Abstract
Postacute sequelae of SARS-CoV2 (PASC) infection is an emerging global health crisis, variably affecting millions worldwide. PASC has no established treatment. We describe 2 cases of PASC in response to opportune administration of over-the-counter antihistamines, with significant improvement in symptoms and ability to perform activities of daily living. Future studies are warranted to understand the potential role of histamine in the pathogenesis of PASC and explore the clinical benefits of antihistamines in the treatment of PASC.
Keywords: antihistamine, COVID-19, long-COVID, long-haul COVID, postacute SARS-CoV-2 infection (PASC), SARS-CoV-2 infection (PASC), treatment
Introduction
Postacute sequelae of SARS-CoV-2 infection (PASC) is a new, ill-defined disease characterized by persistent symptoms that extend beyond the expected resolution time frame. Symptoms also tend to evolve, with more than 200 patient-reported symptoms reflecting multiple organ system involvement.1 PASC is an emerging global health crisis, with an estimated prevalence of 30%.2 PASC is often painful, debilitating, and impairs daily functioning.3 The United States (US) Centers for Disease Control and Prevention (CDC) reports that two-thirds of patients hospitalized with SARS-CoV-2 develop PASC within 6 months after infection.4 Among nonhospitalized patients with COVID-19 in California, Huang et al5 found that 11% continued to report symptoms at least 6 months after infection.
As of August 2021, an estimated 54 million people globally had PASC. There is no treatment for PASC, and the World Health Organization has urgently requested that countries prioritize PASC research and care.6 We present 2 patients, previously healthy middle-aged women who developed signs and symptoms consistent with PASC in early 2020. Both patients report nearly complete resolution of symptoms after administration of over-the-counter histamine antagonists. These case reports are consistent with recent studies showing endothelial injury and aberrant immune response with histamine release during COVID-19.7, 8, 9, 10
Methods
These case reports were deemed exempt by the Indiana University and the University of California, Irvine Institutional Review Boards. Cases were obtained from members of Survivor Corps, a virtual COVID-19 research and advocacy organization hosted on Facebook. Survivor Corps has more than 170,000 members who post about their COVID-19–related experiences, symptoms, and self-management of PASC. In the time frame of March 24, 2020, until July 23, 2021, survivors mentioned using antihistamines on Survivor Corps’ Facebook page more than 900 times (Lambert et al, unpublished data, 2021). To our knowledge, no reports yet document the potential for antihistamine use in managing PASC.
Results
Patient One
Patient 1 is a White woman in her 40s who works in health care. Past medical history includes idiopathic Raynaud phenomenon, polycystic ovarian syndrome, heterozygous factor V Leiden mutation, and an immunoglobulin E-confirmed milk allergy managed with a modified diet. The patient has no history of vascular occlusion, deep vein thrombosis, pulmonary emboli, or stroke. She is a nonsmoker and uses alcohol occasionally. Her body mass index (BMI) is within the reference range for normal weight, and she received annual influenza immunization 3 months before onset of SARS-CoV-2 infection. Before her illness, she engaged in moderate-to-intense physical activity for 1 to 2 hours 4 to 5 times per week.
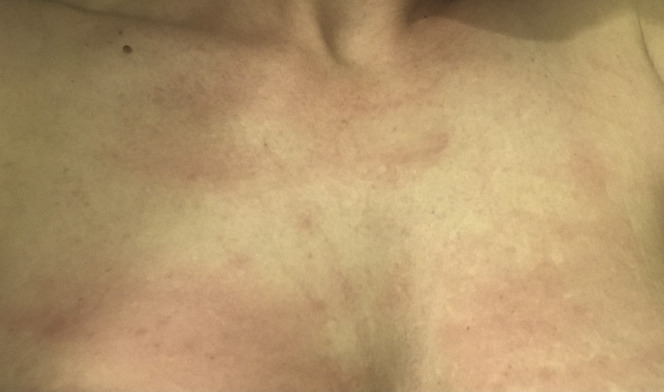
The patient was likely infected with SARS-CoV-2 in early January 2020 while attending a dance festival in southern California. At this time, SARS-CoV-2 testing was limited, and there was no COVID-19 screening protocol in the US. Within 72 hours of probable exposure, the patient developed profound fatigue, malaise, and headache. Within 10 days, she developed a disseminated rash over her anterior and posterior trunk (Figure 1 ). She experienced inspiratory chest pain, bilateral flank pain, dry cough, fever, night sweats, dysgeusia, and ulcerations of the tongue, soft palate, and inside lower lip. The acute illness phase lasted approximately 24 days, at which time she experienced partial resolution of symptoms. To date, however, she experiences persistent rashes in multiple locations as well as flank pain, bilateral chest pain, and right-sided headache.
Figure 1.
Patient 1—Patient with COVID-19 presented with rash on her chest.
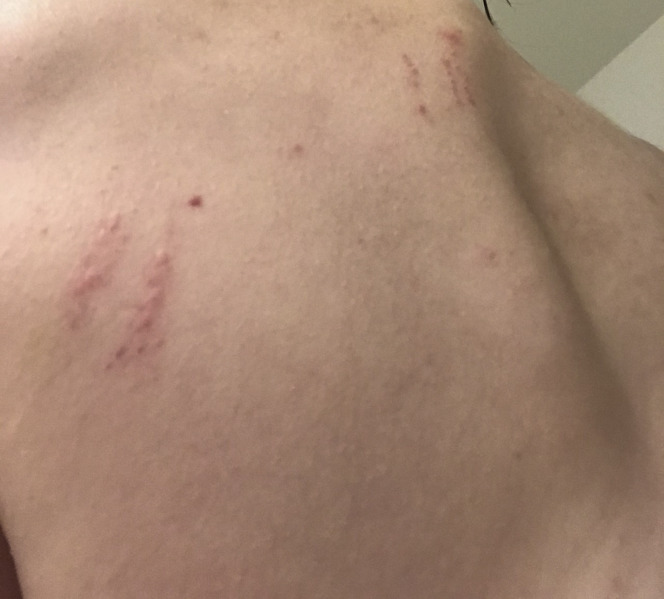
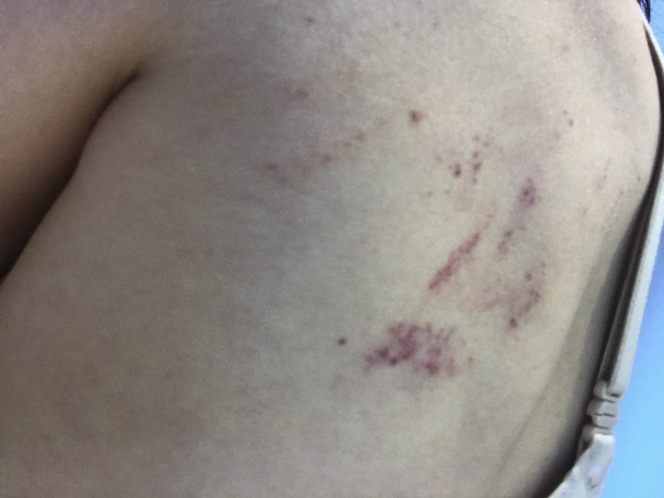
Approximately 2 weeks after the acute illness (January 2020), the patient developed alopecia and noted pinpoint petechiae of the arms, legs, and trunk. Several weeks later (February 2020), she experienced multiple relapses of symptoms with varying degrees of severity. The symptoms tended to occur in clusters. For example, she would first experience bruising, rash, and oral ulcerations, and several days later, develop fatigue and unilateral headache. Other symptoms included nausea, chest pain, flank pain, bruising of arms, legs, and/or trunk, and intermittent rashes (lasting a few hours) on the chest. These were followed by longer-lasting rashes distributed across the body (Figures 2 and 3 ).
Figure 2.
Patient 1—Rash on left upper back and 3 linear rashes on upper back on the spine.
Figure 3.
Patient 1—Rash on left scapula.
In March 2020, the patient reported cognitive impairment (“brain fog”) that occurred concurrently with the aforementioned symptoms. The cognitive impairment also followed a relapsing and remitting pattern as described previously with other symptoms.
Serologic testing for SARS-CoV-2 was obtained when available, in June 2020, 6 months after initial presentation. The serologic testing result was negative.
Multiple specialists evaluated the patient, including internal medicine, neurology, infectious diseases, hematology, dermatology, psychiatry, and rheumatology. Results of magnetic resonance imaging of the head and neck were unremarkable. Other investigations included a 12-lead electrocardiogram, complete blood count, basic metabolic panel, prothrombin time, international normalized ratio, antinuclear antibodies, erythrocyte sedimentation rate, thyroid-stimulating hormone, thyroid panel, rheumatoid factor, C-reactive protein, vitamin B12, zinc, magnesium, and urinalysis with culture and sensitivity. All results were within expected ranges.
Results of ultrasound imaging of the abdomen were also unremarkable. A computed tomography of chest, abdomen, and pelvis in July 2020 revealed right lower-lobe ground-glass opacification. She was referred to pulmonology at this time and was treated with oral antibiotics for 10 days.
More than 1 year after the initial infection, in March 2021, the patient presented to dermatology with oral ulcerations and a rash on the left thumb. Punch biopsies were taken from the mouth and thumb. Results showed mixed lymphocytic and neutrophilic infiltrate at both sites.
Six months after the initial infection (June 2020), the patient ate cheese, to which she has a known allergy. She therefore self-administered an over-the-counter oral histamine antagonist, diphenhydramine HCl 50 mg. The following morning, she noted considerable relief of fatigue and improved ability to concentrate. She did not take additional histamine antagonists for the next 72 hours, during which time she experienced a recurrence of the fatigue and cognitive symptoms. The patient again self-administered diphenhydramine, and observed an improvement in symptoms. After 6 months of daily self-administration of diphenhydramine HCl 50 mg, the patient was prescribed hydroxyzine pamoate 25 mg and instructed to titrate the dose not to exceed 150 mg/d, until relief of symptoms occurred. At 25 mg daily, she experienced partial symptom resolution with decreased rashes and bruising, improved cognition, and reduced fatigue. On follow-up, the pulmonologist also noted these effects.
Because the patient could titrate hydroxyzine pamoate dosage for symptom relief, 4 months after the initial antihistamine administration (in October 2020), the patient increased the dose to 50 mg daily. On a dosing regimen of 50 mg hydroxyzine pamoate daily, the patient had a nearly complete resolution of exercise intolerance, chest pain, fatigue, and brain fog. She has remained on this dose for more than 9 months, with a sustained improvement in symptoms. Additionally, she has experienced significant decreases in headache, rash, and bruises, as well as in frequency and severity of oral ulcerations. She reports achieving approximately 90% of her pre-PASC functioning, fully returning to work, and engaging in moderate-intensity exercise of 1 to 2 hours 5 to 6 times a week.
Patient Two
The second case reports a middle-aged White woman who works as a teacher. Before COVID illness, she was healthy, led an active lifestyle, and had an unremarkable medical history except for asthma and seasonal allergies treated with fexofendine. The patient is a nonsmoker and uses alcohol occasionally, has a BMI within normal range, and received the annual influenza vaccination 4 months before infection with SARS-CoV-2. The patient likely contracted COVID-19 while caring for her child, who was unwell with respiratory symptoms consistent with COVID-19.
Approximately 48 hours after exposure, she developed body aches, an unproductive cough, and a temperature of 99.7°F. A test for SARS-CoV-2 (by reverse transcription polymerase chain reaction) was negative. However, the following day, she developed chills, shortness of breath, and inspiratory chest pain and was given a clinical diagnosis of COVID-19. Over subsequent weeks she developed fever, joint pain, and shortness of breath. These symptoms persisted for approximately 3 months.
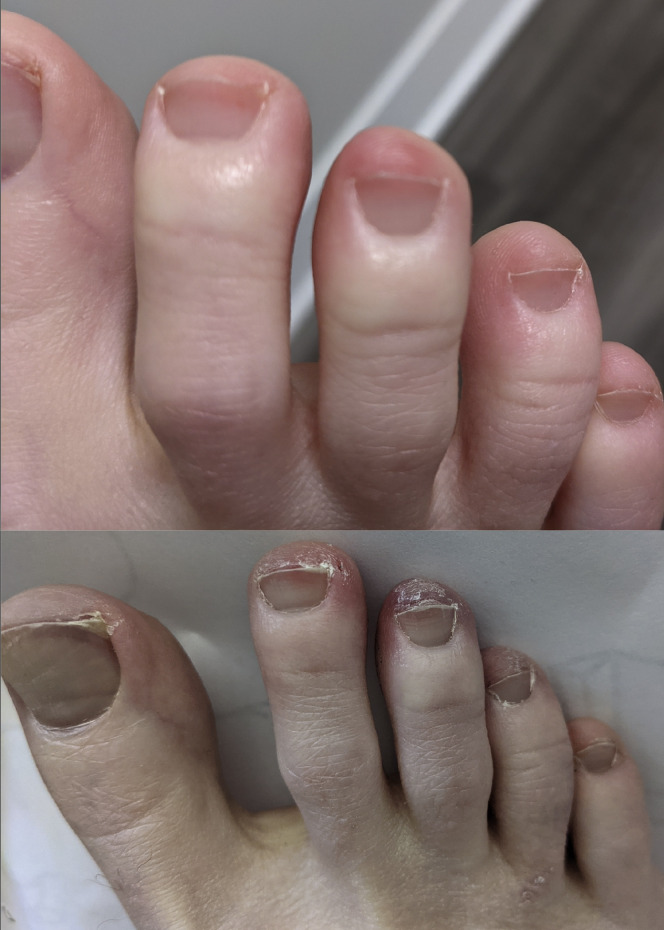
One month after onset of symptoms, the patient developed tachycardia, “COVID-19 toes” (Figure 4 ), significant postexertion fatigue, joint pain, insomnia, intermittent anosmia and dysgeusia, and difficulty concentrating. Three months after symptom onset, an immunoglobulin G antibody test for SARS-CoV-2 was negative.
Figure 4.
Patient 2—COVID toes at 9 months (top) and 11 months (bottom) after symptom onset.
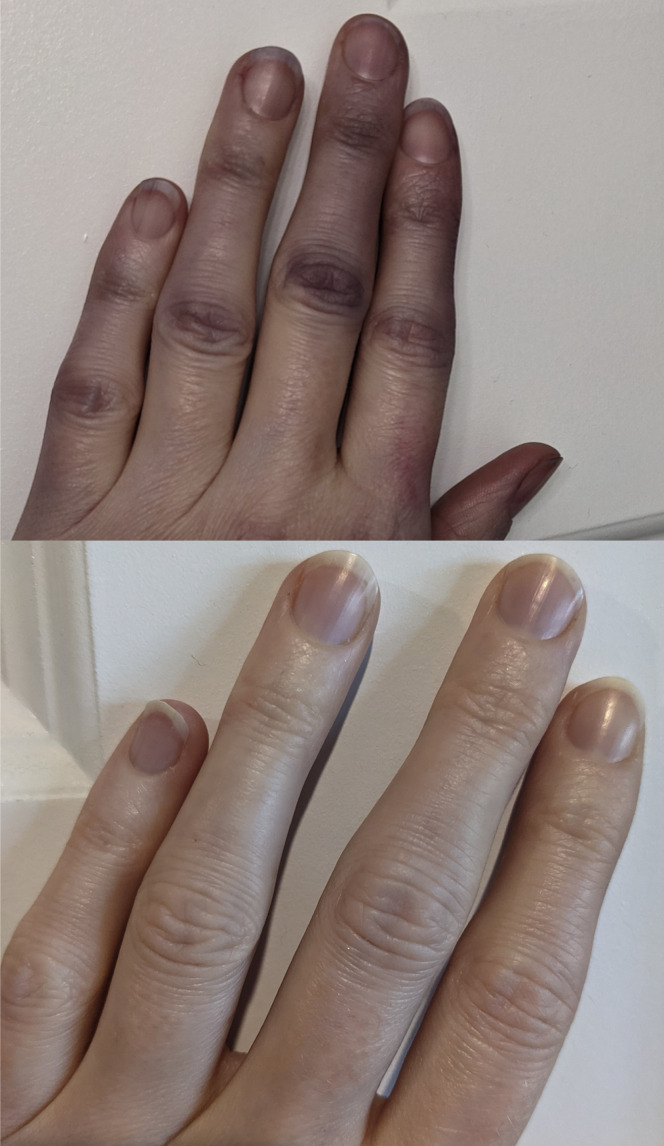
Symptoms persisted for 9 months, with intensification of fatigue and difficulty concentrating as well as ongoing abdominal pain, bloating, and COVID-19 toes. She also developed bilateral acrocyanosis in her hands, from the proximal interphalangeal joint extending to the tips of all 10 fingers (Figure 5 ). The patient sought information online and recognized that her condition could reflect microvascular damage from the initial SARS-CoV-2 infection. She then self-administered 5 baby aspirin daily for 2 days, after which time the acrocyanosis resolved. At this time, the patient began to self-manage her symptoms with a daily regimen of 1 baby aspirin, vitamin D3 (5,000 IU), zinc, and a probiotic supplement.
Figure 5.
Patient 2—Bilateral acrocyanosis of the hands, from the proximal interphalangeal joint extending to the fingertips, of 9 months (top) after onset of symptoms and after resolution (bottom).
Thirteen months after the onset of acute COVID-19 infection, the patient substituted 25 mg diphenhydramine HCl for the fexofenadine as she had run out of the latter. She did not seek guidance for this medication change using an online source. The following morning, she noted resolution of brain fog and fatigue. She therefore continued the diphenhydramine daily, whereupon she noted marked improvement in brain fog, fatigue, and abdominal pain, and abatement of anosmia and dysgeusia. After 2 doses of diphenhydramine 25 mg, the patient was able to resume her preillness exercise regimen. Since this time, the patient has been taking 25 mg diphenhydramine at night and 180 mg fexofenadine in the morning and has attained approximately 95% of preillness functioning, sustained now for more than 60 days.
Discussion
These cases reflect typical presentations of persons with PASC as well as the frequently varying quality and the waxing/waning nature of related symptoms. Both occurred in previously healthy, middle-aged women with normal BMIs, and for whom debilitating symptoms of extreme fatigue and impaired cognition relapsed and remitted for more than 1 year. The clinical presentations described here are consistent with other research on PASC11 and offer anecdotal evidence for treatment of PASC symptoms with a highly accessible over-the-counter histamine antagonist.
Similarly, previous studies7 , 12 , 13 have reported that patients hospitalized with COVID-19 demonstrated symptom improvement after administration of a histamine antagonist. In critically ill patients, histamine antagonists were associated with increased survival and halted COVID-19 symptom progression.12 Further, in a study of 22,000 patients, the histamine agonist famotidine was associated with lower levels of immune markers of severe COVID-19 disease—suggesting that histamine antagonists may mitigate the excessive cytokine release typical of COVID-19.7
Despite these promising findings, it is unclear whether a single histamine antagonist or a combination of first and/or later generations of these drugs can provide optimal treatment for persons experiencing PASC. Observations from the present patients suggest further need to characterize PASC and to investigate the potential role of histamine in its pathogenesis. This may yield insights into potential symptom management strategies.
Conclusion
SARS-CoV-2 binds to angiotensin-converting enzyme 2 receptors abundantly expressed throughout the body, resulting in cellular infection with inflammation and activation of the endothelium.8 As a result, SARS-CoV-2 may affect any organ system. This may be a critical factor in the heterogeneity of PASC signs and symptoms. Nurse practitioners (NPs) are thus likely to see significant variability in PASC symptoms among affected patients, and the trajectory for symptom resolution is unknown. For this reason, NPs can perhaps best support recovery by validating patient experiences and symptom presentations, even in those with negative results on serologic or polymerase chain reaction testing.14, 15
The earliest patients with PASC may not have had access to SARS-CoV-2 confirmatory testing, owing to the lack of reliable and valid testing at the beginning of the US pandemic.16 As a result, some patients with PASC have been dismissed or ignored by medical providers.15 This can leave such patients feeling disempowered and devalued, adversely affecting both well-being and willingness to attend future medical appointments.17 Providers should therefore familiarize themselves with the emerging spectrum of PASC symptoms and provide nonjudgmental and supportive care.
Finally, as understanding of PASC is just beginning to emerge, it is critical that NPs and other providers not conflate its effects with other ill-defined conditions such as myalgic encephalomyelitis and chronic Lyme disease in the absence of adequate scientific evidence.
Acknowledgment
We would like to acknowledge Maria del Pilar Giraldo Herrera, BSN, RN, PHN, DNP student for her thoughtful comments and suggestions for revisions of later drafts of this manuscript.
Biographies
Melissa D. Pinto, PhD, RN, is an associate professor at the University of California, Irvine Sue and Bill Gross School of Nursing and can be contacted at mdpinto@uci.edu
Natalie Lambert, PhD, is an associate professor at Indiana University School of Medicine, Biostatistics and Health Data Sciences, Indianapolis.
Charles A. Downs, PhD, ACNP-BC, is an associate professor at the University of Miami School of Nursing and Health Studies, Coral Gables, FL.
Heather L. Abrahim, MSN, MPA, RN, CCRN, is PhD students at the University of California, Irvine Sue and Bill Gross School of Nursing.
Thomas D. Hughes, MSN, AGACNP-BC, CCRN, is PhD students at the University of California, Irvine Sue and Bill Gross School of Nursing.
Amir M. Rahmani, PhD, is an associate professor at the University of California, Irvine Sue and Bill Gross School of Nursing and Department of Computer Science.
Candace W. Burton, PhD, AFN-BC, AGN-BC, is associate professors at the University of California, Irvine Sue and Bill Gross School of Nursing.
Rana Chakraborty, MD, MSc, is professor and senior associate consult at the Mayo Clinic College of Medicine and Science Department of Pediatrics and Adolescent Medicine, Pediatrics, Immunology, and Obstetrics and Gynecology, Rochester, MN.
Footnotes
In compliance with standard ethical guidelines, the authors report no relationships with business or industry that would pose a conflict of interest.
References
- 1.Davis H.E., Assaf G.S., McCorkell L., et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Logue J.K., Franko N.M., McCulloch D.J., et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert N., Survivor Corps El-Azab S.A., et al. COVID-19 survivors’ report of the timing duration, and health impacts of Post-Acute Sequelae of SARS-CoV-2 (PASC) infection. MedRxiv. 2021 doi: 10.1111/jocn.16541. https://www.medrxiv.org/content/10.1101/2021.03.22.21254026v2 Accessed August 20, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez-Romieu C.A., Serena L., Armand M., et al. Health care utilization and clinical characteristics of non-hospitalized adults in an integrated health care system 28-180 days after COVID-19 diagnosis—Georgia, May 2020–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):644–650. doi: 10.15585/mmwr.mm7017e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y., Pinto M.D., Borelli J.L., et al. COVID symptoms, symptom clusters, and predictors for becoming a long-hauler: Looking for clarity in the haze of the pandemic. MedRxiv. 2021 doi: 10.1177/10547738221125632. https://www.medrxiv.org/content/10.1101/2021.03.03.21252086v1 Accessed August 20, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wise J., Long C.O.V.I.D. WHO calls on countries to offer patients more rehabilitation. BMJ. 2021;372:n405. doi: 10.1136/bmj.n405. [DOI] [PubMed] [Google Scholar]
- 7.Malone R.W., Tisdall P., Fremont-Smith P., et al. COVID-19: gamotidine, histamine, mast cells, and mechanisms. Front Pharmacol. https://doi.org/10.3389/fphar.2021.633680 Published online March 23, 2021. [DOI] [PMC free article] [PubMed]
- 8.Kumar A., Narayan R.K., Kumari C., et al. Sars-Cov-2 cell entry receptor ACE2 mediated endothelial dysfunction leads to vascular thrombosis in COVID-19 patients. Med Hypotheses. 2020;145:110320. doi: 10.1016/j.mehy.2020.110320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patterson B.K., Francisco E.B., Yogendra R., et al. Persistence of SARS CoV-2 S1 protein in CD16+ monocytes in post-acute sequelae of COVID-19 (PASC) up to 15 months post-infection. MedRxiv. 2021 doi: 10.1101/2021.06.25.449905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nabavi N. Long COVID: how to define it and how to manage it. BMJ. 2020;370:M3489. doi: 10.1136/bmj.m3489. [DOI] [PubMed] [Google Scholar]
- 12.Hogan R.B., 2nd, Hogan R.B., 3rd, Cannon T., et al. Dual-histamine receptor blockage with cetirizine-famotidine reduces pulmonary symptoms in COVID-19 patients. Pulm Pharmacol Ther. 2020;63:101942. doi: 10.1016/j.pupt.2020.101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ennis M., Tiligada K. Histamine receptors and COVID-19. Inflamm Res. 2021;70(1):67–75. doi: 10.1007/s00011-020-01422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandenberg O., Martiny D., Rochas O., et al. Considerations for diagnostic COVID-19 testing. Nat Rev Microbiol. 2021;19:171–183. doi: 10.1038/s41579-020-00461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto M.D., Downs C.A., Lambert N., Burton C.W. How an effective response to post-acute sequelae of SAR-CoV2 Infection relies on nursing research. Res Nurs Health. 2021;44(5):743–745. doi: 10.1002/nur.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aucott J.N., Rebman A.W. Long-haul COVID: heed the lessons from other infection triggered illnesses. Lancet. 2021;397(10278):P967–P968. doi: 10.1016/S0140-6736(21)00446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denniston C., Molloy E., Rees C.E. ‘I will never ever go back’: patients written narrative of health care communication. Med Educ. 2018;52:757–771. doi: 10.1111/medu.1361. [DOI] [PubMed] [Google Scholar]