Abstract
We assessed sex differences in amyloid- and tau-PET retention in 119 amyloid positive patients with mild cognitive impairment or Alzheimer’s disease (AD) dementia. Patients underwent 3T-MRI, 11C-PIB amyloid-PET and 18F-Flortaucipir tau-PET. Linear ordinary least squares regression models tested sex differences in Flortaucipir-PET SUVR in a summary temporal region of interest as well as global PIB-PET. No sex differences were observed in demographics, Clinical Dementia Rating Sum of Boxes (CDR-SoB), Mini-Mental State Exam (MMSE), raw episodic memory scores, or cortical thickness. Females had higher global PIB SUVR (ηp2=.043, p=.025) and temporal Flortaucipir SUVR (ηp2=.070, p=.004), adjusting for age and CDR-SoB. Sex differences in temporal Flortaucipir-PET remained significant when controlling additionally for PIB SUVR and APOE4 status (ηp2=.055, p=.013), or when using partial volume-corrected data. No sex differences were present in areas of known Flortaucipir off-target binding. Overall, females demonstrated greater AD regional tau-PET burden than males despite clinical comparability. Further characterization of sex differences will provide insight into AD pathogenesis and support development of personalized therapeutic strategies.
Keywords: Alzheimer’s disease, sex differences, positron emission tomography, amyloid, tau, apolipoprotein E
1. Introduction
Females are disproportionately affected by Alzheimer’s Disease (AD). In the United States, approximately two-thirds of all clinical diagnoses of AD are made in females (“2020 Alzheimer’s disease facts and figures,” 2020), and recent meta-analyses of clinical AD in Europe (Niu et al., 2017) and China (Zhao and Li, 2020) have reported similar overrepresentation of females. This increased prevalence of AD in females may only be partially explained by greater longevity (Buckley et al., 2019b; Davis et al., 2020; Di Carlo et al., 2002; Launer et al., 1999; Oksuzyan et al., 2008). Females diagnosed with mild cognitive impairment (MCI) and AD dementia also demonstrate steeper rates of cognitive decline (Lin et al., 2015; Tschanz et al., 2011) and greater susceptibility to the apolipoprotein E ε4 allele (APOE4) (Altmann et al., 2014; Neu et al., 2017). Collectively, these observations suggest that multiple underlying neurobiological factors likely contribute to sex differences in AD.
Neuropathological studies in samples spanning normal cognition to dementia, including clinic-referred, community-based, and routine autopsy samples have revealed a greater burden of AD neuropathology in females than males, a finding that is more consistently reported for neurofibrillary tangles than amyloid plaques (Barnes et al., 2005; Corder et al., 2004; Filon et al., 2016; Hohman et al., 2018; Liesinger et al., 2018; Oveisgharan et al., 2018; Spina et al., 2021). Analogous differences have been observed with in vivo AD biomarkers. In cross-sectional studies encompassing individuals with normal cognition, MCI, and dementia, cerebrospinal fluid (CSF) total-tau levels have been reported to be higher in females (Hohman et al., 2018), and higher levels of CSF phosphorylated-tau in females have been reported in the presence of APOE4 (Sundermann et al., 2020). Such findings may vary by disease stage (Altmann et al., 2014; Mofrad et al., 2020). A cross-sectional study of cognitively normal older adults from the Harvard Aging Brain Study and Alzheimer’s Disease Neuroimaging Initiative revealed no sex differences in global neocortical amyloid positron emission tomography (PET) retention, but greater entorhinal tau-PET retention in females versus males with higher amyloid-PET retention (Buckley et al., 2019a). It was later found that this sex by amyloid-PET interaction on tau-PET extends to other temporal regions, and a main effect of sex on tau-PET was present beyond the temporal lobe in cognitively normal older adults (Buckley et al., 2020). Cross-sectional findings of greater tau-PET retention in females have been replicated in cognitively normal and community-based samples of middle-aged and older adults (Luchsinger et al., 2020; Palta et al., 2021; Wisch et al., 2020), and some studies have also found increased amyloid-PET retention in females within these populations (Luchsinger et al., 2020; Palta et al., 2021; Rahman et al., 2020). Although these studies may be subject to selection bias and not generalize to the population, the results suggest that sex differences may emerge as early as the preclinical phase of AD. However, cross-sectional studies examining amyloid- and tau-PET retention in patients with MCI and AD dementia have yielded inconsistent results, with studies finding no sex effect on either amyloid or tau-PET (Johnson et al., 2016), higher tau- but not amyloid-PET in females (Digma et al., 2020), higher amyloid-PET in females (Sundermann et al., 2018), and higher amyloid-PET in males (Rowe et al., 2010).
In examining sex differences in MRI biomarkers in samples with MCI and dementia, results from cross-sectional comparisons have been mixed. While several studies have reported no sex differences in cortical thickness (Seo et al., 2011) or volumetric measures (Fjell et al., 2009; Lee et al., 2018; Pennanen et al., 2004), others have found differences such as greater hippocampal volume in females, suggesting they may be less atrophied (Arruda et al., 2020; Sundermann et al., 2016). A sex difference in MRI-observed white matter hyperintensities has also been reported in clinical AD patients, with females expressing greater burden (Sawada et al., 2000). White matter hyperintensities are thought to at least partly reflect cerebrovascular pathology and are commonly found comorbid with AD pathology (Alber et al., 2019), and are therefore an important imaging biomarker to consider in MCI and dementia, despite having rarely been studied with regard to sex differences in these populations.
Recent calls to action have identified the need to replicate and further characterize sex differences that have been identified in aging and dementia studies to date (Nebel et al., 2018; Waters and Laitner, 2021). In light of mixed results particularly in symptomatic patients, the primary goal of this study was to evaluate cross-sectional sex differences in summary indices of tau- and amyloid-PET in patients with MCI or dementia due to AD. Secondary aims included exploratory assessment of whether the effect of sex on AD PET biomarkers differed by APOE4 carrier status, age, or dementia severity, and evaluation of sex differences in cortical thickness, hippocampal volume, and white matter hyperintensity volume.
2. Methods
2.1. Participants
One hundred and nineteen patients from the University of California San Francisco (UCSF) Alzheimer’s Disease Research Center were included in this study. Participants were included if they met clinical criteria for mild cognitive impairment or dementia due to AD (Albert et al., 2011; McKhann et al., 2011), completed a 3T structural MRI, completed a 18F-Flortaucipir (FTP) PET scan to assess in vivo tau pathology, and had all assessments collected within one year of the FTP PET scan. Furthermore, patients were only included if they had a positive 11C-PIB (PIB) PET scan as assessed by both visual read and quantification (see section 2.3.2. below) (Rabinovici et al., 2011). Therefore, all patients were on the AD biological continuum as defined by the 2018 NIA-AA Research Framework (Jack et al., 2018). Each patient received a standard clinical assessment including neurological exam and history intake, study partner interview, and neuropsychological testing. Age of onset was estimated by the clinician during the interview with the patient and study partner in the neurological exam. Diagnosis was determined by consensus among the multi-disciplinary team that met with patient and study partner. All participants gave written consent to participate in study procedures, and the study was approved by the UCSF, University of California Berkeley, and Lawrence Berkeley National Laboratory institutional review boards for human research.
2.2. Genotyping
To assess APOE status, genomic DNA was extracted from peripheral blood using standard protocols. APOE genotyping was carried out by real-time PCR on a LightCycler® 480 System using Taqman SNP Genotyping Assays (#C___3084793_20 and C____904973_10 for rs429358 and rs7412, respectively). Assays were run in duplicate. Genotyping results were available on 115/119 patients.
2.2. Imaging Acquisition
2.2.1. MRI
All patients underwent MRI on a 3T Siemens TIM Trio (n=17 males, n=20 females) or 3T Siemens Prisma FIT (n=40 males, n=42 females) scanner at UCSF. Volumetric MPRAGE sequences were used to acquire T1-weighted images (Sagittal slice orientation; slice thickness = 1.0 mm; slices per slab = 160; in-plane resolution = 1.0×1.0 mm; matrix = 240×256; TR = 2,300 ms; TE = 2.9 ms (Prisma) or TE=2.98 ms (Trio); TI = 900 ms; flip angle = 9°). FLAIR sequences were also collected (slice thickness = 1.00mm; slices per slab = 160 (Trio) or slices per slab = 176 (Prisma); in-plane resolution = 0.98×0.98mm (Trio) or in-plane resolution = 1.0×1.0mm (Prisma); matrix = 256×256; TR = 6000ms (Trio) or TR = 5000ms (Prisma); TE = 388ms (Trio) or TE = 397ms (Prisma); TI = 2100ms (Trio) or TI = 1800ms (Prisma); flip angle = 120°). The average delay between MRI and PET was 46 ± 60 days (47 ± 61 days between MRI and FTP, 46 ± 58 days between MRI and PIB).
2.2.2. PET
PET scans were performed at the Lawrence Berkeley National Laboratory on a Siemens Biograph 6 Truepoint PET/CT scanner in 3D acquisition mode. A low-dose CT/transmission scan was performed for attenuation correction prior to all scans. PIB and FTP PET were synthesized and acquired as previously described (Lehmann et al., 2013; Schöll et al., 2016). Amyloid-PET data was analyzed at 50–70 minutes after injection of approximately 15 mCi of PIB, and tau-PET data was analyzed at 80–100 minutes after injection of approximately 10 mCi of FTP. The average delay between FTP and PIB scans was 10 ± 39 days. PET data were reconstructed using an ordered subset expectation maximization algorithm with weighted attenuation. Images were smoothed with a 4 mm Gaussian kernel with scatter correction and evaluated prior to analysis for patient motion and adequacy of statistical counts.
2.3. Image Processing and Analysis
2.3.1. MRI
The MPRAGE sequences were processed using FreeSurfer 5.3 to obtain patient-specific native-space regions of interest and reference regions for PET processing, based on the Desikan/Killiany atlas.
Mean values of cortical thickness were extracted from fusiform gyrus, parahippocampal gyrus, entorhinal cortex, inferior temporal gyrus, precuneus, and superior parietal lobule for analysis of neurodegeneration. Hippocampal volume was collected as an additional measure of neurodegeneration. Because brain volume varies as a function of head size (Potvin et al., 2017), total intracranial volume (TIV) was collected as a control measure for volumetric analysis. All thickness and volume measurements were extracted from FreeSurfer outputs from patient-specific MPRAGE sequences.
Global white matter hyperintensity (WMH) volume was obtained using native space FLAIR and T1-weighted images in the subset of participants who had both sequences collected on the 3T Siemens Prisma FIT and whose WMH segmentation passed quality control (n=37 females, n=32 males). Segmentation followed a two-step supervised algorithm: the first step provided the WMH prior distribution using a trained linear regression classification, and the second step calculated the posterior distribution using a Hidden Markov Random Field algorithm.
2.3.2. PET
PET images were coregistered to the MPRAGE images using Statistical Parametric Mapping (SPM) Version 12. Standardized uptake value ratios (SUVR) were calculated for the 50–70-min post-injection interval of PIB using mean activity in the cerebellar cortex gray matter as the reference region, and for the 80–100-min post-injection interval of FTP using mean activity in the inferior cerebellar cortex gray matter as the reference region (Maass et al., 2017). While no partial volume correction was done for our main analyses, a supplementary tau-PET region of interest analysis was run using the partial volume correction method described in previous publications (Baker et al., 2017; Maass et al., 2017).
To estimate global amyloid burden, a global PIB index SUVR was calculated for each patient using a composite of frontal, parietal, temporal and cingulate regions known to show high PIB binding in AD (Rabinovici et al., 2010). All patients were amyloid-PET positive per inclusion criteria. Amyloid-PET positivity was based on both visual read (Rabinovici et al., 2011) and a quantitative PIB SUVR cutoff of 1.21 (Villeneuve et al., 2015), both of which have been validated versus post-mortem amyloid burden. Because PIB binding in AD diffusely involves large areas of neocortex (and is highly intercorrelated in these regions (Lockhart et al., 2017)) and is weakly associated with regional neurodegeneration or cognitive decline (Arriagada et al., 1992; Bejanin et al., 2017; La Joie et al., 2012), regional amyloid values were not assessed.
To estimate tau burden, mean values of FTP PET SUVR were extracted from a bilateral temporal meta-ROI that included the fusiform gyrus, parahippocampal gyrus, entorhinal cortex, inferior temporal cortex, and amygdala.
Unlike PIB, the regional pattern of FTP binding is related to cognition and neurodegeneration in AD (Bejanin et al., 2017). Therefore, exploratory FTP PET analyses were performed in individual cortical ROIs (the entorhinal cortex, inferior temporal cortex, precuneus and superior parietal lobule) due to their well-elucidated involvement in AD (Cho et al., 2016; Schöll et al., 2016). In addition, sex differences were assessed in control regions that are prone to “off-target” (i.e. unrelated to tau pathology) binding: the thalamus, putamen, and choroid plexus (Baker et al., 2019).
The temporal meta-ROI and all individual exploratory AD ROIs were also chosen for the sake of comparison to the Buckley et al. study of sex differences in PET biomarkers in cognitively normal older adults (2019a).
2.4. Cognitive and Functional Data
The Clinical Dementia Rating Sum of Boxes (CDR-SoB) was collected as a measure of global clinical severity (Morris, 1993), and the Mini-Mental State Exam (MMSE) was collected as a measure of cognitive mental status (Folstein et al., 1975).
An episodic memory composite combined immediate recall, 30-second and 10-minute delay free recall, and a discrimination calculation derived from delayed recognition of the 9-item California Verbal Learning Test-II (CVLT-II) (Delis et al., 2000), as well as the 10-min delay recall of the Benson Figure (Weintraub et al., 2018). The memory composite score was calculated in two ways. First, individual memory scores were standardized using the mean and standard deviation of the patient sample in order to have a measure approximating average memory performance relative to the study cohort. Second, age-adjusted Z-scores (O’Brien and Dyck, 1995) were calculated using normative data from a group of 293 cognitively normal female and 215 cognitively normal males recruited from UCSF (age: Mfemales = 65.8 ± 6.6 vs Mmales = 68.2 ± 7.2; years of education Mfemales = 17.0 ± 2.0 vs Mmales = 17.4 ± 1.9; MMSE: Mfemales = 29.4 ± 0.8 vs Mmales = 29.2 ± 1.0). Age-adjusted Z-scores were calculated for males and females separately; patients and controls did not differ in age or years of education within each sex for each test. This normative score was calculated to evaluate sex-specific performance relative to controls, as females are reported to have higher memory scores than males, especially in verbal memory (Bolla-Wilson and Bleecker, 1986; Geffen et al., 1990; Kramer et al., 1988; Trahan and Quintana, 1990).
For both methods, the scores were then combined into final composites using a weighted average to have a balanced representation of visual and verbal episodic memory, as previously reported (Bejanin et al., 2017) (Supplementary Table 1). In the presence of any missing scores, composites were calculated on the remaining scores. One patient was unable to complete any episodic memory testing, and thus composites are available on 118/119 patients. While it is possible that calculation of a composite score in the presence of missingness may have introduced bias, there were no significant sex differences in total number of missing test scores (p = .092, d = .313), and individual tests followed similar patterns (Supplementary Table 1).
2.5. Statistical Analyses
All analyses were performed using Jamovi Version 1.1.9.0 (www.jamovi.org). Graphics were created in R Versions 3.5.0 and 4.0.2 with RStudio Versions 1.1.463 and 1.3.1056 (R Core Team, 2020), using the ggplot2, gridExtra, and gtable packages. Results were considered to be statistically significant at p<.05, and 95% confidence intervals are reported for all imaging analyses. Reported p-values have not been corrected for multiple comparisons. All continuous explanatory variables were mean-centered for analyses. Statistical assumptions for all analyses were tested and met. All observations were independent by study design. For t-test and linear regression analyses, response variables data were continuous or interval scaled. Visual inspection of Q-Q plots revealed that error terms were approximately normally distributed. For Chi-squared and Fisher’s exact test, data were categorical (dichotomous) and groups were mutually exclusive and independent.
2.5.1. Demographics
Group differences in demographic characteristics between males and females were assessed using Student’s t-test for continuous data and Fisher’s exact test or a chi-squared test for categorical data.
2.5.2. Cognition and Functional Status
Student’s t-tests were used to assess sex effects on MMSE, episodic memory, and functional impairment as measured by CDR-SoB.
2.5.3. Structural MRI ROIs
General linear models were employed to examine the effect of sex on hippocampal volume and cortical thickness of entorhinal cortex, inferior temporal gyrus, precuneus, and superior parietal lobule, controlling for age and CDR-SoB (n=119; Model 1); age, CDR-SoB and ROI FTP SUVR (n=119, Model 2); and age, CDR-SoB, regional FTP SUVR and global PIB SUVR (n=119, Model 3). For the hippocampal volume analyses, TIV was incorporated as a covariate. Age and CDR-SoB were included as covariates in these and all remaining analyses to determine whether potential sex differences were independent of age and global functional severity (La Joie et al., 2021). Regional FTP SUVR and global PIB SUVR were included as covariates in Models 2 and 3 to evaluate whether potential sex differences in regional volume and cortical thickness were independent of AD PET biomarkers.
As a supplementary analysis, the above models were repeated on age-, sex-, and TIV-adjusted cortical thickness Z-scores using a normative FreeSurfer dataset (Potvin et al., 2017).
2.5.4. Global Amyloid-PET
A series of general linear models was used to analyze sex effects on global amyloid-PET, controlling for age and CDR-SoB (n=119, Model 1); age, CDR-SoB and temporal FTP SUVR (n=119, Model 2); and age, CDR-SoB, temporal FTP SUVR and APOE4 carrier status (n=115, Model 3). Temporal FTP SUVR was included as a covariate in Models 2 and 3 to determine whether differences in tau-PET retention account for sex differences in amyloid. This was done in an effort to evaluate sex effects on each PET biomarker independent of the other, as the relationship between amyloid and tau pathology is yet to be fully elucidated (Jack et al., 2018). As the APOE4 allele has been associated with amyloid accumulation (Verghese et al., 2011), APOE4 status was included as a covariate in Model 3.
2.5.5. Tau-PET ROIs
A series of general linear models was conducted to examine the effect of sex on tau-PET SUVR in the temporal meta-ROI, as well as in exploratory individual ROIs including the entorhinal cortex, inferior temporal gyrus, precuneus, superior parietal lobule, thalamus, putamen, and choroid plexus, controlling for age and CDR-SoB (n=119, Model 1); age, CDR-SoB and PIB SUVR (n=119, Model 2); and age, CDR-SoB, PIB SUVR and APOE4 (n=115, Model 3). Mirroring the previous analysis, global PIB SUVR was included as a covariate to determine whether differences in amyloid-PET burden account for sex differences in tau-PET. Furthermore, APOE4 was included as a covariate in addition to global PIB SUVR in Model 3 to control for potential amyloid-independent effects of APOE4 status on tau (La Joie et al., 2021; Sanchez et al., 2021; Therriault et al., 2020).
As a supplementary analysis, the Model 2 analysis of temporal meta-ROI FTP PET was repeated using partial volume corrected data.
2.5.6. Interaction Models
To determine whether the effect of sex on PET biomarkers of amyloid and tau was conditional on APOE4 status, age, or functional disease severity, a series of general linear models including interaction terms were examined.
We analyzed the interaction of sex and APOE4 status on global PIB PET SUVR, temporal meta-ROI FTP PET SUVR, and entorhinal cortex FTP PET SUVR, controlling for age and CDR-SoB. The entorhinal cortex was examined in addition to the primary PET regions due to evidence suggesting a relatively localized effect of APOE4 on tau PET in the medial temporal cortex (La Joie et al., 2021; Sanchez et al., 2021; Therriault et al., 2020).
Next, we analyzed the interaction of sex and age on global PIB PET SUVR and temporal meta-ROI FTP PET SUVR, controlling for CDR-SoB.
Finally, we analyzed the interaction of sex and CDR-SoB on global PIB PET SUVR and temporal meta-ROI FTP PET SUVR, controlling for age.
2.5.7. White Matter Hyperintensities
It is possible that clinical symptoms could be attributed to pathological processes not detectable by our chosen biomarkers, and that sex differences in AD-related proteinopathy burden could be attributable to cerebrovascular changes. To assess for sex differences in burden of cerebrovascular lesions, global WMH volume was log-transformed and analyzed in a linear regression model with age, CDR-SoB, and TIV as covariates (n=69).
For all sex differences in the analyses from sections 2.5.3–2.5.7, effect size was calculated as partial η2 (ηp2).
3. Results
3.1. Demographic and Clinical Differences
Demographic and clinical data are presented in Table 1. No statistically significant demographic differences were observed between males (n=57) and females (n=62) (d<0.30, φ<0.21). As a whole, the sample was predominantly white (96%) and highly educated (Meducation=16.9 years). The sample was also relatively young (Mage=65.5 years), but with ages ranging from 48 to 95 years in females and 51 to 84 years in males.
Table 1.
Demographic and Clinical Data
| Total [n=119] | Female [n=62] | Male [n=57] | p | Effect Size | |
|---|---|---|---|---|---|
|
| |||||
| Age at PET, y | 65.5 ± 9.4 | 64.2 ± 9.8 | 67.0 ± 8.8 | .11 | d=0.30 |
| Age of Onset, y | 60.4 ± 9.0 [n=111] | 59.2 ± 9.4 [n=57] | 61.7 ± 8.6 [n=54] | .14 | d=0.28 |
| Education, y | 16.9 ± 2.7 | 16.8 ± 2.8 | 17.0 ± 2.6 | .63 | d=0.09 |
| Race (% White) | 96% [n=116] | 92% [n=60] | 100% [n=56] | .06 | φ=0.21 |
| Ethnicity (% Hispanic or Latino) | 2% [n=113] | 2% [n=58] | 2% [n=55] | >.99 | φ<0.01 |
| APOE4+ (%) | 60% [n=115] | 58% [n=59] | 63% [n=56] | .59 | φ=0.05 |
| APOE2+ (%) | 3% [n=115] | 3% [n=59] | 2% [n=56] | >.99 | φ=0.05 |
| APOE4 Homozygotes (%) | 14% [n=115] | 14% [n=59] | 14% [n=56] | .91 | φ=0.01 |
| MMSE, /30 | 21.9 ± 5.7 | 21.8 ± 6.0 | 21.9 ± 5.3 | .93 | d=0.02 |
| MCI (%) | 58% | 58% | 58% | .99 | φ<0.01 |
| CDR-SoB, /18 | 4.0 ± 2.2 | 3.9 ± 1.8 | 4.1 ± 2.5 | .60 | d=0.10 |
| Individual Episodic Memory Tests | - | - | - | - | - |
| CVLT 4-Trial Immediate Free Recall, /36 | 17.5 ± 6.5 [n=116] | 17.8 ± 6.7 [n=61] | 17.2 ± 6.2 [n=55] | .65 | d=0.08 |
| CVLT 30-sec Delay, /9 | 3.7 ± 2.6 [n=116] | 3.7 ± 2.5 [n=61] | 3.7 ± 2.7 [n=55] | >.99 | d<0.01 |
| CVLT 10-min Delay, /9 | 2.4 ± 2.7 [n=116] | 2.3 ± 2.7 [n=61] | 2.6 ± 2.8 [n=55] | .45 | d=0.14 |
| CVLT Recognition Discrimination Index, d’ | 1.7 ± 1.0 [n=114] | 1.7 ± 0.9 [n=60] | 1.8 ± 1.1 [n=54] | .58 | d=0.10 |
| Benson Complex Figure Recall 10-min delay, /17 | 4.8 ± 4.0 [n=114] | 4.2 ± 3.5 [n=60] | 5.6 ± 4.4 [n=54] | .06 | d=0.36 |
| Episodic Memory Composite (within-group Z-score) | 0.0 ± 0.8 [n=118] | −0.1 ± 0.8 [n=61] | 0.1 ± 0.9 [n=57] | .21 | d=0.23 |
| Episodic Memory Composite (age- and sex-adjusted Z-score) | −2.9 ± 1.6 [n=118] | −3.5 ± 1.5 [n=61] | −2.2 ± 1.3 [n=57] | <.001 | d=0.92 |
| TIV (L) | 1.54 ± 0.18 | 1.42 ± 0.10 | 1.67 ± 0.16 | <.001 | d=1.9 |
All continuous numerical data are presented as mean ± SD [subgroup]. Student’s t-test evaluated group differences in continuous data. Fisher’s exact test (race, ethnicity, APOE2+) or a chi-squared test (APOE4+, APOE4 homozygotes, MCI) evaluated group differences in categorical data. Accordingly, effect size was assessed via Cohen’s d or φ coefficient. MCI indicates whether patients were considered MCI as opposed to dementia. APOE4+ indicates an ε4 carrier (homozygous or heterozygous) while APOE2+ indicates an ε2 carrier. Race indicates the patient’s self-reported race, while ethnicity indicates whether the patient reported being of Hispanic or Latino ethnicity (having origins from a primarily Spanish-speaking country in Latin America).
MMSE = Mini Mental State Examination, MCI = Mild Cognitive Impairment, CDR-SoB = Clinical Dementia Rating Sum of Boxes, CVLT = California Verbal Learning Test, TIV = Total Intracranial Volume
Males and females had comparable MMSE scores and were rated similarly on CDR-SoB (Table 1). Regarding episodic memory tests, raw sub-test scores and the standardized raw composite Z-score did not show any significant group difference (d<0.36). However, the age- and sex-adjusted episodic memory Z-scores differed significantly, with female patients showing more impairment (lower age- and sex-adjusted Z-scores) than males. Detailed inspection of the raw memory scores (Supplementary Table 1) showed that in cognitively unimpaired controls, females had higher raw memory scores than males, consistent with prior reports (Bolla-Wilson and Bleecker, 1986; Geffen et al., 1990; Kramer et al., 1988; Trahan and Quintana, 1990), while there was no sex difference in memory scores in the clinically affected group.
3.2. Sex Differences in AD PET Biomarkers
Compared to males, females had higher global PIB SUVR (+0.145 SUVR, 95% CI [0.018, 0.272]) (Figure 1A, Table 2), as well as higher FTP SUVR in the temporal meta-ROI (+0.222 SUVR, 95% CI [0.073, 0.371]) (Figure 1B, Table 3) when correcting for age and CDR-SoB.
Figure 1.
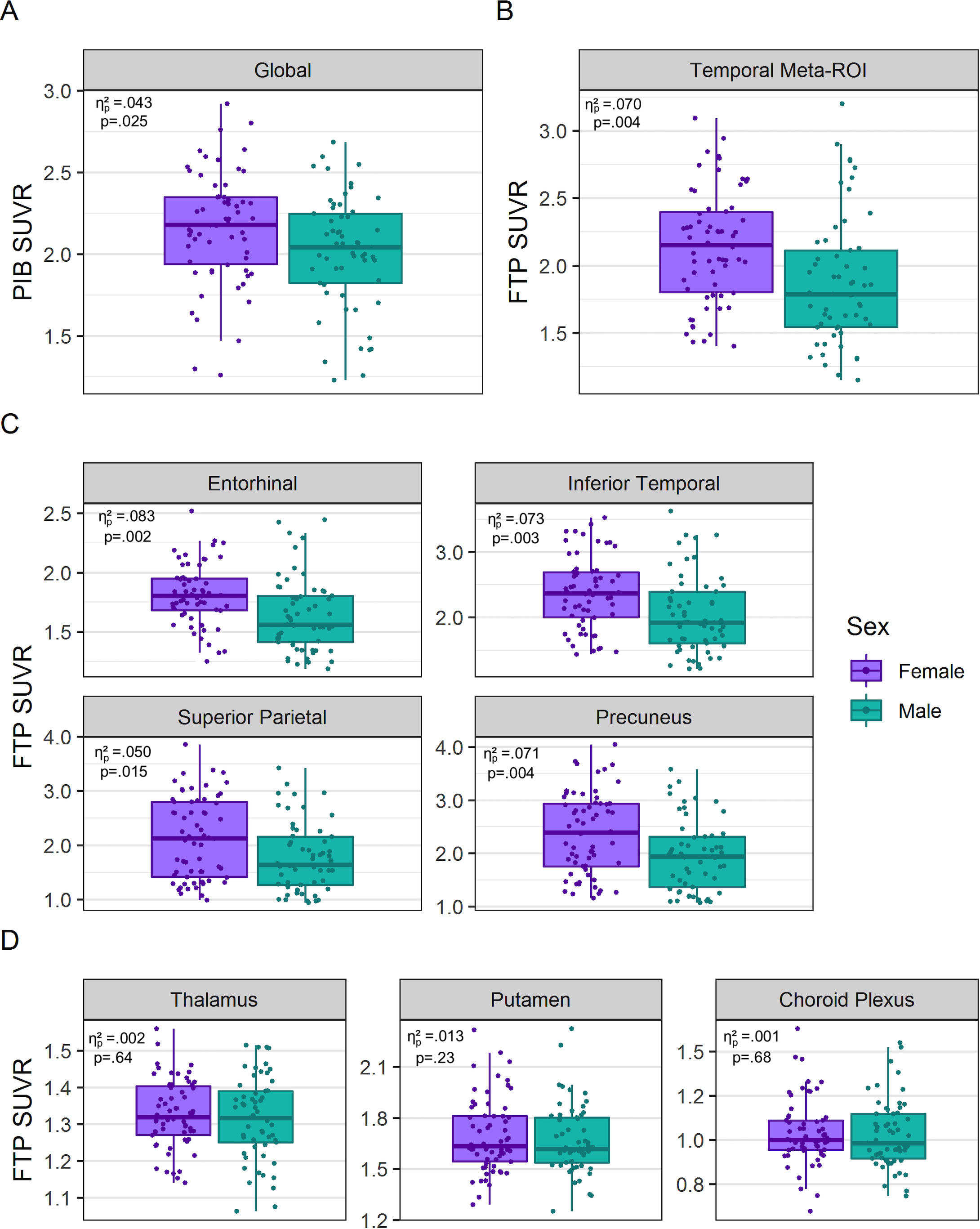
Sex-stratified box plots of (A) global PIB PET, (B) temporal meta-ROI FTP PET, (C) individual AD ROI FTP PET, and (D) regions off-target FTP binding. Partial η2 and p-values of Model 1 group sex differences are reported.
Table 2.
Amyloid PET Analyses
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| ηp2 | Estimate [95% CI] | p | ηp2 | Estimate [95% CI] | p | ηp2 | Estimate [95% CI] | p | |
|
| |||||||||
| Global PIB SUVR | |||||||||
| Sex | .043 | 0.145 [0.018, 0.272] | .025 | .030 | 0.125 [−0.007, 0.256] | .063 | .023 | 0.110 [−0.025, 0.246] | .11 |
| Temporal Meta-ROI FTP SUVR | .012 | 0.094 [−0.064, 0.251] | .24 | .013 | 0.099 [−0.062, 0.260] | .23 | |||
| APOE4 a | .003 | 0.036 [−0.095, 0.168] | .59 | ||||||
All models controlled for age and Clinical Dementia Rating Sum of Boxes. Reference levels are male and ε4 non-carrier for sex and APOE4 status, respectively. ηp2: partial eta-squared.
APOE data available on 56 males and 59 females. APOE4 is coded as either an ε4 carrier or non-carrier.
Models 1 and 2 have a total n=119, while Model 3 has a total n=115.
Table 3.
Tau-PET Analyses
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| ηp2 | Estimate [95% CI] | p | ηp2 | Estimate [95% CI] | p | ηp2 | Estimate [95% CI] | p | |
|
| |||||||||
| Meta-ROI | |||||||||
| Temporal Meta-ROI FTP SUVR | |||||||||
| Sex | .070 | 0.222 [0.073, 0.371] | .004 | .058 | 0.203 [0.051, 0.355] | .009 | .055 | 0.198 [0.042, 0.354] | .013 |
| Global PIB SUVR | .012 | 0.129 [−0.087, 0.345] | .24 | .013 | 0.136 [−0.085, 0.357] | .23 | |||
| APOE4a | <.001 | 0.015 [−0.139, 0.169] | .84 | ||||||
|
Individual ROIs | |||||||||
| Entorhinal FTP SUVR | |||||||||
| Sex | .083 | 0.161 [0.062, 0.260] | .002 | .077 | 0.158 [0.057, 0.260] | .003 | .092 | 0.169 [0.068, 0.270] | .001 |
| Global PIB SUVR | .001 | 0.020 [−0.124, 0.165] | .78 | .001 | 0.029 [−0.114, 0.171] | .69 | |||
| APOE4a | .052 | 0.122 [0.023, 0.222] | .016 | ||||||
| Inferior Temporal FTP SUVR | |||||||||
| Sex | .073 | 0.291 [0.099, 0.483] | .003 | .059 | 0.265 [0.069, 0.461] | .008 | .054 | 0.255 [0.052, 0.458] | .014 |
| Global PIB SUVR | .014 | 0.179 [−0.100, 0.457] | .21 | .015 | 0.184 [−0.102, 0.471] | .21 | |||
| APOE4a | .001 | −0.031 [−0.231, 0.170] | .76 | ||||||
| Precuneus FTP SUVR | |||||||||
| Sex | .071 | 0.279 [0.093, 0.466] | .004 | .043 | 0.207 [0.026, 0.387] | .025 | .041 | 0.198 [0.015, 0.381] | .034 |
| Global PIB SUVR | .12 | 0.500 [0.244, 0.757] | <.001 | .12 | 0.505 [0.246, 0.764] | <.001 | |||
| APOE4a | .002 | −0.044 [−0.225, 0.137] | .63 | ||||||
| Superior Parietal FTP SUVR | |||||||||
| Sex | .050 | 0.247 [0.049, 0.445] | .015 | .028 | 0.176 [−0.017, 0.369] | .074 | .025 | 0.163 [−0.030, 0.355] | .097 |
| Global PIB SUVR | .098 | 0.489 [0.214, 0.763] | <.001 | .11 | 0.497 [0.224, 0.769] | <.001 | |||
| APOE4a | <.001 | −0.016 [−0.206, 0.174] | .87 | ||||||
|
Off-Target ROIs | |||||||||
| Thalamus FTP SUVR | |||||||||
| Sex | .002 | 0.009 [−0.029, 0.046] | .64 | <.001 | 0.003 [−0.035, 0.041] | .88 | .001 | 0.006 [−0.033, 0.044] | .77 |
| Global PIB SUVR | .019 | 0.041 [−0.013, 0.096] | .14 | .022 | 0.043 [−0.012, 0.098] | .12 | |||
| APOE4a | .015 | 0.025 [−0.013, 0.063] | .20 | ||||||
| Putamen FTP SUVR | |||||||||
| Sex | .013 | 0.045 [−0.029, 0.119] | .23 | .009 | 0.038 [−0.038, 0.113] | .32 | .010 | 0.041 [−0.037, 0.119] | .30 |
| Global PIB SUVR | .007 | 0.050 [−0.057, 0.158] | .36 | .009 | 0.054 [−0.056, 0.165] | .33 | |||
| APOE4a | .003 | 0.022 [−0.055, 0.099] | .58 | ||||||
| Choroid Plexus FTP SUVR | |||||||||
| Sex | .001 | 0.014 [−0.054, 0.082] | .68 | .001 | 0.014 [−0.056, 0.084] | .69 | .003 | 0.019 [−0.051, 0.089] | .59 |
| Global PIB SUVR | <.001 | 0.001 [−0.098, 0.100] | .98 | <.001 | 0.008 [−0.091, 0.108] | .87 | |||
| APOE4a | .014 | 0.044 [−0.025, 0.114] | .21 | ||||||
All models controlled for age and Clinical Dementia Rating Sum of Boxes. Reference levels are male and ε4 non-carrier for sex and APOE4 status, respectively. ηp2: partial eta-squared.
APOE data available on 56 males and 59 females. APOE4 is coded as either an ε4 carrier or non-carrier. Models 1 and 2 have a total n=119, while Model 3 has a total n=115.
Both PET measures were further evaluated with the other as a covariate. When controlling additionally for global amyloid-PET retention, the effect of female sex retained significance in FTP SUVR of the temporal meta-ROI (+0.203 SUVR, 95% CI [0.051, 0.355]) (Figure 2, Table 3). However, when correcting additionally for temporal meta-ROI FTP SUVR, the effect of female sex on PIB SUVR no longer reached significance (+0.125 SUVR, 95% CI [−0.007, 0.256]) (Table 2).
Figure 2.
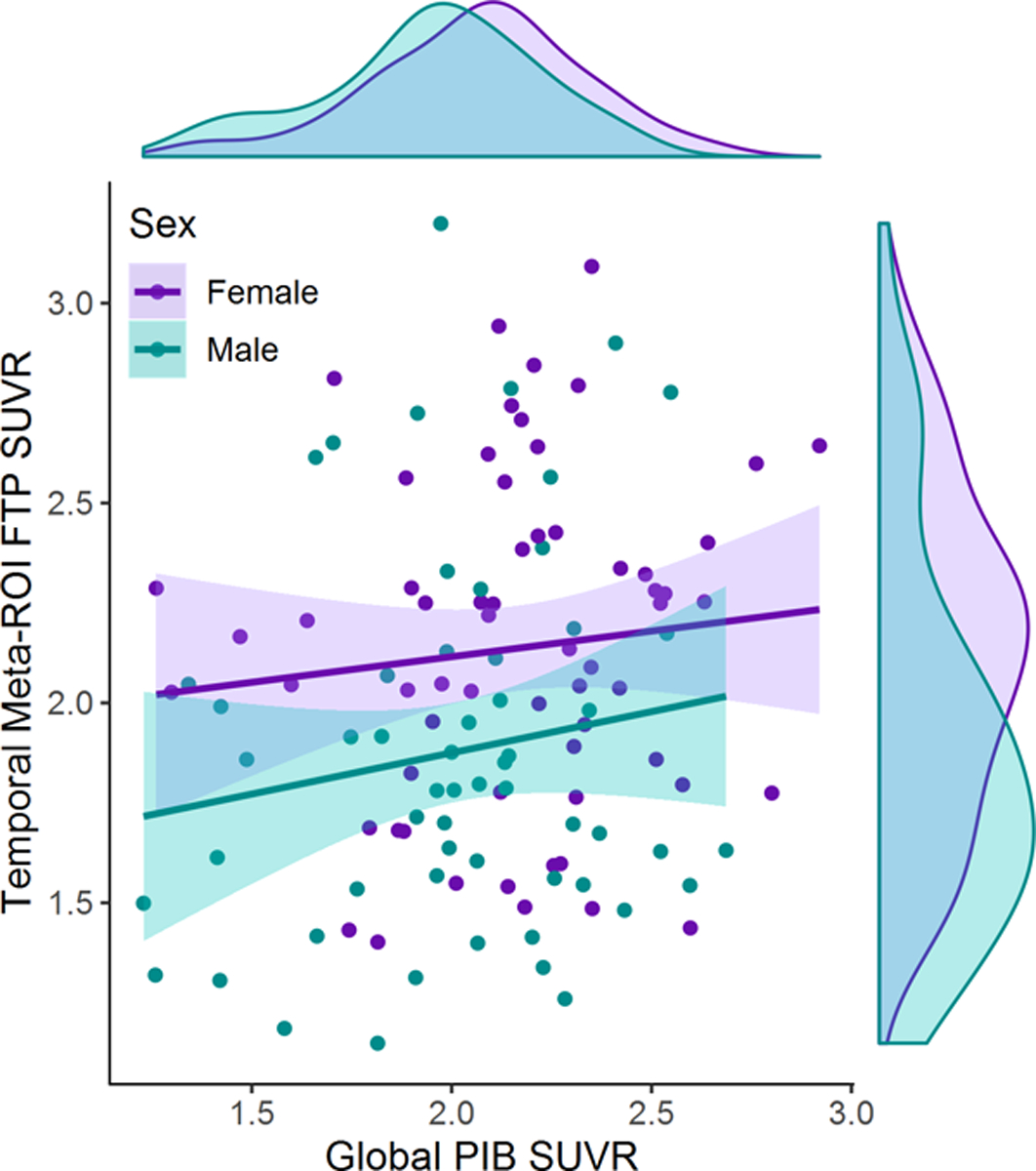
Sex difference in temporal meta-ROI tau PET signal as a function of global amyloid PET signal. All global PIB SUVRs exceeded a value of 1.21 and are therefore in the amyloid-positive range, per study design (Villeneuve et al., 2015). For a given level of amyloid, female AD patients demonstrated greater temporal FTP SUVR than males per Model 2 analysis (ηp2 = .058, p = .009) as described in Table 3. The effect of amyloid on temporal meta-ROI tau PET was not significant (ηp2 = .012, p = .24), and when added to the model, no interaction between sex and global amyloid PET was observed (ηp2 =.004, p =.52).
When APOE4 was added to the models, the effect of female sex on temporal meta-ROI FTP SUVR remained significant (+0.198 SUVR, 95% CI [0.042, 0.354], but the effect of female sex on global PIB SUVR remained non-significant (+0.110 SUVR, 95% CI [−0.025, 0.246]) (Table 2, Table 3). Note that the sample size was slightly reduced (n=115) in these analyses due to missing data on APOE4 status.
Analysis of FTP SUVR was repeated in the temporal meta-ROI using partial volume corrected FTP PET data, while controlling for age, CDR-SoB, and PIB SUVR. FTP SUVR in the temporal meta-ROI with and without partial volume correction were highly correlated (r=0.981, p<0.001) and the effect of female sex remained (+0.385 SUVR, 95% CI [0.056, 0.714]) (Supplementary Figure 1, Supplementary Table 2).
3.3. Sex Differences in Tau-PET Sub-Regions of Interest
Higher FTP SUVR in females was observed across all tested cortical regions: in the entorhinal cortex (+0.161 SUVR, 95% CI [0.062, 0.260]), inferior temporal cortex (+0.291 SUVR, 95% CI [0.099, 0.483]), precuneus (+0.279 SUVR, 95% CI [0.093, 0.466]), and superior parietal lobe (+0.247 SUVR, 95% CI [0.049, 0.445]), correcting for age and CDR-SoB (Figure 1C, Table 3). No sex effects were detected in the thalamus, putamen or choroid plexus, areas of known FTP off-target binding (ηp2s≤.013, ps≥.23) (Figure 1D, Table 3), suggesting that the observed cortical FTP SUVR sex differences were not due to an increase of overall or non-specific tracer binding in females.
When adding PIB SUVR to the model, the effect of sex on FTP SUVR in the entorhinal cortex (+0.158 SUVR, 95% CI [0.057, 0.260]), inferior temporal cortex (+0.265 SUVR, 95% CI [0.069, 0.461]), and precuneus (+0.207 SUVR, 95% CI [0.026, 0.387]) remained significant (Table 3), while the effect on superior parietal FTP SUVR was diminished (+0.176 SUVR, 95% CI [−0.017, 0.369]) and sex effects on FTP SUVR in all areas of off-target binding remained non-significant (ηp2s≤.009, ps≥.32) (Table 3).
When APOE4 was added to the model, the effect of female sex on precuneus FTP SUVR (+0.198 SUVR, 95% CI [0.015, 0.381]) and all temporal FTP SUVR (entorhinal: +0.169 SUVR, 95% CI [0.068, 0.270]; inferior temporal: +0.255 SUVR, 95% CI [0.052, 0.458]) remained significant (Table 3). The sex effect on superior parietal FTP SUVR and FTP SUVR in areas of off-target binding remained non-significant with the addition of APOE4 to the models (ηp2s≤.025, ps≥.097) (Table 3). Note that the sample size was slightly reduced (n=115) in these analyses due to missing data on APOE4 status.
3.4. Interaction Models
We further tested whether the detected sex effects were modulated by APOE4 status, age, or CDR-SoB.
There was a significant sex by APOE4 interaction on FTP SUVR in the temporal meta-ROI, such that greater FTP SUVR in females was only found among ε4 non-carriers (effect of female sex changed by −0.330 SUVR, 95%CI [−0.636, −0.024] in ε4 carriers compared to ε4 non-carriers) (Table 4, Supplementary Figure 2A). However, there was no significant sex by APOE4 interaction on entorhinal FTP SUVR or global PIB SUVR (ηp2s≤.024, ps≥.10) (Table 4, Supplementary Figure 2A). Note that the sample size was slightly reduced (n=115) in these analyses due to missing data on APOE4 status.
Table 4.
Interaction Models
| ηp2 | Estimate [95% CI] | p | |
|---|---|---|---|
|
| |||
| Sex*APOE4 a | |||
|
| |||
| Global PIB SUVR | |||
| Sex | .009 | 0.105 [−0.102, 0.313] | .32 |
| APOE4 | <.001 | 0.015 [−0.176, 0.207] | .87 |
| Sex*APOE4 | .001 | 0.044 [−0.223, 0.310] | .75 |
| Temporal Meta-ROI FTP SUVR | |||
| Sex | .099 | 0.414 [0.176, 0.652] | <.001 |
| APOE4 | .027 | 0.193 [−0.028, 0.413] | .09 |
| Sex*APOE4 | .040 | −0.330 [−0.636, −0.024] | .035 |
| Entorhinal FTP SUVR | |||
| Sex | .10 | 0.272 [0.118, 0.425] | <.001 |
| APOE4 | .072 | 0.209 [0.067, 0.351] | .004 |
| Sex*APOE4 | .024 | −0.165 [−0.362, 0.033] | .10 |
|
Sex*Age b | |||
| Global PIB SUVR | |||
| Sex | .043 | 0.146 [0.018, 0.273] | .025 |
| Age | <.001 | −0.001 [−0.011, 0.010] | .88 |
| Age*Sex | .001 | −0.002 [−0.016, 0.0118] | .77 |
| Temporal Meta-ROI FTP SUVR | |||
| Sex | .070 | 0.221 [0.071, 0.370] | .004 |
| Age | .083 | −0.020 [−0.032, −0.008] | .002 |
| Age*Sex | .004 | 0.006 [−0.011, 0.022] | .50 |
|
Sex*CDR-SoB c | |||
| Global PIB SUVR | |||
| Sex | .043 | 0.146 [0.019, 0.274] | .025 |
| CDR-SoB | <.001 | −0.002 [−0.039, 0.035] | .92 |
| Sex*CDR-SoB | .004 | 0.020 [−0.040, 0.081] | .51 |
| Temporal Meta-ROI FTP SUVR | |||
| Sex | .070 | 0.221 [0.072, 0.370] | .004 |
| CDR-SoB | .132 | 0.054 [0.011, 0.098] | .015 |
| Sex*CDR-SoB | .003 | −0.021 [−0.092, 0.051] | .57 |
Reference levels are male and ε4 non-carrier for sex and APOE4 status, respectively. ηp2: partial eta-squared. CDR-SoB = Clinical Dementia Rating Sum of Boxes.
APOE data available on 56 males and 59 females (total n=115). APOE4 is coded as either an ε4 carrier or non-carrier. Models controlled additionally for age and Clinical Dementia Rating Sum of Boxes.
Models controlled additionally for Clinical Dementia Rating Sum of Boxes.
Models controlled additionally for age.
While there were no sex differences in number of ε2 carriers or homozygous ε4 carriers (Table 1), it is possible that the above interaction results were influenced by these subgroups. Each of these subgroups were too small to allow for further modeling, so sensitivity analyses examined the sex by APOE4 interaction models after excluding ε2 carriers and homozygous ε4 carriers. Though results were non-significant due in part to reduced sample size (n=96) and therefore reduced power, results followed a pattern consistent with the original sex by APOE4 interaction analysis (temporal meta-ROI interaction term: −0.269 SUVR 95%CI [−0.583, 0.045]; entorhinal FTP SUVR and global PIB SUVR interaction terms: ηp2s≤.010, ps≥.34).
No significant sex by age nor sex by CDR-SoB interactions were detected on either global PIB SUVR or temporal meta-ROI FTP SUVR (ηp2s≤.004, ps≥.50) (Table 4, Supplementary Figures 2B and 2C).
3.5. No Main Effect of Sex on Volume or Cortical Thickness
Females and males did not differ on measures of hippocampal volume or cortical thickness in the entorhinal cortex, inferior temporal gyrus, precuneus, or superior parietal lobule in any model (Table 5). Results from the Z-scored cortical thickness analysis did not differ from our main cortical thickness analysis (Supplementary Table 3).
Table 5.
Volume and Cortical Thickness Analyses
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| ηp2 | Estimate [95% CI] | p | ηp2 | Estimate [95% CI] | p | ηp2 | Estimate [95% CI] | p | |
|
| |||||||||
| Volume | |||||||||
| Hippocampal Volume a | |||||||||
|
| |||||||||
| Sex | <.001 | −14.50 [−519.6, 490.6] | .96 | <.001 | 44.12 [−444.6, 532.9] | .86 | <.001 | 56.51 [−437.6, 550.6] | .82 |
| Entorhinal FTP SUVR | .078 | −1042[−1712, −372.4] | .003 | .077 | −1040 [−1712, −367.3] | .003 | |||
| Global PIB SUVR | .002 | −107.7 [−621.0, 405.6] | .68 | ||||||
|
Cortical Thickness | |||||||||
| Entorhinal Thickness | |||||||||
| Sex | .010 | −0.074 [−0.212, 0.064] | .29 | <.001 | −0.012 [−0.151, 0.127] | .86 | .001 | −0.021 [−0.163, 0.121] | .77 |
| Entorhinal FTP SUVR | .076 | −0.383 [−0.631, −0.135] | .003 | .077 | −0.385 [−0.634, −0.136] | .003 | |||
| Global PIB SUVR | .003 | 0.061 [−0.133, 0.255] | .53 | ||||||
| Inferior Temporal Thickness | |||||||||
| Sex | .007 | −0.030 [−0.094, 0.035] | .37 | .002 | 0.013 [−0.048, 0.074] | .66 | .001 | 0.011 [−0.051, 0.073] | .72 |
| Inferior Temporal FTP SUVR | .19 | −0.148 [−0.204, −0.091] | <.001 | .19 | −0.149 [−0.206, −0.092] | <.001 | |||
| Global PIB SUVR | .001 | 0.017 [−0.069, 0.103] | .70 | ||||||
| Precuneus Thickness | |||||||||
| Sex | .012 | −0.035 [−0.093, 0.023] | .24 | .001 | 0.007 [−0.046, 0.060] | .79 | .001 | 0.006 [−0.047, 0.060] | .81 |
| Precuneus FTP SUVR | .23 | −0.151 [−0.201, −0.100] | <.001 | .22 | −0.152 [−0.206, −0.098] | <.001 | |||
| Global PIB SUVR | <.001 | 0.008 [−0.071, 0.087] | .84 | ||||||
| Superior Parietal Thickness | |||||||||
| Sex | .010 | −0.033 [−0.094, 0.029] | .29 | .002 | 0.011 [−0.040, 0.063] | .67 | .001 | 0.008 [−0.044, 0.060] | .76 |
| Superior Parietal FTP SUVR | .33 | −0.178 [−0.225, −0.131] | <.001 | .33 | −0.184 [−0.234, −0.135] | <.001 | |||
| Global PIB SUVR | .006 | 0.032 [−0.045, 0.109] | .41 | ||||||
All models controlled for age and Clinical Dementia Rating Sum of Boxes. Reference level for sex is male. ηp2: partial eta-squared.
Hippocampal volume analysis controlled additionally for total intracranial volume. Models 2 and 3 used entorhinal cortex FTP SUVR as covariate in place of hippocampal FTP SUVR, due to spillover of off-target PET signal from the choroid plexus in the hippocampi.
3.6. No Sex Effect on Cerebrovascular Lesion Volume
Controlling for age, CDR-SoB, and TIV, no sex differences were observed in log-transformed global WMH volume in the subsample with WMH data available for analysis (n=69; Mfemale=3.58±0.4 vs. Mmale=3.60±0.3; p=.18, ηp2=0.028).
4. Discussion
In our sample of amyloid-positive patients with MCI and dementia, females had greater temporoparietal tau-PET and global amyloid-PET retention than males. These results are in line with recent studies showing increased tau-PET retention in females among community sample adults (Luchsinger et al., 2020; Palta et al., 2021), preclinical AD (Buckley et al., 2020, 2019a), and MCI/AD (Digma et al., 2020; Ossenkoppele et al., 2020). The sex effect was most robust on tau-PET retention in temporal regions, remaining significant when controlling for PIB SUVR and APOE4 status in addition to age and CDR-SoB. In contrast, the sex effect on global PIB SUVR was not significant after adjusting for temporal FTP SUVR in addition to age and CDR-SoB. These findings are consistent with pathologic studies of AD which found substantially increased tau burden in females, but no difference or small increases in amyloid burden (Barnes et al., 2005; Liesinger et al., 2018; Oveisgharan et al., 2018). This relatively consistent finding of greater tau pathology in females may suggest that sex-specific genetic and chromosomal (Cáceres and González, 2020; Davis et al., 2020; Fan et al., 2020), hormonal (Sundermann et al., 2020), and cellular (Kodama and Gan, 2019) mechanisms interact to differentially affect accumulation and proliferation of pathology in males and females.
The observed sex differences in PET biomarkers were present despite males and females having similar CDR-SoB and MMSE scores, as well as comparable regional cortical thickness values and hippocampal volumes. Our lack of findings in MRI measures of neurodegeneration is in line with many previous findings (Fjell et al., 2009; Lee et al., 2018; Pennanen et al., 2004; Seo et al., 2011), though not all (Arruda et al., 2020; Sundermann et al., 2016).
These results suggest that female MCI and AD dementia patients may handle a greater burden of pathology without manifesting greater absolute clinical deficits compared to males. Previous studies have shown that females consistently demonstrate higher premorbid verbal memory abilities across the lifespan (Bolla-Wilson and Bleecker, 1986; Geffen et al., 1990; Kramer et al., 1997), which was the trend we observed in our controls (Supplementary Table 1). In contrast, the absence of sex differences in the raw memory abilities of our patient group suggests that over the course of the disease, our female patients may have declined from their premorbid cognitive abilities more than males. This is further corroborated by the observation that females had significantly lower memory scores than males when the memory scores were normatively adjusted for age and the expected female advantage. Thus, this apparent capacity for cognitively impaired females to handle a greater burden of pathology may largely reflect greater premorbid function. This hypothesis is in line with previous research suggesting that a verbal memory advantage in females may mask early cognitive decline and is not inconsistent with results of prior studies. In a cross-sectional examination of neuropathology, Barnes et al. (2005) found that females at lower levels of pathology had a lower likelihood of expressing clinical dementia compared to males. At higher levels of pathology, however, females became more likely to express clinical dementia. Additionally, longitudinal studies have found that females with high levels of AD pathology as measured by CSF and PET experience atrophy and cognitive decline at faster rates (Koran et al., 2017; Rowe et al., 2010). It is clear, then, that the hypothesized capacity for females to better handle brain pathology due to higher premorbid abilities may not equate to better performance over the entire disease course; rather, because premorbid function could act to buffer the effects of neurodegeneration, its advantages may be time-limited. Although this study may have found that relatively mildly impaired females can handle a greater burden of pathology without manifesting greater absolute clinical deficits than males, this may be not be the case at more advanced disease stages. However, not all studies support this hypothesis. For example, one study in a sample that is unbiased towards recruitment of subjects with memory concern has demonstrated that, of subjects who are cognitively normal at baseline, males may be more susceptible to brain volume decline associated with amyloid positivity (Armstrong et al., 2019). Longitudinal studies on sex differences in tau-PET and cognition, spanning the transition from preclinical stage to the dementia stage, are needed.
Cognitive decline and dementia often result from the additive or synergistic contributions of multiple pathologies and pathways (Matthews et al., 2009; Schneider et al., 2007). Thus, it is also possible that the males in our sample have a greater burden of comorbid neuropathologies that could lend another explanation to their similar cognitive and functional performance despite lower amyloid- and tau-PET burden compared to females. To test this, we collected a sensitive measure of WMH volume from FLAIR and T1 imaging in a subsample of participants. WMH are typically reported to be greater in both cognitively healthy (Fatemi et al., 2018) and impaired females (Sawada et al., 2000). However, we found no differences in global WMH volume between males and females. Unfortunately, few other neuropathologies are reliably assessed in vivo at this time. While vascular brain injury as assessed via WMH volume is unlikely to have contributed to our results, it is possible that other neuropathologies may still have contributed to male pathological burden, such as Lewy Body Disease (Barnes et al., 2019). Finally, we should also consider that males in our sample may have less tau burden due to survival bias. Mortality due to cardiovascular factors is increased in males compared to females, especially those aged 45–65 (Chêne et al., 2015). Thus, males surviving to older age may be less likely to express vascular risk factors for dementia, and therefore less likely to develop AD pathology compared to females. However, problems related to cerebrovascular comorbidity and survival bias may be less of a burden in our sample due to the relatively young average age of both males and females.
The present study found that the effect of sex on AD PET biomarkers did not vary by age or clinical severity, and there were no significant interactive effects of sex and APOE4 status on global amyloid-PET SUVR. However, there was a significant interaction between APOE4 status and sex on tau PET retention in the temporal meta-ROI. A previous neuropathological study has found that among routine autopsy cases, female APOE4 carriers develop a greater extent of tau and amyloid pathology (Corder et al., 2004), but a more recent study in a sample of cognitively normal, MCI, and AD autopsy cases did not replicate this relationship (Hohman et al., 2018). Interactions between sex and APOE4 have been more consistently found in CSF phosphorylated tau, with levels higher in clinically-defined MCI (Altmann et al., 2014; Liu et al., 2019) and a sample of both clinically normal and impaired (Hohman et al., 2018) female APOE4 carriers. The interaction of sex and APOE4 on AD PET biomarkers has been more sparsely tested. One study in individuals with clinically diagnosed MCI found a significant sex by APOE4 interaction on tau-PET in multiple ROIs, whereby females carriers demonstrated greater retention (Liu et al., 2019), however such an interaction has also been reported in the opposite direction when examining amyloid-PET in patients with clinical AD (Sundermann et al., 2018). As the present study suggests that greater temporal meta-ROI tau-PET retention in females is pronounced among APOE4 non-carriers only, with no differential effect of APOE4 on greater temporal meta-ROI tau-PET in either sex, our interactive finding deviates from previous reports. However, the study was likely underpowered to detect any interactive effects on AD PET biomarkers. Therefore, results should be interpreted with caution.
Previous research has raised concerns about the biological underpinnings of FTP-PET, suggesting that part of its retention could be “off-target” binding caused by tracer affinity to other compounds (Baker et al., 2019). In particular, enzymes MAO-A and MAO-B are possible sources of off-target binding and levels may vary by sex, notably in the temporal cortex (Saura et al., 1997). Sex differences in other sources of off-target binding in FTP-PET may exist in the brain as well. While we found no sex differences in FTP retention in regions known for off-target binding, it is still possible that sex effects observed in cortical regions could be attributable, in part, to regionally specific off-target tracer retention.
The study has several strengths. We analyzed a relatively large sample with multi-modal imaging, and we limited our analyses to amyloid-positive patients with a diagnosis of MCI or dementia due to AD in order to ensure high likelihood that we are examining underlying AD pathology. As literature in sex differences in this population is mixed, the focus on impaired patients was an additional strength. Furthermore, we added global amyloid-PET burden as a covariate to our tau-PET analyses and demonstrated that the increased tau-PET retention in females was independent of the observed increase in amyloid-PET retention. Finally, the inclusion of regions of “off-target” FTP binding allowed us to test the specificity of the tracer retention to AD tau as opposed to nonspecific binding.
This study also has several limitations. Most prominent, we were unable to distinguish between gender constructs and biological sex. Differences in education, occupational attainment, and occupational exposure to hazards may be gender-role specific and could contribute to reserve and risk. Furthermore, our sample had narrow demographic and clinical representation; our cohort was fairly young and highly educated with a lack of racial and ethnic diversity, was relatively mildly impaired, and was enriched with cases of early-onset AD, which may account for the high frequency of APOE4 carriers. Finally, the present study evaluated cross-sectional differences, but further work is needed to establish longitudinal trajectories.
5. Conclusion
Clinically impaired female patients on the Alzheimer’s continuum demonstrated greater tau PET retention in temporal and parietal ROIs, and less robustly, greater global amyloid PET retention. Additional studies are warranted to replicate these findings in more diverse cohorts and examine longitudinal data. Future research should also seek to understand the differential contributions of gender constructs as opposed to biological sex. Further characterization of sex differences will crucially inform our understanding of AD pathogenesis and support development of personalized strategies for AD prevention, detection, and treatment.
Supplementary Material
Highlights.
In the clinical stages of AD, females had greater tau- and amyloid-PET than males
Greater cortical tau-PET binding in females was observed in temporoparietal regions
Tau-PET sex differences were present when controlling for amyloid-PET, age, and CDR
Tau-PET sex differences were not present in subcortical areas of off-target binding
Acknowledgements
We thank the patients and their caregivers for their participation in the study. Avid Radiopharmaceuticals enabled use of the 18F-Flortaucipir tracer but did not provide direct funding and was not involved in data analysis or interpretation. Funding: This work was supported by the NIH National Institute on Aging [grant numbers R01-AG045611, P50-AG023501, P30-AG062422, P01-AG019724]; the Rainwater Charitable Foundation; the Alzheimer’s Association [grant number AARF-16-443577]; and a gift from Edward and Pearl Fein. Funding sources had no role in study design, data collection, data analysis or interpretation, manuscript writing, or the decision to publish the article.
Footnotes
Declarations of interest: Lauren Edwards, Renaud La Joie, Leonardo Iaccarino, Kaitlin B Casaletto, Yann Cobigo, Harli Grant, Minseon Kim, Taylor J Mellinger, Julie Pham, Amelia Strom, Howard J Rosen, David Soleimani-Meigooni, and Amy Wolf have nothing to disclose. Suzanne L Baker consults for Genentech. Joel H Kramer receives royalties from Pearson, Inc. for the California Verbal Learning Test and serves as a consultant for Biogen. Katherine L Possin received research funding from Quest Diagnostics. Bruce L Miller receives research support from the NIH/NIA and the Centers for Medicare & Medicaid Services (CMS) as grants for the Memory and Aging Center. As an additional disclosure, Dr. Miller serves as Medical Director for the John Douglas French Foundation; Scientific Director for the Tau Consortium; Director/Medical Advisory Board of the Larry L. Hillblom Foundation; Scientific Advisory Board Member for the National Institute for Health Research Cambridge Biomedical Research Centre and its subunit, the Biomedical Research Unit in Dementia (UK); and Board Member for the American Brain Foundation (ABF). Gil D Rabinovici receives research support from Avid Radiopharmaceuticals, GE Healthcare, and Life Molecular Imaging, and has received consulting fees or speaking honoraria from Axon Neurosciences, Avid Radiopharmaceuticals, GE Healthcare, Johnson & Johnson, Roche, Eisai, Genentech, Merck. He is an associate editor of JAMA Neurology.
References
- 2020. Alzheimer’s disease facts and figures, 2020. Alzheimers Dement. 16, 391–460. 10.1002/alz.12068 [DOI] [Google Scholar]
- Alber J, Alladi S, Bae H-J, Barton DA, Beckett LA, Bell JM, Berman SE, Biessels GJ, Black SE, Bos I, Bowman GL, Brai E, Brickman AM, Callahan BL, Corriveau RA, Fossati S, Gottesman RF, Gustafson DR, Hachinski V, Hayden KM, Helman AM, Hughes TM, Isaacs JD, Jefferson AL, Johnson SC, Kapasi A, Kern S, Kwon JC, Kukolja J, Lee A, Lockhart SN, Murray A, Osborn KE, Power MC, Price BR, Rhodius-Meester HFM, Rondeau JA, Rosen AC, Rosene DL, Schneider JA, Scholtzova H, Shaaban CE, Silva NCBS, Snyder HM, Swardfager W, Troen AM, van Veluw SJ, Vemuri P, Wallin A, Wellington C, Wilcock DM, Xie SX, Hainsworth AH, 2019. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities. Alzheimers Dement. Transl. Res. Clin. Interv. 5, 107–117. 10.1016/j.trci.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH, 2011. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann A, Tian L, Henderson VW, Greicius MD, 2014. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol. 75, 563–573. 10.1002/ana.24135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong NM, Huang C-W, Williams OA, Bilgel M, An Y, Doshi J, Erus G, Davatzikos C, Wong DF, Ferrucci L, Resnick SM, 2019. Sex differences in the association between amyloid and longitudinal brain volume change in cognitively normal older adults. NeuroImage Clin. 22, 101769. 10.1016/j.nicl.2019.101769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT, 1992. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42, 631–639. 10.1212/wnl.42.3.631 [DOI] [PubMed] [Google Scholar]
- Arruda F, Rosselli M, Greig MT, Loewenstein DA, Lang M, Torres VL, Vélez-Uribe I, Conniff J, Barker WW, Curiel RE, Adjouadi M, Duara R, 2020. The Association Between Functional Assessment and Structural Brain Biomarkers in an Ethnically Diverse Sample With Normal Cognition, Mild Cognitive Impairment, or Dementia. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 10.1093/arclin/acaa065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SL, Harrison TM, Maass A, Joie R. La, Jagust WJ, 2019. Effect of Off-Target Binding on 18 F-Flortaucipir Variability in Healthy Controls Across the Life Span. J Nucl Med 60, 1444–1451. 10.2967/jnumed.118.224113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SL, Maass A, Jagust WJ, 2017. Considerations and code for partial volume correcting [18F]-AV-1451 tau PET data. Data Brief 15, 648–657. 10.1016/j.dib.2017.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Lamar M, Schneider JA, 2019. Sex differences in mixed neuropathologies in community-dwelling older adults. Brain Res. 1719, 11–16. 10.1016/j.brainres.2019.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA, 2005. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch. Gen. Psychiatry 62, 685–691. 10.1001/archpsyc.62.6.685 [DOI] [PubMed] [Google Scholar]
- Bejanin A, Schonhaut DR, La Joie R, Kramer JH, Baker SL, Sosa N, Ayakta N, Cantwell A, Janabi M, Lauriola M, O’Neil JP, Gorno-Tempini ML, Miller ZA, Rosen HJ, Miller BL, Jagust WJ, Rabinovici GD, 2017. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain 140, 3286–3300. 10.1093/brain/awx243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla-Wilson K, Bleecker ML, 1986. Influence of verbal intelligence, sex, age, and education on the Rey Auditory Verbal Learning Test. Dev. Neuropsychol. 2, 203–211. 10.1080/87565648609540342 [DOI] [Google Scholar]
- Buckley RF, Mormino EC, Rabin JS, Hohman TJ, Landau S, Hanseeuw BJ, Jacobs HIL, Papp KV, Amariglio RE, Properzi MJ, Schultz AP, Kirn D, Scott MR, Hedden T, Farrell M, Price J, Chhatwal J, Rentz DM, Villemagne VL, Johnson KA, Sperling RA, 2019a. Sex Differences in the Association of Global Amyloid and Regional Tau Deposition Measured by Positron Emission Tomography in Clinically Normal Older Adults. JAMA Neurol. 76, 542–551. 10.1001/jamaneurol.2018.4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley RF, Scott MR, Jacobs HIL, Schultz AP, Properzi MJ, Amariglio RE, Hohman TJ, Mayblyum DV, Rubinstein ZB, Manning L, Hanseeuw BJ, Mormino EC, Rentz DM, Johnson KA, Sperling RA, 2020. Sex Mediates Relationships Between Regional Tau Pathology and Cognitive Decline. Ann. Neurol. 88, 921–932. 10.1002/ana.25878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley RF, Waller M, Masters CL, Dobson A, 2019b. To What Extent Does Age at Death Account for Sex Differences in Rates of Mortality From Alzheimer Disease? Am. J. Epidemiol. 188, 1213–1223. 10.1093/aje/kwz048 [DOI] [PubMed] [Google Scholar]
- Cáceres A, González JR, 2020. Female-specific risk of Alzheimer’s disease is associated with tau phosphorylation processes: A transcriptome-wide interaction analysis. Neurobiol. Aging. 10.1016/j.neurobiolaging.2020.08.020 [DOI] [PubMed] [Google Scholar]
- Chêne G, Beiser A, Au R, Preis SR, Wolf PA, Dufouil C, Seshadri S, 2015. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement. 11, 310–320. 10.1016/j.jalz.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Choi JY, Hwang MS, Kim YJ, Lee HM, Lee HS, Lee JH, Ryu YH, Lee MS, Lyoo CH, 2016. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann. Neurol. 80, 247–258. 10.1002/ana.24711 [DOI] [PubMed] [Google Scholar]
- Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H, 2004. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: Modification by age, sex, and APOE polymorphism. Ann. N. Y. Acad. Sci. 1019, 24–28. 10.1196/annals.1297.005 [DOI] [PubMed] [Google Scholar]
- Davis EJ, Broestl L, Abdulai-Saiku S, Worden K, Bonham LW, Miñones-Moyano E, Moreno AJ, Wang D, Chang K, Williams G, Garay BI, Lobach I, Devidze N, Kim D, Anderson-Bergman C, Yu G-Q, White CC, Harris JA, Miller BL, Bennett DA, Arnold AP, De Jager PL, Palop JJ, Panning B, Yokoyama JS, Mucke L, Dubal DB, 2020. A second X chromosome contributes to resilience in a mouse model of Alzheimer’s disease. Sci. Transl. Med. 12, eaaz5677. 10.1126/scitranslmed.aaz5677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA, 2000. California Verbal Learning Test-II, 2nd ed. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Di Carlo A, Baldereschi M, Amaducci L, Lepore V, Bracco L, Maggi S, Bonaiuto S, Perissinotto E, Scarlato G, Farchi G, Inzitari D, 2002. Incidence of dementia, Alzheimer’s disease, and vascular dementia in Italy. The ILSA study. J. Am. Geriatr. Soc. 50, 41–48. 10.1046/j.1532-5415.2002.50006.x [DOI] [PubMed] [Google Scholar]
- Digma LA, Madsen JR, Rissman RA, Jacobs DM, Brewer JB, Banks SJ, Weiner Michael, Aisen P, Petersen R, Jack CR, Jagust W, Trojanowki JQ, Toga AW, Beckett L, Green RC, Saykin AJ, Morris J, Shaw LM, Liu E, Montine T, Thomas RG, Donohue M, Walter S, Gessert D, Sather T, Jiminez G, Harvey D, Donohue M, Bernstein M, Fox N, Thompson P, Schuff N, DeCArli C, Borowski B, Gunter J, Senjem M, Vemuri P, Jones D, Kantarci K, Ward C, Koeppe RA, Foster N, Reiman EM, Chen K, Mathis C, Landau S, Cairns NJ, Householder E, Reinwald LT, Lee V, Korecka M, Figurski M, Crawford K, Neu S, Foroud TM, Potkin S, Shen L, Kelley F, Kim S, Nho K, Kachaturian Z, Frank R, Snyder PJ, Molchan S, Kaye J, Quinn J, Lind B, Carter R, Dolen S, Schneider LS, Pawluczyk S, Beccera M, Teodoro L, Spann BM, Brewer J, Vanderswag H, Fleisher A, Heidebrink JL, Lord JL, Petersen R, Mason SS, Albers CS, Knopman D, Johnson Kris, Doody RS, Meyer JV, Chowdhury M, Rountree S, Dang M, Stern Y, Honig LS, Bell KL, Ances B, Morris JC, Carroll M, Leon S, Householder E, Mintun MA, Schneider S, Oliver A, Griffith R, Clark D, Geldmacher D, Brockington J, Roberson E, Grossman H, Mitsis E, deToledo-Morrell L, Shah RC, Duara R, Varon D, Greig MT, Roberts P, Albert M, Onyike C, D’Agostino D II, Kielb S, Galvin JE, Pogorelec DM, Cerbone B, Michel CA, Rusinek H, de Leon MJ, Glodzik L, De Santi S, Doraiswamy PM, Petrella JR, Wong TZ, Arnold SE, Karlawish JH, Wolk D, Smith CD, Jicha G, Hardy P, Sinha P, Oates E, Conrad G, Lopez OL, Oakley M, Simpson DM, Porsteinsson AP, Goldstein BS, Martin K, Makino KM, Ismail MS, Brand C, Mulnard RA, Thai G, Mc Adams Ortiz C, Womack K, Mathews D, Quiceno M, Arrastia RD, King R, Weiner Myron, Martin Cook K, DeVous M, Levey AI, Lah JJ, Cellar JS, Burns JM, Anderson HS, Swerdlow RH, Apostolova L, Tingus K, Woo E, Silverman DHS, Lu PH, Bartzokis G, Graff Radford NR, Parfitt F, Kendall T, Johnson H, Farlow MR, Hake AM, Matthews BR, Herring S, Hunt C, van Dyck CH, Carson RE, MacAvoy MG, Chertkow H, Bergman H, Hosein C, Black S, Stefanovic B, Caldwell C, Hsiung GYR, Feldman H, Mudge B, Assaly M, Kertesz A, Rogers J, Trost D, Bernick C, Munic D, Kerwin D, Marsel Mesulam M, Lipowski K, Kuo Wu C, Johnson N, Sadowsky C, Martinez W, Villena T, Scott Turner R, Johnson Kathleen, Reynolds B, Sperling RA, Johnson KA, Marshall G, Frey M, Yesavage J, Taylor JL, Lane B, Rosen A, Tinklenberg J, Sabbagh MN, Belden CM, Jacobson SA, Sirrel SA, Kowall N, Killiany R, Budson AE, Norbash A, Johnson PL, Obisesan TO, Wolday S, Allard J, Lerner A, Ogrocki P, Hudson L, Fletcher E, Carmichael O, Olichney J, DeCarli C, Kittur S, Borrie M, Lee TY, Bartha R, Johnson S, Asthana S, Carlsson CM, Potkin SG, Preda A, Nguyen D, Tariot P, Fleisher A, Reeder S, Bates V, Capote H, Rainka M, Scharre DW, Kataki M, Adeli A, Zimmerman EA, Celmins D, Brown AD, Pearlson GD, Blank K, Anderson K, Santulli RB, Kitzmiller TJ, Schwartz ES, Sink KM, Williamson JD, Garg P, Watkins F, Ott BR, Querfurth H, Tremont G, Salloway S, Malloy P, Correia S, Rosen HJ, Miller BL, Mintzer J, Spicer K, Bachman D, Finger E, Pasternak S, Rachinsky I, Rogers J, Kertesz A, Drost D, Pomara N, Hernando R, Sarrael A, Schultz SK, Boles Ponto LL, Shim H, Smith KE, Relkin N, Chaing G, Raudin L, Smith A, Fargher K, Raj BA, 2020. Women can bear a bigger burden: ante- and post-mortem evidence for reserve in the face of tau. Brain Commun. 2. 10.1093/braincomms/fcaa025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan CC, Banks SJ, Thompson WK, Chen C-H, McEvoy LK, Tan CH, Kukull W, Bennett DA, Farrer LA, Mayeux R, Schellenberg GD, Andreassen OA, Desikan R, Dale AM, 2020. Sex-dependent autosomal effects on clinical progression of Alzheimer’s disease. Brain 143, 2272–2280. 10.1093/brain/awaa164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi F, Kantarci K, Graff-Radford J, Preboske GM, Weigand SD, Przybelski SA, Knopman DS, Machulda MM, Roberts RO, Mielke MM, Petersen RC, Jack CR, Vemuri P, 2018. Sex differences in cerebrovascular pathologies on FLAIR in cognitively unimpaired elderly. Neurology 90, e466–e473. 10.1212/WNL.0000000000004913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filon JR, Intorcia AJ, Sue LI, Vazquez Arreola E, Wilson J, Davis KJ, Sabbagh MN, Belden M, Caselli RJ, Adler CH, Woodruff BK, Rapscak SZ, Ahern GL, Burke AD, Jacobson S, Shill HA, Driver-Dunckley E, Chen K, Reiman EM, Beach TG, Serrano GE, 2016. Gender differences in Alzheimer disease: Brain atrophy, histopathology burden, and cognition. J. Neuropathol. Exp. Neurol. 75, 748–754. 10.1093/jnen/nlw047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve N, Fischl B, Dale AM, Walhovd KB, 2009. Minute Effects of Sex on the Aging Brain: A Multisample Magnetic Resonance Imaging Study of Healthy Aging and Alzheimer’s Disease. J. Neurosci. 29, 8774–8783. 10.1523/JNEUROSCI.0115-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, 1975. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Geffen G, Moar KJ, O’hanlon AP, Clark CR, Geffen LB, 1990. Performance measures of 16- to 86-year-old males and females on the auditory verbal learning test. Clin. Neuropsychol. 4, 45–63. 10.1080/13854049008401496 [DOI] [PubMed] [Google Scholar]
- Hohman TJ, Dumitrescu L, Barnes LL, Thambisetty M, Beecham G, Kunkle B, Gifford KA, Bush WS, Chibnik LB, Mukherjee S, De Jager PL, Kukull W, Crane PK, Resnick SM, Keene CD, Montine TJ, Schellenberg GD, Haines JL, Zetterberg H, Blennow K, Larson EB, Johnson SC, Albert M, Bennett DA, Schneider JA, Jefferson AL, Blennowe K, Abner E, Adams P, Albin R, Apostolova L, Arnold S, Asthana S, Atwood C, Baldwin C, Barber R, Barral S, Beach T, Becker J, Beekly D, Bigio E, Bird T, Blacker D, Boeve B, Bowen J, Boxer A, Burke J, Burns J, Buxbaum J, Cairns N, Cantwell L, Cao C, Carlson C, Carlsson C, Carney R, Carrasquillo M, Chui H, Crane P, Cribbs D, Crocco E, Cruchaga C, DeCarli C, Dick M, Dickson D, Doody R, Duara R, Ertekin-Taner N, Evans D, Faber K, Fairchild T, Fallon K, Fardo D, Farlow M, Farrer L, Ferris S, Foroud T, Frosch M, Galasko D, Gearing M, Geschwind D, Ghetti B, Gilbert J, Goate A, Graff-Radford N, Green R, Growdon J, Hakonarson H, Hamilton R, Hamilton-Nelson K, Hardy J, Harrell L, Honig L, Huebinger R, Huentelman M, Hulette C, Hyman B, Jarvik G, Jin LW, Jun G, Kamboh MI, Karydas A, Katz M, Kauwe J, Kaye J, Kim R, Kowall N, Kramer J, Kuzma A, LaFerla F, Lah J, Leverenz J, Levey A, Li G, Lieberman A, Lipton R, Lopez O, Lunetta K, Lyketsos C, Malamon J, Marson D, Martin E, Martiniuk F, Mash D, Masliah E, Mayeux R, McCormick W, McCurry S, McDavid A, McDonough S, McKee A, Mesulam M, Miller B, Miller C, Miller J, Morris J, Myers A, Naj A, O’Bryant S, Olichney J, Parisi J, Paulson H, Pericak-Vance M, Peskind E, Petersen R, Pierce A, Poon W, Potter H, Qu L, Quinn J, Raj A, Raskind M, Reiman E, Reisberg B, Reisch J, Reitz C, Ringman J, Roberson E, Rogaeva E, Rosen H, Rosenberg R, Royall D, Sager M, Sano M, Saykin A, Schneider L, Seeley W, Smith A, Sonnen J, Spina S, St George-Hyslop P, Stern R, Swerdlow R, Tanzi R, Trojanowski J, Troncoso J, Tsuang D, Valladares O, Van Deerlin V, Van Eldik L, Vardarajan B, Vinters H, Vonsattel JP, Wang LS, Weintraub S, Welsh-Bohmer K, Wilhelmsen K, Williamson J, Wingo T, Woltjer R, Wright C, Wu CK, Younkin S, Yu CE, Yu L, Zhao Y, 2018. Sex-specific association of apolipoprotein e with cerebrospinal fluid levels of tau. JAMA Neurol. 75, 989–998. 10.1001/jamaneurol.2018.0821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Contributors, 2018. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. J. Alzheimers Assoc. 14, 535–562. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, Mormino E, Chhatwal J, Amariglio R, Papp K, Marshall G, Albers M, Mauro S, Pepin L, Alverio J, Judge K, Philiossaint M, Shoup T, Yokell D, Dickerson B, Gomez-Isla T, Hyman B, Vasdev N, Sperling R, 2016. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann. Neurol. 79, 110–119. 10.1002/ana.24546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama L, Gan L, 2019. Do Microglial Sex Differences Contribute to Sex Differences in Neurodegenerative Diseases? Trends Mol. Med. 25, 741–749. 10.1016/j.molmed.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koran MEI, Wagener M, Hohman TJ, 2017. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav. 11, 205–213. 10.1007/s11682-016-9523-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Delis DC, Daniel M, 1988. Sex differences in verbal learning. J. Clin. Psychol. 44, 907–915. [DOI] [Google Scholar]
- Kramer JH, Delis DC, Kaplan E, O’Donnell L, Prifitera A, 1997. Developmental sex differences in verbal learning. Neuropsychology 11, 577–584. 10.1037//0894-4105.11.4.577 [DOI] [PubMed] [Google Scholar]
- La Joie R, Perrotin A, Barre L, Hommet C, Mezenge F, Ibazizene M, Camus V, Abbas A, Landeau B, Guilloteau D, de La Sayette V, Eustache F, Desgranges B, Chetelat G, 2012. Region-Specific Hierarchy between Atrophy, Hypometabolism, and -Amyloid (A) Load in Alzheimer’s Disease Dementia. J. Neurosci. 32, 16265–16273. 10.1523/JNEUROSCI.2170-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Joie R, Visani AV, Lesman-Segev OH, Baker SL, Edwards L, Iaccarino L, Soleimani-Meigooni DN, Mellinger T, Janabi M, Miller ZA, Perry DC, Pham J, Strom A, Gorno-Tempini ML, Rosen HJ, Miller BL, Jagust WJ, Rabinovici GD, 2021. Association of APOE4 and Clinical Variability in Alzheimer Disease With the Pattern of Tau- and Amyloid-PET. Neurology 96, e650–e661. 10.1212/WNL.0000000000011270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, Amaducci LA, Brayne C, Copeland JRM, Dartigues JF, Kragh-Sorensen P, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A, 1999. Rates and risk factors for dementia and Alzheimer’s disease: Results from EURODEM pooled analyses. Neurology 52, 78–84. 10.1212/wnl.52.1.78 [DOI] [PubMed] [Google Scholar]
- Lee Juyoun, Cho H, Jeon S, Kim HJ, Kim YJ, Lee Jeongmin, Kim ST, Lee J-M, Chin J, Lockhart SN, Lee AY, Na DL, Seo SW, 2018. Sex-Related Reserve Hypothesis in Alzheimer’s Disease: Changes in Cortical Thickness with a Five-Year Longitudinal Follow-Up. J. Alzheimers Dis. JAD 65, 641–649. 10.3233/JAD-180049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Ghosh PM, Madison C, Laforce R, Corbetta-Rastelli C, Weiner MW, Greicius MD, Seeley WW, Gorno-Tempini ML, Rosen HJ, Miller BL, Jagust WJ, Rabinovici GD, 2013. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain 136, 844–858. 10.1093/brain/aws327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesinger AM, Graff-Radford NR, Duara R, Carter RE, Hanna Al-Shaikh FS, Koga S, Hinkle KM, DiLello SK, Johnson MF, Aziz A, Ertekin-Taner N, Ross OA, Dickson DW, Murray ME, 2018. Sex and age interact to determine clinicopathologic differences in Alzheimer’s disease. Acta Neuropathol. (Berl.) 136, 873–885. 10.1007/s00401-018-1908-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM, 2015. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement. Transl. Res. Clin. Interv. 1, 103–110. 10.1016/j.trci.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Paranjpe MD, Zhou X, Duy PQ, Goyal MS, Benzinger TLS, Lu J, Wang R, Zhou Y, 2019. Sex modulates the ApoE ε4 effect on brain tau deposition measured by 18F-AV-1451 PET in individuals with mild cognitive impairment. Theranostics 9, 4959–4970. 10.7150/thno.35366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart SN, Schöll M, Baker SL, Ayakta N, Swinnerton KN, Bell RK, Mellinger TJ, Shah VD, O’Neil JP, Janabi M, Jagust WJ, 2017. Amyloid and tau PET demonstrate region-specific associations in normal older people. NeuroImage 150, 191–199. 10.1016/j.neuroimage.2017.02.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Palta P, Rippon B, Soto L, Ceballos F, Pardo M, Laing K, Igwe K, Johnson A, Tomljanovic Z, He H, Reitz C, Kreisl W, Razlighi Q, Teresi J, Moreno H, Brickman AM, 2020. Sex Differences in in vivo Alzheimer’s Disease Neuropathology in Late Middle-Aged Hispanics. J. Alzheimers Dis. 74, 1243–1252. 10.3233/JAD-191183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Landau S, Horng A, Lockhart SN, Rabinovici GD, Jagust WJ, Baker SL, La Joie R, 2017. Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer’s disease. NeuroImage 157, 448–463. 10.1016/j.neuroimage.2017.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P, 2009. Epidemiological pathology of dementia: Attributable-risks at death in the medical research council cognitive function and ageing study. PLoS Med. 6. 10.1371/journal.pmed.1000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH, 2011. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 263–269. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mofrad RB, Tijms BM, Scheltens P, Barkhof F, van der Flier WM, Am Sikkes S, Teunissen CE, 2020. Sex differences in CSF biomarkers vary by Alzheimer’s disease stage and APOE ε4 genotype. Neurology. 10.1212/WNL.0000000000010629 [DOI] [PubMed] [Google Scholar]
- Morris JC, 1993. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43, 2412–2414. 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- Nebel RA, Aggarwal NT, Barnes LL, Gallagher A, Goldstein JM, Kantarci K, Mallampalli MP, Mormino EC, Scott L, Yu WH, Maki PM, Mielke MM, 2018. Understanding the impact of sex and gender in Alzheimer’s disease: A call to action. Alzheimers Dement. J. Alzheimers Assoc. 14, 1171–1183. 10.1016/j.jalz.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, Wang LS, Romero K, Arneric SP, Redolfi A, Orlandi D, Frisoni GB, Au R, Devine S, Auerbach S, Espinosa A, Boada M, Ruiz A, Johnson SC, Koscik R, Wang JJ, Hsu WC, Chen YL, Toga AW, 2017. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: A meta-analysis. JAMA Neurol. 74, 1178–1189. 10.1001/jamaneurol.2017.2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Álvarez-Álvarez I, Guillén-Grima F, Aguinaga-Ontoso I, 2017. Prevalence and incidence of Alzheimer’s disease in Europe: A meta-analysis. Neurol. Engl. Ed. 32, 523–532. 10.1016/j.nrleng.2016.02.009 [DOI] [PubMed] [Google Scholar]
- O’brien PC, Dyck PJ, 1995. Procedures for setting normal values. Neurology. 10.1212/WNL.45.1.17 [DOI] [PubMed] [Google Scholar]
- Oksuzyan A, Juel K, Vaupel JW, Christensen K, 2008. Men: Good health and high mortality. Sex differences in health and aging. Aging Clin. Exp. Res. 10.1007/BF03324754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Lyoo CH, Jester-Broms J, Sudre CH, Cho H, Ryu YH, Choi JY, Smith R, Strandberg O, Palmqvist S, Kramer J, Boxer AL, Gorno-Tempini ML, Miller BL, Joie RL, Rabinovici GD, Hansson O, 2020. Assessment of Demographic, Genetic, and Imaging Variables Associated With Brain Resilience and Cognitive Resilience to Pathological Tau in Patients With Alzheimer Disease. JAMA Neurol. 77, 632–642. 10.1001/jamaneurol.2019.5154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA, 2018. Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathol. (Berl.) 136, 887–900. 10.1007/s00401-018-1920-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palta P, Rippon B, Tahmi M, Pardo M, Johnson A, Tomljanovic Z, He H, Laing K, Razlighi Q, Teresi JA, Moreno H, Brickman AM, Kreisl W, Luchsinger JA, 2021. Sex differences in in-vivo tau neuropathology in a multi-ethnic sample of late middle-aged adults. Neurobiol. Aging. 10.1016/j.neurobiolaging.2021.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hänninen T, Laakso MP, Hallikainen M, Vanhanen M, Nissinen A, Helkala E-L, Vainio P, Vanninen R, Partanen K, Soininen H, 2004. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol. Aging 25, 303–310. 10.1016/S0197-4580(03)00084-8 [DOI] [PubMed] [Google Scholar]
- Potvin O, Dieumegarde L, Duchesne S, 2017. Normative morphometric data for cerebral cortical areas over the lifetime of the adult human brain. NeuroImage 156, 315–339. 10.1016/j.neuroimage.2017.05.019 [DOI] [PubMed] [Google Scholar]
- R Core Team, 2020. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Rabinovici GD, Furst AJ, Alkalay A, Racine CA, O’Neil JP, Janabi M, Baker SL, Agarwal N, Bonasera SJ, Mormino EC, Weiner MW, Gorno-Tempini ML, Rosen HJ, Miller BL, Jagust WJ, 2010. Increased metabolic vulnerability in early-onset Alzheimer’s disease is not related to amyloid burden. Brain 133, 512–528. 10.1093/brain/awp326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Rosen HJ, Alkalay A, Kornak J, Furst AJ, Agarwal N, Mormino EC, O’Neil JP, Janabi M, Karydas A, Growdon ME, Jang JY, Huang EJ, DeArmond SJ, Trojanowski JQ, Grinberg LT, Gorno-Tempini ML, Seeley WW, Miller BL, Jagust WJ, 2011. Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology 77, 2034–2042. 10.1212/WNL.0b013e31823b9c5e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Schelbaum E, Hoffman K, Diaz I, Hristov H, Andrews R, Jett S, Jackson H, Lee A, Sarva H, Pahlajani S, Matthews D, Dyke J, de Leon MJ, Isaacson RS, Brinton RD, Mosconi L, 2020. Sex-driven modifiers of Alzheimer risk: A multimodality brain imaging study. Neurology 95, e166–e178. 10.1212/WNL.0000000000009781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O’Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins R, Masters CL, Ames D, Villemagne VL, 2010. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol. Aging 31, 1275–1283. 10.1016/j.neurobiolaging.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Sanchez JS, Becker JA, Jacobs HIL, Hanseeuw BJ, Jiang S, Schultz AP, Properzi MJ, Katz SR, Beiser A, Satizabal CL, O’Donnell A, DeCarli C, Killiany R, Fakhri GE, Normandin MD, Gómez-Isla T, Quiroz YT, Rentz DM, Sperling RA, Seshadri S, Augustinack J, Price JC, Johnson KA, 2021. The cortical origin and initial spread of medial temporal tauopathy in Alzheimer’s disease assessed with positron emission tomography. Sci. Transl. Med. 13. 10.1126/scitranslmed.abc0655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura J, Andrés N, Andrade C, Ojuel J, Eriksson K, Mahy N, 1997. Biphasic and region-specific MAO-B response to aging in normal human brain. Neurobiol. Aging 18, 497–507. 10.1016/S0197-4580(97)00113-9 [DOI] [PubMed] [Google Scholar]
- Sawada H, Udaka F, Izumi Y, Nishinaka K, Kawakami H, Nakamura S, Kameyama M, 2000. Cerebral white matter lesions are not associated with apoE genotype but with age and female sex in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 68, 653–656. 10.1136/jnnp.68.5.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA, 2007. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69, 2197–2204. 10.1212/01.wnl.0000271090.28148.24 [DOI] [PubMed] [Google Scholar]
- Schöll M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, Baker SL, Vogel JW, Faria J, Schwimmer HD, Rabinovici GD, Jagust WJ, 2016. PET Imaging of Tau Deposition in the Aging Human Brain. Neuron 89, 971–982. 10.1016/j.neuron.2016.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SW, Im K, Lee J-M, Kim ST, Ahn HJ, Go SM, Kim S-H, Na DL, 2011. Effects of demographic factors on cortical thickness in Alzheimer’s disease. Neurobiol. Aging 32, 200–209. 10.1016/j.neurobiolaging.2009.02.004 [DOI] [PubMed] [Google Scholar]
- Spina S, La Joie R, Petersen C, Nolan AL, Cuevas D, Cosme C, Hepker M, Hwang J-H, Miller ZA, Huang EJ, Karydas AM, Grant H, Boxer AL, Gorno-Tempini ML, Rosen HJ, Kramer JH, Miller BL, Seeley WW, Rabinovici GD, Grinberg LT, 2021. Comorbid neuropathological diagnoses in early versus late-onset Alzheimer’s disease. Brain J. Neurol. 10.1093/brain/awab099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann EE, Biegon A, Rubin LH, Lipton RB, Mowrey W, Landau S, Maki PM, 2016. Better verbal memory in women than men in MCI despite similar levels of hippocampal atrophy. Neurology 86, 1368–1376. 10.1212/WNL.0000000000002570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann EE, Maki P, Biegon A, Lipton RB, Mielke MM, Machulda M, Bondi MW, Alzheimer’s Disease Neuroimaging Initiative, 2019. Sex-specific norms for verbal memory tests may improve diagnostic accuracy of amnestic MCI. Neurology 93, e1881–e1889. 10.1212/WNL.0000000000008467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann EE, Panizzon MS, Chen X, Andrews M, Galasko D, Banks SJ, for the Alzheimer’s Disease Neuroimaging Initiative, 2020. Sex differences in Alzheimer’s-related Tau biomarkers and a mediating effect of testosterone. Biol. Sex Differ. 11, 33. 10.1186/s13293-020-00310-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann EE, Tran M, Maki PM, Bondi MW, 2018. Sex differences in the association between apolipoprotein E ε4 allele and Alzheimer’s disease markers. Alzheimers Dement. Diagn. Assess. Dis. Monit. 10, 438–447. 10.1016/j.dadm.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therriault J, Benedet AL, Pascoal TA, Mathotaarachchi S, Chamoun M, Savard M, Thomas E, Kang MS, Lussier F, Tissot C, Parsons M, Qureshi MNI, Vitali P, Massarweh G, Soucy J-P, Rej S, Saha-Chaudhuri P, Gauthier S, Rosa-Neto P, 2020. Association of Apolipoprotein E ε4 With Medial Temporal Tau Independent of Amyloid-β. JAMA Neurol. 77, 470–479. 10.1001/jamaneurol.2019.4421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trahan DE, Quintana JW, 1990. Analysis of gender effects upon verbal and visual memory performance in adults. Arch. Clin. Neuropsychol. 5, 325–334. 10.1016/0887-6177(90)90012-E [DOI] [PubMed] [Google Scholar]
- Tschanz JT, Corcoran CD, Schwartz S, Treiber K, Green RC, Norton MC, Mielke MM, Piercy K, Steinberg M, Rabins PV, Leoutsakos JM, Welsh-Bohmer KA, Breitner JCS, Lyketsos CG, 2011. Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with alzheimer dementia: The cache county dementia progression study. Am. J. Geriatr. Psychiatry 19, 532–542. 10.1097/JGP.0b013e3181faec23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Holtzman DM, 2011. Roles of Apolipoprotein E in Alzheimer’s Disease and Other Neurological Disorders. Lancet Neurol. 10, 241–252. 10.1016/S1474-4422(10)70325-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, Madison C, Ayakta N, Ghosh PM, La Joie R, Arthur-Bentil SK, Vogel JW, Marks SM, Lehmann M, Rosen HJ, Reed B, Olichney J, Boxer AL, Miller BL, Borys E, Jin LW, Huang EJ, Grinberg LT, Decarli C, Seeley WW, Jagust W, 2015. Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: Statistical and pathological evaluation. Brain 138, 2020–2033. 10.1093/brain/awv112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters A, Laitner MH, 2021. Biological sex differences in Alzheimer’s preclinical research: A call to action. Alzheimers Dement. Transl. Res. Clin. Interv. 7, e12111. 10.1002/trc2.12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Besser L, Dodge HH, Teylan M, Ferris S, Goldstein FC, Giordani B, Kramer J, Loewenstein D, Marson D, Mungas D, Salmon D, Welsh-Bohmer K, Zhou X-H, Shirk SD, Atri A, Kukull WA, Phelps C, Morris JC, 2018. Version 3 of the Alzheimer Disease Centers’ Neuropsychological Test Battery in the Uniform Data Set (UDS). Alzheimer Dis. Assoc. Disord. 32, 10–17. 10.1097/WAD.0000000000000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisch JK, Meeker KL, Gordon BA, Flores S, Dincer A, Grant EA, Benzinger TL, Morris JC, Ances BM, 2020. Sex-related Differences in Tau Positron Emission Tomography (PET) and the Effects of Hormone Therapy (HT). Alzheimer Dis. Assoc. Disord. 10.1097/WAD.0000000000000393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Li X, 2020. The prevalence of Alzheimer’s disease in the Chinese Han population: a meta-analysis. Neurol. Res. 42, 291–298. 10.1080/01616412.2020.1716467 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


