : Molecular phenotypes of acute kidney injury in kidney transplants. J Am Soc Nephrol 23: 948–958, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta G, Moinuddin I, Kamal L, King AL, Winstead R, Demehin M, et al. : Correlation of donor-derived cell-free DNA with histology and molecular diagnoses of kidney transplant biopsies [published online ahead of print May 28, 2021]. Transplantation 2021 [DOI] [PubMed] [Google Scholar]
- 34.Loupy A, Duong Van-Huyen JP, Hidalgo LG, Reeve J, Racape M, Aubert O, et al. : Gene expression profiling for the identification and classification of antibody-mediated heart rejection. Am J Transplant 17: 284, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Halloran PF, Chang J, Famulski K, Hidalgo LG, Salazar ID, Merino Lopez M, et al. : Disappearance of T cell-mediated rejection despite continued antibody-mediated rejection in late kidney transplant recipients. J Am Soc Nephrol 26: 1711–1720, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madill-Thomsen KSB, Böhmig GA, Bromberg J, Einecke G, Eskandary F, Gupta G, et al. ; INTERCOMEX Investigators : Donor-specific antibody is associated with increased expression of rejection transcripts in renal transplant biopsies classified as no rejection. J Am Soc Nephrol 32: 2743–2758, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.