Summary
The centromere performs a universally conserved function, to accurately partition genetic information upon cell division. Yet, centromeres are among the most rapidly evolving regions of the genome and are bound by varying assortments of centromere-binding factors that are themselves highly divergent at the protein sequence level. A common thread in most species is the dependence on the centromere-specific histone variant CENP-A for the specification of the centromere site. However, CENP-A is not universally required in all species or cell types, making the identification of a general mechanism for centromere specification challenging. In this review, we examine our current understanding of the mechanisms of centromere specification in CENP-A-dependent and independent systems, focusing primarily on recent work.
eTOC blurb
In this review, Mellone and Fachinetti highlight diverse mechanisms that control centromere specification in CENP-A-dependent and -independent systems, with emphasis on recent work. They discuss the possible roles of different components, from centromeric DNA to its associated factors (including transcripts), in centromere identity focusing on multicellular species.
Introduction
Cell division is an essential process in the life of all organisms: it contributes to growth and development, wound repair, response to infection, and cell turnover. When a cell divides, it needs to accurately distribute its chromosomes, the molecular basis of genetic heredity.
The centromere plays a key function in this process. It is the chromosomal docking site for the assembly of the kinetochore, a multimeric protein machinery responsible for the formation of load-bearing spindle-fiber attachments. The centromere and the kinetochore also mediate sensing and regulatory functions for microtubule attachment and chromosome separation during cell division (reviewed by Santaguida and Musacchio 1). Centromeres can be positioned at single sites (in monocentric species) or be scattered along the entire chromosome (in holocentric species). Defects in centromere formation or kinetochore assembly lead to chromosome mis-segregation and consequently to numerical (aneuploidy) and structural chromosome alterations in the daughter cells (reviewed by Santaguida and Amon 2). The centromeres of multicellular organisms are complex DNA–protein structures frequently built on repetitive DNA sequences and typically composed of a specialized type of chromatin embedded within large flanking heterochromatic regions that form the so-called pericentromere. In addition, centromeres can form de novo at genomic locations not usually associated with centromere activity (also known as neocentromeres), with important implications for speciation (reviewed by Murillo-Pineda and Jansen 3). The existence of neocentromeres as well as functional dicentric chromosomes (chromosomes containing two centromere satellite arrays with only one functionally active; reviewed by Sullivan and Sullivan 4) suggests that centromeres are epigenetically regulated chromatin loci (reviewed by Allshire and Karpen 5). During the last two decades substantial effort has gone into understanding what defines and maintains the centromere position. Central to these efforts was the identification of CENP-A (Centromeric Protein A), a histone H3 variant6,7 that acts as the epigenetic mark required to maintain centromere position and function (reviewed by Fukagawa and Earnshaw 8). CENP-A has been observed at the centromeres of species from all kingdoms of life, as well as at human neocentromeres. In these species, CENP-A is essential for centromere formation and assembly through the recruitment of additional centromeric proteins (referred to as the ‘constitutive centromere-associated network’, or CCAN, which includes CENP-C, CENP-N, CENP-T and CENP-I in human cells) and stably maintains the centromeric position throughout generations (reviewed by McKinley and Cheeseman 9 and briefly discussed in this review).
Although CENP-A plays a key role in centromere specification, other components upstream of CENP-A and/or uncoupled from CENP-A activity have also been implicated in regulating centromere identity. In this review, we focus on aspects of centromere specification that function in parallel, intersect or are independent of the CENP-A pathway.
CENP-A chromatin distribution and organization
CENP-A localizes to centromeres forming distinct foci, visible cytologically in both interphase and mitosis. However, CENP-A is not the only protein present at centromeric sites10,11 and other H3 variants such as histone H3.1 and H3.3 are also associated with centromeric chromatin12,13. Estimates of the number of CENP-A molecules found at individual centromeres are surprisingly low relative to the overall size of centromeric DNA: the fission yeast Schizosaccharomyces pombe only contains 26; Drosophila melanogaster 84; chicken DT-40 cells; and human cells ~400. These estimates raise a few questions about how the presence of CENP-A nucleosomes may specify centromere identity.
Analysis of extended chromatin fibers in human and Drosophila cells revealed that CENP-A nucleosomes exhibit discontinuous staining, visible as several ‘spots’ of CENP-A alternating with ‘spots’ of histone H3 12 At D. melanogaster’s centromeres, the CENP-A domain ranges in size between 101-171 kb, depending on the centromere, and extended chromatin fibers show ~18 CENP-A spots on average18. If each Drosophila centromere contains an estimated average of 42 nucleosomes/centromere15, we can infer that each ‘spot’ consists only of approximately 3 nucleosomes in the span of 6-9 kb (the estimated length of each ‘spot’)18. Similarly, the CENP-A domain for human centromere 8 is 632-kb long19. Therefore, the estimated 200 CENP-A nucleosomes10 would represent only ~6 % of all nucleosomes occupying this centromere (since approximately 3,200 nucleosomes are expected to occupy a region this size). Although more studies are needed to illuminate the precise distribution of CENP-A nucleosomes at the centromere, it appears that just few CENP-A nucleosomes are sufficient to mark the site of centromere formation in both flies and humans. Even though only approximately one fifth of human CENP-A localizes to the centromere, CENP-A is still ~50 fold enriched at the centromere relative to the rest of the genome10. Thus, on average, no other regions of the genome harbors comparable local enrichment of CENP-A than centromeres.
Two models have proposed ways in which the sparse CENP-A nucleosomes within centromeric chromatin may fold during cell division to promote inner and outer kinetochore nucleation, both implicating the involvement of highly ordered fibers12,16,20. In human cells, rosette like-structures comprising several CENP-A nucleosomes and spanning up to 300 nm were revealed with super resolution during interphase21. Such compacted structures could be mediated by CENP-C through cross-array clustering22 or by CENP-N through stacking of CENP-A nucleosomes23,24. Given that CENP-A nucleosomes are likely to be quite spaced from one another in vivo, their assembly into stacked and compacted CENP-A nucleosomes would require that they be bundled through the formation of chromatin loops. Such CCAN-mediated clustering of CENP-A nucleosomes could contribute to centromere identity by differentiating centromeric from non-centromeric CENP-A nucleosomes (Figure 1A) (reviewed by Nagpal and Fierz 25). These bundled loops (observed during interphase) could then reorganize to form more ordered structures during cell division8,12,16,20.
Figure 1. Genetic and epigenetic factors control centromere position.
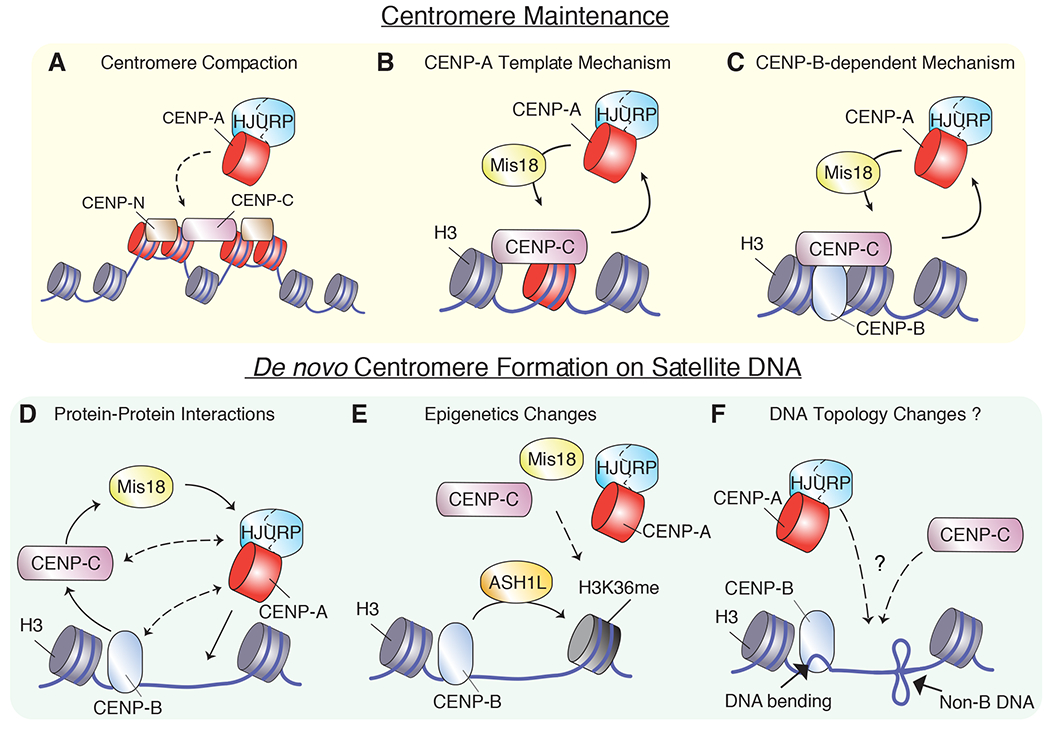
(A-C) Models for maintenance of human centromeres via CENP-A nucleosomes clustering (A), via a CENP-A self-assembly template mechanism (B) and via CENP-B/CENP-C interaction (C). (D-E) Models for de novo centromere formation of human centromeres via protein/protein interactions (D), changes in the epigenetics landscape (E) and presence of secondary structures and/or topological changes (F). Dashed lines denote proposed interactions, question marks represent untested interactions.
CENP-A stabilization via multiple mechanisms (reviewed by Mitra et al. 26) and, possibly, bundling of CENP-A nucleosomes at centromeric regions, could be an important mechanism to restrict centromere activity at its native position, enabling kinetochore assembly only at these stable sites. An additional model for centromere specification was suggested by a recent study that monitored CENP-A stability during DNA replication, a time when histones disassemble from the parental chromosome and are subsequently re-assembled onto sister chromatids27. Nucleosomes are either maintained, ensuring the inheritance of epigenetic marks throughout replication, or assembled de novo. CENP-A is maintained by the centromeric protein ‘Holliday junction recognition protein’ (HJURP) via a direct interaction with the replication machinery11,28 and CENP-A itself is required for proper centromere replication29. Nechemia-Arbely et al. observed that the DNA replication machinery evicts the previously deposited, non-centromeric CENP-A and retains only the centromeric pool through a protective mechanism enacted by CENP-C and other CCAN members 11. This data demonstrates that DNA replication can act as an error correction mechanism to restrict CENP-A accumulation to the centromere and to preserve centromere identity.
Given that CENP-A nucleosomes can be found at non-centromeric genomic regions, it is tempting to speculate that CENP-A post-translational modifications (PTMs) found exclusively at the centromere could be another way to restrict CENP-A’s function to centromeric regions. However, the function of most CENP-A PTMs remains controversial and to date no specific PTM associated with only centromeric bound CENP-A have been identified (reviewed by Srivastava et al. 30). Nonetheless, a special importance for correct kinetochore formation has been attributed to mono-methylation of lysine 20 of histone H4; this modification is enriched in CENP-A nucleosomes, but not in pre-nucleosomal CENP-A–H4 complexes or canonical H3 nucleosome due to allosteric inhibition 31,32.
New CENP-A assembly at the centromere has been proposed to be directed by the presence of preexisting CENP-A nucleosomes though an epigenetic self-assembly loop (Figure 1B) (reviewed by Musacchio and Desai 33). Analyses of extended chromatin fibers led to the observation that CENP-A deposition at native centromeres occurs in nucleosomes that are in close proximity to preexisting CENP-A nucleosomes34 with flanking pericentric heterochromatin acting as a regional boundary35. These observations suggest a model where new CENP-A deposition is limited by the availability of paired CENP-A-H3 di-nucleosomes, which are connected through CENP-C (and possibly other CCAN proteins)33. In this model, once all H3 nucleosomes of all di-nucleosomes are replaced, new CENP-A deposition is terminated in G1 phase. During this stage, it has been proposed that part of the Mis18 complex (a key component required for CENP-A deposition, see below) dissociates from the centromere, possibly via ubiquitination36, halting CENP-A reloading37,38. However, the extent to which hetero-di-nucleosomes are important for new CENP-A deposition remains to be tested.
Recent findings in human cells suggest that the number of centromeric CENP-A molecules is quantitatively determined by the total pool of CENP-A available39, but is independent of both the number of CENP-A molecules previously present at centromeric sites33 and any specific post-translational modifications on pre-deposited CENP-A40 (Figure 1C). This model was further supported by observations in a subpopulation of temporally quiescent CD4+ T cells, which lack CENP-A yet are capable of rapidly loading CENP-A at native centromeres39. Interestingly, a complete turnover of all preexisting CENP-A molecules was previously observed in the holocentric species C. elegans41. These findings suggest that additional factors other than CENP-A provide key marks of centromere identity necessary to maintain certain levels of CENP-A at centromeres.
Deposition of CENP-A by specific assembly factors
CENP-A deposition is controlled by Cyclin Dependent Kinases 1 and 2 (CDK1/2) and Plk1 and mediated by specific chaperones. To date, three CENP-A chaperones have been identified: HJURP in tetrapods, Scm3 in yeast, and CAL1 in Drosophila (reviewed by Westhorpe and Straight 42). HJURP and CAL1 bind pre-nucleosomal CENP-A/H4 dimers preventing tetramerization of CENP-A/H4 43–45 and mediating new CENP-A deposition at mitotic exit (for more details see review by McKinley and Cheeseman 9).
HJURP is also required to retain CENP-A at the centromere during DNA replication11,28 and could be critical for CENP-C46 and CENP-T assembly47. However, the molecular mechanisms underlying both events remain poorly understood. Targeting HJURP to an ectopic location is sufficient to trigger CENP-A assembly both at native48,49 and artificial chromosomes50. De novo centromere formation via tethering of CAL1 was also demonstrated in vivo51. These studies highlight that CENP-A assembly factors are the driving force for centromere formation.
In vertebrate cells, HJURP binding to centromeres requires the Mis18 complex, an octameric complex of two M18BP1 (Mis18 Binding Protein 1), four Mis18α, and two Mis18b subunits52–54 (Figure 2). Both HJURP and the Mis18 complex are phospho-regulated to temporally restrict their binding to centromere to prevent premature CENP-A deposition (for more details see review by McKinley and Cheeseman 9). The Mis18 complex is highly conserved and essential for CENP-A assembly; notably, it has not been identified in Drosophila, suggesting the evolution of distinct CENP-A reloading pathways (reviewed by Zasadzińska and Foltz 55).
Figure 2. Protein/protein interactions govern centromere identity.

Schematic map of reported protein-protein interactions between HJURP, CENP-B, CENP-C, CENP-A, M18BP1, Mis18α and Mis18β. Numbers indicate amino acid (aa) positions for each protein relative to their domains. Specific aa residues are also marked to highlight a particular PTM or interaction. HIKLMN are CENP proteins, CATD = CENP-A targeting domain, HCTD1/2 = HJURP C-terminal domain, SANT-A = SANT associated domain, green and red P = phosphorylation that lead to protein activation or inhibition, respectively. Top left inset: model for new CENP-A deposition based on an interplay of two CENP-C molecules to assemble a new CENP-A nucleosome at the centromere during early G1 phase.
As the Mis18 complex is upstream of CENP-A and HJURP deposition, a key question is how it is recruited to the centromeres. In human cells, this complex does not require HJURP 48 nor CENP-A or CENP-B for its recruitment39, while in chicken cells and Xenopus egg extracts, M18BP1 directly connects to CENP-A nucleosomes through residues that are, however, not conserved in humans56,57. Converging evidence in multiple species indicates that M18BP1 recognizes centromeres via the direct binding to the constitutive centromeric components CENP-C 39,49,58,59 and CENP-I 49, as well as binding to other factors such as MgcRacGAP 60 and KAT7 61, both of which transiently localize to centromeres (Figure 2). This suggests that M18BP1 is the connecting factor between the centromeric regions and the pre-nucleosomal CENP-A/H4/HJURP complex. Mis18β also directly interacts with the C-terminus of CENP-C 62. Remarkably, tethering M18BP1 to an ectopic site is not sufficient to recruit CENP-A in human cells49,63, suggesting that, in addition to the presence of the Mis18 complex, human HJURP also requires components of the CCAN such as CENP-C and CENP-I to successfully assemble CENP-A de novo 49,58,64,65. It remains to be understood which factor is responsible for initiating CENP-A deposition at the centromere.
Is CENP-C a simple reader of centromeric chromatin or a marker for centromere formation?
CENP-C is a constitutive centromere component that acts as a “blueprint” of the CCAN network, mediating its assembly and promoting kinetochore formation via an interaction with Mis12 66,67 (reviewed by Hara and Fukagawa 68). CENP-C depletion leads to a strong reduction of CCAN proteins levels, but not to their immediate and complete loss69. CENP-C was proposed to act as a “reader” of CENP-A nucleosomes (reviewed by Ali-Ahmad and Sekulić 70). Its replenishment follows rapidly after CENP-A reloading in G1/S phase69,71 and CENP-C binding to CENP-A nucleosomes is regulated by PTMs72,73. CENP-C binds to CENP-A nucleosomes through two binding sites (Figure 2), with its central region showing higher affinity (reviewed by Ali-Ahmad and Sekulić 70) and is required for CENP-A stabilization along with CENP-N 74,75.
When bound to an ectopic site, CENP-C is sufficient to trigger the incorporation of endogenous CENP-A at this locus, albeit inefficiently (~30%), in chicken, human and fly cells49,64,76. At endogenous centromeres, CENP-C is a key factor to mediate de novo CENP-A assembly: following co-depletion and subsequent re-expression of both endogenous CENP-A and CENP-C, only CENP-C had the ability, although limited, to reinstate centromere memory39. This observation suggests that de novo centromere formation is likely initiated by a critical level of CENP-C rather than by CENP-A (Figure 1D). Once CENP-A is deposited, centromere position is efficiently maintained indefinitely by the CENP-A-dependent self-assembly loop39.
How CENP-C is capable of promoting de novo centromere formation still remains to be tested. A likely possibility is that in human cells this ability is mediated by interacting with and recruiting the Mis18 complex to the centromeres39,49,58,59 (Figure 1 and 2). In contrast, in chicken cells and Xenopus egg extracts, CENP-C is not sufficient for the assembly of the Mis18 complex56,57. In Drosophila, where the Mis18 complex is absent, CENP-C was shown to directly interact with CAL115,77. Interestingly, an interaction of HJURP with CENP-C fragments (but not the full-length protein) was also observed in human cells46. This raises the possibility that HJURP may also be important for CENP-C recruitment (and vice versa), although a transient interaction with the Mis18 complex could play a role in new CENP-A reloading. It is important to note that CENP-C not only binds to CENP-A nucleosomes and Mis18, but it also connects to chromatin in other ways: indirectly via CENP-I/H/K/M/L/N/T complexes (reviewed by Ali-Ahmad and Sekulić 70) and via CENP-B 69,78,79 and, directly, through an interaction with DNA (Figure 1 and 2). Although its DNA sequence specificity remains uncertain, CENP-C has DNA binding activity80–82, which could potentially initiate CENP-C loading during neocentromere formation.
Emerging roles of CENP-B
Many species including mammals, fission yeast, and insects have evolved sequence-specific DNA-binding proteins (CENP-B or CENP-B-related proteins) originating from the domestication of pogo-like transposases (reviewed by Gamba and Fachinetti83). CENP-B is a 2-helix-turn-helix, DNA-binding protein that binds to a 17-bp DNA-sequence motif called the CENP-B box84, which is present at all human centromeres except the Y chromosome85. Although pogo-like transposases are found in several different species, CENP-B-box-like motifs display a certain degree of conservation, being found only in vertebrates (reviewed by Gamba and Fachinetti83).
Contrary to all other components that constitute the core centromere, CENP-B is not essential, as CENP-B KO mice are viable and CENP-B-deficient functional centromeres exist (e.g. the Y chromosome and neocentromeres in humans, and centromeres of species such as certain equids and some primates) (reviewed by Fukagawa and Earnshaw 8, Dumont and Fachinetti 86, and Giulotto et al 87). Paradoxically, CENP-B evolved to bind centromeric DNA, is widely conserved in vertebrates, and plays functional roles at the centromere.
CENP-B interacts with CENP-A 79,88 and CENP-C 78,79 (Figure 2) and it was shown to be sufficient for preserving kinetochore assembly and for the maintenance of chromosome segregation fidelity69. Further, the enrichment of CENP-B boxes, CENP-B and other centromeric/kinetochore components is associated with a lower rate of chromosome mis-segregation89. However, it is important to note that while CENP-A/B/C are interacting partners, about half of CENP-B molecules do not engage in these interactions90. This non-CENP-A/C-interacting CENP-B pool could participate in heterochromatin formation91,92 via the recruitment of the DAXX chaperone93. CENP-B binding to repetitive DNA has been proposed to be regulated by PTM on CENP-B itself94,95 and by DNA methylation96 and facilitated by Nap1 97 and INMAP 98 (Figure 2). However, further studies are required to test if and how these PTMs regulate the interaction of CENP-B with CENP-C.
In addition to its active role in kinetochore function, CENP-B is also required for centromere formation and maintenance. ‘Bottom-up’ assembly strategies, in which large fragments of centromeric DNA of a minimum length of 30-kb are introduced into cells, demonstrated that CENP-B boxes and CENP-B facilitate centromere formation on human artificial chromosome (HACs) (reviewed by Ohzeki et al 99). These findings indicate that CENP-B may act as a mark for the centromere identity upstream of CENP-A91. Along the same line, it was recently shown that under temporal centromere inactivation in which CENP-A level is compromised, CENP-B can specify the ‘memory’ of centromere position on native human centromeres by preserving a critical level of CENP-C and, possibly, by preventing neocentromere formation39 (Figure 1C). The finding that CENP-B enables CENP-A recruitment via CENP-C 39 leads to a possible model for the temporal events occurring during centromere formation, at least at centromeres normally bound by CENP-B: CENP-B-mediated HAC formation occurs first through the recruitment of CENP-C and subsequently of CENP-A (Figure 1D). Notably, a direct interaction between CENP-A and CENP-B has been observed79,88,91, even though this interaction appears to be insufficient to load and stabilize CENP-A in the absence of CENP-C 39,75 (Figure 2). Masumoto and colleagues also proposed that CENP-B favors CENP-A incorporation by promoting the formation of a permissive chromatin environment through the direct recruitment of ASH1L, a H3K36 methylase92 (Figure 1E).
An alternative and more speculative model to explain such CENP-B-dependent CENP-A deposition arises from studying the topology of centromeric chromatin. The presence of CENP-B at the centromere has been proposed to affect centromere organization by dictating, but not directly preserving, nucleosome positioning along centromere DNA100–102 and by remodeling centromere architecture101,103. In addition, the binding of CENP-B to centromeric DNA induces conformational changes to centromeric chromatin by generating a ~60° bending of the DNA104–106. The ability of CENP-B to bend DNA may be important at centromeres that do not contain DNA sequences able to fold into non-B DNA structures, which have been proposed as possible marks of centromere identity107 (Figure 1F). The specific structure and biological relevance of the chromatin changes induced by CENP-B still need to be determined.
Centromeric DNA composition and its potential roles
Two nearly universal characteristics of centromeric DNA are its repetitive nature and the low degree of sequence conservation. Despite the striking sequence divergence, a recurring feature of regional centromeres in fungi, plants and animals is the presence of both transposable elements and repeated arrays of satellite DNA that span from several kilobases to megabases and could provide favorable conditions for centromere formation and/or function (reviewed by Gamba and Fachinetti83 and Talbert and Henikoff108; Figure 3).
Figure 3. Centromere organization in model species.

Schematic showing the DNA organization 183for three multicellular species with fully sequenced centromeres, Drosophila melanogaster centromere 318, Zea Mays centromere 10 183, and Homo sapiens centromere 8 19. A) Drosophila melanogaster centromere 3 is composed of blocks of 10-mer (Prodsat) and 12-mer (dodeca) satellites flanking an island of complex DNA, named Giglio, containing the centromere-enriched enriched retroelement G2/Jockey-3 and the Ribosomal Intergenic Spacer (IGS). B) Z. mays centromere 10 includes two CentC enriched-clusters, called C1 and C2, separated by a retroelement enriched region devoid of CentC (NC). CRs are Centromeric Retroelements 1-6. C) H. sapiens centromere 8 is composed of flanking monomeric α-sat interspersed with Long Interspersed Elements (LINEs), Short Interspersed Elements (SINEs) and other human satellites surrounding a region containing α-sat Higher Order Repeats (HORs). Dotted lines represent intervening sequences with the same organization of those shown. The brackets demarcate the region of the centromere that contains CENP-A and its corresponding length.
In addition to form on repetitive DNA, naturally-occurring centromeres can also emerge de novo, albeit rarely. Such de novo centromeres have been observed in human patients (neocentromeres; reviewed by Marshall et al. 109) and during speciation in plants and mammals (evolutionary new centromeres or ENCs110–112). However, neocentromeres display defective segregation, error correction and cohesion in cells-based assays79,113,114 and ENCs typically accumulate centromeric satellites after their inception during evolution115. These findings suggest that certain intrinsic features of centromeric DNA may facilitate centromere stability or function. Yet, knowledge of what such advantageous features may be has remained elusive in part because of the difficulties associated with functionally and phylogenetically analyze such large, complex, and inaccessible regions.
In humans, all conventional centromeres are positioned on α-satellite (α-sat) DNA, an AT-rich monomer 171 bp long that is repeated head-to-tail116. α-sat DNA is specific to primates and has two basic organizations: 1) monomeric α-sat, which displays variable organization and sequence and 2) ‘higher-order repeats’ (HORs) consisting of divergent monomers forming a larger repeating unit. HORs are tandemly arranged to form large arrays up to several megabases and are thought to evolve through concerted evolution117. HORs arrays differ in sequence and organization between chromosomes and the high degree of polymorphism observed across individuals demonstrates they are rapidly evolving (reviewed by Miga 118 ). Each centromere spans multiple arrays, yet the kinetochore only forms on a single HOR on each chromosomes that is referred to as ‘active’ (reviewed by McNulty and Sullivan 119).
The recent advent of long-read sequencing and assembly technologies has allowed unprecedented access to the DNA sequences of complex centromeres in maize120, fission yeast121, Drosophila18, and humans19,122,123. Twenty years since the announcement of the sequencing of the human genome, full centromere assemblies were recently revealed for the sex chromosomes and for chromosome 8, and the remaining centromeres are described in a recent preprint124. While the X and Y centromeres contain arrays composed of canonical chromosome-specific HORs122,123, centromere 8 consists of an HOR array (D8Z2) composed of four distinct HOR types of different lengths. Interestingly, the CENP-A region of centromere 8 shows a high degree of HOR subtype mixing, suggesting that this region is undergoing optimization. While the centromere 8 core is composed of HORs, the flanking satellite blocks are composed of monomeric α-sat DNA displaying more sequence variation19. HORs make up the centromere 8 of Great Apes as well, whereas in both humans and Great Apes monomeric α-sat is typically relegated to the pericentromere. Interestingly, monomeric α-sat is retained at the centromeres of lower primates19, suggesting a very recent reorganization of centromeric α-sat through homogenization (reviewed by Balzano and Giunta 125). A recent preprint reported the sequences associated with each of the active human centromeres, confirming that CENP-A associates with a single HOR on each chromosome that is depleted of CpG methylation and showing that CENP-A consistently associates with the most recent HOR haplotypes126.
In addition to satellite DNA, retroelements (REs) are also associated with centromeres across widely divergent taxa such as fungi, plants, flies, and mammals. In Drosophila melanogaster, the centromeres form on complex DNA ‘islands’ enriched in REs and flanked by asymmetric large arrays of simple satellites18 (Figure 3). The centromeres of maize and rice are composed of satellite repeats and interspersed centromeric REs120,127,128 and REs invade maize neocentromeres after their formation129. In mammals, REs have been found associated with the centromeres of species such as gibbons130,131 and the koala132, to name just two. Computational models of human centromeres have uncovered REs associated with most centromeres (reviewed by Klein and O’Neill 133). Complete maps for the three centromeres available at the time of this review identified a single LINE (long interspersed nuclear element — a retroelement) present at the active X centromere123, no retroelement insertions at the Y centromere122, and LINEs and SINEs (short interspersed nuclear element — a non-autonomous retroelement) scattered at centromere 8, but only within the outer monomeric alpha-satellite region19. Examination of the phylogeny of pericentromeric and centromeric LINE repeats at the human X centromere supports a model whereby the functional centromere has evolved more recently compared to flanking monomeric α-sat DNA, which might have preceded HORs as the functional primate centromere117. Ongoing sequencing and annotation efforts will further shed light on the prominence of these elements within active centromere regions.
Why REs are common at centromeres is still unexplained. One model is that their activity may spur the formation of de novo centromeres. This model is consistent with the findings that LINEs (along with endogenous retroviruses) are enriched at evolutionary breakpoints with latent centromere activity in marsupials134, at the 10q25 neocentromere found on a chromosome 10-derived marker chromosome mardel(10)135, and at all ENCs found in donkey115. Furthermore, the presence of LINE elements interspersed with monomeric α-sat at what are considered the ancestral centromere sequences of primates, now relegated to the flanking pericentromere19,117, is consistent with the possibility that REs contribute to centromere inception, although they later become ‘extradited’ to the pericentromere during satellite homogenization and optimization (reviewed by Wong and Choo 136).
Centromeric REs have also been implicated in satellite ontogenesis (reviewed by Klein and O’Neill 133 and Presting 137) and have been proposed to promote centromere integrity by maintaining centromeric chromatin transcriptionally active to either facilitate CENP-A maintenance or by generating non-coding RNAs with structural roles (reviewed by Klein and O’Neill 133). In Drosophila embryos, the centromere-enriched LINE-like element G2/Jockey-3 is transcribed at low levels18, and transcripts emanating from a single, transcriptionally active LINE at the human 10q25 neocentromere influence CENP-A levels and neocentromere function135. Although these findings are consistent with the proposed roles for retroelements transcription in centromere integrity, more studies are necessary to better establish these correlations across species.
The vast degree of sequence variation observed at the centromeres across species is puzzling, given the highly conserved function that centromeres perform. However, cell biological and genetic evidence support the notion that centromeres represent battlegrounds of conflict, spurring their rapid evolution, a phenomenon named “centromere drive” (reviewed by Henikoff and Malik 138, Rosin and Mellone 139, and Lampson and Black 140). Nonetheless, satellite DNA and transposable elements are nearly ubiquitous, hinting at some type of ‘universal’ role that these elements may confer. What could such shared sequence features be, given the extraordinary divergence of centromeric DNA? Using a computational approach, a study identified the recurrence of dyad symmetries in small (<10bp) centromeric DNA sequence units that are predicted to adopt energetically stable non-B-form DNA structures such as DNA melting and cruciform extrusions, which may induce centromere formation107 (Figure 1F). Interestingly, in humans these dyad-symmetries were only found at centromeres that lack CENP-B boxes, such as neocentromeres and the Y centromere, suggesting that at all other centromeres the DNA-bending properties of CENP-B may induce secondary structures that mimic non-B DNA. ‘I-motifs’, a type of non-B-form DNA, were observed in vitro for a Drosophila centromeric satellite using NMR spectroscopy 141,142. Larger topological structures such as DNA loops forming during DNA replication were observed by electron microscopy in a heterologous Xenopus egg extract system with a BAC containing human centromeric DNA143. A functional implication of centromeric DNA loops has been proposed during mitosis, where they may act as molecular springs to absorb the microtubule pulling force exerted on centromeric chromatin144,145. Interestingly, in S. pombe, centromeric DNA inherently destabilizes H3 nucleosomes even when relocated to non-centromeric ectopic sites, presenting yet another mechanism for how centromere sequences could favor centromere formation/propagation: through programmed H3 eviction during the cell cycle146.
In summary, whether such secondary DNA structures exist in vivo, and what their biological relevance may be remains uncertain, as non-B-form DNA is unlikely to be unique to centromeric regions. The emergence of full centromere sequences for multicellular species are paving the way for the types of functional and evolutionary studies needed to gain new insights into the contributions of centromeric repeats to centromere organization and identity.
Centromere Transcription, Transcripts or Both?
Although it is clear that a single sequence does not dictate the identity of complex centromeres, species-specific assortments of centromeric repeats may evolve to attain an optimal genomic landscape for centromere chromatin homeostasis127,133. Several lines of evidence support the notion that centromeric repeats are transcriptionally active (see Corless et al. 147 and Liu et al. 148 for recent reviews). However, the questions of why centromeres are transcribed — and what the role of centromere-emanating transcripts may be — have yet to be answered.
In fission yeast, while pericentric repeats are processed into small interfering RNAs (siRNAs) via the RNA interference pathway involving Dicer149, centromere-derived transcripts are immediately degraded by the exosome, suggesting that they are mere byproducts of centromeric transcription unlikely to play a structural role as non-coding RNAs150. The central core region of S. pombe’s centromere 2 displays extensive low quality RNAPII (RNA polymerase II) transcription151 and its sequence can be extensively altered and still function as a centromere as long as it contains cryptic transcription start sites151. In G2 phase of the cell cycle, a sequence of centromere 2 is ‘programmed’ to lose histone H3 coinciding with the time of Cnp1/CENP-A deposition. Interestingly, this programmed loss of H3 occurs in parallel with the recruitment of elongating RNAPII 146. Furthermore, a GATA-like transcription factor, Ams2, is required for proper CENP-A accumulation at centromeres in fission yeast152.
Transcription was also observed at ectopic sites upon de novo centromere formation resulting from DNA tethering of CAL1 to an ectopic location in Drosophila S2 cells. CAL1 promotes this transcription through an interaction with both RNAPII and the histone chaperone FACT 153 (Figure 4A). Similarly, an increase in transcription was observed at the site of neocentromere formation in human cultured cells114. While transcription may facilitate the removal of histone H3 in exchange for CENP-A at the centromere, it also runs the risk of removing pre-deposited CENP-A; however, such transcription-coupled loss of CENP-A is prevented by the conserved elongation factor Spt6154. Collectively, these studies point to a role for transcription in the chromatin reorganization necessary for stable CENP-A incorporation into nucleosomes at centromeres147,148,153.
Figure 4. Centromere transcription.
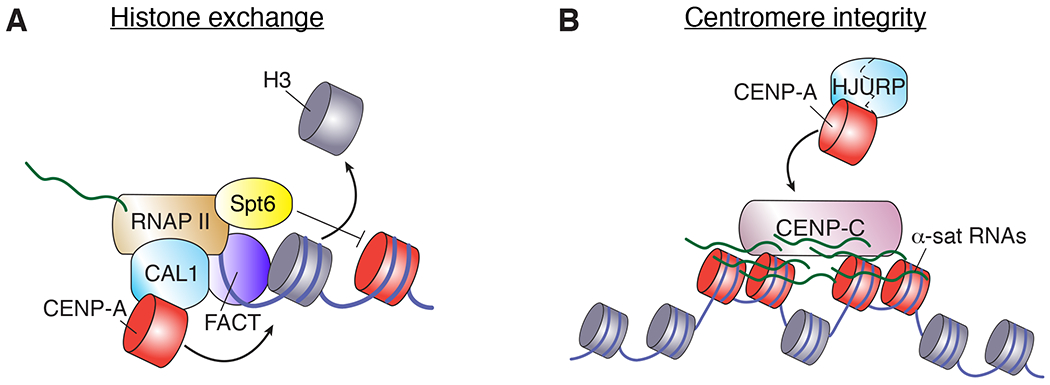
Diagram depicting two ways that transcription has been shown to influence centromere function. A) In Drosophila, transcription facilitates histone exchange and CENP-A deposition through the destabilizing effect of transcribing RNA polymerase II (RNAPII) and the histone chaperone FACT, which are recruited by the CENP-A assembly factor CAL1. The elongation factor Spt6 prevents loss of pre-existing CENP-A. Analogous findings have been shown in S. pombe and human cells. B) In human cells, α-satellite (α-sat) transcripts localize to their site of origin in cis, promoting centromere integrity and the deposition of new CENP-A.
As mentioned previously in reference to the possible roles of centromeric retroelements, transcription has been implicated in centromere assembly and integrity also through the formation of centromere-derived non-coding RNAs (Figure 4B). Transcripts emanating from centromere-associated sequences have now been described for a large number of species spanning multiple taxa (reviewed by Grenfell et al. 155 and Chan and Wong 156). In human cells, α-sat transcripts are produced from both the centromere and the pericentromere – with those derived from the active HORs being more stable – and they have been observed localized to their cognate DNA sequences157. α-sat RNAs do not depend on centromere activity157, yet removal of some centromeric components leads to a moderate increase in centromeric α-sat transcripts158, particularly at replicating centromeres29. Importantly, depletion of array-specific α-sat transcripts results in defective new CENP-A centromeric assembly at the targeted centromere, reduced level of centromeric CENP-C and, consequently, mitotic defects157,159. These findings, along with the physical interaction observed between α-sat RNAs and centromere proteins, prompted the speculation that these transcripts function in cis as non-coding RNAs, with an intrinsic sequence-specific role in centromere integrity157. In contrast, another study that detected α-sat transcripts using single-molecule RNA FISH emphasized instead a role for transcription itself rather than for α-sat non-coding RNAs158. More work is needed to discriminate between these models and to extend these observations to other species.
Recent genomic analysis of nascent RNAs from a hydatiform human cell line shows very low ongoing transcription coming from α-sat160, suggesting that α-sat RNAs accumulate and remain stably associated with the centromere157. During mitosis, α-sat transcription has been proposed to maintain centromeric cohesion161 and to facilitate chromosome segregation in a manner dependent on R-loops162. Elongating RNA Polymerase II (RNAPII) has been observed co-localizing with the centromeres of metaphase chromosomes in human and Drosophila cells, and in Xenopus egg extracts159,163–166. However, in human cells, RNAPII retention seems to result from persistent cohesion at the centromere rather than from active recruitment of the transcriptional machinery167. What is puzzling is that RNAPII is presumably lost from centromeres upon removal of cohesion in anaphase167, yet alpha-satellite transcripts are present throughout the cell cycle157, including during G1 when new CENP-A is deposited. This suggests that either RNAPII is re-loaded at the centromeres in G1 or that new CENP-A loading at this time is merely regulated by the presence of α-sat transcripts rather than by ongoing active transcription.
It’s unclear if the function of non-coding RNAs and of transcription per se work in synergy or are mutually exclusive mechanisms in the same context156. One possibility is that transcription provides an optimal chromatin environment for de novo CENP-A deposition and maintenance during centromere establishment, but that centromere-derived transcripts acquire non-coding RNA functions during evolution through, for instance, the establishment of protein-RNA interactions that further stabilize a newly formed centromere133.
Non-canonical centromere specification mechanisms
Centromere divergence reaches well beyond the variation in the primary sequences of centromeric DNA and centromere-binding proteins. The reliance on CENP-A for centromere specification is all but universal, and examples of CENP-A-independent modes of centromere specification and function continue to emerge. Such CENP-A-independent centromere function has been observed in specialized tissues of organisms that otherwise rely on CENP-A for mitotic centromere function as well as in species that display evolutionarily ‘re-invented’ centromere conformations entirely lacking CENP-A orthologs.
In C. elegans, both oocyte meiotic divisions involve a reorganization of the kinetochore in cup-like structures that assemble and mediate chromosome segregation in a CENP-A- and CENP-C-independent manner168. In Arabidopsis thaliana, expression and localization studies revealed the presence of CENP-A at the centromeres of sperm cells but not at those of egg cells. Following zygote formation, paternal CENP-A is removed, and CENP-A is expressed from both paternal and maternal copies of the gene and deposited at centromeres de novo 169.
Several orders of holocentric insects, including Lepidoptera (e.g. butterflies and moths), completely lack CENP-A orthologs170. Many of these lineages also lack CENP-C, yet contain several members of the CCAN, including the histone-fold domain containing protein CENP-T171. Interestingly, chromatin profiling approaches uncovered a mutually exclusive relationship between the position of centromere sites and transcriptional activity in the silkworm Bombyx mori. Perturbations of transcriptional states and analysis of differentially expressed genes in a related species revealed that centromere occupancy can switch for a given position, suggesting a sequence-independent centromere formation mechanism172.
Holocentric insects are not the only species not relying on CENP-A for centromere formation. Mucor circinelloides, an opportunistic human pathogen, displays monocentric chromosome containing conserved centromere (CENP-T but not CENP-C) and kinetochore proteins (Dsn1 and Mis12) over a short AT-rich DNA sequence motif surrounded by a novel type of retrotransposon. Remarkably, this organism lacks CENP-A or any other centromeric-specific histone variants173. As their centromeres share features with both point and regional yeast centromeres, the authors proposed that the centromeres of M. circinelloides are mosaic, a new type of centromere configuration that emerged in early-diverging fungi.
Even more remarkably, the kinetoplastid parasite Trypanosoma brucei encodes for entirely evolutionarily distinct kinetochore proteins (KKT1-25), that even lack the nearly universal CENP-C and Ndc80 proteins174. T. brucei displays monocentric chromosomes that are large and repetitive175. Although microtubule-binding proteins that might perform the role of Ndc80 have been identified176, a protein that may specify centromere position similarly to CENP-A is not yet known. Histone H3 variants in T. brucei are not specific to the centromere177. A recent study reports the characterization of two of the six proteins that constitutively localize to T. brucei’s centromeres called KKT2 and KKT3 178. Both proteins harbor a kinase domain and a polo-like box and have redundant functions in recruiting several other proteins to the kinetochore. The central region of KKT2/3 contains two zinc finger domains, suggesting it can bind DNA directly178. Together, these examples underscore the diversity of centromere specification mechanisms behind the canonical view centered around CENP-A.
Concluding remarks
The centromere has been the focus of fascination for biologists since its discovery as the primary site of chromosome constriction by Walter Flemming in the 19th century179. Today, the centromere is recognized as a fundamental player in genome inheritance, well beyond a simple constriction on the chromosome. The centromere biology field has made tremendous progress in isolating and functionally characterizing centromere-bound proteins and, more recently, in identifying the centromeric DNA sequences of multicellular species. However, many aspects of how the centromere site is specified remain elusive, and since in nature centromeres rarely form de novo, scientists have resorted to several strategies to experimentally create de novo centromeres. Deleting centromeric DNA (reviewed by Murillo-Pineda and Jansen 3, and Hori and Fukagawa 180), depleting centromeric components (reviewed by Hoffmann and Fachinetti 71), creating HACs (reviewed by Ohzeki et al. 99), or mis-targeting centromeric proteins (reviewed by Hori and Fukagawa 180) are just a few examples of how scientists are trying to understand the ‘rules’ of what defines a functional and heritable centromere. Even though CENP-A is the most recognized pillar of centromere specification, how it is faithfully directed to centromeres is turning out to be very complex, involving several protein networks and even centromeric RNAs or other epigenetic or genetic features (reviewed by Talbert and Henikoff 108,181 and Scelfo and Fachinetti 182). The existence of organisms that do not rely on CENP-A at all underlies the fact that no factor is universally conserved, as is true for centromeric DNA. Thus, centromeres are figuratively and literally ‘always on the move’ and still hold many secrets that will keep scientists busy for years to come.
Acknowledgements.
The authors would like to thank Sebastian Hoffmann and Lars Jansen for help with Figure 2, Prachi Tandale, Gernod Presting, and Rachel O’Neill for help with Figure 3, and Sylvia Erhardt for suggestions. Figure 3 was created with Biorender.com. D.F. receives salary support from the CNRS and I. Curie. B.M. is supported by NIH award R35 GM131868. We apologize to the authors whose contributions could not be highlighted in this review due to space and reference limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- 1.Santaguida S, and Musacchio A (2009). The life and miracles of kinetochores. The EMBO Journal 28, 2511–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santaguida S, and Amon A (2015). Short- and long-term effects of chromosome mis-segregation and aneuploidy. Nature Reviews Molecular Cell Biology 16, 473–485. [DOI] [PubMed] [Google Scholar]
- 3.Murillo-Pineda M, and Jansen LET (2020). Genetics, epigenetics and back again: Lessons learned from neocentromeres. Experimental Cell Research 389, 111909. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan LL, and Sullivan BA (2020). Genomic and functional variation of human centromeres. Experimental Cell Research 389, 111896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allshire RC, and Karpen GH (2008). Epigenetic regulation of centromeric chromatin: old dogs, new tricks? Nature Reviews Molecular Cell Biology 9, 923–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Earnshaw WC, and Rothfield N (1985). Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma 91, 313–321. [DOI] [PubMed] [Google Scholar]
- 7.Palmer D, O’Day K, Wener M, Andrews B, and Margolis R (1987). A 17-kD centromere protein (CENP-A) copurifies with nucleosome core particles and with histones. The Journal of Cell Biology 104, 805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukagawa T, and Earnshaw WC (2014). The centromere: chromatin foundation for the kinetochore machinery. Developmental Cell 30, 496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKinley KL, and Cheeseman IM (2016). The molecular basis for centromere identity and function. Nature Reviews Molecular Cell Biology 17, 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodor DL, Mata JF, Sergeev M, David AF, Salimian KJ, Panchenko T, Cleveland DW, Black BE, Shah JV, and Jansen LE (2014). The quantitative architecture of centromeric chromatin. eLife 3, e02137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nechemia-Arbely Y, Miga KH, Shoshani O, Aslanian A, McMahon MA, Lee AY, Fachinetti D, Yates JR, Ren B, and Cleveland DW (2019). DNA replication acts as an error correction mechanism to maintain centromere identity by restricting CENP-A to centromeres. Nature Cell Biology 21, 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blower MD, Sullivan BA, and Karpen GH (2002). Conserved Organization of Centromeric Chromatin in Flies and Humans. Developmental Cell 2, 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunleavy EM, Almouzni G, and Karpen GH (2011). H3.3 is deposited at centromeres in S phase as a placeholder for newly assembled CENP-A in G1 phase. Nucleus 2, 146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lando D, Endesfelder U, Berger H, Subramanian L, Dunne PD, McColl J, Klenerman D, Carr AM, Sauer M, Allshire RC, et al. (2012). Quantitative single-molecule microscopy reveals that CENP-ACnp1 deposition occurs during G2 in fission yeast. Open Biol. 2, 120078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schittenhelm RB, Althoff F, Heidmann S, and Lehner CF (2010). Detrimental incorporation of excess Cenp-A/Cid and Cenp-C into Drosophila centromeres is prevented by limiting amounts of the bridging factor Call. J. Cell Sci 123, 3768–3779. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro SA, Vagnarelli P, Dong Y, Hori T, McEwen BF, Fukagawa T, Flors C, and Earnshaw WC (2010). A super-resolution map of the vertebrate kinetochore. PNAS 107, 10484–10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston K, Joglekar A, Hori T, Suzuki A, Fukagawa T, and Salmon ED (2010). Vertebrate kinetochore protein architecture: protein copy number. The Journal of Cell Biology 189, 937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang C-H, Chavan A, Palladino J, Wei X, Martins NMC, Santinello B, Chen C-C, Erceg J, Beliveau BJ, Wu C-T, et al. (2019). Islands of retroelements are major components of Drosophila centromeres. Plos Biol. 17, e3000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logsdon GA, Vollger MR, Hsieh P, Mao Y, Liskovykh MA, Koren S, Nurk S, Mercuri L, Dishuck PC, Rhie A, et al. (2021). The structure, function and evolution of a complete human chromosome 8. Nature 593, 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall OJ, Marshall AT, and Choo KHA (2008). Three-dimensional localization of CENP-A suggests a complex higher order structure of centromeric chromatin. The Journal of Cell Biology 183, 1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andronov L, Ouararhni K, Stoll I, Klaholz BP, and Hamiche A (2019). CENP-A nucleosome clusters form rosette-like structures around HJURP during G1. Nat. Commun 10, 4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melters DP, Pitman M, Rakshit T, Dimitriadis EK, Bui M, Papoian GA, and Dalal Y (2019). Intrinsic elasticity of nucleosomes is encoded by histone variants and calibrated by their binding partners. PNAS 116, 24066–24074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou K, Gebala M, Woods D, Sundararajan K, Edwards G, Krzizike D, Wereszczynski J, Straight AF, and Luger K (2021). CENP-N promotes the compaction of centromeric chromatin. Biorxiv, 2021.06.14.448351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pentakota S, Zhou K, Smith C, Maffini S, Petrovic A, Morgan GP, Weir JR, Vetter IR, Musacchio A, and Luger K (2017). Decoding the centromeric nucleosome through CENP-N. eLife 6, e33442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagpal H, and Fierz B (2020). The elusive structure of centro-chromatin: molecular order or dynamic heterogenetity? J. Mol. Biol 433, 166676. [DOI] [PubMed] [Google Scholar]
- 26.Mitra S, Srinivasan B, and Jansen LET (2020). Stable inheritance of CENP-A chromatin: Inner strength versus dynamic control. The Journal of Cell Biology 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacAlpine DM, and Almouzni G (2013). Chromatin and DNA Replication. Cold Spring Harbor Perspectives in Biology 5, a010207–a010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zasadzińska E, Huang J, Bailey AO, Guo LY, Lee NS, Srivastava S, Wong KA, French BT, Black BE, and Foltz DR (2018). Inheritance of CENP-A Nucleosomes during DNA Replication Requires HJURP. Developmental Cell 47, 348–362.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giunta S, Hervé S, White RR, Wilhelm T, Dumont M, Scelfo A, Gamba R, Wong CK, Rancati G, Smogorzewska A, et al. (2021). CENP-A chromatin prevents replication stress at centromeres to avoid structural aneuploidy. PNAS 118, e2015634118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srivastava S, Zasadzińska E, and Foltz DR (2018). Posttranslational mechanisms controlling centromere function and assembly. Current Opinion in Cell Biology 52, 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bailey AO, Panchenko T, Shabanowitz J, Lehman SM, Bai DL, Hunt DF, Black BE, and Foltz DR (2016). Identification of the Post-translational Modifications Present in Centromeric Chromatin*. Mol. Cell Proteomics 15, 918–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arimura Y, Tachiwana H, Takagi H, Hori T, Kimura H, Fukagawa T, and Kurumizaka H (2019). The CENP-A centromere targeting domain facilitates H4K20 monomethylation in the nucleosome by structural polymorphism. Nat. Commun 10, 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Musacchio A, and Desai A (2017). A Molecular View of Kinetochore Assembly and Function. Biology 6, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross JE, Woodlief KS, and Sullivan BA (2016). Inheritance of the CENP-A chromatin domain is spatially and temporally constrained at human centromeres. Epigenetics & Chromatin 9, 20–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lam AL, Boivin CD, Bonney CF, Rudd MK, and Sullivan BA (2006). Human centromeric chromatin is a dynamic chromosomal domain that can spread over noncentromeric DNA. PNAS 103, 4186–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim IS, Lee M, Park JH, Jeon R, Baek SH, and Kim KI (2014). βTrCP-mediated ubiquitylation regulates protein stability of Mis18β in a cell cycle-dependent manner. Biochem. Bioph. Res. Co 443, 62–67. [DOI] [PubMed] [Google Scholar]
- 37.Nardi IK, Zasadzińska E, Stellfox ME, Knippler CM, and Foltz DR (2016). Licensing of Centromeric Chromatin Assembly through the Mis18α-Mis18β Heterotetramer. Molecular Cell 61, 774–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stankovic A, Guo LY, Mata JF, Bodor DL, Cao X-J, Bailey AO, Shabanowitz J, Hunt DF, Garcia BA, Black BE, et al. (2017). A Dual Inhibitory Mechanism Sufficient to Maintain Cell-Cycle-Restricted CENP-A Assembly. Molecular Cell 65, 231–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann S, Izquierdo HM, Gamba R, Chardon F, Dumont M, Keizer V, Hervé S, McNulty SM, Sullivan BA, Manel N, et al. (2020). A genetic memory initiates the epigenetic loop necessary to preserve centromere position. The EMBO Journal 39, nrg2466–937–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niikura Y, Kitagawa R, and Kitagawa K (2016). CENP-A Ubiquitylation Is Inherited through Dimerization between Cell Divisions. Cell reports 15, 61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gassmann R, Rechtsteiner A, Yuen KW, Muroyama A, Egelhofer T, Gaydos L, Barron F, Maddox P, Essex A, Monen J, et al. (2012). An inverse relationship to germline transcription defines centromeric chromatin in C. elegans. Nature 484, 534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Westhorpe FG, and Straight AF (2014). The Centromere: Epigenetic Control of Chromosome Segregation during Mitosis. Csh. Perspect. Biol 7, a015818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu H, Liu Y, Wang M, Fang J, Huang H, Yang N, Li Y, Wang J, Yao X, Shi Y, et al. (2011). Structure of a CENP-A-histone H4 heterodimer in complex with chaperone HJURP. Genes & Development 25, 901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medina- Pritchard B, Lazou V, Zou J, Byron O, Abad MA, Rappsilber J, Heun P, and Jeyaprakash AA (2020). Structural basis for centromere maintenance by Drosophila CENP- A chaperone CAL1. The EMBO Journal 39, e103234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mellone BG, Grive KJ, Shteyn V, Bowers SR, Oderberg I, and Karpen GH (2011). Assembly of Drosophila Centromeric Chromatin Proteins during Mitosis. Plos Genetics 7, e1002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tachiwana H, Müller S, Blümer J, Klare K, Musacchio A, and Almouzni G (2015). HJURP involvement in de novo CenH3(CENP-A) and CENP-C recruitment. Cell reports 11, 22–32. [DOI] [PubMed] [Google Scholar]
- 47.Ding M, Jiang J, Yang F, Zheng F, Fang J, Wang Q, Wang J, Yao W, Liu X, Gao X, et al. (2019). Holliday junction recognition protein interacts with and specifies the centromeric assembly of CENP-T. J. Biol. Chem 294, 968–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barnhart MC, Kuich PHJL, Stellfox ME, Ward JA, Bassett EA, Black BE, and Foltz DR (2011). HJURP is a CENP-A chromatin assembly factor sufficient to form a functional de novo kinetochore. The Journal of Cell Biology 194, 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shono N, Ohzeki J, Otake K, Martins NMC, Nagase T, Kimura H, Larionov V, Earnshaw WC, and Masumoto H (2015). CENP-C and CENP-I are key connecting factors for kinetochore and CENP-A assembly. Journal of Cell Science 128, 4572–4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Logsdon GA, Gambogi CW, Liskovykh MA, Barrey EJ, Larionov V, Miga KH, Heun P, and Black BE (2019). Human Artificial Chromosomes that Bypass Centromeric DNA. Cell 178, 624–639.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palladino J, Chavan A, Sposato A, Mason TD, and Mellone BG (2020). Targeted De Novo Centromere Formation in Drosophila Reveals Plasticity and Maintenance Potential of CENP-A Chromatin. Developmental Cell 52, 379–394.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujita Y, Hayashi T, Kiyomitsu T, Toyoda Y, Kokubu A, Obuse C, and Yanagida M (2007). Priming of Centromere for CENP-A Recruitment by Human hMis18α, hMis18β, and M18BP1. Developmental Cell 12, 17–30. [DOI] [PubMed] [Google Scholar]
- 53.Pan D, Klare K, Petrovic A, Take A, Walstein K, Singh P, Rondelet A, Bird AW, and Musacchio A (2017). CDK-regulated dimerization of M18BP1 on a Mis18 hexamer is necessary for CENP-A loading. eLife 6, e02137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spiller F, Medina- Pritchard B, Abad MA, Wear MA, Molina O, Earnshaw WC, and Jeyaprakash AA (2017). Molecular basis for Cdk1- regulated timing of Mis18 complex assembly and CENP- A deposition. EMBO Rep 18, 894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zasadzińska E, and Foltz DR (2017). Orchestrating the Specific Assembly of Centromeric Nucleosomes. In Centromeres and Kinetochores. (Springer International Publishing; ), pp. 165–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.French BT, Westhorpe FG, Limouse C, and Straight AF (2017). Xenopus laevis M18BP1 Directly Binds Existing CENP-A Nucleosomes to Promote Centromeric Chromatin Assembly. Developmental Cell 42, 190–199.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hori T, Shang W-H, Hara M, Ariyoshi M, Arimura Y, Fujita R, Kurumizaka H, and Fukagawa T (2017). Association of M18BP1/KNL2 with CENP-A Nucleosome Is Essential for Centromere Formation in Non-mammalian Vertebrates. Developmental Cell 42, 181–189.e3. [DOI] [PubMed] [Google Scholar]
- 58.Dambacher S, Deng W, Hahn M, Sadic D, Fröhlich J, Nuber A, Hoischen C, Diekmann S, Leonhardt H, and Schotta G (2012). CENP-C facilitates the recruitment of M18BP1 to centromeric chromatin. Nucleus 3, 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moree B, Meyer CB, Fuller CJ, and Straight AF (2011). CENP-C recruits M18BP1 to centromeres to promote CENP-A chromatin assembly. The Journal of Cell Biology 194, 855–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lagana A, Dorn JF, Rop VD, Ladouceur A-M, Maddox AS, and Maddox PS (2010). A small GTPase molecular switch regulates epigenetic centromere maintenance by stabilizing newly incorporated CENP-A. Nature Cell Biology 12, 1186–1193. [DOI] [PubMed] [Google Scholar]
- 61.Ohzeki J, Shono N, Otake K, Martins NMC, Kugou K, Kimura H, Nagase T, Larionov V, Earnshaw WC, and Masumoto H (2016). KAT7/HBO1/MYST2 Regulates CENP-A Chromatin Assembly by Antagonizing Suv39h1-Mediated Centromere Inactivation. Developmental Cell 37, 413–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stellfox ME, Nardi IK, Knippler CM, and Foltz DR (2016). Differential Binding Partners of the Mis18α/β YIPPEE Domains Regulate Mis18 Complex Recruitment to Centromeres. Cell reports 15, 2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohzeki J, Larionov V, Earnshaw WC, and Masumoto H (2019). De novo formation and epigenetic maintenance of centromere chromatin. Current Opinion in Cell Biology 58, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hori T, Shang W-H, Takeuchi K, and Fukagawa T (2013). The CCAN recruits CENP-A to the centromere and forms the structural core for kinetochore assembly. The Journal of Cell Biology 200, 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okada M, Cheeseman IM, Hori T, Okawa K, McLeod IX, Yates JR, Desai A, and Fukagawa T (2006). The CENP-H-I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nature Cell Biology 8, 446–457. [DOI] [PubMed] [Google Scholar]
- 66.Foltz DR, Jansen LET, Black BE, Bailey AO, Yates JR, and Cleveland DW (2006). The human CENP-A centromeric nucleosome-associated complex. Nature Cell Biology 8, 458–469. [DOI] [PubMed] [Google Scholar]
- 67.Klare K, Weir JR, Basilico F, Zimniak T, Massimiliano L, Ludwigs N, Herzog F, and Musacchio A (2015). CENP-C is a blueprint for constitutive centromere–associated network assembly within human kinetochores. The Journal of Cell Biology 210, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hara M, and Fukagawa T (2020). Dynamics of kinetochore structure and its regulations during mitotic progression. Cell Mol Life Sci 77, 2981–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoffmann S, Dumont M, Barra V, Ly P, Nechemia-Arbely Y, McMahon MA, Hervé S, Cleveland DW, and Fachinetti D (2016). CENP-A Is Dispensable for Mitotic Centromere Function after Initial Centromere/Kinetochore Assembly. Cell reports 17, 2394–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ali-Ahmad A, and Sekulić N (2020). CENP-A nucleosome—a chromatin-embedded pedestal for the centromere: lessons learned from structural biology. Essays Biochem 64, 205–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoffmann S, and Fachinetti D (2018). Real-Time De Novo Deposition of Centromeric Histone-Associated Proteins Using the Auxin-Inducible Degradation System. Methods in molecular biology (Clifton, N.J.) 1832, 223–241. [DOI] [PubMed] [Google Scholar]
- 72.Ariyoshi M, Makino F, Watanabe R, Nakagawa R, Kato T, Namba K, Arimura Y, Fujita R, Kurumizaka H, Okumura E, et al. (2021). Cryo- EM structure of the CENP- A nucleosome in complex with phosphorylated CENP- C. The EMBO Journal 40, e105671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watanabe R, Hara M, Okumura E, Hervé S, Fachinetti D, Ariyoshi M, and Fukagawa T (2019). CDK1-mediated CENP-C phosphorylation modulates CENP-A binding and mitotic kinetochore localization. The Journal of Cell Biology 218, 4042–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo LY, Allu PK, Zandarashvili L, McKinley KL, Sekulic N, Dawicki-McKenna JM, Fachinetti D, Logsdon GA, Jamiolkowski RM, Cleveland DW, et al. (2017). Centromeres are maintained by fastening CENP-A to DNA and directing an arginine anchor-dependent nucleosome transition. Nat. Commun 8, 15775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Falk SJ, Guo LY, Sekulic N, Smoak EM, Mani T, Logsdon GA, Gupta K, Jansen LET, Duyne GDV, Vinogradov SA, et al. (2015). Chromosomes. CENP-C reshapes and stabilizes CENP-A nucleosomes at the centromere. Science 348, 699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen C-C, Dechassa ML, Bettini E, Ledoux MB, Belisario C, Heun P, Luger K, and Mellone BG (2014). CAL1 is the Drosophila CENP-A assembly factor. The Journal of Cell Biology 204, 313–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roure V, Medina-Pritchard B, Lazou V, Rago L, Anselm E, Venegas D, Jeyaprakash AA, and Heun P (2019). Reconstituting Drosophila Centromere Identity in Human Cells. Cell Reports 29, 464–479.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suzuki N, Nakano M, Nozaki N, Egashira S, Okazaki T, and Masumoto H (2004). CENP-B interacts with CENP-C domains containing Mif2 regions responsible for centromere localization. The Journal of biological chemistry 279, 5934–5946. [DOI] [PubMed] [Google Scholar]
- 79.Fachinetti D, Han JS, McMahon MA, Ly P, Abdullah A, Wong AJ, and Cleveland DW (2015). DNA Sequence-Specific Binding of CENP-B Enhances the Fidelity of Human Centromere Function. Developmental Cell 33, 314–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang CH, Tomkiel J, Saitoh H, Johnson DH, and Earnshaw WC (1996). Identification of overlapping DNA-binding and centromere-targeting domains in the human kinetochore protein CENP-C. Molecular and Cellular Biology 16, 3576–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Politi V, Perini G, Trazzi S, Pliss A, Raska I, Earnshaw WC, and Valle GD (2002). CENP-C binds the alpha-satellite DNA in vivo at specific centromere domains. J. Cell Sci 115, 2317–27. [DOI] [PubMed] [Google Scholar]
- 82.Sugimoto K, Yata H, Muro Y, and Himeno M (1994). Human Centromere Protein C (CENP-C) Is a DNA-Binding Protein Which Possesses a Novel DNA-Binding Motif1. J Biochem. 116, 877–881. [DOI] [PubMed] [Google Scholar]
- 83.Gamba R, and Fachinetti D (2020). From evolution to function: Two sides of the same CENP-B coin? Experimental Cell Research 390, 111959. [DOI] [PubMed] [Google Scholar]
- 84.Muro Y, Masumoto H, Yoda K, Nozaki N, Ohashi M, and Okazaki T (1992). Centromere protein B assembles human centromeric alpha-satellite DNA at the 17-bp sequence, CENP-B box. The Journal of Cell Biology 116, 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Earnshaw W, Sullivan K, Machlin P, Cooke C, Kaiser D, Pollard T, Rothfield N, and Cleveland D (1987). Molecular cloning of cDNA for CENP-B, the major human centromere autoantigen. The Journal of Cell Biology 104, 817–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dumont M, and Fachinetti D (2017). Centromeres and Kinetochores, Discovering the Molecular Mechanisms Underlying Chromosome Inheritance. Prog. Mol. Subcell Biology 56, 305–336. [DOI] [PubMed] [Google Scholar]
- 87.Giulotto E, Raimondi E, and Sullivan KF (2017). Centromeres and Kinetochores, Discovering the Molecular Mechanisms Underlying Chromosome Inheritance. Prog. Mol. Subcell Biology 56, 337–354. [DOI] [PubMed] [Google Scholar]
- 88.Fujita R, Otake K, Arimura Y, Horikoshi N, Miya Y, Shiga T, Osakabe A, Tachiwana H, Ohzeki J, Larionov V, et al. (2015). Stable complex formation of CENP-B with the CENP-A nucleosome. Nucleic acids research 43, 4909–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dumont M, Gamba R, Gestraud P, Klaasen S, Worrall JT, Vries SGD, Boudreau V, Luypaert CS, Maddox PS, Lens SM, et al. (2020). Human chromosome- specific aneuploidy is influenced by DNA- dependent centromeric features. The EMBO Journal 39, 1132–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cooke CA, Bernat RL, and Earnshaw WC (1990). CENP-B: a major human centromere protein located beneath the kinetochore. The Journal of Cell Biology 110, 1475–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Okada T, Ohzeki J, Nakano M, Yoda K, Brinkley WR, Larionov V, and Masumoto H (2007). CENP-B Controls Centromere Formation Depending on the Chromatin Context. Cell 131, 1287–1300. [DOI] [PubMed] [Google Scholar]
- 92.Otake K, Ohzeki J, Shono N, Kugou K, Okazaki K, Nagase T, Yamakawa H, Kouprina N, Larionov V, Kimura H, et al. (2020). CENP-B creates alternative epigenetic chromatin states permissive for CENP-A or heterochromatin assembly. Journal of Cell Science 133, jcs243303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morozov VM, Giovinazzi S, and Ishov AM (2017). CENP-B protects centromere chromatin integrity by facilitating histone deposition via the H3.3-specific chaperone Daxx. Epigenetics & Chromatin 10, 63–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dai X, Otake K, You C, Cai Q, Wang Z, Masumoto H, and Wang Y (2013). Identification of Novel α-N-Methylation of CENP-B That Regulates Its Binding to the Centromeric DNA. Journal of proteome research 12, 4167–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maalouf JE, Texier P, Erliandri I, Cohen C, Corpet A, Catez F, Boutell C, and Lomonte P (2018). CENP-B dynamics at centromeres is regulated by a SUMOylation/ubiquitination and proteasomal-dependent degradation mechanism involving the SUMO-targeted ubiquitin E3 ligase RNF4. Biorxiv, 245597. [Google Scholar]
- 96.Tanaka Y, Kurumizaka H, and Yokoyama S (2005). CpG methylation of the CENP- B box reduces human CENP- B binding. FEBS Journal 272, 282–289. [DOI] [PubMed] [Google Scholar]
- 97.Tachiwana H, Miya Y, Shono N, Ohzeki J, Osakabe A, Otake K, Larionov V, Earnshaw WC, Kimura H, Masumoto H, et al. (2013). Nap1 regulates proper CENP-B binding to nucleosomes. Nucleic acids research 41, 2869–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tan T, Chen Z, Lei Y, Zhu Y, and Liang Q (2014). A Regulatory Effect of INMAP on Centromere Proteins: Antisense INMAP Induces CENP-B Variation and Centromeric Halo. Plos One 9, e91937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ohzeki J, Otake K, and Masumoto H (2020). Human artificial chromosome: Chromatin assembly mechanisms and CENP-B. Experimental Cell Research 389, 111900. [DOI] [PubMed] [Google Scholar]
- 100.Hasson D, Panchenko T, Salimian KJ, Salman MU, Sekulic N, Alonso A, Warburton PE, and Black BE (2013). The octamer is the major form of CENP-A nucleosomes at human centromeres. Nature Structural Molecular Biology 20, 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yoda K, Ando S, Okuda A, Kikuchi A, and Okazaki T (1998). In vitro assembly of the CENP- B/α- satellite DNA/core histone complex: CENP- B causes nucleosome positioning. Genes Cells 3, 533–548. [DOI] [PubMed] [Google Scholar]
- 102.Nechemia-Arbely Y, Fachinetti D, Miga KH, Sekulic N, Soni GV, Kim DH, Wong AK, Lee AY, Nguyen K, Dekker C, et al. (2017). Human centromeric CENP-A chromatin is a homotypic, octameric nucleosome at all cell cycle points. The Journal of Cell Biology 216, 607–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tawaramoto MS, Park S-Y, Tanaka Y, Nureki O, Kurumizaka H, and Yokoyama S (2003). Crystal Structure of the Human Centromere Protein B (CENP-B) Dimerization Domain at 1.65-Å Resolution. The Journal of biological chemistry 278, 51454–51461. [DOI] [PubMed] [Google Scholar]
- 104.Tanaka Y, Nureki O, Kurumizaka H, Fukai S, Kawaguchi S, Ikuta M, Iwahara J, Okazaki T, and Yokoyama S (2001). Crystal structure of the CENP-B protein-DNA complex: the DNA-binding domains of CENP-B induce kinks in the CENP-B box DNA. The EMBO Journal 20, 6612–6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yoda K, Kitagawa K, Masumoto H, Muro Y, and Okazaki T (1992). A human centromere protein, CENP-B, has a DNA binding domain containing four potential alpha helices at the NH2 terminus, which is separable from dimerizing activity. The Journal of Cell Biology 119, 1413–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tanaka Y, Tachiwana H, Yoda K, Masumoto H, Okazaki T, Kurumizaka H, and Yokoyama S (2005). Human centromere protein B induces translational positioning of nucleosomes on alpha-satellite sequences. The Journal of biological chemistry 280, 41609–41618. [DOI] [PubMed] [Google Scholar]
- 107.Kasinathan S, and Henikoff S (2018). Non-B-Form DNA Is Enriched at Centromeres. Molecular biology and evolution 26, 1301–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Talbert PB, and Henikoff S (2020). What makes a centromere? Experimental Cell Research 389, 111895. [DOI] [PubMed] [Google Scholar]
- 109.Marshall OJ, Chueh AC, Wong LH, and Choo KHA (2008). Neocentromeres: New Insights into Centromere Structure, Disease Development, and Karyotype Evolution. 82, 261–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang L, Zeng Z, Zhang W, and Jiang J (2014). Three Potato Centromeres Are Associated with Distinct Haplotypes with or Without Megabase-Sized Satellite Repeat Arrays. Genetics 196, 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Montefalcone G, Tempesta S, Rocchi M, and Archidiacono N (1999). Centromere Repositioning. Genome Res 9, 1184–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nergadze SG, Piras FM, Gamba R, Corbo M, Cerutti F, McCarter JGW, Cappelletti E, Gozzo F, Harman RM, Antczak DF, et al. (2018). Birth, evolution, and transmission of satellite-free mammalian centromeric domains. Genome Research 28, 789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bassett EA, Wood S, Salimian KJ, Ajith S, Foltz DR, and Black BE (2010). Epigenetic centromere specification directs aurora B accumulation but is insufficient to efficiently correct mitotic errors. The Journal of Cell Biology 190, 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Murillo-Pineda M, Valente LP, Dumont M, Mata JF, Fachinetti D, and Jansen LET (2021). Induction of spontaneous human neocentromere formation and long-term maturation. The Journal of Cell Biology 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Piras FM, Nergadze SG, Magnani E, Bertoni L, Attolini C, Khoriauli L, Raimondi E, and Giulotto E (2010). Uncoupling of Satellite DNA and Centromeric Function in the Genus Equus. PLoS Genetics 6, e1000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.MANUELIDIS L, and WU JC (1978). Homology between human and simian repeated DNA. Nature 276, 92–94. [DOI] [PubMed] [Google Scholar]
- 117.Schueler MG, Higgins AW, Rudd MK, Gustashaw K, and Willard HF (2001). Genomic and Genetic Definition of a Functional Human Centromere. Science 294, 109–115. [DOI] [PubMed] [Google Scholar]
- 118.Miga KH (2019). Centromeric Satellite DNAs: Hidden Sequence Variation in the Human Population. Genes 10, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McNulty SM, and Sullivan BA (2018). Alpha satellite DNA biology: finding function in the recesses of the genome. Chromosome Res. 26, 115–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wolfgruber TK, Nakashima MM, Schneider KL, Sharma A, Xie Z, Albert PS, Xu R, Bilinski P, Dawe RK, Ross-Ibarra J, et al. (2016). High Quality Maize Centromere 10 Sequence Reveals Evidence of Frequent Recombination Events. Frontiers in Plant Science 7, 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tong P, Pidoux AL, Toda NRT, Ard R, Berger H, Shukla M, Torres-Garcia J, Müller CA, Nieduszynski CA, and Allshire RC (2019). Interspecies conservation of organisation and function between nonhomologous regional centromeres. Nature communications 10, 2343–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jain M, Olsen HE, Turner DJ, Stoddart D, Bulazel KV, Paten B, Haussler D, Willard HF, Akeson M, and Miga KH (2018). Linear assembly of a human centromere on the Y chromosome. Nature Biotechnology 36, 321–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miga KH, Koren S, Rhie A, Vollger MR, Gershman A, Bzikadze A, Brooks S, Howe E, Porubsky D, Logsdon GA, et al. (2020). Telomere-to-telomere assembly of a complete human X chromosome. Nature 585, 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nurk S, Koren S, Rhie A, Rautiainen M, Bzikadze AV, Mikheenko A, Vollger MR, Altemose N, Uralsky L, Gershman A, et al. (2021). The complete sequence of a human genome. Biorxiv, 2021.05.26.445798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Balzano E, and Giunta S (2020). Centromeres under Pressure: Evolutionary Innovation in Conflict with Conserved Function. Genes-basel 11, 912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Altemose N, Logsdon GA, Bzikadze AV, Sidhwani P, Langley SA, Caldas GV, Hoyt SJ, Uralsky L, Ryabov FD, Shew CJ, et al. (2021). Complete genomic and epigenetic maps of human centromeres. Biorxiv, 2021.07.12.452052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gent JI, Wang N, and Dawe RK (2017). Stable centromere positioning in diverse sequence contexts of complex and satellite centromeres of maize and wild relatives. Genome Biol. 18, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cheng Y, Guo W, and Deng X (2003). Molecular characterization of cytoplasmic and nuclear genomes in phenotypically abnormal Valencia orange (Citrus sinensis) + Meiwa kumquat (Fortunella crassifolia) intergeneric somatic hybrids. Plant Cell Rep 21, 445–451. [DOI] [PubMed] [Google Scholar]
- 129.Schneider KL, Xie Z, Wolfgruber TK, and Presting GG (2016). Inbreeding drives maize centromere evolution. PNAS 113, E987–E996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Carbone L, Harris RA, Mootnick AR, Milosavljevic A, Martin DIK, Rocchi M, Capozzi O, Archidiacono N, Konkel MK, Walker JA, et al. (2012). Centromere Remodeling in Hoolock leuconedys (Hylobatidae) by a New Transposable Element Unique to the Gibbons. Genome Biol. Evol 4, 648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hartley GA, Okhovat M, O’Neill RJ, and Carbone L (2021). Comparative analyses of gibbon centromeres reveal dynamic genus specific shifts in repeat composition. Mol. Biol. Evol, msab148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Johnson RN, O’Meally D, Chen Z, Etherington GJ, Ho SYW, Nash WJ, Grueber CE, Cheng Y, Whittington CM, Dennison S, et al. (2018). Adaptation and conservation insights from the koala genome. Nature Genetics 50, 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Klein SJ, and O’Neill RJ (2018). Transposable elements: genome innovation, chromosome diversity, and centromere conflict. Chromosome Res. 26, 5–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Longo MS, Carone DM, Program NCS, Green ED, O’Neill MJ, and O’Neill RJ (2009). Distinct retroelement classes define evolutionary breakpoints demarcating sites of evolutionary novelty. Bmc Genomics 10, 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chueh AC, Northrop EL, Brettingham-Moore KH, Choo KHA, and Wong LH (2009). LINE retrotransposon RNA is an essential structural and functional epigenetic component of a core neocentromeric chromatin. PLoS Genetics 5, e1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wong LH, and Choo KHA (2004). Evolutionary dynamics of transposable elements at the centromere. Trends Genetics 20, 611–616. [DOI] [PubMed] [Google Scholar]
- 137.Presting GG (2018). Centromeric retrotransposons and centromere function. Curr. Opin. Genet. Dev 49, 79–84. [DOI] [PubMed] [Google Scholar]
- 138.Henikoff S, and Malik HS (2002). Centromeres: selfish drivers. Nature 417, 227. [DOI] [PubMed] [Google Scholar]
- 139.Rosin LF, and Mellone BG (2017). Centromeres Drive a Hard Bargain. Trends Genetics 33, 101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lampson MA, and Black BE (2017). Cellular and Molecular Mechanisms of Centromere Drive. Cold Spring Harbor Symposia on Quantitative Biology 82, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gallego J, Chou S-H, and Reid BR (1997). Centromeric pyrimidine strands fold into an intercalated motif by forming a double hairpin with a Novel T:G:G:T tetrad: solution structure of the d(TCCCGTTTCCA) dimer11 Edited by I Tinoco. J. Mol. Biol 273, 840–856. [DOI] [PubMed] [Google Scholar]
- 142.Garavís M, Méndez-Lago M, Gabelica V, Whitehead SL, González C, and Villasante A (2015). The structure of an endogenous Drosophila centromere reveals the prevalence of tandemly repeated sequences able to form i-motifs. Scientific reports 5, 13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aze A, Sannino V, Soffientini P, Bachi A, and Costanzo V (2016). Centromeric DNA replication reconstitution reveals DNA loops and ATR checkpoint suppression. Nature Cell Biology 18, 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bloom KS (2014). Centromeric heterochromatin: the primordial segregation machine. Annual Review of Genetics 48, 457–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rosandić M, Glunčić M, Paar V, and Basar I (2008). The role of alphoid higher order repeats (HORs) in the centromere folding. J. Theor. Biol 254, 555–560. [DOI] [PubMed] [Google Scholar]
- 146.Shukla M, Tong P, White SA, Singh PP, Reid AM, Catania S, Pidoux AL, and Allshire RC (2018). Centromere DNA Destabilizes H3 Nucleosomes to Promote CENP-A Deposition during the Cell Cycle. Current Biology 28, 3924–3936.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Corless S, Höcker S, and Erhardt S (2020). Centromeric RNA and its function at and beyond centromeric chromatin. J. Mol. Biol 432, 4257–4269. [DOI] [PubMed] [Google Scholar]
- 148.Liu Q, Liu Y, Shi Q, Su H, Wang C, Birchler JA, and Han F (2021). Emerging roles of centromeric RNAs in centromere formation and function. Genes Genom. 43, 217–226. [DOI] [PubMed] [Google Scholar]
- 149.Volpe TA, Kidner C, Hall IM, Teng G, Grewal SIS, and Martienssen RA (2002). Regulation of Heterochromatic Silencing and Histone H3 Lysine-9 Methylation by RNAi. Science 297, 1833–1837. [DOI] [PubMed] [Google Scholar]
- 150.Choi ES, Strålfors A, Castillo AG, Durand-Dubief M, Ekwall K, and Allshire RC (2011). Identification of Noncoding Transcripts from within CENP-A Chromatin at Fission Yeast Centromeres*. J. Biological Chem 286, 23600–23607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Catania S, Pidoux AL, and Allshire RC (2015). Sequence features and transcriptional stalling within centromere DNA promote establishment of CENP-A chromatin. PLoS Genetics 11, e1004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen ES, Saitoh S, Yanagida M, and Takahashi K (2003). A Cell Cycle-Regulated GATA Factor Promotes Centromeric Localization of CENP-A in Fission Yeast. Molecular Cell 11, 175–187. [DOI] [PubMed] [Google Scholar]
- 153.Chen C-C, Bowers S, Lipinszki Z, Palladino J, Trusiak S, Bettini E, Rosin L, Przewloka MR, Glover DM, O’Neill RJ, et al. (2015). Establishment of Centromeric Chromatin by the CENP-A Assembly Factor CAL1 Requires FACT-Mediated Transcription. Developmental Cell 34, 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bobkov GOM, Huang A, Berg S.J.W. van den, Mitra S, Anselm E, Lazou V, Schunter S, Feederle R, Imhof A, Lusser A, et al. (2020). Spt6 is a maintenance factor for centromeric CENP-A. Nature Communications 11, 2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Grenfell AW, Strzelecka M, and Heald R (2016). Transcription brings the complex(ity) to the centromere. Cell Cycle 16, 00–00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chan FL, and Wong LH (2012). Transcription in the maintenance of centromere chromatin identity. Nucleic Acids Research 40, 11178–11188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.McNulty SM, Sullivan LL, and Sullivan BA (2017). Human Centromeres Produce Chromosome-Specific and Array-Specific Alpha Satellite Transcripts that Are Complexed with CENP-A and CENP-C. Developmental Cell 42, 226–240.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bury L, Moodie B, Ly J, McKay LS, Miga KH, and Cheeseman IM (2020). Alpha-satellite RNA transcripts are repressed by centromere-nucleolus associations. Elife 9, e59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chan FL, Marshall OJ, Saffery R, Kim BW, Earle E, Choo KHA, and Wong LH (2012). Active transcription and essential role of RNA polymerase II at the centromere during mitosis. PNAS 109, 1979–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hoyt SJ, Storer JM, Hartley GA, Grady PGS, Gershman A, Lima L.G. de, Limouse C, Halabian R, Wojenski L, Rodriguez M, et al. (2021). From telomere to telomere: the transcriptional and epigenetic state of human repeat elements. Biorxiv, 2021.07.12.451456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen Y, Zhang Q, Teng Z, and Liu H (2021). Centromeric transcription maintains centromeric cohesion in human cells. The Journal of Cell Biology 220, e202008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kabeche L, Nguyen HD, Buisson R, and Zou L (2017). A mitosis-specific and R loop–driven ATR pathway promotes faithful chromosome segregation. Science 115, eaan6490–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Blower MD (2016). Centromeric Transcription Regulates Aurora-B Localization and Activation. Cell Reports 15, 1624–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Molina O, Vargiu G, Abad MA, Zhiteneva A, Jeyaprakash AA, Masumoto H, Kouprina N, Larionov V, and Earnshaw WC (2016). Epigenetic engineering reveals a balance between histone modifications and transcription in kinetochore maintenance. Nature communications 7, 13334–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bobkov GOM, Gilbert N, and Heun P (2018). Centromere transcription allows CENP-A to transit from chromatin association to stable incorporation. The Journal of Cell Biology 134, jcb.201611087–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rošić S, Köhler F, and Erhardt S (2014). Repetitive centromeric satellite RNA is essential for kinetochore formation and cell division. The Journal of Cell Biology 207, 335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Perea-Resa C, Bury L, Cheeseman IM, and Blower MD (2020). Cohesin Removal Reprograms Gene Expression upon Mitotic Entry. Molecular Cell 78, 127–140.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Monen J, Maddox PS, Hyndman F, Oegema K, and Desai A (2005). Differential role of CENP-A in the segregation of holocentric C. elegans chromosomes during meiosis and mitosis. Nature Cell Biology 7, 1248–1255. [DOI] [PubMed] [Google Scholar]
- 169.Ingouff M, Rademacher S, Holec S, Šoljić L, Xin N, Readshaw A, Foo SH, Lahouze B, Sprunck S, and Berger F (2010). Zygotic Resetting of the HISTONE 3 Variant Repertoire Participates in Epigenetic Reprogramming in Arabidopsis. Current Biology 20, 2137–2143. [DOI] [PubMed] [Google Scholar]
- 170.Drinnenberg IA, deYoung D, Henikoff S, and Malik HS (2014). Recurrent loss of CenH3 is associated with independent transitions to holocentricity in insects. Elife 3, e03676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cortes-Silva N, Ulmer J, Kiuchi T, Hsieh E, Cornilleau G, Ladid I, Dingli F, Loew D, Katsuma S, and Drinnenberg IA (2020). CenH3-Independent Kinetochore Assembly in Lepidoptera Requires CCAN, Including CENP-T. Current Biology 30, 561–572.e10. [DOI] [PubMed] [Google Scholar]
- 172.Senaratne AP, Muller H, Fryer KA, Kawamoto M, Katsuma S, and Drinnenberg IA (2020). Formation of the CenH3-Deficient Holocentromere in Lepidoptera Avoids Active Chromatin. Current Biology 31, 173–181.e7. [DOI] [PubMed] [Google Scholar]
- 173.Navarro-Mendoza MI, Pérez-Arques C, Panchal S, Nicolés FE, Mondo SJ, Ganguly P, Pangilinan J, Grigoriev IV, Heitman J, Sanyal K, et al. (2019). Early Diverging Fungus Mucor circinelloides Lacks Centromeric Histone CENP-A and Displays a Mosaic of Point and Regional Centromeres. Current Biology 29, 3791–3802.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Akiyoshi B (2016). The unconventional kinetoplastid kinetochore: from discovery toward functional understanding. Biochem. Soc. T 44, 1201–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Obado SO, Bot C, Nilsson D, Andersson B, and Kelly JM (2007). Repetitive DNA is associated with centromeric domains in Trypanosoma brucei but not Trypanosoma cruzi. Genome Biol 8, R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Llauró A, Hayashi H, Bailey ME, Wilson A, Ludzia P, Asbury CL, and Akiyoshi B (2018). The kinetoplastid kinetochore protein KKT4 is an unconventional microtubule tip–coupling protein. The Journal of Cell Biology 217, 3886–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lowell JE, and Cross GAM (2004). A variant histone H3 is enriched at telomeres in Trypanosoma brucei. J. Cell Sci 117, 5937–5947. [DOI] [PubMed] [Google Scholar]
- 178.Marcianò G, Ishii M, Nerusheva OO, and Akiyoshi B (2021). Kinetoplastid kinetochore proteins KKT2 and KKT3 have unique centromere localization domains. The Journal of Cell Biology 220, e202101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Flemming W (1882). Zellsubstanz, Kern und Zelltheilung. [Google Scholar]
- 180.Hori T, and Fukagawa T (2020). Artificial generation of centromeres and kinetochores to understand their structure and function. Experimental Cell Research 389, 111898. [DOI] [PubMed] [Google Scholar]
- 181.Talbert PB, and Henikoff S (2018). Transcribing Centromeres: Noncoding RNAs and Kinetochore Assembly. Trends Genetics 34, 587–599. [DOI] [PubMed] [Google Scholar]
- 182.Scelfo A, and Fachinetti D (2019). Keeping the Centromere under Control: A Promising Role for DNA Methylation. Cells 8, 912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wolfgruber TK, Sharma A, Schneider KL, Albert PS, Koo D-H, Shi J, Gao Z, Han F, Lee H, Xu R, et al. (2009). Maize Centromere Structure and Evolution: Sequence Analysis of Centromeres 2 and 5 Reveals Dynamic Loci Shaped Primarily by Retrotransposons. Plos Genetics 5, e1000743. [DOI] [PMC free article] [PubMed] [Google Scholar]


