Abstract
Background
Surgical simulation is a valuable educational tool for trainees to practice in a safe, standardized, and controlled environment. Interactive feedback-based virtual reality (VR) has recently moved to the forefront of spine surgery training, with most commercial products focusing on instrumentation. There is a paucity of learning tools directed at decompression principles. The purpose of this study was to evaluate the efficacy of VR simulation and its educational role in learning spinal anatomy and decompressive techniques.
Methods
A VR simulation module was created with custom-developed software. Orthopaedic and neurosurgical trainees were prospectively enrolled and interacted with patient-specific 3D models of lumbar spinal stenosis while wearing a headset. A surgical toolkit allowed users to perform surgical decompression, specifically removing soft tissues and bone. The module allowed users to perform various techniques in posterior decompressions and comprehend anatomic areas of stenosis. Pre- and post-module testing, and utility questionnaires were administered to provide both quantitative and qualitative evaluation of the module as a learning device.
Results
28 trainees were enrolled (20-orthopaedic, 8-neurosurgery) in the study. Pre-test scores on anatomic knowledge progressively improved and showed strong positive correlation with year-in-training (Pearson's r = 0.79). Following simulation, the average improvement in post-test scores was 11.4% in junior trainees (PGYI-III), and 1.0% in senior trainees (PGYIII-Fellows). Knowledge improvement approached statistical significance amongst junior trainees (p = 0.0542). 89% of participants found the VR module useful in understanding and learning the pathology of spinal stenosis. 71% found it useful in comprehending decompressive techniques. 96% believed it had utility in preoperative planning with patient-specific models.
Conclusions
Our original VR spinal decompression simulation has shown to be overwhelmingly positively received amongst trainees as both a learning module of patho-anatomy and patient-specific preoperative planning, with particular benefit for junior trainees.
Keywords: Virtual Reality, Surgical Education, Spine Surgery Training, Spinal Stenosis
Introduction
Medical education delivered via computer simulation has gained popularity, as it allows novice trainees to practice skills with a steep learning curve without the risk of training-related complications in acute medical scenarios [1,2]. Virtual Reality (VR) is simulation using a computer-generated multimedia environment that allows trainees to interact with 3-dimensional models. VR has been used in a variety of training scenarios including orthopaedic surgery, arthroscopic surgery [3], endoscopy, ophthalmology, neurosurgery, as well as spine surgery [4,5]. VR training is attractive as it can improve practitioner performance including cognitive [6] and procedural, conferring performance improvements in speed and accuracy across a wide range of procedures [7], [8], [9]. Furthermore, VR training modules are independent of patient, cadaver, or synthetic model availability allowing a cost-effective, minimal-risk approach to surgical training with graded, unlimited practice opportunities [9], [10], [11], [12].
The field of spinal surgery has also recently seen a marked increase in commercially available VR training tools, with a specific focus on minimally invasive spine surgery and pedicle screw instrumentation [4,5]. Recent reports on the efficacy of VR tools for this use are optimistic. Randomized studies using VR pedicle screw training simulators comparing performance between VR-trained and traditionally trained groups found that those training with VR outperformed those in traditional learning groups [13,14]. Similarly, residents and fellows improved in cervical lateral mass screw placements in sawbones and cadavers following VR simulation training [15]. While the variety of simulation types are limited, current simulation models have demonstrated validity as useful training models and improved performance [4].
Despite the surge of interest and tools available for training specific aspects of spinal surgery such as those for pedicle screw placements and vertebroplasties, no learning tools have been developed that specifically focus on teaching patho-anatomy and the principles underlying decompressive surgery. Spinal surgical anatomy due to its complex three-dimensional nature can be difficult to conceptualize by trainees and limited opportunities exist for translation from textbook to in vivo application [[16], [17], [18]]. The shift towards minimally invasive techniques further makes comprehension of key anatomical landmarks difficult given the limited surgical exposures and visualization. Spinal decompressions, with or without instrumentation, requires a thorough understanding of spinal anatomy, as neural element compression can occur at multiple sites (i.e., central, lateral recess, foramen), and occur due to a multitude of degenerative anatomical changes. VR simulation training offers an opportunity to learn anatomy without the limitation of exposure, patient risk, and time constraints of the operating room. With the pedagogical shift towards competency-based curricula [19], augmenting traditional learning models with VR simulation may provide further benefit for trainees gaining essential competencies.
The quality of decompression is the key element to successful lumbar spine surgery for leg dominant symptoms and there is little incentive for industry support for development of simulation models solely for decompression. To our knowledge no simulation model exists to teach spinal anatomy and the principles of decompression surgery. The aim of this investigation is twofold: (1) to develop a VR educational tool with a 3-D interactive model of the spine that can be used to teach the surgical anatomy of spinal stenosis and the principles of decompression; and (2) to evaluate the efficacy and acceptability by trainees of this VR simulation model in the context of competency-based surgical education.
Methods
Study Overview
This prospective cohort study was conducted at the Orthopedic Biomechanics Laboratory at a large level-I trauma center. Orthopaedic Surgery and Neurosurgery residents were prospectively recruited and instructed to perform various decompressive techniques within the VR module. Each participant was categorized by post-graduate year and underwent quantitative pre- and post-module testing on spine knowledge examination, as well as a qualitative assessment of VR module utility.
Research ethics review was not required because the study met criterial for exemption from such a review based on our institutional process for confirming that the project was deemed improvement in quality and not human subject research.
Patient specific model development
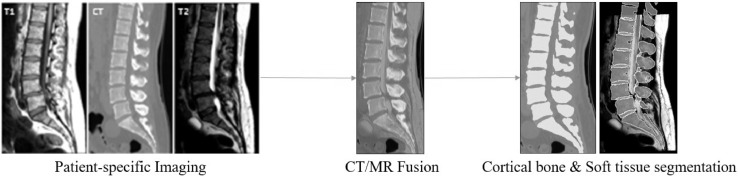
A patient-specific, 3-dimensional model of a stenotic lumbar spine was constructed using a custom module and workflow embedded in the 3D Slicer platform, an open-source medical image processing, analysis and visualization software [20]. Patient-specific 3D computed tomography (CT) and magnetic resonance imaging (MRI) studies were obtained to build a lumbar spinal stenosis model. These CT and MRI volumes were loaded into the simulator workflow for creation of appropriate geometry. CT and MRI data were fused using an initial fiducial based registration. Fiducials were roughly placed in the center of each vertebrae in the MRI and CT volumes. Initial alignment was followed by an intensity-based affine multi-modality (Mattes mutual information metric based) registration. CT and MRI information allowed for identification and visualization of the relative position of both soft and hard tissues.
Bone segmentation was performed upon the CT images, applying a user specified threshold based on a custom slider tool embedded in 3D Slicer. Soft tissue segmentation was performed using T1 and T2 weighted MRI images using a semi-automated process, starting with manually placed seed points, Simple Region Growing Segmentation followed by manual correction, allowing differentiation between neural elements, ligaments and spinal discs. The interface allows easy reference and blending of all 3 image volumes when manually adjusting the soft tissue segmentations. CT images and bone segmentations were used to further verify soft tissue segmentations (Fig. 1).
Fig. 1.
Processing of patient-specific cross-sectional CT and MRI information allowed for identification and visualization of the relative position of both soft and hard tissues, and creation into a 3-dimensional spinal model.
Virtual reality simulation
The user wore a VR headset for audio-visual simulation of the operation, and two handsets were provided to manipulate the 3D spine model. The simulator communicates with the headset through OpenVR and the SlicerVirtualReality plugin. The workflow was compatible with most virtual reality headsets and was tested with the Occulus Rift and the Samsung Odyssey headsets. The study was conducted with the Samsung Odyssey Headset as it has inside out tracking, allowing quicker setup and a larger volume of use. The simulator with Virtual Reality headset attached were run on a computer with a modern processor (AMD Ryzen 72nd Gen) and video card (Nvidia RTX 2070). Tissue-resecting tools for both soft tissue and bone (Kerrison rongeur, high speed burr and woodson elevator) were created within this interface to allow user interaction with the spine model in a virtually simulated 3D environment. (Fig. 2).
Fig. 2.
Example of a virtual reality simulation of lumbar laminotomy using a Kerrison rongeur (left), and completion of decompression and removal of ligamentum flavum, exposing the compressed irregular dura (right).
Assessment of learning
For quantitative assessment of learning of the anatomical principles of spinal stenosis, each participant underwent a standardized pre-test on lumbar spinal anatomical knowledge and the pathophysiology of spinal stenosis. Each participant was then oriented to the VR simulator by a research assistant. Instructions were provided on how to manipulate and position the spine model, select, and use the tools available within the simulation.
Once familiarized, the participant was instructed on the steps for performance of spinal decompression in virtual reality, beginning with central laminectomy, followed by lateral recess decompression as well as foraminotomy. Free manipulation of the spinal model allowed the user to fully visualize and comprehend the areas of neural compression often not fully appreciated in live cases (Fig. 3). The participant then completed the task of a one level decompression (Fig. 4). The same standardized test was then again administered to evaluate knowledge acquisition post-procedure. A second exit questionnaire was also administered for qualitative purposes to assess acceptability and satisfaction of the VR simulator.
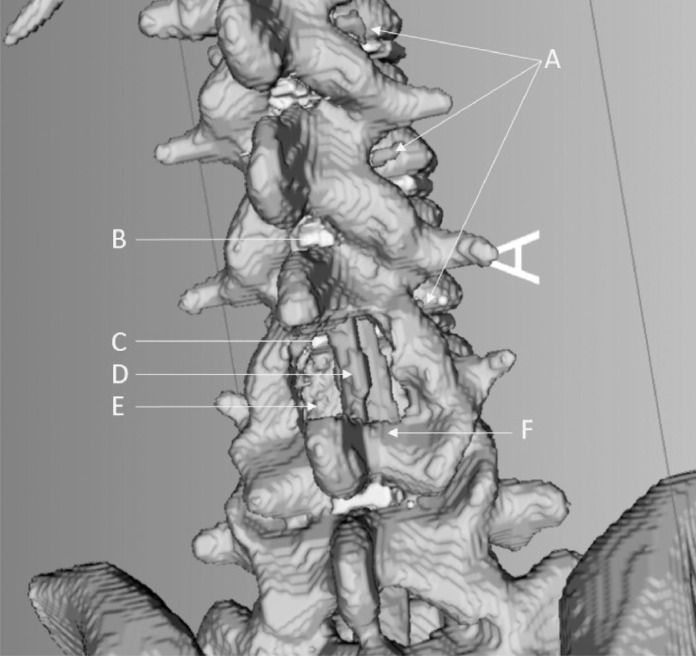
Fig. 3.
Demonstration and identification of bony and soft tissue, and neural elements. A – Nerve roots, B – Ligamentum Flavum, C – Intervertebral Disc, D – Re-expanded Dura, E – Area of decompression, F – Retained Lamina
Fig. 4.
Representative example of a surgical trainee interacting with the virtual reality module.
Data analysis
Descriptive and inferential statistics were performed on standardized subject pre-test and post-test scores following simulation session. Data was aggregated across training levels and trends were analyzed via paired sample t-test to assess differences in performance between junior and senior trainees. Pearson's r testing was applied to assess strength of correlation between pre-test knowledge and trainee level. Similarly, descriptive statistics were applied to subject questionnaire answers and aggregated for review.
Results
Participant demographics
A total of 28 trainee participants were prospectively enrolled in the virtual reality simulation from orthopaedics and neurosurgery over the course of a six month period. Six PGY-I (4 orthopaedic, 2 neurosurgery), six PGY-II (6 orthopaedic, 0 neurosurgery), nine PGY-III (7 orthopaedic, 2 neurosurgery), two PGY-IVs (1 orthopaedic, 1 neurosurgery), one PGY-V residents (0 orthopaedic, 1 neurosurgery), and four spine fellows (2 orthopaedic, 2 neurosurgery) participated (Table 1).
Table 1.
Total trainee participants separated by specialty and training level.
| Orthopaedic Surgery | Neurosurgery | |
|---|---|---|
| PGY-I | 4 | 2 |
| PGY-II | 6 | 0 |
| PGY-III | 7 | 2 |
| PGY-IV | 1 | 1 |
| PGY-V | 0 | 1 |
| Fellows | 2 | 2 |
All neurosurgical trainees had experience with spine surgery given the subjects integration from the beginning of residency. All orthopaedic trainees from PGYII and up had undergone at minimum a three-month spine rotation. All orthopaedic PGY-Is had minimal to no experience with spine surgery.
Quantitative assessment - pre and post-simulation knowledge assessment
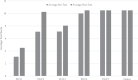
Pre-simulation test scores demonstrated a strong positive correlation to level in training (Pearson's R = 0.792). Junior (PGY-I, PGY-II, and PGY-III) residents exhibited the strongest improvement in test scores following utilization of the virtual reality simulation module, with a combined improvement of 11.4% on post-module examination (Table 2). The score improvement approached statistical significance on inferential analysis (N = 21, t = -1.984, p = 0.054).
Table 2.
Comparison of trainee pre-simulation and post-simulation test scores on spinal anatomy and pathology, stratified by year in training. Total score is out of 15 points. Statistical difference (p < 0.05) was reached amongst the PGY-2 cohort in pre- and post-testing.
 |
Seniors trainees (PGY-IV – Fellows) demonstrated a minimal change in test scores following VR testing, with an average improvement of 1.0% on post-module examination. Statistical significance was not found amongst the senior trainees (N = 7, t = -0.2325, p = 0.82).
Post-simulation participant questionnaire
A large majority of our participants agreed with the utility of the virtual reality decompression module, stating that it was useful in learning the patho-anatomy of spinal stenosis (89%), and was useful for learning the concepts of performing a laminectomy (71%). Participants found it particularly helpful in preoperative planning if utilized with patient-specific cross-sectional imaging (96%).
Statements regarding the effectiveness of the learning tool for developing surgical efficiency (46%) and the specific technical details (33%) were less agreed upon (Table 3).
Table 3.
Post-module questionnaire results demonstrating level of agreement amongst trainees to various statements regarding the utility of the virtual reality simulation module.
| Questionnaire Statement | Agree or Strongly Agree |
|---|---|
| I feel more comfortable with spinal anatomy after using this tool | 53.57% |
| Useful tool in learning the pathology of spinal stenosis | 89.29% |
| Useful tool in learning how to perform a spinal decompression | 71.43% |
| Tool will be useful in preoperative planning if usedwith patient specific models | 96.43% |
| Tool will make my surgical workflow more efficient | 45.83% |
| More comfortable performing a lumbar decompression after this session | 33.33% |
Discussion & conclusions
Our training tool is the first virtual reality-based simulation and learning tool designed uniquely for posterior lumbar decompressions. This educational module has the ability to automate the processing of patient-specific cross-sectional imaging (Computed Tomography and Magnetic Resonance Imaging) to produce accurate 3-dimensional spine models. These models are accurate in both bony and soft tissue patho-anatomy, and our study has demonstrated their use in resident education. Pre-operative planning and anatomical understanding of any individual case can be facilitated using our model.
Emphasis up until now for simulation in lumbar spinal surgery has been with pedicle screw placement. The risks with instrumentation are well recognized and has been the impetus for the numerous platforms used for simulation involving pedicle screws [21]. Our study and simulator platform for decompression is an extension of previous work at our institution where CT based patient specific simulation has been used for pedicle screw insertion [22]. Decompression requires a different skill set and anatomical understanding of the three dimensional nature of the spinal canal than is required for pedicle screw insertion. Our Virtual Realty simulator for decompression can also be used with pedicle screw simulation such that both components of the surgical procedure can be practised. However, the current study limits its focus to the decompression aspect of simulation which has not been previously evaluated for its efficacy and acceptability as a learning medium.
This study has demonstrated that the greatest educational benefit using a Virtual Reality spinal decompression module can be achieved at the level of junior residents, who have either no or limited prior experience with spine surgery. With even a short learning session and interaction with the module, junior trainees can improve their understanding of spinal stenosis, critical neuroanatomy, and basic concepts of performing decompressions. This module also allowed trainees to experience and perform various types of posterior decompressions (laminectomy, laminotomy, hemi-laminotomy, unilateral laminectomy for bilateral decompression, foraminotomy) and better appreciate the extent of such techniques. There was a strong support for the value of this teaching tool in learning the patho-anatomy of spinal stenosis (89%), useful for learning the concepts of performing a laminectomy (71%).
We had originally aimed to investigate if any difference in knowledge existed between orthopaedic and neurosurgical residents of various levels. However, given our small neurosurgical sample size available for testing, our results would likely be statistically inaccurate and difficult to make any definitive conclusions. With enough statistical power, we suspect junior neurosurgical trainees would achieve a higher pre-test knowledge score by virtue of an earlier immersion of spine training in residency.
This model can be built based on patient specific scans and used as a preoperative aid in surgical planning for both the decompression and instrumentation components of a particular procedure. There was near-unanimous agreement amongst trainees that this system would be useful in pre-operative planning for upcoming cases. Such a VR experience would allow a trainee to enter live surgical cases with a greater degree of familiarity, confidence, and preparedness.
Our simulation module at present is in continued evolution, and the first phase of this project involved software development and assessment of a trainee's ability to learn the 3-dimensional anatomy of the spinal canal, understand the components that contribute to the pathology, and virtually do the steps of a decompression while understanding the different tissues that contribute to the pathology. The end goal is to improve the execution of the actual surgical procedure, and this will require an intraoperative evaluation of learning metrics comparing prior VR simulation exposure with our model to no prior exposure.
Not unexpectedly in its current form, the simulation tool was not felt to be of high value for the technical aspects of surgery. Like learning modules developed for spinal instrumentation, the identification of landmarks and trajectory is best established by simulation models, however there is no substitution for actual in vivo practise with real haptic feedback for appreciating the forces required to safely use surgical tools. The simulator should improve the trainees understanding of the steps and anatomy and be better able to focus on the actual technique once inside the operating room.
Surgical education and training will continue to evolve as competency-based curriculums become more prevalent as well as with the enforcement of mandatory work-hour restrictions. During times of a viral pandemic as is being experienced at the time of this manuscript preparation (COVID-19), elective surgical caseloads and volume can become unpredictable and can further compromise learning opportunities. While there is no replacement for true hands-on experience in the operating theater, this study in addition to previous studies in the literature reinforce that the operative experience can be further enhanced through training outside of the operating room [13,14]. Virtual reality is one of many learning tools that is additive to more common and often passive forms of learning such as reading or videos. Interactive learning is more effective if made enjoyable, and such surgical simulations provides one further medium that can complement the more traditional learning methods.
Funding disclosures
Study funding was provided by the Feldberg Chair in Spinal Surgery at Sunnybrook Health Sciences. There are no study-specific conflicts of interest to disclose.
Declarations of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Patient Informed Consent Statement
The authors declare that informed patient consent was taken from all the patients.
References
- 1.Ziv A, Small SD, Wolpe PR. Patient safety and simulation-based medical education. Med Teach. 2000;22(5):489–495. doi: 10.1080/01421590050110777. [DOI] [PubMed] [Google Scholar]
- 2.Rosen KR. The history of medical simulation. J Crit Care. 2008;23(2):157–166. doi: 10.1016/j.jcrc.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Aïm F, Lonjon G, Hannouche D, Nizard R. Effectiveness of virtual reality training in orthopaedic surgery. Arthrosc J Arthrosc Relat Surg. 2016;32(1):224–232. doi: 10.1016/j.arthro.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Pfandler M, Lazarovici M, Stefan P, Wucherer P, Weigl M. Virtual reality-based simulators for spine surgery: a systematic review. Spine J. 2017;17(9):1352–1363. doi: 10.1016/j.spinee.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Yoo JS, Patel DS, Hrynewycz NM, Brundage TS, Singh K. The utility of virtual reality and augmented reality in spine surgery. Ann Transl Med. 2019;7(S5):S171. doi: 10.21037/atm.2019.06.38. S171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyaw BM, Saxena N, Posadzki P, et al. Virtual reality for health professions education: systematic review and meta-analysis by the digital health education collaboration. J Med Internet Res. 2019;21(1):e12959. doi: 10.2196/12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGaghie WC. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2012;86(6):706–711. doi: 10.1097/ACM.0b013e318217e119.Does. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality training improves operating room performance. Ann Surg. 2002;236(4):458–464. doi: 10.1097/01.SLA.0000028969.51489.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Visser H, Watson M, Salvado O, Passenger J. Progress in virtual reality simulators for surgical training and certification. Med J Aust. 2011;194(4):38–40. doi: 10.5694/j.1326-5377.2011.tb02942.x. [DOI] [PubMed] [Google Scholar]
- 10.Aziz MA, Mckenzie JC, Wilson JS, Cowie RJ, Ayeni SA, Dunn BK. The human cadaver in the age of biomedical informatics. Anat Rec. 2002;269(1):20–32. doi: 10.1002/ar.10046. [DOI] [PubMed] [Google Scholar]
- 11.Bridges M, Diamond DL. The financial impact of teaching surgical residents in the operating room. Am J Surg. 1999;177(1):28–32. doi: 10.1016/S0002-9610(98)00289-X. [DOI] [PubMed] [Google Scholar]
- 12.Tan SSY, Sarker SK. Simulation in surgery: a review. Scott Med J. 2011;56(2):104–109. doi: 10.1258/smj.2011.011098. [DOI] [PubMed] [Google Scholar]
- 13.Gasco J, Patel A, Ortega-Barnett J, et al. Virtual reality spine surgery simulation: an empirical study of its usefulness. Neurol Res. 2014;36(11):968–973. doi: 10.1179/1743132814Y.0000000388. [DOI] [PubMed] [Google Scholar]
- 14.Shi J, Hou Y, Lin Y, Chen H, Yuan W. Role of visuohaptic surgical training simulator in resident education of orthopedic surgery. World Neurosurg. 2018;111:e98–e104. doi: 10.1016/j.wneu.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Gottschalk MB, Yoon ST, Park DK, Rhee JM, Mitchell PM. Surgical training using three-dimensional simulation in placement of cervical lateral mass screws: a blinded randomized control trial. Spine J. 2015;15(1):168–175. doi: 10.1016/j.spinee.2014.08.444. [DOI] [PubMed] [Google Scholar]
- 16.Tomlinson SB, Hendricks BK, Cohen-Gadol A. Immersive three-dimensional modeling and virtual reality for enhanced visualization of operative neurosurgical anatomy. World Neurosurg. 2019;131:313–320. doi: 10.1016/j.wneu.2019.06.081. [DOI] [PubMed] [Google Scholar]
- 17.Atesok K, Mabrey JD, Jazrawi LM, Egol KA. Surgical simulation in orthopaedic skills training. J Am Acad Orthop Surg. 2012;20(7):410–422. doi: 10.5435/JAAOS-20-06-410. [DOI] [PubMed] [Google Scholar]
- 18.Chikwe J, de Souza AC, Pepper JR. No time to train the surgeons. BMJ. 2004;328(February):418–419. doi: 10.1136/bmj.328.7437.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris KA, Nousiainen MT, Reznick R Competency-based resident education—The Canadian perspective. Surg (United States). 2019;167(4):681-684. doi:10.1016/j.surg.2019.06.033 [DOI] [PubMed]
- 20.Kikinis R, Pieper SD, Vosburgh K. In: Intraoperative Imaging Image-Guided Therapy. Jolesz Ferenc A., editor. 2014. 3D Slicer: a platform for subject-specific image analysis, visualization, and clinical support. Editor 3(19):277–289 ISBN: 978-1-4614-7656-6 (Print) 978-1-4614-7657-3 (Online) [Google Scholar]
- 21.Xiang L, Zhou Y, Wang H, Zhang H, Song G, Zhao Y, Han J, Liu J. Significance of preoperative planning simulator for junior surgeons' training of pedicle screw insertion. J Spinal Disord Tech. 2015;Vol 28(1):E25–E29. doi: 10.1097/BSD.0000000000000138. [DOI] [PubMed] [Google Scholar]
- 22.Klein S, Whyne C, Rush R, Ginsberg H. CT- based patient specific simulation software for pedicle screw insertion. J spinal Disord Tech. 2009;22(7):502–506. doi: 10.1097/BSD.0b013e31819877fd. [DOI] [PubMed] [Google Scholar]