Abstract
Background
Temperature elevation, a classic marker of infection and local temperature elevation, might be a useful predictor of early infection. However temperature measurement around the spine is not readily accessible. The purpose of this study was to explore whether a temperature sensing implant might reproducibly detect local temperature change associated with peri-implant wound infection, in a rabbit model.
Methods
Twelve adult rabbits were implanted with a spinal screw-rod construct. Temperature probes were placed at the implantation site as well as at a separate scapular site away from the surgical site to serve as control. Animals were inoculated with S. aureus: group 1 (saline control), group 2 (low dose 1 × 102 CFU/site), group 3 (medium dose 1 × 104 CFU/site), and group 4 (high dose 1 × 106 CFU/site) and monitored for 7 days prior to euthanasia.
Results
The scapular control temperature and implant site temperature in the non-infected animals remained similar throughout the study period. Both the scapular control and implant site temperatures were elevated in the infected animals compared to the non-infected animals. There was a statistically significant difference in the scapular control temperature and implant site temperature in all infected animals but not in the non-infected animals. Difference in temperature elevation between implant site and control scapular site were greatest for the animals with worst clinical appearance during the post-mortem evaluation.
Conclusions
This rabbit model demonstrates that local temperature measured in proximity to a spinal implant is elevated in the presence of infection with greater elevations associated with worse infections. Availability of an implantable temperature sensor may yield valuable information for the assessment and treatment of suspected spinal wound infection in the clinical setting.
Keywords: Spine infection, Animal model, Local temperature change, Implantable temperature sensor
Background
Wound infection is a serious complication of spinal surgery, particularly in the presence of spinal implants [1,2]. Early identification of infection is often difficult as markers of infection such as mildly elevated WBC or CRP, mild wound erythema, minimal wound drainage, or increased pain may all be part of a normal post-op course [3]. It is also frequently difficult to determine whether an infection may be superficial, or more consequential, subfascial with concomitant involvement of the implants [4]. This often leaves surgeons with difficulty in determining whether a “possible” infection should be observed, or treated with oral antibiotics, inpatient observation or even surgical exploration. The advent of “smart” implants with internal sensors presents an opportunity to improve detection capabilities
Temperature elevation is a classic marker of infection, although application to spinal wound evaluation has been problematic. Systemic temperature elevation is easily measured but is uncommon with early spinal wound infection [5,6]. It is more typically seen with severe infection, suggesting an element of systemic sepsis. Local temperature elevation, on the other hand, might be a useful predictor of early infection. However, temperature measurement around the spine is not readily accessible. Beyond the potential for early identification of infection, elevated local temperature surrounding the implants might also be useful in differentiating between superficial and deep wound infection [7].
The goal of this study was to explore whether a temperature sensing implant might reproducibly detect local temperature change associated with peri-implant wound infection, in a rabbit model [8,9]. In particular, we sought to differentiate between infected and non-infected animals and between infected and non-infected sites within the same animal.
Methods
After receiving Institutional Animal Care and Use Committee Approval, twelve adult New Zealand white rabbits weighing at least 3kg were implanted with a multi-axial bone screw anchored in the L6 transverse process, a set screw and a titanium rod. A separate incision approximately 5” away from the surgical site at the scapula was also created to serve as a control site. Each animal had two separate intramuscular probes (IPTT-300, Bio Medic Data Systems, Seaford, DE) measuring temperature at these two discrete sites. Prior to closure, implant constructs in the “infected” group were inoculated with Staphylococcus aureus and the “non-infected” group were inoculated with saline. The animals were divided into 4 groups of three: group 1 (non-infected control with saline), group 2 (low dose of 1 × 102 CFU/site), group 3 (medium dose of 1 × 104 CFU/site), and group 4 (high dose of 1 × 106 CFU/site). No prophylactic antibiotics were administered. All animals were monitored for 7 days after implantation prior to euthanasia. Temperature readings were collected from both sites three times per day. White blood cell (WBC) counts and C-reactive protein (CRP) serum levels were determined at day zero and day six.
Post-mortem analysis included observational analysis of infection severity as graded by the veterinary staff. The severity of the infection was scored from 0 = no infection to 4 = severe infection (Table 1). Measurement of total colony forming units (CFU) from each wound site was also performed. Samples for CFU counts were taken from peri-implant tissue, spinal hardware and the temperature probe. CFU data was analyzed with HardyCHROM UTI agar (Hardy Diagnostics, Santa Maria, CA) and Tryptic Soy Agar (TSA).
Table 1.
Clinical Wound Infection Score.
| Score | Percentage of Area Covered in White Matter |
|---|---|
| 0 | 0 |
| 1 | > 0 to 25 % |
| 2 | > 25 to 50 % |
| 3 | > 50 to 75 % |
| 4 | >75 to 100 % |
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 27.0 (Armonk, NY). Repeated measures ANOVA was used to compare temperatures across the four animal groups over time. One way ANOVA was used to compare serum markers at Day 1 and Day 7 among the four groups followed by Dunnett's Method to compare the Non-infected animals to the Infected animals.
Results
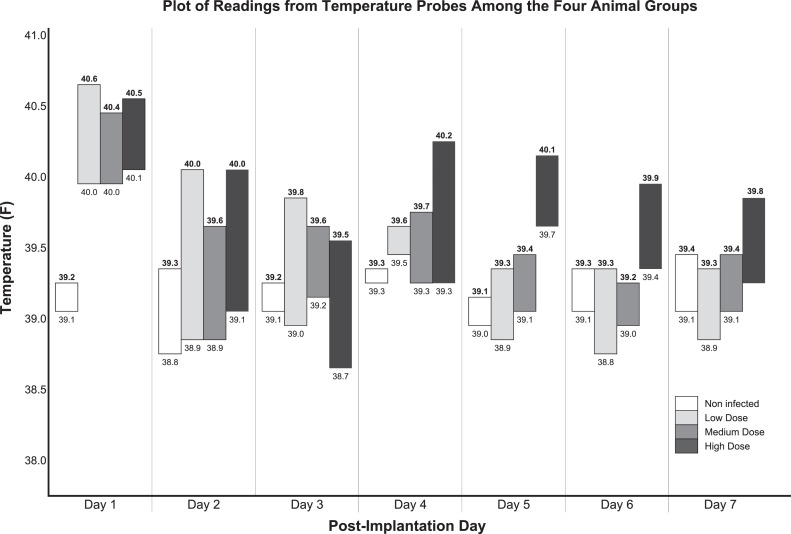
Non-infected animals remained asymptomatic during the course of the study, while infected animals inoculated with S. aureus had elevated temperatures at both the control scapula and implant site. Elevated temperatures peaked on Day 4 in all animals at all sites except for the control scapula in High Dose infected animals where the elevated temperature peaked at Day 5. Temperatures returned to normal on Day 6 (Fig. 1).
Fig. 1.
Plot of readings from temperature probes from the four animal groups. Numbers in bold font are temperatures at the implant site. Numbers in regular font are temperatures at the control scapular site. Bars signify the difference between the temperatures in the implant site compared to the scapular control site.
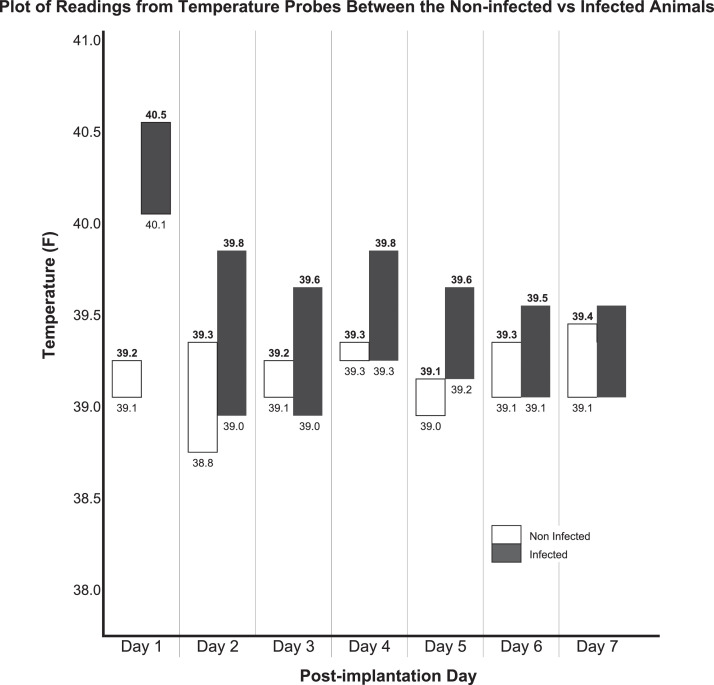
The scapular control temperature and implant site temperature in the non-infected animals remained similar throughout the study period. Both the scapular control and implant site temperatures were elevated in the infected animals compared to the non-infected animals (Fig. 2). Repeated measures ANOVA showed that there was no difference in the scapular site temperature over time between the infected and the non-infected animals (p=0.380) but that there was a significant difference in implant site temperature over time between the infected and non-infected animals (p=0.007).
Fig. 2.
Plot of readings from temperature probes from the non-infected vs infected animals. Numbers in bold font are temperatures at the implant site. Numbers in regular font are temperatures at the control scapular site. Bars signify the difference between the temperatures in the implant site compared to the scapular control site.
White blood cell counts increased by 7% in the non-infected animals and increased 50% in the infected animals (Table 2) regardless of inoculation dose. Non-infected animals had a 30% increase in neutrophils compared to an 80% increase in the infected animals across all infected animals. CRP was elevated in all animals prior to euthanasia compared to pre-implantation. CRP levels were similar between the non-infected animals and the Low Dose infected animals. Post-hoc analysis showed that there was a difference in CRP levels between Medium and High Dose infected animals and the non-infected animals at termination. No bacteria were recovered from the implant site in the non-infected animals. TSA and HardyChrom™ UTI showed similar bacterial recovery across all infected animals regardless of initial inoculation dose.
Table 2.
Serum Markers.
| Inoculation Dose | |||||
|---|---|---|---|---|---|
| Non Infected | Low Dose | Medium Dose | High Dose | p-value§ | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Leukocyte count, K/µL | |||||
| Day 1 | 5.17 (0.74) | 4.20 (0.62) | 4.10 (0.86) | 5.07 (0.91) | 0.280 |
| Day 7 | 5.56 (1.7)* | 8.08 (1.68) | 8.83 (1.88) | 10.89 (0.68) | 0.019 |
| Neutrophil Count, K/µL | |||||
| Day 1 | 1.61 (0.19) | 1.36 (0.22) | 1.3 (0.09) | 1.61 (0.52) | 0.468 |
| Day 7 | 2.38 (0.43)* | 5.64 (2.09) | 6.68 (1.96) | 7.96 (0.71) | 0.010 |
| Percentage Neutrophil, % | |||||
| Day 1 | 31.32 (3.63) | 32.5 (5.15) | 32.34 (5.48) | 31.39 (5.02) | 0.985 |
| Day 7 | 44.24 (9.01)* | 68.25 (12.2) | 74.85 (6.08) | 73.03 (2.07) | 0.006 |
| CRP,ng/mL x 103 | |||||
| Day 1 | 18.04 (14.7) | 10.34 (4.7) | 11.63 (6.07) | 24.13 (14.23) | 0.434 |
| Day 7 | 50.75 (70.45) | 246.6 (175.69) | 357.67 (106.46) | 284.33 (128.43) | 0.082 |
| CFU/g sample x 106 | |||||
| HardyChrom | 9.88 (7.61) | 19.74 (15.49) | 21.67 (10.34) | 0.098 | |
| TSA | 11.47 (8.45) | 21.73 (19.13) | 23 (10.79) | 0.136 | |
p-value from One way ANOVA comparing variable among the four groups.
Values in non-infected group were statistically significantly different from the three infected groups based on post-hoc analysis using Dunnett's method.
Clinical Wound Infection Scores for each of the animals based on clinical appearance during the post-mortem evaluation and maximum temperature recorded over the study period is summarized in Table 3. CWIS depended on whether the animal was inoculated with S. aureus independent of the initial inoculation dose. Animals with the highest CWIS also had the highest maximum temperature over the study period. Scapular microchip incision site was macroscopically normal with no fluid or white matter across all groups.
Table 3.
Clinical Wound Infection Score and Maximum Implant Site Temperature for each animal.
| Inoculation Dose | Animal ID | White Matter Score | Maximum Implant Site Temperature (°F) |
|---|---|---|---|
| Non Infected | 59780 | 0 | 39.4 |
| 59781 | 0 | 39.4 | |
| 59782 | 0 | 39.3 | |
| Low Dose | 59783 | 4 | 40.9 |
| 59784 | 4 | 40.6 | |
| 59785 | 2 | 40.4 | |
| Medium Dose | 59786 | 3 | 40.4 |
| 59787 | 1 | 40.2 | |
| 59788 | 4 | 40.2 | |
| High Dose | 59789 | 1 | 40.4 |
| 59790 | 3 | 40.6 | |
| 59791 | 4 | 40.6 |
Discussion
This experimental rabbit model serves as proof of concept that local temperature measured in proximity to a spinal implant is elevated in the presence of infection. Local temperature elevation was clearly different between infected versus non-infected animals and also between the infected spinal implant site and a control site within the infected animals. The largest between group difference (infected versus non-infected animals) was seen on Day 1 which may be reflective of an early systemic inflammatory response to the inoculum superimposed on the catabolic effect of the surgery [10, 11].
Not only were both the scapular control and implant site temperatures elevated in the infected animals compared to the non-infected animals, the implant site temperature was significantly higher compared to the scapular control temperature in all infected animals. This finding suggests that implant site temperature may differentiate site of infection versus systemic temperature elevation or as compared to a non-infected surgical site. The largest within animal temperature difference was seen on Day 2, suggesting that local temperature elevation may be a marker of early infection.
Although leukocyte and neutrophil counts were elevated similarly across all infected animals regardless of initial inoculation dose, CRP levels on Day 7 were higher in the infected animals with medium and large initial inoculation doses compared to those with no or small initial inoculation doses. This suggests a dose dependent association between initial bacterial inoculation and level of CRP at termination. The temperature differential between the spinal implant site and the control scapular site was greatest in those animals with the most severe infection as graded by clinical appearance (CWIS = 4).
Manifestations of infection in this model were consistent with clinical experience [2, 6]. Severity of infection is not adequately evaluated by a single marker, as clinical presentation is modulated by local factors such as soft tissue injury, and systemic factors such as immune response. In the clinical setting, WBC elevation might indicate either a more severe infection or a more robust clinical response. Minimal wound erythema might indicate a minor infection or potentially host immunodeficiency.
Particularly for patients with spinal implants, determining the severity of infection and specifically the presence of subfascial involvement may be difficult, but is critically important. Deep wound exploration is a substantial procedure which surgeons would certainly avoid if it were not necessary. On the other hand, untreated deep wound infection presents a substantial risk, potentially leading to osteomyelitis with loss of fixation and the need for a dramatically more complex management strategy. For this reason, an additional data from implant site temperature might enhance a surgeon's diagnostic capability and could have significant clinical value. Recent advances in nanotechnology, seen clinically in the cardiovascular space, make this a potential near-term capability for spinal implants [12, 13].
Limitations of this model are firstly the question of clinical relevance. Larger animal studies might be helpful, but only human trials would fully demonstrate utility. Alterations in the experimental design, such as the use of systemic temperature as the internal control would present a more relevant clinical comparison. Also, an antibiotic treatment arm might give some indication as to whether early identification of deep infection might facilitate treatment short of surgical exploration.
Conclusions
Despite these shortcomings, this study demonstrates the technical feasibility of local temperature measurement as a marker of peri-implant spinal wound infection in an animal model. It presents at least the possibility that the technique could be applied clinically and might yield valuable information for the assessment and treatment of suspected spinal wound infection in the clinical setting.
Declarations of Competing Interests
SDG receives consulting fees from Medtronic. OA, NMB, PL and ASB are Medtronic employees. SDG and LYC- Institution received research funds from Medtronic.
Footnotes
One or more authors declare potential competing financial interests or personal relationships as specified on required ICMJE-NASSJ Disclosure Forms
Summary: In a rabbit model, local temperature measured in proximity to a spinal implant was elevated in the presence of infection compared to a control site at the scapula; with greater elevations associated with worse infections.
References
- 1.Kurtz SM, Lau E, Ong KL, et al. Infection risk for primary and revision instrumented lumbar spine fusion in the Medicare population. J Neurosurg Spine. 2012;17(4):342–347. doi: 10.3171/2012.7.SPINE12203. Oct. [DOI] [PubMed] [Google Scholar]
- 2.Radcliff KE, Neusner AD, Millhouse PW, et al. What is new in the diagnosis and prevention of spine surgical site infections. Spine J. 2015;15(2):336–347. doi: 10.1016/j.spinee.2014.09.022. Feb. [DOI] [PubMed] [Google Scholar]
- 3.Abdul-Jabbar A, Berven SH, Hu SS, et al. Surgical site infections in spine surgery: identification of microbiologic and surgical characteristics in 239 cases. Spine (Phila Pa 1976) 2013;38(22):E1425–E1431. doi: 10.1097/BRS.0b013e3182a42a68. Oct 15. [DOI] [PubMed] [Google Scholar]
- 4.Chahoud J, Kanafani Z, Kanj SS. Surgical site infections following spine surgery: eliminating the controversies in the diagnosis. Front Med (Lausanne) 2014;1:7. doi: 10.3389/fmed.2014.00007. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seo J, Park JH, Song EH, et al. Postoperative Nonpathologic Fever After Spinal Surgery: Incidence and Risk Factor Analysis. World Neurosurg. 2017;103:78–83. doi: 10.1016/j.wneu.2017.03.119. Jul. [DOI] [PubMed] [Google Scholar]
- 6.Mayo BC, Haws BE, Bohl DD, et al. Postoperative Fever Evaluation Following Lumbar Fusion Procedures. Neurospine. 2018;15(2):154–162. doi: 10.14245/ns.1836026.013. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chanmugam A, Langemo D, Thomason K, et al. Relative Temperature Maximum in Wound Infection and Inflammation as Compared with a Control Subject Using Long-Wave Infrared Thermography. Adv Skin Wound Care. 2017;30(9):406–414. doi: 10.1097/01.ASW.0000522161.13573.62. Sep. [DOI] [PubMed] [Google Scholar]
- 8.Poelstra KA, Barekzi NA, Grainger DW, et al. A novel spinal implant infection model in rabbits. Spine (Phila Pa 1976) 2000;25(4):406–410. doi: 10.1097/00007632-200002150-00003. Feb. [DOI] [PubMed] [Google Scholar]
- 9.Laratta JL, Shillingford JN, Hardy N, et al. A Dose-Response Curve for a Gram-Negative Spinal Implant Infection Model in Rabbits. Spine (Phila Pa 1976) 2017;42(21):E1225–E1230. doi: 10.1097/BRS.0000000000002205. Nov. [DOI] [PubMed] [Google Scholar]
- 10.Andres BM, Taub DD, Gurkan I, Wenz JF. Postoperative fever after total knee arthroplasty: the role of cytokines. Clin Orthop Relat Res. 2003;(415):221–231. doi: 10.1097/01.blo.0000093914.26658.55. Oct PMID: 14612649. [DOI] [PubMed] [Google Scholar]
- 11.Kenan S, Liebergall M, Simchen E, Porat S. Fever following orthopedic operations in children. J Pediatr Orthop. 1986;6(2):139–142. doi: 10.1097/01241398-198603000-00003. Mar-Apr PMID: 3958164. [DOI] [PubMed] [Google Scholar]
- 12.Cheung CC, Deyell MW. Remote Monitoring of Cardiac Implantable Electronic Devices. Can J Cardiol. 2018;34(7):941–944. doi: 10.1016/j.cjca.2018.01.003. Jul Epub 2018 Jan 8. PMID: 29691097. [DOI] [PubMed] [Google Scholar]
- 13.Tallaj JA, Singla I, Bourge RC. Implantable hemodynamic monitors. Cardiol Clin. 2011;29(2):289–299. doi: 10.1016/j.ccl.2011.03.002. May PMID: 21459250. [DOI] [PubMed] [Google Scholar]