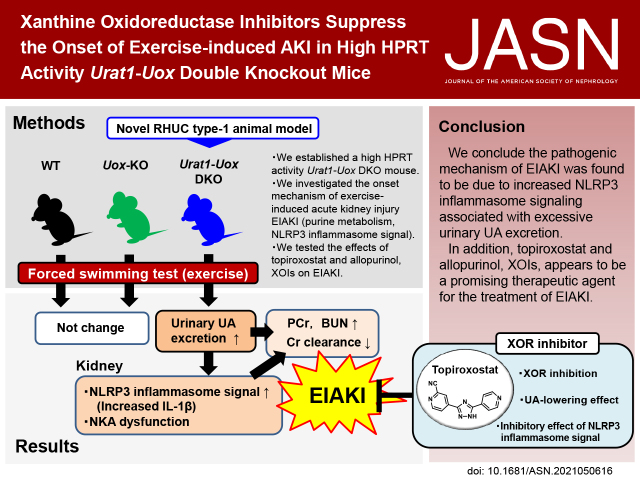
Significance Statement
Hereditary renal hypouricemia type 1 (RHUC1) is caused by URAT1/SLC22A12 dysfunction, resulting in urolithiasis and exercise-induced AKI (EIAKI). However, the precise pathophysiologic mechanisms underlying EIAKI have yet to be elucidated. We investigated the cause of EIAKI and the therapeutic effect of xanthine oxidoreductase inhibitors by establishment of a high HPRT activity Urat1-Uox double knockout mouse. The onset of EIAKI associated with RHUC1 was related to increased excessive urinary urate excretion brought on by exercise, and inflammatory signals via NLRP3 inflammasome activation in the kidney. We showed xanthine oxidoreductase inhibitors (topiroxostat and allopurinol) to be effective for the treatment of EIAKI with RHUC1 in this mouse model.
Keywords: renal hypouricemia (RHUC), urate transporter 1 (URAT1), exercise-induced acute kidney injury (EIAKI), hypoxanthine phosphoribosyltransferase (HPRT), xanthine oxidoreductase inhibitor (XOI), mice, knockout, renal tubular transport, inborn errors, urolithiasis
Visual Abstract
Abstract
Background
Hereditary renal hypouricemia type 1 (RHUC1) is caused by URAT1/SLC22A12 dysfunction, resulting in urolithiasis and exercise-induced AKI (EIAKI). However, because there is no useful experimental RHUC1 animal model, the precise pathophysiologic mechanisms underlying EIAKI have yet to be elucidated. We established a high HPRT activity Urat1-Uox double knockout (DKO) mouse as a novel RHUC1 animal model for investigating the cause of EIAKI and the potential therapeutic effect of xanthine oxidoreductase inhibitors (XOIs).
Methods
The novel Urat1-Uox DKO mice were used in a forced swimming test as loading exercise to explore the onset mechanism of EIAKI and evaluate related purine metabolism and renal injury parameters.
Results
Urat1-Uox DKO mice had uricosuric effects and elevated levels of plasma creatinine and BUN as renal injury markers, and decreased creatinine clearance observed in a forced swimming test. In addition, Urat1-Uox DKO mice had increased NLRP3 inflammasome activity and downregulated levels of Na+-K+-ATPase protein in the kidney, as Western blot analysis showed. Finally, we demonstrated that topiroxostat and allopurinol, XOIs, improved renal injury and functional parameters of EIAKI.
Conclusions
Urat1-Uox DKO mice are a useful experimental animal model for human RHUC1. The pathogenic mechanism of EIAKI was found to be due to increased levels of IL-1β via NLRP3 inflammasome signaling and Na+-K+-ATPase dysfunction associated with excessive urinary urate excretion. In addition, XOIs appear to be a promising therapeutic agent for the treatment of EIAKI.
Renal hypouricemia (RHUC) is a hereditary disease characterized by low levels of plasma urate (UA).1 RHUC type 1 (OMIM #220150, RHUC1) is caused by mutations of the SLC22A12 gene which encodes the reabsorptive urate transporter 1 (URAT1).2 Interestingly, allele frequency of dysfunctional variants SLC22A12 p.T467M and p.L415_G417 is higher in the European Roma population, which exceeds that of the Asian prevalent variant p.W258X.3–5 RHUC1 often complicates urolithiasis and exercise-induced AKI (EIAKI), a condition associated with acute renal failure and severe loin pain after anerobic exercise such as sprinting or competitive swimming, and is often seen in young men.1,6,7 However, the precise pathophysiologic mechanisms underlying EIAKI have yet to be elucidated unlike hyperuricemic nephropathy.8
Studies of RHUC1 are severely hampered by the lack of a suitable rodent model. With this in mind, we previously established a Urat1-knockout (KO) mouse.9 However, although Urat1-KO mice showed increased urinary UA excretion, there was no decrease in the level of plasma UA as observed in RHUC patients.9 It is known that there are species-specific differences in purine metabolism between human and rodents. Unlike humans, rodents such as mice possess the enzyme uricase (UOX), which converts UA to allantoin (AL). Thus, instead of UA, the final metabolic product of purine catabolism in rodents is AL, which is eliminated through urine.10 Therefore, we went on to establish and evaluate Urat1-Uox double knockout (DKO) mice to simulate human purine metabolism. However, the Urat1-Uox DKO mice also did not show a significant decrease in plasma UA levels.11
Compared with human, rodents such as mice (e.g., C57BL/6J strain) are known to have a low activity of hypoxanthine phosphoribosyltransferase (HPRT), which contributes to the purine salvage pathway.12 Interestingly, there are subspecies differences of HPRT in mice. HPRT in mice comprises HPRT A protein with high activity and HPRT B protein with low activity.13,14 Wild-type (WT)-derived strains of mice (e.g., Mus musculus molossinus) have HPRT A protein similar to that found in humans, which is more highly active than C57BL/6J mice with HPRT B protein.13,15 Urat1-KO and Urat1-Uox DKO mice reported so far have a pure C57BL/6J background, and HPRT activity of these mice is lower than normal mice (e.g., C57BL/6J).9,11,13 Therefore, we reasoned that it is necessary to alter purine metabolism in our model mice to resemble more closely that found in human with relatively high HPRT activity. In previous studies, we established and reported a novel mouse model of hyperuricemia with high HPRT activity as found in humans. The HPRT activity in these model mice is approximately 30-fold higher than that of WT mice (C57BL/6J).16
Thus, we hypothesized that high HPRT activity Urat1-Uox DKO mice could be an experimental animal model for human RHUC1. In this study, we established high HPRT activity Urat1-Uox DKO mice as a novel RHUC1 animal model and investigated the precise pathophysiologic mechanisms underlying EIAKI. We also investigated the therapeutic effect of xanthine oxidoreductase inhibitors (XOIs) on EIAKI using the model mice.
Methods
Animals
Animal procedures were approved by the Teikyo University Ethics Committee for Animal Experiments (numbers 15–052 and 20–018). Male C57BL/6J mice as WT were purchased from Sankyo Labo. Service Corporation. B6-ChrXCMSM mice (with the central part of the X chromosome derived from M. m. molossinus) were purchased with a C57BL/6J genetic background from the National Institute of Genetics (Shizuoka, Japan).17 Uox-KO mice with low HPRT activity were purchased from the Jackson Laboratory (Bar Harbor, ME) and then backcrossed to a genetic background of C57BL/6J. High HPRT activity Uox-KO mice were established by mating B6-ChrXCMSM mice (high HPRT activity) with Uox-KO mice.16 Low HPRT activity Urat1-Uox DKO mice were established by mating Urat1-KO mice9 with Uox-KO mice and then backcrossed to a genetic background of C57BL/6J.11 High HPRT activity Urat1-Uox DKO mice were established by mating high HPRT activity Uox-KO mice with low HPRT activity Urat1-Uox DKO mice. Mice were housed in sterile and ventilated cages, kept in an air-conditioned room with a standard 12-hour light/dark cycle. The mice were fed a standard diet and water ad libitum throughout the acclimatization and experimental periods.
Genotyping of the Hprt, Urat1, and Uox Genes
Allelic discrimination of the Hprt gene was conducted using TaqMan GTXpress Master Mix (4401892; Thermo Fisher Scientific)15 with the following oligonucleotide primers and minor groove binder (MGB) probes: (FAM)-cctcctcagaccgctttttg; R-tctgctggagtccccttg; (VIC)-cccgtcatgccgac-(MGB); (FAM)-cccgtcatggcgac-MGB, where (FAM)- and (VIC)- represent fluorescein amidite and 4,7,2′-trichloro-7′-phenyl-6-carboxyfluorescein, respectively. PCR amplifications were carried out in a final volume of 10 μl consisting of a master mix, 0.9 μmol/L of each primer, and 0.2 μmol/L of each labeled probe. The holding stage before PCR was performed at 25°C for 1 minute. PCR cycling was conducted initially at 95°C for 20 seconds, followed by 40 cycles at 95°C for 3 seconds each, and finally at 60°C for 30 seconds. The holding stage after PCR was performed at 25°C for 1 minute.
Genotyping of the Urat1 gene was performed by PCR of genomic DNA templates extracted from an ear punch according to the dilution protocol of Phire Animal Tissue Direct PCR Kit (F140WH; Thermo Fisher Scientific).11 To avoid carryover contamination, TaKaRa PCR Carryover Prevention Kit (6088; Takara Bio, Inc.) was used. The PCR mixture contained 0.8 μl of dU plus dNTP mixture, 0.3 μl of 25 mmol/L MgCl2, 0.1 μl of UNG (reagents from TaKaRa PCR Carryover Prevention Kit), 1 μl of 10× Standard Taq Reaction buffer, 0.2 μl of Hot Start Taq DNA polymerase (NEB), 0.5 μl of template, 0.3 μl of three mixed primers, and 6.8 μl of distilled water in a total volume of 10 μl. Using a common sense primer (5′-ccctcttctattaaatggctcctag-3′) and antisense primers (5′-ccatgggtttctcctgggtacc-3′ for the WT allele and 5′-gggtgttgggtcgtttgttcgg-3′ for the targeted allele), we amplified 362 bp and 274 bp amplicons from WT and targeted Urat1 alleles, respectively.
Genotyping of the Uox gene was performed by PCR of genomic DNA templates extracted from an ear punch using the same procedure as described for the genotyping of the Urat1 gene. The PCR mixture contained 0.8 μl of dU plus dNTP mixture, 0.3 μl of 25 mmol/L MgCl2, 0.1 μl of UNG (reagents from TaKaRa PCR Carryover Prevention Kit), 1 μl of 10× Standard Taq Reaction buffer, 0.2 μl of Hot Start Taq DNA polymerase (NEB), 0.5 μl of template, 0.3 μl of three mixed primers, and 6.8 μl of distilled water in a total volume of 10 μl. Using sense primers (5′-tcagaaacatcgagacctttgc-3′ for the WT allele and 5′-atcgccttctatcgccttctt-3′ for the targeted allele) and a common antisense primer (5′-taccgtttctcatctgctccac-3′), we amplified 291 bp and 158 bp amplicons from WT and targeted Uox alleles, respectively.
Western Blotting
Kidney tissues from mice were rapidly dissected and frozen in liquid nitrogen. Kidneys were homogenized in lysis buffer and 1% protease inhibitor cocktail. The homogenate was centrifuged at 12,000× g for 10 minutes at 4°C, and the supernatants were recovered as tissue lysates. Plasma membrane fractions were purified using a Minute Plasma Membrane Protein Isolation Kit (Invent Biotechnologies).18 The protein content of tissue lysates and plasma membrane were analyzed using a TaKaRa BCA Protein Assay Kit (Takara Bio), and samples containing equal amounts of protein were heated in Laemmli buffer. Samples were run on Mini-PROTEAN TGX Gels 4%–15% (Bio-Rad) and transferred to Trans-Blot Turbo polyvinylidene fluoride (PVDF) membranes (Bio-Rad). Stainfree technology (Bio-Rad) was used to determine equal loading of protein. Membranes were blocked in 5% BSA for 1 hour at room temperature and exposed to primary antibodies overnight at 4°C, followed by incubation with appropriate horseradish peroxidase–conjugated secondary antibodies (Cell Signaling) and visualization with Clarity Western ECL Substrate (Bio-Rad) on film or a Molecular Imager ChemiDoc XRS Plus system (Bio-Rad). The following antibodies were used: URAT1 (created a primary antibody against rat URAT1),19 β-actin (4967S; Cell Signaling Technology), NLRP3 (NBP2-12446; Novus Biologicals, LLC), ASC (NBP1-78977; Novus Biologicals, LLC), Caspase-1 (GTX101322; GeneTex, Inc.), IL-1β (bs-0812R; Bioss, Inc.), and Na+-K+-ATPase (NKA) (3010S; Cell Signaling Technology). The primary antibody was used at 1:1000 dilution in Tris-buffered saline with Tween 20.
In Vivo Experimental Protocols
For the in vivo studies, we used male WT mice weighing 20–25 g (10 weeks old), high HPRT activity Uox-KO mice weighing 23–38 g (8–12 weeks old), and high HPRT activity Urat1-Uox DKO mice weighing 25–40 g (8–12 weeks old). Study 1: Male WT mice (n=9) and Uox-KO mice (n=8) and Urat1-Uox DKO mice (n=9) were fed standard powder chow (CRF-1). Urine (spot or pooled) was collected in a metabolic cage, and blood was collected (10–20 μl) from the tail vein using a fixed cage without anesthesia. Study 2: Male WT (n=8), Uox-KO (n=8), and Urat1-Uox DKO mice (n=9) fed CRF-1 were used in the forced swimming test. Pooled urine was collected in a metabolic cage and blood was collected (10–20 μl) from the tail vein using a fixed cage without anesthesia both before and after the forced swimming test. Study 3: Male high HPRT activity Uox-KO mice were fed CRF-1 (control, n=6) for 7 days. Male high HPRT activity Urat1-Uox DKO mice were randomly divided into three groups and fed CRF-1 (n=5) and topiroxostat (0.3 and 1 mg/kg per day, n=5–6, respectively) for 7 days. The blood and spot urine samples were collected on the seventh day after administration of each drug. Study 4: Male high HPRT activity Urat1-Uox DKO mice were randomly divided into two groups for assessment of topiroxostat: control (vehicle, n=10) and topiroxostat (1 mg/kg, n=10). Topiroxostat was orally administered. In each case, the drug volume administered corresponded to 10 ml/kg body weight. Control mice received the same volume of drug vehicle (0.5% methylcellulose) via the same administration route. A forced swimming test was performed 15 minutes after the administration of drug to these model mice. Pooled urine was collected in a metabolic cage and blood was collected (10–20 μl) from the tail vein using a fixed cage without anesthesia both before and after the forced swimming test. Study 5: Male high HPRT activity Urat1-Uox DKO mice were randomly divided into two groups for assessment of allopurinol: control (vehicle, n=6) and allopurinol (30 mg/kg, n=6). Allopurinol was orally administered. In each case, the drug volume administered corresponded to 10 ml/kg body weight. Control mice received the same volume of drug vehicle (0.5% methylcellulose) via the same administration route. A forced swimming test was performed 15 minutes after the administration of drug to these model mice. Pooled urine was collected in a metabolic cage and blood was collected (10–20 μl) from the tail vein using a fixed cage without anesthesia both before and after the forced swimming test.
HPLC Analysis of Metabolites (Oxypurine and AL) and Creatinine
Purine metabolites and creatinine (Cr) in plasma and urine were measured using an HPLC method as described previously.16 A 1–2 μl aliquot of the separated plasma was deproteinized with 80% acetonitrile by centrifugation at 12,000× g, 4°C for 4 minutes using an Ultrafree-MC 0.22-μm PVDF membrane filter unit (Millipore, Billerica, MA). The deproteinized sample was resuspended with 10 μl of HPLC mobile phase (10 mmol/L ammonium formate) after evaporation. Pooled and spot urine samples were collected and diluted 50 times in HPLC mobile phase (10 mmol/L ammonium formate). Separation of the resuspended and the 50-fold diluted samples was achieved at a flow rate of 0.2 ml/min on a 250 mm × 2 mm, 5-μm particle size octadecyl silica column, Unison US-C18 (US026; Imtakt Corporation) at 25°C using a Hitachi pump. Peaks corresponding to AL, oxypurine (OP) (comprising UA, hypoxanthine [HX], and xanthine [XA], i.e., UA+HX+XA), and Cr were detected at 234 nm. Urinary AL/Cr ratio, urinary UA/Cr ratio, urinary HX+XA/Cr ratio, urinary OP/Cr ratio, fractional excretion of UA (FEUA), fractional excretion of HX+XA (FEHX+XA), fractional excretion of OP (FEOP), Cr clearance (CLCr), and UA clearance (CLUA) were then calculated.16
Mouse Erythrocyte HPRT Activity Assay
Mouse erythrocyte HPRT activity was measured by an HPLC method as described previously.16 Assay mixtures comprised 2 mmol/L 5-phosphoribosyl-1-pyrophosphate (PRPP), 6 mmol/L MgCl2, and 100 μmol/L HX in 50 mmol/L Tris-HCl buffer (pH 7.4). The reaction was initiated by addition of 20 mg/ml erythrocyte extract containing HPRT to each mixture at 37°C for 5 minutes. The reaction was then quenched and the mixtures deproteinized by addition of 80% acetonitrile. Samples were subjected to centrifugation at 20,000× g, 4°C for 15 minutes and the supernatant was collected. The supernatant was further clarified by centrifugation at 12,000× g, 4°C for 4 minutes using an Ultrafree-MC 0.22-μm PVDF membrane filter unit (Millipore) to remove proteins. The deproteinized sample was resuspended with 10 μl of HPLC mobile phase (50 mmol/L ammonium formate, pH 4.1) after evaporation. Assay samples were analyzed by HPLC at a flow rate of 0.25 ml/min on a 250 mm × 2 mm, 5-μm particle size octadecyl silica column, using a Unison US-C18 system (US026; Imtakt Corporation) at 40°C using a Hitachi pump. The peak corresponding to inosine monophosphate (IMP) was detected at 260 nm. Each HPRT activity was expressed as product nanomoles per minute per milligram erythrocytes.
Measurement of BUN
BUN in plasma was measured using a BUN Colorimetric Detection Kit (K024-H1; Arbor Assays).
Forced Swimming Test
The forced swimming load test was carried out with some modifications based on the report of Matsukawa et al. 2017.20 The forced swimming tests were carried out under a nonfasted state to avoid energy depletion before performing the swimming exercise. Mice performed the swimming exercise for 5 minutes with a load corresponding to 5% of their body weight attached to their tails in a tank (30×30×40 cm) filled with water to a depth of 25 cm, maintained at 25±1°C. This exercise schedule was implemented five times in total with a 1-minute rest in between. Urine and blood were collected before and after the forced swimming test. After completion of the test, blood was collected from the inferior vena cava using a 24-gauge needle and a heparinized syringe under isoflurane inhalation anesthesia. The mice were fully exsanguinated and organs, such as the kidney, removed.
Assessment of Kidney Injury
The kidneys from the mice were collected immediately after euthanasia. Kidney injury was assessed by analyzing 4% formaldehyde-fixed paraffin-embedded tissue sections (2-mm-thick) stained with periodic acid–Schiff reagent.21 The kidney sections were observed by two investigators in a blinded manner.
Analysis of UA Crystal Deposits in the Urine
Urinary crystal sections were obtained from absolute ethanol-fixed urinary deposits. UA crystal deposits were visualized under polarized light.22 In addition, urinary deposits were dissolved in HPLC mobile phase (10 mmol/L ammonium formate) to 50-fold dilution and then analyzed by HPLC. The UA peak was detected at 295 nm.
Drugs and Reagents
Topiroxostat was manufactured and supplied by Fuji Yakuhin Co., Ltd. (Saitama, Japan). Allopurinol was obtained from FUJIFILM Wako Pure Chemical Corporation (Tokyo, Japan). For in vivo studies, topiroxostat was adjusted to 0.3 and 1 mg topiroxostat/100 g feed. The topiroxostat and allopurinol were suspended in 0.5% methylcellulose solution and administered at a constant volume of 10 ml/kg body weight.
Statistical Analyses
All data were expressed as the mean±SEM. Data were analyzed using unpaired t test, 1-way ANOVA, or 2-way ANOVA followed by Tukey’s or Dunnett’s post hoc test. A P value less than 0.05 was considered statistically significant.
Results
Establishment of High HPRT Activity Urat1-Uox DKO Mice
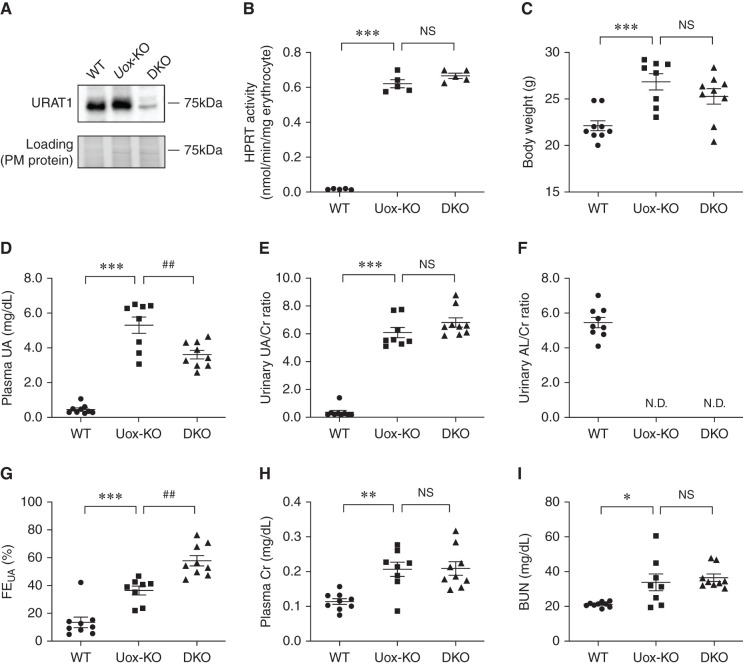
Firstly, we investigated the construction of high HPRT activity Urat1-Uox DKO mice. These model mice were established by mating a high HPRT activity Uox-KO mouse with a low HPRT activity Urat1-Uox DKO mouse. The gender rate of mice born in the high HPRT activity Urat1-Uox DKO group was 50.4% male (121 of 240) and 49.6% female (119 of 240). Growth curves for both the male and female mice from 6 to 20 weeks were similar to the high HPRT activity Uox-KO and Urat1-Uox DKO group (Supplemental Figure 1). To investigate the effect of Uox and Urat1 knockout and HPRT activity, we measured the level of URAT1 protein in kidney, AL, UA, Cr in plasma and urine, and erythrocyte HPRT activity in male WT mice (C57BL/6J), high HPRT activity Uox-KO mice, and Urat1-Uox DKO mice. The level of Urat1 protein dramatically decreased in Urat1-Uox DKO mice kidney compared with WT and Uox-KO mice as determined by Western blotting (Figure 1A). The urinary AL/Cr ratio for WT mice was 5.45±0.29, but urinary AL was not detected in either of these model mice (Figure 1F). This observation confirmed that Uox was successfully knocked out. Moreover, HPRT activity in the erythrocytes of Uox-KO and Urat1-Uox DKO mice was markedly elevated by approximately 33-fold compared with WT mice (Figure 1B). Next, we examined the profile of purine metabolism and renal functional markers in high HPRT activity Urat1-Uox DKO mice. Compared with male WT mice, the plasma UA was significantly increased for both Uox-KO and Urat1-Uox DKO mice, and the plasma UA of Urat1-Uox DKO mice was significantly decreased compared with that of Uox-KO mice (Figure 1D). The urinary UA/Cr ratio was significantly elevated for Uox-KO and Urat1-Uox DKO mice compared with WT mice, but there was no significant difference in urinary UA/Cr ratio for Urat1-Uox DKO mice compared with Uox-KO mice (Figure 1E). The FEUA were significantly increased for Uox-KO and Urat1-Uox DKO mice compared with WT mice, and the FEUA for Urat1-Uox DKO mice was also significantly elevated compared with that for Uox-KO mice (Figure 1G). In the low HPRT activity group, no significant changes were observed for plasma UA and FEUA of Urat1-Uox DKO mice compared with those of the Uox-KO mice (Supplemental Figure 2). The plasma Cr and BUN were significantly increased for Uox-KO and Urat1-Uox DKO mice compared with WT mice, but there was no difference between Uox-KO and Urat1-Uox DKO mice (Figure 1, H and I).
Figure 1.
Establishment of male high HPRT activity Urat1-Uox DKO mice. (A) Expression of URAT1 protein in the kidney plasma membrane of male WT (C57BL/6J), high HPRT activity Uox-KO, and Urat1-Uox DKO mice (n=3 per group). (B) HPRT activity of mouse erythrocytes in male WT, high HPRT activity Uox-KO, and Urat1-Uox DKO mice (n=5 per group). (C) Body weight, (D) plasma UA, (E) urinary UA/Cr ratio, (F) urinary AL/Cr ratio, (G) FEUA, (H) plasma Cr, and (I) BUN in male WT (B6, n=9), high HPRT activity Uox-KO (n=8), and Urat1-Uox DKO mice (n=9). Data represent means±SEM; data were analyzed using 1-way ANOVA with Tukey’s post hoc test in (B–I). Significantly different from WT (*P<0.05, **P<0.01, ***P<0.001) and Uox-KO (##P<0.01). N.D., not detected; NS, not significant (P>0.05).
EIAKI on High HPRT Activity Urat1-Uox DKO Mice
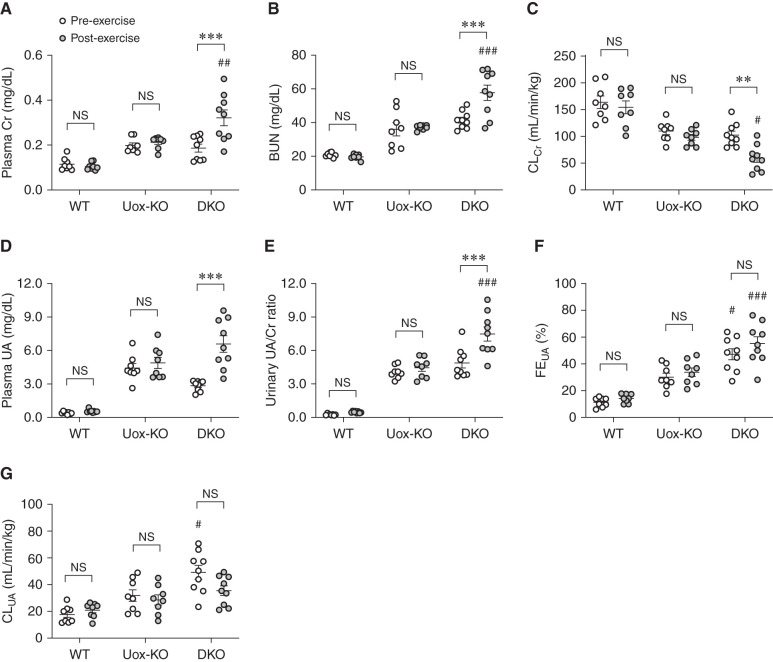
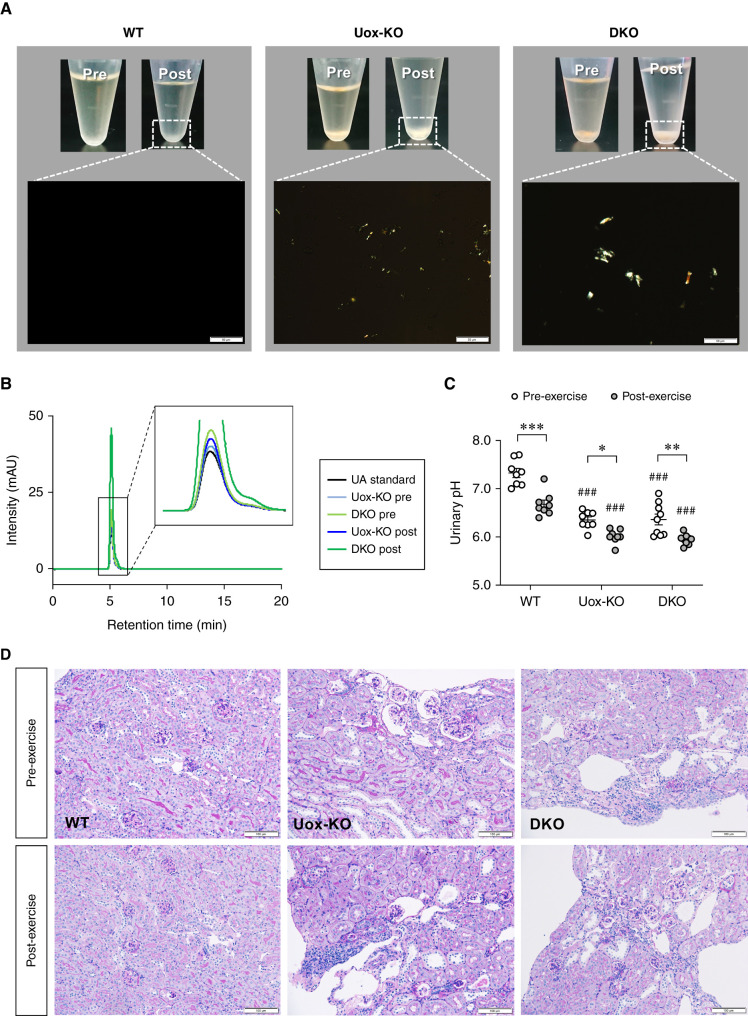
We investigated EIAKI by using WT, high HPRT activity Uox-KO, and Urat1-Uox DKO mice in a forced swimming test as exercise. For male WT and Uox-KO mice, no significant difference was detected in the plasma Cr, BUN, CLCr, plasma UA, urinary UA/Cr ratio, and FEUA between pre- and post-exercise (Figure 2, A–F). For male Urat1-Uox DKO mice, however, the plasma Cr, BUN, plasma UA, and urinary UA/Cr ratio in the post-exercise group were elevated compared with those of the pre-exercise group (Figure 2, A, B, D, and E), whereas CLCr for the post-exercise group was significantly decreased compared with that for the pre-exercise group (Figure 2C). The CLUA was not significantly different pre- and post-exercise, but the clearance tended to decrease (Figure 2G). Female Urat1-Uox DKO mice were also observed, which showed elevated plasma Cr and decreased CLCr in the post-exercise group (Supplemental Figure 3). In the Urat1-Uox DKO group, renal functional parameters (plasma Cr, BUN, and CLCr) were positively or negatively correlated with UA parameters (plasma UA, urinary UA/Cr ratio, FEUA, and CLUA) (Supplemental Figure 4). In addition, analysis of the urine from Uox-KO and Urat1-Uox DKO mice both pre- and post-exercise indicated UA crystalluria, which was absent in WT mice, and a lower urinary pH (Figure 3, A–C). Next, we performed periodic acid–Schiff staining of the kidney sections of these model mice both pre- and post-exercise to examine any renal pathologic changes. Histologic impairments in the kidneys of Uox-KO and Urat1-Uox DKO mice included dilated Bowman’s spaces and tubules, and collapsed and necrotic nephrons (as previously reported22,23), but not observed in changes between pre- and post-exercise groups of these model mice (Figure 3D).
Figure 2.
EIAKI of high HPRT activity Urat1-Uox DKO mice. (A) Plasma Cr, (B) BUN, (C) CLCr, (D) plasma UA, (E) urinary UA/Cr ratio, (F) FEUA, and (G) CLUA in WT (B6, n=8), high HPRT activity Uox-KO (n=8), and Urat1-Uox DKO mice (n=9) pre- and post-exercise. Data represent means±SEM; data were analyzed using 2-way ANOVA with Tukey’s post hoc test in (A–G). Significantly different from pre-exercise group (**P<0.01, ***P<0.001) and change in Uox-KO group (#P<0.05, ##P<0.01, ###P<0.001). NS, not significant (P>0.05).
Figure 3.
Renal histology and UA crystalluria of high HPRT activity Urat1-Uox DKO mice. (A) Urine was collected from WT, Uox-KO, and Urat1-Uox DKO mice pre- and post-exercise. Bright signals in the lower images are urinary deposits. Urinary crystals were observed under polarized light (scale bar=50 μm). (B) A peak corresponding to UA was detected in solution after redissolving urinary crystals in HPLC buffer (refer to Supplemental Methods for details). (C) Urinary pH in WT (B6, n=8), Uox-KO (n=8), and Urat1-Uox DKO mice (n=9) pre- and post-exercise. (D) Periodic acid-Schiff staining of the kidney of WT, Uox-KO, and Urat1-Uox DKO mice pre- and post-exercise (scale bar =100 μm). Data represent means±SEM; data were analyzed using 2-way ANOVA with Tukey’s post hoc test in (C). Significantly different from pre-exercise group (*P<0.05, **P<0.01, ***P<0.001) and WT mice group (###P<0.001).
Activation of NLRP3 Inflammasome and Downregulation of NKA in EIAKI
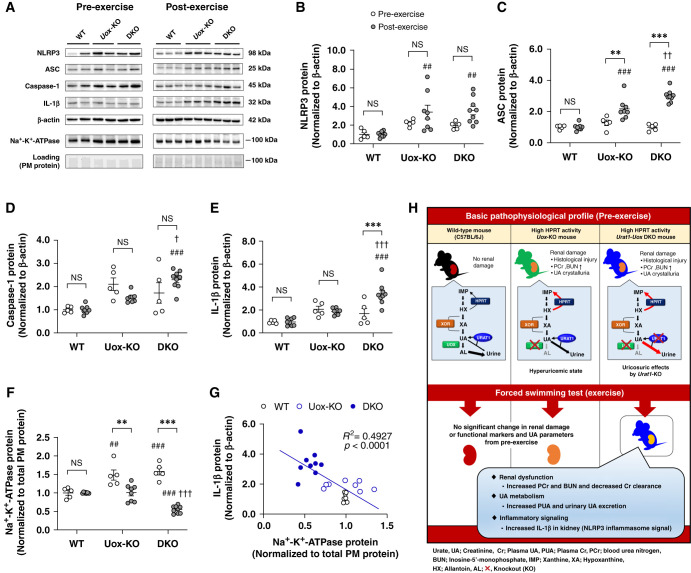
It has been previously reported that NLRP3 inflammasome and NKA signaling failure may contribute to hyperuricemic nephropathy associated with elevated urinary UA excretion.8,24 To investigate whether NLRP3 inflammasome activation and impaired NKA signaling contributed to EIAKI, we measured NLRP3 inflammasome signals and NKA levels in the kidney of WT, high HPRT activity Uox-KO, and Urat1-Uox DKO mice pre- and post-exercise by Western blot analysis (Figure 4, A-G). In the pre-exercise group, NKA levels for the Uox-KO and Urat1-Uox DKO mice were significantly elevated compared with WT mice (Figure 4F), whereas NLRP3, ASC, Caspase-1, and IL-1β for the Uox-KO and Urat1-Uox DKO mice were not significantly different (Figure 4, B–E). In the post-exercise group, the level of NLRP3 and ASC for Uox-KO mice and Urat1-Uox DKO mice was significantly elevated compared with WT mice (Figure 4, B and C). The levels of Caspase-1 and IL-1β in Uox-KO and Urat1-Uox DKO mice were significantly elevated compared with WT mice (Figure 4, D and E). Moreover, the levels of ASC, Caspase-1, and IL-1β in Urat1-Uox DKO mice were significantly increased compared with Uox-KO mice (Figure 4, C–E). There was no significant difference in the level of NKA between Uox-KO mice and WT mice. By contrast, the level of NKA in Urat1-Uox DKO mice was significantly decreased compared with WT and Uox-KO mice (Figure 4F). In addition, IL-1β was negatively correlated with NKA protein (Figure 4G). IL-1β and NKA protein were also positively and negatively correlated with renal functional and UA parameters, respectively (Supplemental Figure 5).
Figure 4.
Expression of NLRP3 inflammasome related proteins and NKA alternated in kidney of high HPRT activity Urat1-Uox DKO mice pre- and post-exercise. (A) Representative Western blots of NLRP3, ASC, Caspase-1, IL-1β, β-actin, and NKA α1 in pre- and post-exercise. Protein expression of (B) NLRP3, (C) ASC, (D) Caspase-1, and (E) IL-1β in kidney of WT (pre, n=5; post, n=8), Uox-KO (pre, n=5; post, n=8), and Urat1-Uox DKO mice (pre, n=5; post, n=9) pre- and post-exercise as determined by Western blot analysis. Protein expression of (F) NKA α1 in the kidney plasma membrane-enriched (PM) protein of WT (pre, n=5; post, n=8), Uox-KO (pre, n=5; post, n=8), and Urat1-Uox DKO mice (pre, n=5; post, n=9) pre- and post-exercise as determined by Western blot analysis. (G) The correlation of IL-1β with NKA PM protein in kidney of WT, Uox-KO, and Urat1-Uox DKO mice after exercise. Dot plot graphs show the results of densitometric quantitation. Data represent means±SEM; data were analyzed using 2-way ANOVA with Tukey’s post hoc test in (B–F). Significantly different from pre-exercise group (**P<0.01, ***P<0.001), WT mice group (##P<0.01, ###P<0.001), and Uox-KO mice group (†P<0.05, ††P<0.01, †††P<0.001). NS, not significant (P>0.05). (H) Schematic representation of the pathophysiologic features of model mice.
Effect of Topiroxostat on Purine Metabolism of High HPRT Activity Urat1-Uox DKO Mice
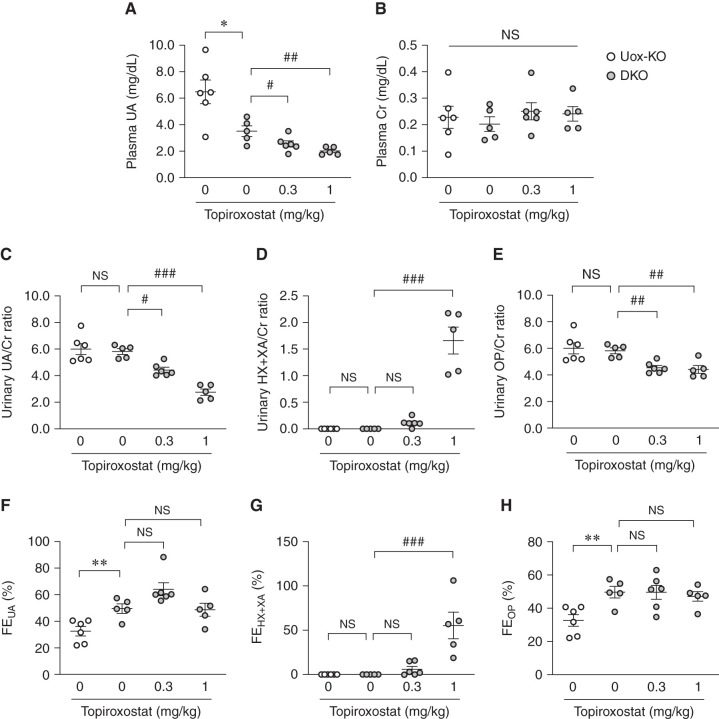
We measured purine metabolites in the plasma and urine of male high HPRT activity Uox-KO mice and Urat1-Uox DKO mice treated with topiroxostat (0.3 and 1 mg/kg per day) for 7 days. Urat1-Uox DKO mice were observed to undergo a significant decrease in plasma UA and increase in FEUA compared with Uox-KO mice (Figure 5, A and F). Topiroxostat elicited a dose-dependent decrease in plasma UA and urinary UA/Cr ratio for Urat1-Uox DKO mice (Figure 5, A and C), whereas the urinary HX+XA/Cr ratio and FEHX+XA increased (Figure 5, D and G). The urinary OP/Cr ratio in the topiroxostat-treated Urat1-Uox DKO group was significantly decreased compared with the nontreated Urat1-Uox DKO group (Figure 5E). FEUA and FEOP in the topiroxostat-treated groups were not significantly different compared with the nontreated Urat1-Uox DKO group (Figure 5, F and H). No significant difference was observed in plasma Cr (Figure 5B).
Figure 5.
Effects of topiroxostat, a nonpurine-type XOI, on purine metabolism of high HPRT activity Urat1-Uox DKO mice. (A) Plasma UA, (B) plasma Cr, (C) urinary UA/Cr ratio, (D) urinary HX+XA/Cr ratio, (E) urinary OP/Cr ratio, (F) FEUA, (G) FEHX+XA, and (H) FEOP in high HPRT activity Uox-KO (n=6) and Urat1-Uox DKO mice (DKO control, n=5; topiroxostat 0.3 mg/kg, n=6; topiroxostat 1 mg/kg, n=5). Data represent means±SEM; data were analyzed using unpaired t test (Uox-KO versus DKO control) and 1-way ANOVA with Dunnett’s post hoc test (DKO control versus topiroxostat-treated groups) in (A–H). Significantly different from Uox-KO group (*P<0.05, **P<0.01) and Urat1-Uox DKO group (#P<0.05, ##P<0.01, ###P<0.001). NS, not significant (P>0.05).
Effects of XOIs on EIAKI of High HPRT Activity Urat1-Uox DKO Mice
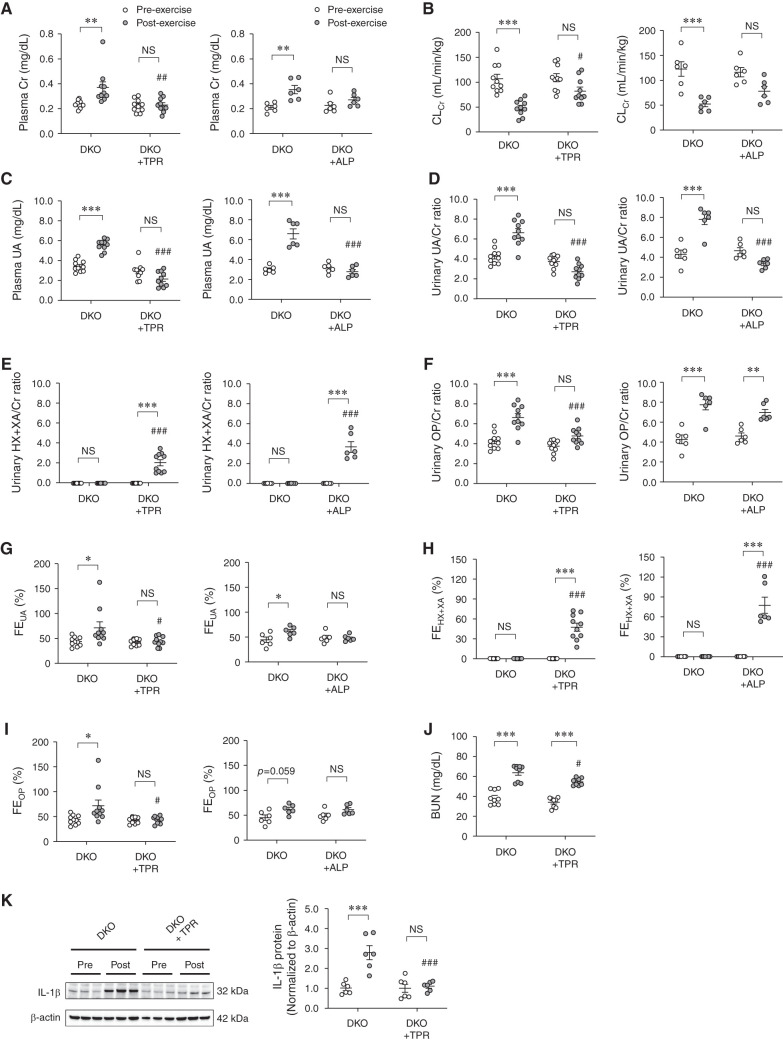
We investigated the effect of topiroxostat (nonpurine-type XOI) and allopurinol (purine-type XOI) on EIAKI using high HPRT activity Urat1-Uox DKO mice. post-exercise plasma Cr and BUN in the topiroxostat group was significantly decreased compared with the control group (Figure 6, A and J). In contrast, post-exercise CLCr in the topiroxostat group was significantly elevated compared with the control group (Figure 6B). The plasma UA, urinary UA/Cr ratio, and OP/Cr ratio in the post-exercise topiroxostat group were significantly decreased compared with the control group (Figure 6, C, D, and F). The urinary HX+XA/Cr ratio and FEHX+XA of the post-exercise topiroxostat group were significantly increased compared with the control group (Figure 6, E and H). However, FEUA and FEOP in the post-exercise topiroxostat group were significantly decreased compared with the control group (Figure 6, G and I). post-exercise, IL-1β levels in the kidneys of the topiroxostat group were significantly decreased compared with those of the control group (Figure 6K). In addition, allopurinol was also found to improve renal function (plasma Cr and CLCr) and UA related parameters (plasma UA, urinary UA/Cr ratio, and FEUA) in the post-exercise Urat1-Uox DKO group (Figure 6, A–D and G).
Figure 6.
Effects of topiroxostat and allopurinol, XOIs, on EIAKI of high HPRT activity Urat1-Uox DKO mice. (A) Plasma Cr, (B) CLCr, (C) plasma UA, (D) urinary UA/Cr ratio, (E) urinary HX+XA/Cr ratio, (F) urinary OP/Cr ratio, (G) FEUA, (H) FEHX+XA, and (I) FEOP in Urat1-Uox DKO control group (DKO, n=10) and topiroxostat-treated Urat1-Uox DKO group (DKO+TPR, n=10) or Urat1-Uox DKO control group (DKO, n=6) and allopurinol-treated Urat1-Uox DKO group (DKO+ALP, n=6) pre- and post-exercise. (J) BUN in Urat1-Uox DKO control group (DKO, n=10) and topiroxostat-treated Urat1-Uox DKO group (DKO+TPR, n=10) pre- and post-exercise. (K) IL-1β in the kidney of the Urat1-Uox DKO control group (DKO, n=6) and topiroxostat-treated Urat1-Uox DKO group (DKO+TPR, n=6) pre- and post-exercise as determined by Western blot analysis. Data represent means±SEM; data were analyzed using 2-way ANOVA with Tukey’s post hoc test in (A–K). Significantly different from pre-exercise groups (*P<0.05, **P<0.01, ***P<0.001) and Urat1-Uox DKO control group (#P<0.05, ##P<0.01, ###P<0.001). NS, not significant (P>0.05). ALP, allopurinol; TPR, topiroxostat.
Discussion
RHUC1 patients often present with complications of urolithiasis and EIAKI, a condition associated with acute renal failure and characterized by severe loin pain after anerobic exercise, such as sprinting or competitive swimming.1,6,7 However, the number of RHUC patients is small, and there are no useful pathologic model animals to study this condition. Hence, the onset mechanism of EIAKI remains unclear. In this study, we established a high HPRT activity Urat1-Uox DKO mouse as a novel RHUC1 animal model. Indeed, the novel Urat1-Uox DKO mice showed remarkable uricosuric effects associated with the Urat1 knockout, which mimic the high activity of HPRT in human. Conventional Urat1-Uox DKO mice did not display these characteristics (Figure 1 and Supplemental Figure 2). In addition, using the newly established male novel Urat1-Uox DKO mice, we investigated the onset mechanism of EIAKI brought on by a forced swimming test. With novel Urat1-Uox DKO mice we observed an elevation in the level of plasma Cr and BUN and a decrease of CLCr after the forced swimming test, whereas no such fluctuation in these renal damage parameters was observed with Uox-KO mice (Figure 2, A–C). Indeed, in clinical cases it has been reported that EIAKI of RHUC1 resulted in increased serum Cr and BUN as renal damage markers.25–29 Thus, our results suggest that novel Urat1-Uox DKO mice induce EIAKI similar to that observed for RHUC1 patients. In addition, Urat1-Uox DKO mice had elevated plasma UA, urinary UA/Cr ratio, and FEUA after exercise (Figure 2, D–F). Interestingly, it has been reported that patients with xanthinuria type 1 (OMIM #278330), which is characterized by the isolated deficiency of XDH, present with hypouricemia but do not develop EIAKI.30 Thus, our findings suggest that patients with EIAKI with RHUC1 undergo a temporary increase in plasma UA and excessive urinary UA excretion during anoxic exercise, thereby inducing renal damage. Numerous UA crystal precipitates were found in the urine of Uox-KO and Urat1-Uox DKO mice, which exhibited a lower pH than WT mice (Figure 3, A–C). Moreover, the urinary pH of Uox-KO and Urat1-Uox DKO mice was further decreased after exercise (Figure 3C). Indeed, urine containing UA precipitates and a low pH are known risk factors for renal dysfunction associated with CKD.31,32 Moreover, RHUC1 patients often present with complications of urolithiasis.1 Therefore, we speculate that the kidneys of post-exercise RHUC1 patients are not only saturated with soluble levels of UA due to excessive urinary UA excretion, but are also prone to UA crystal formation, which may be involved in renal dysfunction (Figure 4H).
Soluble UA and monosodium UA crystals are known to induce an inflammatory response through activation of NLRP3 inflammasomes and cytotoxicity of proximal tubular cells.33–37 Xiao et al. reported observing NLRP3 inflammasome activity, time-dependent NKA activity, and ATP consumption induced by high UA stimulation in human proximal tubular cells, resulting in reduced NKA activity and cell damage via mitochondria disorder.24 Therefore, we hypothesized that EIAKI was due to NLRP3 inflammasome activity and impaired NKA signaling in the kidney. To test this hypothesis, we quantified the protein level of NLRP3 inflammasome and NKA in the kidney after exercise by Western blot analysis. We found a significant increase in the level of NLRP3 inflammasome related proteins (NLRP3, ASC, Caspase-1, and IL-1β) and a decrease in NKA in the kidneys of Urat1-Uox DKO mice compared with Uox-KO mice (Figure 4, B–F). In particular, there was a markedly increased level of IL-1β in the kidneys of Urat1-Uox DKO mice after exercise (Figure 4E). In addition, it has been suggested that IL-1β induces renal dysfunction and decrease of renal NKA expression.38 Thus, our results suggest that elevated IL-1β through NLRP3 inflammasome activation and impaired NKA signaling in kidney via excessive urinary UA excretion are factors related to the mechanism of EIAKI on RHUC1.
XOIs as UA-lowering drugs have been used to reduce urinary UA excretion for the treatment or prevention of urolithiasis and EIAKI associated with hypouricemia.1,39–41 These XOIs are classified as purine-type (allopurinol) and nonpurine-type (febuxostat and topiroxostat) drugs. Nonpurine-type XOIs elicit a more selective and stronger xanthine oxidoreductase (XOR) inhibitory effect than purine-type XOIs. Moreover, nonpurine-type XOIs can be used without dose adjustment even in patients with moderate CKD.42–46 Therefore, in this study we selected topiroxostat, a nonpurine-type XOI, and evaluated its effects for purine metabolism and EIAKI on Urat1-Uox DKO mice. Topiroxostat was found to have a dose-dependent and significant plasma and urinary UA-lowering effect on Urat1-Uox DKO mice (Figure 5, A and C). Moreover, there was no significant change in the level of plasma Cr by administrating the drug. (Figure 5B). Therefore, we assume that the dose of topiroxostat used in this test will not have any adverse effects on the kidney of Urat1-Uox DKO mice. We also found that topiroxostat significantly suppressed UA production and excessive urinary UA excretion after the forced swimming test (Figure 6, C and D). In addition, topiroxostat improved not only the levels of plasma Cr, CLCr, and BUN as renal functional markers but also the levels of IL-1β in renal tissue for EIAKI (Figure 6, A, B, J, and K). EIAKI is suppressed in RHUC1 patients after administration of allopurinol as an XOI.40,41 Our findings also showed that allopurinol improved renal function and UA-related parameters of EIAKI in Urat1-Uox DKO mice (Figure 6, A–D). Therefore, we postulate that XOIs may alleviate renal injury by suppressing excessive UA production via XOR inhibition.
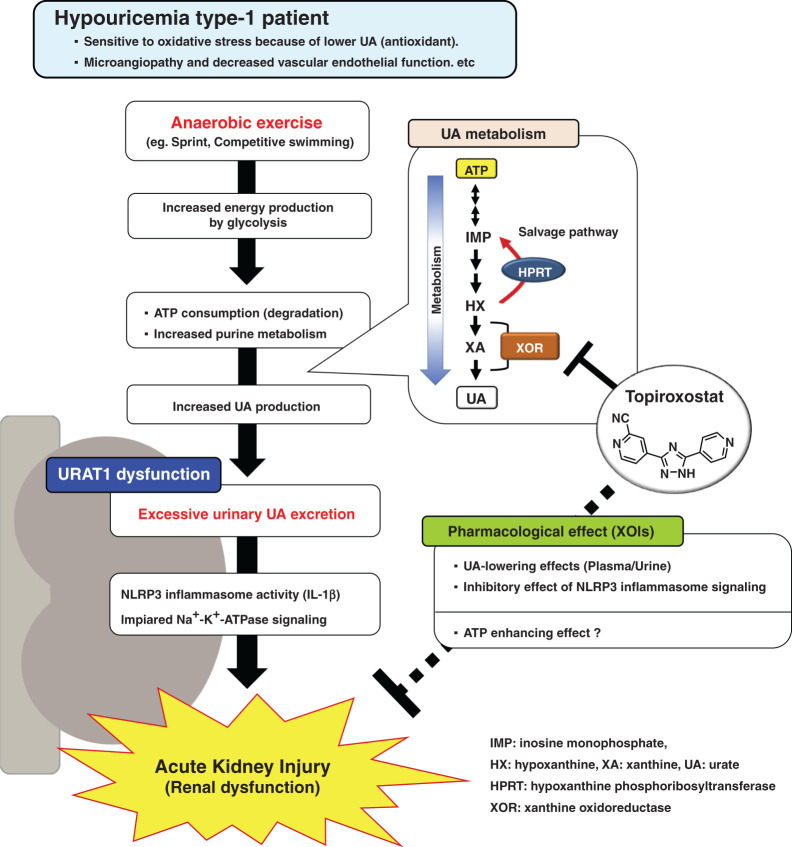
In this study, we showed that topiroxostat not only decreased urinary UA/Cr ratio and FEUA but also urinary OP/Cr ratio and FEOP in post-exercise Urat1-Uox DKO mice (Figure 6, D, F, G, and I). These observations suggest increased amounts of HX, resulting from inhibition of XOR, are converted via the purine salvage enzyme HPRT, resulting in reduction of urinary OP excretion. The XOI-induced increase in the level of HX is reported to be important in promoting ATP production via the purine salvage pathway.47 Moreover, Johnson et al. performed clinical studies where combined therapy with nonpurine-type XOI and inosine was found to be beneficial for disorders with ATP deficiency by efficiently increasing ATP production via the purine salvage pathway.48 Interestingly, recent studies have shown that XOIs improve AKI by bringing about an ATP-enhancing action via the salvage pathway in a renal ischemia-reperfusion model.49,50 Furthermore, it has also been reported that prolonged ATP depletion and downregulation of NKA occur in the kidney of renal ischemia-reperfusion model animals.49,51–53 Taken together with the findings from this study, we speculate that increased ATP biosynthesis via the salvage pathway induced by XOIs preserves, to some extent, the function of the NKA, thereby preventing renal damage (Figure 7). Thus, we believe that XOIs (topiroxostat and allopurinol) may be useful for treating EIAKI in RHUC1 patients. Nonetheless, further researches will be needed in the future to investigate the safety of XOIs in RHUC1 patients and the possibility of other factors in the pathogenesis of human EIAKI.
Figure 7.
Schematic representation of the mechanism of EIAKI on RHUC1 together with the therapeutic action of XOIs.
In conclusion, high HPRT activity Urat1-Uox DKO mice were established as novel RHUC1 animal model. The pathogenic mechanism of EIAKI was found to be due to increased IL-1β via NLRP3 inflammasome signaling and NKA dysfunction associated with excessive urinary UA excretion. In addition, XOIs appear to be a promising therapeutic agent for the treatment of EIAKI.
Disclosures
T. Hosoya was an employee of Fuji Yakuhin at the time of this study. M. Hosoyamada reports research funding from Fuji Yakuhin. K. Matsumoto reports current employer is Fuji Yakuhin. S. Shibata reports research funding with Bayer, Chugai Pharma, Daiichi-Sankyo, Fuji Yakuhin, Kissei, Kyowa-Kirin, Otsuka, Kenkyusho Sanwa Kagaku Taisho-Toyama, Tanabe-Mitsubishi, Teijin Pharma, and Torii Pharma; reports honoraria with AstraZeneca, Bayer, Daiichi-Sankyo, Kissei, Kyowa-Kirin, Mochida, Sanwa Kagaku Kenkyusho, and Tanabe-Mitsubishi; reports scientific advisor or membership with JASN, Hypertension Research, and Scientific Reports. S. Uchida reports honoraria with Fuji Yakuhin, Mochida, Sanwa Kagaku Kenkyusho, and Teijin Pharma. The remaining author has nothing to disclose.
Funding
This study was partly supported by Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science grant 26460347 (to M. Hosoyamada) and Joint Research Expenses from Fuji Yakuhin Co., Ltd. (to M. Hosoyamada).
Supplementary Material
Acknowledgments
T. Hosoya and M. Hosoyamada conceived and designed the study; T. Hosoya, K. Matsumoto, and N.H.Tomioka performed experiments and data acquisition; T. Hosoya and N.H.Tomioka analyzed and interpreted the data; T. Hosoya drafted the paper; M. Hosoyamada, N.H.Tomioka, K. Matsumoto, S. Shibata, and S. Uchida performed critical revision. Topiroxostat was manufactured and supplied by Fuji Yakuhin. We thank Mr. Naoki Kurita, Dr. Naoki Ashizawa, and Dr. Takashi Iwanaga (of Fuji Yakuhin) for their invaluable advice. We thank Ms. Hiromi Yamaguchi and Ms. Kazumi Miyamoto for their experimental supports.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021050616/-/DCSupplemental.
Supplemental Methods. In vivo experimental protocols.
Supplemental Figure 1. Growth curves of male and female high HPRT activity Uox-KO and Urat1-Uox DKO mice.
Supplemental Figure 2. UA kinetic profiling of male low HPRT activity Urat1-Uox DKO mice.
Supplemental Figure 3. EIAKI of female high HPRT activity Urat1-Uox DKO mice.
Supplemental Figure 4. Correlation of renal functional makers with UA kinetic parameters in high HPRT activity Uox-KO and Urat1-Uox DKO mice before (pre) and after (post) the forced swimming test.
Supplemental Figure 5. Correlation of IL-1β and NKA protein with UA kinetic and renal injury parameters in the post-exercise group.
References
- 1.Nakayama A, Matsuo H, Ohtahara A, Ogino K, Hakoda M, Hamada T, et al. : Clinical practice guideline for renal hypouricemia (1st edition). Hum Cell 32: 83–87, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, et al. : Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417: 447–452, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Ichida K, Hosoyamada M, Hisatome I, Enomoto A, Hikita M, Endou H, et al. : Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol 15: 164–173, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Ichida K, Hosoyamada M, Kamatani N, Kamitsuji S, Hisatome I, Shibasaki T, et al. : Age and origin of the G774A mutation in SLC22A12 causing renal hypouricemia in Japanese. Clin Genet 74: 243–251, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Vidanapathirana DM, Jayasena S, Jasinge E, Stiburkova B: A heterozygous variant in the SLC22A12 gene in a Sri Lanka family associated with mild renal hypouricemia. BMC Pediatr 18: 210, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishikawa I: Acute renal failure with severe loin pain and patchy renal ischemia after anaerobic exercise in patients with or without renal hypouricemia. Nephron 91: 559–570, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa I, Nakagawa M, Hayama S, Yoshida S, Date T: Acute renal failure with severe loin pain and patchy renal ischaemia after anaerobic exercise (ALPE) (exercise-induced acute renal failure) in a father and child with URAT1 mutations beyond the W258X mutation. Nephrol Dial Transplant 20: 1015, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Wen L, Yang H, Ma L, Fu P: The roles of NLRP3 inflammasome-mediated signaling pathways in hyperuricemic nephropathy. Mol Cell Biochem 476: 1377–1386, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Hosoyamada M, Takiue Y, Morisaki H, Cheng J, Ikawa M, Okabe M, et al. : Establishment and analysis of SLC22A12 (URAT1) knockout mouse. Nucleosides Nucleotides Nucleic Acids 29: 314–320, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Álvarez-Lario B, Macarrón-Vicente J: Uric acid and evolution. Rheumatology (Oxford) 49: 2010–2015, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Hosoyamada M, Tsurumi Y, Hirano H, Tomioka NH, Sekine Y, Morisaki T, et al. : Urat1-Uox double knockout mice are experimental animal models of renal hypouricemia and exercise-induced acute kidney injury. Nucleosides Nucleotides Nucleic Acids 35: 543–549, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Tax WJ, Veerkamp JH, Trijbels JM: Activity of purine phosphoribosyltransferases and of two enzymes of pyrimidine biosynthesis in erythrocytes of ten mammalian species. Comp Biochem Physiol B 54: 209–212, 1976 [DOI] [PubMed] [Google Scholar]
- 13.Johnson GG, Chapman VM: Altered turnover of hypoxanthine phosphoribosyltransferase in erythroid cells of mice expressing Hprt a and Hprt b alleles. Genetics 116: 313–320, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson GG, Kronert WA, Bernstein SI, Chapman VM, Smith KD: Altered turnover of allelic variants of hypoxanthine phosphoribosyltransferase is associated with N-terminal amino acid sequence variation. J Biol Chem 263: 9079–9082, 1988 [PubMed] [Google Scholar]
- 15.Watanabe T, Tomioka NH, Watanabe S, Suzuki Y, Tsuchiya M, Hosoyamada M: The mechanism of false in vitro elevation of uric acid level in mouse blood. Biol Pharm Bull 39: 1081–1084, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Hosoya T, Uchida S, Shibata S, Tomioka NH, Hosoyamada M: Perfecting a high hypoxanthine phosphoribosyltransferase activity-uricase KO mice to test the effects of purine- and non-purine-type xanthine dehydrogenase (XDH) inhibitors. Br J Pharmacol 177: 2274–2285, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takada T, Mita A, Maeno A, Sakai T, Shitara H, Kikkawa Y, et al. : Mouse inter-subspecific consomic strains for genetic dissection of quantitative complex traits. Genome Res 18: 500–508, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishizawa K, Wang Q, Li J, Xu N, Nemoto Y, Morimoto C, et al. : Inhibition of sodium glucose cotransporter 2 attenuates the dysregulation of Kelch-like 3 and NaCl cotransporter in obese diabetic mice. J Am Soc Nephrol 30: 782–794, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toyoki D, Shibata S, Kuribayashi-Okuma E, Xu N, Ishizawa K, Hosoyamada M, et al. : Insulin stimulates uric acid reabsorption via regulating urate transporter 1 and ATP-binding cassette subfamily G member 2. Am J Physiol Renal Physiol 313: F826–F834, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Matsukawa T, Motojima H, Sato Y, Takahashi S, Villareal MO, Isoda H: Upregulation of skeletal muscle PGC-1α through the elevation of cyclic AMP levels by cyanidin-3-glucoside enhances exercise performance. Sci Rep 7: 44799, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sellmayr M, Hernandez Petzsche MR, Ma Q, Krüger N, Liapis H, Brink A, et al. : Only hyperuricemia with crystalluria, but not asymptomatic hyperuricemia, drives progression of chronic kidney disease. J Am Soc Nephrol 31: 2773–2792, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu J, Hou X, Yuan X, Cui L, Liu Z, Li X, et al. : Knockout of the urate oxidase gene provides a stable mouse model of hyperuricemia associated with metabolic disorders. Kidney Int 93: 69–80, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Wu X, Wakamiya M, Vaishnav S, Geske R, Montgomery C Jr, Jones P, et al. : Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc Natl Acad Sci U S A 91: 742–746, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao J, Zhang X, Fu C, Yang Q, Xie Y, Zhang Z, et al. : Impaired Na+-K+-ATPase signaling in renal proximal tubule contributes to hyperuricemia-induced renal tubular injury. Exp Mol Med 50: e452, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda Y, Abe A, Nakanishi S, Umezu M, Hirano K, Hayakawa H, et al. : Two cases of nephrotic syndrome (NS)-induced acute kidney injury (AKI) associated with renal hypouricemia. Clin Nephrol 76: 78–82, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Shimizu Y, Wakabayashi K, Totsuka A, Hayashi Y, Nitta S, Hara K, et al. : Exercise-induced acute kidney injury in a police officer with hereditary renal hypouricemia. Case Rep Nephrol Dial 9: 92–101, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aomura D, Sonoda K, Harada M, Hashimoto K, Kamijo Y: A case of acute kidney injury in a patient with renal hypouricemia without intense exercise. Case Rep Nephrol Dial 10: 26–34, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka M, Itoh K, Matsushita K, Matsushita K, Wakita N, Adachi M, et al. : Two male siblings with hereditary renal hypouricemia and exercise-induced ARF. Am J Kidney Dis 42: 1287–1292, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Stiburkova B, Sebesta I, Ichida K, Nakamura M, Hulkova H, Krylov V, et al. : Novel allelic variants and evidence for a prevalent mutation in URAT1 causing renal hypouricemia: Biochemical, genetics and functional analysis. Eur J Hum Genet 21: 1067–1073, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iguchi A, Sato T, Yamazaki M, Tasaki K, Suzuki Y, Iino N, et al. : A case of xanthinuria type I with a novel mutation in xanthine dehydrogenase. CEN Case Rep 5: 158–162, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maalouf NM, Sakhaee K, Parks JH, Coe FL, Adams-Huet B, Pak CY: Association of urinary pH with body weight in nephrolithiasis. Kidney Int 65: 1422–1425, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Cameron MA, Maalouf NM, Adams-Huet B, Moe OW, Sakhaee K: Urine composition in type 2 diabetes: Predisposition to uric acid nephrolithiasis. J Am Soc Nephrol 17: 1422–1428, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J: Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Xiao J, Zhang XL, Fu C, Han R, Chen W, Lu Y, et al. : Soluble uric acid increases NALP3 inflammasome and interleukin-1β expression in human primary renal proximal tubule epithelial cells through the Toll-like receptor 4-mediated pathway. Int J Mol Med 35: 1347–1354, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Braga TT, Forni MF, Correa-Costa M, Ramos RN, Barbuto JA, Branco P, et al. : Soluble uric acid activates the NLRP3 inflammasome. Sci Rep 7: 39884, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong XY, Bai L, Bai SJ, Wang YK, Ji T: Uric acid induced epithelial-mesenchymal transition of renal tubular cells through PI3K/p-Akt signaling pathway. J Cell Physiol 234: 15563–15569, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Wang YJ, Chen YY, Hsiao CM, Pan MH, Wang BJ, Chen YC, et al. : Induction of autophagy by pterostilbene contributes to the prevention of renal fibrosis via attenuating NLRP3 inflammasome activation and epithelial-mesenchymal transition. Front Cell Dev Biol 8: 436, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt C, Höcherl K, Schweda F, Kurtz A, Bucher M: Regulation of renal sodium transporters during severe inflammation. J Am Soc Nephrol 18: 1072–1083, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Klinenberg JR, Goldfinger SE, Seegmiller JE: The effectiveness of the xanthine oxidase inhibitor allopurinol in the treatment of gout. Ann Intern Med 62: 639–647, 1965 [DOI] [PubMed] [Google Scholar]
- 40.Yeun JY, Hasbargen JA: Renal hypouricemia: Prevention of exercise-induced acute renal failure and a review of the literature. Am J Kidney Dis 25: 937–946, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Bhasin B, Stiburkova B, De Castro-Pretelt M, Beck N, Bodurtha JN, Atta MG: Hereditary renal hypouricemia: A new role for allopurinol? Am J Med 127: e3–e4, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto K, Okamoto K, Ashizawa N, Nishino T: FYX-051: A novel and potent hybrid-type inhibitor of xanthine oxidoreductase. J Pharmacol Exp Ther 336: 95–103, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Valladares I, Khan T, Espinoza LR: Efficacy and safety of febuxostat in patients with hyperuricemia and gout. Ther Adv Musculoskelet Dis 3: 245–253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamatani N, Fujimori S, Hada T, Hosoya T, Kohri K, Nakamura T, et al. : An allopurinol-controlled, multicenter, randomized, open-label, parallel between-group, comparative study of febuxostat (TMX-67), a non-purine-selective inhibitor of xanthine oxidase, in patients with hyperuricemia including those with gout in Japan: Phase 2 exploratory clinical study [published correction appears in J Clin Rheumatol 20: E3, 2014 10.1097/RHU.0b013e31821d352f]. J Clin Rheumatol 17[Suppl 2]: S44–S49, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Hosoya T, Ogawa Y, Hashimoto H, Ohashi T, Sakamoto R: Comparison of topiroxostat and allopurinol in Japanese hyperuricemic patients with or without gout: A phase 3, multicentre, randomized, double-blind, double-dummy, active-controlled, parallel-group study. J Clin Pharm Ther 41: 290–297, 2016 [DOI] [PubMed] [Google Scholar]
- 46.Cutolo M, Cimmino MA, Perez-Ruiz F: Potency on lowering serum uric acid in gout patients: A pooled analysis of registrative studies comparing febuxostat vs. allopurinol. Eur Rev Med Pharmacol Sci 21: 4186–4195, 2017 [PubMed] [Google Scholar]
- 47.Kato S, Kato M, Kusano T, Nishino T: New strategy that delays progression of amyotrophic lateral sclerosis in G1H-G93A transgenic mice: Oral administration of xanthine oxidoreductase inhibitors that are not substrates for the purine salvage pathway. J Neuropathol Exp Neurol 75: 1124–1144, 2016 [DOI] [PubMed] [Google Scholar]
- 48.Johnson TA, Jinnah HA, Kamatani N: Shortage of cellular ATP as a cause of diseases and strategies to enhance ATP. Front Pharmacol 10: 98, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujii K, Kubo A, Miyashita K, Sato M, Hagiwara A, Inoue H, et al. : Xanthine oxidase inhibitor ameliorates postischemic renal injury in mice by promoting resynthesis of adenine nucleotides. JCI Insight 4: e124816, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tani T, Okamoto K, Fujiwara M, Katayama A, Tsuruoka S: Metabolomics analysis elucidates unique influences on purine / pyrimidine metabolism by xanthine oxidoreductase inhibitors in a rat model of renal ischemia-reperfusion injury. Mol Med 25: 40, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kristensen ML, Kierulf-Lassen C, Nielsen PM, Krag S, Birn H, Nejsum LN, et al. : Remote ischemic perconditioning attenuates ischemia/reperfusion-induced downregulation of AQP2 in rat kidney. Physiol Rep 4: e12865, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sampaio LS, Iannotti FA, Veneziani L, Borelli-Tôrres RT, De Maio F, Piscitelli F, et al. : Experimental ischemia/reperfusion model impairs endocannabinoid signaling and Na+/K+ ATPase expression and activity in kidney proximal tubule cells. Biochem Pharmacol 154: 482–491, 2018 [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto S, Yamamoto M, Nakamura J, Mii A, Yamamoto S, Takahashi M, et al. : Spatiotemporal ATP dynamics during AKI predict renal prognosis. J Am Soc Nephrol 31: 2855–2869, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.