Abstract
Introduction
Neuropilin 1 (NRP-1) is a novel co-receptor promoting SARS-CoV-2 infectivity. Animal data indicate a role in trans-endothelial lipid transport and storage. As human data are sparse, we aimed to assess the role of NRP-1 in 2 metabolic active tissues in human obesity and in the context of weight loss-induced short- and long-term metabolic changes.
Methods
After a standardized 12-week weight reduction program, 143 subjects (age >18; body mass index ≥27 kg/m<sup>2</sup>, 78% female) were randomized to a 12-month lifestyle intervention or a control group using a stratified randomization scheme. This was followed by 6-month follow-up without any intervention. Phenotyping was performed before and after weight loss, after 12-month intervention and after subsequent 6 months of follow-up. Tissue-specific insulin sensitivity was estimated by HOMA-IR (whole body and mostly driven by liver), insulin sensitivity index (ISI)Clamp (predominantly skeletal muscle), and free fatty acid (FFA) suppression during hyperinsulinemic-euglycemic clamp (FFASupp) (predominantly adipose tissue). NRP-1 mRNA expression was measured in subcutaneous adipose tissue (NRP-1AT) and skeletal muscle (NRP-1SM) before and after weight loss.
Results
NRP-1 was highly expressed in adipose tissue (7,893 [7,303–8,536] counts), but neither NRP-1AT nor NRP-1SM were related to estimates of obesity. Higher NRP-1AT was associated with stronger FFASupp (r = −0.343, p = 0.003) and a tendency to higher ISIClamp (r = 0.202, p = 0.085). Weight loss induced a decline of NRP-1AT but not NRP-1SM. This was more pronounced in subjects with stronger reduction of adipose ACE-2 mRNA expression (r = 0.250; p = 0.032) but was not associated with short- and long-term improvement of FFASupp and ISIClamp.
Conclusion
NRP-1AT is related to adipose insulin sensitivity in obesity. Weight loss-induced decline of NRP-1AT seems not to be involved in metabolic short- and long-term improvements after weight loss. However, weight loss-induced reduction of both NRP-1AT and ACE-2AT indicates a lower susceptibility of adipose tissue for SARS-CoV-2 after body weight reduction.
Keywords: Adipose tissue, Insulin sensitivity, Obesity, Neuropilin 1, Weight loss
Introduction
Numerous data indicate a substantial association of metabolic and cardiovascular morbidity with an unfavorable clinical course of COVID-19 disease [1, 2]. As several of these detrimental comorbidities are frequently seen in obesity, increased fat mass (FM) is assumed to promote an inauspicious course of Coronavirus disease. Accordingly, increased disease severity [3, 4, 5] and worse clinical outcome [6, 7, 8] of COVID-19 disease are reported in subjects with higher body mass index (BMI) or visceral adiposity. Although increased airway resistance, impaired respiratory muscle function followed by disturbed respiratory mechanic and gas exchange [9], hyperinflammatory state due to adipose tissue dysfunction [10], and elevation of circulating phospholipase A2 activity might [11] be relevant in this context, numerous other endogenous factors are supposed to play a crucial role in the interaction of obesity and COVID-19 disease. We have demonstrated a decrease of adipose ACE-2 receptor expression by weight loss and an inverse relationship to insulin sensitivity [12]. As the binding of spike protein of the SARS-CoV-2 to ACE-2 receptor is an essential process for virus entry, these findings may also be relevant in the context of COVID-19 disease.
Very recently, neuropilin 1 (NRP-1) has been described as a novel co-receptor facilitating the binding of SARS-CoV-2 to ACE-2 [13]. NRP-1 is abundantly expressed by endothelial cells and serves as a co-receptor for the vascular endothelial growth factor (VEGF) [14]. However, it can also be found in other cell types like vascular smooth muscle cells, mesenchymal stem cells, and adipose tissue macrophages [15, 16]. The presence of NRP-1 on the cell surface seems to be crucial in the context of the COVID-19 pandemic. It potentiates the entry and infectivity of the SARS-CoV-2 virus [13, 17] though not affecting cell surface attachment of the spike protein. In detail, after proteolytic cleavage of surface glycoprotein S of the coronavirus by furin into S1 and S2, the C-end terminal rule motif of the S1 subunit binds to NRP-1 which enables infection of the cells by SARS-CoV-2 virus [13, 17]. Accordingly, clinical data demonstrate enriched NRP-1 expression in lung tissue of COVID-19 patients [18]. However, available data strongly suggest that NRP-1 alone is not sufficient to allow infection of cells by SARS-CoV-2. Only co-expression of ACE-2 and NRP-1 markedly increase viral infectivity [13].
Beside the role in virus infectivity, several metabolic properties of NRP-1 are known. Binding of VEGF-B to both VEGF receptor 1 and the co-receptor NRP-1 increases trans-endothelial lipid transport as well as peripheral lipid uptake and storage [19]. By this means, NRP-1 is involved in the regulation of free fatty acid (FFA) availability for ß-oxidation [15]. Accordingly, NRP-1 deficient adipose tissue macrophages are less efficient at internalizing lipids and shift their metabolism toward glucose catabolism [15]. This promotes a rather pro-inflammatory M1-like phenotype contributing to an impaired metabolism. Moreover, NRP-1 deficiency provokes an increase of large hypertrophic adipocytes with raised lipolytic activity augmenting the risk of fatty liver disease [15]. Vice versa, preserved NRP-1 expression in adipose tissue macrophages is required for angiogenesis preventing tissue hypoxia and inflammation. Furthermore, the NRP-1 mediated restoration of the impaired FFA oxidation contributes to improved insulin sensitivity in mice [15].
Given the relevance of NRP-1 in lipid metabolism and during SARS-CoV-2 infection, we aimed to test our hypotheses that (1) NRP-1 expression in the metabolic active tissue compartments skeletal muscle and adipose tissue is related to metabolic alterations seen in moderate obesity and (2) weight loss modifies NRP-1 expression in these tissues. These analyses will increase current knowledge regarding NRP-1 expression and metabolism in humans. Most interestingly, as current data are sparse in humans and NRP-1 may also represent a therapeutic target in prevention of tissue-specific SARS-CoV-2 infections [20], in particular the regulation of NRP-1 by lifestyle-based weight loss interventions may be a crucial mechanism decreasing the susceptibility to SARS-CoV-2 infections in obesity.
Methods
Participants
A total of 156 subjects characterized by overweight or obesity (120 female and 36 male) (BMI ≥27 kg/m2) were enrolled in a 12-week multimodal weight loss intervention. All subjects who lost at least 8% of initial body weight (n = 143) were subsequently included in a 12-month randomized controlled weight maintenance intervention followed by 6 months of follow-up. Details of the performed weight loss-weight maintenance trial (Maintain-Adult, ClinicalTrials.gov NCT00850629) were already described [21, 22]. The study was performed between 2010 and 2016 at a University Center.
Study Design
The major characteristics of the trial are shown in online supplementary Figure S1 (for all online suppl. material, see www.karger.com/doi/10.1159/000520419). After the initial weight loss period, the effects of a 12-month multimodal lifestyle intervention to maintain body weight was compared with a control group within a randomized controlled trial.
Pre-Trial Weight Loss Phase
A structured weight reduction program was performed to reduce body weight by at least 8%. This includes caloric restriction using a very low energy diet and nutritional counseling, physical exercises, and psychological advices [22].
Twelve-Month Randomized Weight Maintenance Phase
All subjects (n = 143, 112 female and 31 male) were randomly assigned to an intervention or control group. Subjects of the intervention group underwent a 12-month multimodal lifestyle intervention based on nutritional counseling, physical exercises, and psychological advices. No energy restriction was intended during this maintenance period [21, 22]. No structured counseling was performed in the control group. Details of the study protocol are described in the online supplement.
Follow-Up Period
After 12 months, both groups underwent a 6-month free living period. No further active intervention was performed during this follow-up period.
Randomization and Masking
Randomization was performed by the study team using a stratified randomization list. Stratification considered body weight at baseline (3 BMI strata) and sex. Subjects could not be blinded to group assignment as no intervention was performed in the control group.
Outcomes
The primary outcome defined as weight regain after 18 months (absolute change of BMI from T0 to T18 [kg/m2]) was reported previously [21]. Predefined secondary outcomes were the analysis of hormonal, transcriptional, and metabolic predictive markers of body weight regain, metabolic improvement, and cardiovascular risk factors. Within this exploratory analysis, we report the data of adipose and myocellular mRNA expression of NRP-1 in the context of obesity, weight loss, and weight loss-induced long-term changes of insulin resistance.
Power Calculation
Weight change was used for power calculation. This considered the fact that the variance of any causal endocrine parameter should be smaller than that of body weight, since the latter is also affected by other parameters such as socioeconomic or lifestyle factors, which do not necessarily modify endocrine circuits. We aimed to identify a weight difference of 1.15% between intervention and control group. This reflects 30% of a previously described effect after 6 months [23]. Given a variance of 1.96% in the control group, we estimated a sample size of 46 individuals per treatment arm would be required to provide 80% power with an α-error rate of 5% (query 7.0). We assumed a 20% drop out rate during the initial weight loss period and about 15% drop outs during the randomized intervention period. Therefore, at least 144 adults had to be included in the weight reduction period (T-3).
Phenotyping
During the 3 days preceding phenotyping a dietary recommendation of a balanced energy intake was given to all participants. Phenotyping focusing on anthropometric, hormonal, and metabolic evaluation was performed before (T-3) and after (T0) weight loss, 12 months (T12) after randomization, and after the subsequent 6-month follow-up period (T18). Phenotyping procedures were performed following a 10-h overnight fast at the endocrine trial center of the Charité Medical School at 8:00 a.m. To avoid interactions between the study procedures, the phenotyping procedures were planned and carried out at intervals of at least 2 days. This includes body impedance analysis using AKERN BIA 101 (SMT medical GmbH & Co. KG, Würzburg, Germany). Waist circumference (WC) was measured 3 times and the means were calculated. Fasting blood samples were taken between 8.00 and 9:00 a.m. The oral glucose tolerance test was performed at 9:00 a.m. A hyperinsulinemic-euglycemic clamp was conducted on a separate day at T-3, T0, and T12 as previously described [21, 24]. Finally, adipose tissue biopsies were taken at T-3 and T0 from different sides to avoid an effect of previous biopsy on mRNA expression at T0. Biopsy samples (0.5–1.0 g) were obtained from periumbilical abdominal subcutaneous adipose tissue by repeated needle biopsies using a 12 G biopty-cut needle (CR Bard GmbH, Karlsruhe, Germany). Muscle biopsies were taken from the gastrocnemius muscle using the same approach at T-3 and T0. Fat and muscle samples were subsequently snap-frozen in liquid nitrogen and stored at −80°C. Blood samples were centrifuged, and plasma and serum samples were frozen immediately at −80°C until further analyses.
Laboratory Analyses
Standard laboratory analyses are described in the online supplement. Tissue samples were analyzed by RNA sequencing using the HiSeq2000 system (TruSeq SBS Kit-Hs 200 cycles; Illumina, San Diego, CA, USA) (details, see online supplement).
Statistics and Calculations
Assessment of myocellular insulin sensitivity based on hyperinsulinemic-euglycemic clamp by dividing the average glucose infusion rate (mg glucose/min) during the steady state by the body weight (M-value). Subsequently, insulin sensitivity index (ISIClamp) was calculated as ratio of M-value to the serum insulin concentration (I, mU/L) during steady state of the clamp. HOMA-IR was calculated to assess whole body insulin sensitivity [25]. Effects of insulin on lipolysis was calculated by the suppression of FFA during hyperinsulinemic-euglycemic clamp and expressed as relative changes compared to fasting FFA levels (FFASupp) [12, 26, 27].
Weight loss-induced changes (T-3 to T0) of specific parameters (BMI, FM, WC, HOMA-IR, FFASupp, ISIClamp, and NRP-1 mRNA expression (NRP-1AT and NRP-1SM)) were expressed as percentage of baseline values at T-3 (ΔBMI, ΔFM, ΔWC, ΔHOMA-IR, ΔFFASuppT3T0, ΔISIClampT3T0, ΔNRP-1AT, and ΔNRP-1SM). Changes of FFASupp, and ISIClamp between T-3 and T12 were expressed as percentage of baseline values at T-3 (ΔFFASuppT3T12 and ΔISIClampT3T12).
Statistical procedures were performed using SPSS version 25.0 (SPSS Inc., Chicago, IL, USA) and SAS software, version 9.4 (SAS Institute). The data reported here reflect secondary analyses and are based on per protocol analysis including data of all available participants at the corresponding time point. Comparisons were made via paired Student's t test for normally distributed data and Wilcoxon test for skewed data. Correlations between variables were investigated by Pearson's correlation coefficient for normally distributed data or Spearman's rank correlation coefficient for skewed data. Data were presented as median and limits of the interquartile range (25th–75th percentile). Raw values were plotted unless stated otherwise. Results were considered significant, if the 2-sided α was below 0.05.
Multivariate linear regression models were used to analyze the impact of weight loss-induced changes of NRP-1AT on weight loss-induced relative changes of ISIClamp (ΔISIClampT3T0) and ΔFFASupp (ΔFFASuppT3T0) as well as long-term improvement of both estimates of insulin sensitivity (ΔISIClampT3T12 and ΔFFASuppT3T12). These models included age, sex, and concomitant decline of BMI as potential confounders. As the hyperinsulinemic-euglycemic clamp was only performed at T-3, T0, and T12, we have chosen these time points for calculation. Given the effect of the 12-month intervention on BMI [21] at T12, the models regarding long-term improvement were also adjusted for treatment group and concomitant BMI changes.
Study Approval
The study protocol was approved by the Institutional Review Board of the Charité Medical School and all subjects gave written informed consent. The trial was registered at ClinicalTrials.gov (NCT00850629).
Results
As previously reported, 143 middle-aged subjects with overweight or obesity were analyzed within this study before and after weight loss [22]. 122 and 112 subjects could be re-evaluated after the randomized intervention period (T12) and after additional 6 months of follow-up (T18), respectively. Baseline characteristics of the participants have been reported previously and are shown in Table 1 [22].
Table 1.
Basal characteristics of the participants
| Parameter | Participants, n | Before weight loss |
|
|---|---|---|---|
| median | (IQR) | ||
| Females, n (%) | 112 (78) | ||
| Postmenopausal females, n (%) | 58 (51) | ||
| Age, year | 143 | 50.5 | (41.7–60.8) |
| BMI, kg/m2 | 143 | 35.6 | (32.9–41.0) |
| FM, % | 126 | 37.4 | (32.6–40.0) |
| WC, cm | 143 | 106.5 | (97.0–117.0) |
| Total cholesterol, mg/dL | 143 | 200.0 | (176.0–233.0) |
| HDL-cholesterol, mg/dL | 143 | 49.3 | (40.6–61.3) |
| LDL-cholesterol, mg/dL | 143 | 123.1 | (103.2–146.8) |
| Triacylglycerol, mg/dL | 143 | 126.0 | (85.0–169.0) |
| HOMA-IR | 142 | 2.2 | (1.4–3.4) |
| ISIClamp, mg kg−1 min−1/(mU L−1) | 139 | 0.06 | (0.04–0.08) |
| FFASupp, % | 139 | −91.5 | (−85.7–[–94.4]) |
| ACE-2AT, counts | 75 | 29 | (20–47) |
Metabolic and anthropometric parameters of the randomized participants before weight loss. Results are presented as median and IQR. IQR, interquartile range; FM, fat mass; WC, waist circumference; ISI, insulin sensitivity index; FFA, free fatty acid; BMI, body mass index.
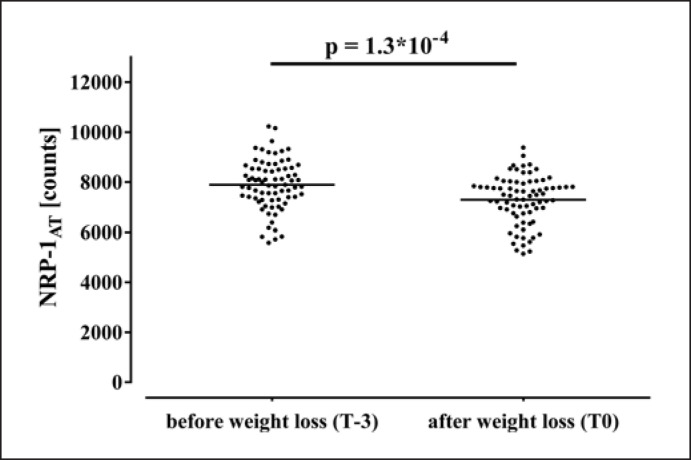
At baseline, abundant NRP-1 mRNA expression (NRP-1AT) was detected in subcutaneous adipose tissue (7,893 [7,303–8,536] counts), which was higher in females than males (8,088 [7,436–8,672] vs. 7,409 [6,800–7,724] counts; p = 0.038). In contrast, NRP-1 was expressed to a much lower degree in skeletal muscle (NRP-1SM) (2,040 [1,818–2,306] counts) (p for tissue comparison = 1.6 × 10−49) and did not differ between male and female subjects (2,002 [1,867–2,313] vs. 2,050 [1,757–2,307] counts; p = 0.712). In contrast to NRP-1AT (r = 0.092, p = 0.434), NRP-1SM declined with age (r = −0.219, p = 0.042).
Neither NRP-1AT nor NRP-1SM expression were related to estimates of obesity (BMI: r = 0.079, p = 0.498 and r = −0.040, p = 0.712; FM: r = 0.045, p = 0.714 and r = 0.055, p = 0.633; WC: r = 0.103, p = 0.379 and r = −0.082, p = 0.448, respectively) as well as whole body insulin sensitivity (HOMA-IR: r = −0.178, p = 0.127 and r = −0.092, p = 0.394, respectively). Accordingly, presence of type 2 diabetes or metabolic syndrome did not affect expression of both, NRP-1AT or NRP-1SM (Table 2). Focusing on tissue-specific effects, a stronger insulin-mediated suppression of FFAs (FFASupp) reflecting adipose tissue insulin sensitivity was associated with higher NRP-1AT expression (r = −0.343, p = 0.003). The relationship between myocellular insulin sensitivity (ISIClamp) and higher NRP-1AT or NRP-1SM expression marginally failed to be significant (r = 0.202, p = 0.085 and r = 0.189, p = 0.083). Although both ACE-2 (ACE-2AT) [12] (online suppl. Table S1) and NRP-1 were highly expressed in subcutaneous adipose tissue, no relationship between NRP-1AT and ACE-2AT could be revealed (r = 0.167, p = 0.151).
Table 2.
Metabolic state and NRP-1 mRNA expression in subcutaneous adipose tissue and skeletal muscle
| Subjects | NRP-1AT |
NRP-1SM |
||
|---|---|---|---|---|
| median | (IQR) | median | (IQR) | |
| With type 2 diabetes | 7,666 | (7,464–8,005) | 1,975 | (1,456–2,161) |
| Without type 2 diabetes | 8,067 | (7,273–8,643) | 2,040 | (1,819–2,325) |
| With metabolic syndrome | 7,862 | (7,254–8,681) | 2,045 | (1,770–2,308) |
| Without metabolic syndrome | 8,005 | (7,327–8,481) | 2,019 | (1,844–2,316) |
NRP-1 mRNA expression in subcutaneous adipose tissue and skeletal muscle before weight loss. Results are presented as median and IQR. NRP-1, neuropilin 1; IQR, interquartile range.
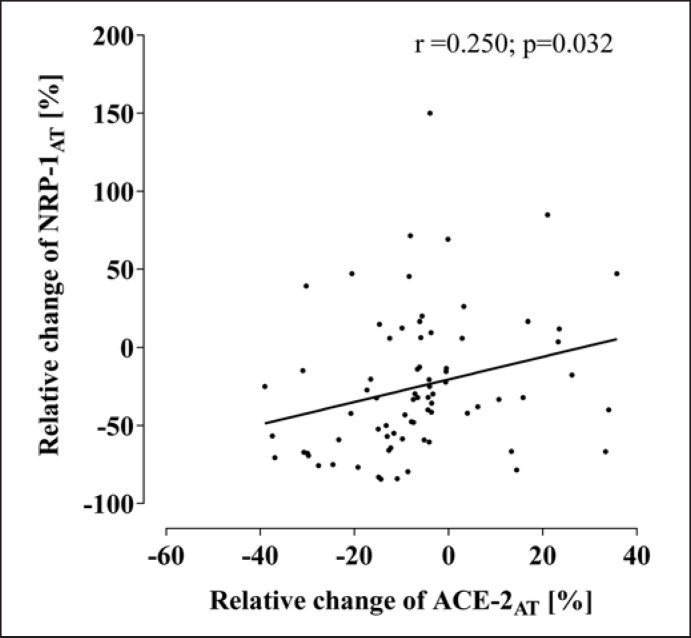
Diet-induced reduction of BMI (−4.6 [4.3–4.9] kg/m2) [28] was accompanied by a decline of NRP-1AT as shown in Figure 1, while NRP-1SM was not modified (2,040 [1,818–2,306] vs. 1,994 [1,719–2,258] counts; p = 0.193). Previously described improvements of estimates of obesity (BMI, FM, and WC) [21] and insulin resistance (HOMA-IR, ISIClamp, and FFASupp) (online suppl. Table S1) [12] were not associated with the decline of NRP-1AT. However, reduction of adipose ACE-2AT was more pronounced in subjects also demonstrating a stronger decline of NRP-1AT (r = 0.250, p = 0.032) as shown in Figure 2.
Fig. 1.
Effects of weight loss on adipose NRP-1 mRNA expression. NRP-1, neuropilin 1.
Fig. 2.
Association of weight loss-induced changes of ACE-2 and NRP-1 in subcutaneous adipose tissue. NRP-1, neuropilin 1.
Weight loss-induced changes of NRP-1AT were neither related to observed short- and long-term improvement of FFASupp (FFASuppT3T0 and FFASuppT3T12) nor ISIClamp (ISIClampT3T0 and ISIClampT3T12). This is in line with our previous findings regarding ACE-2AT [12].
Discussion
The surface protein NRP-1 is a known cofactor of endothelial VEGF receptor 1 involved in lipid metabolism. The presence of this factor could facilitate the effect of VEGF-B on trans-endothelial lipid uptake, transport, and storage [19]. Impaired lipid metabolism represents a crucial element in regulation of FM and obesity, which is thought to be devastating in the course of COVID-19 disease. Interestingly, very recent experimental data indicate that NRP-1 is also a crucial co-receptor of ACE-2 promoting virus entry and infectivity in COVID-19 [13, 17]. This is of high interest, as hepatic expression of other cofactors like transmembrane serine protease 2 is reported to be increased in subjects with obesity and fatty liver disease [29]. Nevertheless, data concerning NRP-1 in humans are still rather sparse. Thus, characterization of NRP-1 in obesity is of immense relevance in humans.
In accordance with the described effect on lipid utilization and FM regulation, we revealed an abundant expression of NRP-1 in subcutaneous adipose tissue in obese humans. This was related to insulin-mediated FFA-suppression, which can be considered as a parameter of adipose tissue insulin sensitivity [26, 27]. Thus, high NRP-1 abundance might have beneficial effects on local metabolism in adipose tissue. However, available data do not indicate a direct interaction of NRP-1/VEGF system and insulin signaling so far. Recently, it was shown that downregulation of NRP-1 in macrophages is associated with exacerbated insulin resistance in mice. This is potentially driven by activation of nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (Nlrp3) inflammasome resulting in increased systemic inflammation and insulin resistance [30]. Vice versa, Nlrp3 ablation in mice prevents obesity-induced inflammasome activation in fat depots and enhances insulin signaling [31]. Thus, current evidence rather indicates an indirect interaction of NRP-1 expression and insulin sensitivity via reduced Nlrp3 activity. This is supported by our human findings. However, it might be primarily relevant for adipose tissue insulin signaling, as only a borderline or no relationship with ISIClamp or HOMA-IR, mostly reflecting myocellular or hepatic insulin sensitivity, respectively, was seen. Although the exact nature of this is currently unknown, the selective accumulation of NRP-1-positive macrophages in adipose tissue but not in circulation, seen in mice during diet-induced obesity [15], may indicate a specific role of adipose tissue macrophages. However, the situation seems to be more complex, as Dai et al. [30] reported no modification of NRP-1 expression in subcutaneous adipose tissue of high fat-fed mice. To the best of our knowledge, the cause of this discrepancy is currently not known. Alternative mechanisms seem to modulate the relationship between obesity and NRP-1 in adipose tissue. This might also explain why we did not reveal any association with estimates of general and central obesity in our subjects. Actually, the degree of obesity might play a crucial role in this context. Indeed, we could not detect any effect of the presence or absence of metabolic syndrome or type 2 diabetes in our cohort with moderate obesity. However, recently published data of Frühbeck et al. [32] indicate an increased NRP-1 expression in subcutaneous adipose tissue only in subjects with morbid obesity who also suffered from impaired glucose metabolism, but not in those with morbid obesity and normal glucose metabolism. Even if our results might be affected by the low rate of type 2 diabetics in our cohort (app. 10%), future research is clearly warranted to investigate this discrepancy.
Nevertheless, weight loss resulted in a specific decline of NRP-1 expression in adipose tissue, while no effect could be revealed in skeletal muscle. The metabolic relevance of this attenuation remains unclear, as the reduction of NRP-1AT was neither associated with short- nor long-term improvements of estimates of obesity and insulin resistance. This is in contrast to the weight loss-induced decline of ACE-2AT, which was associated with smaller short- and long-term improvement of insulin sensitivity [12]. Given the impact of NRP-1 co-expression on SARS-CoV-2 infectivity [13] the downregulation of both, ACE-2AT and NRP-1AT might however be crucial to drop down the internalization rate of active viruses in adipose tissue compartment after weight loss. As NRP-1 could not promote virus entry by itself, the concomitant effect on ACE-2 will be required. Even though the direct effect of weight loss on susceptibility for SARS-CoV-2 infection is not yet shown in humans, weight loss induced by bariatric surgery seems to be associated with less severe COVID-19 disease [33]. This might potentially involve modulation of adipose ACE-2AT and NRP-1AT expression.
Actually, there is no clear evidence for SARS-CoV-2 infection of adipose tissue and detected virus level in peripheral blood samples were rather low [34]. Nevertheless, the frequent occurrence of extrapulmonary manifestations demonstrates the systemic character of SARS-CoV-2 infections [35]. The presence of ACE-2 and NRP-1 in adipose tissue and the fact that adipose tissue is a proven target tissue for multiple viruses [36] make it plausible that adipose tissue could be also targeted by SARS-CoV-2. In fact, a reservoir function for SARS-CoV-2 is currently discussed for adipose tissue [36].
Some caveats limit the interpretation of our data as most of the results are based on associations. However, numerous experimental and animal findings published previously are in accordance with our human data and provide potential molecular explanations. Given mentioned data highlighting the metabolic role of NRP-1 expression in adipose tissue macrophages [15], cell-specific analysis of NRP-1 expression in adipocytes, and adipose tissue macrophages is of high relevance. However, our data focused on mRNA expression measured in total adipose tissue. Therefore, future research is required. Behavioral and environmental factors are known to modify the effects of dietary weight loss interventions [37, 38]. Especially behavioral factors were not considered in our current analysis. Although we aimed to standardize the dietary intake and physical activity during the group sessions, we cannot exclude that our result might be affected by these factors. Moreover, numerous techniques are described to assess adipose tissue insulin sensitivity. Hyperinsulinemic-euglycemic clamp is considered as a well-established analytical approach, although different insulin infusion rates (4–80 mU/m2, partly used stepwise) [27, 39, 40, 41] and outcome measures (suppression of plasma FFAs, suppression of plasma glycerol or glycerol and FFA rates of appearance using tracer dilution technique) [27, 39, 41, 42] were reported. Even if tracer dilution technique is widely accepted to assess adipose tissue lipolysis, suppression of FFA during hyperinsulinemic-euglycemic clamp is also frequently used [26, 42, 43, 44, 45]. Therefore, we believe that we used a valid approach, even if adipose tissue microdialysis was not performed in this study. Finally, recent data indicating a detrimental effect of obesity in COVID-19 also highlighted the role of visceral adipose tissue mass [6]. Weight loss-induced changes of NRP-1 expression in this compartment might differ from subcutaneous adipose tissue. Therefore, our findings are not simply transferable to visceral adipose tissue.
On the other hand, numerous strengths of the current trial should be mentioned. These include the large sample size, the long duration of a highly standardized intervention, subsequent observation, and the comprehensive phenotyping including detailed assessment of adipose and myocellular insulin sensitivity by hyperinsulinemic-euglycemic clamp and tissue biopsies in a large cohort.
Conclusion
Taken together, in accordance to the facilitating role of NRP-1 regarding VEGF-B-mediated trans-endothelial lipid uptake, transport, and storage, NRP-1 is abundantly expressed in adipose tissue and is related to the antilipolytic effects of insulin. Although the metabolic short- and long-term impact of weight loss-induced decline of NRP-1AT is currently not known, the decline of both, subcutaneous adipose NRP-1 and ACE-2 expression may potentially mediate beneficial effects regarding SARS-CoV-2 infectivity. This might be of high relevance in obesity.
Statement of Ethics
The study protocol was approved by the Ethical Review Board of the Charité Medical School (EA1/140/12). The trial was registered at ClinicalTrials.gov (NCT00850629). All subjects gave written informed consent.
Conflict of Interest Statement
We declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding Sources
This research was supported by the Deutsche Forschungsgemeinschaft (DFG KFO 218/1), the German Diabetes Society (DDG), and the German Ministry for Education and Research (BMBF) by support of the German Center for Cardiovascular Research (DZHK; BER5.1).
Author Contributions
D.S., F.B., K.M., and J.S. researched data and wrote the manuscript; K.M., L.L., D.S., and L.S. were responsible for data analysis. All authors contributed to interpretation of the results. All authors critically read and edited several drafts before submission. All authors read and approved the submitted version.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary Material
Supplementary data
Acknowledgments
We thank K. Simon, B. Horchler, N. Huckauf, and C. Kalischke for excellent technical assistance as well as A. Reisshauer for the support regarding physical activity intervention. We thank Nestlé HealthCare Nutrition GmbH, Frankfurt am Main, Germany, for the opportunity to purchase the Optifast 2® diet at a reduced price. We thank all participants of the clinical trial.
References
- 1.Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31((6)):1068–77.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argenziano MG, Bruce SL, Slater CL, Tiao JR, Baldwin MR, Barr RG, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalligeros M, Shehadeh F, Mylona EK, Benitez G, Beckwith CG, Chan PA, et al. Association of obesity with disease severity among patients with COVID-19. Obesity. 2020;28((7)):1200–4. doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28((7)):1195–9. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao M, Piernas C, Astbury NM, Hippisley-Cox J, O'Rahilly S, Aveyard P, et al. Associations between body-mass index and COVID-19 severity in 6.9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2021;9((6)):350–9. doi: 10.1016/S2213-8587(21)00089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe M, Caruso D, Tuccinardi D, Risi R, Zerunian M, Polici M, et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. 2020;111:154319. doi: 10.1016/j.metabol.2020.154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe M, Risi R, Tuccinardi D, Baquero CJ, Manfrini S, Gnessi L. Obesity and SARS-CoV-2: a population to safeguard. Diabetes Metab Res Rev. 2020:e3325. doi: 10.1002/dmrr.3325. [DOI] [PubMed] [Google Scholar]
- 8.Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murugan AT, Sharma G. Obesity and respiratory diseases. Chron Respir Dis. 2008;5((4)):233–42. doi: 10.1177/1479972308096978. [DOI] [PubMed] [Google Scholar]
- 10.Fruhbeck G, Catalan V, Rodriguez A, Ramirez B, Becerril S, Salvador J, et al. Involvement of the leptin-adiponectin axis in inflammation and oxidative stress in the metabolic syndrome. Sci Rep. 2017;7((1)):6619. doi: 10.1038/s41598-017-06997-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snider JM, You JK, Wang X, Snider AJ, Hallmark B, Zec MM, et al. Group IIA secreted phospholipase A2 is associated with the pathobiology leading to COVID-19 mortality. J Clin Invest. 2021;131((19)):e149236. doi: 10.1172/JCI149236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Spranger L, Soll D, Beer F, Brachs M, Spranger J, et al. Metabolic impact of weight loss induced reduction of adipose ACE-2: potential implication in COVID-19 infections? Metabolism. 2020;113:154401. doi: 10.1016/j.metabol.2020.154401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370((6518)):856–60. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92((6)):735–45. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 15.Wilson AM, Shao Z, Grenier V, Mawambo G, Daudelin JF, Dejda A, et al. Neuropilin-1 expression in adipose tissue macrophages protects against obesity and metabolic syndrome. Sci Immunol. 2018;3((21)):eaan4626. doi: 10.1126/sciimmunol.aan4626. [DOI] [PubMed] [Google Scholar]
- 16.Kofler N, Simons M. The expanding role of neuropilin: regulation of transforming growth factor-beta and platelet-derived growth factor signaling in the vasculature. Curr Opin Hematol. 2016;23((3)):260–7. doi: 10.1097/MOH.0000000000000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daly JL, Simonetti B, Klein K, Chen KE, Williamson MK, Anton-Plagaro C, et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370((6518)):861–5. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383((2)):120–8. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464((7290)):917–21. doi: 10.1038/nature08945. [DOI] [PubMed] [Google Scholar]
- 20.Chekol Abebe E, Mengie Ayele T, Tilahun Muche Z, Asmamaw Dejenie T. Neuropilin 1: a novel entry factor for SARS-CoV-2 infection and a potential therapeutic target. Biologics. 2021;15:143–52. doi: 10.2147/BTT.S307352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mai K, Brachs M, Leupelt V, Jumpertz-von Schwartzenberg R, Maurer L, Gruters-Kieslich A, et al. Effects of a combined dietary, exercise and behavioral intervention and sympathetic system on body weight maintenance after intended weight loss: results of a randomized controlled trial. Metabolism. 2018;83:60–7. doi: 10.1016/j.metabol.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Mai K, Li L, Wiegand S, Brachs M, Leupelt V, Ernert A, et al. An integrated understanding of the molecular mechanisms of how adipose tissue metabolism affects long-term body weight maintenance. Diabetes. 2019;68((1)):57–65. doi: 10.2337/db18-0440. [DOI] [PubMed] [Google Scholar]
- 23.Woo J, Sea MM, Tong P, Ko GT, Lee Z, Chan J, et al. Effectiveness of a lifestyle modification programme in weight maintenance in obese subjects after cessation of treatment with Orlistat. J Eval Clin Pract. 2007;13((6)):853–9. doi: 10.1111/j.1365-2753.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- 24.Mai K, Andres J, Biedasek K, Weicht J, Bobbert T, Sabath M, et al. Free fatty acids link metabolism and regulation of the insulin-sensitizing fibroblast growth fctor-21. Diabetes. 2009;58((7)):1532–8. doi: 10.2337/db08-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28((7)):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Magkos F, Fabbrini E, Conte C, Patterson BW, Klein S. Relationship between adipose tissue lipolytic activity and skeletal muscle insulin resistance in nondiabetic women. J Clin Endocrinol Metab. 2012;97((7)):E1219–23. doi: 10.1210/jc.2012-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ter Horst KW, van Galen KA, Gilijamse PW, Hartstra AV, de Groot PF, van der Valk FM, et al. Methods for quantifying adipose tissue insulin resistance in overweight/obese humans. Int J Obes. 2017;41((8)):1288–94. doi: 10.1038/ijo.2017.110. [DOI] [PubMed] [Google Scholar]
- 28.Brachs M, Wiegand S, Leupelt V, Ernert A, Kintscher U, Jumpertz von Schwarzenberg R, et al. ANP system activity predicts variability of fat mass reduction and insulin sensitivity during weight loss. Metabolism. 2016;65((6)):935–43. doi: 10.1016/j.metabol.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Fondevila MF, Mercado-Gomez M, Rodriguez A, Gonzalez-Rellan MJ, Iruzubieta P, Valenti V, et al. Obese patients with NASH have increased hepatic expression of SARS-CoV-2 critical entry points. J Hepatol. 2021;74((2)):469–71. doi: 10.1016/j.jhep.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai X, Okon I, Liu Z, Bedarida T, Wang Q, Ramprasath T, et al. Ablation of neuropilin 1 in myeloid cells exacerbates high-fat diet-induced insulin resistance through Nlrp3 inflammasome in vivo. Diabetes. 2017;66((9)):2424–35. doi: 10.2337/db17-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17((2)):179–88. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fruhbeck G, Catalan V, Valenti V, Moncada R, Gomez-Ambrosi J, Becerril S, et al. FNDC4 and FNDC5 reduce SARS-CoV-2 entry points and spike glycoprotein S1-induced pyroptosis, apoptosis, and necroptosis in human adipocytes. Cell Mol Immunol. 2021;18:2457–9. doi: 10.1038/s41423-021-00762-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchesi F, Valente M, Ricco M, Rottoli M, Baldini E, Mecheri F, et al. Effects of bariatric surgery on COVID-19: a Multicentric Study from a high incidence area. Obes Surg. 2021;31((6)):2477–88. doi: 10.1007/s11695-020-05193-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323((18)):1843–4. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carvalho T. Extrapulmonary SARS-CoV-2 manifestations. Nat Med. 2020;26((12)):1806. doi: 10.1038/s41591-020-01162-z. [DOI] [PubMed] [Google Scholar]
- 36.Ryan PM, Caplice NM. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity. 2020;28((7)):1191–4. doi: 10.1002/oby.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299((10)):1139–48. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 38.Del CP, Bryan DR, Garvey WT, Gower BA, Hunter GR. Dietary adherence during weight loss predicts weight regain. Obesity. 2011;19((6)):1177–81. doi: 10.1038/oby.2010.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jocken JW, Goossens GH, Boon H, Mason RR, Essers Y, Havekes B, et al. Insulin-mediated suppression of lipolysis in adipose tissue and skeletal muscle of obese type 2 diabetic men and men with normal glucose tolerance. Diabetologia. 2013;56((10)):2255–65. doi: 10.1007/s00125-013-2995-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JY, Nasr A, Tfayli H, Bacha F, Michaliszyn SF, Arslanian S. Increased lipolysis, diminished adipose tissue insulin sensitivity, and impaired β-cell function relative to adipose tissue insulin sensitivity in obese youth with impaired glucose tolerance. Diabetes. 2017;66((12)):3085–90. doi: 10.2337/db17-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stumvoll M, Jacob S, Wahl HG, Hauer B, Loblein K, Grauer P, et al. Suppression of systemic, intramuscular, and subcutaneous adipose tissue lipolysis by insulin in humans. J Clin Endocrinol Metab. 2000;85((10)):3740–5. doi: 10.1210/jcem.85.10.6898. [DOI] [PubMed] [Google Scholar]
- 42.Campos GM, Rabl C, Havel PJ, Rao M, Schwarz JM, Schambelan M, et al. Changes in post-prandial glucose and pancreatic hormones, and steady-state insulin and free fatty acids after gastric bypass surgery. Surg Obes Relat Dis. 2014;10((1)):1–8. doi: 10.1016/j.soard.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lomonaco R, Bril F, Portillo-Sanchez P, Ortiz-Lopez C, Orsak B, Biernacki D, et al. Metabolic impact of nonalcoholic steatohepatitis in obese patients with type 2 diabetes. Diabetes Care. 2016;39((4)):632–8. doi: 10.2337/dc15-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haugaard SB, Andersen O, Pedersen SB, Dela F, Fenger M, Richelsen B, et al. Tumor necrosis factor alpha is associated with insulin-mediated suppression of free fatty acids and net lipid oxidation in HIV-infected patients with lipodystrophy. Metabolism. 2006;55((2)):175–82. doi: 10.1016/j.metabol.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 45.Curry TB, Roberts SK, Basu R, Basu A, Schroeder D, Joyner MJ, et al. Gastric bypass surgery is associated with near-normal insulin suppression of lipolysis in nondiabetic individuals. Am J Physiol Endocrinol Metab. 2011;300((4)):E746–51. doi: 10.1152/ajpendo.00596.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.