Abstract
Background
Renin-angiotensin aldosterone system inhibitors (RAASi) are commonly used among patients hospitalized with a severe acute respiratory syndrome coronavirus 2 infection coronavirus disease 2019 (COVID-19). We evaluated whether continuation versus discontinuation of RAASi were associated with short term clinical or biochemical outcomes.
Methods
The RAAS-COVID-19 trial was a randomized, open label study in adult patients previously treated with RAASi who are hospitalized with COVID-19 (NCT04508985). Participants were randomized 1:1 to discontinue or continue RAASi. The primary outcome was a global rank score calculated from baseline to day 7 (or discharge) incorporating clinical events and biomarker changes. Global rank scores were compared between groups using the Wilcoxon test statistic and the negative binomial test (using incident rate ratio [IRR]) and the intention-to-treat principle.
Results
Overall, 46 participants were enrolled; 21 participants were randomized to discontinue RAASi and 25 to continue. Patients’ mean age was 71.5 years and 43.5% were female. Discontinuation of RAASi, versus continuation, resulted in a non-statistically different mean global rank score (discontinuation 6 [standard deviation [SD] 6.3] vs continuation 3.8 (SD 2.5); P = .60). The negative binomial analysis identified that discontinuation increased the risk of adverse outcomes (IRR 1.67 [95% CI 1.06-2.62]; P = .027); RAASi discontinuation increased brain natriuretic peptide levels (% change from baseline: +16.7% vs -27.5%; P = .024) and the incidence of acute heart failure (33% vs 4.2%, P = .016).
Conclusion
RAASi continuation in participants hospitalized with COVID-19 appears safe; discontinuation increased brain natriuretic peptide levels and may increase risk of acute heart failure; where possible, RAASi should be continued.
Background
As infection rates for severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) continue to rise globally, there is a growing need to understand factors influencing coronavirus disease 2019 (COVID-19) outcomes. Observational data suggest that patients with pre-existing cardiovascular (CV) disease or CV risk factors are at increased risk for severe COVID-19 manifestations.1., 2., 3. COVID-19 appears to promote the development of cardiovascular complications, particularly in patients with pre-existing CV disease,4 with higher rates of myocardial injury, myocarditis, acute coronary syndrome, heart failure, and arrhythmias.5., 6., 7., 8.
While the mechanisms underlying the development of cardiovascular injury are unclear, there is a growing understanding that upregulation of the renin-angiotensin aldosterone system (RAAS) may contribute to underlying pathogenicity from SARS-COV-2.9 , 10 Among patients with underlying CV disease or CV risk factors, angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blocker (ARB) are among the most commonly prescribed RAASi.11 Emerging literature suggests that continuing RAAS inhibitors (RAASi) – such as ACEi/ARBs - may be associated with better outcomes in patients with COVID-19.12 , 13 The Blockers of Angiotensin Receptor and Angiotensin-Converting Enzyme inhibitors suspension in hospitalized patients with coronavirus infection (BRACE-CORONA) trial randomized 659 participants to either continuation or discontinuation of ACEi/ARBs, and suggested that continuation of ACEi/ARB is safe and associated with a numeric, non-statistically significant, reduction in risk of CV events.12 Prior studies have demonstrated safety of continuing RAASi in the COVID-19 clinical severity at 30 days; however, in exploratory analyses, lower organ dysfunction rates was observed in the discontinued RAASi arms, thereby highlighting the need for more studies in the acute COVID-19 setting.14 Consensus guidelines have suggested continuing RAASi in patients infected with COVID-19, yet they urge for the need for further evidence for RAASi use in patients with COVID-19.15 , 16
Given the disproportionate impact of SARS-COV-2 infection in patients with CV disease or CV risk factors,1 , 9 , 17 more evidence on the CV impact of discontinuation or continuation of RAASi among patients hospitalized with COVID-19 are needed. The RAAS-COVID-19 trial was conducted to evaluate whether an upfront strategy of temporary discontinuation of RAASi versus continuation impacts short term clinical and biomarker outcomes among patients admitted to hospital with COVID-19.
Methods
Study design
The RAAS-COVID-19 trial is a prospective, randomized, open label trial at three large referral hospitals in Montreal. The trial was designed to evaluate whether continuation or discontinuation of RAASi were associated with worse short term clinical or biochemical outcomes. The trial protocol was approved by the McGill institutional ethics review board, and all patients provided informed consent prior to enrollment. Details of the rational and trial design have been previously described18 and additional details are included in the Appendix. The RAAS-COVID-19 trial is registered with Clinicaltrials.gov, NCT04508985. Individual participant level data from this trial will not be made publicly available.
Participants
Hospitalized patients aged 18 years or older were eligible for the trial if they had a diagnosis of COVID-19 confirmed microbiologically by PCR testing within 96 hours of admission. Participants were included if they were chronically treated (duration ≥1 month) with an ACEi or ARB as an outpatient prior to admission. Participants were excluded if they had a clinical indication to stop ACEi or ARB treatment during hospitalization including hypotension or shock or immediate requirement for ventilation. Participants were further excluded if they had a history of heart failure with reduced ejection fraction (ejection fraction <40%) or recent heart failure admission in the past 3 months; use of five or more antihypertensive drugs at baseline, history of chronic kidney disease with an estimated glomerular filtration rate (eGFR) <45 mL/min/1.73m2; or had an anticipated discharge less than 24 hours. The full list of inclusion and exclusion criteria are included in the Appendix. None of our patients were vaccinated or received tocilizumab.
Study intervention
Eligible patients were randomized in a 1:1 ratio to a strategy of temporarily holding versus continuing the RAASi. Randomization was performed with an electronic database system at the time of enrollment using a random number generator. Neither participant, study team, nor treating team were blinded to study intervention. Reviewers for adjudicating heart failure events were blinded to randomization strategy.
Procedure
All participants were randomly assigned, and their treating physician was asked to keep the patient on the assigned treatment unless an important clinical change occurred before discharge. The withdrawn medication could be re-initiated any point after day 7 or on the day of discharge (if the participant was discharged prior to day 7) at the clinical discretion of the treating team. The study team communicated with the treating team daily to indicate that the medication had been withdrawn and to consider restarting the medication after day 7 or at the day of discharge. Among participants who were randomized to the intervention arm, a list of additional anti-hypertensive agents were provided to the treating team (Supplementary Table I). Clinicians were blinded to the follow-up blood work including natriuretic peptides and troponins unless they had ordered these tests for a clinical indication.
Demographic and clinical data were collected from participants at baseline and at day 7 (or at time of discharge) and supplemented by information from the electronic health record. Routine data during hospitalization were obtained and included: information on vital status (date and cause of death), length of stay, receipt of other treatments for COVID-19, vital signs (heart rate, blood pressure and respiratory rate), adverse cardiovascular events (including myocardial infarction, new atrial fibrillation, ischemic or hemorrhagic stroke, or acute decompensated heart failure), transfer to intensive care unit (and indication for transfer), and receipt of invasive ventilatory support. Biochemical data including high sensitivity troponin, brain natriuretic peptide (BNP), eGFR, c-reactive protein (CRP), and absolute lymphocyte count were obtained at baseline, day 4, and day 7 of admission (or prior to discharge). Discharge information including the length of stay, and RAASi re-initiation during admission (if beyond 7 days of hospitalization) or re-prescription at the time of discharge was also recorded. Patients were followed until 30-days post discharge to determine vital status and hospital readmission. We utilized definition of mild versus moderate COVID-19 as per previous publications and trials and included the following: Mild: blood oxygen saturation ≥94% and lung infiltrates ≤50%; Moderate: blood oxygen saturation <94% or lung infiltrates > 50%, or ratio of partial pressure of arterial oxygen to fraction of inspired oxygen < 300.12 , 19
Outcomes
The primary outcome was a global rank score in which all participants were assigned hierarchies of clinical and biomarker outcomes from baseline to day 7 (or discharge), with higher weighting given to endpoints of greatest clinical importance. In the context of the current trial, death was considered to be the most clinically meaningful endpoint, and therefore had the highest score. This was followed by admission to the intensive care unit (ICU), need for mechanical ventilation, and non-fatal major adverse cardiovascular events including acute decompensated heart failure. The lowest scores were assigned to biomarker changes (Table I ).
Table I.
Global rank hierarchy for the primary endpoint
| Item (from Randomization to day 4 for primary outcome) | Points |
|---|---|
| Death | 7 |
| Transfer to ICU for invasive ventilation | 6 |
| Transfer to ICU for other indication | 5 |
| Non-fatal MACE (Any of the following - MI, Stroke, Acute HF, new onset Afib) | 4 |
| Length of stay > 4 days | 3 |
| Development of acute kidney injury (>40% decline in eGFR or doubling of serum Cr) | 2 |
| Urgent intravenous treatment for high blood pressure/hypertensive crisis | 2 |
| >30% increase in baseline high sensitivity troponin | 1 |
| >30% increase in baseline BNP | 1 |
| Increase in baseline CRP >30% | 1 |
| Lymphocyte count drop >30% | 1 |
Afib, atrial fibrillation; BNP, brain natriuretic peptide; CRP, c-reactive protein; eGFR, estimated glomerular filtration rate calculated by the Modification of Diet in Renal Disease Study (MDRD) equation; ICU, intensive care unit; MACE, major adverse cardiac event; MI, myocardial infarction.
Components of the global rank score included: death; transfer to ICU for invasive ventilation or other indication; non-fatal major adverse cardiovascular events (any of: myocardial infarction, ischemic or hemorrhagic stroke, acute decompensated heart failure, or new onset atrial fibrillation); length of stay >4 days; development of acute kidney injury (>40% decline in eGFR or doubling of serum Cr from baseline); urgent intravenous treatment for high blood pressure/hypertensive crisis; or changes in biomarkers from baseline: >30% increase in high sensitivity troponin from baseline, >30% increase in BNP; >30% increase in CRP; or lymphocyte count drop >30%. Participants who died during the seventh day of the study were ranked based on all events occurring before their death and also including the fatal event in the score. Based on earlier literature published, CRP and lymphocyte count were demonstrated to have prognostic impacts in patients with COVID-19, hence was included within the hierarchical outcome.1 , 19., 20., 21.
Participants who did not die but were transferred to ICU for invasive ventilation were ranked based on all the events occurring before the ICU entry and also including the ICU admission in the score. Those participants who neither died nor were transferred to ICU for invasive ventilation were ranked based on the remaining outcomes.
Secondary endpoints were individual components of the primary global rank score. Exploratory endpoints included each individual component of the primary global rank score assessed from baseline up to day 7 or day of discharge, length of hospitalization, RAASi re-initiation during admission or at time of discharge, hospital readmission, or mortality within 30 days post discharge.
The ability to clearly define heart failure events in the context of a patient in hospital with an active COVID-19 infection remains challenging.22 The limited ability to conduct accurate physical examination, the overlapping symptoms of worsening COVID-19 infection and acute heart failure, and the overlap of features of the chest x-ray in patients with COVID-19 and acute heart failure challenges the ability to define a heart failure events. In light of this, we had initially defined a heart failure event, if the treating team clinically identified a heart failure event during the hospitalization. This approach has been demonstrated to have reasonable validity when compared to central blinded adjudication in heart failure trials.23 , 24 However, to further provide confidence in the ascertainment of outcomes, we conducted a post-hoc adjudication for heart failure events. We leveraged the standardized heart failure event definition as recommended by Hicks et al25 which is now the standard heart failure definition used widely across many heart failure clinical trials. We modified the Hicks et al definition to account for the fact that patients were already admitted in hospital (see Supplementary Table II). Re-adjudication was conducted by two team members (H.A. and M.A.) with any discrepancies evaluated by A.S. Adjudication was blinded to the initial identification of heart failure events and randomization allocation thereby the adjudication process was unbiased.
Sample size calculation
Using a global rank sum score strategy enables basing an endpoint decision on the totality of observed trends across multiple clinical and biomarker domain, and this strategy has been validated in several CV trials. Given prior rates of outcomes from COVID-19 literature, we estimated the following among participants admitted with COVID-19: 27% would require an ICU admission, 25% would require mechanical ventilation, and 28% would die.1 , 20 , 26 Additional CV and biomarker based outcomes in CV trials and initial publications from COVID-19 data.1 , 2 , 17 , 27 , 28 Based on these assumptions, we estimated a mean of 16 points in the control group and a reduction to 12 points in the experimental group with a standard deviation of 5 points. To meet these assumptions 40 participants were required to have an 80% chance of detecting (at the 5% level), a decrease in the primary outcome from 16 to 12 points (as above described).
Statistical analysis
All analyses were conducted using the intention-to-treat principle and included all randomized participants. The primary prespecified analysis of the global rank score is based on the Wilcoxon test statistic to compare the global rank score by treatment groups. The global rank score will be described as mean (with standard deviation [SD]) and median (with interquartile range [IQR]). The primary outcome was also analyzed by means of a post-hoc non-parametric test (negative binomial model) with the count of events as outcome, the treatment indication as independent variable and the duration of hospital stay (log of time in days) as exposure variable. The rationale for the negative binomial is that it enables the totality of events that occurred throughout the period of follow-up there by enabling a more comprehensive view of the hospitalization period.
Medians, 25th and 75th percentiles are presented for continuous variables; the number and percentage of participants in each category are presented for categorical variables. For all endpoints a P-value <.05 were considered statistically significant. Appropriate statistical models were used to examine the effect of the withdrawal intervention on both the primary, secondary, and exploratory outcomes adjusting for relevant covariates. Adjustment variables included age, sex, body-mass index and ethnicity. For continuous endpoint variables, conventional general linear models was used. For endpoints where the response is dichotomous (binary), the logistic regression model were used. Exploratory analysis of vitals and laboratory values monitored during duration of hospitalization were done by comparing the median percent change from baseline with the associated 25th and 75th percentile and by analysis of covariance. The results of the negative binomial test are described as an incident rate (IR; with 95% CI) and the difference between treatment arms is described as an incidence rate ratio (IRR; with 95% CI). For the primary analysis, we conducted several sensitivity analyses including adjustment for whether an individual was co-enrolled in another trials and removal of individuals who are co-enrolled in additional trials. All analyses were conducted using SAS version 9.4 (Cary, NC). This study was funded by the McGill Interdisciplinary Initiative in Infection and Immunity. The authors are solely responsible for the design and conduct of this study, all study analyses and drafting and editing of the paper.
Results
Baseline demographics
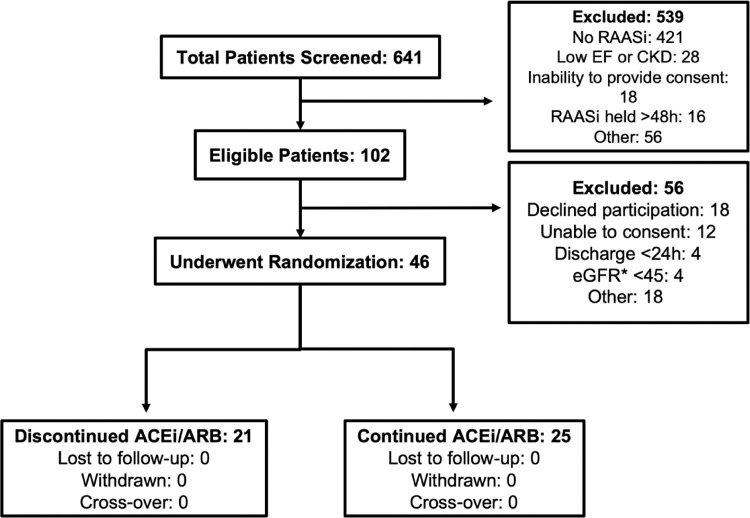
Between October 2020 and March 2021, 46 participants were enrolled. Overall, 21 patients were randomly assigned to discontinuation of RAASi and 25 patients to continuation of RAASi (Figure 1 ). The mean age of all participants was 71.5 years, 20 (43.5%) were female, the median number of days since COVID diagnosis was 2 days. In total, 29 (63.0%) of patients had mild COVID-19 infection while the remaining 17 (37.0%) had moderate COVID-19 infection. The most frequent ethnicities enrolled include White (19 [41.3%]), Central Asian (9 [19.6%]), and East Asian (8 [917.4%]). Overall, 18 (39.1%) of patients were on an ACEi, while 28 (60.9%) of patients were on an ARB. No patient was on a mineralocorticoid receptor agonist on admission (Table II ). Nearly one half of patients were treated with dexamethasone during hospitalization. There was no statistical difference in the overall baseline vital signs and laboratory biomarkers between groups (Table III ).
Figure 1.
Clinical trial flow diagram. ACEi, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; EF, ejection fraction; eGFR, estimated glomerular filtration rate (mL/min/1.73m2); RAASi, renin-angiotensin-aldosterone system inhibitors.
Table II.
Baseline characteristics by randomization arm
| Overall N = 46 | Discontinuation of RAAS inhibitor (N = 21) | Continuation of RAAS inhibitor (N = 25) | |
|---|---|---|---|
| Age at admission, years (SD), median (Q1, Q3) | 71.5 (12.9) | 69.4 (13.1) | 73.3 (12.7) |
| 71 (61, 84) | 65 (59, 81) | 73 (65, 84) | |
| Female, % | 20 (43.5) | 6 (28.6) | 14 (56.0) |
| Ethnicity, % | |||
| Black or African American | 4 (8.7) | 4 (19.1) | 0 (0.0) |
| Central Asian | 9 (19.6) | 3 (14.3) | 6 (24.0) |
| East Asian | 8 (17.4) | 4 (19.1) | 4 (16.0) |
| Southeast Asian | 4 (8.7) | 0 (0.0) | 4 (16.0) |
| White or Caucasian | 19 (41.3) | 10 (47.6) | 9 (36.0) |
| Not Reported or Refused to respond | 2 (4.4) | 0 (0.0) | 2 (8.0) |
| Number of days since COVID diagnosis, days, median (Q1, Q3)* | 2 (1, 6) | 2 (1, 8) | 2 (1, 3) |
| Mild COVID-19 | 29 (63.0) | 15 (71.4) | 14 (56.0) |
| Moderate COVID-19 | 17 (37.0) | 6 (28.6) | 11 (44.0) |
| BMI (Kg/m2) | 28.2 (6.0) | 29.2 (7.9) | 27.3 (3.8) |
| Prior history of heart failure, % | 5 (10.9) | 3 (14.3) | 2 (8.0) |
| Coronary artery disease (any coronary occlusion>/=50%), % | 15 (32.6) | 6 (28.6) | 9 (36.0) |
| Previous revascularization (coronary artery bypass grafting or percutaneous coronary intervention), % | 11 (23.9) | 3 (14.3) | 8 (32.0) |
| Hypertension, % | 46 (100.0) | 21 (100.0) | 25 (100.0) |
| Atrial fibrillation/flutter, % | 7 (15.2) | 3 (14.3) | 4 (16.0) |
| Stroke or transient ischemic attack, % | 3 (6.5) | 1 (4.8) | 2 (8.0) |
| Diabetes Mellitus, % | 20 (43.5) | 8 (38.1) | 12 (48.0) |
| Smoking, % | 1 (2.2) | 0 (0.0) | 1 (4.0) |
| COPD, % | 2 (4.4) | 1 (4.8) | 1 (4.0) |
| Sleep Apnea, % | 5 (10.9) | 4 (19.1) | 1 (4.0) |
| Depression, % | 1 (2.2) | 0 (0.0) | 1 (4.0) |
| Dyslipidemia, % | 27 (58.7) | 10 (47.6) | 17 (68.0) |
| Cancer requiring chemotherapy or radiation, % | 3 (6.5) | 3 (14.3) | 0 (0.0) |
| Chronic liver disease, % | 2 (4.4) | 0 (0.0) | 2 (8.0) |
| Chronic kidney disease (eGFR < 60 mL/kg/1.73m2), % | 9 (19.6) | 4 (19.1) | 5 (20.0) |
| Renin-Angiotensin System Inhibitors, % | |||
| Angiotensin converting enzyme inhibitor | 18 (39.1) | 8 (38.1) | 10 (40.0) |
| Angiotensin receptor blockers | 28 (60.9) | 13 (61.9) | 15 (60.0) |
| Beta-Adrenergic Receptor Blockers, % | 24 (52.2) | 9 (42.9) | 15 (60.0) |
| Additional Therapies % | |||
| Any calcium channel antagonist | 22 (47.8) | 8 (38.1) | 14 (56.0) |
| Aspirin | 23 (50.0) | 11 (52.4) | 12 (48.0) |
| Warfarin | 1 (2.2) | 1 (4.8) | 0 (0.0) |
| Any direct oral anti-coagulant | 10 (21.7) | 6 (28.6) | 4 (16.0) |
| Any statins | 34 (73.9) | 15 (71.4) | 19 (76.0) |
| Any additional anti-hypertensive medication | 14 (30.4) | 4 (19.0) | 10 (40.0) |
| Chronic NSAID | 2 (4.3) | 0 (0.0) | 2 (8.0) |
| Additional treatments for COVID-19, % | |||
| Dexamethasone | 22 (47.8) | 11 (52.4) | 11 (44.0) |
BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; NSAID, non-steroidal anti-inflammatory drugs; RAAS, renin-angiotensin aldosterone system; SD, standard deviation. Q1, 25th percentile; Q3, 75th percentile.
Data presented are frequency (%), mean (SD) or median (Q1, Q3). Group difference for continuous variables was evaluated using analysis of variance unless otherwise stated. Group difference for categorical variables was evaluated using Chi-square/Fisher's exact test.
Data are reported as median (Q1, Q3). Group difference is evaluated using Wilcoxon rank-sum test.
Table III.
Baseline vital signs and biomarkers
| Discontinuation of RAAS inhibitor (N = 21) | Continuation of RAAS inhibitor (N = 25) | |
|---|---|---|
| Heart rate, BPM (SD) | 77.7 (10.2) | 75.0 (11.4) |
| Resting systolic blood pressure, mmHg (SD) | 136.7 (17.0) | 135.7 (19.2) |
| Resting diastolic blood pressure, mmHg (SD) | 71.0 (11.8) | 71.6 (9.6) |
| Resting respiratory rate, respirations/minute, median (Q1, Q3)* | 20 (20, 20) | 20 (18, 20) n = 24 |
| Serum Hemoglobin, g/L (SD) | 12.2 (1.8) | 12.6 (2.0) |
| Serum Sodium, mmol/L (SD) | 137.8 (3.5) | 137.0 (4.6) |
| Serum Potassium, mmol/L (SD) | 4.2 (0.5) | 4.1 (0.5) |
| Blood Urea Nitrogen, mmol/L, median (Q1, Q3)* | 7.5 (6.4, 9.7) | 7.4 (4.8, 10.5) |
| Serum Glucose, mmol/L, median (Q1, Q3)* | 5.9 (5.0, 8.8) | 7.0 (4.9, 9.3) |
| C-reactive protein, mg/L, median (Q1, Q3)* | 37.5 (15.1, 65.9) | 32.7 (16.8, 91.4) |
| Lymphocyte count x 109, median (Q1, Q3)* | 0.87 (0.74, 1.23) | 1.11 (0.57, 1.30) |
| High-sensitivity Troponin I ng/L, median (Q1, Q3)* | 11.0 (7.9, 22.2) | 9.9 (7.4, 19.2) |
| Natriuretic Peptides BNP, pg/mL, median (Q1, Q3)* | 132.5 (47, 249.5) | 92.0 (60.0, 152.0) |
| eGFR, mL/min/1.73 m2, median (Q1, Q3)* | 92.5 (58.0, 101.6) | 60.9 (47.8, 95.8) |
BNP, brain natriuretic peptide; BPM, beats per minute; eGFR, estimated glomerular filtration rate; RAAS, renin-angiotensin aldosterone system; SD, standard deviation. Q1, 25th percentile; Q3, 75th percentile.
Data are reported as median (Q1, Q3). Group difference is evaluated using Wilcoxon rank-sum test and no differences in baseline characteristics were identified. Standard range values are as follows: Blood urea nitrogen 2.1-8.5 mmol/L; glucose 3.9-11.0 mmol/L; C-reactive protein 0-5 mg/L; lymphocyte count 1.0-4.80 × 109; High-sensitivity Troponin I </= 17.5 ng/L; BNP < 100 pg/mL.
Primary outcome
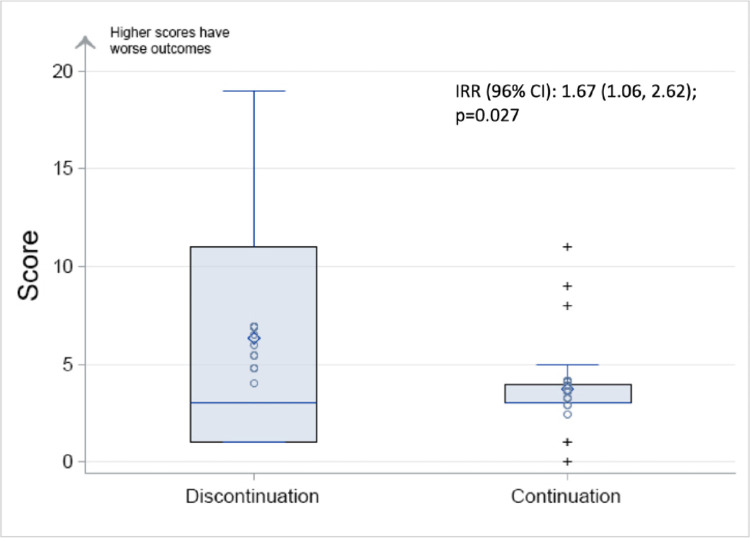
The mean of the global rank score was numerically higher with a greater distribution of higher scores in participants who discontinued ACEi/ARB compared to those who continued ( Figure 2 ). Despite the “right shift” is score distribution, in the primary intention-to-treat analysis, there was no statistically significant difference in the mean global rank score between participants discontinuing vs continuing ACEi/ARB: the mean global score was 6.3 (SD 6.3) for discontinuation vs 3.8 (SD 2.5) for continuation; median global score was 3 (IQR 1, 11) for continuation and 3 (IQR 3, 4) for discontinuation (P-value for comparison using Wilcoxon test P = .60; Figure 2). Comparing ACEi/ARBs discontinuation vs continuation using the negative binomial test, showed a higher rate of events among participants discontinuing ACEi/ARBs: discontinuation IR = 2.98 (95% CI 2.17-4.10) vs continuation IR = 1.79 (95% CI 1.30-2.46); with a corresponding IRR = 1.67 (95% CI 1.1-2.6; P = .027) (Figure 3 ; Table IV ). These results remained statistically significant after adjusting for age, sex, body-mass index and ethnicity (group difference P-value = .04).
Figure 2.
Distribution of global rank scores between treatment arms. Lower score indicates clinical stability, higher scores indicate worsened outcomes. Boxes indicate the percentage of individuals at end of follow up with a specific score. Components of the global rank score includes: Components of the global rank score included: death; transfer to ICU for invasive ventilation or other indication; non-fatal major adverse cardiovascular events (any of: myocardial infarction, ischemic or hemorrhagic stroke, acute decompensated heart failure, or new onset atrial fibrillation); length of stay >4 days; development of acute kidney injury (>40% decline in eGFR or doubling of serum Cr from baseline); urgent intravenous treatment for high blood pressure/hypertensive crisis; or changes in biomarkers from baseline: >30% increase in high sensitivity troponin from baseline, >30% increase in BNP; >30% increase in CRP; or lymphocyte count drop >30%. The P-value for comparison using Wilcoxon test .6; P-value for comparison between the treatment arms with negative binomial .027. BNP, brain natriuretic peptide; CRP, c-reactive protein; eGFR, estimated glomerular filtration rate; ICU, intensive care unit.
Figure 3.
Box plot of score by randomized group. Lower score indicates clinical stability, higher scores indicate worsened outcomes. Vertical line through the box indicates the median. Edge of the box indicates the quartiles. Whiskers indicate minimum and maximum. CI, confidence interval; IRR, incident rate ratio.
Table IV.
Primary and secondary outcomes
| Discontinuation of RAAS inhibitor (N = 21) | Continuation of RAAS inhibitor (N = 25) | Group difference P-value | |
|---|---|---|---|
| Mean score (SD) | 6.3 (6.3) | 3.8 (2.5) | .598* |
| Median score (IQR) | 3 (1, 11) | 3 (3, 4) | |
| Negative binomial (IR)† | 2.98 (2.17, 4.10) | 1.79 (1.30, 2.46) | IRR (95% CI) = 1.667 (1.060, 2.620) P = .027 |
| Individual components of the endpoint: | |||
| Death | 2 (9.5) | 1 (4.0) | .585 |
| Transfer to ICU for Invasive ventilation | 2 (9.5) | 1 (4.0) | .585 |
| Transfer to ICU for other indication | 1 (4.8) | 0 (0.0) | .457 |
| Acute myocardial infarction | 3 (14.3) | 0 (0.0) | .088 |
| Stroke | 0 (0.0) | 0 (0.0) | NA |
| Acute decompensated heart failure | 7 (33.3) | 1 (4.0) | .016 |
| New onset atrial fibrillation | 1 (4.8) | 0 (0.0) | .457 |
| Length of stay > 4 days | 12 (57.1) | 18 (72.0) | .527 |
| Development of acute kidney injury (>40% decline in eGFR or doubling of serum Cr) | 1 (4.8) | 1 (4.0) | 1.000 |
| Urgent intravenous treatment for high blood pressure/hypertensive crisis | 0 (0.0) | 0 (0.0) | NA |
| >30% increase in baseline high sensitivity troponin | 6 (28.6) | 6 (24.0) | .749 |
| >30% increase in baseline BNP | 6 (28.6) | 6 (24.0) | .749 |
| Increase in baseline CRP >30% | 5 (23.8) | 4 (16.0) | .711 |
| Lymphocyte count drop >30% | 3 (14.3) | 5 (20.0) | .710 |
BNP, brain natriuretic peptides; CRP, c-reactive protein; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; IQR, interquartile range; IR, incidence rate; IRR, incidence rate ratio; RAAS, renin-angiotensin aldosterone system; SD, standard deviation.
Group difference was evaluated using Fisher's exact test unless otherwise stated.
Group difference was evaluated using Wilcoxon rank-sum test.
Incident rate and incident rate ratio presented with 95% confidence interval.
Secondary and exploratory outcomes
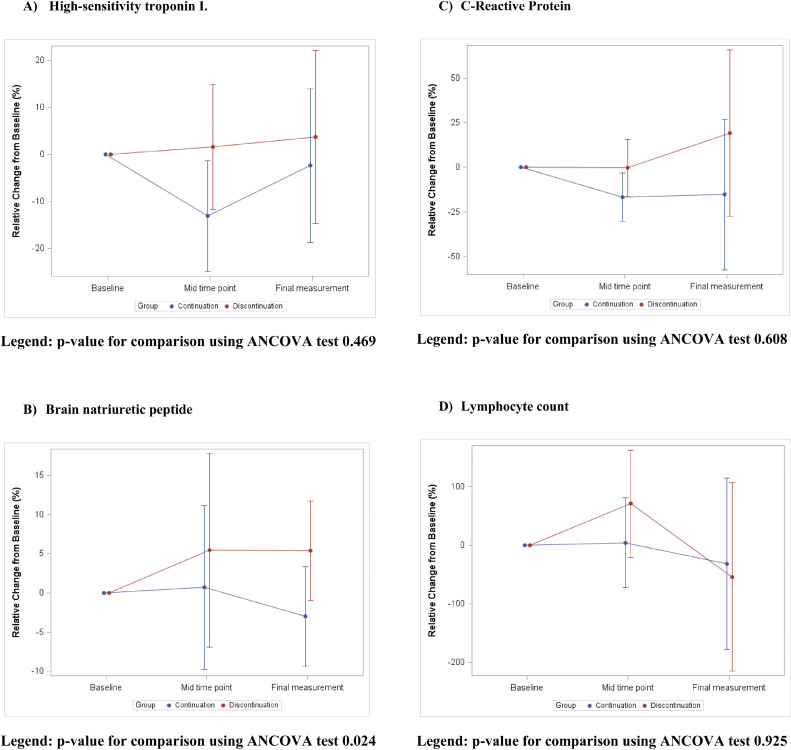
In the secondary analysis, participants who discontinued ACEi/ARB had a significantly higher percent change in serum BNP during hospitalization (median percent change: 16.7% [IQR 4.9% to -35.0%] vs -27.5% ([IQR 40.0% to -38.9%], P = .024) (Figure 4 ). These results persisted after adjusting for age and sex (P = .01) (Table IV). Compared with continuing ACEi/ARB, participants who discontinued therapy had a higher incidence of acute decompensated heart failure episodes (33.3% vs 4.0%, P = .016) (Table IV).
Figure 4.
Relative change in biomarker changes across randomization groups, A) High-sensitivity troponin I, P-value for comparison using ANCOVA test .469. B) Brain natriuretic peptide, P-value for comparison using ANCOVA test .024. C) C-Reactive Protein, P-value for comparison using ANCOVA test .608. D) Lymphocyte count, P-value for comparison using ANCOVA test .925. ANCOVA, analysis of covariance.
The incidence of death, transfer to ICU, acute myocardial infarction, new onset atrial fibrillation, length of stay >4 days, development of acute kidney injury, treatment for high blood pressure or hypertensive crisis, >30% increase in baseline high sensitivity troponin, BNP or CRP, and lymphocyte count drop >30% were not significantly different between groups ( Table IV ).
Regarding safety events, there was no difference in incidence of adverse events such as hypotension, hyperkalemia, or acute kidney injury in participants randomized to ACEi/ARB continuation (Table V ). Serum potassium was lower in patients who discontinued ACEi/ARB compared to patients who continued (median percent change: -4.1% [IQR -7.7% to 12.8%] vs 8.3% [IQR 2.3%-16.3%]) (Table V ).
Table V.
Change from baseline to last available measurements of vital signs and blood measurements
| Percentage change, % |
|||
|---|---|---|---|
| Discontinuation of RAAS inhibitor (N = 21) | Continuation of RAAS inhibitor (N = 25) | Group difference P-value* | |
| Vital signs | |||
| Heart rate, BPM | -10.1 (-13.1, 6.3) | 0.0 (-10.3, 15.3) | .486 |
| Resting systolic blood pressure, mmHg | -2.5 (-13.0, 11.5) | -0.8 (-10.2, 5.9) | .686 |
| Resting diastolic blood pressure, mmHg | 10.8 (-8.8, 17.1) | 7.3 (0, 18.3) | .942 |
| Resting respiratory rate, respirations/minute | 0.0 (-10.0, 0.0) | 0.0 (0.0, 2.8) | .277 |
| Biomarkers | |||
| Serum Hemoglobin, mmol/L | -3.1 (-6.5, 3.3) | -0.9 (-4.8, 2.5) | .769 |
| Serum Sodium, mmol/L | 0.0 (-1.4, 2.2) | 0.7 (-1.4, 1.5) | .517 |
| Serum Potassium, mmol/L | -4.1 (-7.7, 12.8) | 8.3 (2.3, 16.3) | .065 |
| Blood Urea Nitrogen, mmol/L | 23.8 (8.9, 51.8) | 18.4 (-11.2, 54.0) | .728 |
| Serum Glucose, mmol/L | -1.7 (-39.0, 24.1) | 1.9 (-16.7, 24.4) | .435 |
| C-reactive protein, mg/L | -40.7 (-70.1, 17.7) | -50.0 (-71.3, 6.7) | .608 |
| Lymphocyte count x 109 | 38.1 (-11.5, 80.0) | 4.7 (-14.2, 62.0) | .925 |
| High-sensitivity Troponin I† | -20.3 (-48.7, 31.3) | -14.1 (-35.6, 16.1) | .469 |
| Natriuretic Peptides BNP, pg/mL | 16.7 (4.9, 35.0) | -27.5 (-40.0, 38.9) | .024 |
| eGFR, mL/min/1.73 m2 | -1.5 (-12.4, 12.3) | 0.0 (-6.5, 10.8) | .746 |
BNP, brain natriuretic peptide; BPM, beats per minute; eGFR, estimated glomerular filtration rate; RAAS, renin-angiotensin aldosterone system.
Data are reported as median with interquartile range (Q1, Q3).
Group difference was evaluated on log transformed data.
There was no difference in length of stay between patients who discontinued compared to participants who continued their RAASi (median days: discontinued 6.5 [IQR 3.5, 14] vs continue 6 [IQR 4, 14]). At discharge an ACEi/ARB was re-prescribed in 14 (out of 19 surviving; 73.7%) in the discontinuation arm, compared to 22 (out of surviving 24; 92.0%) in the continuation arm. The length of stay for people who experienced a heart failure event was median 18 (IQR 11, 49) days. The length of stay for people who experienced any cardiovascular event (non-fatal myocardial infarction, stroke, heart failure, atrial fibrillation) was 15 (IQR 7, 34) days. In comparison, the length of stay for people who did not experience any cardiovascular event was 6 (IQR 4, 10) days.
Post discharge outcomes
One person in each randomization arm died and compared to patients who continued their RAASi, there no difference in readmission 30-days following discharge from hospital (3 [8.0%] vs 2 [14.3%]).
Sensitivity analysis
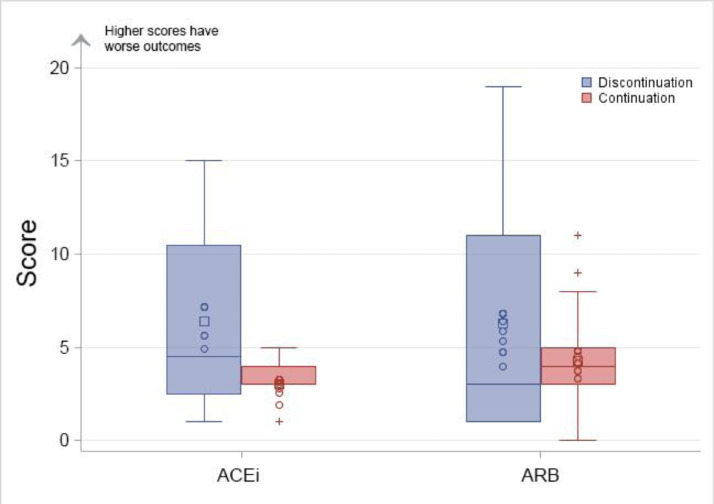
There was no difference in the global rank scores when stratified by ACEi/ARB at baseline ( Figure 5 , Supplementary Table III). There were 3 patients (1 in discontinue and 2 in continue) co-enrolled in additional COVID-19 therapeutic intervention studies. When these participants were excluded, the magnitude and significance of the results aligned with the overall cohort for the primary outcome (global rank score median: discontinue 3 [IQR 1, 14] vs continue 3 [IQR 4, 4.5] P = .93) or the negative binomial test (IR: discontinue 3.0 [95% CI 2.2-4.3] vs continue P = .03). Furthermore, there was no difference in the identification of heart failure when evaluated with site-defined heart failure versus adjudicated events. There was no interaction between the use of dexamethasone and clinical outcomes as reflected by the negative binomial test (interaction P = .44).
Figure 5.
Distribution of global rank score by randomized groups and ACEi/ARB at baseline. ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Discussion
In this open label, randomized study of 46 adults hospitalized with COVID-19, discontinuation of RAASi, compared to continuation, was associated with a higher risk for short-term adverse events related to COVID-19 as assessed using a score of clinical and biomarker outcomes. Patients who discontinued RAASi had a higher incidence of acute decompensated heart failure events during hospitalization, and this was accompanied by a rise in serum BNP. While requiring verification in larger clinical trials, our results support the continued use of RAASi in patients admitted to hospital with COVID-19 and further suggests the possibility for a protective effect of RAASi on CV outcomes.
Vertebrate models of zebrafish indicate that RAASi exposure increases expression of ACE2, highlighting the possible interrelationship between RAASi, ACE2 and COVID-19.29 While reassuring that the use of guideline directed cardiovascular therapies did not decrease during the COVID-19 pandemic,30 the specific role of RAASi in the context of COVID-19 infections have been explored observational studies31 , 32 and randomized control trials.12 , 13 These studies have collectively shown no increase in the risk for COVID-19 severity in patients who continued compared to those who discontinued RAASi therapy. Our results expand on these findings to highlight the increased acute heart failure risk when RAASi are stopped and where possible support the continuation of RAASi during COVID-19 hospitalization.
The BRACE-CORONA trial was a registry-based trial of 659 participants admitted to hospital for mild to moderate COVID-19 in Brazil. The results of the study suggested no significant difference in the primary outcome of mean number of days alive and out of hospital within 30 days in patients who continued compared to discontinued RAASi (mean ratio: 0.95, 95% CI: 0.90-1.01).12 Importantly, BRACE-CORONA demonstrated a numeric increase in the risk of myocardial infarction with discontinuation vs continuation (7.5% vs 4.6%), but new or worsening heart failure events were not significantly different between arms (4.2% vs 4.9%). Our results extend these findings by demonstrating the safety of continuing ACEi/ARB and by identifying an increased risk of acute heart failure accompanied by a rise in BNP levels among patients who had their RAASi therapy discontinued. The RAAS-COVID-19 trial differed from BRACE-CORONA in a number of ways, which may be reflected in the differences in results. Our trial enrolled participants who were older compared to BRACE-CORONA (median 71.5 vs 55.0 years of age). As age is an important risk factor for heart failure development, our population could have a higher heart failure risk which allowed to ascertain the benefit of continuing RAASi in this population. Furthermore, half of the patients were treated by dexamethasone, thereby possibly reducing competing risk of COVID-19 specific respiratory outcomes.
The Elimination or Prolongation of ACE Inhibitors and ARB in Coronavirus Disease 2019 (REPLACE COVID) trial was a prospective randomized trial of 152 patients that evaluated whether continuing versus discontinuing RAASi affected outcomes in patients admitted to hospital with COVID-19. Compared with discontinuation of RAASi, the study found that continuation of RAASi had no effect on a composite global rank score as a marker for COVID-19 severity (median rank 73 [IQR 40-110] for continuation vs 81 [38-117] for discontinuation; β-coefficient 8 [95% CI −13 to 29]).13 The incidence of heart failure events was balanced between the two study arms (continuation n = 2 [2.7%] vs discontinuation n = 1 [1.3%]). The results seen in our study may have differed from REPLACE COVID as the population enrolled in the RAAS-COVID-19 was older (71 vs 62 years of age) and had a higher proportion of people with prior ischemic heart disease (32.6% vs 12%) leading to a population at higher risk of acute heart failure. Across clinical settings, observational recent real-world population level studies of 165,355 patients in Sweden33 and 659,180 patients in the Veteran's Affairs system in the United States34 demonstrated that use of RAASi did not increase the risk of severe COVID-19 infection, further demonstrating the safety of RAASi in patients admitted with acute COVID-19.
Our results highlight the novel finding of an overall higher rate of acute decompensated heart failure events in patients who discontinued RAASi, which was further supported by a rise in BNP among patients in whom RAASi were discontinued, while among those in whom RAASi were continued the BNP fell. While the identification of heart failure events in patients with acutely infected COVID-19, the concordance of our site-identified heart failure events compared to adjudicated events provides further confidence in our results. Natriuretic peptides have strong prognostic utility in patients admitted with COVID-19, and have recently shown to independently predict in-hospital death and clinical outcomes including patients without cardiovascular disease.8 Furthermore, some reviews have uncovered rates of symptomatic heart failure developing in up to 40% of patients with COVID-19, especially in the context of uncovering previously unknown heart failure 35and while early case reports described impaired cardiac systolic function from myocarditis, emerging data has suggested a much higher risk for heart failure exacerbation with preserved ejection fraction (HFpEF).7 , 35 , 36 As we identified, there was no significant change in the systolic or diastolic blood pressure between randomization arms from baseline to follow-up. In addition, there was no events of hypertensive urgency/emergency requiring urgent use of intravenous antihypertensive agents in both arms. These findings suggest that worsening hypertension was not a driver of the heart failure events between study arms. Myocardial inflammation or ischemia is common in COVID-19, and in a recent cardiac MRI study of 148 patients with severe COVID-19 infection, myocarditis like injury or ischemia was found in more than half of patients following hospitalization.7 Echocardiographic findings from case series of patients with COVID-19 have demonstrated primarily preserved ejection fraction in more than 90% of hospitalized patients, with nearly 20% demonstrating evidence of left ventricular diastolic dysfunction.25 The development of HFpEF in these patients could be mediated through direct viral infiltration, inflammation, or cardiac fibrosis leading to diastolic dysfunction. Severe COVID-19 has been associated with inflammation as seen on cardiac MRI,7 and attenuation of inflammatory pathway with RAASi may prevent clinical heart failure events. Alternatively, COVID-19 may serve as a physiological stressor to unmask subclinical HFpEF in a vulnerable myocardium.37 This maybe particularly true in our population of patients, most of whom were at an elevated cardiovascular risk. While results from our secondary analysis are hypothesis generating, they suggest the potential protective role for RAASi in development of heart failure events and should be validated in larger clinical studies.
Our trial occurred later during the COVID-19 pandemic, and this was evident with nearly half of our patients receiving dexamethasone treatment during hospitalization. Lower rates of death and ICU admission could be explained by employment increased use of corticosteroids for COVID-19 and more experience managing these patients.38 Furthermore, there was no evidence of increased kidney injury, hyperkalemia, or hypotension associated with continuation of ACEi/ARB. These findings are important as clinicians often worry about these adverse events for patients hospitalization for COVID-19.39
Limitations
Our results should be interpreted in the context of several limitations. Firstly, our study is limited by a small sample size of patients and a limited number of clinical events. Secondly, this was an open-labeled trial and providers caring for the patient were aware of which group the patient was assigned to, which may have introduced bias in the treatment of patients; however, objective markers such as changes in natriuretic peptides corroborated the clinical findings of acute heart failure. As most patients did not have a baseline history of HFrEF, most did not have a measured left ventricular ejection fraction in our system at baseline. The exclusion of more critically ill patients, which may reflect a more severe COVID-19 infection, reflects the inclusion of a more stable population in our study. There was an imbalance in some covariates in the baseline features of the trial; however, adjustment did not impact the overall results. Quality of life measurements were not conducted in this study. Understanding the clinical significance of hierarchical points remains challenging. As the clinical practice changed morbidity and mortality profiles changed, and thereby may have limited the power to assess our endpoints. The endpoints for the clinical outcome events were identified through clinical site documentations; however, for heart failure events we conducted a blinded adjudication as defined in the methods.
The adjudication process for heart failure was conducted in a post-hoc manner but reviewers were blinded to randomization treatment strategy. The primary reason to decline participation was lack of interest in being a part of a research study. As a result, the population enrolled represents a more motivated population and therefore may not be generalizable to the broader population of patients admitted with COVID-19. Our study only evaluated shorter term outcomes and longer term evaluation will be needed to ascertain the impact of ACEi/ARB management in patients admitted with COVID-19. None of our patients had COVID-19 vaccination. Our results are still however relevant as the vast majority of current admission in hospitals are occurring in patients who are unvaccinated.40
Conclusion
Among patients with COVID-19, continuation of the RAASi, while safe, did not improve outcomes as indicated by our primary outcome. Despite the non-statistically different differences in the mean and median rank sum scores, the present trial shows that in patients hospitalized with COVID-19, discontinuation of RAASi may increase the risk of short term adverse events using a “count” (negative binomial) model, which were related to a higher incidence of acute decompensated heart failure events during hospitalization and a rise in serum BNP. Our results support the continued use of RAASi in patients hospitalized with COVID-19 and further suggests the possibility for a protective effect for heart failure events. In patients with a clinical indication, RAASi should be continued.
Funding
The RAAS-COVID-19 Randomized Controlled Trial was funded by the McGill Interdisciplinary Initiative in Infection and Immunity (MI4) and the Division of Cardiology at McGill University.
Disclosures
A.S. reports receiving support from the Fonds de Recherche Santé Quebec (FRSQ) Junior 1 clinician scholars program, Canada Institute for Health Research (CIHR grant #175095); Roche Diagnostics, Boeringer-Ingelheim, Novartis, and Takeda. APA is supported by a Mentored Patient-Oriented Research Career Development Award (K23HL150159) through the National Heart, Lung, and Blood Institute, has received relevant research support through grants to his institution from Amarin Pharma, Inc, Abbott, and Novartis, and modest reimbursement for travel from Novartis. M. C is funded from a NSERC Alliance COVID-19 Grant ALLRP 554923 – 20 and NSERC Discovery Grant RGPIN-2018-04546. JPF has no conflicts of interest regarding the present trial.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ahj.2022.01.015.
Appendix. Supplementary materials
References
- 1.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phelps M, Christensen DM, Gerds T, et al. Cardiovascular comorbidities as predictors for severe COVID-19 infection or death. Eur Hear J - Qual Care Clin Outcomes. 2021;7:172–180. doi: 10.1093/ehjqcco/qcaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azevedo RB, Botelho BG, Hollanda JVG de, et al. Covid-19 and the cardiovascular system: a comprehensive review. J Hum Hypertens. 2020;35:4–11. doi: 10.1038/s41371-020-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranard LS, Fried JA, Abdalla M, et al. Approach to acute cardiovascular complications in COVID-19 infection. Circ Hear Fail. 2020;13:4–11. doi: 10.1161/CIRCHEARTFAILURE.120.007220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotecha T, Knight DS, Razvi Y, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021;42:1866–1878. doi: 10.1093/EURHEARTJ/EHAB075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham JW, Claggett BL, Jering KS, et al. Prognostic value of natriuretic peptides and cardiac troponins in COVID-19. Circulation. 2021;144:177–179. doi: 10.1161/CIRCULATIONAHA.121.054969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishiga M, Wang DW, Han Y, et al. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni W, Yang X, Yang D, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24:1–10. doi: 10.1186/S13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuentes AV, Pineda MD, Venkata KCN. Comprehension of top 200 prescribed drugs in the US as a resource for pharmacy teaching, training and practice. Pharm J Pharm Educ Pract. 2018;6:43. doi: 10.3390/PHARMACY6020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopes RD, Macedo AVS, De Barros E Silva PGM, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA - J Am Med Assoc. 2021;325:254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen JB, Hanff TC, William P, et al. Continuation versus discontinuation of renin–angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021;9:275–284. doi: 10.1016/S2213-2600(20)30558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer A, Schreinlechner M, Sappler N, et al. Discontinuation versus continuation of renin-angiotensin-system inhibitors in COVID-19 (ACEI-COVID): a prospective, parallel group, randomised, controlled, open-label trial. Lancet Respir Med. 2021;9:863–872. doi: 10.1016/S2213-2600(21)00214-9/ATTACHMENT/62CE994F-BF2A-4464-BD32-A04FD084B6C8/MMC1.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bozkurt B, Kovacs R, Harrington B. Joint HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19. J Card Fail. 2020;26:370. doi: 10.1016/J.CARDFAIL.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang [accessed 29 March 2021].
- 17.Grover A, Oberoi M. A systematic review and meta-analysis to evaluate the clinical outcomes in COVID-19 patients on angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Eur Hear J - Cardiovasc Pharmacother. 2021;7:148–157. doi: 10.1093/ehjcvp/pvaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aflaki M, Flannery A, Ferreira JP, et al. Management of Renin-Angiotensin-Aldosterone System blockade in patients admitted to hospital with confirmed coronavirus disease (COVID-19) infection (The McGill RAAS-COVID- 19): a structured summary of a study protocol for a randomized controlled trial. Trials. 2021;22:115. doi: 10.1186/s13063-021-05080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;2019(3-6) doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 20.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;19:19–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abraham WT, Fiuzat M, Psotka MA, O'Connor CM. Heart failure collaboratory statement on clinical trials in the landscape of COVID-19. JACC Hear Fail. 2020;8:423–425. doi: 10.1016/J.JCHF.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyl B, Lopez Sendon J, Borer JS, et al. Comparison of outcome adjudication by investigators and by a central end point committee in heart failure trials. Circ Hear Fail. 2020;13:123–131. doi: 10.1161/CIRCHEARTFAILURE.119.006720. [DOI] [PubMed] [Google Scholar]
- 24.Carson P, Fiuzat M, O'Connor C, et al. Determination of hospitalization type by investigator case report form or adjudication committee in a large heart failure clinical trial (β-Blocker Evaluation of Survival Trial [BEST]) Am Heart J. 2010;160:649–654. doi: 10.1016/j.ahj.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Hicks KA, Mahaffey KW, Mehran R, et al. 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation. 2018;137:961–972. doi: 10.1161/CIRCULATIONAHA.117.033502. [DOI] [PubMed] [Google Scholar]
- 26.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington State. JAMA. 2020;4720:2019–2021. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felker GM, Maisel AS. A global rank end point for clinical trials in acute heart failure. Circ Heart Fail. 2010;3:643–646. doi: 10.1161/CIRCHEARTFAILURE.109.926030. [DOI] [PubMed] [Google Scholar]
- 28.Sun H, Davison BA, Cotter G, et al. Evaluating treatment efficacy by multiple end points in phase ii acute heart failure clinical trials analyzing data using a global method. Circ Hear Fail. 2012;5:742–749. doi: 10.1161/CIRCHEARTFAILURE.112.969154. [DOI] [PubMed] [Google Scholar]
- 29.Kim GJ, Melgoza A, Jiang F, Guo S. The effect of renin-angiotensin-aldosterone system inhibitors on organ-specific ace2 expression in zebrafish and its implications for COVID-19. Sci Rep. 2021;11:23670. doi: 10.1038/S41598-021-03244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaduganathan M, Li D, Van Meijgaard J, Warraich HJ. Prescription filling patterns of evidence-based medical therapies for heart failure during the COVID-19 pandemic in the United States. J Card Fail. 2021 doi: 10.1016/j.cardfail.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds HR, Adhikari S, Pulgarin C, et al. Renin–Angiotensin–Aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancia G, Rea F, Ludergnani M, et al. Renin–Angiotensin–Aldosterone system blockers and the risk of Covid-19. 2020;382:2431-2440. doi: 10.1056/NEJMOA2006923. [DOI] [PMC free article] [PubMed]
- 33.Loader J, Lampa E, Gustafsson S, et al. Renin-Angiotensin Aldosterone system inhibitors in primary prevention and COVID-19. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandhu AT, Kohsaka S, Lin S, et al. Renin-angiotensin-aldosterone system inhibitors and SARS-CoV-2 infection: an analysis from the veteran's affairs healthcare system. Am Heart J. 2021;240:46–57. doi: 10.1016/J.AHJ.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freaney PM, Shah SJ, Khan SS. COVID-19 and heart failure with preserved ejection fraction. JAMA. 2020;324:1499–1500. doi: 10.1001/JAMA.2020.17445. [DOI] [PubMed] [Google Scholar]
- 36.Sandoval Y, Januzzi JL, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol. 2020;76:1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szekely Y, Lichter Y, Taieb P, et al. Spectrum of cardiac manifestations in COVID-19. Circulation. 2020;142:342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Group TRC Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krahn A. COVID-19 and Concerns Regarding Use of ACEi/ARB/ARNi Medications for Heart Failure or Hypertension. https://www.acc.org/latest-in-cardiology/features/accs-coronavirus-disease-2019- [accessed 28 March 2021].
- 40.Vasileiou E, Simpson CR, Shi T, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397:1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.