Abstract
A rapid drug susceptibility test to measure the susceptibility of Mycobacterium tuberculosis to isoniazid (INH) and rifampin (RIF) using clinical isolates and a newly defined mycolic acid index (MAI) was evaluated. A total of 200 clinical isolates of M. tuberculosis were tested for susceptibility or resistance to INH and RIF by the MAI susceptibility and indirect-proportion methods. Overall, there was agreement between the two methods for 398 (99.5%) of the 400 total tests. Specifically, the sensitivity of the MAI susceptibility method for INH and RIF was 97.6 and 100%, respectively. The specificity and positive predictive value were 100% for both drugs, and the negative predictive value for INH and RIF was 98.3 and 100%, respectively. In conclusion, the MAI susceptibility method described here can be used for rapid drug susceptibility testing of M. tuberculosis clinical isolates within 5 days after clinical isolates are incubated in the presence or absence of an antituberculosis drug.
Tuberculosis (TB) is still a worldwide public health problem. Its high incidence is partly due to the emergence of drug-resistant Mycobacterium tuberculosis strains. This problem is compounded because drug susceptibility tests of M. tuberculosis usually used in clinical laboratories are based on growth of the microorganisms on solid or liquid medium in the presence or absence of anti-TB drugs for a minimum of 3 weeks before results are obtained, which is too long for adherence to the Centers for Disease Control and Prevention guidelines for mycobacterial laboratory efficiency (10). For this reason, new rapid and accurate susceptibility tests of M. tuberculosis are essential and should be used for TB control.
Mycolic acid analysis using high-performance liquid chromatography (HPLC) and p-bromophenacyl bromide derivatizing reagent for UV detection is a well-established method for identification of mycobacterial strains isolated from clinical specimens (1–3). A modification of this method that uses a coumarin as the fluorescent derivatizing agent of mycolic acids (8) allowed the detection of mycobacteria directly from clinical specimens (6, 8), since the use of the fluorescence increases the sensitivity of detection to a level at least 200-fold that usual with UV detection. In addition, our group (5) described a linear relationship between the total area under the mycolic acid (TAMA) chromatographic peaks of a culture of M. tuberculosis and log CFU per milliliter, suggesting the possibility of using TAMA as a good estimator of mycobacterial growth and also as a means of susceptibility testing of M. tuberculosis. The development of the new derivatization method of mycolic acids allowed the possibility of improving the use of TAMA for susceptibility testing of M. tuberculosis, since the chromatographic signal of the mycolic acids was increased, thus increasing the difference between cultivated strains in the presence or absence of an anti-TB drug and reducing the volume of the liquid medium needed (11). Although the feasibility of using TAMA for susceptibility testing of M. tuberculosis was demonstrated (5), the results were obtained only with the M. tuberculosis H37Ra strain. Therefore, in the present work we set out to prove that TAMA can be used for rapid testing of the susceptibility of M. tuberculosis clinical isolates to isoniazid (INH) and rifampin (RIF) and we established a new resistance criterion for each drug from mycolic acid analysis.
A total of 393 clinical specimens (bronchial washes or sputum) were received from the Dr. José Eleuterio González University Hospital in Monterrey, Mexico; clinical isolates were characterized from these specimens by conventional biochemical tests (9) and by their mycolic acid patterns (2, 3, 6) as M. tuberculosis (64 cultures) and mycobacteria other than tuberculosis (8 cultures). Although most patients were suspected of having TB based on clinical symptoms and/or a positive stain for acid-fast bacilli, the other clinical specimens did not show growth after 8 weeks of incubation. The 64 M. tuberculosis strains were used for this study, together with 60 clinical isolates obtained from the State Laboratory of the Department of Health of Nuevo León, Monterrey, Mexico, and 76 clinical isolates from the collection strains of the Regional Center of Infectious Disease Control of the School of Medicine of the Autonomous University of Nuevo León, Guadalupe, Mexico.
The indirect-proportion method (4), following the recommendations of the National Committee for Clinical Laboratory Standards (10), was used to determine the percentage of M. tuberculosis organisms resistant to 0.2 μg of INH/ml or 1.0 μg of RIF/ml. An isolate was considered susceptible to an antimycobacterial agent if the number of colonies that grew on the drug-containing plate was smaller than 1% of the number of colonies that grew on the drug-free control; otherwise, it was considered as resistant isolate. Of the 200 clinical isolates tested by the indirect-proportion method, 110 were susceptible to INH and RIF, 17 were resistant to INH but not to RIF, 6 were resistant to RIF but not to INH, and 67 were resistant to both anti-TB drugs.
By using a procedure described previously (11), two screw-cap tubes (13 by 100 mm) containing 1 ml of Middlebrook 7H9 broth without anti-TB drug, one tube containing broth plus 0.2 μg of INH/ml, and one containing broth plus 2.0 μg of RIF/ml were each inoculated with 100 μl of a suspension of each clinical isolate prepared with growth from a glass bead-homogenized Lowenstein-Jensen slant culture previously adjusted to be equivalent to the McFarland 0.5 turbidity standard. The drug-containing tubes and one of the drug-free tubes were incubated for 5 days at 37°C. Afterwards, mycolic acid analyses were carried out by HPLC using fluorescence detection and all the broth in each tube. Control strain M. tuberculosis H37Rv (ATCC 27294) was tested by both methods as a quality control. The results obtained for all isolates by both methods were blinded to laboratory workers.
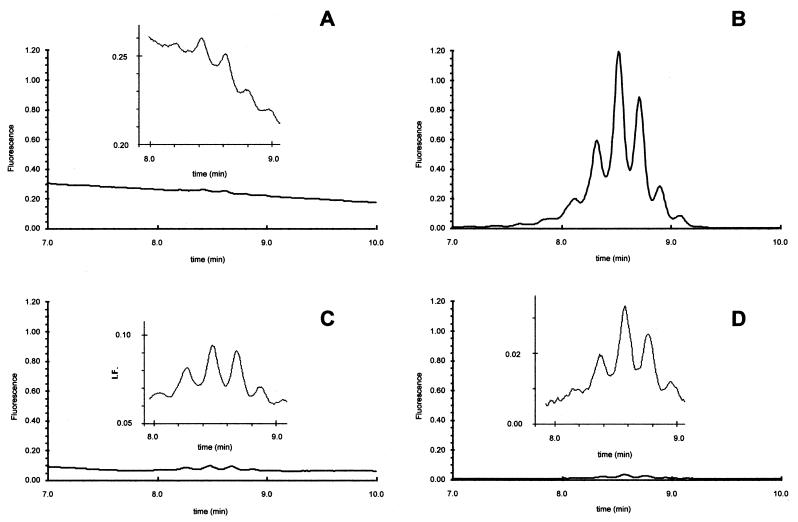
Figure 1 shows four chromatograms of the mycolic acid pattern of a clinical isolate obtained from a drug-free tube before incubation (Fig. 1A) and after 5 days of incubation in the absence of anti-TB drug (Fig. 1B), in the presence of INH (Fig. 1C), or in the presence of RIF (Fig. 1D). It is worth noting that only the tube incubated for 5 days in the absence of anti-TB drug gave a significant signal corresponding to mycolic acids, indicating in a qualitative form that this clinical isolate was susceptible to both drugs.
FIG. 1.
Mycolic acid pattern of a clinical isolate obtained from a drug-free tube before incubation (A) and from tubes after 5 days of incubation in the absence of anti-TB drug (B), in the presence of INH (C), or in the presence of RIF (D). The four chromatograms are drawn to the same scale with a zoom in the zone of interest.
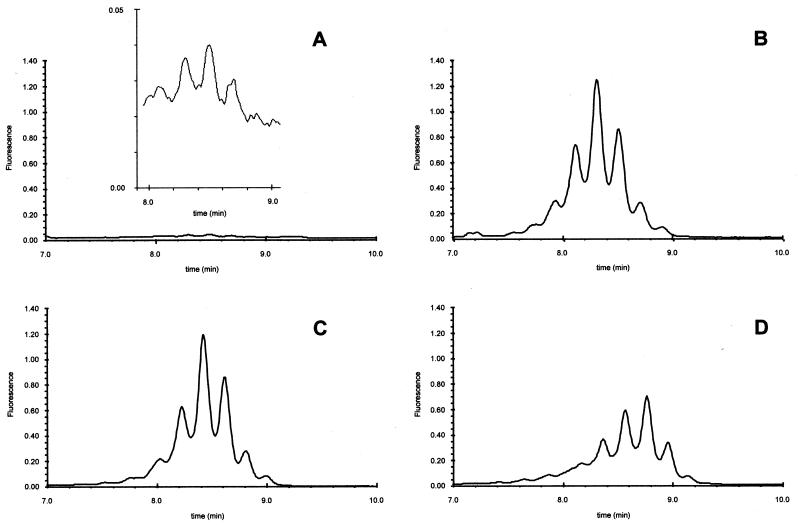
Figure 2 shows four chromatograms similar to those in Fig. 1 but from another clinical isolate. It can be seen that a high mycolic acid signal was detected in the incubated drug-free sample (Fig. 2B) and in the two incubated drug-containing samples (Fig. 2C and D), indicating that this clinical isolate was resistant to both drugs.
FIG. 2.
Mycolic acid pattern of a clinical isolate (different from that in Fig. 1) obtained from a drug-free tube before incubation (A) and from tubes after 5 days of incubation in the absence of anti-TB drug (B), in the presence of INH (C), or in the presence of RIF (D). The four chromatograms are drawn to the same scale with a zoom in the zone of interest.
To carry out this susceptibility test in a quantitative form, the mycolic acid index (MAI) at 5 days for INH and RIF, defined by the ratio of the increase in mycolic acid concentration during incubation in the presence of a drug and the increase in mycolic acid concentration during incubation in the absence of the drug, was used as described previously (11). The susceptibility results showed that 82 of the 84 INH-resistant isolates had a MAI to INH at 5 days greater than 0.50 and all the RIF-resistant isolates had a MAI greater than 0.30, while all the INH- or RIF-susceptible isolates had a MAI less than 0.10 and 0.15, respectively, indicating that the MAI is a very efficient way of determining the drug susceptibility of M. tuberculosis clinical isolates. For the two INH-resistant clinical isolates that did not show agreement of results between the two methods, the percentage of organisms resistant to INH was 1.1 and 2.3%, respectively. With these results, we considered a clinical isolate of M. tuberculosis susceptible to INH if the MAI was 0.15 or less, and a similar interpretation was made for RIF. Furthermore, the reproducibility of the MAI susceptibility method was evaluated using one susceptible, one INH-resistant, and one RIF-resistant strain and tested three times, obtaining a coefficient of variation less than 5%.
We demonstrated previously that susceptibility testing of M. tuberculosis, specifically with the M. tuberculosis H37Ra strain, could be accomplished rapidly by using a mycolic acid analysis (5). The results of the MAI susceptibility method described in this paper were accessible clearly within 5 days after the clinical isolates were incubated in the presence or absence of an anti-TB drug. The MAI susceptibility method is based on the ability of M. tuberculosis to synthesize mycolic acids during growth. In nonviable mycobacteria or mycobacteria susceptible to anti-TB drugs, synthesis of new mycolic acids is reduced due to the absence or decreased metabolic activity of the organisms. We tested 200 clinical isolates of M. tuberculosis for susceptibility or resistance to INH and RIF by the MAI susceptibility and indirect-proportion methods. Overall, there was agreement between the two methods for 398 (99.5%) of the 400 total tests. Specifically, if it is assumed that the proportion method is correct and with the resistance-susceptibility cutoff selected for the MAI susceptibility method, the sensitivity of the MAI susceptibility method for INH and RIF was 97.6 and 100%, respectively, the specificity and positive predictive value were 100% for both drugs, and the negative predictive value for INH and RIF was 98.3 and 100%, respectively.
Besides the rapidity and objectivity of the MAI susceptibility method and the fact that the use of mycolic acid analysis is increasing (2, 3), a major advantage of this method is its ability to confirm the identification of M. tuberculosis and the diagnosis of TB. However, there is an important shortcoming in the use MAI for susceptibility testing of M. tuberculosis. Although the test is rapid, accurate, and reproducible, many clinical laboratories do not have the facilities to perform the procedure. Nevertheless, the test could be used by public health laboratories or large reference laboratories with a biosafety level 3 laboratory. Another concern is the need to take care of mycolic acid carryover. It is recommended that after an injection into the HPLC system, the syringe be cleaned at least five times with HPLC-grade methylene chloride and the injector loop be cleaned one time with 1 ml of the same solvent; it is also recommended that a blank injection be used between samples when the prior mycolic acid signal is high. In spite of the high cost of an HPLC system, the system can be used for both mycobacterial identification and the MAI susceptibility method, reducing its cost per procedure. Furthermore, the reagents and supplies for HPLC are cheap compared with those needed for other rapid-susceptibility methods such as the Bactec radiometric method (Bactec TB system; Becton Dickinson Diagnostic Instrument Systems, Sparks, Md.) or methods for detecting mutations in specific genes. The Bactec and MAI methods should be able to obtain results after the same length of time because they use almost the same culture medium; however the Bactec method does not allow estimation of the percentage of resistant organisms and is vulnerable to false susceptibility or resistance results due to the possibility of mixed populations of mycobacterial species (7); these deficiencies are not a problem in our MAI susceptibility method. Since mycolic acid analysis can be done at present directly with young cultures (8), the MAI susceptibility method could be carried out in a direct form instead of the indirect form described here. In addition, the MAI susceptibility method could be used together with other analytic techniques useful for determining the amounts of mycolic acids in M. tuberculosis cultures that could be developed in the future. In conclusion, the MAI susceptibility method described here can be used to perform susceptibility testing of M. tuberculosis clinical isolates and shows great promise as a rapid, effective, accurate, and reliable susceptibility method for a mycobacteriology laboratory.
Acknowledgments
We acknowledge the importance of grants 970402004 from “Sistema de Investigación Alfonso Reyes” and SA093-98 from “Programa de Apoyo a la Investigación Cientifica y Tecnológica” of the Universidad Autónoma de Nuevo León for this work. We also thank Secretaria de Salud del Estado de Nuevo León for financial support.
We thank Hospital Universitario José Eleuterio González, Monterrey, Mexico, and Laboratorio Estatal de Salud, Guadalupe, Mexico, for providing samples; Maria de la Luz Acevedo-Duarte for technical support; and R. M. Chandler-Burns for stylistic suggestions in the preparation of the manuscript.
REFERENCES
- 1.Butler W R, Jost K C, Jr, Kilburn J O. Identification of mycobacteria by high-performance liquid chromatography. J Clin Microbiol. 1991;29:2468–2472. doi: 10.1128/jcm.29.11.2468-2472.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler W R, Floyd M M, Silcox V, Cage G, Desmond E, Duffey P S, Guthertz L S, Gross W, Jost K C, Ramos L S, Thibert L, Warren N, editors. Steering Committee, HPLC Users Group. Standardized method for HPLC identification of mycobacteria. Washington, D.C.: U.S. Department of Health and Human Services; 1996. [Google Scholar]
- 3.Butler W R, Floyd M M, Silcox V, Cage G, Desmond E, Duffey P S, Guthertz L S, Gross W, Jost K C, Ramos L S, Thibert L, Warren N, editors. Steering Committee, HPLC Users Group. Mycolic acid pattern standards for HPLC identification of mycobacteria. Washington, D.C.: U.S. Department of Health and Human Services; 1999. [Google Scholar]
- 4.Canetti G, Frosman S, Grosset J H, Hauduroy P, Langerova M, Mahler H T, Meissner G, Mitchison D A, Sula L. Mycobacteria: laboratory methods for testing drug sensitivity and resistance. Bull W H O. 1963;29:565–578. [PMC free article] [PubMed] [Google Scholar]
- 5.Garza-González E, Guerrero-Olazarán M, Tijerina-Menchaca R, Viader-Salvadó J M. Determination of drug susceptibility of Mycobacterium tuberculosis through mycolic acid analysis. J Clin Microbiol. 1997;35:1287–1289. doi: 10.1128/jcm.35.5.1287-1289.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garza-González E, Guerrero-Olazarán M, Tijerina-Menchaca R, Viader-Salvadó J M. Identification of mycobacteria by mycolic acid pattern. Arch Med Res. 1998;29:303–306. [PubMed] [Google Scholar]
- 7.Inderlied C B, Salfinger M. Antimycobacterial agents and susceptibility tests. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. pp. 1601–1623. [Google Scholar]
- 8.Jost K C, Jr, Dunbar D F, Barth S S, Headley V L, Elliott L B. Identification of Mycobacterium tuberculosis and M. avium complex directly from smear-positive sputum specimens and BACTEC 12B cultures by high-performance liquid chromatography with fluorescence detection and computer-driven pattern recognition models. J Clin Microbiol. 1995;33:1270–1277. doi: 10.1128/jcm.33.5.1270-1277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kent P T, Kubica G P. Public health mycobacteriology: a guide for the level III laboratory, U.S. Department of Health and Human Services publication 86–8230. U.S. Washington, D.C.: Department of Health and Human Services; 1985. [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Antimycobacterial susceptibility testing for Mycobacterium tuberculosis. Proposed standard M24-T. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 11.Viader-Salvadó J M, Guerrero-Olazarán M, Garza-González E, Tijerina-Menchaca R. Drug susceptibility of Mycobacterium tuberculosis through the mycolic acid index. Methods Mol Med. 2000;48:13–19. doi: 10.1385/1-59259-077-2:13. [DOI] [PubMed] [Google Scholar]