Significance Statement
Receptor-interacting protein kinase 3 (RIPK3), a key necroptosis pathway protein, may have an independent role in inflammation. The authors explored RIPK3′s role in kidney inflammation occurring in the presence or absence of kidney cell death and AKI, identifying RIPK3—bone marrow RIPK3 specifically—as a driver of kidney inflammation, even in the absence of tubular cell death or kidney failure. Experiments in chimeric mice and cell culture identified IL-6 as a key RIPK3-regulated mediator and showed that RIPK3 expression by bone marrow cells recruits proinflammatory responses in tubular cells. These findings identify bone marrow RIPK3 as a key mediator and potential therapeutic target in conditions characterized by kidney inflammation. Strategies aimed at targeting bone marrow RIPK3 may preserve therapeutic efficacy while decreasing potential systemic consequences of RIPK3 inhibition.
Keywords: acute kidney injury, kidney, inflammation, bone marrow-derived leukocytes, RIPK3, TWEAK, NLRP3
Visual Abstract
Abstract
Background
Receptor-interacting protein kinase 3 (RIPK3), a component of necroptosis pathways, may have an independent role in inflammation. It has been unclear which RIPK3-expressing cells are responsible for the anti-inflammatory effect of overall Ripk3 deficiency and whether Ripk3 deficiency protects against kidney inflammation occurring in the absence of tubular cell death.
Methods
We used chimeric mice with bone marrow from wild-type and Ripk3-knockout mice to explore RIPK3′s contribution to kidney inflammation in the presence of folic acid–induced acute kidney injury AKI (FA-AKI) or absence of AKI and kidney cell death (as seen in systemic administration of the cytokine TNF-like weak inducer of apoptosis [TWEAK]).
Results
Tubular and interstitial cell RIPK3 expressions were increased in murine AKI. Ripk3 deficiency decreased NF-κB activation and kidney inflammation in FA-AKI but did not prevent kidney failure. In the chimeric mice, RIPK3-expressing bone marrow–derived cells were required for early inflammation in FA-AKI. The NLRP3 inflammasome was not involved in RIPK3′s proinflammatory effect. Systemic TWEAK administration induced kidney inflammation in wild-type but not Ripk3-deficient mice. In cell cultures, TWEAK increased RIPK3 expression in bone marrow–derived macrophages and tubular cells. RIPK3 mediated TWEAK-induced NF-κB activation and inflammatory responses in bone marrow–derived macrophages and dendritic cells and in Jurkat T cells; however, in tubular cells, RIPK3 mediated only TWEAK-induced Il-6 expression. Furthermore, conditioned media from TWEAK-exposed wild-type macrophages, but not from Ripk3-deficient macrophages, promoted proinflammatory responses in cultured tubular cells.
Conclusions
RIPK3 mediates kidney inflammation independently from tubular cell death. Specific targeting of bone marrow–derived RIPK3 may limit kidney inflammation without the potential adverse effects of systemic RIPK3 targeting.
The incidence of AKI is increasing as an aging population is subjected to complex medical procedures.1 Inflammation, parenchymal cell loss, and eventual nephron loss are AKI features that may lead to tubulointerstitial fibrosis, and there is a bidirectional relationship between AKI and chronic kidney disease (CKD).2,3 Currently, no satisfactory treatment attenuates AKI or accelerates recovery. Tubular cell death is the main histologic characteristic of AKI, representing an early event that is accompanied by an inflammatory response. Regulated necrosis contributes to the loss of tubular cells in AKI.4,5 Cells dying from regulated necrosis (e.g., by ferroptosis) release inflammatory molecules and damage-associated molecular patterns that amplify the inflammatory response and activate a secondary inflammation-dependent cell death wave (e.g., necroptosis).5–7
Receptor-interacting protein kinase 3 (RIPK3) is a key necroptosis pathway protein, which may be involved in necroptosis-independent inflammatory processes.8 RIPK1 and RIPK3 may mediate inflammation in response to LPS in vivo and have been related to NLRP3 inflammasome activation in macrophages.9–11 Moreover, RIPK3 mediates the expression of tissue repair cytokines during experimental colitis.12 RIPK3 has been involved in kidney injury in sepsis, but different mechanisms have been suggested by various studies.13,14 RIPK3 may also drive experimental kidney fibrosis in long-term models.15,16
The cytokine TNF-like weak inducer of apoptosis (TWEAK) is a key mediator of kidney injury.17,18 During AKI, TWEAK promotes NF-κB activation, release of inflammatory mediators by kidney parenchymal cells, downregulation of anti-inflammatory and nephroprotective factors, modulation of epigenetic regulators, and increased fibroblast proliferation.17–20 The systemic administration of TWEAK elicits the kidney expression of inflammatory mediators and infiltration by inflammatory cells, but this is not associated with kidney cell death or AKI. In fact, TWEAK induction of cell death requires the simultaneous presence of other inflammatory cytokines (e.g., TNF and IFN-γ).21–23
We previously demonstrated that overall Ripk3 deficiency does not prevent early (48-hour) kidney failure in folic acid–induced AKI (FA-AKI), although it did decrease kidney Mcp-1 mRNA expression and infiltration by inflammatory cells.5 It remained unclear which RIPK3-expressing cells were responsible for the anti-inflammatory effect of overall Ripk3 deficiency and whether Ripk3 deficiency protected from kidney inflammation occurring in the absence of tubular cell death. We now report that RIPK3 from bone marrow (BM) cells is required to activate inflammatory responses in tubular cells and to promote early kidney inflammation in FA-AKI. Additionally, RIPK3 is required for kidney inflammation elicited by the systemic administration of TWEAK, a condition not associated with kidney cell death. In this regard, proinflammatory responses elicited by RIPK3 differed in tubular cells and inflammatory cells. These results identify BM RIPK3 as a therapeutic target in kidney inflammation.
Methods
Animal Models
All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory and were approved by the animal ethics committee of IIS-FJD (PROEX 070/17).
FA-AKI
Folic acid nephropathy is a classic model of kidney tubulointerstitial injury and inflammation characterized by acute renal failure, tubular cell death, interstitial leukocyte infiltration, and subsequent tubular regeneration21 that has been reported in humans.24 Wild-type (WT) C57BL/6 mice from Charles River (Wilmington, MA), Ripk3-knockout (KO) 12- to 14-week-old mice on the C57BL/6 background (provided by Kim Newton and Vishva Dixit, Genentech, San Francisco, CA), and Nlrp3-KO (provided by Pablo Pelegrin, Instituto Murciano de Investigación Biosanitaria, Murcia, Spain) were studied.25,26 Mice received a single intraperitoneal injection of 250 mg/kg folic acid (Sigma-Aldrich, St. Louis, MO) in 0.3 mol/L sodium bicarbonate or vehicle and were euthanized at 48 hours. Thus, we studied the acute phase of the disease, not the chronic fibrotic phase that usually requires repeated folic acid injections and weeks-long follow-up.
Systemic TWEAK Administration
Twenty-five milligrams per kilogram TWEAK (Millipore) or its vehicle (saline) was administered intraperitoneally to WT or Ripk3-KO mice, which were euthanized 24 hours later. The dose of TWEAK was calculated from prior in vitro dose-response experiments for an extracellular volume of 6.5 ml per mouse and was previously shown to induce biologic responses in the kidneys, including increased expression of inflammatory cytokines and inflammatory cell infiltration, in the absence of tubular cell death or decreased kidney function.21
Generation of BM Chimera Mice
WT and Ripk3-KO receptor mice were irradiated with 4.5 Gy on 2 consecutive days to deplete the autologous BM. BM was extracted from the femur and tibia of donor WT or Ripk3-KO mice, and 107 cells were transferred to irradiated receptor mice by intravenous injection. After 1 month, FA-AKI was induced. The efficacy of BM reconstitution was tested by PCR analysis on blood samples with the same primers used for tail Ripk3 genotyping.27
In all experimental models, plasma samples were collected at the time of euthanasia. Kidneys were perfused in situ with cold saline before removal. One kidney was snap frozen in liquid nitrogen for RNA and protein studies, and the other was fixed and paraffin embedded for histologic studies.28
RNA Extraction and Real-Time PCR
Total RNA was extracted by the TRI Reagent method (Invitrogen, Carlsbad, CA), and 1 µg RNA was reverse transcribed with the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Quantitative PCR was performed in a 7500 Real Time PCR System with the Prism 7000 System SDS software using predeveloped primers (Applied Biosystems), and RNA expression of different genes was corrected for GAPDH expression.21
Western Blot
Tissue samples were homogenized in lysis buffer (50 mM Tris⋅HCl, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 0.2% Triton X-100, 0.3% NP-40, 0.1 mM PMSF, and 1 µg/ml pepstatin A) and then separated by 10% SDS-PAGE under reducing conditions. For western blot of RIPK3, tissue samples were homogenized in T-PER Tissue Protein Extraction Reagent (Thermo Scientific). After electrophoresis, samples were transferred to PVDF membranes (Millipore); blocked with 5% skimmed milk in PBS/0.5% vol/vol Tween 20 for 1 hour; washed with PBS/Tween; and incubated with anti-RIPK3 (1:1000; ProSci), anticleaved caspase 1 (1:500; Adipogen), and anti-NLRP3 (1:500; Cell Signaling) antibodies diluted in 5% milk PBS/Tween. Blots were washed with PBS/Tween, incubated with appropriate horseradish peroxidase–conjugated secondary antibody (1:5000; GE Healthcare, Aylesbury, United Kingdom), developed with the chemiluminescence method (ECL; Fisher Scientific), and probed with mouse monoclonal anti–α-tubulin (1:10,000; Sigma-Aldrich) or anti-GAPDH (1:5000; Millipore) antibodies. Levels of expression were corrected for minor differences in loading.
Immunohistochemistry
Immunohistochemistry was performed as described previously in paraffin-embedded tissue sections that were 4-µm thick. Primary antibodies were rat polyclonal anti-F4/80 (1:50; BioRad, Hercules, CA), anti-CD3 (ready to use; DAKO, Glostrup, Denmark), rat monoclonal anti-Ly6G (1:100; BioXCell, Lebanon, NH), and rabbit polyclonal anti-p65 (1:80; Santa Cruz Biotechnology). Sections were counterstained with Carazzi hematoxylin. Negative controls included incubation with a nonspecific Ig of the same isotype as the primary antibody. The total number of positive cells was quantitated in 20 randomly chosen fields (×40) using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). Samples were examined in a blinded manner.
Immunofluorescence
RIPK3 immunofluorescence in murine and human tissue sections was performed using anti-RIPK3 antibody (1:200; ProSci) and the Tyramide SuperBoost Kits with Alexa Fluor Tyramides, as recommended by the manufacturer (Invitrogen). Nuclei were counterstained with DAPI (1:10,000; Sigma-Aldrich). Human kidney tissue was from two patients with AKI (woman/man, 55/52 years, and 8.7/8.1 mg/dl serum creatinine, respectively), and normal kidney from a nephrectomy specimen (man, 76 years, and 0.9 mg/dl serum creatinine) was obtained from the IIS-Fundacion Jimenez Diaz Biobank. Images were visualized by fluorescence microscopy (Nikon E600).
For leukocyte colocalization, RIPK3 staining was followed by incubation overnight at 4°C with anti-F4/80 (1:50; BioRad), anti-CD3 (1:200; Invitrogen-Thermo Scientific), rat monoclonal anti-Ly6G (1:100; BioXCell), or anti-CD68 (1:200; DAKO); slides were then washed with PBS and incubated with anti-rat Alexa 633–conjugated secondary antibodies (1:200; Invitrogen). To colocalize with different tubular cell types, RIPK3 staining was followed by incubation for 30 min with lotus tetragonolobus lectin for proximal tubules (1:100; Vector Laboratories) and with dolichos biflorus agglutinin lectin for distal tubules (1:100; Vector Laboratories). Nuclei were counterstained with DAPI.
For p65 immunofluorescence, macrophages were fixed in 4% paraformaldehyde, and Jurkat cells were collected onto slides by Rotofix 32 A Cytocentrifuge (Hettich, Tuttlingen, Germany) and fixed in methanol at −20°C. Then, cells were permeabilized in 0.2% Triton X-100/PBS, washed in PBS, and incubated with anti-p65 (1:80; Santa Cruz Biotechnology) followed by Alexa 488–conjugated secondary antibody (1:200; Invitrogen). Nuclei were counterstained with DAPI.
Images of colocalization and p65 were obtained using a confocal fluorescence microscope (LEICA TCS SP5 II) and the imaging software “LAS AF” (Leica).
Cells and Reagents
MCT cells are a proximal tubular cell line harvested originally from the renal cortex of SJL mice and have been extensively characterized.29 They were cultured in RPMI 1640 (GIBCO, Grand Island, NY), 10% heat-inactivated FBS, 2 mM glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin in 5% CO2 at 37°C.30 Jurkat cells and immortalized T lymphocytes were grown in RPMI-1640 medium plus 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin in 5% CO2 at 37°C.31
BM cells were collected from femur and tibia of WT and Ripk3-KO mice. After euthanasia with CO2, tibia and femur were isolated and flushed with RPMI 1640 10% FBS. BM cells were cultured for 4 days in RPMI 1640 supplemented with 10% FCS, 2 mM glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. To this media, 5 ng/ml M-CSF (Biolegend, San Diego, CA) was added for bone marrow–derived macrophage (BMDM) cultures, whereas 5 ng/ml GM-CSF (Biolegend) and 5 ng/ml IL-4 (R&D Systems) were added for bone marrow dendritic cell (BMDC) culture.
Primary tubular epithelial cells (TECs) were obtained from WT and Ripk3-KO mice. After euthanasia with CO2, the kidney cortex was decapsulated, dissected from the medulla, sliced, minced, and digested with 1.25 mg/ml collagenase type I (GIBCO) at 37°C for 20 minutes. The rubber end of a syringe plunger was used to filter the pellet through a 70-μm cell strainer over a 50-ml conical tube by washing with Medium 199 1× (GIBCO). After depleting red blood cells with erythrocyte lysis buffer (154 mM NH4Cl, 10 mM KHCO3, and 0.5M EDTA), the pellet was passed through a 40-µm cell strainer (FALCON) and resuspended in RPMI 1640 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, and ITS (Thermo Fisher); cells were seeded in gelatin type B–coated plates. After 7 days, cells were reseeded in the corresponding plates for experiments.
Subconfluent cells were rested in serum-free medium overnight, and 100 ng/ml TWEAK (Merck) was added. Necrostatin-1 (Nec1; 30 µM; Sigma-Aldrich) and GSK872 (1 µM; Biovision) were added 1 hour before TWEAK. The concentration is on the basis of prior studies.21
To test the effect of the BMDM secretome on MCT cells, WT and Ripk3-KO BMDMs were stimulated with 100 ng/ml TWEAK for 3 hours. Then, the media were replaced by fresh media, and cells were further incubated for 24 hours. Then, MCT cells were cultured in BMDM-conditioned media for 24 hours.
siRNA Transfection
Cells seeded in six-well plates were transfected 24 hours later with 20 nM scrambled siRNA or siRNA against RIPK3 (Invitrogen) using lipofectamine (Invitrogen; Thermo Fisher), following previously described methods.6 After 48 hours, transfected cells were stimulated with 100 ng/ml TWEAK.
ELISA
BMDMs were stimulated with 100 ng/ml TWEAK for 3 hours, and murine MCP-1, IL-6 (BD Pharmingen, San Diego, CA), and IL-1β (R&D Systems) in the supernatants were determined by ELISA.
NF-κB Luciferase Reporter Gene Assay
Cells plated in six-well plates for 24 hours were transfected with Lipofectamine (Invitrogen; Thermo Fisher) according to the manufacturer’s instructions. pNFκB-Luc (Stratagene, La Jolla, CA) and pRLTK vectors that contain the Renilla luciferase gene (Promega, Madison, WI) were used in a 10:1 ratio. The medium was replaced with RPMI without serum 4 hours post-transfection, and cells were stimulated with 100 ng/ml TWEAK for 3 hours. Luciferase activity was determined by a luciferase assay (Promega) in a luminometer (Berthold, Nashua, NH) and normalized to Renilla activity to control for differences in transfection efficiency.
Statistical Analyses
Results are expressed as means ± SDs. Differences between groups were evaluated using one-way ANOVA with Tukey post hoc tests using the Prism software (Graphpad 7.04). For pairs of samples, data were analyzed using t tests. A P value of 0.05 was considered statistically significant.
Results
RIPK3 Expression Is Increased in Tubular Cells and Leukocytes in AKI
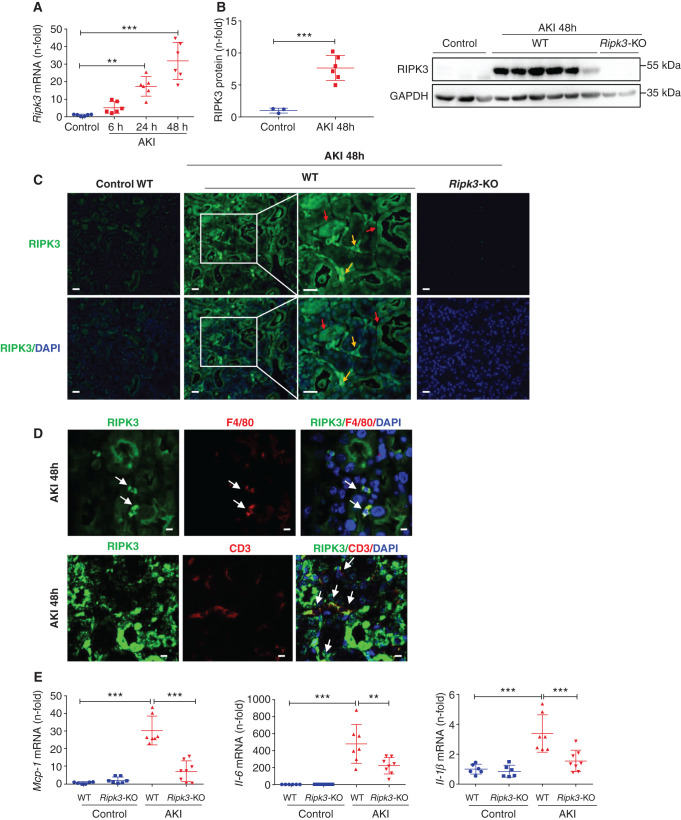
We have explored the mechanisms underlying the dissociation between inflammation and kidney failure in FA-AKI in Ripk3-deficient mice. First, we explored RIPK3 expression during FA-AKI and identified cells expressing RIPK3. Whole-kidney RIPK3 mRNA and protein expression increased from very early during FA-AKI (Figure 1, A and B). Thus, Ripk3 mRNA was already increased 6 hours following folic acid administration. Immunofluorescence identified RIPK3 expression during FA-AKI in both tubular and interstitial cells (Figure 1C). The macrophage marker F4/80 and T lymphocyte marker CD3 colocalized with RIPK3 in interstitial cells during AKI, whereas the neutrophil marker Ly6G did not (Figure 1D, Supplemental Figure 1A). Moreover, RIPK3 colocalized with proximal (lotus tetragonolobus lectin) and distal (dolichos biflorus agglutinin) tubular cell markers (Supplemental Figure 1B). Increased tubular and interstitial cell RIPK3 expression was also observed in human AKI, where it colocalized with CD68-positive macrophages (Supplemental Figure 2). Thus, macrophages, T lymphocytes, and tubular cells express RIPK3 during AKI.
Figure 1.
RIPK3 deficiency prevented the increase in kidney inflammatory mediators in FA-AKI. (A) Time course of kidney RIPK3 mRNA expression in AKI. Mean ± SD of six animals per group. **P<0.01; ***P<0.001. (B) Quantification and representative western blot of RIPK3 expression in AKI at 48 hours. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. Mean ± SD of three to six animals per group. ***P<0.001. (C) RIPK3 immunofluorescence in AKI at 48 hours. Increased RIPK3 expression is observed in both tubules (red arrows) and interstitial infiltrating cells (yellow arrows). Representative images are shown. Magnification, ×200; ×400 in detail. Scale bars, 50 μm. Cell nuclei were counterstained with DAPI. (D) Colocalization of RIPK3 with the macrophage marker F4/80 and the lymphocyte marker CD3 in AKI at 48 hours. Confocal microscopy. Magnification, ×800. Scale bars, 5 μm. (E) Kidney Mcp-1, IL-6, and Il-1β mRNA levels are lower in Ripk3-KO than in WT mice in FA-AKI at 48 hours. Mean ± SD of six to eight animals per group. **P<0.01; ***P<0.001.
Anti-Inflammatory Effect of Ripk3 Deficiency in FA-AKI
Next, we further characterized the inflammatory response in WT and Ripk3-KO mice during AKI. In addition to Mcp-1 mRNA,5 the kidney expression of other proinflammatory molecules, such as Il-6 and Il-1β, was also increased in WT mice with FA-AKI and was lower in Ripk3-deficient mice with FA-AKI (Figure 1E). Thus, the anti-inflammatory effect of Ripk3 deficiency is not limited to a specific inflammatory mediator, and overall Ripk3 deficiency results in decreased proinflammatory responses during AKI.
RIPK3 from BM Cells Is Key for Kidney Inflammation in FA-AKI
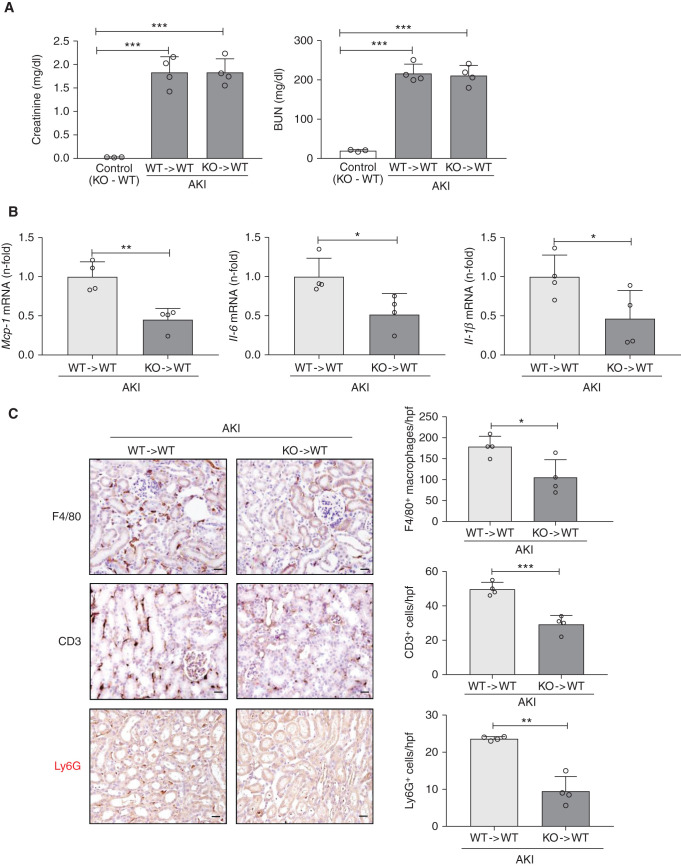
Given that kidney interstitial macrophages and T lymphocytes expressed RIPK3 during FA-AKI, we analyzed the role of BM-derived cells RIPK3 in BM chimera mice. WT mice were lethally irradiated and reconstituted with BM from either Ripk3-KO (KO → WT) or WT donors (WT → WT). Following induction of FA-AKI and consistent with prior results obtained in constitutive Ripk3-deficient mice,5 both chimera developed kidney failure of similar severity (Figure 2A). However, BM Ripk3 deficiency decreased the kidney expression of proinflammatory cytokines, such as Mcp-1, Il-6, and Il-1β (Figure 2B). Moreover, BM Ripk3 deficiency decreased kidney leukocyte infiltration (F4/80+ macrophages, CD3+ lymphocytes, and Ly6G+ neutrophils) (Figure 2C). Additionally, irradiated Ripk3-KO mice were reconstituted with Ripk3-KO (KO → KO) or WT (WT → KO) BM. After FA-AKI induction, both chimeras developed kidney failure, but reconstitution with WT BM increased the expression of proinflammatory cytokines and the kidney leukocyte infiltration (Supplemental Figure 3). These results suggest that RIPK3 from BM-derived cells plays a key proinflammatory role in FA-AKI, although we cannot discard a role of tubular cells because WT → KO mice did not differ from KO → KO in Mcp-1 expression (Supplemental Figure 3B) and inflammatory cell infiltrates were overall lower in WT → KO mice than in WT → WT mice (Figure 2C, Supplemental Figure 3C), despite similar severity of kidney failure (Figure 2A, Supplemental Figure 3A).
Figure 2.
RIPK3 deficiency in BM-derived cells reduces kidney inflammation during FA-AKI. (A) Renal function was assessed by plasma creatinine and BUN levels at 48 hours after AKI induction in BM chimera mice. WT mice with WT BM are represented as WT → WT, and those with Ripk3-KO BM are represented as KO → WT. (B) Kidney Mcp-1, IL-6, and Il-1β mRNA levels assessed by RT-PCR at 48 hours of AKI in BM chimera mice. (A and B) Mean ± SD of four mice per group. (C) Kidney interstitial infiltration by F4/80-positive interstitial macrophages, CD3+ T cells, and Ly6G+ cells in FA-AKI at 48 hours in BM chimera mice. Representative images and quantification. Magnification, ×200. Scale bars, 50 μm. *P<0.05; **P<0.01; ***P<0.001.
The NLRP3 Inflammasome Is Not Involved in RIPK3-Induced Kidney Inflammation in AKI
RIPK3 can promote cell death and/or inflammation through inflammasome activation.10,32 In this sense, kidney NLRP3 expression and caspase 1 cleavage were increased in FA-AKI, and both were reduced in Ripk3-KO mice, suggesting that RIPK3 may also activate the NLRP3 inflammasome in FA-AKI (Supplemental Figure 4A). To study the role of the NLRP3 inflammasome, FA-AKI was induced in Nlrp3-deficient mice. Like Ripk3-deficient mice, Nlrp3-deficient mice were not protected from AKI as assessed by serum creatinine and BUN (Supplemental Figure 4B). Contrary to Ripk3-deficient mice, the expression of inflammatory molecules was not reduced in Nlrp3-deficient mice with FA-AKI (Supplemental Figure 4C). Western blot of cleaved caspase 1 confirmed that Nlrp3 deficiency efficiently blocked NLRP3 inflammasome activation in FA-AKI (Supplemental Figure 4D). Thus, despite the evidence for decreased NLRP3 inflammasome activation in Ripk3-deficient mice, decreased NLRP3 inflammasome activation did not explain the decreased kidney inflammation observed in Ripk3-deficient mice.
BM-Derived RIPK3 Promotes NF-κB Activation in Kidney Tubular Cells
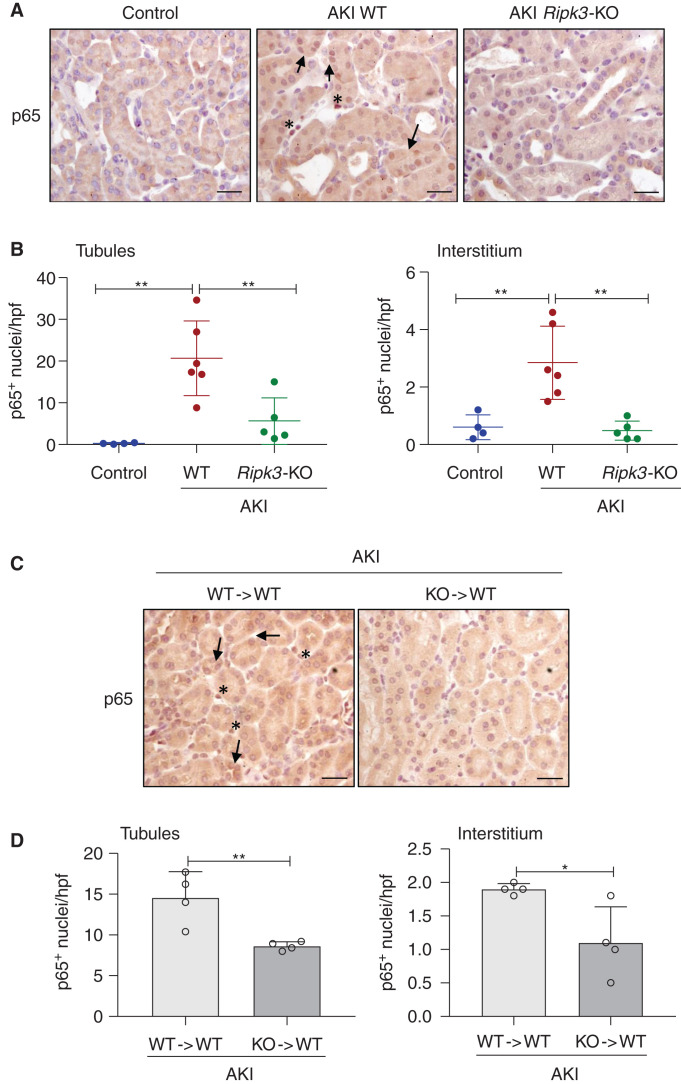
NF-κB is a master regulator of inflammatory gene expression.33 Moreover, RIPK3 may induce NF-κB activation.9,12 NF-κB activation was assessed by immunohistochemistry of nuclear translocation of the p65 NF-κB subunit. Kidney p65 nuclear translocation was decreased in overall Ripk3-deficient mice compared with WT mice with AKI (Figure 3A). Indeed, nuclear p65 staining was reduced in both tubular and interstitial cells, supporting a role of inflammatory cells in RIPK3-mediated inflammation (Figure 3B). In this regard, p65 nuclear translocation was also reduced in tubular cells and in interstitial cells in chimera mice with Ripk3-KO BM compared with mice with WT BM (Figure 3, C and D). Thus, decreased NF-κB activation may be one of the mechanisms of decreased kidney inflammation in Ripk3-deficient mice. Consistent with this view, BM Ripk3 deficiency resulted in decreased NF-κB activation in WT tubular cells, suggesting crosstalk between different cell types.
Figure 3.
RIPK3 mediates NF-κB activation in FA-AKI. (A and C) Kidney NF-κB p65 immunohistochemistry 48 hours following AKI induction comparing (A) WT mice with whole Ripk3-KO mice and (D) BM chimera mice. WT mice with WT BM are represented as WT → WT, and those with Ripk3-KO BM are represented as KO → WT. Nuclear NF-κB p65 is observed in renal tubules (arrows) and in interstitial cells (asterisks) in AKI mice. Original magnification, ×400. Scale bars, 50 µm. (B and D) Quantification of NF-κB p65 nuclear translocation in tubules and in interstitial cells. Mean ± SD of four to six animals pre group. *P<0.05; **P<0.01.
RIPK3 Mediates TWEAK-Induced Kidney Inflammation
Next, to address whether RIPK3-mediated kidney inflammation requires cell death, we explored the role of RIPK3 in TWEAK-induced kidney inflammation. A single TWEAK injection in mice induces a kidney inflammatory response,21 but TWEAK administration did not modify kidney function at 24 hours in either WT or Ripk3-deficient mice (Figure 4A). However, TWEAK increased the kidney expression of RIPK3 and of proinflammatory factors, such as Mcp-1, Il-6, and Il-1β, and this increase was milder in Ripk3-deficient mice than in WT mice (Figure 4, B and C). Moreover, Ripk3 deficiency decreased TWEAK-induced macrophage infiltration (Figure 4D). These results indicate that RIPK3 may mediate kidney inflammation even in the absence of kidney cell death or AKI and specifically, that RIPK3 mediates kidney inflammation induced by TWEAK, a key proinflammatory cytokine that amplifies AKI.
Figure 4.
RIPK3 mediates TWEAK-induced kidney inflammation in healthy mice. (A) Renal function was assessed by plasma creatinine and BUN levels at 24 hours following TWEAK injection. Mean ± SD of four to five animals per group. (B) Kidney Ripk3 mRNA expression assessed by RT-PCR at 24 hours after TWEAK injection. (C) Kidney Mcp-1, Il-6, and Il-1β mRNA levels are lower in Ripk3-KO than in WT mice injected with TWEAK. (D) Interstitial infiltration of F4/80-positive interstitial macrophage. Image representative. Magnification, ×400. Scale bars, 50 µm. (B–D) Mean ± SD of four to ten animals per group. *P<0.05; **P<0.01; ***P<0.001.
TWEAK-Induced IL-6 Expression in Tubular Cells Is Mediated by RIPK3
Next, we analyzed the specific role of RIPK3 in TWEAK-induced proinflammatory responses in kidney tubular cells. First, we observed that TWEAK increases Ripk3 mRNA expression in cultured proximal tubular MCT cells and in primary TECs (Supplemental Figure 5A). In MCT cells, GSK´872, a chemical inhibitor of RIPK3 kinase activity, reduced TWEAK-induced Il-6 overexpression and IL-6 protein release (Figure 5, A and B), whereas Mcp-1 expression was unchanged (Figure 5A). Furthermore, similar results were observed in primary TEC from WT and Ripk3-deficient mice; Il-6 was the proinflammatory factor induced by TWEAK that depends on RIPK3 activation in tubular cells (Figure 5C).
Figure 5.
RIPK3 inhibition or deficiency reduces TWEAK-induced IL-6 expression in cultured tubular cells. (A and B) MCT cells were pretreated with RIPK3 inhibitor GSK´872 before stimulation with 100 ng/ml TWEAK for 3 hours. (A) Mcp-1 and Il-6 mRNA was assessed by RT-PCR. (B) IL-6 released to cell supernatants was measured by ELISA. (C) Mcp-1 and Il-6 mRNA in WT or Ripk3-KO primary tubular cells (TECs) stimulated with 100 ng/ml TWEAK for 3 and 6 hours. (D) NF-κB transcriptional activity was measured by luciferase reporter gene assay in TECs stimulated with 100 ng/ml TWEAK for 3 hours. Luciferase values were normalized to Renilla activity. (A–D) Mean ± SD of three independent experiments. *P<0.05; **P<0.01; ***P<0.001; ##P<0.01 versus TWEAK alone.
We next explored factors upstream or downstream of RIPK3 potentially contributing to TWEAK-induced, RIPK3-mediated Il-6 upregulation. Because the RIPK1 kinase is upstream of RIPK3, we explored RIPK1 involvement in TWEAK-induced Il-6 expression. However, the RIPK1 inhibitor Nec1 did not reduce Il-6 expression in TWEAK-stimulated MCT cells (Supplemental Figure 6A). Next, we focused on events downstream of RIPK3. Thus, we explored whether RIPK3 modulates TWEAK-induced NF-κB activation in tubular cells. For this, we took advantage of a luciferase reporter gene assay in WT and Ripk3-deficient TECs. TWEAK-induced NF-κB activation was not modulated by Ripk3 deficiency in tubular cells (Figure 5D), as expected by the observation that expression of Mcp-1, a canonical NF-κB target, was not responsive to Ripk3 deficiency (Figure 5C). In this regard, TWEAK-induced upregulation of MCP-1 expression has been repeatedly shown to be NF-κB dependent.21,34,35
These findings suggest that the kinase activity of RIPK3 is necessary to induce expression of IL-6 in tubular cells in response to TWEAK but not to induce the expression of additional proinflammatory factors that were RIPK3 dependent in vivo. Thus, two different RIPK3 proinflammatory activities were identified, one directly exerted within tubular cells in response to TWEAK, leading to NF-κB–independent IL-6 secretion, and a second activity extrinsic to tubular cells, leading to the expression of additional chemokines in the kidneys in vivo.
RIPK3 Mediates TWEAK-Induced Inflammation in Both BMDMs and BMDCs
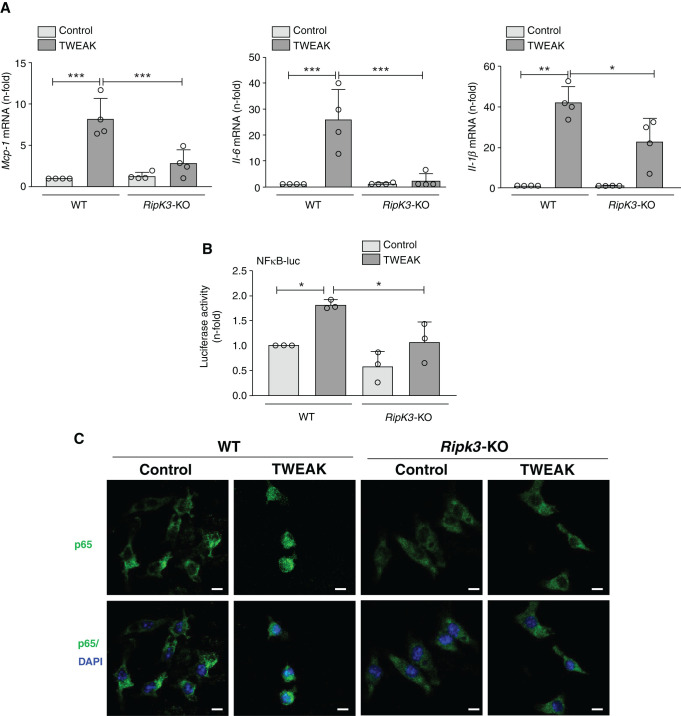
RIPK3 targeting in cultured tubular cells did not prevent the expression of many proinflammatory mediators that were induced by TWEAK and responsive to Ripk3 deficiency in vivo. Additionally, kidney inflammation in AKI was partially dependent on RIPK3 expression by BM cells. Thus, we explored the role of RIPK3 in TWEAK-induced inflammatory responses in BMDM and BMDC.
As in tubular cells, TWEAK also increased RIPK3 expression in BMDM (Supplemental Figure 5B). However, the pattern of the proinflammatory response to TWEAK differed in WT tubular cells (in which the increase in Mcp-1 expression was severalfold higher than the increase in Il-6 expression at the time points studied) and WT BMDMs (in which the increase in Mcp-1 expression was severalfold lower than the increase in Il-6 expression) (Figures 5A and 6A). Additionally and different from tubular cells, TWEAK-stimulated BMDMs from Ripk3-deficient mice (BMDM-KO) had a milder inflammatory response to TWEAK than those from WT mice (BMDM-WT) that involved decreased expression of all mediators explored (Mcp-1, Il-6, and Il-1β), although like in tubular cells, the most dramatic difference between BMDM-WT and BMDM-KO corresponded to IL-6 (Figure 6A).
Figure 6.
RIPK3 mediates TWEAK-induced cytokine expression in BMDMs. (A) WT and Ripk3-KO BMDMs were stimulated with 100 ng/ml TWEAK for 3 hours, and gene expression of Mcp-1, Il-6, and Il-1β was assessed by RT-PCR. (B) NF-κB transcriptional activity was measured by the luciferase reporter gene assay in BMDM stimulated with 100 ng/ml TWEAK for 3 hours. Luciferase values were normalized to Renilla activity. (A and B) Mean ± SD of three to four independent experiments. *P<0.05; **P<0.01; ***P<0.001. (C) Immunofluorescence of NF-κB p65 in WT and Ripk3-KO BMDM stimulated with 100 ng/ml TWEAK for 3 hours. Cell nuclei were counterstained with DAPI. Confocal microscopy. Magnification, ×800. Scale bars, 5 µm.
The proinflammatory effect of TWEAK was milder in BMDCs, although Ripk3 deficiency also reduced Il-6 and IL-1β expression (Supplemental Figure 7A). In these cells, TWEAK did not upregulate Mcp-1.
Next, we explored potential upstream and downstream contributors to the proinflammatory effect of RIPK3 in BMDM. As in tubular cells, RIPK1 was not involved in TWEAK-induced inflammation in BMDM because Nec1 did not reduce cytokine expression (Supplemental Figure 6B). Next, we tested the role of NF-κB in RIPK3-mediated TWEAK-induced proinflammatory responses in BMDM. Luciferase gene reporter assays showed that TWEAK promotes NF-κB transcriptional activity in BMDM, and contrary to findings in tubular cells, this is dependent on RIPK3 (Figure 6B). NF-κB p65 immunofluorescence confirmed TWEAK induction of p65 nuclear translocation in BMDM-WT but not in BMDM-KO (Figure 6C).
RIPK3 Mediates TWEAK-Induced Inflammation in Jurkat Cells
As T lymphocytes also expressed RIPK3 in AKI (Figure 1D), we explored the role of RIPK3 in TWEAK-induced inflammatory responses in the Jurkat T lymphocyte cell line. TWEAK stimulation for 3 hours induced the expression of Mcp-1 and Il-6 and promoted nuclear NF-κB p65 translocation in Jurkat cells. As in BMDM, the increase in Mcp-1 expression was severalfold lower than the increase in Il-6 expression. Additionally, the RIPK3 inhibitor GSK´872 prevented TWEAK-induced inflammation in Jurkat cells, suggesting that T lymphocytes may also contribute to RIPK3-mediated inflammation (Supplemental Figure 7, B and C).
The TWEAK-Stimulated BMDM Secretome Promotes Tubular Cell Proinflammatory Responses
Because intracellular RIPK3 did not account for the increased tubular cell expression of diverse chemokines in response to TWEAK, we next analyzed whether TWEAK-stimulated BMDMs promote proinflammatory responses in tubular cells that may contribute to amplify kidney inflammation. TWEAK stimulation resulted in BMDM secretion of IL-6 and MCP-1 (Figure 7A). However, TWEAK failed to induce IL-1β secretion, and this is in accordance with results in vivo because TWEAK did not induce caspase 1 processing in vivo, a step prior to IL-1β release (Supplemental Figure 8). To assess the effect of the BMDMs secretome on tubular cells, we stimulated MCT cells with conditioned medium from BMDM-WT or BMDM-KO stimulated or not with TWEAK. Conditioned medium from TWEAK-stimulated BMDM-WT induced the expression of inflammatory cytokines in MCT cells, whereas this was not observed for conditioned media from BMDM-KO (Figure 7B). These results support the concept that RIPK3 expression by BM-derived cells contributes to TWEAK-induced inflammation and potentially, to AKI by recruiting and amplifying inflammatory responses in tubular cells.
Figure 7.
RIPK3 is involved in macrophages-induced inflammatory responses in tubular cells. (A) MCP-1, IL-6, and IL-1β levels were measured by ELISA in supernatants of BMDM stimulated with 100 ng/ml TWEAK for 3 hours. (B) MCT cells were cultured with conditioned medium from BMDM, which had been stimulated with 100 ng/ml TWEAK for 3 hours, before a change of medium and further incubation without TWEAK for 24 hours. Tubular cell Mcp-1 and Il-6 mRNA was measured by RT-PCR. Mean ± SD of four independent experiments. *P<0.05; **P<0.01; ***P<0.001.
Discussion
The main finding of this study is that during AKI, early kidney inflammation mediated by RIPK3 is dependent on BM-derived cells, potentially including macrophage, dendritic cells, and T lymphocytes. RIPK3 from macrophages promotes NF-κB activation and NF-κB–dependent release of proinflammatory cytokines that, in turn, promote a macrophage-tubular crosstalk that recruits inflammatory responses from tubular cells (Figure 8A). Additionally, tubular cell RIPK3 has direct NF-κB–independent stimulatory effects on IL-6 secretion. This proinflammatory role of RIPK3 in AKI seems to be independent of necroptosis and of NLRP3. In this regard, RIPK3-dependent kidney inflammation was also observed following the systemic administration of TWEAK, a kidney stressor that does not cause tubular cell death. However, RIPK3-induced kidney inflammation may likely contribute to the second wave of cell death mediated by necroptosis that takes place during the amplification phase of kidney injury at later time points5,6 (Figure 8B).
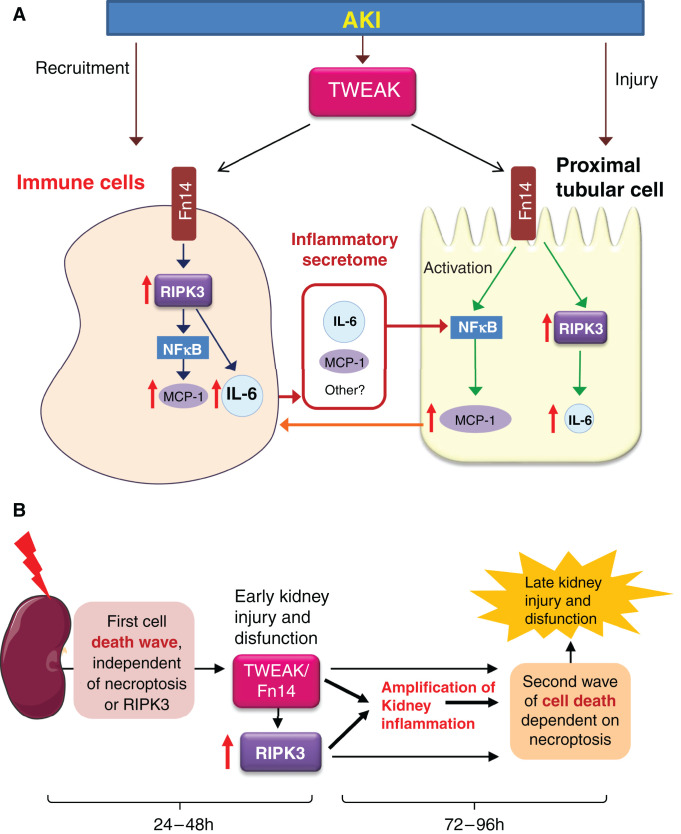
Figure 8.
RIPK3 role in kidney inflammation with or without associated tubular cell death. (A) AKI is characterized by tubular cell injury and death; upregulation of TWEAK and above all, its receptor Fn14; and macrophage recruitment to the kidneys. In vivo studies in WT and Ripk3-deficient mice disclosed a proinflammatory role of RIPK3, which was independent from its role in cell death in early AKI stages. Thus, Ripk3-deficient mice with AKI displayed milder kidney inflammation in the presence of a similar severity of kidney failure and tubular cell death. Moreover, the systemic administration of TWEAK, a key cytokine in AKI that by itself induced kidney inflammation but not tubular cell death or AKI, also induced RIPK3-dependent kidney inflammation. TWEAK had been shown to promote the amplification of AKI inflammation. Chimeric mice studies pinpointed a key role for BM-derived cells in the proinflammatory effect of RIPK3 in the kidney. Moreover, cell cultures identified macrophages, dendritic cells, and T lymphocytes as key BM-derived cells. Indeed, the macrophage secretome in response to TWEAK induced the release of proinflammatory mediators from tubular cells (burgundy arrows) in an RIPK3-dependent manner. By contrast, RIPK3 deficiency or inhibition only had a marginal effect on the inflammatory response to TWEAK in cultured tubular cells, which was mainly driven by RIPK3-independent NF-κB activation. Thus, only TWEAK-induced IL-6 secretion was RIPK3 dependent in tubular cells, but this was not the key proinflammatory response in tubular cells; in WT tubular cells, the increase in MCP-1 expression was severalfold higher than the increase in IL-6 expression, whereas in WT BMDM, the increase in MCP-1 expression was severalfold lower than the increase in IL-6 expression. In any case, secretion of MCP-1 and other chemokines by tubular cells would attract macrophages (orange arrow) to close the vicious circle of inflammation. The NLRP3 inflammasome (not shown) did not appear to be a key contributor to RIPK3-induced kidney inflammation. (B) Conceptual depiction of two sequential waves of cell death in AKI and the contribution of RIPK3 to inflammation in the early stages (up to 48 hours) and to tubular cell death in more advanced stages (up to 96 hours). AKI results in tubular cell death, local release of multiple inflammatory mediators including TWEAK, and recruitment of inflammatory cells. By contrast, administration of TWEAK alone triggers a local inflammatory response without cell death, as at the time of TWEAK binding to Fn14, there is no local inflammatory milieu. The initial wave of cell death is RIPK3 independent in AKI, and in fact, it is driven by ferroptosis, as previously reported.5 We now report on the TWEAK-RIPK3 interaction to amplify kidney inflammation. Kidney inflammation results in a second wave of necroptotic cell death that magnifies AKI and is evident at 96 hours, as previously reported.6
AKI is characterized by tubular cell death and local inflammation. Indeed, tubular cell death is a hallmark of human AKI.36 A sequential contribution of diverse forms of cell death to FA-AKI was recently characterized.5,6 Thus, the first wave of tubular cell death (first 48 hours) is mediated by ferroptosis and triggers an inflammatory response, whereas necroptosis and apoptosis do not contribute to FA-AKI in this early time point and are recruited later in response to inflammatory mediators. Despite the absence of necroptosis involvement, Ripk3-deficient mice displayed milder inflammation than WT mice at this early time point (48 hours).5 These findings are in line with the recently published inflammatory role of RIPK3 in experimental colitis and aortic abdominal aneurysm.9,12,37 Although RIPK3 may be involved in kidney cell death in murine sepsis13,14 and in kidney fibrosis,15,16,38 the role and regulation of RIPK3 in early inflammation during AKI and following systemic TWEAK-induced inflammation had not been explored.
TWEAK is a cytokine of the TNF superfamily that activates Fn14 and plays a key role in kidney injury. In fact, TWEAK induces kidney inflammation and cell death in an inflammatory environment and different strategies of TWEAK or Fn14 targeting protected from different preclinical models of AKI, including FA-AKI.17 Now, we characterized the proinflammatory role of RIPK3 in AKI independent from necroptosis because RIPK3-dependent inflammation was not prevented by the necroptosis inhibitor Nec1. In addition, Ripk3 deficiency also reduced kidney inflammation following TWEAK administration, a condition not associated with tubular cell death or AKI, thus confirming that RIPK3 mediates inflammation independent of necroptosis.22,23 Because TWEAK is a key amplifier of AKI, these findings may explain Ripk3 deficiency protection from inflammation-dependent AKI amplification as at later FA-AKI time points (96 hours), Ripk3-deficient mice showed better preserved kidney function and less cell death.6 The protection afforded by Nec1 at this later time points suggests at least a partial role for decreased necroptosis in the protection offered by Ripk3 deficiency at later time points.6 In FA-AKI, kidney function recovers by day 7, but at this time point, there is still residual histologic injury that may progress to fibrosis and CKD. In this regard, our data suggest that the beneficial effect of RIPK3 inhibition over kidney fibrosis at day 2816 may result from the anti-inflammatory effect of RIPK3 blockade at early time points, as in these studies, RIPK3 blockade was started 24 hours after folic acid administration and maintained for 28 days.
Despite increased RIPK3 expression in tubular cells and myeloid cells during AKI, suggesting that both cell types could contribute to RIPK3-induced kidney inflammation, myeloid cell Ripk3 deficiency was enough to decrease inflammation after AKI. However, reduction of inflammation did not protect from kidney disfunction at 48 hours. Moreover, in TWEAK-stimulated cultured tubular and myeloid cells, RIPK3 targeting had an anti-inflammatory effect, although in tubular cells, NF-κB was activated in an RIPK3-independent manner and drove the expression of inflammatory factors independently from RIPK3. In this regard, we identified two different RIPK3 proinflammatory pathways, one in tubular cells leading to IL-6 secretion and another in myeloid cells leading to the expression of additional multiple NF-κB–dependent chemokines that recruit inflammatory responses in tubular cells. The key role of macrophages and dendritic cells in RIPK3-mediated inflammation is consistent with data in liver fibrosis, lung injury, arthritis, and colitis.10,12,32,39–41
The mechanisms of RIPK3-induced kidney inflammation are poorly characterized. RIPK3 may activate NF-κB and the inflammasome.10–12,32,33,37,39 However, the role of inflammasomes in kidney diseases is unclear.42,43 In this regard, Ripk3 deficiency prevented NF-κB activation in tubular and interstitial cells in AKI at 48 hours, and this was partially mediated by RIPK3 from myeloid cells. Cultured cell experiments showed that in BMDM, NF-κB and MCP-1 expressions are downstream of RIPK3 activation, whereas in tubular cells, NF-κB activation and MCP-1 expression are not influenced by RIPK3. Thus, RIPK3 may not directly activate NF-κB in tubular cells in vivo; rather, tubular cell NF-κB activation could be a consequence of RIPK3-induced cytokine production by macrophages. In this regard, conditioned medium from TWEAK-stimulated macrophages promoted inflammatory factor production in tubular cells. This was abrogated by Ripk3 deficiency in macrophages. Regarding inflammasomes, our results suggest that RIPK3 mediates inflammasome activation in AKI because NLRP3 and cleaved caspase 1 levels were reduced in Ripk3-deficient mice. Similar results were observed in the fibrotic stage of FA-AKI at 28 days and in diabetic nephropathy where kidney NLRP3 expression was lower in Ripk3-KO, although these studies did not perform a functional analysis of inflammasome activation.15,16 However, we now show that NLRP3 deficiency neither prevents kidney injury nor inflammation at 48 hours after AKI induction, suggesting that RIPK3-induced kidney inflammation is inflammasome independent.
Ripk3 deficiency was previously reported to prevent progression of AKI at later time point6 The current study focused on early events in AKI and does not address the mechanisms of later protection, i.e. it does not address whether protection from AKI progression solely depends on decreased inflammation at early stages or whether it requires direct inhibition of necroptosis pathways within tubular cells. Inhibition of necroptosis through interference of molecular mechanisms within tubular cells would not be dependent on macrophage Ripk3 deficiency.
In conclusion, we have characterized the cellular and molecular mechanisms of the proinflammatory role of RIPK3 in FA-AKI and in non-AKI kidney inflammation triggered by TWEAK. In FA-AKI, Ripk3 deficiency reduced kidney inflammation but did not prevent early kidney injury or cell death. The key role of RIPK3 expressed by BM-derived leukocytes, like macrophages and T cells, in kidney inflammation suggests that the development of drugs that specifically target RIPK3 in myeloid cells may retain the anti-inflammatory effect of Ripk3 deficiency in kidney disease but display milder adverse effects than systemic RIPK3 inhibition.
Disclosures
S. Carrasco reports consultancy agreements with Instituto de Investigacion Sanitaria Fundacion Jimenez Diaz; ownership interest in Instituto de Investigacion Sanitaria Fundacion Jimenez Diaz; research funding from Instituto de Investigacion Sanitaria Fundacion Jimenez Diaz; and honoraria from Instituto de Investigacion Sanitaria Fundacion Jimenez Diaz. J. Guerrero-Mauvecin reports consultancy agreements with Hospital Fundación Jiménez Díaz and Universidad Autónoma de Madrid; ownership interest in Hospital Fundación Jiménez Díaz and Universidad Autónoma de Madrid; research funding from Universidad Autónoma de Madrid; honoraria from Hospital Fundación Jiménez Díaz and Universidad Autónoma de Madrid; and other interests/relationships with Hospital Fundación Jiménez Díaz and Universidad Autónoma de Madrid. A. Ortiz reports consultancy agreements with Genzyme Sanofi and Retrophin; research funding from AstraZeneca, Mundipharma, and Sanofi Genzyme; honoraria from Advicciene, Alexion, Amgen, Amicus, Astellas, AstraZeneca, Bayer, Chiesi, Fresenius Medical Care, Idorsia, Kyowa Kirin, Menarini, Otsuka, Sanofi Genzyme, and Vifor Fresenius Medical Care Renal Pharma; scientific advisor or membership with Clinical Kidney Journal (Editor-in-Chief), the Dutch Kidney Foundation Scientific Advisory Board, board of directors of the IIS-Fundacion Jimenez Diaz UAM, JASN (editorial board), Journal of Nephrology (editorial board), PDI (editorial board), SOMANE and ERA Councils, and the Spanish Society of Nephrology (member); and speakers bureau for (received honoraria for speaker engagements) Advicciene, Alexion, Amgen, Amicus, Astellas, AstraZeneca, Bayer, Chiesi, Fresenius Medical Care, Idorsia, Kyowa Kirin, Menarini, Otsuka, Sanofi Genzyme, and Vifor Fresenius Medical Care Renal Pharma. M.D. Sanchez-Niño reports honoraria from Sanofi-Genzyme. All remaining authors have nothing to disclose.
Funding
This research was funded by Instituto de Salud Carlos III (ISCIII)-Fondo de Investigacion Sanitaria (FIS)-Fondo Europeo de Desarrollo Regional FEDER grants PI19/00588 and PI19/00815 and by Sociedad Española de Nefrología grant 9749/004. Salary support was from ISCIII Miguel Servet (to N. Mendez-Barbero), ISCIII predoctoral contract of Training in Investigation in Health PFIS (to A.M. Lopez-Diaz), Ministerio de Ciencia, Innovación y Universidades (to J. Guerrero-Mauvecin), Red de Investigacion Renal (REDINREN) grant RD016/0009 (to M. Fontecha-Barriuso), and Ramon y Cajal program (to M.D. Sanchez-Niño and A.B. Sanz). We acknowledge Comunidad de Madrid en Biomedicina grant B2017/BMD-3686 CIFRA2-CM; FRIAT; IIS-Fundacion Jimenez Diaz Biobank grant PT17/0015/0006; and Instituto de Salud Carlos III FIS/FEDER grants PI17/00257, PI17/00119, PI20/00140, PI18/01366, PI20/00639, PI21/01126, DTS18/00032, European Research Area-PerMed-JTC2018 KIDNEY ATTACK AC18/00064 and Personalized treatment in IgA Nephropathy (PERSTIGAN) AC18/00071 and ISCIII-Redes temáticas de investigación cooperativa en salud (RETICS) REDinREN RD016/0009.
Supplementary Material
Acknowledgments
We acknowledge Dr. Elena Almarza Novoa, Ms. Edilia Ines de Almeida, and Mr. Jesus Martinez Palacio for technical support with mice irradiation (Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas, Madrid, Spain). We thank Miss Irene Cuevas, Miss Marta Ricote, Mr. Carlos Carnero, and Mr. Carlos Castilla for technical support with animal models (animal facility, Instituto de Investigacion Sanitaria-Fundacion Jimenez Diaz).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021030383/-/DCSupplemental.
Supplemental Figure 1. Colocalization of RIPK3 with neutrophils and tubular segments in mice.
Supplemental Figure 2. RIPK3 immunostaining in human AKI and control kidney.
Supplemental Figure 3. Transfer of WT bone marrow promotes inflammation in Ripk3-KO mice with AKI.
Supplemental Figure 4. NLRP3 is not involved in RIPK3-induced kidney inflammation at 48 hours of AKI.
Supplemental Figure 5. Ripk3 expression is upregulated by TWEAK in cultured tubular cells and in BMDM.
Supplemental Figure 6. RIPK1 inhibition with Nec1 does not prevent TWEAK-induced cytokine gene expression in MCT cells or BMDMs.
Supplemental Figure 7. RIPK3 partially mediates TWEAK-induced cytokine expression in BMDCs and in Jurkat cells.
Supplemental Figure 8. TWEAK injection does not increase cleaved caspase 1 in healthy kidneys.
Supplemental Figure 9. Uncropped gel scans merged with molecular weight images for all presented Western blots. (A) Corresponds to Figure 1C. (B–D) Corresponds to Supplemental Figure 4.
Supplemental Table 1. Clinical characteristics of patients.
References
- 1.Martin-Cleary C, Molinero-Casares LM, Ortiz A, Arce-Obieta JM: Development and internal validation of a prediction model for hospital-acquired acute kidney injury. Clin Kidney J 14: 309–316, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellomo R, Kellum JA, Ronco C: Acute kidney injury. Lancet 380: 756–766, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Siew ED, Parr SK, Abdel-Kader K, Eden SK, Peterson JF, Bansal N, et al. : Predictors of recurrent AKI. J Am Soc Nephrol 27: 1190–1200, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, et al. : Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A 111: 16836–16841, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin-Sanchez D, Ruiz-Andres O, Poveda J, Carrasco S, Cannata-Ortiz P, Sanchez-Niño MD, et al. : Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. J Am Soc Nephrol 28: 218–229, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin-Sanchez D, Fontecha-Barriuso M, Carrasco S, Sanchez-Niño MD, Mässenhausen AV, Linkermann A, et al. : TWEAK and RIPK1 mediate a second wave of cell death during AKI. Proc Natl Acad Sci U S A 115: 4182–4187, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linkermann A, Stockwell BR, Krautwald S, Anders HJ: Regulated cell death and inflammation: An auto-amplification loop causes organ failure. Nat Rev Immunol 14: 759–767, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Moriwaki K, Chan FK: The inflammatory signal adaptor RIPK3: Functions beyond necroptosis. Int Rev Cell Mol Biol 328: 253–275, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Najjar M, Saleh D, Zelic M, Nogusa S, Shah S, Tai A, et al. : RIPK1 and RIPK3 kinases promote cell-death-independent inflammation by toll-like receptor 4. Immunity 45: 46–59, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D’Cruz AA, et al. : RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun 6: 6282, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawlor KE, Feltham R, Yabal M, Conos SA, Chen KW, Ziehe S, et al. : XIAP loss triggers RIPK3- and caspase-8-driven IL-1β activation and cell death as a consequence of TLR-MyD88-induced cIAP1-TRAF2 degradation. Cell Rep 20: 668–682, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Moriwaki K, Balaji S, McQuade T, Malhotra N, Kang J, Chan FK: The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair. Immunity 41: 567–578, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sureshbabu A, Patino E, Ma KC, Laursen K, Finkelsztein EJ, Akchurin O, et al. : RIPK3 promotes sepsis-induced acute kidney injury via mitochondrial dysfunction. JCI Insight 3: e98411, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Li R, Dong W, Yang H, Zhang L, Chen Y, et al. : RIPK3 mediates renal tubular epithelial cell apoptosis in endotoxin-induced acute kidney injury. Mol Med Rep 20: 1613–1620, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Huang C, Zhao Y, Cao Q, Yi H, Chen X, et al. : RIPK3 blockade attenuates tubulointerstitial fibrosis in a mouse model of diabetic nephropathy. Sci Rep 10: 10458, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y, Huang C, Yi H, Cao Q, Zhao Y, Chen J, et al. : RIPK3 blockade attenuates kidney fibrosis in a folic acid model of renal injury. FASEB J 34: 10286–10298, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Sanz AB, Ruiz-Andres O, Sanchez-Niño MD, Ruiz-Ortega M, Ramos AM, Ortiz A: Out of the TWEAKlight: Elucidating the role of Fn14 and TWEAK in acute kidney injury. Semin Nephrol 36: 189–198, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Sanz AB, Izquierdo MC, Sanchez-Niño MD, Ucero AC, Egido J, Ruiz-Ortega M, et al. : TWEAK and the progression of renal disease: Clinical translation. Nephrol Dial Transplant 29[Suppl 1]: i54–i62, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Moreno JM, Fontecha-Barriuso M, Martín-Sánchez D, Sánchez-Niño MD, Ruiz-Ortega M, Sanz AB, et al. : The contribution of histone crotonylation to tissue health and disease: Focus on kidney health. Front Pharmacol 11: 393, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz-Andres O, Sanchez-Niño MD, Moreno JA, Ruiz-Ortega M, Ramos AM, Sanz AB, et al. : Downregulation of kidney protective factors by inflammation: Role of transcription factors and epigenetic mechanisms. Am J Physiol Renal Physiol 311: F1329–F1340, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Sanz AB, Justo P, Sanchez-Niño MD, Blanco-Colio LM, Winkles JA, Kreztler M, et al. : The cytokine TWEAK modulates renal tubulointerstitial inflammation. J Am Soc Nephrol 19: 695–703, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanz AB, Sanchez-Niño MD, Izquierdo MC, Jakubowski A, Justo P, Blanco-Colio LM, et al. : Tweak induces proliferation in renal tubular epithelium: A role in uninephrectomy induced renal hyperplasia. J Cell Mol Med 13[9B]: 3329–3342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Justo P, Sanz AB, Sanchez-Niño MD, Winkles JA, Lorz C, Egido J, et al. : Cytokine cooperation in renal tubular cell injury: The role of TWEAK. Kidney Int 70: 1750–1758, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Metz-Kurschel U, Kurschel E, Wagner K, Aulbert E, Graben N, Philipp T: Folate nephropathy occurring during cytotoxic chemotherapy with high-dose folinic acid and 5-fluorouracil. Ren Fail 12: 93–97, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J: Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Newton K, Sun X, Dixit VM: Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol 24: 1464–1469, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, et al. : RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci U S A 111: 7753–7758, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontecha-Barriuso M, Martín-Sanchez D, Martinez-Moreno JM, Cardenas-Villacres D, Carrasco S, Sanchez-Niño MD, et al. : Molecular pathways driving omeprazole nephrotoxicity. Redox Biol 32: 101464, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haverty TP, Kelly CJ, Hines WH, Amenta PS, Watanabe M, Harper RA, et al. : Characterization of a renal tubular epithelial cell line which secretes the autologous target antigen of autoimmune experimental interstitial nephritis. J Cell Biol 107: 1359–1368, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanz AB, Sanchez-Niño MD, Izquierdo MC, Jakubowski A, Justo P, Blanco-Colio LM, et al. : TWEAK activates the non-canonical NFkappaB pathway in murine renal tubular cells: Modulation of CCL21. PLoS One 5: e8955, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández-Sánchez A, Baragaño Raneros A, Carvajal Palao R, Sanz AB, Ortiz A, Ortega F, et al. : DNA demethylation and histone H3K9 acetylation determine the active transcription of the NKG2D gene in human CD8+ T and NK cells. Epigenetics 8: 66–78, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Wang S, Fu R, Zhou M, Zhang T, Pan W, et al. : RIP3 dependent NLRP3 inflammasome activation is implicated in acute lung injury in mice. J Transl Med 16: 233, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanz AB, Sanchez-Niño MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, et al. : NF-kappaB in renal inflammation. J Am Soc Nephrol 21: 1254–1262, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Poveda J, Sanz AB, Carrasco S, Ruiz-Ortega M, Cannata-Ortiz P, Sanchez-Niño MD, et al. : Bcl3: A regulator of NF-κB inducible by TWEAK in acute kidney injury with anti-inflammatory and antiapoptotic properties in tubular cells. Exp Mol Med 49: e352, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poveda J, Sanz AB, Rayego-Mateos S, Ruiz-Ortega M, Carrasco S, Ortiz A, et al. : NFκBiz protein downregulation in acute kidney injury: Modulation of inflammation and survival in tubular cells. Biochim Biophys Acta 1862: 635–646, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Solez K, Morel-Maroger L, Sraer JD: The morphology of “acute tubular necrosis” in man: Analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine (Baltimore) 58: 362–376, 1979 [PubMed] [Google Scholar]
- 37.Wang Q, Liu Z, Ren J, Morgan S, Assa C, Liu B: Receptor-interacting protein kinase 3 contributes to abdominal aortic aneurysms via smooth muscle cell necrosis and inflammation. Circ Res 116: 600–611, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imamura M, Moon JS, Chung KP, Nakahira K, Muthukumar T, Shingarev R, et al. : RIPK3 promotes kidney fibrosis via AKT-dependent ATP citrate lyase. JCI Insight 3: e94979, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei S, Zhou H, Wang Q, Zhou S, Li C, Liu R, et al. : RIP3 deficiency alleviates liver fibrosis by inhibiting ROCK1-TLR4-NF-κB pathway in macrophages. FASEB J 33: 11180–11193, 2019 [DOI] [PubMed] [Google Scholar]
- 40.Dominguez S, Montgomery AB, Haines GK 3rd, Bloomfield CL, Cuda CM: The caspase-8/RIPK3 signaling axis in antigen presenting cells controls the inflammatory arthritic response. Arthritis Res Ther 19: 224, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen H, Li Y, Wu J, Li G, Tao X, Lai K, et al. : RIPK3 collaborates with GSDMD to drive tissue injury in lethal polymicrobial sepsis. Cell Death Differ 27: 2568–2585, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komada T, Muruve DA: The role of inflammasomes in kidney disease. Nat Rev Nephrol 15: 501–520, 2019 [DOI] [PubMed] [Google Scholar]
- 43.Mulay SR: Multifactorial functions of the inflammasome component NLRP3 in pathogenesis of chronic kidney diseases. Kidney Int 96: 58–66, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.