Abstract
The medical applications of glucagon‐like peptide‐1 receptor (GLP‐1R) agonists is evergrowing in scope, highlighting the urgent need for a comprehensive understanding of the mechanisms through which GLP‐1R activation impacts physiology and behaviour. A new area of research aims to elucidate the role GLP‐1R signalling in glia, which play a role in regulating energy balance, glycemic control, neuroinflammation and oxidative stress. Once controversial, existing evidence now suggests that subsets of glia (e.g. microglia, tanycytes and astrocytes) and infiltrating macrophages express GLP‐1Rs. In this review, we discuss the implications of these findings, with particular focus on the effectiveness of both clinically available and novel GLP‐1R agonists for treating metabolic and neurodegenerative diseases, enhancing cognition and combating substance abuse.
LINKED ARTICLES
This article is part of a themed issue on GLP1 receptor ligands (BJP 75th Anniversary). To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v179.4/issuetoc
Keywords: astrocyte, beta cell, blood glucose, exendin, food intake, gliosis, inflammation, liraglutide, macrophage, microglia, neuroprotection, semaglutide
Abbreviations
- AP
area postrema
- BBB
blood–brain barrier
- DMV
dorsal motor nucleus of the vagus
- EAAT1, aka GLAST and SLC1A3
excitatory amino acid transporter
- EAAT2, aka GLT‐1 and SLC1A2
excitatory amino acid transporter 2
- GLP‐1
glucagon‐like peptide‐1
- GLP‐1R
glucagon‐like peptide‐1 receptor
- IOP
intraocular pressure
- NTS
nucleus tractus solitarius
1. INTRODUCTION
Glia denotes several groups of nonneuronal cell types (e.g. astrocytes, microglia, tanycytes and oligodendrocytes) traditionally understudied in the many disciplines of neuroscience, including in the context of energy balance and glycaemic control. With respect to glucagon‐like peptide‐1 (GLP‐1) physiology, an up‐to‐date PubMed search using keywords ‘GLP‐1 and glia’ returned fewer than 100 papers, a tiny portion of the 17,000+ publications containing the keyword ‘GLP‐1’. Recently, however, interests in understanding the role glia plays in the physiology and pharmacology of GLP‐1 have intensified. Accumulating evidence overwhelmingly support the assertion that glia are cellular substrates modulated by GLP‐1 signalling, whether through direct ligand action and/or through indirect recruitment. GLP‐1 receptor (GLP‐1R) agonists have been shown to exert antiapoptotic and neuroprotective effects (Li et al., 2009; McClean et al., 2011; Perry et al., 2002; Sterling et al., 2020b), while reducing β‐amyloid plaque accumulation (Li et al., 2009; McClean et al., 2011; Perry et al., 2003), enhancing neuronal progenitor cell differentiation (Hamilton et al., 2011; McClean et al., 2011) and modulating LTP and synaptic plasticity (Kobayashi et al., 2013; McClean et al., 2010) through glia‐mediated mechanisms. Fittingly, GLP‐1R agonists are presently being investigated as a means to improve cognitive function, reduce depressive behaviours and, as a potential treatment for alcohol and drug abuse (Erreger et al., 2012; Graham et al., 2013; Hayes et al., 2014; Hsu et al., 2015; Isacson et al., 2011; Shirazi, Dickson, & Skibicka, 2013; Skibicka, 2013; Sorensen et al., 2015; Wang et al., 2010). This brief review highlights recent evidence exploring the neuroprotective potential of GLP‐1R agonists in neuroinflammation and neurologic disorders, and the contribution of GLP‐1R signalling in astrocytes as a putative means to regulate ingestive behaviour and body weight. Where relevant, discussions involve exploring the role glia plays in mediating the physiological effects of central GLP‐1 signalling, the limitations of existing studies and the ongoing challenges facing the GLP‐1 field in the effort to understand GLP‐1–glia interactions.
2. ROLE OF GLIA IN CNS TRAFFICKING GLP‐1R LIGANDS
Astrocytes occupy a strategic position between the capillary endothelial cells and neurons to help form the blood–brain barrier (BBB). Astrocytes that contribute perivascular endfeet to the BBB have a unique role in ionic, amino acid, neurotransmitter, neuropeptide and water homeostasis in the brain. This subset of astrocytes are ideally positioned to detect circulating neuroendocrine signals, pharmacological ligands and modulate the processing of neural circuitries relevant to energy balance (Hermann & Rogers, 2009; J. G. Kim et al., 2014; McDougal, Hermann, & Rogers, 2013; McDougal, Viard, et al., 2013). Another intriguing but understudied glial population relevant to CNS ligand trafficking are tanycytes. Tanycytes are specialized, polarized ependymocytes that line the floor of the third ventricle in the median eminence and the subpostrema subnuclei that connects the area postrema (AP) to the nucleus tractus solitarius (NTS) in the caudal brainstem (Guillebaud et al., 2017; Langlet et al., 2013; Liberini et al., 2020; Prevot et al., 2013). These unique cells allow for trafficking of circulating signals relevant to food intake and energy balance control to adjacent neurons in the basal hypothalamic arcuate nucleus and the AP/NTS, respectively.
A subset of astrocytes and tanycytes both express the GLP‐1R and the GLP‐1R is internalized along with its ligand following binding (Gabery et al., 2020; Reiner et al., 2016; Secher et al., 2014), suggesting that glial cells facilitate the trafficking of GLP‐1 ligands across the BBB (Gabery et al., 2020). Multiple studies from both pharma and academia have begun to map the neuroanatomical distribution of GLP‐1R agonists in rodent models (Fortin et al., 2020; Gabery et al., 2020; Hernandez et al., 2018; Reiner et al., 2016; Secher et al., 2014). Such studies clearly demonstrate GLP‐1R agonist accumulation in circumventricular nuclei of the AP and median eminence, as well as in adjacent nuclei of the basal hypothalamus and NTS. To a lesser degree, GLP‐1R agonists were also present but more sparsely in distributed in nuclei throughout the brain, depending in part on what GLP‐1R ligand is being analysed (Gabery et al., 2020; Secher et al., 2014). Because many existing GLP‐1R agonists have prolonged half‐lives, often of the order of days, what is understudied is whether ligand distribution changes following acute versus chronic weekly treatment. What is also unknown is whether glia‐facilitated penetration of GLP‐1 ligands into the CNS is altered by chronic GLP‐1R agonist treatment, changes in metabolic and neurodegenerative disease states or with aging in general.
3. CONTRIBUTION OF GLIA IN MEDIATING THE EFFECT OF GLP‐1 ON ENERGY BALANCE
Acknowledging that a subset of glia, including a heterogeneous group of astrocytes, express GLP‐1Rs and/or are responsive to GLP‐1 pharmacology (C. H. Lee et al., 2018; Gong et al., 2014; Reiner et al., 2016; Sterling et al., 2020b; Yun et al., 2018), along with the idea that glia may facilitate the transport of GLP‐1R ligands into specific nuclei of the CNS, necessitates a discussion of the role of glia in controlling energy balance. Although multiple studies have shown that intraparenchymal delivery of GLP‐1R agonists to distributed CNS nuclei suppresses food intake and body weight, modulates reward, and/or produces behavioural measures of malaise (Kanoski et al., 2016), the contribution of glia in mediating GLP‐1R agonist's action has not been investigated in the majority of these nuclei.
Astrocytes are critical for the modulation of l‐glutamic acid in the extracellular space via two subtypes of astrocytic l‐glutamic acid transporters, excitatory amino acid transporter 2 (EAAT2 aka GLT‐1 and SLC1A2) and excitatory amino acid transporter 1 (EAAT1 aka GLAST and SLC1A3) (Danbolt, 2001; Perego et al., 2000). The idea that GLP‐1R ligands may act directly on astrocytes in nuclei that receive glutamatergic inputs relevant to food intake and body weight regulation is supported by circumstantial evidence. The NTS of the dorsal vagal complex (DVC; composed of the NTS, AP and dorsal motor nucleus of the vagus [DMV]) is the first central nucleus to receive and process within‐meal information, vagally mediated glutamatergic signals arising from the gastrointestinal (GI) tract (Grill & Hayes, 2009; Moran, 2006). The DVC expresses the GLP‐1R (Hayes, 2012; Hayes, De Jonghe, & Kanoski, 2010; Merchenthaler et al., 1999; Reiner et al., 2016) and also acts as a critical sensor for circulating endocrine factors and nutrients (Blouet & Schwartz, 2012; Filippi et al., 2012; Hayes, Skibicka, et al., 2010; Huo et al., 2007; Marty et al., 2005; R. C. Ritter et al., 1981; S. Ritter et al., 2006). Not only are axons of GLP‐1 producing preproglucagon (PPG) neurons in close apposition with NTS astrocytes, approximately one third of NTS astrocytes respond to GLP‐1R agonists by intracellular calcium signalling (Reiner et al., 2016). In addition, pharmacological blockade of NTS astrocytes has been shown to attenuate the intake and body weight‐suppressive effects of GLP‐1R agonists (Reiner et al., 2016). It is important to point out that these data do not suggest that astrocytes are the cellular population required for all of the metabolic effects of GLP‐1 signalling but rather are likely to be one of many cellular substrates by which GLP‐1 and GLP‐1R ligands control food intake and body weight. Indeed, even within the NTS, recent reports have clearly indicated that glutamatergic (Adams et al., 2018) and GABAergic (Fortin et al., 2020) neurons expressing GLP‐1Rs are both needed to observe full intake suppression following GLP‐1 ligand delivery. The evergrowing body of literature collectively suggests that multiple cell types, including glial subtypes, in multiple nuclei relevant to energy balance express GLP‐1Rs and facilitate the anorectic response to GLP‐1 ligands. What remains to be determined is the unique mechanisms by which glia modulate neurotransmission to contribute to energy balance control.
Despite the long‐standing appreciation that astrocytes are the most abundant cells within the CNS, only recently have scientists begun to embrace the idea that astrocytes serve a critical role in regulating neuronal excitability and synaptic plasticity (Agulhon et al., 2013; Halassa & Haydon, 2010; J. G. Kim et al., 2014). In fact, a single astrocyte may connect thousands of synapses and, along with presynaptic terminals and postsynaptic neurons, form a tripartite synapse (Araque, Parpura, et al., 1999; Araque, Sanzgiri, et al., 1999; Halassa & Haydon, 2010). Like neurons, astrocytes are activated by neurotransmitters released from presynaptic terminals and gliotransmitters released by other astrocytes. Importantly, astrocytes also express receptors for and are activated by other non‐GLP‐1‐circulating signals of energy availability (e.g. leptin and ghrelin) (Chowen et al., 1999; Iwai et al., 2006; J. G. Kim et al., 2014; Kobayashi et al., 2013; Marina et al., 2017; McDougal, Hermann, & Rogers, 2013; Stein et al., 2020). Astrocytic activation increases calcium signalling, stimulating the release of gliotransmitters such as l‐glutamic acid, ATP and d‐serine (Araque et al., 2001; Coco et al., 2003; Halassa & Haydon, 2010; Mothet et al., 2000; Parpura et al., 1994).
In the case of l‐glutamic acid‐mediated astrocyte–neuron signalling, astrocytes are predominately responsible for the clearance of glutamate from the synapse by EAAT1 and EAAT2 (Danbolt, 2001; Perego et al., 2000). Interestingly, an increase in cAMP signalling within astrocytes reduces the expression of these glutamate transporters (Lim et al., 2005) and enhances synaptic glutamatergic signalling. Consistent with our previous research examining cAMP/PKA signalling in GLP‐1R‐expressing neurons (Hayes et al., 2011), GLP‐1R activation in rat astrocytes results similarly in a dose‐dependent increase in cAMP (Reiner et al., 2016). Likewise, in a coronal brainstem slice preparation, exendin‐4 produced a robust and sustained live Ca++ signalling response in NTS astrocytes (Reiner et al., 2016). In summary, the collective evidence supports the hypothesis that within the NTS, GLP‐1R activation of astrocytes may enhance vagal glutamatergic transmission of GI‐derived satiation signals, possibly through a down‐regulation of synaptic l‐glutamic acid clearance and/or gliotransmission (see Figure 1 for a basic theoretic working model of astrocytic contribution to GLP‐1 signalling in the NTS).
FIGURE 1.
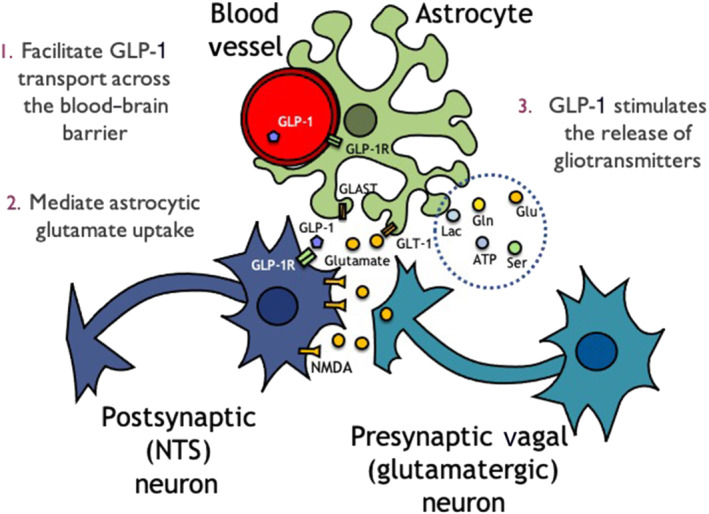
The role of astrocytes providing a supportive role to neurons has been investigated heavily over the past 30 years. What we know now in the context of glucagon‐like peptide‐1 (GLP‐1) signalling is that first, astrocytes could theoretically help facilitate the transport of GLP‐1 across the blood–brain barrier, due to the internalization of the receptor when bound (1). GLP‐1 could modulate synaptic signalling indirectly by regulating astrocytic glutamate transporters GLT‐1 and GLAST (2) or through the release of gliotransmitters (3). GLP‐1R, glucagon‐like peptide‐1 receptor; NTS, nucleus tractus solitarius. Abbreviations, Lac, lactate; Gln, glutamine; Glu, glutamate; Ser, serine
In addition to activating classic downstream intracellular signalling pathways to mediate hypophagic effects (e.g. cAMP/PKA, MAPK, AMPK and Akt) (Hayes et al., 2011; Rupprecht et al., 2013), hindbrain GLP‐1R activation also increases interleukin (IL) signalling (Shirazi, Palsdottir, et al., 2013). Intriguingly, ILs can block the ability of astrocytes to clear l‐glutamic acid from the synapses (Takahashi et al., 2003). Furthermore, cytokine signalling sensitizes vagal afferent signalling (Hermann & Rogers, 2008) and presumably modulates other presynaptic glutamatergic signalling in GLP‐1R‐expressing astrocytes throughout the CNS. Another glia‐specific mechanism that may contribute to GLP‐1R‐mediated suppression of intake and body weight could be microglia GLP‐1R‐mediated increase of brain derived neurotrophic factor (BDNF) expression, as seen in human glia cultures (Spielman et al., 2017). Indeed, BDNF activation of the TrkB receptor has been well characterized to suppress food intake and body weight (B. Xu et al., 2003; Nakagawa et al., 2003; Spaeth et al., 2012; Tsao et al., 2008). In short, a concerted effort is underway to uncover the numerous mechanisms by which glia‐derived GLP‐1R activation could modulate the neuronal excitability in the tripartite synapse to influence energy balance. Discussed in more detail below, it is important to note that the complexity of GLP‐1‐glia signalling with relevance to energy balance control is altered in various energy states such as obesity.
4. GLP‐1 IN NEURODEGENERATIVE DISEASES AND GLAUCOMA
GLP‐1R activation initiates a signalling cascade that inhibits the release of pro‐inflammatory cytokines and astrocyte transformation to a neurotoxic (A1) phenotype, both key contributors to the pathogenesis of Parkinson's and Alzheimer's neurodegeneration (Athauda & Foltynie, 2016). Indeed, an evergrowing body of literature shows that GLP‐1R agonists exert an assortment of anti‐inflammatory effects to induce neuroprotection in multiple in vitro and animal models of Parkinson's and Alzheimer's diseases (see Figure 2). Specifically, GLP‐1R agonists, such as exenatide (aka exendin‐4), liraglutide and lixisenatide, have been shown to prevent dopamine neuronal degeneration in multiple studies utilizing toxin‐induced nigrostriatal degeneration, resulting in improved motor function (Bertilsson et al., 2008; Harkavyi et al., 2008; Li et al., 2009; Liu et al., 2015; S. Kim et al., 2009). GLP‐1 analogues also reduce amyloid β deposition and improve cognition in animal models (Gengler et al., 2012; Hamilton et al., 2011; Han et al., 2013; Hsu et al., 2018; Li et al., 2010; McClean et al., 2010; McGovern et al., 2012; Perry et al., 2003; Porter et al., 2010; Wang et al., 2010). In human trials, exenatide improved both motor and nonmotor deficits in patients with Parkinson's disease (Athauda et al., 2017) and liraglutide improved amyloid β accumulation (Gejl et al., 2016) and cognitive function in Alzheimer's disease (Femminella et al., 2019).
FIGURE 2.
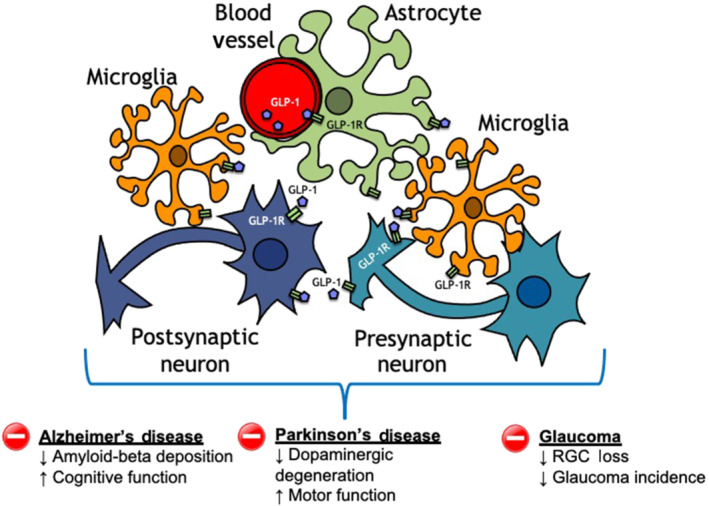
A growing body of preclinical evidence is supporting the hypothesis that glucagon‐like peptide‐1 receptor (GLP‐1R) agonism may be a novel therapeutic tool to treat and/or prevent the onset of multiple neurodegenerative diseases. The accumulating evidence is suggestive of a complex putative multicellular anti‐inflammatory and neuroprotective action of GLP‐1R agonists on neurons, astrocytes and microglia. GLP‐1, glucagon‐like peptide‐1; RGC, retinal ganglion cell
Recently, a novel GLP‐1R agonists, NLY01 (Neuraly, Germantown, MD), prevented neurodegeneration and improved behavioural deficits in a mouse model of Parkinson's disease (Yun et al., 2018). Indeed, NLY01 is a pegylated form of exendin‐4 with a long half‐life in both non‐human primates (88 h) and mice (38 h), where it can efficiently penetrate the BBB resulting in high concentration in the CNS (Yun et al., 2018). In mouse models of Parkinson's disease and in culture, NLY01 was shown to prevent neuron death by reducing IL‐1α, TNF‐α and C1q release from microglia, thereby preventing astrocyte conversion to a neurotoxic (A1) phenotype (Yun et al., 2018). Clinical trials examining the safety and efficacy of NLY01 in treating early Parkinson's and Alzheimer's diseases are ongoing (clinical trial identifiers: NCT04154072 and NCT03672604).
The anti‐inflammatory and neuroprotective effects of GLP‐1R agonists suggest that they may be similarly beneficial in other disease processes with a neurodegenerative component. Glaucoma is an example of such a neurodegenerative disease and is characterized by retinal ganglion cell (RGC) degeneration and optic nerve atrophy, resulting in progressive and permanent loss of vision. It is the leading cause of irreversible blindness worldwide and has been projected to affect more than 100 million people by 2040. Regardless of the glaucoma subtype, all available treatment modalities for glaucoma rely on intraocular pressure (IOP) reduction through either decreased aqueous production or increased outflow. Intraocular pressure lowering, however proves insufficient to prevent disease progression in a significant number of patients and intraocular pressure‐independent treatment options are urgently needed for this blinding disease.
In the retina, intraocular pressure elevation stimulates microglia/macrophages (CD11b+ cells) to produce IL‐1α, TNF‐α and C1q, a trio of pro‐inflammatory cytokines necessary and sufficient to induce A1 astrocyte transformation and retinal ganglia cell death (Guttenplan et al., 2020; Liddelow et al., 2017; Sterling et al., 2020a). Knocking out the cytokine genes or neutralizing antibodies to these cytokines rescued retinal ganglia cells in a microbead‐induced mouse model of acute, hypertensive glaucoma (Guttenplan et al., 2020; Sterling et al., 2020a). In a recent study by our group, we showed that treatment with NLY01 reduced production of all three pro‐inflammatory cytokines by microglia/macrophages in this mouse model of glaucoma (Sterling et al., 2020a). Further, NLY01 treatment prevented A1 astrocyte transformation and rescued retinal ganglia cells in these animals (Sterling et al., 2020a). GLP‐1R agonists reduce blood–retina barrier (BRB) permeability by down‐regulating pro‐inflammatory cytokines and protect tight junctions in rodent models of diabetes (Simo & Hernandez, 2017). However, it is unknown whether NLY01's ability to rescue retinal ganglia cells involves modulating infiltration of myeloid cells through the blood–retina barrier. In support of this possibility, GLP‐1R agonists have been shown to modulate macrophage phenotypes and decrease macrophage infiltration in rodent models of diabetes (Y. S. Lee et al., 2012), atherosclerosis and nephropathy (Y. S. Lee & Jun, 2016), and multiple sclerosis (Chiou et al., 2019). The use of GLP‐1R agonists has also been associated with decreased glaucoma risk in diabetic patients (Sterling et al., 2021). However, because diabetes is an independent risk factor for glaucoma, it is not known whether GLP‐1R agonists directly alter glaucoma progression and/or if this is a secondary outcome of improved glycaemic control following GLP‐1R agonist administration.
In addition to macrophage and microglia, Müller cells are another important class of glia that spans the entire thickness of the retina to provide structural support, establish cellular homeostasis and maintain the blood–retina barrier. Increased glial fibrillary acidic protein (GFAP) expression, signalling Müller activation and gliosis have been demonstrated in animal models of glaucoma and human glaucomatous retinas (Lam et al., 2003; Tezel et al., 2003), whereas decreased l‐glutamic acid uptake by Müller cells resulting in excitotoxicity has been postulated in glaucoma pathogenesis (Kawasaki et al., 2000). Pertinent to this review, GLP‐1R agonists have been shown to exert a beneficial effect in animal models of diabetic retinopathy by limiting Müller reactivity and promoting survival resulting in improved blood–retina barrier integrity (Fan et al., 2014a, 2014b; Ren et al., 2020). Although direct evidence of reduced Müller reactivity following GLP‐1R agonist treatment has not been similarly demonstrated in glaucoma, given the potential pathogenic role of Müller activation in glaucoma pathogenesis, this may be another area in which GLP‐1R agonists are of benefit.
Notably, it remains to be seen whether GLP‐1R agonists are beneficial in models of other forms of glaucoma, either those with chronic and progressive intraocular pressure elevation or for so‐called normotensive glaucoma, where optic degeneration occurs without elevated intraocular pressure. Our group is presently working to answer one of these questions by evaluating the effect of NLY01 in the DBA/2J mouse model of pigmentary glaucoma and chronic, progressive intraocular pressure elevation. Collectively, existing data clearly highlight GLP‐1R agonists as promising drug targets for treating neurodegenerative diseases such as Alzheimer's and hypertensive glaucoma through putative GLP‐1‐glia/macrophage‐mediated mechanisms.
5. THE CHALLENGES IN MOVING THE GLP‐1 FIELD FORWARD IN GLIA RESEARCH
The concept that astrocytes, tanycytes and microglia, in addition to neurons, facilitate the effects mediated by GLP‐1R activation is rapidly gaining traction. We believe that multidisciplinary evaluation of this idea portends widespread implications beyond improving treatments for diabetes and obesity, but must overcome a number of unique challenges, a few of which are highlighted below.
5.1. Multiple neuroscience disciplines have embraced glia as a focus in their research; the obesity and diabetes fields are just joining the fray
The bulk of research on GLP‐1 has focused on the metabolic diseases of obesity and diabetes. To the average diabetologist or obesity expert, the idea that glia contributes to normal glycaemic control or energy balance regulation is likely a foreign concept or, at best, one that is understood as being under investigation. Although the therapeutic potential of GLP‐1's activity on glia has been predominately investigated in nonmetabolic diseases, similar studies are desperately needed in the metabolic field. Investigation into the contribution of glia in modulating neuronal processing of satiety signals is essential, as is investigating glia‐mediated synaptic pruning with regard to neural pathways of relevance to ingestive behaviour. Although GLP‐1 and GLP‐1R ligands clearly act on glia, it is also clear that the obesity and diabetes field has not yet devoted a wealth of resources to the study of these interactions. Nonetheless, investigating the cellular substrates that mediate GLP‐1's latent potential to treat neurological and metabolic diseases through glia‐mediated mechanisms is likely to uncover additional therapeutic targets that can be martialled to treat these same diseases. Although historically the tools have been lacking for interrogating glia in vivo, multiple recent advancements are likely to be of interest to the GLP‐1 research community and should be utilized (Yu et al., 2020).
5.2. Rat versus mouse and possible glia‐specific difficulties with transgenic reporter lines
In the GLP‐1 field, there are notable differences between species in both GLP‐1 physiology and behavioural and metabolic effects produced by GLP‐1 pharmacology (Huo et al., 2008; Lachey et al., 2005; Perez‐Tilve, 2010). Of relevance to this review, GLP‐1R expression on astrocytes may differ between mice (Cork et al., 2015) and rats (Kobayashi et al., 2013; Marina et al., 2017; Mora et al., 1992; Reiner et al., 2016). In light of these differences, a logical follow‐up question would be ‐ which species is the appropriate model(s) for understanding GLP‐1 physiology in humans? Although primary human microglia and astrocytes have been shown to express GLP‐1Rs in culture (Spielman et al., 2017), we believe that these data will require confirmation using additional methods for reasons described in detail below. It is also worth stating that because no reliable or validated antibody for the GLP‐1R is commercially available, progress in basic anatomical approaches has been limited. Instead, as bulk single‐nuclei transcriptomic analyses with 10× technology in human post‐mortem brain tissue become more widely available, we believe this technique will shed light on the question of which animal model best recapitulates human GLP‐1 physiology. At present, however, existing single‐nuclei transcriptomic data throughout the human brain are not available to answer this question.
In the near term, the notion of species differences between mice and rats for CNS GLP‐1R cellular expression may not be as marked as once thought. This is supported by mounting evidence in both mice and rats showing that a subset of glia, including astrocytes, express GLP‐1Rs and/or are responsive to GLP‐1 pharmacology (C. H. Lee et al., 2018; Gong et al., 2014; Reiner et al., 2016; Sterling et al., 2020b; Yun et al., 2018). The confusion in the literature regarding this difference may be traced back to an initial reliance on a Cre recombinase‐based reporter mouse for the GLP‐1R (Cork et al., 2015). Importantly, and not limited to the GLP‐1R‐Cre mouse, a reliance on Cre recombinase can produce false‐positive and false‐negative expressions (Song & Palmiter, 2018). Indeed, in the initial creation of the GLP‐1R–Cre founder strains, Richards et al. (2014) reported that although one founder strain displayed expected GLP‐1R expression, the other strain showed sparse expression in pancreatic islets. This is a clear example of a false‐negative scenario for GLP‐1R–Cre in one of the two founder strains. In the report by Cork et al. (2015), the fact that the heterozygote mouse expressing Cre recombinase under the Glp1r promoter did not show expression of GLP‐1R on GFAP‐positive cells may be the result of such an unintended false‐negative scenario. Another important consideration when using heterozygote mice is that one cannot rule out possible unknown haploinsufficiency for the CNS GLP‐1R expression. Further, GFAP is only expressed by a subset of astrocytes and should not be relied upon as a ubiquitous marker for astrocytes (Bushong et al., 2002; J. Xu, 2018; Walz & Lang, 1998; Zhang et al., 2019). Collectively, and in line with a growing body of literature in not only rats, but also in mice showing GLP‐1R expression and/or GLP‐1R agonism in glia (C. H. Lee et al., 2018; Gong et al., 2014; Sterling et al., 2020b; Yun et al., 2018), reliance on the Cre reporter line may have lead the field to mistakenly conclude that absence of evidence is equal to evidence of absence with respect to GLP‐1R–glia expression. For each CNS nucleus of interest, a triangulation of analyses in mice, rats and human tissue using immunohistochemistry, in situ hybridization and bulk single‐nuclei RNAseq 10× transcriptomic may be necessary to better delineate GLP‐1R expression and understand putative direct actions of GLP‐1 on glia.
5.3. The potential implications of intraparenchymal injections on macrophage recruitment
Microglia are resident macrophages of the CNS and are unique among macrophages in that they self‐renew from their original yolk sac lineage in adulthood (Ajami et al., 2007; Bruttger et al., 2015; Elmore et al., 2014; Epelman et al., 2014; Ginhoux et al., 2010; Hoeffel et al., 2015; Mildner et al., 2007; Sheng et al., 2015). Peripheral macrophages, in contrast, derive not only from the primordial yolk sac (Alliot et al., 1999; Ginhoux & Merad, 2011) but also from foetal monocytes and haematopoietic stem cells (Epelman et al., 2014; Hoeffel et al., 2015; Sheng et al., 2015). After infiltrating the CNS in response to injury, disease and microglia depletion, peripheral macrophages demonstrate morphology and expression profiles similar to resident microglia (Ajami et al., 2011; Bennett et al., 2018; Varvel et al., 2012). Yolk sac‐derived macrophages, in particular, were shown to express many microglia signature genes including Tmem119, Fcrls, Hexb and Olfml3 when injected into the brain of microglia‐deficient mice, complicating efforts to differentiate resident microglia from infiltrating macrophages (Bennett et al., 2018). Nevertheless, peripheral infiltration versus local activation implicates important differences in disease pathogenesis and necessitates accurate characterization. Similarities between macrophages and microglia are of particular concern to the study of GLP‐1 action on glia. The neuroscience field relies heavily on stereotaxically guided intraparenchymal implantation of electrodes, fibre optics, adeno‐associated viruse (AAV)‐mediated transfections and indwelling cannula. All techniques that begin with an experimenter‐induced brain injury in their execution and may potentiate macrophage infiltration. Because GLP‐1Rs are expressed on infiltrating macrophages (Shiraishi et al., 2012), a challenge for the GLP‐1 field will be to differentiate between macrophage‐mediated and glia‐mediated GLP‐1 function when using these approaches.
5.4. In vitro, in situ, in vivo … the devil is in the details when studying GLP‐1‐glia signalling
As research efforts examining GLP‐1 action on astrocytes and microglia intensify, and with respect to experiments looking at the contribution of glia to energy balance control, it is important to remember that glia are an unique and dynamic group of cells in constant states of transcriptomic and morphological flux. In the context of energy balance, study results have clearly shown that perturbations to diet and/or energy states can influence the in situ cytoarchitecture of hypothalamic and brainstem DVC astrocytes, as well as microglia morphology and activity (Fuente‐Martin et al., 2012; Garcia‐Caceres et al., 2011; J. G. Kim et al., 2014; Liberini et al., 2020; MacDonald et al., 2020; Stein et al., 2020). Interpretation of GLP‐1 action on glia is therefore affected by multiple factors that include, but are not limited to, age, diet and energy states (i.e. fasted, fed, overfed and obese) of the animal model or humans under investigation. Further, glia are ‘supporting cells’ of the CNS, and it is necessary to appreciate that when cultured in isolation, their transcriptomes and functions change (Bohlen et al., 2019; Collins & Bohlen, 2018; Gosselin et al., 2017), thus making it difficult to interpret physiological or pharmacological GLP‐1 signalling on glia in vitro. The best approaches for studying the role of glia in mediating GLP‐1 function will be ones that involve various assays to include in situ analyses and, when possible, in vivo physiological and behavioural assessments in addition to in vitro assays.
6. CONCLUSIONS
The old saying ‘less is more’ does not apply to GLP‐1. Indeed, GLP‐1 is a hormonal axis that keeps on giving when it comes to combating not only diabetes and obesity but also potentially other diseases such as neurodegenerative diseases and substance abuse. The more the neuroscience community as a whole investigates GLP‐1 physiology and pharmacology, the more we are likely to be rewarded with insights into the innerworkings of both the peripheral and central GLP‐1 systems. Highlighted here is the emerging literature showing a complex role for glia in putatively trafficking GLP‐1 ligands into the CNS, as well as mediating beneficial effects of GLP‐1R signalling in neuroprotection, reducing oxidative stress and leading to food intake and weight loss suppression. Additional research is needed to interrogate the details through which each of these glia‐mediated mechanisms is targeted and influenced by GLP‐1 pharmacology and to understand how metabolic and neurodegenerative diseases impact the overall glia‐GLP‐1 landscape.
6.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY http://www.guidetopharmacology.org and are permanently archived in the Concise Guide to PHARMACOLOGY 2021/22 (Alexander et al., 2021).
AUTHOR CONTRIBUTIONS
All the authors prepared, edited and approved the final version of the manuscript.
CONFLICT OF INTEREST
M.R.H. receives research funding from Boehringer Ingelheim and Eli Lilly and Company that was not used in support of these studies. M.R.H. is an owner of Cantius Therapeutics, LLC that pursues biological work unrelated to the current study. All other authors have no conflicts of interest to report.
ACKNOWLEDGEMENT
This work was supported by the National Institutes of Health (NIH‐EY029765 [Q.N.C.] and NIH‐DK115762 [M.R.H.]).
Cui, Q. N. , Stein, L. M. , Fortin, S. M. , & Hayes, M. R. (2022). The role of glia in the physiology and pharmacology of glucagon‐like peptide‐1: implications for obesity, diabetes, neurodegeneration and glaucoma. British Journal of Pharmacology, 179(4), 715–726. 10.1111/bph.15683
Funding information National Institutes of Health, Grant/Award Numbers: NIH‐DK115762, NIH‐EY029765
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article because no new data were created or analysed in this study.
REFERENCES
- Adams, J. M. , Pei, H. , Sandoval, D. A. , Seeley, R. J. , Chang, R. B. , Liberles, S. D. , & Olson, D. P. (2018). Liraglutide modulates appetite and body weight through glucagon‐like peptide 1 receptor‐expressing glutamatergic neurons. Diabetes, 67(8), 1538–1548. 10.2337/db17-1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agulhon, C. , Boyt, K. M. , Xie, A. X. , Friocourt, F. , Roth, B. L. , & McCarthy, K. D. (2013). Modulation of the autonomic nervous system and behaviour by acute glial cell Gq protein‐coupled receptor activation in vivo. The Journal of Physiology, 591(Pt 22), 5599–5609. 10.1113/jphysiol.2013.261289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajami, B. , Bennett, J. L. , Krieger, C. , McNagny, K. M. , & Rossi, F. M. (2011). Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nature Neuroscience, 14(9), 1142–1149. 10.1038/nn.2887 [DOI] [PubMed] [Google Scholar]
- Ajami, B. , Bennett, J. L. , Krieger, C. , Tetzlaff, W. , & Rossi, F. M. (2007). Local self‐renewal can sustain CNS microglia maintenance and function throughout adult life. Nature Neuroscience, 10(12), 1538–1543. 10.1038/nn2014 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , & CGTP Collaborators . (2021). The Concise Guide to PHARMACOLOGY 2019/20: Introduction and Other Protein Targets. Br J Pharmacol, 176(Suppl 1), S1–S20. 10.1111/bph.14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliot, F. , Godin, I. , & Pessac, B. (1999). Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Research. Developmental Brain Research, 117(2), 145–152. 10.1016/s0165-3806(99)00113-3 [DOI] [PubMed] [Google Scholar]
- Araque, A. , Carmignoto, G. , & Haydon, P. G. (2001). Dynamic signaling between astrocytes and neurons. Annual Review of Physiology, 63, 795–813. 10.1146/annurev.physiol.63.1.795 [DOI] [PubMed] [Google Scholar]
- Araque, A. , Parpura, V. , Sanzgiri, R. P. , & Haydon, P. G. (1999). Tripartite synapses: Glia, the unacknowledged partner. Trends in Neurosciences, 22(5), 208–215. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10322493 [DOI] [PubMed] [Google Scholar]
- Araque, A. , Sanzgiri, R. P. , Parpura, V. , & Haydon, P. G. (1999). Astrocyte‐induced modulation of synaptic transmission. Canadian Journal of Physiology and Pharmacology, 77(9), 699–706. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10566947 [PubMed] [Google Scholar]
- Athauda, D. , & Foltynie, T. (2016). The glucagon‐like peptide 1 (GLP) receptor as a therapeutic target in Parkinson's disease: Mechanisms of action. Drug Discovery Today, 21(5), 802–818. 10.1016/j.drudis.2016.01.013 [DOI] [PubMed] [Google Scholar]
- Athauda, D. , Maclagan, K. , Skene, S. S. , Bajwa‐Joseph, M. , Letchford, D. , Chowdhury, K. , Hibbert, S. , Budnik, N. , Zampedri, L. , Dickson, J. , Li, Y. , Aviles‐Olmos, I. , Warner, T. T. , Limousin, P. , Lees, A. J. , Greig, N. H. , Tebbs, S. , & Foltynie, T. (2017). Exenatide once weekly versus placebo in Parkinson's disease: A randomised, double‐blind, placebo‐controlled trial. Lancet, 390(10103), 1664–1675. 10.1016/S0140-6736(17)31585-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, F. C. , Bennett, M. L. , Yaqoob, F. , Mulinyawe, S. B. , Grant, G. A. , Hayden Gephart, M. , Plowey, E. D. , & Barres, B. A. (2018). A combination of ontogeny and CNS environment establishes microglial identity. Neuron, 98(6), 1170–1183 e1178. 10.1016/j.neuron.2018.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertilsson, G. , Patrone, C. , Zachrisson, O. , Andersson, A. , Dannaeus, K. , Heidrich, J. , Kortesmaa, J. , Mercer, A. , Nielsen, E. , Rönnholm, H. , & Wikstrom, L. (2008). Peptide hormone exendin‐4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson's disease. Journal of Neuroscience Research, 86(2), 326–338. 10.1002/jnr.21483 [DOI] [PubMed] [Google Scholar]
- Blouet, C. , & Schwartz, G. J. (2012). Brainstem nutrient sensing in the nucleus of the solitary tract inhibits feeding. Cell Metabolism, 16(5), 579–587. 10.1016/j.cmet.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen, C. J. , Bennett, F. C. , & Bennett, M. L. (2019). Isolation and culture of microglia. Current Protocols in Immunology, 125(1), e70. 10.1002/cpim.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruttger, J. , Karram, K. , Wortge, S. , Regen, T. , Marini, F. , Hoppmann, N. , Klein, M. , Blank, T. , Yona, S. , Wolf, Y. , & Mack, M. (2015). Genetic cell ablation reveals clusters of local self‐renewing microglia in the mammalian central nervous system. Immunity, 43(1), 92–106. 10.1016/j.immuni.2015.06.012 [DOI] [PubMed] [Google Scholar]
- Bushong, E. A. , Martone, M. E. , Jones, Y. Z. , & Ellisman, M. H. (2002). Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. The Journal of Neuroscience, 22(1), 183–192. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11756501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou, H. C. , Lin, M. W. , Hsiao, P. J. , Chen, C. L. , Chiao, S. , Lin, T. Y. , Chen, Y. C. , Wu, D. C. , & Lin, M. H. (2019). Dulaglutide modulates the development of tissue‐infiltrating Th1/Th17 cells and the pathogenicity of encephalitogenic Th1 cells in the central nervous system. International Journal of Molecular Sciences, 20(7), 1584. 10.3390/ijms20071584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowen, J. A. , de Fonseca, F. R. , Alvarez, E. , Navarro, M. , Garcia‐Segura, L. M. , & Blazquez, E. (1999). Increased glucagon‐like peptide‐1 receptor expression in glia after mechanical lesion of the rat brain. Neuropeptides, 33(3), 212–215. 10.1054/npep.1999.0757 [DOI] [PubMed] [Google Scholar]
- Coco, S. , Calegari, F. , Pravettoni, E. , Pozzi, D. , Taverna, E. , Rosa, P. , Matteoli, M. , & Verderio, C. (2003). Storage and release of ATP from astrocytes in culture. The Journal of Biological Chemistry, 278(2), 1354–1362. 10.1074/jbc.M209454200 [DOI] [PubMed] [Google Scholar]
- Collins, H. Y. , & Bohlen, C. J. (2018). Isolation and culture of rodent microglia to promote a dynamic ramified morphology in serum‐free medium. Journal of Visualized Experiments, 133, e57122. 10.3791/57122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork, S. C. , Richards, J. E. , Holt, M. K. , Gribble, F. M. , Reimann, F. , & Trapp, S. (2015). Distribution and characterisation of glucagon‐like peptide‐1 receptor expressing cells in the mouse brain. Molecular Metabolism, 4(10), 718–731. 10.1016/j.molmet.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt, N. C. (2001). Glutamate uptake. Progress in Neurobiology, 65(1), 1–105. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11369436 [DOI] [PubMed] [Google Scholar]
- Elmore, M. R. , Najafi, A. R. , Koike, M. A. , Dagher, N. N. , Spangenberg, E. E. , Rice, R. A. , Kitazawa, M. , Matusow, B. , Nguyen, H. , West, B. L. , & Green, K. N. (2014). Colony‐stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron, 82(2), 380–397. 10.1016/j.neuron.2014.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelman, S. , Lavine, K. J. , Beaudin, A. E. , Sojka, D. K. , Carrero, J. A. , Calderon, B. , Brija, T. , Gautier, E. L. , Ivanov, S. , Satpathy, A. T. , Schilling, J. D. , Schwendener, R. , Sergin, I. , Razani, B. , Forsberg, E. C. , Yokoyama, W. M. , Unanue, E. R. , Colonna, M. , Randolph, G. J. , & Mann, D. L. (2014). Embryonic and adult‐derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity, 40(1), 91–104. 10.1016/j.immuni.2013.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger, K. , Davis, A. R. , Poe, A. M. , Greig, N. H. , Stanwood, G. D. , & Galli, A. (2012). Exendin‐4 decreases amphetamine‐induced locomotor activity. Physiology & Behavior, 106(4), 574–578. 10.1016/j.physbeh.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, Y. , Liu, K. , Wang, Q. , Ruan, Y. , Ye, W. , & Zhang, Y. (2014a). Exendin‐4 alleviates retinal vascular leakage by protecting the blood–retinal barrier and reducing retinal vascular permeability in diabetic Goto‐Kakizaki rats. Experimental Eye Research, 127, 104–116. 10.1016/j.exer.2014.05.004 [DOI] [PubMed] [Google Scholar]
- Fan, Y. , Liu, K. , Wang, Q. , Ruan, Y. , Zhang, Y. , & Ye, W. (2014b). Exendin‐4 protects retinal cells from early diabetes in Goto‐Kakizaki rats by increasing the Bcl‐2/Bax and Bcl‐xL/Bax ratios and reducing reactive gliosis. Molecular Vision, 20, 1557–1568. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25489228 [PMC free article] [PubMed] [Google Scholar]
- Femminella, G. D. , Frangou, E. , Love, S. B. , Busza, G. , Holmes, C. , Ritchie, C. , Lawrence, R. , McFarlane, B. , Tadros, G. , Ridha, B. H. , Bannister, C. , Walker, Z. , Archer, H. , Coulthard, E. , Underwood, B. R. , Prasanna, A. , Koranteng, P. , Karim, S. , Junaid, K. , … Edison, P. (2019). Evaluating the effects of the novel GLP‐1 analogue liraglutide in Alzheimer's disease: Study protocol for a randomised controlled trial (ELAD study). Trials, 20(1), 191. 10.1186/s13063-019-3259-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi, B. M. , Yang, C. S. , Tang, C. , & Lam, T. K. (2012). Insulin activates Erk1/2 signaling in the dorsal vagal complex to inhibit glucose production. Cell Metabolism, 16(4), 500–510. 10.1016/j.cmet.2012.09.005 [DOI] [PubMed] [Google Scholar]
- Fortin, S. M. , Lipsky, R. K. , Lhamo, R. , Chen, J. , Kim, E. , Borner, T. , Schmidt, H. D. , & Hayes, M. R. (2020). GABA neurons in the nucleus tractus solitarius express GLP‐1 receptors and mediate anorectic effects of liraglutide in rats. Science Translational Medicine, 12, eaay8071. 10.1126/scitranslmed.aay8071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuente‐Martin, E. , Garcia‐Caceres, C. , Granado, M. , de Ceballos, M. L. , Sanchez‐Garrido, M. A. , Sarman, B. , Liu, Z. W. , Dietrich, M. O. , Tena‐Sempere, M. , Argente‐Arizón, P. , & Díaz, F. (2012). Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. The Journal of Clinical Investigation, 122(11), 3900–3913. 10.1172/JCI64102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabery, S. , Salinas, C. G. , Paulsen, S. J. , Ahnfelt‐Ronne, J. , Alanentalo, T. , Baquero, A. F. , Buckley, S. T. , Farkas, E. , Fekete, C. , Frederiksen, K. S. , & Hogendorf, W. F. (2020). Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight, 5(6). e133429. 10.1172/jci.insight.133429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Caceres, C. , Fuente‐Martin, E. , Burgos‐Ramos, E. , Granado, M. , Frago, L. M. , Barrios, V. , Horvath, T. , Argente, J. , & Chowen, J. A. (2011). Differential acute and chronic effects of leptin on hypothalamic astrocyte morphology and synaptic protein levels. Endocrinology, 152(5), 1809–1818. 10.1210/en.2010-1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gejl, M. , Gjedde, A. , Egefjord, L. , Moller, A. , Hansen, S. B. , Vang, K. , Rodell, A. , Brændgaard, H. , Gottrup, H. , Schacht, A. , & Møller, N. (2016). In Alzheimer's disease, 6‐month treatment with GLP‐1 analog prevents decline of brain glucose metabolism: Randomized, placebo‐controlled, double‐blind clinical trial. Frontiers in Aging Neuroscience, 8, 108. 10.3389/fnagi.2016.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengler, S. , McClean, P. L. , McCurtin, R. , Gault, V. A. , & Holscher, C. (2012). Val(8)GLP‐1 rescues synaptic plasticity and reduces dense core plaques in APP/PS1 mice. Neurobiology of Aging, 33(2), 265–276. 10.1016/j.neurobiolaging.2010.02.014 [DOI] [PubMed] [Google Scholar]
- Ginhoux, F. , Greter, M. , Leboeuf, M. , Nandi, S. , See, P. , Gokhan, S. , Mehler, M. F. , Conway, S. J. , Ng, L. G. , Stanley, E. R. , Samokhvalov, I. M. , & Merad, M. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science, 330(6005), 841–845. 10.1126/science.1194637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux, F. , & Merad, M. (2011). Microglia arise from extra‐embryonic yolk sac primitive progenitors. Medical Science (Paris), 27(8–9), 719–724. 10.1051/medsci/2011278013 [DOI] [PubMed] [Google Scholar]
- Gong, N. , Xiao, Q. , Zhu, B. , Zhang, C. Y. , Wang, Y. C. , Fan, H. , Ma, A. N. , & Wang, Y. X. (2014). Activation of spinal glucagon‐like peptide‐1 receptors specifically suppresses pain hypersensitivity. The Journal of Neuroscience, 34(15), 5322–5334. 10.1523/JNEUROSCI.4703-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin, D. , Skola, D. , Coufal, N. G. , Holtman, I. R. , Schlachetzki, J. C. M. , Sajti, E. , Jaeger, B. N. , O'Connor, C. , Fitzpatrick, C. , Pasillas, M. P. , & Pena, M. (2017). An environment‐dependent transcriptional network specifies human microglia identity. Science, 356(6344). eaal3222. 10.1126/science.aal3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, D. L. , Erreger, K. , Galli, A. , & Stanwood, G. D. (2013). GLP‐1 analog attenuates cocaine reward. Molecular Psychiatry, 18(9), 961–962. 10.1038/mp.2012.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill, H. J. , & Hayes, M. R. (2009). The nucleus tractus solitarius: A portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. International Journal of Obesity, 33(Suppl 1), S11–S15. 10.1038/ijo.2009.10 [DOI] [PubMed] [Google Scholar]
- Guillebaud, F. , Girardet, C. , Abysique, A. , Gaige, S. , Barbouche, R. , Verneuil, J. , Jean, A. , Leprince, J. , Tonon, M. C. , Dallaporta, M. , & Lebrun, B. (2017). Glial endozepines inhibit feeding‐related autonomic functions by acting at the brainstem level. Frontiers in Neuroscience, 11, 308. 10.3389/fnins.2017.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttenplan, K. A. , Stafford, B. K. , El‐Danaf, R. N. , Adler, D. I. , Munch, A. E. , Weigel, M. K. , Huberman, A. D. , & Liddelow, S. A. (2020). Neurotoxic reactive astrocytes drive neuronal death after retinal injury. Cell Reports, 31(12), 107776. 10.1016/j.celrep.2020.107776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa, M. M. , & Haydon, P. G. (2010). Integrated brain circuits: Astrocytic networks modulate neuronal activity and behavior. Annual Review of Physiology, 72, 335–355. 10.1146/annurev-physiol-021909-135843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, A. , Patterson, S. , Porter, D. , Gault, V. A. , & Holscher, C. (2011). Novel GLP‐1 mimetics developed to treat type 2 diabetes promote progenitor cell proliferation in the brain. Journal of Neuroscience Research, 89(4), 481–489. 10.1002/jnr.22565 [DOI] [PubMed] [Google Scholar]
- Han, W. N. , Holscher, C. , Yuan, L. , Yang, W. , Wang, X. H. , Wu, M. N. , & Qi, J. S. (2013). Liraglutide protects against amyloid‐β protein‐induced impairment of spatial learning and memory in rats. Neurobiology of Aging, 34(2), 576–588. 10.1016/j.neurobiolaging.2012.04.009 [DOI] [PubMed] [Google Scholar]
- Harkavyi, A. , Abuirmeileh, A. , Lever, R. , Kingsbury, A. E. , Biggs, C. S. , & Whitton, P. S. (2008). Glucagon‐like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson's disease. Journal of Neuroinflammation, 5, 19. 10.1186/1742-2094-5-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, M. R. (2012). Neuronal and intracellular signaling pathways mediating GLP‐1 energy balance and glycemic effects. Physiology & Behavior, 106(3), 413–416. 10.1016/j.physbeh.2012.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, M. R. , De Jonghe, B. C. , & Kanoski, S. E. (2010). Role of the glucagon‐like‐peptide‐1 receptor in the control of energy balance. Physiology & Behavior, 100(5), 503–510. 10.1016/j.physbeh.2010.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, M. R. , Leichner, T. M. , Zhao, S. , Lee, G. S. , Chowansky, A. , Zimmer, D. , de Jonghe, B. C. , Kanoski, S. E. , Grill, H. J. , & Bence, K. K. (2011). Intracellular signals mediating the food intake‐suppressive effects of hindbrain glucagon‐like peptide‐1 receptor activation. Cell Metabolism, 13(3), 320–330. 10.1016/j.cmet.2011.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, M. R. , Mietlicki‐Baase, E. G. , Kanoski, S. E. , & De Jonghe, B. C. (2014). Incretins and amylin: Neuroendocrine communication between the gut, pancreas, and brain in control of food intake and blood glucose. Annual Review of Nutrition, 34, 237–260. 10.1146/annurev-nutr-071812-161201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, M. R. , Skibicka, K. P. , Leichner, T. M. , Guarnieri, D. J. , DiLeone, R. J. , Bence, K. K. , & Grill, H. J. (2010). Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metabolism, 11(1), 77–83. 10.1016/j.cmet.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann, G. E. , & Rogers, R. C. (2008). TNF α: A trigger of autonomic dysfunction. The Neuroscientist, 14(1), 53–67. 10.1177/1073858407305725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann, G. E. , & Rogers, R. C. (2009). TNF activates astrocytes and catecholaminergic neurons in the solitary nucleus: Implications for autonomic control. Brain Research, 1273, 72–82. 10.1016/j.brainres.2009.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez, N. S. , Ige, K. Y. , Mietlicki‐Baase, E. G. , Molina‐Castro, G. C. , Turner, C. A. , Hayes, M. R. , & Schmidt, H. D. (2018). Glucagon‐like peptide‐1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats. Neuropsychopharmacology, 43(10), 2000–2008. 10.1038/s41386-018-0010-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffel, G. , Chen, J. , Lavin, Y. , Low, D. , Almeida, F. F. , See, P. , Beaudin, A. E. , Lum, J. , Low, I. , Forsberg, E. C. , Poidinger, M. , Zolezzi, F. , Larbi, A. , Ng, L. G. , Chan, J. K. Y. , Greter, M. , Becher, B. , Samokhvalov, I. M. , Merad, M. , & Ginhoux, F. (2015). C‐Myb+ erythro‐myeloid progenitor‐derived fetal monocytes give rise to adult tissue‐resident macrophages. Immunity, 42(4), 665–678. 10.1016/j.immuni.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, T. M. , Hahn, J. D. , Konanur, V. R. , Lam, A. , & Kanoski, S. E. (2015). Hippocampal GLP‐1 receptors influence food intake, meal size, and effort‐based responding for food through volume transmission. Neuropsychopharmacology, 40(2), 327–337. 10.1038/npp.2014.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, T. M. , Noble, E. E. , Liu, C. M. , Cortella, A. M. , Konanur, V. R. , Suarez, A. N. , Reiner, D. J. , Hahn, J. D. , Hayes, M. R. , & Kanoski, S. E. (2018). A hippocampus to prefrontal cortex neural pathway inhibits food motivation through glucagon‐like peptide‐1 signaling. Molecular Psychiatry, 23(7), 1555–1565. 10.1038/mp.2017.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo, L. , Gamber, K. M. , Grill, H. J. , & Bjorbaek, C. (2008). Divergent leptin signaling in proglucagon neurons of the nucleus of the solitary tract in mice and rats. Endocrinology, 149(2), 492–497. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17974623. 10.1210/en.2007-0633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo, L. , Maeng, L. , Bjorbaek, C. , & Grill, H. J. (2007). Leptin and the control of food intake: Neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology, 148(5), 2189–2197. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd%3DRetrieve%26db%3DPubMed%26dopt%3DCitation%26list%5Fuids%3D17317774. 10.1210/en.2006-1572 [DOI] [PubMed] [Google Scholar]
- Isacson, R. , Nielsen, E. , Dannaeus, K. , Bertilsson, G. , Patrone, C. , Zachrisson, O. , & Wikstrom, L. (2011). The glucagon‐like peptide 1 receptor agonist exendin‐4 improves reference memory performance and decreases immobility in the forced swim test. European Journal of Pharmacology, 650(1), 249–255. 10.1016/j.ejphar.2010.10.008 [DOI] [PubMed] [Google Scholar]
- Iwai, T. , Ito, S. , Tanimitsu, K. , Udagawa, S. , & Oka, J. (2006). Glucagon‐like peptide‐1 inhibits LPS‐induced IL‐1β production in cultured rat astrocytes. Neuroscience Research, 55(4), 352–360. 10.1016/j.neures.2006.04.008 [DOI] [PubMed] [Google Scholar]
- Kanoski, S. E. , Hayes, M. R. , & Skibicka, K. P. (2016). Glp‐1 and weight loss: Unraveling the diverse neural circuitry. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 310, R885–R895. 10.1152/ajpregu.00520.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki, A. , Otori, Y. , & Barnstable, C. J. (2000). Muller cell protection of rat retinal ganglion cells from glutamate and nitric oxide neurotoxicity. Investigative Ophthalmology & Visual Science, 41(11), 3444–3450. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11006237 [PubMed] [Google Scholar]
- Kim, J. G. , Suyama, S. , Koch, M. , Jin, S. , Argente‐Arizon, P. , Argente, J. , Liu, Z. W. , Zimmer, M. R. , Jeong, J. K. , Szigeti‐Buck, K. , Gao, Y. , Garcia‐Caceres, C. , Yi, C. X. , Salmaso, N. , Vaccarino, F. M. , Chowen, J. , Diano, S. , Dietrich, M. O. , Tschöp, M. H. , & Horvath, T. L. (2014). Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nature Neuroscience, 17(7), 908–910. 10.1038/nn.3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. , Moon, M. , & Park, S. (2009). Exendin‐4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase‐3 expression in an animal model of Parkinson's disease. The Journal of Endocrinology, 202(3), 431–439. 10.1677/JOE-09-0132 [DOI] [PubMed] [Google Scholar]
- Kobayashi, K. , Iwai, T. , Sasaki‐Hamada, S. , Kamanaka, G. , & Oka, J. (2013). Exendin (5‐39), an antagonist of GLP‐1 receptor, modulates synaptic transmission via glutamate uptake in the dentate gyrus. Brain Research, 1505, 1–10. 10.1016/j.brainres.2013.01.012 [DOI] [PubMed] [Google Scholar]
- Lachey, J. L. , D'Alessio, D. A. , Rinaman, L. , Elmquist, J. K. , Drucker, D. J. , & Seeley, R. J. (2005). The role of central glucagon‐like peptide‐1 in mediating the effects of visceral illness: Differential effects in rats and mice. Endocrinology, 146(1), 458–462. 10.1210/en.2004-0419 [DOI] [PubMed] [Google Scholar]
- Lam, T. T. , Kwong, J. M. , & Tso, M. O. (2003). Early glial responses after acute elevated intraocular pressure in rats. Investigative Ophthalmology & Visual Science, 44(2), 638–645. 10.1167/iovs.02-0255 [DOI] [PubMed] [Google Scholar]
- Langlet, F. , Mullier, A. , Bouret, S. G. , Prevot, V. , & Dehouck, B. (2013). Tanycyte‐like cells form a blood–cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. The Journal of Comparative Neurology, 521(15), 3389–3405. 10.1002/cne.23355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. H. , Jeon, S. J. , Cho, K. S. , Moon, E. , Sapkota, A. , Jun, H. S. , Ryu, J. H. , & Choi, J. W. (2018). Activation of glucagon‐like peptide‐1 receptor promotes neuroprotection in experimental autoimmune encephalomyelitis by reducing neuroinflammatory responses. Molecular Neurobiology, 55(4), 3007–3020. 10.1007/s12035-017-0550-2 [DOI] [PubMed] [Google Scholar]
- Lee, Y. S. , & Jun, H. S. (2016). Anti‐inflammatory effects of GLP‐1‐based therapies beyond glucose control. Mediators of Inflammation, 2016, 11, 3094642. 10.1155/2016/3094642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. S. , Park, M. S. , Choung, J. S. , Kim, S. S. , Oh, H. H. , Choi, C. S. , Ha, S. Y. , Kang, Y. , Kim, Y. , & Jun, H. S. (2012). Glucagon‐like peptide‐1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia, 55(9), 2456–2468. 10.1007/s00125-012-2592-3 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Duffy, K. B. , Ottinger, M. A. , Ray, B. , Bailey, J. A. , Holloway, H. W. , Tweedie, D. , Perry, T. A. , Mattson, M. P. , Kapogiannis, D. , Sambamurti, K. , Lahiri, D. K. , & Greig, N. H. (2010). GLP‐1 receptor stimulation reduces amyloid‐β peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. Journal of Alzheimer's Disease, 19(4), 1205–1219. 10.3233/JAD-2010-1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Perry, T. , Kindy, M. S. , Harvey, B. K. , Tweedie, D. , Holloway, H. W. , Powers, K. , Shen, H. , Egan, J. M. , Sambamurti, K. , Brossi, A. , Lahiri, D. K. , Mattson, M. P. , Hoffer, B. J. , Wang, Y. , & Greig, N. H. (2009). GLP‐1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and parkinsonism. Proceedings of the National Academy of Sciences of the United States of America, 106(4), 1285–1290. 10.1073/pnas.0806720106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberini, C. G. , Ghidewon, M. , Ling, T. , Lhamo, R. , Juntereal, N. , Stein, L. M. , & Hayes, M. R. (2020). Early life overnutrition impairs plasticity of non‐neuronal brainstem cells and drives obesity in offspring across development in rats. International Journal of Obesity, 44(12), 2405–2418. 10.1038/s41366-020-00658-5 [DOI] [PubMed] [Google Scholar]
- Liddelow, S. A. , Guttenplan, K. A. , Clarke, L. E. , Bennett, F. C. , Bohlen, C. J. , Schirmer, L. , Bennett, M. L. , Münch, A. E. , Chung, W. S. , Peterson, T. C. , Wilton, D. K. , Frouin, A. , Napier, B. A. , Panicker, N. , Kumar, M. , Buckwalter, M. S. , Rowitch, D. H. , Dawson, V. L. , Dawson, T. M. , … Barres, B. A. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 541(7638), 481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, G. , Wang, S. , & Mao, J. (2005). cAMP and protein kinase A contribute to the downregulation of spinal glutamate transporters after chronic morphine. Neuroscience Letters, 376(1), 9–13. 10.1016/j.neulet.2004.11.016 [DOI] [PubMed] [Google Scholar]
- Liu, W. , Jalewa, J. , Sharma, M. , Li, G. , Li, L. , & Holscher, C. (2015). Neuroprotective effects of lixisenatide and liraglutide in the 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine mouse model of Parkinson's disease. Neuroscience, 303, 42–50. 10.1016/j.neuroscience.2015.06.054 [DOI] [PubMed] [Google Scholar]
- MacDonald, A. J. , Holmes, F. E. , Beall, C. , Pickering, A. E. , & Ellacott, K. L. J. (2020). Regulation of food intake by astrocytes in the brainstem dorsal vagal complex. Glia, 68(6), 1241–1254. 10.1002/glia.23774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina, N. , Turovsky, E. , Christie, I. N. , Hosford, P. S. , Hadjihambi, A. , Korsak, A. , Ang, R. , Mastitskaya, S. , Sheikhbahaei, S. , Theparambil, S. M. , & Gourine, A. V. (2017). Brain metabolic sensing and metabolic signaling at the level of an astrocyte. Glia, 66(6), 1185–1199. 10.1002/glia.23283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty, N. , Dallaporta, M. , Foretz, M. , Emery, M. , Tarussio, D. , Bady, I. , Binnert, C. , Beermann, F. , & Thorens, B. (2005). Regulation of glucagon secretion by glucose transporter type 2 (glut2) and astrocyte‐dependent glucose sensors. The Journal of Clinical Investigation, 115(12), 3545–3553. 10.1172/JCI26309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean, P. L. , Gault, V. A. , Harriott, P. , & Holscher, C. (2010). Glucagon‐like peptide‐1 analogues enhance synaptic plasticity in the brain: A link between diabetes and Alzheimer's disease. European Journal of Pharmacology, 630(1–3), 158–162. 10.1016/j.ejphar.2009.12.023 [DOI] [PubMed] [Google Scholar]
- McClean, P. L. , Parthsarathy, V. , Faivre, E. , & Holscher, C. (2011). The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. The Journal of Neuroscience, 31(17), 6587–6594. 10.1523/JNEUROSCI.0529-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal, D. H. , Hermann, G. E. , & Rogers, R. C. (2013). Astrocytes in the nucleus of the solitary tract are activated by low glucose or glucoprivation: Evidence for glial involvement in glucose homeostasis. Frontiers in Neuroscience, 7, 249. 10.3389/fnins.2013.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal, D. H. , Viard, E. , Hermann, G. E. , & Rogers, R. C. (2013). Astrocytes in the hindbrain detect glucoprivation and regulate gastric motility. Autonomic Neuroscience, 175(1–2), 61–69. 10.1016/j.autneu.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern, S. F. , Hunter, K. , & Holscher, C. (2012). Effects of the glucagon‐like polypeptide‐1 analogue (Val8)GLP‐1 on learning, progenitor cell proliferation and neurogenesis in the C57B/16 mouse brain. Brain Research, 1473, 204–213. 10.1016/j.brainres.2012.07.029 [DOI] [PubMed] [Google Scholar]
- Merchenthaler, I. , Lane, M. , & Shughrue, P. (1999). Distribution of pre‐pro‐glucagon and glucagon‐like peptide‐1 receptor messenger RNAs in the rat central nervous system. The Journal of Comparative Neurology, 403(2), 261–280. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9886047. [DOI] [PubMed] [Google Scholar]
- Mildner, A. , Schmidt, H. , Nitsche, M. , Merkler, D. , Hanisch, U. K. , Mack, M. , Heikenwalder, M. , Brück, W. , Priller, J. , & Prinz, M. (2007). Microglia in the adult brain arise from Ly‐6ChiCCR2+ monocytes only under defined host conditions. Nature Neuroscience, 10(12), 1544–1553. 10.1038/nn2015 [DOI] [PubMed] [Google Scholar]
- Mora, F. , Exposito, I. , Sanz, B. , & Blazquez, E. (1992). Selective release of glutamine and glutamic acid produced by perfusion of GLP‐1 (7–36) amide in the basal ganglia of the conscious rat. Brain Research Bulletin, 29(3‐4), 359–361. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1356598 [DOI] [PubMed] [Google Scholar]
- Moran, T. H. (2006). Gut peptide signaling in the controls of food intake. Obesity (Silver Spring), 14(Suppl 5), 250S–253S. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17021376 [DOI] [PubMed] [Google Scholar]
- Mothet, J. P. , Parent, A. T. , Wolosker, H. , Brady, R. O. Jr. , Linden, D. J. , Ferris, C. D. , Rogawski, M. A. , & Snyder, S. H. (2000). d‐serine is an endogenous ligand for the glycine site of the N‐methyl‐d‐aspartate receptor. Proceedings of the National Academy of Sciences of the United States of America, 97(9), 4926–4931. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10781100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, T. , Ogawa, Y. , Ebihara, K. , Yamanaka, M. , Tsuchida, A. , Taiji, M. , Noguchi, H. , & Nakao, K. (2003). Antiobesity and antidiabetic effects of brain‐derived neurotrophic factor in rodent models of leptin resistance. International Journal of Obesity and Related Metabolic Disorders, 27(5), 557–565. 10.1038/sj.ijo.0802265 [DOI] [PubMed] [Google Scholar]
- Parpura, V. , Basarsky, T. A. , Liu, F. , Jeftinija, K. , Jeftinija, S. , & Haydon, P. G. (1994). Glutamate‐mediated astrocyte–neuron signalling. Nature, 369(6483), 744–747. 10.1038/369744a0 [DOI] [PubMed] [Google Scholar]
- Perego, C. , Vanoni, C. , Bossi, M. , Massari, S. , Basudev, H. , Longhi, R. , & Pietrini, G. (2000). The GLT‐1 and GLAST glutamate transporters are expressed on morphologically distinct astrocytes and regulated by neuronal activity in primary hippocampal cocultures. Journal of Neurochemistry, 75(3), 1076–1084. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10936189 [DOI] [PubMed] [Google Scholar]
- Perez‐Tilve, D. (2010). Exendin‐4 increases blood glucose levels acutely in rats by activation of the sympathetic nervous system. American Journal of Physiology. Endocrinology and Metabolism, 298, E1088–E1096. 10.1152/ajpendo.00464.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, T. , Haughey, N. J. , Mattson, M. P. , Egan, J. M. , & Greig, N. H. (2002). Protection and reversal of excitotoxic neuronal damage by glucagon‐like peptide‐1 and exendin‐4. The Journal of Pharmacology and Experimental Therapeutics, 302(3), 881–888. 10.1124/jpet.102.037481 [DOI] [PubMed] [Google Scholar]
- Perry, T. , Lahiri, D. K. , Sambamurti, K. , Chen, D. , Mattson, M. P. , Egan, J. M. , & Greig, N. H. (2003). Glucagon‐like peptide‐1 decreases endogenous amyloid‐β peptide (Aβ) levels and protects hippocampal neurons from death induced by Aβ and iron. Journal of Neuroscience Research, 72(5), 603–612. 10.1002/jnr.10611 [DOI] [PubMed] [Google Scholar]
- Porter, D. W. , Kerr, B. D. , Flatt, P. R. , Holscher, C. , & Gault, V. A. (2010). Four weeks administration of liraglutide improves memory and learning as well as glycaemic control in mice with high fat dietary‐induced obesity and insulin resistance. Diabetes, Obesity & Metabolism, 12(10), 891–899. 10.1111/j.1463-1326.2010.01259.x [DOI] [PubMed] [Google Scholar]
- Prevot, V. , Langlet, F. , & Dehouck, B. (2013). Flipping the tanycyte switch: How circulating signals gain direct access to the metabolic brain. Aging (Albany NY), 5(5), 332–334. 10.18632/aging.100557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner, D. J. , Mietlicki‐Baase, E. G. , McGrath, L. E. , Zimmer, D. J. , Bence, K. K. , Sousa, G. L. , Konanur, V. R. , Krawczyk, J. , Burk, D. H. , Kanoski, S. E. , Hermann, G. E. , Rogers, R. C. , & Hayes, M. R. (2016). Astrocytes regulate GLP‐1 receptor‐mediated effects on energy balance. The Journal of Neuroscience, 36(12), 3531–3540. 10.1523/JNEUROSCI.3579-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, X. , Sun, L. , Wei, L. , Liu, J. , Zhu, J. , Yu, Q. , Kong, H. , & Kong, L. (2020). Liraglutide up‐regulation thioredoxin attenuated Muller cells apoptosis in high glucose by regulating oxidative stress and endoplasmic reticulum stress. Current Eye Research, 45(10), 1283–1291. 10.1080/02713683.2020.1737137 [DOI] [PubMed] [Google Scholar]
- Richards, P. , Parker, H. E. , Adriaenssens, A. E. , Hodgson, J. M. , Cork, S. C. , Trapp, S. , Gribble, F. M. , & Reimann, F. (2014). Identification and characterization of GLP‐1 receptor‐expressing cells using a new transgenic mouse model. Diabetes, 63(4), 1224–1233. 10.2337/db13-1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter, R. C. , Slusser, P. G. , & Stone, S. (1981). Glucoreceptors controlling feeding and blood glucose: Location in the hindbrain. Science, 213(4506), 451–452. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6264602. 10.1126/science.6264602 [DOI] [PubMed] [Google Scholar]
- Ritter, S. , Dinh, T. T. , & Li, A. J. (2006). Hindbrain catecholamine neurons control multiple glucoregulatory responses. Physiology & Behavior, 89(4), 490–500. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd%3DRetrieve%26db%3DPubMed%26dopt%3DCitation%26list%5Fuids%3D16887153. 10.1016/j.physbeh.2006.05.036 [DOI] [PubMed] [Google Scholar]
- Rupprecht, L. E. , Mietlicki‐Baase, E. G. , Zimmer, D. J. , McGrath, L. E. , Olivos, D. R. , & Hayes, M. R. (2013). Hindbrain GLP‐1 receptor‐mediated suppression of food intake requires a PI3K‐dependent decrease in phosphorylation of membrane‐bound Akt. American Journal of Physiology. Endocrinology and Metabolism, 305(6), E751–E759. 10.1152/ajpendo.00367.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secher, A. , Jelsing, J. , Baquero, A. F. , Hecksher‐Sorensen, J. , Cowley, M. A. , Dalboge, L. S. , Hansen, G. , Grove, K. L. , Pyke, C. , Raun, K. , & Schäffer, L. (2014). The arcuate nucleus mediates GLP‐1 receptor agonist liraglutide‐dependent weight loss. The Journal of Clinical Investigation, 124(10), 4473–4488. 10.1172/JCI75276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng, J. , Ruedl, C. , & Karjalainen, K. (2015). Most tissue‐resident macrophages except microglia are derived from fetal hematopoietic stem cells. Immunity, 43(2), 382–393. 10.1016/j.immuni.2015.07.016 [DOI] [PubMed] [Google Scholar]
- Shiraishi, D. , Fujiwara, Y. , Komohara, Y. , Mizuta, H. , & Takeya, M. (2012). Glucagon‐like peptide‐1 (GLP‐1) induces M2 polarization of human macrophages via STAT3 activation. Biochemical and Biophysical Research Communications, 425(2), 304–308. 10.1016/j.bbrc.2012.07.086 [DOI] [PubMed] [Google Scholar]
- Shirazi, R. , Palsdottir, V. , Collander, J. , Anesten, F. , Vogel, H. , Langlet, F. , Jaschke, A. , Schurmann, A. , Prevot, V. , Shao, R. , Jansson, J. O. , & Skibicka, K. P. (2013). Glucagon‐like peptide 1 receptor induced suppression of food intake, and body weight is mediated by central IL‐1 and IL‐6. Proceedings of the National Academy of Sciences of the United States of America, 110(40), 16199–16204. 10.1073/pnas.1306799110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi, R. H. , Dickson, S. L. , & Skibicka, K. P. (2013). Gut peptide GLP‐1 and its analogue, Exendin‐4, decrease alcohol intake and reward. PLoS ONE, 8(4), e61965. 10.1371/journal.pone.0061965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo, R. , & Hernandez, C. (2017). GLP‐1R as a target for the treatment of diabetic retinopathy: Friend or foe? Diabetes, 66(6), 1453–1460. 10.2337/db16-1364 [DOI] [PubMed] [Google Scholar]
- Skibicka, K. P. (2013). The central GLP‐1: Implications for food and drug reward. Frontiers in Neuroscience, 7, 181. 10.3389/fnins.2013.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, A. J. , & Palmiter, R. D. (2018). Detecting and avoiding problems when using the Cre–lox system. Trends in Genetics, 34(5), 333–340. 10.1016/j.tig.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen, G. , Reddy, I. A. , Weikop, P. , Graham, D. L. , Stanwood, G. D. , Wortwein, G. , Galli, A. , & Fink‐Jensen, A. (2015). The glucagon‐like peptide 1 (GLP‐1) receptor agonist exendin‐4 reduces cocaine self‐administration in mice. Physiology & Behavior, 149, 262–268. 10.1016/j.physbeh.2015.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaeth, A. M. , Kanoski, S. E. , Hayes, M. R. , & Grill, H. J. (2012). TrkB receptor signaling in the nucleus tractus solitarius mediates the food intake‐suppressive effects of hindbrain BDNF and leptin. American Journal of Physiology. Endocrinology and Metabolism, 302(10), E1252–E1260. 10.1152/ajpendo.00025.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman, L. J. , Gibson, D. L. , & Klegeris, A. (2017). Incretin hormones regulate microglia oxidative stress, survival and expression of trophic factors. European Journal of Cell Biology, 96(3), 240–253. 10.1016/j.ejcb.2017.03.004 [DOI] [PubMed] [Google Scholar]
- Stein, L. M. , Lhamo, R. , Cao, A. , Workinger, J. , Tinsley, I. , Doyle, R. P. , Grill, H. J. , Hermann, G. E. , Rogers, R. C. , & Hayes, M. R. (2020). Dorsal vagal complex and hypothalamic glia differentially respond to leptin and energy balance dysregulation. Translational Psychiatry, 10(1), 90. 10.1038/s41398-020-0767-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling, J. K. , Adetunji, M. , Guttha, S. , Bargoud, A. , Uyhazi, K. , Ross, A. G. , Dunaief, J. L. , & Cui, Q. N. (2020a). GLP‐1R agonist NLY01 reduces retinal inflammation, astrocyte reactivity, and retinal ganglion cell death secondary to ocular hypertension. Biorxiv. 10.1101/2020.06.18.146720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling, J. K. , Adetunji, M. O. , Guttha, S. , Bargoud, A. R. , Uyhazi, K. E. , Ross, A. G. , Dunaief, J. L. , & Cui, Q. N. (2020b). GLP‐1 receptor agonist NLY01 reduces retinal inflammation and neuron death secondary to ocular hypertension. Cell Reports, 33(5), 108271. 10.1016/j.celrep.2020.108271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling, J. K. , Hua, P. , Dunaief, J. L. , Cui, Q. N. , & VanderBeek, B. L. (2021). Exposure to glucagon‐like peptide 1 receptor (GLP‐1R) agonists reduces glaucoma risk. MedRxiv. 10.1101/2021.01.16.21249949 [DOI] [Google Scholar]
- Takahashi, J. L. , Giuliani, F. , Power, C. , Imai, Y. , & Yong, V. W. (2003). Interleukin‐1β promotes oligodendrocyte death through glutamate excitotoxicity. Annals of Neurology, 53(5), 588–595. 10.1002/ana.10519 [DOI] [PubMed] [Google Scholar]
- Tezel, G. , Chauhan, B. C. , LeBlanc, R. P. , & Wax, M. B. (2003). Immunohistochemical assessment of the glial mitogen‐activated protein kinase activation in glaucoma. Investigative Ophthalmology & Visual Science, 44(7), 3025–3033. 10.1167/iovs.02-1136 [DOI] [PubMed] [Google Scholar]
- Tsao, D. , Thomsen, H. K. , Chou, J. , Stratton, J. , Hagen, M. , Loo, C. , Garcia, C. , Sloane, D. L. , Rosenthal, A. , & Lin, J. C. (2008). TrkB agonists ameliorate obesity and associated metabolic conditions in mice. Endocrinology, 149(3), 1038–1048. 10.1210/en.2007-1166 [DOI] [PubMed] [Google Scholar]
- Varvel, N. H. , Grathwohl, S. A. , Baumann, F. , Liebig, C. , Bosch, A. , Brawek, B. , Thal, D. R. , Charo, I. F. , Heppner, F. L. , Aguzzi, A. , Garaschuk, O. , Ransohoff, R. M. , & Jucker, M. (2012). Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proceedings of the National Academy of Sciences of the United States of America, 109(44), 18150–18155. 10.1073/pnas.1210150109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz, W. , & Lang, M. K. (1998). Immunocytochemical evidence for a distinct GFAP‐negative subpopulation of astrocytes in the adult rat hippocampus. Neuroscience Letters, 257(3), 127–130. 10.1016/s0304-3940(98)00813-1 [DOI] [PubMed] [Google Scholar]
- Wang, X. H. , Li, L. , Holscher, C. , Pan, Y. F. , Chen, X. R. , & Qi, J. S. (2010). Val8‐glucagon‐like peptide‐1 protects against Aβ1–40‐induced impairment of hippocampal late‐phase long‐term potentiation and spatial learning in rats. Neuroscience, 170(4), 1239–1248. 10.1016/j.neuroscience.2010.08.028 [DOI] [PubMed] [Google Scholar]
- Xu, B. , Goulding, E. H. , Zang, K. , Cepoi, D. , Cone, R. D. , Jones, K. R. , Tecott, L. H. , & Reichardt, L. F. (2003). Brain‐derived neurotrophic factor regulates energy balance downstream of melanocortin‐4 receptor. Nature Neuroscience, 6(7), 736–742. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12796784. 10.1038/nn1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. (2018). New insights into GFAP negative astrocytes in calbindin D28k immunoreactive astrocytes. Brain Sciences, 8(8), 143. 10.3390/brainsci8080143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X. , Nagai, J. , & Khakh, B. S. (2020). Improved tools to study astrocytes. Nature Reviews. Neuroscience, 21(3), 121–138. 10.1038/s41583-020-0264-8 [DOI] [PubMed] [Google Scholar]
- Yun, S. P. , Kam, T. I. , Panicker, N. , Kim, S. , Oh, Y. , Park, J. S. , Kwon, S. H. , Park, Y. J. , Karuppagounder, S. S. , Park, H. , Kim, S. , Oh, N. , Kim, N. A. , Lee, S. , Brahmachari, S. , Mao, X. , Lee, J. H. , Kumar, M. , An, D. , … Ko, H. S. (2018). Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease. Nature Medicine, 24(7), 931–938. 10.1038/s41591-018-0051-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Ma, Z. , Zou, W. , Guo, H. , Liu, M. , Ma, Y. , & Zhang, L. (2019). The appropriate marker for astrocytes: Comparing the distribution and expression of three astrocytic markers in different mouse cerebral regions. BioMed Research International, 2019, 9605265. 10.1155/2019/9605265 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no new data were created or analysed in this study.


