Significance Statement
Biallelic pathogenic variants in SLC12A3, encoding the thiazide-sensitive sodium chloride cotransporter NCC, cause Gitelman syndrome. Gitelman patients suffer from hypokalemic alkalosis, hypomagnesemia, and salt wasting. A subset of Gitelman syndrome cases remains genetically unsolved. This paper describes the identification of pathogenic mitochondrial DNA (mtDNA) variants in the genes encoding the transfer RNAs for phenylalanine (MT-TF) and isoleucine (MT-TI) in 13 families with a Gitelman-like phenotype. Six families were additionally affected by progressive CKD. Mitochondrial dysfunction was demonstrated in patient-derived fibroblasts and linked to defective sodium reabsorption by NCC in vitro. These findings advocate for screening for mtDNA variants in unexplained Gitelman syndrome patients and influence genetic counseling of affected families. Furthermore, they provide insight into the physiology of renal sodium handling.
Keywords: epithelial sodium transport, genetic renal disease, human genetics, Na transport, ion transport, mitochondria, Gitelman-s syndrome, blood pressure, chronic kidney disease, chronic kidney failure
Visual Abstract
Abstract
Background
Gitelman syndrome is the most frequent hereditary salt-losing tubulopathy characterized by hypokalemic alkalosis and hypomagnesemia. Gitelman syndrome is caused by biallelic pathogenic variants in SLC12A3, encoding the Na+-Cl− cotransporter (NCC) expressed in the distal convoluted tubule. Pathogenic variants of CLCNKB, HNF1B, FXYD2, or KCNJ10 may result in the same renal phenotype of Gitelman syndrome, as they can lead to reduced NCC activity. For approximately 10 percent of patients with a Gitelman syndrome phenotype, the genotype is unknown.
Methods
We identified mitochondrial DNA (mtDNA) variants in three families with Gitelman-like electrolyte abnormalities, then investigated 156 families for variants in MT-TI and MT-TF, which encode the transfer RNAs for phenylalanine and isoleucine. Mitochondrial respiratory chain function was assessed in patient fibroblasts. Mitochondrial dysfunction was induced in NCC-expressing HEK293 cells to assess the effect on thiazide-sensitive 22Na+ transport.
Results
Genetic investigations revealed four mtDNA variants in 13 families: m.591C>T (n=7), m.616T>C (n=1), m.643A>G (n=1) (all in MT-TF), and m.4291T>C (n=4, in MT-TI). Variants were near homoplasmic in affected individuals. All variants were classified as pathogenic, except for m.643A>G, which was classified as a variant of uncertain significance. Importantly, affected members of six families with an MT-TF variant additionally suffered from progressive chronic kidney disease. Dysfunction of oxidative phosphorylation complex IV and reduced maximal mitochondrial respiratory capacity were found in patient fibroblasts. In vitro pharmacological inhibition of complex IV, mimicking the effect of the mtDNA variants, inhibited NCC phosphorylation and NCC-mediated sodium uptake.
Conclusion
Pathogenic mtDNA variants in MT-TF and MT-TI can cause a Gitelman-like syndrome. Genetic investigation of mtDNA should be considered in patients with unexplained Gitelman syndrome-like tubulopathies.
Gitelman syndrome (GS) is a recessively inherited renal tubulopathy caused by pathogenic variants in SLC12A3, which encodes the thiazide-sensitive Na+-Cl− cotransporter (NCC). NCC mediates reabsorption of sodium and chloride in the distal convoluted tubule (DCT).1 GS is characterized by distal tubular salt wasting with secondary hyperaldosteronism, hypochloremic metabolic alkalosis, hypokalemia, hypomagnesemia, and hypocalciuria. Common clinical manifestations of GS include muscle cramps, paresthesias, nocturia, salt craving, muscle weakness, and fatigue.2
GS may be phenocopied by a number of genetic and nongenetic conditions. Nongenetic causes include diuretic abuse, chronic laxative abuse, and chronic vomiting. The most important genetic differential diagnosis to pathogenic variants in SLC12A3 is the presence of biallelic pathogenic variants in CLCNKB, which encodes the distal tubular basolateral chloride channel ClC-Kb. Such variants can be found in approximately 3% of patients with a GS-like tubulopathy.3 Additionally, pathogenic variants in KCNJ10, FXYD2, and HNF1B may result in a similar biochemical phenotype, but typically cause additional symptoms such as sensorineural deafness, epilepsy, ataxia, intellectual disability, diabetes, or renal cysts.4–6 Still, 10% of patients with clinical characteristics of GS do not have a pathogenic variant in SLC12A3 or other genes currently associated with a GS-like tubulopathy, suggesting that not all genetic causes for GS have been identified.3
Mitochondrial diseases form a heterogenous group of hereditary disorders characterized by mitochondrial dysfunction.7 Interestingly, a small group of mitochondrial diseases has been associated with distal tubular dysfunction.8–11 For instance, a large family carrying a variant in the mitochondrial transfer RNA (tRNA) (mt-tRNA) gene for isoleucine (MT-TI) was affected by hypokalemia and hypomagnesemia in addition to arterial hypertension and hypercholesterolemia.9 To date, all reports report extrarenal manifestations in addition to the GS-like electrolyte abnormalities.
In this study, we describe three large families with genetically unexplained GS. The presumed maternal inheritance pattern led to the identification of mitochondrial DNA (mtDNA) variants in mt-tRNAs for isoleucine and phenylalanine (encoded by MT-TI and MT-TF, respectively). We subsequently screened two cohorts of patients with hypomagnesemia or a clinical diagnosis of GS, and identified ten more families with variants in MT-TI and MT-TF. We analyzed the clinical phenotype of these patients, characterized mitochondrial function in patient-derived fibroblasts, and assessed the effect of mitochondrial dysfunction on NCC-mediated sodium transport.
Methods
Inclusion and Ethical Approval
The maternal inheritance pattern in families 1, 2, and 3 prompted an analysis of the mitochondrial genome. The identification of three mtDNA candidate variants in MT-TI and MT-TF encouraged us to screen for variants in these two genes in additional families with unexplained hypomagnesemia or a clinical suspicion of GS (156 families). This led to the identification of variants in MT-TI or MT-TF in eight more families (families 4–12). Family 13 was known to have a pathogenic variant in MT-TF and has been published before as Pedigree III by Connor et al.12
The study was performed in accordance with the Declaration of Helsinki, and informed consent was obtained from all patients before inclusion into the study. Where needed, ethical approval was provided by the institutional review board of Arnhem-Nijmegen (study reference 2019–5749).
DNA Sequencing
In family 1, the initial diagnosis was made on DNA isolated from cells in urine, with amplification through long-template PCR and sequencing with the Ion Torrent PGM. The obtained mtDNA sequence was screened for rearrangements and mismatches. The presence of the variant was later confirmed in DNA isolated from blood by Sanger sequencing using the MT-TI sequencing primers listed in Supplemental Table 1. In family 2, DNA was isolated from whole blood. Exome enrichment was done with SureSelectXT Automated Target Enrichment and sequencing by a HiSeq4000 platform (Illumina) with 2×75-bp paired-end reads. Sequence reads were aligned to the Human Genome Reference Assembly GCRh37/hg19 using Burrows-Wheeler Alignment (BWA) version 0.7.1213 and indexed using SAMtools version 1.6.14 SNVs and indels were subsequently called by the Genome Analysis Toolkit (GATK) HaplotypeCaller version 3.4–46. The candidate variant was identified by targeted reanalysis of the mtDNA covered by the exome sequencing data. Family 3 underwent whole-genome 150-bp paired-end sequencing using an Illumina HiSeq X platform as part of the 100,000 Genomes Project, and were processed on the Illumina North Star Version 4 Whole Genome Sequencing Workflow (NSV4, version 2.6.53.23), comprising the iSAAC Aligner (version 03.16.02.19) and Starling Small Variant Caller (version 2.4.7). Samples were aligned to the Homo Sapiens NCBI GRCh38 assembly. The candidate variant was identified after targeted reanalysis of mtDNA. In all three families, results were confirmed by Sanger sequencing in an extended set of family members for segregation (primers are listed in Supplemental Table 1).
Families 4–9 were ascertained through screening for variants in MT-TI and MT-TF by Sanger sequencing of DNA obtained from whole blood. The variants in families 10 and 12 were identified by analysis of the complete mitochondrial genome with a long-range PCR followed by circular consensus sequencing on a Sequel (Pacific Biosystems). The variant in family 11 was identified by multigene panel analysis (Bioscientia). This multigene tubulopathy panel used Roche/Nimblegen enrichment and sequencing on an Illumina platform. The variant in family 13 was identified by sequencing of the mitochondrial genome.12 In all families except for family 13, the diagnostic trajectory had included a screen for pathogenic variants in the coding regions of SLC12A3, CLCNKB, and several other tubulopathy genes by a multigene panel or by exome sequencing.
To exclude other genetic causes of reduced GFR, two genome sequencing panels were analyzed in family 3 (panel names: “unexplained kidney failure in young people” and “tubulointerstitial kidney disease”15). In family 10, a >300-gene-containing exome sequencing panel for kidney diseases was used. In family 13, targeted genetic analysis of UMOD, HNF1B, REN, and MUC1 was performed, including SNaPshot minisequencing of MUC1 and MLPA for HNF1B (as described for family 6 in the study by Ekici et al.16).
Determination of Heteroplasmy
Heteroplasmy levels were determined in fibroblasts and/or whole blood from nine families using single-molecule molecular inversion probes. A variant was considered homoplasmic if coverage at the variant position was at least 300 and the percentage of reads with the variant was >99%. A detailed description can be found in the Supplemental Material.
Identification, Selection, and Assessment of Candidate Variants
Very rare mtDNA variants (population frequency <0.1%) in families 1, 2, and 3 were considered candidate variants. Variant population frequencies were obtained from MITOMAP, HelixMTdb, and gnomAD. Furthermore, MITOTIP and PON-mt-tRNA were used to predict pathogenicity of candidate variants. The secondary structures of the mt-tRNAs for isoleucine (mt-tRNAIle) and phenylalanine (mt-tRNAPhe) were modeled using rtools, CentroidHomFold. For conservation analysis of MT-TI and MT-TF (encoding mt-tRNAIle and mt-tRNAPhe, respectively), we selected the species suggested by Yarham et al.17 and aligned sequences using clustal O followed by manual curation.
Pathogenicity of mtDNA variants was evaluated by using the criteria proposed by Wong et al.,18 similar to the ACGS criteria.19
Clinical Data
Clinical data, including renal biopsy specimens, were obtained as part of routine clinical care at the respective local centers. Electrolyte measurements were performed in serum samples in some centers, and in plasma samples in others. For simplicity, we will henceforth refer to all as “serum” measurements. Reference values for the measurements presented were very similar across centers. Urinary calcium excretion was normalized to the upper limit of normal to enable comparison between children and adults (Supplemental Table 2). The eGFR was calculated using serum creatinine and the CKD-EPI (adults) or Schwartz formula (children). Hypertension was defined as a systolic BP >140 mm Hg or a diastolic BP >90 mm Hg. In family 12, sexes were left out in some individuals and some unaffected siblings were added for pseudonymization purposes.
Thiazide tests were performed in three families as described in the Supplementary Material, according to previously described protocols.20,21
Fibroblasts
In families 3 and 4, fibroblasts were grown from a skin biopsy sample. In families 6 and 11, fibroblasts were obtained by nasal brush (Cytobrush Plus, Cooper Surgical, #176291). Culture conditions are described in the Supplemental Material. In addition to one family control (unaffected relative on the paternal line in family 6, control 1), two control cell lines of unrelated individuals were included. Both had been shown to have normal mitochondrial function in earlier experiments. One fibroblast line was obtained commercially (ATCC PCS-201–012, lot #61683453, from a 40-year-old woman, control 2); the other was derived from a skin biopsy done at the Radboudumc, Nijmegen (control 3).
Oxidative Phosphorylation Activity Measurements
Measurements of the activity of the mitochondrial oxidative phosphorylation (OXPHOS) complexes were performed per clinical routine as described previously (additional information in the Supplemental Material).22
Mitochondrial Respiration by the Seahorse XFe96 Analyzer
Oxygen consumption rate (OCR) was measured in a Cell Mito Stress Test by the Seahorse XFe96 Analyzer as described earlier.23 Citrate synthase activity was measured in all wells after the stress test for normalization purposes (Supplemental Material).
Seahorse XFe96 Data Analysis
Wave Desktop Software version 2.3 (Agilent) was used to read Seahorse data, to remove background signal, and to normalize for citrate synthase activity. However, use of this program for subsequent analysis has several disadvantages. Firstly, there is no automated way to exclude wells that did not respond to the Mito Stress Test (which can occur if one of the drugs was not injected correctly). Secondly, the larger variation observed with larger OCR values (i.e., heteroscedasticity) violates the assumptions underlying many statistical tests, including ANOVA.24 Lastly, interplate variation can be significant.24 To improve the validity of the data, an R-script was developed to analyze the data (Supplemental Material). The code is publicly available on GitHub (https://github.com/DaanViering/Seahorse-analyzeR).
Effect of Complex IV Inhibition on NCC-Mediated 22Na+ Uptake
HEK293 cells were transfected with either 0.5 µg of DNA construct containing NCC (pCIneo-NCC-IRES-GFP) or 0.5 µg of construct without NCC (pCIneo-IRES-GFP, hereafter indicated with “mock”). Two days after transfection, samples were put on hypotonic-low-chloride or isotonic buffer with or without 100 µmol/L thiazide, and with either 1 mmol/L potassium cyanide (KCN, experimental condition) or as a control 1 mmol/L potassium chloride (KCl). After half an hour of incubation, samples were put on isotonic buffer containing both 22Na+ and inhibitors of other sodium transporters and channels (i.e., amiloride 100 µmol/L, bumetanide 100 µmol/L, and ouabain 1 mmol/L). After half an hour in the 22Na+, cells were lysed and radioactivity was measured on a liquid scintillation counter (Hidex 600SL). NCC expression was assessed by immunoblotting, following the same protocol as described below. Culturing and the 22Na+ uptakes were done in triplicate; the complete experiment was performed four times (Supplemental Material).
Effect of Complex IV Inhibition on NCC Phosphorylation
Seeding of HEK293 cells and transfection were similar to what is described above for the 22Na+ uptake experiments. Samples were subsequently put on hypotonic-low-chloride or isotonic buffer with or without 100-µmol/L hydrochlorothiazide, and with either 1-mmol/L KCN (experimental condition) or as a control 1-mmol/L KCl. Culturing was done in duplicate; the complete experiment was performed three times. To investigate the effect of complex IV inhibition on other sodium transporters, its effect on the phosphorylation of the sodium-potassium-chloride cotransporter NKCC2 was assessed using an analogous protocol (Supplemental Material).
Immunoblotting
SDS-PAGE immunoblotting was performed with the following primary antibodies: rabbit anti-NCC (1:2000, Millipore, #AB3553), and rabbit anti–pT58-NCC (NCC phosphorylated at human position p.Thr60, 1:2000, kind gift from Robert Fenton25). Primary antibodies were targeted with the following secondary antibody: peroxidase anti–rabbit-IgG (1:10,000, Sigma Aldrich, #A4914). Imaged blots were subjected to densitometric analysis of band intensities (Supplemental Material).
Statistical Analyses
GraphPad Prism 8.4.3 was used for statistical analyses. For the Seahorse XFe96 experiments, Welch’s ANOVA test was applied with the null hypothesis that maximal mitochondrial respiration was not different for any of the variants compared with the control fibroblasts. Correction for multiple testing was performed using Dunnett T3 testing. Additionally, we assessed for each patient whether the maximum mitochondrial respiratory capacity in their fibroblasts was significantly different from the fibroblasts of control 1, again using Welch’s ANOVA test (for this, fibroblasts from 5.I.2 were excluded because only one measurement was available) with Dunnett T3 correction.
To assess the difference between the KCN and KCl conditions during the 22Na+ absorption, we used an unpaired t test. To assess the difference between the KCN and KCl conditions in the immunoblotting experiments, we used multiple t tests with Holm–Sidak correction for multiple testing. Statistical significance was defined as P<0.05 unless stated otherwise.
Results
Identification of Four mtDNA Variants in MT-TI and MT-TF
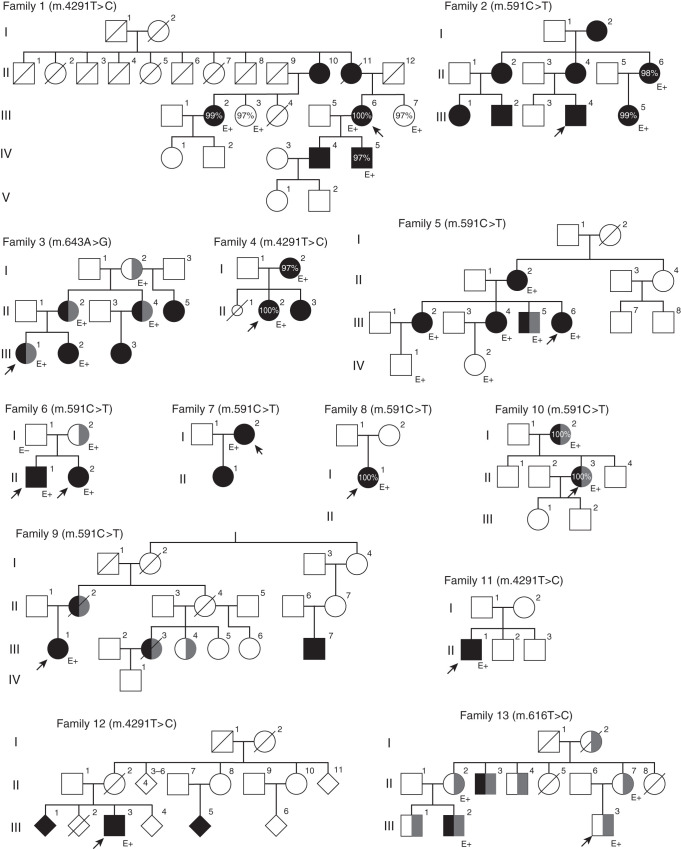
Three large families with an unexplained GS-like electrolyte constellation showed pedigrees compatible with maternal inheritance (families 1, 2, and 3 in Figure 1). No pathogenic variants in SLC12A3 and CLCNKB were found in any of these families, nor in other known tubulopathy genes.26 Analysis of mtDNA revealed three candidate pathogenic variants: m.4291T>C in the mt-tRNA for isoleucine (MT-TI) in family 1, and two variants in the mt-tRNA for phenylalanine (MT-TF) in families 2 and 3 (m.591C>T and m.643A>G, respectively). The variants cosegregated with the GS phenotype in all three families. Individuals were shown to carry the variant at (near) homoplasmy (97%–100% of reads carried the variant in all three families). We subsequently screened for variants in MT-TF and MT-TI in 156 additional families and individual patients with an unexplained GS phenotype or unexplained hypomagnesemia. This screening identified three more families/individual patients with the m.4291T>C variant and six more families/individual patients with the m.591C>T variant. Lastly, a family with the m.616T>C variant in MT-TF (family 13, described previously as “Pedigree III” by Connor et al.12) was also shown to have GS-like electrolyte abnormalities.
Figure 1.
Pedigrees of the 13 affected families demonstrate maternal inheritance pattern. Black filling denotes tubulopathy. Probands are denoted with arrows; CKD (any stage) is denoted by gray filling. Percentages indicate heteroplasmy level of the variant in blood. E+, the presence of the variant as confirmed by genetic testing; E−, the exclusion of the variant.
Heteroplasmy levels in blood and fibroblasts ranged from 97% to 100% (homoplasmic) in all tested patients (Table 1, Supplemental Table 3). Pedigrees can be found in Figure 1.
Table 1.
Summary of clinical data
| Patient | Sex | Age,a yr | Variant | Gene | Heteroplasmy, (% in Blood | Fibroblasts | Serum Magnesium, mmol/L | Fractional Magnesium Excretion | Serum Potassium, mmol/L | eGFR, ml/min per 1.73 m2 | BP, mm Hg | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference range | 0.7–1.1 | <4.0b | 3.6–5.2 | >90 | <140/90 | ||||||
| 1.III.6 | F | 39 | m.4291T>C | MT-TI | 100 | | 0.40 | 6.7 | 3.6 | 82 | 140/80 | Primary hyperparathyroidism |
| 1.III.2 | F | m.4291T>C | MT-TI | 99 | | |||||||
| 1.IV.4 | M | 36 | m.4291T>C | MT-TI | 0.67 | 2.9 | 3.7 | >90 | |||
| 1.IV.5 | M | 34 | m.4291T>C | MT-TI | 97 | | 0.56 | 8.3 | 3.4 | >90 | ||
| 2.II.6 | F | m.591C>T | MT-TF | 98 | | 0.86c | 3.3c | 65 | 122/80 | |||
| 2.III.1 | F | 5 | m.591C>T | MT-TF | 0.73 | 3.2 | >90 | ||||
| 2.III.2 | M | 10 | m.591C>T | MT-TF | 0.55 | 9.0 | 3.0 | >90 | 125/71 | ||
| 2.III.4 | M | 8 | m.591C>T | MT-TF | 0.71c | 13 | 3.6 | >90 | 151/89 | ||
| 2.III.5 | F | m.591C>T | MT-TF | 99 | | 0.48 | 7.8 | 3.0 | >90 | 114/65 | ||
| 3.I.2 | F | 50 | m.643A>G | MT-TF | 0.70 | 7.9 | 3.6 | 35 | |||
| 3.II.2 | F | 22 | m.643A>G | MT-TF | 0.52 | 4.9 | 3.3 | 32 | 120/73 | Albuminuria | |
| 3.II.4 | F | 21 | m.643A>G | MT-TF | 0.66 | 4.2 | 3.1 | 55 | |||
| 3.II.5 | F | 14 | m.643A>G | MT-TF | 2.9 | ||||||
| 3.III.1 | F | 2 | m.643A>G | MT-TF | 0.76c | 8.2 | 4.1 | 30 | 98/54 | Albuminuria and elevated RBP. Renal biopsy performed | |
| 3.III.2 | F | 1 | m.643A>G | MT-TF | 0.48 | 8.5 | 3.0 | >90 | Albuminuria and elevated RBP | ||
| 3.III.3 | F | m.643A>G | MT-TF | 0.61 | 4.3 | ||||||
| 4.I.2 | F | m.4291T>C | MT-TI | 97 | 100 | 0.52 | 4.3 | 3.4 | >90 | 125/78 | ||
| 4.II.2 | F | 15 | m.4291T>C | MT-TI | 100 | 100 | 0.42c | 5.3 | 4.2 | >90 | 113/72 | |
| 5.III.6 | F | 3 | m.591C>T | MT-TF | 0.10 | 10.1 | 3.6 | 75 | 123/67 | Requires subcutaneous magnesium supplementation. Salt craving | |
| 5.II.1 | M | 70 | m.591C>T | MT-TF | 0.40 | 4.9 | 65 | ||||
| 5.III.2 | F | 34 | m.591C>T | MT-TF | 0.40 | 6.4 | 3.1 | >90 | 149/91 | ||
| 5.III.4 | F | 32 | m.591C>T | MT-TF | 0.73 | 2.8 | 3.7 | >90 | 157/98 | ||
| 5.III.5 | M | 42 | m.591C>T | MT-TF | 0.30 | 12.1 | 2.4 | 77 | 197/105 | ||
| 5.IV.1 | F | m.591C>T | MT-TF | 0.70 | 3.9 | >90 | 133/80 | ||||
| 5.IV.2 | M | m.591C>T | MT-TF | 0.80 | 3 | 3.9 | >90 | 149/63 | |||
| 6.I.2 | F | m.591C>T | MT-TF | 26 | Salt and spicy food craving | ||||||
| 6.II.1 | M | 12 | m.591C>T | MT-TF | 0.44c | 9.5 | 3.1 | >90 | 120/80 | Salt and spicy food craving. High renin and aldosterone | |
| 6.II.2 | F | 10 | m.591C>T | MT-TF | 0.63c | 8.1 | 3.3c | >90 | 110/70 | Salt and spicy food craving. High renin and aldosterone | |
| 7.I.2 | F | 33 | m.591C>T | MT-TF | 0.56 | 5.5 | 2.8 | >90 | 128/82 | High renin | |
| 7.II.1 | F | 8 | m.591C>T | MT-TF | 0.74 | 3.3 | >90 | 101/66 | |||
| 8.II.1 | F | 18 | m.591C>T | MT-TF | 100 | | 0.54c | 2.7c | 113/65 | High renin | ||
| 9.III.1 | F | 40 | m.591C>T | MT-TF | 0.51 | 4.0c | >90 | 100/50 | Orthostatic hypotension, m. Winiwater–Buerger, migraine, Wolff–Parkinson–White syndrome | ||
| 10.II.3 | F | 39 | m.591C>T | MT-TF | 100 | | 0.59c | 5 | 3.2 | 50 | 122/70 | Transient mild thrombopenia. Osteopenia |
| 10.I.2 | F | m.591C>T | MT-TF | 0.68 | 4 | 4.5 | 24 | Primary hyperparathyroidism. Mild thrombopenia. CVA (2×). BP controlled with three antihypertensives. Osteopenia | |||
| 11.II.1 | F | m.4291T>C | MT-TI | 100 | | 0.52 | 7.5 | 3.4c | >90 | 142/95 | ||
| 12.II.1 | M | 46 | m.4291T>C | MT-TI | 0.56 | 8.3 | 4.5 | >90 | |||
| 13.II.3 | M | 27 | m.616T>C | MT-TF | 0.54c | 4.0 | 39 | 120/60 | |||
| 13.III.2 | M | 21 | m.616T>C | MT-TF | 0.60c | 3.8c | 56 |
Summary of clinical data of patients with causative mtDNA variants in MT-TI or MT-TF. If values were outside measurement limits, the value was set equal to the measurement limit. If multiple measurements were available, the first measurement was taken in the case of serum magnesium, serum potassium, and FEMg, whereas the last available measurement was taken in the case of eGFR. eGFR was calculated with CKD-EPI, except for individuals below the age of 19, in which case the Schwartz formula was used. For conversion of serum magnesium (mmol/L) to (mg/dl), multiply by 2.43. FEMg is calculated by: serum creat×urinary Mg/(serum Mg×urinary creat)×100%.
Age at presentation.
Upper limit of normal for FEMg applies to hypomagnesemic individuals only and is on the basis of Elisaf et al.75
With supplementation of magnesium or potassium.
Assessment of Variant Pathogenicity
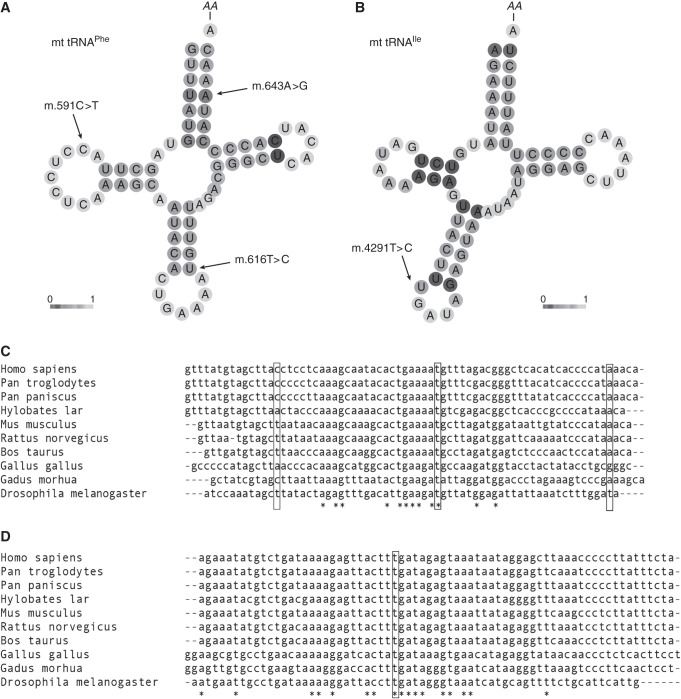
The m.591C>T, m.616T>C, and m.643A>G variants are all located in the MT-TF gene encoding mt-tRNAPhe (Figure 2A). The m.4291T>C variant is positioned in mt-tRNAIle, encoded by the MT-TI gene (Figure 2B). In the MITOMAP Genbank, HelixMTdb, and gnomAD population databases (together comprising 304,824 individuals), homoplasmic occurrences have been observed for the m.643A>G variant (three homoplasmic occurrences, one heteroplasmic occurrence), but not for the other variants (Supplemental Table 4). Furthermore, finding the m.591C>T variant in six of 156 screened families is unlikely to have occurred by chance (corrected P value 6.6×10−16). Finding the m.4291T>C variant in three of 156 screened families is also unlikely to have occurred by chance (corrected P value 1.7×10−9). Evolutionary conservation ranged from well conserved (m.4291T>C and m.616T>C, conserved to fruit flies) to poorly conserved (m.591C>T, conserved only to chimpanzees), as shown in Figure 2, C and D. Computational evidence was conflicting on pathogenicity of the variants (Supplemental Table 4). Application of the criteria by Wong et al.18 resulted in classification of the m.591C>T, m.616T>C, and m.4291T>C variants as pathogenic and the m.643A>G variant as a variant of uncertain significance (Supplemental Table 5). Variant classifications were submitted to ClinVar.
Figure 2.
In silico prediction analysis of variants. In silico prediction analysis of variants in the mt-tRNAs for phenylalanine and isoleucine (mt-tRNAPhe and mt-tRNAIle, respectively). (A and B) CentroidHomFold predictions of secondary structure of the two tRNAs. The grayscale indicates pseudo base-pairing probabilities; light shading represents a low probability and dark shading a high probability. Bold letters indicate anticodons. AA indicates amino acid binding position. (A) Predicted secondary structure of mt-tRNAPhe; the locations of the patient variants m.591C>T, m.616T>C, and m.643A>G are indicated. (B) Predicted secondary structure of mt-tRNAIle; the location of the patient variant m.4291T>C is indicated. (C) MT-TF and (D) MT-TI nucleotide sequences in a standard set of species.17 Fully conserved residues are indicated by stars (aligned with clustal O).
Clinical Phenotype
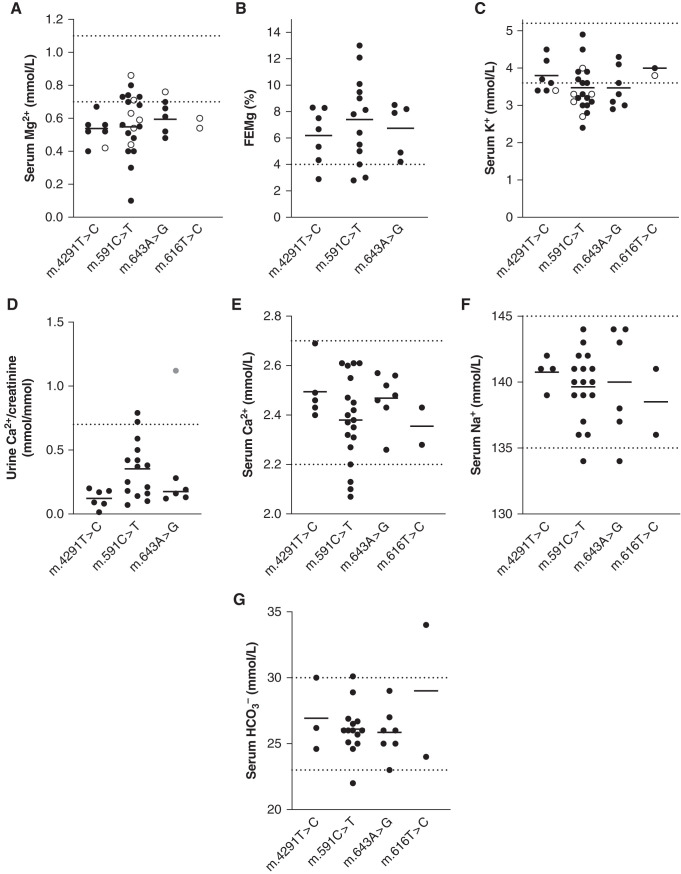
To better characterize the clinical phenotype associated with the four variants in MT-TI and MT-TF, phenotypical data from all patients were collected (Table 1, Figure 3, Supplemental Table 3). Ten index patients presented with hypomagnesemia-related symptoms, such as tetany, tremor, paresthesia, muscle fatigue, joint complaints (chondrocalcinosis), or cerebral seizures at the initial visit. In two other index patients, hypokalemia or hypomagnesemia was discovered as an incidental finding. Of the investigated individuals on the maternal lineage of each family, 31 of 36 had hypomagnesemia (86%). A significant degree of variation in serum magnesium was present among individuals, with patient 5.II.1 having an immeasurably low serum magnesium (<0.1 mmol/L); she receives supplementation of magnesium with a subcutaneous pump system. A high fractional magnesium excretion in 21 of 25 patients with available data (average 6.9%, range 2.8%–12%) implicated renal magnesium wasting as the cause of the hypomagnesemia. The average serum potassium level was at the lower border of normal (3.5 mmol/L) and hypokalemia was present in 26 of 41 family members on the maternal lineage (63%).
Figure 3.
Patients with variants in MT-TI or MT-TF have Gitelman syndrome-like electrolyte abnormalities. (A–G) Serum and urinary electrolyte values in patients by pathogenic variant. Dotted lines represent upper and lower limits of normal. For the fractional excretion of magnesium (FEMg) and urinary calcium excretion, lower limits of normal were not available; therefore, only the upper limit of normal is depicted in panels (B) and (D). Upper limit of normal for FEMg applies to hypomagnesemic individuals only and these are on the basis of Elisaf et al.75 Black circles (•), without supplementation; open circles (^), with supplementation; gray circle in panel (D), a child (individual 3.III.2); upper limit of normal for this age is 2.2 mmol/mmol Ca2+/creatinine. FEMg is calculated by: serum creatinine×urinary magnesium/(serum magnesium×urinary creatinine)×100%.
Activation of the renin-angiotensin-aldosterone system is common in GS. In four of eight individuals in whom renin levels were measured, renin was elevated (families 6, 7, and 8). Additionally, aldosterone levels were elevated in two of them. Furthermore, five individuals from families 5, 6, and 9 reported salt and/or spicy food craving.
Increased renal echogenicity was observed in three patients, of whom two had CKD (6.II.1, 3.III.1, and 13.II.3; Supplemental Table 3). Renal ultrasound was unremarkable in three other patients (1.III.6, 6.II.2, 9.II.1, and 13.III.2). One or more renal cysts were present in two patients (10.II.3 and 10.I.2).
Patients with GS have a markedly blunted response to thiazide diuretics.21 Thiazide tests were performed in five patients (1.III.6, 4.I.2, 4.II.2, 12.III.3, and 5.II.6). Hydrochlorothiazide (50 mg) induced a maximal increase of fractional chloride excretion (maximal ΔFECl) of 4.39%, 2.52%, 2.38%, 2.35%, and 0.16%, respectively (Supplemental Figure 1). Whereas the first four patients with the m.4291T>C variant (1.III.6, 4.I.2, 4.II.2, 12.III.3) demonstrated a relatively preserved response to hydrochlorothiazide, the response was completely blunted in individual 5.II.6 with the m.591C>T variant (cut-off value used for the diagnosis of GS is 2.3%).21
Hypertension was present in eight of 27 individuals (30%), which is comparable to the general adult population.27,28
CKD in Several Families with MT-TF Variants
The GFR is usually normal in patients with GS.29 In contrast, a high prevalence of reduced eGFR (eGFR<90 ml/min per 1.73 m2) was observed in six families (families 3, 5, 6, 9, 10, and 13; Table 1, Figure 1, Supplemental Table 3). Interestingly, affected members of all of these families carried a variant in MT-TF. Elaborate screening for other genetic causes of reduced GFR by different gene panels was negative in tested families (families 3, 10, and 13).
In family 3 (m.643A>G), eGFR was impaired in four individuals (median eGFR 34 ml/min per 1.73 m2, ranging 30–55). The only individual in this family with a currently normal eGFR (100 ml/min per 1.73 m2) was a 2-year-old girl (3.III.2). The older sibling (3.III.1) developed ESKD necessitating kidney transplantation at the age of 9 years. We observed mild albuminuria (8–22.7 mg/mmol creatinine) in three patients (3.II.2, 3.III.1, and 3.III.2). In family 5, a mild decrease in eGFR was observed in three individuals (5.II.1, 5.III.5, and 5.III.6; eGFR between 60 and 90 ml/min per 1.73 m2). In family 6, individual 6.I.2 had reached CKD stage 4 (eGFR 15–30 ml/min per 1.73 m2) at the time of study, and she reported having a sister diagnosed with CKD and early onset diabetes. On the basis of a family history in family 9, three individuals were affected by CKD, of whom 9.III.3 is on hemodialysis and 9.III.4 has received her second kidney transplantation. Individuals 10.I.2 and 10.II.3 had an eGFR of 24 and 50 ml/min per 1.73 m2, respectively. Lastly, family 13 (m.616T>C) was diagnosed initially with autosomal dominant tubulointerstitial kidney disease12 on the basis of a KDIGO consensus report.30 Data were available for ten individuals in the maternal lineage. Eight had a decreased eGFR, of whom two are included in this study on the basis of their electrolyte abnormalities. Four of these eight individuals currently receive RRT.
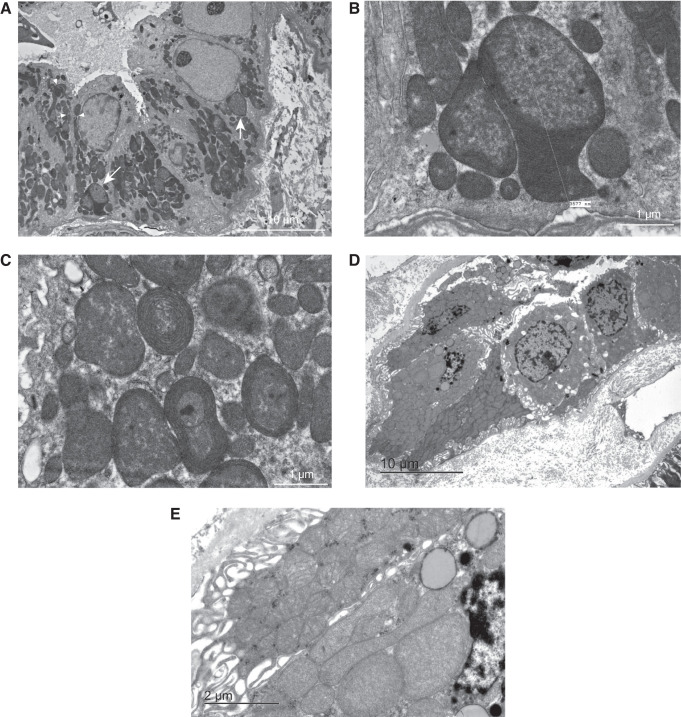
Kidney biopsy specimens have been taken in families 3, 10, and 13. The kidney biopsy performed in patient 3.III.1 to investigate CKD showed localized cortical scarring with tubular atrophy, glomerulosclerosis, interstitial fibrosis, and a chronic interstitial infiltrate. After identification of the m.643A>G variant in this individual, electron microscopy was performed and showed abnormal mitochondria, especially in the distal tubule (Figure 4, A–C). Notably, proximal convoluted tubules demonstrated well-developed apical microvilli and normal mitochondria. Other cells, including blood vessel smooth muscle and endothelial cells, showed no apparent mitochondrial irregularity. The kidney biopsy specimen of patient 10.II.3 contained sclerosed glomeruli (20%) together with interstitial fibrosis and tubular atrophy (10%). Examination of the distal tubule by electron microscopy showed evidently abnormal mitochondria (Figure 4, D and E). In this patient, mitochondria appeared abnormal in both proximal tubule and distal tubule, although mitochondria in proximal tubules had better cristae structures than those in the distal tubule (Supplemental Figure 2, A and B). In this patient, electron microscopy also showed subtle signs resembling those normally seen in chronic thrombotic microangiopathy. Kidney biopsy specimens in family 13 showed isolated interstitial fibrosis with tubular atrophy. Unfortunately, biopsy samples and images could not be retrieved for this family as they were taken several decades ago.
Figure 4.
Renal biopsies of two MT-TF patients show abnormal mitochondria in distal parts of the nephron. (A–C) Transmission electron microscopy of the renal biopsy sample of patient 3.III.1. (D and E) Transmission electron microscopy of a percutaneous renal biopsy sample of patient 10.II.3. (A) Representative image of a perpendicular cross-section of the distal tubule, with a large number of abnormally shaped and sized mitochondria (two examples indicated with white arrows). Cristae profiles appear distorted, including some mitochondria with no discernable cristae. Nanotunneling visible (three examples indicated with white arrowheads). Magnification, ×1000. (B) Close-up of atypical giant mitochondrion of >3 µm in length (same as indicated by the left arrow in panel [A]). Note the large size and compartmentalization. Magnification, ×6000. (C) Close-up of atypical mitochondria (not in panel [A]). Note the concentric cristae (onion-like appearance). Magnification, ×6000. (D) Representative image of a perpendicular cross-section of the distal tubule; enlarged mitochondria are visible. (E) A close-up of panel (D) shows an almost complete lack of cristae structure in most mitochondria.
Apparent Absence of Extrarenal Disease
In contrast to patients with other mitochondrial diseases, serum lactate was normal in all patients who were tested. Furthermore, no signs of proximal tubular disease were seen, because urinary amino acid analyses were unremarkable in the seven patients tested (families 3 and 6). Proteinuria was absent in the tested healthy individuals (seven patients from families 2, 4, 5, 7, 11, and 12), but was present in 10.II.3. Newcastle Mitochondrial Disease Adult Scale scores were low and would concur with calling the two patients “asymptomatic” (4.I.2 and 4.II.1). No obvious abnormalities were seen on cholesterol levels (total cholesterol, HDL, and LDL; Supplemental Table 3).
Mitochondrial Function in Fibroblasts
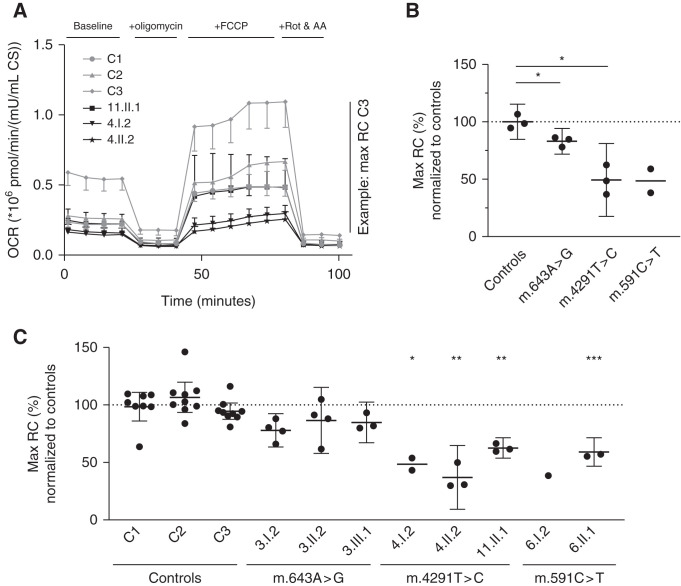
To confirm that the mtDNA variants are indeed associated with mitochondrial dysfunction, we isolated fibroblasts from eight patients and assessed mitochondrial function. The Mito Stress Test on a Seahorse XFe96 (Figure 5A) showed decreased maximal mitochondrial respiration in fibroblasts from patients compared with controls (Figure 5B). The average decreases for the respective mutants were: a 17% decrease for m.643A>G (P=0.046), a 51% decrease for m.4291T>C (P=0.02), and a 51% decrease for m.591C>T (not significant, P=0.22). Welch’s ANOVA showed a significant difference between all individuals (P<0.0001), with a Dunnett T3 test showing that the maximal respiratory capacity in fibroblasts of 6.II.1, 4.I.2, 1.II.2, and 11.II.1 differed significantly from maximal respiratory capacity in control 1 (corresponding to the unaffected father, 6.I.1; Figure 5C).
Figure 5.
Mitochondrial maximal respiratory capacity is reduced in patient fibroblasts. Mitochondrial function assessed by the Seahorse XFe96 analyzer. (A) Representative OCR plot of a Mito Stress Test of fibroblasts from three patients with the m.4291T>C variant and three controls (n=6 wells for each measurement point). Error bars denote + or − SD. (B) Average maximal mitochondrial respiration for the different mtDNA variants. Each point represents the average of all independent experiments for one individual (n=1–9, depending on the individual, as can be seen in panel [C]). (C) Average maximal mitochondrial respiration for each individual. Each point represents the average of all replicate wells on one Seahorse plate (n=6). (B and C) Means are represented by horizontal bars, error bars denote the 95% confidence interval, and a one-way ANOVA with Dunnett T3 was used to calculate significance. OCR is in pmol O2/min per mU/ml citrate synthase. AA, antimycin A; CS, citrate synthase activity; FCCP, carbonyl cyanide 4‐(trifluoromethoxy)phenylhydrazone; RC, respiratory capacity; Rot, rotenone. *P<0.05; **P<0.005; ***P<0.0005.
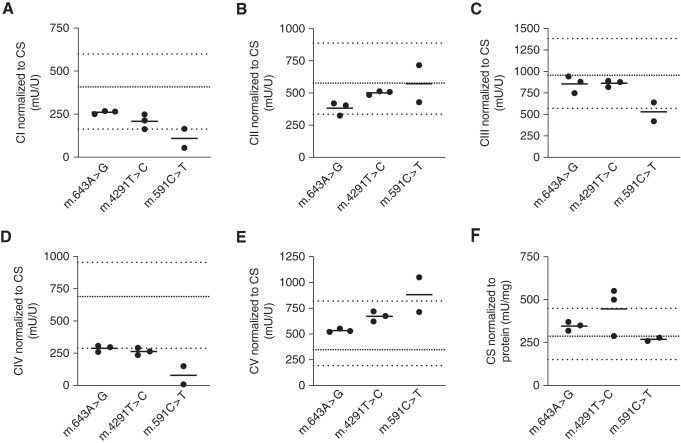
We hypothesized that the defect in mitochondrial respiration would be caused by dysfunction of one or more OXPHOS complexes, and measured their activity. Patient fibroblasts showed an average 67% reduction in the activity of OXPHOS complex IV (also known as cytochrome C oxidase) compared with reference values of healthy individuals (Figure 6D). The individual with the greatest reduction in maximal mitochondrial respiration also showed the largest impairment in complex IV activity (6.I.2). OXPHOS complex I activity was low to borderline normal in patients 6.I.2, 6.II.2, and 11.II.1 (Figure 6A). Activity of OXPHOS complexes II, III, and V was within the reference range in all patients, except for patients 6.I.2 and 3.III.1 (Figure 6, B, C, and E). Citrate synthase activity was within the reference ranges in all patients, except for individuals 4.I.2 and 4.II.2 (m.4291T>C), who had elevated activity (11% and 23% above the upper boundary of normal; Figure 6F).
Figure 6.
OXPHOS complex IV activity is reduced in patient mitochondria. (A–E) Activities of the five OXPHOS complexes (CI–CV), and (F) citrate synthase activity. All measurements were performed in isolated mitochondria from patient-derived fibroblasts. Thick, dotted lines represent the reference range from our center; thin, dotted lines represent the means of control individuals. CI to CV, OXPHOS complexes I to V; CS, citrate synthase activity.
Complex IV does not have a particularly high content of phenylalanine and isoleucine, as can be seen in Supplemental Table 6.
Complex IV Inhibition Reduces NCC-Mediated Sodium Absorption in HEK293 Cells
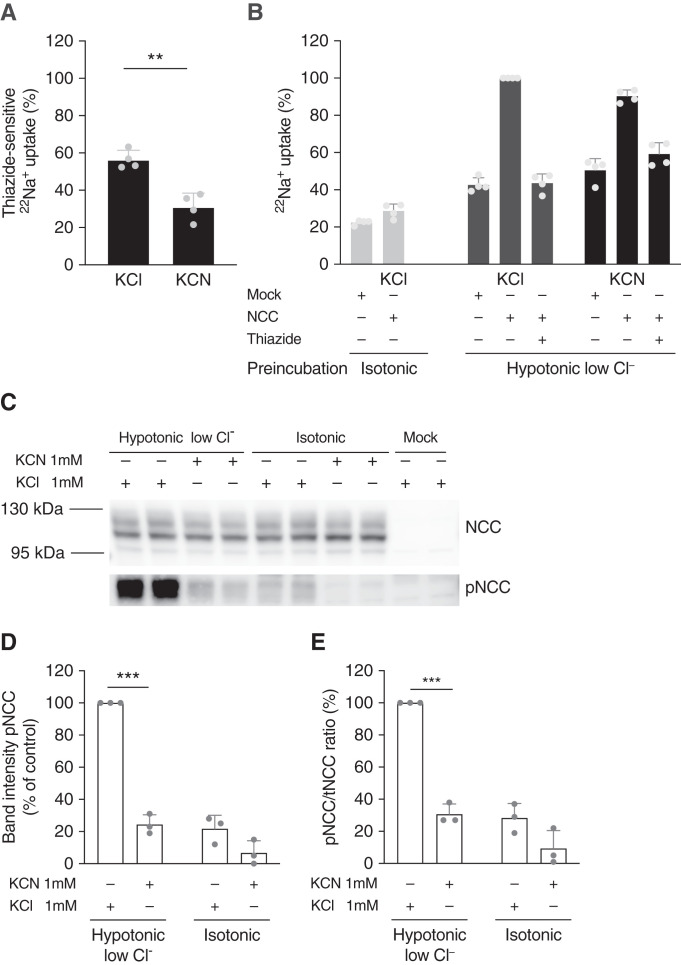
Because of the clinical similarities with GS and the abnormal mitochondria in the distal tubule observed with electron microscopy, we hypothesized that inhibition of OXPHOS complex IV would reduce NCC-mediated sodium uptake. Indeed, inhibition of complex IV with the specific inhibitor31,32 KCN reduced thiazide-sensitive 22Na+ absorption in NCC-transfected HEK293 cells by 45% (P=0.001; Figure 7A, Supplemental Figure 3). Even when adjusting for the observation that KCN induced an increase in 22Na+ uptake in mock-transfected cells and hydrochlorothiazide-treated cells, KCN still reduced 22Na+ uptake by 10% (Figure 7B). Furthermore, KCN blunted the response on NCC phosphorylation that is normally observed after 30 minutes of incubation in hypotonic-low-chloride buffer (adjusted P=0.00006; Figure 7, C–E, Supplemental Figure 4). A similar effect was observed for KCN on NKCC2 phosphorylation in hypotonic-low-chloride buffer (P=0.007; Supplemental Figures 5 and 6).
Figure 7.
NCC-mediated sodium uptake and NCC-phosphorylation are reduced with complex IV inhibition. (A and B) 22Na+ uptake in HEK293 cells transfected with NCC or mock, with or without inhibition of OXPHOS complex IV with KCN. KCN 1 mmol/L or KCl 1 mmol/L (control) was added during both preincubation and the uptake period, as indicated; the same applies to hydrochlorothiazide (HCTZ) 100 µmol/L. Bars represent mean with SD. (A) HCTZ-sensitive 22Na+ uptake of NCC-transfected cells over a period of 30 minutes. Data in (A) are on the basis of (B). Significance was assessed with an unpaired t test. (B) 22Na+ uptake in 30 minutes after preincubation with hypotonic-low-chloride buffer or isotonic buffer. Cells were transfected with NCC or mock and treated with KCl or KCN (n=4 of triplicates in each experiment). (C) Representative immunoblots showing NCC and phosphorylated NCC after a 30-minute incubation in hypotonic-low-chloride or isotonic buffer, with either KCN or KCl treatment. The mock condition has been incubated in hypotonic-low-chloride buffer as well. (D–E) Densitometry analysis of pNCC band intensity, and pNCC/tNCC ratio (n=3 duplicates in each experiment). Significance was assessed with unpaired t tests and corrected for multiple testing. pNCC, NCC phosphorylated at Thr60. **P<0.005; ****P<0.00005.
Discussion
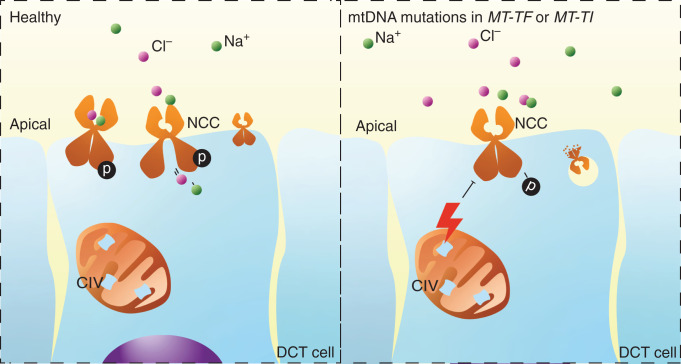
To date, approximately 10% of cases with clinical characteristics of GS remain genetically unsolved.3 Here, we show that variants in two mt-tRNAs can lead to a GS-like syndrome, even in the apparent absence of other manifestations of mitochondrial disease. Thirteen families are described with hypokalemia and hypomagnesemia caused by renal magnesium wasting together with elevated renin levels. Nine families carry a variant in MT-TF, which encodes the mt-tRNA for phenylalanine. Four families carry a variant in MT-TI, encoding the tRNA for isoleucine. The variants in MT-TF were associated with the development of CKD in 19 individuals on the maternal lineage of six families. Electron microscopy of kidney biopsy specimens from two individuals with a pathogenic variant in MT-TF and CKD demonstrated tubulointerstitial kidney disease and abnormal mitochondria in the distal tubule. Cells in the DCT have the largest number of mitochondria per unit length of the nephron and would therefore be sensitive to mitochondrial dysfunction.33 In line with these findings, patient-derived fibroblasts were found to exhibit a disturbed mitochondrial OXPHOS, putatively caused by a significant impairment of complex IV that was observed in patient mitochondria. In HEK293 cells, pharmacologic inhibition of complex IV was shown to result in a reduction in NCC-mediated sodium reabsorption. We propose that the mitochondrial variants result in reduced NCC activity (Figure 8).
Figure 8.
Induction of Gitelman-like syndrome by pathogenic mtDNA variants, proposed mechanism. Proposed mechanism of Gitelman-like syndrome induced by pathogenic mtDNA variants in the genes encoding the isoleucine and phenylalanine tRNAs (MT-TI and MT-TF, respectively). The m.4291T>C, m.591C>T, m.616T>C, and m.643A>G variants lead to complex IV dysfunction and reduced maximal respiration. This leads to a decrease in the phosphorylation of NCC and sodium transport. Reduced sodium transport in the DCT leads to reduced magnesium transport in the DCT and increased sodium transport in the collecting duct. Increased sodium reabsorption in the collecting duct leads to increased potassium excretion through ROMK (not shown here). CIV, OXPHOS complex IV.
In total, we identified three different variants in MT-TF in nine families, of which m.591C>T has not been described before, and describe the m.4291T>C variant in MT-TI in three other families. Heteroplasmy levels ranged from 97% to 100% (homoplasmy) in all affected individuals, suggesting that a large proportion of mtDNA copies needs to be affected before a clinically overt phenotype manifests itself.
The four mtDNA variants described here were associated with hypomagnesemia, hypokalemia, and activation of renin production, hallmarks of GS.9 Two other symptoms of GS, hypocalciuria and metabolic alkalosis, were only ascertained in a subset of patients. Interestingly, none of the 13 families had clinically overt extrarenal manifestations of mitochondrial dysfunction at the moment of this study. This is in contrast to what is normally observed with pathogenic variants in mtDNA or nuclear-encoded mitochondrial genes.8–10,34 Systematic evaluation of symptoms associated with mitochondrial dysfunction should be performed to definitively exclude the presence of rare or subclinical extrarenal manifestations. Yet, specific mtDNA variants have been described that can result in diseases that affect only a single organ, such as in Leber hereditary optic neuropathy and nonsyndromic hearing loss.7 Our results show that mt-tRNA variants can cause a maternally inherited GS-like syndrome. Moreover, mt-tRNA variants may explain other familial and sporadic individual cases, because systematic screening of our cohort identified pathogenic mtDNA variants in nine of 156 families with characteristics of GS.
Genetic heterogeneity and pleiotropy are common phenomena in mitochondrial disorders. For example, the MELAS phenotype (mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes) can be caused by pathogenic variants in the mt-tRNA for leucine (MT-TL1), phenylalanine (MT-TF), and histidine (MT-TH).35–37 On the other hand, different pathogenic variants in one mt-tRNA can lead to different clinical manifestations. For instance, variants in MT-TI and MT-TF have already been associated with cardiomyopathy,38 Leigh syndrome,39 nonsyndromic hearing loss,40 chronic progressive external ophthalmoplegia,41 and MELAS.35
The specific base pair affected by the variant thus appears to be important, as demonstrated by the finding of multiple families with the m.591C>T and m.4291T>C variants in our study. Interestingly, variants in MT-TF have previously been associated with renal phenotypes, especially tubulointerstitial kidney disease with progressive kidney failure.12,42–45 Indeed, also in our cohort, CKD was observed in affected members of two families with the m.643A>G and m.616T>C variants and members of four of the seven families with the m.591C>T variant (all in MT-TF). In contrast, CKD is not a hallmark of classic GS, nor did any of the patients with the m.4291T>C variant (affecting MT-TI) have CKD. Thus, the different mtDNA variants might confer a varying predisposition to tubulointerstitial kidney disease and loss of GFR.
The clinical similarity with GS and the finding of abnormal mitochondria in the distal tubule on a renal biopsy specimen suggested that the mitochondrial dysfunction reported here is responsible for a defect in sodium and magnesium reabsorption in this tubular segment. The electrolyte abnormalities in GS are caused by loss of function of NCC, the electroneutral sodium-chloride cotransporter expressed apically in the DCT. Notably, other genetic diseases have further highlighted the link between reduced NCC function and GS-like electrolyte abnormalities.46–48 For instance, pathogenic variants in three DCT-localized proteins, namely ClC-Kb (CLCNKB), Kir4.1 (KCNJ10), and Kir5.1 (KCNJ16), give rise to very similar electrolyte abnormalities as observed in GS.49–51 Kir4.1 is essential for K+ extrusion at the basolateral membrane and for recycling K+ imported by the Na+-K+-ATPase.49 Loss of function of Kir4.1 thus reduces basolateral negative membrane potential.52 This inhibits Cl− extrusion through ClC-Kb, a basolateral chloride channel encoded by CLCNKB.53 A subsequent rise in intracellular chloride is sensed by WNK4 and thus reduces activation of SPAK/OSR1.54,55 Finally, this will lower NCC phosphorylation and consequently NCC-mediated sodium transport.56
Here, we provide evidence that pathogenic variants in MT-TI and MT-TF also impair NCC function. Patient-derived fibroblasts showed impairment of mitochondrial function, especially of OXPHOS complex IV. Given the difficulty of introducing mtDNA mutations in a cell model, we decided to take a pharmacologic approach at inhibiting complex IV. This indeed resulted in lower NCC phosphorylation and lower NCC-mediated sodium transport.
Reabsorption of sodium is a process with a high energy demand, and even more so in the DCT because of the smaller osmotic gradient.57 To accomplish this, the DCT is dependent on aerobic energy production.58–60 Consequently, mitochondrial dysfunction here might lead to diminished function of the Na+-K+-ATPase, which needs ATP to maintain basolateral membrane potential, a prerequisite for sodium and magnesium transport.61,62 Supporting this mechanism, pathogenic variants in FXYD2, ATP1A1, and HNF1B are also known to cause a GS-like phenotype.5,63–66 FXYD2 and ATP1A1 encode two different subunits of the Na+-K+-ATPase, whereas HNF1B has been shown to regulate FXYD2 and KCNJ16 mRNA expression.67,68
Whereas one patient showed a blunted response to the administration of hydrochlorothiazide, three patients demonstrated a larger increase in maximal ΔFECl than observed in patients with GS, suggesting residual NCC function in these patients. This is in line with other GS-like tubulopathies, such as those caused by pathogenic variants in CLCNKB or HNF1B. In these patients also, thiazide tests did not show the same level of reduction in thiazide response as has been established for GS.21,69,70
The penetrance of hypocalciuria is lower in our patients than in GS. Interestingly, urinary calcium excretion in CLCNKB patients, HNF1B patients, and FXYD2 patients does not seem to be as low as in GS, either.65,66,71 Metabolic alkalosis seems to be more pronounced in classic GS, too.65,66 For these diseases, it is thought that the phenotypic differences arise from an additional dysfunction of other segments, e.g., thick ascending limb dysfunction in CLCNKB, in addition to the effects in the DCT. We cannot exclude that MT-TI/MT-TF-associated GS-like syndrome also affects the connecting tubule72,73 or the thick ascending limb. Indeed, our in vitro data demonstrate reduced NKCC2 phosphorylation.
The m.4291T>C variant has been reported before in a large family with a 50% penetrance of hypertension, hypercholesterolemia, and hypomagnesemia,9 and in a family with congenital cataract.74 In the 13 families with an mtDNA variant reported here, no individuals were affected by congenital cataract. Hypercholesterolemia and hypertension were not more frequent than in the general population, although this conclusion might not be generalizable to all four variants. Incomplete penetrance of clinically significant hypomagnesemia was noticed in a few cases too, even with (near) homoplasmic presence of the variant, although we cannot exclude the presence of subclinical symptoms. Systematic analysis of a larger number of patients and families will be required to identify subclinical symptoms and determine the penetrance of additional disease manifestations in the different variants. The m.616T>C variant has been reported before in three families,12,44,45 but none of these had reported GS-like electrolyte abnormalities. However, it should be noted that these symptoms could have been missed initially, as in family 13, or could develop later in life.12
The m.643A>G variant has been reported only once before,18 and was classified in this study as a variant of uncertain significance toward the pathogenic side of the spectrum.19 Future studies might provide decisive evidence on its pathogenicity.
Current next-generation sequencing analyses usually do not report mitochondrial variants. Not all exome sequencing kits cover the mitochondrial genome well, and if they provide adequate coverage, pipelines often focus on variants in or near protein coding sequences in nuclear DNA. Nevertheless, identification of pathogenic near homoplasmic mtDNA variants has important clinical and genetic implications. First of all, clinicians should be aware of the combination of GS-like electrolyte abnormalities and progressive CKD in patients with MT-TF variants. Secondly, our findings stress that clinicians treating patients with mitochondrial disorders should appreciate the possibility of electrolyte abnormalities, because hypomagnesemia might sometimes explain part of the muscle complaints. Furthermore, finding mtDNA variants in patients with unexplained GS has important consequences for genetic counseling, given the different inheritance pattern. Thus, specific testing for pathogenic variants in the mitochondrial genome, or including the mitochondrial genome in analyses of next-generation sequencing approaches, is warranted. Lastly, the fact that the variant was found in (near) homoplasmic state in all families suggests that genetic testing can be performed on DNA isolated from blood (in contrast to many other mitochondrial disorders, where affected tissue is needed to avoid false negative results).
In conclusion, MT-TI and MT-TF variants can cause a Gitelman-like syndrome. In all patients evaluated for a genetic cause of hypomagnesemia or hypokalemia, clinicians should consider screening MT-TI and MT-TF for pathogenic variants by next-generation sequencing or specific mtDNA testing. Importantly, pathogenic variants in MT-TF also confer a significant risk for the development of CKD.
Disclosures
C. Bergmann reports Ownership Interest in Medizinische Genetik Mainz; Research Funding from Limbach; Honoraria from Alexion, Atheneum, Kyowa Kirin, Merck, Otsuka, PTC, Sanofi; Scientific Advisor or Membership via medical and scientific board of German PKD Foundation, advisory boards of PTC, and Alexion; and Other Interests/Relationships as member of the kidney community (GPN, DGfN, ERA/EDTA, IPNA, ESPN, ASN). D. Bockenhauer reports Consultancy Agreements with Advicenne, Avrobio, Otsuka, Sanofi; Honoraria from Advicenne, Recordati; and Scientific Advisor or Membership as associate editor of Pediatric Nephrology, NDT, and editorial board of JASN. P. Houillier reports Consultancy Agreements with Amgen, Shire/Takeda, KyowaKirin; Research Funding from Amgen, Takeda/Shire; Honoraria from KyowaKirin, Takeda/Shire; Scientific Advisor or Membership with NCCR Kidney.CH (Switzerland); Member of the editorial board of JASN; and Other Interests/Relationships via working group on calcium and bone for the European Society of Endocrinology. G. Klaus reports Consultancy Agreements: Fresenius Medical Care as Oral presentation at ESPN 2021, Vifor Fresenius Medical Care Renal Pharma Ltd (stopped 2020); Honoraria from Fresenius Medical Care for Oral presentation at ESPN 2021, Vifor Fresenius Medical Care Renal Pharma Ltd (stopped 2020); Other Interests/Relationships with KfH-Kuratorium für Dialyse und Nierentransplantation, and Gesellschaft für Pädiatrische Nephrologie (GPN) Speaker for medical education. N. Knoers reports Honoraria from ErasmusMC, The Netherlands, for SEP evaluation; Scientific Advisor or Membership via different research advisory committees without any financial compensation; and Other Interests/Relationships as Chair board ERA-EDTA WGIKD, Chair Task Force Molecular Diagnostics, European Reference Network of Rare Renal Diseases, ERKNET. M. Konrad reports Consultancy Agreements with Otsuka. A. Mallett reports Research Funding from Otsuka, Sanofi-Genzyme; Scientific Advisor or Membership with Otsuka; Other Interests/Relationships as Local Site Trial Investigator for Achillion, Dicerna, Novotech, Reata, Sanofi-Genzyme; and Research Fellowships with RACP Jacquot Research Establishment Fellowship and MNHHS Clinical Research Fellowship. T. Nijenhuis reports Research Funding from the Dutch Kidney Foundation, Radboudumc; Scientific Advisor or Membership via Scientific board of the Dutch Society for Nephrology, Scientific advisory board of the Dutch Kidney Foundation, Co-chair of the Tubulopathies Expert Working Group, European Rare Kidney Disorders Network (ERKNet), and Board member of ERA-EDTA Working Group Inherited Kidney Disorders. A. van Eerde reports Other Interests/Relationships with ERA-EDTA, Working Group Inherited Kidney Diseases Board, ERKNet, WG chair receives funding from the Dutch Kidney Foundation. R. Vargas-Poussou reports Scientific Advisor or Membership with Advicenne. J. de Baaij reports Research Funding from the Dutch Kidney Foundation and the Dutch Diabetes Research Foundation. All remaining authors have nothing to disclose.
Funding
This work was financially supported by the IMAGEN project which is cofunded by the PPP Allowance made available by Health∼Holland, Top Sector Life Sciences & Health, to stimulate public-private partnerships (IMplementation of Advancements in GENetic Kidney Disease, LSHM20009) and the Dutch Kidney Foundation (Nierstichting) (20OP+018). Additionally, we received support from ZonMW under the frame of EJPRD, the European Joint Programme on Rare Diseases (EJPRD2019-40). This project has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under the EJP RD COFUND-EJP N° 825575 and the Netherlands Organization for Scientific Research (Nederlandse Organisatie voor Wetenschappelijk Onderzoek) (NWO Veni 016.186.012). The 100,000 Genomes Project is managed by Genomics England Limited (a wholly owned company of the Department of Health and Social Care). The 100,000 Genomes Project is funded by the National Institute for Health Research and NHS England. The Wellcome Trust, Cancer Research UK, and the Medical Research Council have also funded research infrastructure of the 100,000 Genomes Project.
Supplementary Material
Acknowledgments
The authors are grateful to the patients for their participation in this study. We thank Caro Bos, Larissa Govers, Edwin van Kaauwen, Dr. Bert van den Heuvel, Frans van den Brandt, and Gijs Franken for their excellent technical support. We thank Thijs Landman and Joeri van Strien for help with the conception of Seahorse_analyzeR. We thank Prof. dr. Robert Fenton for kindly providing the anti-pT58-NCC antibodies. This research includes data generated by the 100,000 Genomes Project. The 100,000 Genomes Project uses data provided by patients and collected by the National Health Service as part of their care and support.
Günter Klaus, Jean-Marie Coulibaly, Marion Vallet, Solenne Pelletier, Stéphane Decramer, Martin Kömhoff, Rolf Beetz, Mohan Shenoy, Karl P. Schlingmann, Detlef Bockenhauer, Rosa Vargas-Poussou, Pascal Houillier, Martin Konrad, Robert Kleta, André van Beek, Tom Nijenhuis, Andrew Mallett, and Chirag Patel were responsible for patient inclusion. Karl P. Schlingmann, Jeroen H.F. de Baaij, Detlef Bockenhauer, Melanie M.Y. Chan, Rosa Vargas-Poussou, Carsten Bergmann, Nine V.A.M. Knoers, André van Beek, Ernie M.H.F. Bongers, Albertien M. van Eerde, Tom Nijenhuis, Andrew Mallett, Marguerite Hureaux, Daan Viering, and Genomics England Research Consortium carried out the genetic investigations. Glenn Anderson and Eric J. Steenbergen performed histologic investigations. Daan Viering, Jeroen H.F. de Baaij, and Daan Panneman performed cell experiments. Karl P. Schlingmann, Jeroen H.F. de Baaij, Detlef Bockenhauer, Rosa Vargas-Poussou, René Bindels, Richard J. Rodenburg, Nine Knoers, Tom Nijenhuis, and Robert Kleta supervised the project. Jeroen H.F. de Baaij and Daan Viering drafted the manuscript. All authors were responsible for revision and approval of the manuscript.
Several authors of this publication are members of the European Reference Network for Rare Kidney Diseases (ERKNet)—Project ID No. 739532. Andrew Mallett was supported by an Royal Australasian College of Physicians Jacquot Research Establishment Fellowship and an Metro North Hospital and Health Service Clinical Research Fellowship.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021050596/-/DCSupplemental.
Supplemental Table 1. Primer and smMIP sequences.
Supplemental Table 2. Age-specific reference for urinary calcium/creatinine ratio.
Supplemental Table 3. Full clinical data.
Supplemental Table 4. Additional variant information.
Supplemental Table 5. Criteria for pathogenicity in each of the variants.
Supplemental Table 6. Number of phenylalanine and isoleucine residues in the mitochondrial genes.
Supplemental Figure 1. Thiazide test.
Supplemental Figure 2. Electron microscopy of proximal tubular cells.
Supplemental Figure 3. Control immunoblots 22Na+ uptake experiments.
Supplemental Figure 4. Full immunoblot NCC phosphorylation experiments.
Supplemental Figure 5. NKCC2 phosphorylation with complex IV inhibition.
Supplemental Figure 6. Full immunoblot NKCC2 phosphorylation experiments.
References
- 1.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, et al. : Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Blanchard A, Bockenhauer D, Bolignano D, Calò LA, Cosyns E, Devuyst O, et al. : Gitelman syndrome: consensus and guidance from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 91: 24–33, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Vargas-Poussou R, Dahan K, Kahila D, Venisse A, Riveira-Munoz E, Debaix H, et al. : Spectrum of mutations in Gitelman syndrome. J Am Soc Nephrol 22: 693–703, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Downie ML, Lopez Garcia SC, Kleta R, Bockenhauer D: Inherited tubulopathies of the kidney: insights from genetics. Clin J Am Soc Nephrol 16: 620–630, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viering DHHM, de Baaij JHF, Walsh SB, Kleta R, Bockenhauer D: Genetic causes of hypomagnesemia, a clinical overview. Pediatr Nephrol 32: 1123–1135, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Made CI, Hoorn EJ, de la Faille R, Karaaslan H, Knoers NV, Hoenderop JG, et al. : Hypomagnesemia as first clinical manifestation of ADTKD-HNF1B: a case series and literature review. Am J Nephrol 42: 85–90, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Chinnery PF: Primary Mitochondrial disorders overview. In: GeneReviews®, edited by Adam MP AH, Pagon RA, et al.: Seattle, Washington, University of Washington, 2000 [Google Scholar]
- 8.Goto Y, Itami N, Kajii N, Tochimaru H, Endo M, Horai S: Renal tubular involvement mimicking Bartter syndrome in a patient with Kearns-Sayre syndrome. J Pediatr 116: 904–910, 1990 [DOI] [PubMed] [Google Scholar]
- 9.Wilson FH, Hariri A, Farhi A, Zhao H, Petersen KF, Toka HR, et al. : A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science 306: 1190–1194, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giordano C, Powell H, Leopizzi M, De Curtis M, Travaglini C, Sebastiani M, et al. : Fatal congenital myopathy and gastrointestinal pseudo-obstruction due to POLG1 mutations. Neurology 72: 1103–1105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Zhong C, Yang Q, Zhang G, Yang H, Li Q, et al. : Novel SARS2 variants identified in a Chinese girl with HUPRA syndrome. Mol Genet Genomic Med 9: e1650, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connor TM, Hoer S, Mallett A, Gale DP, Gomez-Duran A, Posse V, et al. : Mutations in mitochondrial DNA causing tubulointerstitial kidney disease. PLoS Genet 13: e1006620, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Durbin R: Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26: 589–595, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. ; 1000 Genome Project Data Processing Subgroup : The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin AR, Williams E, Foulger RE, Leigh S, Daugherty LC, Niblock O, et al. : PanelApp crowdsources expert knowledge to establish consensus diagnostic gene panels. Nat Genet 51: 1560–1565, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Ekici AB, Hackenbeck T, Morinière V, Pannes A, Buettner M, Uebe S, et al. : Renal fibrosis is the common feature of autosomal dominant tubulointerstitial kidney diseases caused by mutations in mucin 1 or uromodulin. Kidney Int 86: 589–599, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Yarham JW, McFarland R, Taylor RW, Elson JL: A proposed consensus panel of organisms for determining evolutionary conservation of mt-tRNA point mutations. Mitochondrion 12: 533–538, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong LC, Chen T, Wang J, Tang S, Schmitt ES, Landsverk M, et al. : Interpretation of mitochondrial tRNA variants. Genet Med 22: 917–926, 2020 [DOI] [PubMed] [Google Scholar]
- 19.Ellard S, Baple E, Callaway A, Berry I, Forrester N, Turnbull C, et al. : ACGS Best Practice Guidelines for Variant Classification in Rare Disease 2020, ACGS, 2020. Accessed March 1, 2021. [Google Scholar]
- 20.Bech AP, Wetzels JFM, Nijenhuis T: Reference values of renal tubular function tests are dependent on age and kidney function. Physiol Rep 5: e13542, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colussi G, Bettinelli A, Tedeschi S, De Ferrari ME, Syrén ML, Borsa N, et al. : A thiazide test for the diagnosis of renal tubular hypokalemic disorders. Clin J Am Soc Nephrol 2: 454–460, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Rodenburg RJT: Biochemical diagnosis of mitochondrial disorders. J Inherit Metab Dis 34: 283–292, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panneman DM, Wortmann SB, Haaxma CA, van Hasselt PM, Wolf NI, Hendriks Y, et al. : Variants in NGLY1 lead to intellectual disability, myoclonus epilepsy, sensorimotor axonal polyneuropathy and mitochondrial dysfunction. Clin Genet 97: 556–566, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yépez VA, Kremer LS, Iuso A, Gusic M, Kopajtich R, Koňaříková E, et al. : OCR-Stats: robust estimation and statistical testing of mitochondrial respiration activities using Seahorse XF Analyzer. PLoS One 13: e0199938, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen NB, Hofmeister MV, Rosenbaek LL, Nielsen J, Fenton RA: Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int 78: 160–169, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Ashton EJ, Legrand A, Benoit V, Roncelin I, Venisse A, Zennaro MC, et al. : Simultaneous sequencing of 37 genes identified causative mutations in the majority of children with renal tubulopathies. Kidney Int 93: 961–967, 2018 [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization : Global Status Report on Noncommunicable Diseases 2014, 2014. Available at: http://apps.who.int/iris/bitstream/handle/10665/148114/9789241564854_eng.pdf;jsessionid=51B6533AF532336F82399400471FA2AD?sequence=1. Accessed July 12, 2021. [Google Scholar]
- 28.World Health Organization : Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks, 2009. Available at: https://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf.. Accessed July 12, 2021. [Google Scholar]
- 29.Walsh PR, Tse Y, Ashton E, Iancu D, Jenkins L, Bienias M, et al. : Clinical and diagnostic features of Bartter and Gitelman syndromes. Clin Kidney J 11: 302–309, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.et al. Kidney Disease: Improving Global Outcomes: Autosomal dominant tubulointerstitial kidney disease: Diagnosis, classification, and management. https://kdigo.org/wp-content/uploads/2017/02/KDIGO_ADTKD-2015.pdf. Accessed March 15, 2021 [Google Scholar]
- 31.Kunz WS, Kudin A, Vielhaber S, Elger CE, Attardi G, Villani G: Flux control of cytochrome c oxidase in human skeletal muscle. J Biol Chem 275: 27741–27745, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Gnaiger E: Mitochondrial Pathways and Respiratory Control: An Introduction to OXPHOS Analysis. Mitochondr Physiol Network 19.12, Innsbruck, OROBOROS MiPNet Publications, 2014 [Google Scholar]
- 33.Reilly RF, Ellison DH: Mammalian distal tubule: Physiology, pathophysiology, and molecular anatomy. Physiol Rev 80: 277–313, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Belostotsky R, Ben-Shalom E, Rinat C, Becker-Cohen R, Feinstein S, Zeligson S, et al. : Mutations in the mitochondrial seryl-tRNA synthetase cause hyperuricemia, pulmonary hypertension, renal failure in infancy and alkalosis, HUPRA syndrome. Am J Hum Genet 88: 193–200, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanna MG, Nelson IP, Morgan-Hughes JA, Wood NW: MELAS: A new disease associated mitochondrial DNA mutation and evidence for further genetic heterogeneity. J Neurol Neurosurg Psychiatry 65: 512–517, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goto Y, Nonaka I, Horai S: A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 348: 651–653, 1990 [DOI] [PubMed] [Google Scholar]
- 37.Melone MA, Tessa A, Petrini S, Lus G, Sampaolo S, di Fede G, et al. : Revelation of a new mitochondrial DNA mutation (G12147A) in a MELAS/MERFF phenotype. Arch Neurol 61: 269–272, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Giordano C, Perli E, Orlandi M, Pisano A, Tuppen HA, He L, et al. : Cardiomyopathies due to homoplasmic mitochondrial tRNA mutations: Morphologic and molecular features. Hum Pathol 44: 1262–1270, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Cox R, Platt J, Chen LC, Tang S, Wong LJ, Enns GM: Leigh syndrome caused by a novel m.4296G>A mutation in mitochondrial tRNA isoleucine. Mitochondrion 12: 258–261, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Gutiérrez Cortés N, Pertuiset C, Dumon E, Börlin M, Hebert-Chatelain E, Pierron D, et al. : Novel mitochondrial DNA mutations responsible for maternally inherited nonsyndromic hearing loss. Hum Mutat 33: 681–689, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Schaller A, Desetty R, Hahn D, Jackson CB, Nuoffer JM, Gallati S, et al. : Impairment of mitochondrial tRNAIle processing by a novel mutation associated with chronic progressive external ophthalmoplegia. Mitochondrion 11: 488–496, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tzen C-Y, Tsai J-D, Wu T-Y, Chen B-F, Chen M-L, Lin S-P, et al. : Tubulointerstitial nephritis associated with a novel mitochondrial point mutation. Kidney Int 59: 846–854, 2001 [DOI] [PubMed] [Google Scholar]
- 43.D’Aco KE, Manno M, Clarke C, Ganesh J, Meyers KE, Sondheimer N: Mitochondrial tRNA(Phe) mutation as a cause of end-stage renal disease in childhood. Pediatr Nephrol 28: 515–519, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lorenz R, Ahting U, Betzler C, Heimering S, Borggrafe I, Lange-Sperandio B: Homoplasmy of the mitochondrial DNA mutation m.616T>C leads to mitochondrial tubulointerstitial kidney disease and encephalopathia. Nephron 144:156-160, 2020 [DOI] [PubMed] [Google Scholar]
- 45.Riedhammer KM, Braunisch MC, Günthner R, Wagner M, Hemmer C, Strom TM, et al. : Exome sequencing and identification of phenocopies in patients with clinically presumed hereditary nephropathies. Am J Kidney Dis 76: 460–470, 2020 [DOI] [PubMed] [Google Scholar]
- 46.Zhang C, Wang L, Zhang J, Su XT, Lin DH, Scholl UI, et al. : KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1). Proc Natl Acad Sci U S A 111: 11864–11869, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang MX, Cuevas CA, Su XT, Wu P, Gao ZX, Lin DH, et al. : Potassium intake modulates the thiazide-sensitive sodium-chloride cotransporter (NCC) activity via the Kir4.1 potassium channel. Kidney Int 93: 893–902, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, et al. : Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, et al. : Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360: 1960–1970, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Konrad M, Vollmer M, Lemmink HH, VAN DEN Heuvel LPWJ, Jeck N, Vargas-Poussou R, et al. : Mutations in the chloride channel gene CLCNKB as a cause of classic Bartter syndrome. J Am Soc Nephrol 11: 1449–1459, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Schlingmann KP, Renigunta A, Hoorn EJ, Forst AL, Renigunta V, Atanasov V, et al. : Defects in KCNJ16 cause a novel tubulopathy with hypokalemia, salt wasting, disturbed acid-base homeostasis, and sensorineural deafness. J Am Soc Nephrol 32: 1498–1512, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cuevas CA, Su X-T, Wang M-X, Terker AS, Lin D-H, McCormick JA, et al. : Potassium sensing by renal distal tubules requires Kir4.1. J Am Soc Nephrol 28: 1814–1825, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janssen AG, Scholl U, Domeyer C, Nothmann D, Leinenweber A, Fahlke C: Disease-causing dysfunctions of barttin in Bartter syndrome type IV. J Am Soc Nephrol 20: 145–153, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J-C, Lo Y-F, Lin Y-W, Lin S-H, Huang C-L, Cheng C-J: WNK4 kinase is a physiological intracellular chloride sensor. Proc Natl Acad Sci U S A 116: 4502–4507, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grimm PR, Coleman R, Delpire E, Welling PA: Constitutively active SPAK causes hyperkalemia by activating NCC and remodeling distal tubules. J Am Soc Nephrol 28: 2597–2606, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang S-S, Fang Y-W, Tseng M-H, Chu P-Y, Yu IS, Wu H-C, et al. : Phosphorylation regulates NCC stability and transporter activity in vivo. J Am Soc Nephrol 24: 1587–1597, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hansell P, Welch WJ, Blantz RC, Palm F: Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension. Clin Exp Pharmacol Physiol 40: 123–137, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCormick JA, Ellison DH: Distal convoluted tubule. Compr Physiol 5: 45–98, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hall AM, Rhodes GJ, Sandoval RM, Corridon PR, Molitoris BA: In vivo multiphoton imaging of mitochondrial structure and function during acute kidney injury. Kidney Int 83: 72–83, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bagnasco S, Good D, Balaban R, Burg M: Lactate production in isolated segments of the rat nephron. Am J Physiol 248: F522–F526, 1985 [DOI] [PubMed] [Google Scholar]
- 61.Meij IC, Koenderink JB, De Jong JC, De Pont JJ, Monnens LA, Van Den Heuvel LP, et al. : Dominant isolated renal magnesium loss is caused by misrouting of the Na+,K+-ATPase γ-subunit. Ann N Y Acad Sci 986: 437–443, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Franken GAC, Adella A, Bindels RJM, de Baaij JHF: Mechanisms coupling sodium and magnesium reabsorption in the distal convoluted tubule of the kidney. Acta Physiol (Oxf) 231: e13528, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Geven WB, Monnens LA, Willems HL, Buijs WC, ter Haar BG: Renal magnesium wasting in two families with autosomal dominant inheritance. Kidney Int 31: 1140–1144, 1987 [DOI] [PubMed] [Google Scholar]
- 64.Schlingmann KP, Bandulik S, Mammen C, Tarailo-Graovac M, Holm R, Baumann M, et al. : Germline de novo mutations in ATP1A1 cause renal hypomagnesemia, refractory seizures, and intellectual disability. Am J Hum Genet 103: 808–816, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Baaij JH, Dorresteijn EM, Hennekam EA, Kamsteeg EJ, Meijer R, Dahan K, et al. : Recurrent FXYD2 p.Gly41Arg mutation in patients with isolated dominant hypomagnesaemia. Nephrol Dial Transplant 30: 952–957, 2015 [DOI] [PubMed] [Google Scholar]
- 66.Adalat S, Hayes WN, Bryant WA, Booth J, Woolf AS, Kleta R, et al. : HNF1B mutations are associated with a Gitelman-like tubulopathy that develops during childhood. Kidney Int Rep 4: 1304–1311, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kompatscher A, de Baaij JHF, Aboudehen K, Hoefnagels APWM, Igarashi P, Bindels RJM, et al. : Loss of transcriptional activation of the potassium channel Kir5.1 by HNF1β drives autosomal dominant tubulointerstitial kidney disease. Kidney Int 92: 1145–1156, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferrè S, Veenstra GJ, Bouwmeester R, Hoenderop JG, Bindels RJ: HNF-1B specifically regulates the transcription of the γa-subunit of the Na+/K+-ATPase. Biochem Biophys Res Commun 404: 284–290, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Bech AP, Wetzels JF, Bongers EMHF, Nijenhuis T: Thiazide responsiveness testing in patients with renal magnesium wasting and correlation with genetic analysis: A diagnostic test study. Am J Kidney Dis 68: 168–170, 2016 [DOI] [PubMed] [Google Scholar]
- 70.Nozu K, Iijima K, Kanda K, Nakanishi K, Yoshikawa N, Satomura K, et al. : The pharmacological characteristics of molecular-based inherited salt-losing tubulopathies. J Clin Endocrinol Metab 95: E511–E518, 2010 [DOI] [PubMed] [Google Scholar]
- 71.Jeck N, Konrad M, Peters M, Weber S, Bonzel KE, Seyberth HW: Mutations in the chloride channel gene, CLCNKB, leading to a mixed Bartter-Gitelman phenotype. Pediatr Res 48: 754–758, 2000 [DOI] [PubMed] [Google Scholar]
- 72.Reilly RF, Huang CL: The mechanism of hypocalciuria with NaCl cotransporter inhibition. Nat Rev Nephrol 7: 669–674, 2011 [DOI] [PubMed] [Google Scholar]
- 73.Kovacikova J, Winter C, Loffing-Cueni D, Loffing J, Finberg KE, Lifton RP, et al. : The connecting tubule is the main site of the furosemide-induced urinary acidification by the vacuolar H+-ATPase. Kidney Int 70: 1706–1716, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Roshan M, Kabekkodu SP, Vijaya PH, Manjunath K, Graw J: Analysis of mitochondrial DNA variations in Indian patients with congenital cataract. Mol Vis 18: 181–193, 2012 [PMC free article] [PubMed]
- 75.Elisaf M, Panteli K, Theodorou J, Siamopoulos KC: Fractional excretion of magnesium in normal subjects and in patients with hypomagnesemia. Magnes Res 10: 315–320, 1997 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.