Abstract
Type 2 diabetes mellitus and the associated desensitisation of insulin signalling has been identified as a risk factor for progressive neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease and others. Glucagon‐like peptide 1 (GLP‐1) is a hormone that has growth factor‐like and neuroprotective properties. Several clinical trials have been conducted, testing GLP‐1 receptor agonists in patients with Alzheimer's disease, Parkinson's disease or diabetes‐induced memory impairments. The trials showed clear improvements in Alzheimer's disease, Parkinson's disease and diabetic patients. Glucose‐dependent insulinotropic polypeptide/gastric inhibitory peptide (GIP) is the ‘sister’ incretin hormone of GLP‐1. GIP analogues have shown neuroprotective effects in animal models of disease and can improve on the effects of GLP‐1. Novel dual GLP‐1/GIP receptor agonists have been developed that can enter the brain at an enhanced rate. The improved neuroprotective effects of these drugs suggest that they are superior to single GLP‐1 receptor agonists and could provide disease‐modifying care for Alzheimer's disease and Parkinson's disease patients.
LINKED ARTICLES
This article is part of a themed issue on GLP1 receptor ligands (BJP 75th Anniversary). To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v179.4/issuetoc
Keywords: Alzheimer, brain, epilepsy, incretins, inflammation, Parkinson, stroke
Abbreviations
- ADAS‐Cog
Alzheimer's Disease Assessment Scale–Cognitive Subscale
- ADCS‐ADL
Alzheimer's Disease Cooperative Study‐Activities of Daily Living
- 6‐OHDA
6‐hydroxydopamine
- GLP‐1
Glucagon‐like peptide 1
- GIP
Glucose‐dependent insulinotropic polypeptide/gastric inhibitory peptide
- MCAO
Middle cerebral artery occlusion
- MDS
Movement Disorder Society
- SN
Substantia nigra
- UPDRS
Unified Parkinson's Disease Rating Scale
1. INTRODUCTION
Type 2 diabetes mellitus is one of the risk factors for neurodegenerative disorders such as Alzheimer's disease and Parkinson's disease. Numerous patient cohort studies have demonstrated that people with type 2 diabetes mellitus are at a much higher risk of developing Alzheimer's disease (Akimoto et al., 2020; An et al., 2018; Kellar & Craft, 2020; Liu, Hu, et al., 2015) or Parkinson's disease (Athauda & Foltynie, 2016; Hu et al., 2007; Kuan et al., 2017; Rhee et al., 2020; Sergi et al., 2019; Svenningsson et al., 2016) later on in life. As one of the drivers behind this observation, impaired insulin signalling in the brains of people with Alzheimer's disease or Parkinson's disease had been identified (Athauda & Foltynie, 2016; Cheong et al., 2020; Craft, 2005; Freiherr et al., 2013; Hölscher, 2011; Moroo et al., 1994; Steen et al., 2005; Talbot et al., 2012). As early as the 1970s, Hoyer and colleagues described impaired glucose metabolism and energy turnover as a key feature of Alzheimer's disease, but it had been ignored as the finding did not fit in with the amyloid hypothesis that dominated Alzheimer's disease research until recently (Hoyer, 1998, 2004; Hoyer et al., 1988). Importantly, insulin signalling impairments in the brain has also been described in people who do not have type 2 diabetes mellitus (Craft, 2005; Steen et al., 2005; Talbot, 2014). An important driver of insulin desensitisation in these cases is most likely the chronic inflammation response that is always present in the brains of people with Alzheimer's disease (Akiyama et al., 2000; Clark & Vissel, 2014), Parkinson's disease (Hirsch & Hunot, 2009; Tansey & Goldberg, 2010), stroke (Endres et al., 2008), epilepsy (Varvel et al., 2016), Huntington chorea (Ghasemi, Dargahi, et al., 2013) and others. During the chronic inflammation response, microglia will release pro‐inflammatory cytokines, which downregulate growth factor signalling (Clark et al., 2012; Hölscher, 2020b; Santos & Ferreira, 2018).
In the brain, insulin acts as a growth factor and is critical for energy metabolism, gene expression, cell growth, cell repair, synaptic activity, inhibition of apoptosis and other key processes that keep neurons healthy and functional (Frolich et al., 1999; Ghasemi, Haeri, et al., 2013; Hölscher, 2014; Schubert et al., 2003; Talbot et al., 2012). It is therefore easy to see why continuous impairment of insulin signalling in the brain will increase the risk for developing neurodegenerative disorders.
2. NASAL APPLICATION OF INSULIN IMPROVES SYMPTOMS IN ALZHEIMER'S DISEASE
In order to test the hypothesis that normalising insulin signalling in the brain has beneficial effects in the brain in Alzheimer's disease, Craft and colleagues conducted a series of clinical trials in which patients were given insulin via a nasal spray (Kellar & Craft, 2020). The rationale behind this is that it is not possible to give insulin i.v. or subcutaneously (s.c.) to non‐diabetic people, which would reduce peripheral glucose levels and cause a hypoglycaemic shock. To avoid this, the insulin is applied by a nasal spray and enters the brain via nasal epithelia with little reaching the peripheral bloodstream (Freiherr et al., 2013). A range of pilot studies consistently showed that insulin or long‐acting insulin analogues improve memory, cognition and uptake of glucose in the brain as shown by 18FDG‐PET imaging, reduced Alzheimer's disease biomarkers in the central nervous system and more (Freiherr et al., 2013; Hölscher, 2020a; Kellar & Craft, 2020). For example, in a 4‐month‐long placebo‐controlled trial of 104 patients with mild to moderate Alzheimer's disease patients, intra‐nasal treatment with 20 IU of insulin daily improved memory and both doses of insulin (20 and 40 IU) preserved general cognition as assessed by the Alzheimer's Disease Assessment Scale–Cognitive Subscale (ADAS‐Cog) score and functional abilities as assessed by the Alzheimer's Disease Cooperative Study‐Activities of Daily Living (ADCS‐ADL) scale. The decrease in 18FDG‐PET uptake in brain scans seen in placebo‐treated patients over time was not visible in the drug‐treated patients. Two months after drug treatment had stopped, the improvements in memory were still present (Craft et al., 2012). Biochemical analyses of exosomes that originate from the brain showed that in patients treated with 20 IU insulin, biomarkers of insulin resistance (pS312‐IRS‐1, pY‐IRS‐1) were improved and showed strong positive correlations with ADAS‐Cog changes (Mustapic et al., 2019). This result is a proof of concept that overcoming insulin resistance in the brain does indeed reduce disease progression in Alzheimer's disease and that this is a viable target for research.
3. INSULIN SIGNALLING IS IMPAIRED IN THE BRAINS OF PARKINSON'S DISEASE PATIENTS
Type 2 diabetes has been identified as a risk factor for Parkinson's disease, too. A large‐scale cohort analysis of patient databases showed that the presence of diabetes increased the risk of Parkinson's disease regardless of co‐morbidities such as cardiovascular, cerebrovascular and chronic kidney diseases (Rhee et al., 2020). In a meta‐analysis of available studies, it was found that type 2 diabetes mellitus can increase progression and likelihood of developing motor and cognitive impairments (Chohan et al., 2021) (see also Cheong et al., 2020; Sergi et al., 2019). The analysis of exosomes derived from the brain demonstrated impaired insulin signalling and treatment with exendin‐4 improved this (Athauda et al., 2019). Measurements of insulin desensitisation in brain tissue showed that in the striatum of Parkinson's disease brains, insulin signalling was found to be impaired (Talbot, 2021). Importantly, a pilot study testing the effects of nasally applied insulin in Parkinson's disease patients showed improvement of Parkinson's disease severity as measured by the modified Hoehn and Yahr scale and Unified Parkinson's Disease Rating Scale (UPDRS)‐Motor (Part III) tests (Novak et al., 2019).
4. ARE ALL DRUGS THAT RESENSITISE INSULIN SIGNALLING NEUROPROTECTIVE?
Treating Alzheimer's disease patients with insulin is not a sensible approach as it can enhance insulin desensitisation and eventually accelerate Alzheimer's disease disease progression. A clinical trial using nasal insulin did show worsening of disease progression in a subgroup, indicating that there is a threshold after which insulin no longer improves disease progression (Claxton et al., 2015). In addition, studies of cohorts of patients taking different medications for treating type 2 diabetes mellitus showed that prolonged use of insulin actually increased the risk of developing Alzheimer's disease (Bohlken et al., 2018). This effect mirrors the observations made in type 2 diabetes mellitus patients that are treated with insulin. Prolonged treatment will eventually lead to insulin desensitisation (Dailey, 2007). Different drugs to treat type 2 diabetes mellitus that can improve insulin sensitivity are on the market. Metformin is a widely prescribed drug that has been tested for its potential to reduce the risk for developing Alzheimer's disease. The results from preclinical studies are mixed (Hölscher, 2020b) and a Phase II clinical trial that tested metformin in Alzheimer's disease patients showed no improvement (Luchsinger et al., 2016). Another very popular and effective drug class is the glucagon‐like peptide 1 (GLP‐1) receptor agonist group that currently has several different drugs on the market for the treatment of type 2 diabetes mellitus (Dhillon, 2018; Müller et al., 2019; Schmidt et al., 2014). Activating the GLP‐1 receptor can resensitise insulin signalling (Campbell & Drucker, 2013; Hölscher, 2019; Long‐Smith et al., 2013; Madsbad et al., 2011; Zhou et al., 2019). In addition, GLP‐1 analogues do not affect blood glucose levels in normoglycaemic people (Wadden et al., 2013) and therefore can be safely given to non‐diabetic patients with chronic neurodegenerative disorders.
5. GLUCAGON‐LIKE PROTEIN 1
GLP‐1 is a peptide hormone containing 30–31 amino acids. It plays important physiological signalling roles to control cell metabolism and energy utilisation (Baggio & Drucker, 2007). The G‐protein‐coupled GLP‐1 receptor belongs to the Class B receptor family. The other receptors for glucagon, the GLP‐2 receptor and the glucose‐dependent insulinotropic polypeptide/gastric inhibitory polypeptide (GIP) receptor belong to the same group. Agonist binding to the receptor activates an adenylyl cyclase, increases IP3 levels and activates the associated classic growth factor second messenger signalling pathways (see Figure 1) (Baggio & Drucker, 2007; Doyle & Egan, 2007; Hölscher, 2020a). The GLP‐1 receptor is expressed on neurons and found in most areas of the brain, indicating that GLP‐1 plays a key signalling role (Cork et al., 2015; Darsalia et al., 2012, 2013; During et al., 2003; Graham et al., 2020; Hamilton & Holscher, 2009; Lee et al., 2011; Li et al., 2009; Merchenthaler et al., 1999; Mora et al., 1992; Teramoto et al., 2011). The receptor is normally not expressed in glial cells at a high level, but receptor densities increase after an inflammation response is triggered in the brain, indicating that it plays a role in controlling inflammation (Chowen et al., 1999; Lee et al., 2011; Ohshima et al., 2015). GLP‐1 is expressed in glial cells and has anti‐inflammatory properties. In fact, GLP‐1 is considered to be an anti‐inflammatory cytokine and reduces release of pro‐inflammatory cytokines (Iwai et al., 2006; Kappe et al., 2012). In patients with type 2 diabetes mellitus, the GLP‐1 mimetic exendin‐4 (exenatide) reduced levels of reactive oxygen species (ROS) and nuclear factor‐κB (NF‐κB) and the mRNA expression of the pro‐inflammatory cytokines TNF‐α and IL‐1ß in mononuclear cells in the blood (Chaudhuri et al., 2012).
FIGURE 1.
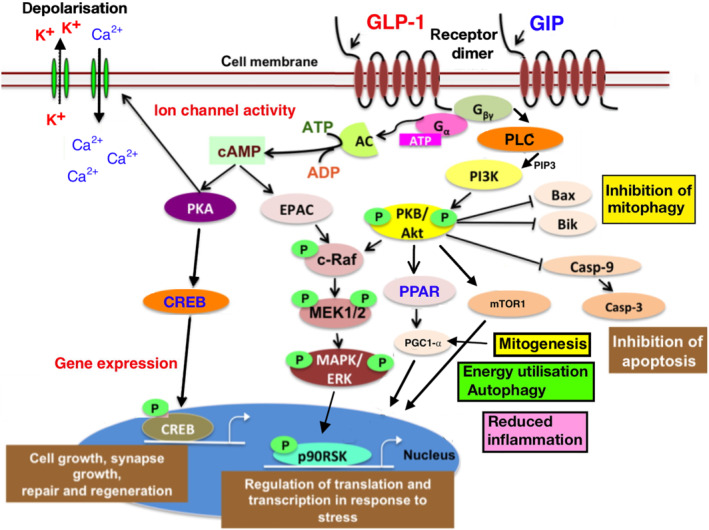
GLP‐1 and GIP induced second messenger cell signalling pathways, which control energy utilisation, mitogenesis, mitophagy, gene expression, autophagy, inhibition of apoptosis, modulation of ion channels, cell growth and repair and the cellular response to oxidative stress. Neuronal function and synaptic activity as well as the inflammation response by microglia is influenced by these processes. Adapted from (Hölscher, 2018). Abbreviations: GLP‐1R, glucagon like peptide 1 receptor; GIPR, glucose‐dependent insulinotropic polypeptide/gastric inhibitory peptide receptor; PKA, protein kinase A; PLC, phospholipase C; PI3K , phosphoinositide 3 kinase; PKB, protein kinase B; AC, adenylate cyclase; EPAC, exchange proteins directly activated by cAMP; MAPK, mitogen‐activated protein kinase; mTOR, mammalian target of rapamycin; ERK, extracellular signal‐regulated kinase; CREB, cyclic AMP response element binding protein; P90RSK, ribosomal S6 kinase; PPAR, peroxisome proliferator‐activated receptor family; MEK1/2, MAPK or ERK kinases; PGC‐1α , peroxisome proliferator‐activated receptor γ coactivator 1‐α; c‐Raf, rapidly accelerated fibrosarcoma (Raf) proto‐oncogene serine/threonine‐protein kinase Mcl1, myeloid cell leukaemia protein‐1; Casp‐9, caspase 9; Casp‐3, caspase 3; Bax, Bik, Bcl2‐interacting killer
6. GLP‐1 MIMETICS HAVE ANTI‐INFLAMMATORY PROPERTIES
Progressive neurodegenerative diseases as well as stroke induce a chronic inflammation response in the brain (Clark & Vissel, 2018; de Oliveira Manoel & Macdonald, 2018; Ferrari & Tarelli, 2011; Lukiw & Bazan, 2000). This secondary downstream process causes further neurodegenerative effects via the activation of immune cells such as microglia in the brain. These cells release pro‐inflammatory cytokines and free radicals such as nitric oxide (NO), which is neurotoxic (Ayasolla et al., 2004). The neurodegenerative effects of chronic inflammation play a major role in disease progression (Arnon & Aharoni, 2009) and research for anti‐inflammatory drugs for such conditions is ongoing (Aisen, 2002; Cole et al., 2004; Griffin, 2008; Lee et al., 2010). It is therefore of great interest to note that GLP‐1 mimetics have anti‐inflammatory properties. Several studies demonstrated that both activated microglia and activated astrocytes, which take part in the immune/inflammation response, induce GLP‐1 receptor expression. GLP‐1 treatment prevented an endotoxin‐induced release of IL‐1β by these cells (Chowen et al., 1999; Iwai et al., 2006; Ohshima et al., 2015). IL‐1ß is pro‐inflammatory and reduces neuronal transmission while increasing apoptosis‐related signalling (Rothwell & Hopkins, 1995). Furthermore, exendin‐4 can reduce monocyte adhesion to the aortic endothelium in an inflammation response in atherosclerosis and also prevents lipopolysaccharide (LPS)‐induced cytokine and chemokine release (Arakawa et al., 2010) and can prevent an increase in microvascular permeability (Dozier et al., 2009). We tested the effects of the GLP‐1 analogue liraglutide in the APP/PS1 mouse model of Alzheimer's disease, which develops a chronic inflammation response in the brain. The liraglutide reduced the numbers of activated microglia and astroglia (McClean et al., 2011; McClean & Holscher, 2014b). As this may be an indirect effect due to the reduction of amyloid in the brain that can reduce the inflammation response, we followed up this study with a second study that measured the effects of liraglutide on inflammation only. X‐ray exposure is known to induce an inflammation response. The expression of pro‐inflammatory cytokines and nitric oxide synthase after x‐ray exposure to the brains of mice was significantly reduced by liraglutide (Parthsarathy & Holscher, 2013b). Furthermore, liraglutide reduced the level of activated micro‐ and astroglia and the levels of the pro‐inflammatory cytokines in an inflammation study induced by intracerebroventricular (i.c.v.) injection of palmitate (Barreto‐Vianna et al., 2017). Another study testing liraglutide in the 5xFAD mouse model of Alzheimer's disease showed clear anti‐inflammatory effects by reducing activated glia levels (Paladugu et al., 2021). Importantly, liraglutide showed clear anti‐inflammatory properties in a primate study where amyloid oligomers had been injected into the cerebral ventricle to induce an inflammatory response. Treatment with liraglutide reduced the inflammation, reduced loss of synapses, improved cognition and re‐sensitised insulin signalling (Batista et al., 2018; Lourenco et al., 2013). In animal models of Parkinson's disease, GLP‐1 receptor agonists show the same anti‐inflammatory properties. In the MPTP mouse model of Parkinson's disease, we and others found that the activation of microglia and the increase of pro‐inflammatory cytokines in the brain were much reduced by GLP‐1 receptor agonists (Feng et al., 2018; Liu, Jalewa, et al., 2015; Zhang et al., 2015, 2018, 2019). In the 6‐hydroxydopamine (6‐OHDA) rat model of Parkinson's disease, we furthermore found a reduction of the inflammation response induced by the toxin (Jalewa et al., 2017; Zhang et al., 2020).
7. GLP‐1 MIMETICS ARE NEUROPROTECTIVE IN ANIMAL MODELS OF ALZHEIMER'S DISEASE
In several rodent models of Alzheimer's disease, GLP‐1 receptor agonists were found to be neuroprotective. The GLP‐1 receptor agonist exendin‐4 (exenatide) showed protective effects in a triple transgenic mouse model that expresses human mutated amyloid beta precursor protein (APP), presenilin‐1 (PSEN1) and microtubule associated protein tau (MAPT) genes that are related to early‐onset Alzheimer's disease and frontotemporal dementia (FTD) (Li et al., 2010). Liraglutide (Victoza) (Courrèges et al., 2008) displayed neuroprotective effects in the tgAPP/PS1 mouse model of Alzheimer's disease. Memory loss, impaired synaptic transmission (long‐term poterntiastion; LTP) in the hippocampus, synapse loss, chronic inflammation in the brain, the amyloid plaque load in the cortex and total amyloid levels in the cortex were much reduced (McClean et al., 2011). In a triple tgAPP/PS1/tau mouse model, liraglutide improved learning and memory, decreased levels of hyperphosphorylated tau and tangles, increased ERK phosphorylation and decreased JNK phosphorylation, both kinases that are involved in inflammation. Liraglutide furthermore decreased the number of degenerative neurons in the hippocampus and cortex (Chen et al., 2017). In other studies, liraglutide had neuroprotective effects in 14‐ to 16‐month‐old APP/PS1 mice, indicating that treatment even at more progressed stages of Alzheimer's disease may still have benefits (McClean & Holscher, 2014a). In a chronic 8‐month‐long study, liraglutide reduced key pathological markers of Alzheimer's disease such as memory impairment, synaptic loss, reduced load of amyloid plaques and chronic inflammation in the brain and therefore has the potential to be used as a prophylactic treatment (McClean et al., 2015). Other studies were able to reproduce the protective effects of liraglutide in mouse models of Alzheimer's disease (Holubova et al., 2018; Parthsarathy & Holscher, 2013a; Qi et al., 2016; Salles et al., 2020). The GLP‐1 receptor agonist lixisenatide (Lyxumia®) has comparable protective effects in the APP/PS1 model (McClean & Holscher, 2014b). Liraglutide furthermore showed protective effects in the APP/PS1/tau Alzheimer's disease model and in a rat model where amyloid is injected into the brain (Cai et al., 2014). One study failed to find neuroprotective effects of liraglutide in two Alzheimer's disease mouse models. The reason for this may be that the study contained a number of flaws. For instance, a transgenic mouse model that expresses the London APP mutation was used, which develops predominately intracellular amyloid aggregates and very few extracellular plaques (Dewachter et al., 2000). Unfortunately, the authors measured only amyloid plaques and no biomarkers for inflammation or for growth factor signalling in this model and found that liraglutide had no effects on the plaque load (Hansen et al., 2016). Liraglutide showed protective effects in the human P301L mutated tau gene‐expressing mouse, a model of frontotemporal lobe dementia. Liraglutide reduced motor impairments and the amount of tangles and hyperphosphorylated tau in the brain (Hansen, Fabricius, et al., 2015). In the accelerated senescence SAMP8 mouse model, liraglutide improved memory formation and reduced neuronal loss in the hippocampus (Hansen, Barkholt, et al., 2015). Liraglutide furthermore improved insulin desensitisation and chronic inflammation in the brain induced by the injection of amyloid oligomers into the cortex of cynomolgus monkeys. The level of synaptic markers was also protected from the effects of amyloid in the brain, indicating that synaptic loss was prevented (Batista et al., 2018; Lourenco et al., 2013). Importantly, GLP‐1 receptor agonists are able to normalise neuronal progenitor cell proliferation and neurogenesis in the hippocampus of mice (During et al., 2003; Hamilton et al., 2011; Hunter & Holscher, 2012; Li et al., 2010; McClean et al., 2011; Parthsarathy & Holscher, 2013a; Porter, Irwin, et al., 2010; Porter, Kerr, et al., 2010). Another important physiological role of GLP‐1 mimetics is that it protects cells against endoplasmic reticulum stress toxicity and autophagy impairments (Panagaki et al., 2017; Sharma et al., 2013).
8. GLP‐1 MIMETICS SHOW PROTECTIVE EFFECTS IN ANIMAL MODELS OF PARKINSON'S DISEASE
The GLP‐1 mimetic exendin‐4 showed good protective effects in several animal models of Parkinson's disease. In the 6‐OHDA lesion model in the rat, the drug protected dopamine neurons and improved motor activity (Bertilsson et al., 2008; Harkavyi et al., 2008). Exendin‐4 had similar protective effects in the MPTP mouse model of Parkinson's disease (Kim et al., 2009; Li et al., 2009). In a separate study, exendin‐4 had good protective effects in the rotenone rat model of Parkinson's disease. Rotenone is a pesticide that can induce Parkinson's disease in humans (Aksoy et al., 2017). Both liraglutide and lixisenatide are protective in the MPTP mouse model of Parkinson's disease. Motor co‐ordination was improved and neurons in the substantia nigra (SN) were protected by both drugs. Pro‐apoptotic mitochondrial BAX/BAD levels were reduced, whereas insulin‐related second messenger signalling was normalised (Liu, Jalewa, et al., 2015). Recently, the long‐acting protease‐resistant GLP‐1 analogue semaglutide (Ozempic®) has been brought to the market as a treatment for type 2 diabetes mellitus (Dhillon, 2018). In the MPTP mouse model of Parkinson's disease, semaglutide was found to have good neuroprotective properties on motor activity, dopamine levels, dopamine neurons in the SN and reducing inflammation as well as the levels of α‐synuclein (Zhang et al., 2018; Zhang et al., 2019). These encouraging preclinical results suggest that GLP‐1 analogues are a viable strategy to treat Parkinson's disease (Bae & Song, 2017; Candeias et al., 2015; Hölscher, 2018; Wiciński et al., 2019).
9. GLP‐1 MIMETICS ARE PROTECTIVE IN ANIMAL MODELS OF EPILEPSY
We tested the GLP‐1 analogue liraglutide in the lithium–pilocarpine animal model of epilepsy. Treatment once daily for 7 days after the induction of epilepsy reduced the chronic inflammation response in the brain as shown by reduced numbers of activated microglia and astrocytes and reduced levels of TNF‐a and IL‐1ß in the hippocampus. The marker for mitochondrial apoptosis BAX (Bcl‐2‐like protein 4) was reduced and the mitochondrial survival factor anti‐apoptotic protein (Bcl‐2) was enhanced by liraglutide (Wang et al., 2018). Another study tested liraglutide in two different animal models of epilepsy, the mouse intrahippocampal kainic acid (KA) model of temporal lobe epilepsy and the WAG/Rij rat model of absence epileptogenesis. Liraglutide reduced the development of spontaneous seizures in the kainate‐induced epilepsy. Memory impairment and anxiety‐like behaviour in the open field were improved. In the forced swim test, liraglutide displayed antidepressant effects. Liraglutide did not modify the epileptogenic process underlying the development of absence seizures in WAG/Rij rats but showed antidepressant in the forced swim test (Citraro et al., 2019).
Another study compared the antiepileptic drug levetiracetam with the effects of liraglutide, either in separate groups or in combination. In the pentylenetetrazol (PTZ) kindling model, levetiracetam had anti‐epileptic properties as expected, but enhanced depressive‐like behaviour in rats. Levetiracetam furthermore induced a pro‐depressant effect and impaired avoidance‐memory retention in non‐ pentylenetetrazol‐treated controls. Liraglutide delayed but did not prevent full epilepsy. The liraglutide prevented the depressive‐like behaviour induced by pentylenetetrazol kindling and by pentylenetetrazol + levetiracetam treatment. The levetiracetam+ liraglutide combination protected against pentylenetetrazol‐induced anxiety and impairments in locomotion and cognition. The levetiracetam + liraglutide combination furthermore had anti‐oxidative and anti‐inflammatory effects and reduced nitrite levels and lipid peroxidation in the brain, while increasing levels of reduced glutathione. Liraglutide on its own or levetiracetam + liraglutide as a combination increased hippocampal brain‐derived neurotrophic factor (BDNF) levels (de Souza et al., 2019). In a separate study testing the effects of liraglutide in the pentylenetetrazol kindling mouse model, pretreatment with liraglutide prevented the seizure severity, normalised behavioural activity and cognition, reduced oxidative stress and altered levels of neurotransmitters such as glutamate, dopamine/noradrenaline and serotonin in mouse brains. The expression of the GLP‐1 receptor in the brain was upregulated, too (Koshal & Kumar, 2016b). The same group tested liraglutide in a different model of epilepsy, the corneal mouse model, where kindling was induced by electrical stimulation. Measuring the same parameters as in their first study, they found the same profile of improvements and neuroprotective effects in the brain (Koshal & Kumar, 2016a). In a Dravet syndrome mouse model, which is a refractory form of epilepsy typically caused by heterozygous mutations of the Scn1a gene for the voltage‐gated sodium channel Nav1.1, liraglutide significantly alleviated seizures recorded in the electroencephalogram (EEG). Cognitive impairments were improved and the number of necrotic neurons in the hippocampus was reduced by the drug. The apoptosis kinase caspase‐3 was downregulated and mTOR activity improved. This demonstrates that apoptosis was reduced and growth factor signalling improved. In addition, mitochondria were protected by lowering BAX levels and enhancing Bcl‐2 levels (Liu et al., 2020). See Koshal et al. (2018) for a review on this subject.
10. GLP‐1 EFFECTS IN STROKE AND REPERFUSION INJURY
A good body of evidence exists in the literature that GLP‐1 receptor agonists have protective effects on the cardiovascular system and on stroke and ischaemia. The anti‐inflammatory properties and the neuroprotective effects of these drugs indicate that these drugs may be useful in treating stroke victims. Exendin‐4 showed good neuroprotection in a transient middle cerebral artery occlusion (MCAO) stroke model in rats. It was found that exendin‐4 reduced the brain area that degenerated after the stroke had been induced. In a functional score of motor activity, the drug‐treated group performed better (Li et al., 2009). In a transient cerebral ischaemia model in gerbils, the effect of exendin‐4 treatment was measured in the hippocampal CA1 region. It was found that GLP‐1 receptor expression was increased after 1 day and GLP‐1 receptor immunoreactivity was found not only in pyramidal neurons but also in astrocytes and GABA interneurons. Exendin‐4 reversed the ischaemia‐induced hyperactivity, reduced neuronal loss and also reduced microglial inflammatory activation in a dose‐dependent manner (Lee et al., 2011). In a rat MCAO stroke reperfusion study, both semaglutide and liraglutide were tested. Liraglutide injected as a bolus reduced brain infarct size by up to 90% and improved neurological scores in a dose‐dependent manner. Semaglutide and liraglutide when administered s.c. reduced the brain infarct size by 63% and 48%, respectively and improved motor scores at 72‐h post‐surgery (Basalay et al., 2019). In diabetic rats, an upregulation of protein level of inducible nitric oxide synthase (iNOS) and NADPH oxidase and a suppression of endothelial nitric oxide synthase (eNOS) expression were found in carotid arteries of diabetic stroke model rats. Lixisenatide was able to reduce the inflammation response and upregulate eNOS expression. The expression of iNOS and NADPH oxidase was reduced and neurological tests showed an improvement in motor skills (Abdel‐Latif et al., 2018). A further study tested the neuroprotective effect of exendin‐4 after focal cerebral ischaemia induction. The drug reduced infarct volume and improved the motor impairment. It also reduced oxidative stress, induction of an inflammation response and neuronal death after reperfusion (Teramoto et al., 2011). In a MCAO stroke study testing the effects of exendin‐4 in diabetic rats, neuronal death in the cortex was much reduced by the drug. Additionally, there were a reduction in microglial infiltration and an increase of stroke‐induced neural stem cell proliferation and neuroblast formation (Darsalia et al., 2012). A separate study confirmed these results (Li et al., 2009). Exendin‐4 furthermore was protective when applied post‐MCAO stroke even in healthy and in diabetic mice. The inflammation response in the brain was reduced, too (Darsalia et al., 2014). Human recombinant GLP‐1 had been tested in the same model and showed similar protective effects (Jiang et al., 2016). A study testing exendin‐4 and liraglutide in a MCAO stroke model in diabetic db/db mice also showed good neuroprotective effects (Li, Liu, Jou, & Wang, 2016). In a study testing the effects of exendin‐4 in a MCAO stroke mouse model, animals were treated in addition with the coagulation inhibitor warfarin. Neurodegeneration by MCAO induce stroke was much reduced and warfarin‐associated haemorrhagic transformation was reduced in the mice, too. Activation of microglia and levels of pro‐inflammatory cytokines in the brain was much reduced by the drug. In addition, the PI3K/Akt/GSK‐3β second messenger signalling cascade that is activated by insulin was functionally improved (Chen et al., 2016). Liraglutide had comparable protective properties in a MCAO stroke rat model. Apoptosis and oxidative stress was reduced in the brain, liraglutide normalised Akt and extracellular signal‐regulated kinases (ERK) activity and kinases associated with inflammation c‐jun‐NH2‐terminal kinase (JNK) and p38 were reduced in activity (Zhu et al., 2016). We tested semaglutide in the MCAO rat model. Semaglutide‐treated animals showed reduced scores of neurological impairments in several motor and grip strength tasks. The cerebral infarction size was reduced and the loss of neurons in the hippocampal areas CA1 and CA3 and the dentate gyrus was much reduced. Chronic inflammation as seen in levels of activated microglia and in the activity of the p38 MAPK/MKK/c‐Jun/NF‐κB p65 inflammation signalling pathway was reduced. In addition, improved growth factor signalling as shown in levels of activated ERK1 and IRS‐1 and a reduction in the apoptosis signalling pathway C‐raf, ERK2, Bcl‐2/BAX and caspase‐3 were observed. Neurogenesis had also been normalised in the dentate gyrus (Yang et al., 2019). Importantly, the effects of GLP‐1 on cardiovascular parameters were found to be independent of blood glucose levels. In a study testing liraglutide along a metformin group in diabetic rats, it was found that metformin did not show comparable neuroprotective properties as liraglutide did, even though both drugs effectively controlled blood glucose levels (Filchenko et al., 2018).
11. CLINICAL TRIALS TESTING CARDIOVASCULAR RISK FACTORS
In a double‐blind, placebo‐controlled clinical trial testing liraglutide in people with type 2 diabetes mellitus and cardiovascular risks (LEADER trial), the effects on cardiovascular events were tested. A total of 9340 patients were observed for 3.8 years. Fewer patients died from cardiovascular causes in the liraglutide group (Marso et al., 2016). In a separate double‐blind, placebo‐controlled clinical trial testing the GLP‐1 receptor agonist dulaglutide (REWIND trial), 9900 people with type 2 diabetes mellitus and cardiovascular risk factors were monitored for 2 years and tested every 6 months for the composite primary outcome of stroke, myocardial infarction or death from cardiovascular or unknown causes. The trial showed reduced risk for developing cardiovascular impairments and as a secondary outcome, the risk of developing cognitive impairment was reduced by 14% by dulaglutide (Cukierman‐Yaffe et al., 2020). For further details on this topic, please consult the reviews (Darsalia et al., 2018; Erbil et al., 2019; Groeneveld et al., 2016; Maskery et al., 2021).
In conclusion, when considering the detailed information on molecular changes induced by GLP‐1 receptor agonists observed in animal studies and the range of neuroprotective properties in stroke and ischaemia found in clinical studies, the evidence is strong that such drugs may be helpful in reducing the cytotoxic effects that evolve in the brain after stroke.
12. CLINICAL TRIALS IN ALZHEIMER'S DISEASE AND PARKINSON'S DISEASE PATIENTS
As there are several GLP‐1 receptor agonists on the market to treat type 2 diabetes mellitus, it is relatively straightforward to test these drugs in the clinic in patients with Alzheimer's disease or Parkinson's disease. Based on the encouraging results of preclinical studies, clinical trials have been conducted or are on the way to investigate the neuroprotective effects of exendin‐4, liraglutide, semaglutide or other GLP‐1 mimetics in Parkinson's disease or Alzheimer's disease patients. First results from clinical trials demonstrate that the preclinical results translate to the clinic.
13. PARKINSON'S DISEASE
A pilot trial testing exendin‐4 (exenatide, Byetta, Bydureon) in Parkinson's disease patients had been conducted. This clinical trial tested exenatide in an open‐label trial in 45 non‐diabetic patients. The average time since diagnosis was 10 years, which means that Parkinson's disease had already progressed. Exendin‐4 was administered for 12 months and patients were retested 2 months after the trial had stopped. Drug‐treated patients showed an improvement of 2.7 points on the Movement Disorder Society (MDS)‐UPDRS test battery of motor activity, whereas control patients declined 2.2 points. In addition, patients were assessed in the Mattis DRS‐2 cognitive test battery, as late‐stage Parkinson's disease patients often develop cognitive impairments, too. There was a clear improvement in the exendin‐4 group, whereas the control group deteriorated rapidly (Aviles‐Olmos et al., 2013). After the trial had finished, patients were tested again 12 months later. The drug group had not deteriorated in motor skill tests or in the cognitive assessment since the beginning of the trial 24 months ago, whereas the control group had deteriorated continuously as expected for Parkinson's disease patients. This demonstrates that the exendin‐4 effect was not short lived as it is with L‐DOPA treatment, but stopped disease progression in particular in cognitive measures (Aviles‐Olmos et al., 2014). However, the pilot study did not incorporate a placebo control group, questioning the validity of the results. Hence, a follow‐up Phase II double‐blind, placebo‐controlled trial had been conducted. These patients were not as progressed in Parkinson's disease and the period since diagnosis was around 6 years. In the MDS‐UPDRS Part 3 test battery, the drug group improved after 48 weeks compared with the placebo group. Twelve weeks after the trial had stopped, patients were retested and the difference between groups was still statistically significant. The outcome confirmed the first pilot study. It showed disease modification by exendin‐4 treatment, as improvements were still visible even when the drug was no longer present in the body. Tests of cerebrospinal fluid (CSF) samples demonstrated that the drug is able to enter the brain and that when people were retested after the trial had finished, no drug had remained in the CSF (Athauda et al., 2017). In order to investigate the underlying mechanism of action, exosomes were analysed from blood plasma. These exosomes originate from the brain. The content of the exosomes showed that drug treatment normalised insulin signalling in neurons, as predicted from preclinical studies. When analysing the levels of the insulin receptor‐activated second messenger cascade by measuring phosphorylated IRS‐1, Akt and mTOR, it was shown that the insulin desensitisation was much reduced by the drug (Athauda et al., 2019). This is a proof of concept that GLP‐1 mimetics can normalise insulin signalling in the brain and modify disease progression.
Several other clinical trials are currently ongoing, testing the drugs lixisenatide (clinical trials identifier NCT03439943), liraglutide (NCT02953665), semaglutide (NCT03659682) and the PEGylated version of exendin‐4 (NLY01) in Parkinson's disease patients (NCT04154072). Further clinical trials are in planning. The group that tested exendin‐4 in a pilot study and a Phase II trial will test exendin‐4 in a larger Phase II trial, testing 200 patients over 2 years and extending the range of biomarkers to be measured (NCT04232969). The company Peptron developed a novel formulation for administering exendin‐4 and plans a clinical trial, testing their product (NCT04269642). The trial will recruit 99 patients and will continue for 60 weeks.
The range of different trial in Parkinson's disease demonstrates that the strategy of activating GLP‐1 receptor in the brain has evolved into a fully‐fledged research and drug development area.
14. ALZHEIMER'S DISEASE
A pilot study testing the effects of liraglutide had been conducted in Alzheimer's disease patients. This double‐blind, randomised, placebo‐controlled trial included memory tests and 18FDG‐PET brain imaging and PIB‐PET imaging to estimate amyloid plaque load (Egefjord et al., 2012). The low number of 38 patients in this trial meant that the trial was underpowered for the cognition tests, which require much higher numbers to reach the statistical power to show a drug effect. Additionally, drug treatment only lasted for 6 months, which is too short for the placebo control group to deteriorate sufficiently to allow a drug effect on disease progression to become visible. However, there was a clear drug effect in the 18FDG‐PET brain scans. Whereas the placebo control group showed a up to 20% of reduced 18FDG‐PET activity, the drug group showed a stable 18FDG‐PET signal over time, with some brain regions even showing higher signals. This demonstrates that glucose utilisation and neuronal activity in the cortex did not deteriorate in the drug group (Gejl et al., 2016). This result is what one would expect from a drug that re‐sensitises insulin signalling in the brain. Another double‐blind placebo‐controlled pilot study testing liraglutide in cognitively impaired patients showed a drug effect in fMRI brain scans after 1 year of treatment. Brain activity and the connection between different active brain areas were reduced in placebo‐treated subjects, but not in the drug‐treated patients, suggesting that the drug prevented disease progression (Watson et al., 2019).
A placebo‐controlled double‐blind Phase II clinical trial has been conducted, testing liraglutide in over 200 MCI/Alzheimer's disease patients for 1 year (ELAD study). It analysed the effects on cognition (ADAScog and ADASexec tests), 18FDG‐PET activity, brain volume changes as measured by MRI brain scans, content of exosomes that originate from the brain and microRNA harvested from blood plasma (Femminella et al., 2019). First results have been published at the CTAD conference in 2020. It was found that liraglutide reduces cognitive impairment in ADASexec tests (P < .001) and that brain temporal lobe volumes shrank less than in the placebo group (P < .001) and total grey matter cortical volume shrank a lot less, too (P = .002), indicating that neuronal loss has been reduced by the drug. Other biochemical marker results have not been published yet (Edison et al., 2020). The result is a proof of concept for the use of GLP‐1 mimetics to treat Alzheimer's disease and clearly demonstrates that liraglutide is neuroprotective in the brain and can reduce disease progression.
In addition, the company Novo Nordisk announced in December 2020 that a Phase III clinical trial in Alzheimer's disease patients will be conducted, testing their GLP‐1 analogue semaglutide in its oral formulation (Rybelsus), which is currently on the market to treat type 2 diabetes mellitus. The trial will recruit 3700 patients and drug treatment will go on for 2 years (https://www.globenewswire.com/news-release/2020/12/16/2146164/0/en/Novo-Nordisk-to-enter-phase-3-development-in-Alzheimer-s-disease-with-oral-semaglutide.html).
15. GLUCOSE‐DEPENDENT INSULINOTROPIC POLYPEPTIDE/GASTRIC INHIBITORY POLYPEPTIDE (GIP)
GIP is the ‘sister’ incretin hormone of GLP‐1 and their physiological roles are closely related (see Figure 1) (Baggio & Drucker, 2007; Finan et al., 2016). It is a 42‐amino acid long peptide hormone that is expressed in a range of cells, including neurons (Nyberg et al., 2007). The GIP receptor is a seven membrane‐spanning G‐protein‐coupled receptor of the glucagon‐type family that enhances cAMP levels when activated (Park et al., 2013). GIP receptor expression has been observed on large neurons such as the pyramidal neurons in the cortex and hippocampus, granule neurons in the dentate gyrus, Purkinje cells in the cerebellum and basal brain areas (Kaplan & Vigna, 1994; Nyberg et al., 2005; Usdin et al., 1993).
16. GIP ANALOGUES ARE PROTECTIVE IN ANIMAL MODELS OF ALZHEIMER'S DISEASE
We have tested protease‐resistant long‐acting GIP analogues in the APP/PS1 mouse model of Alzheimer's disease. D‐Ala2GIP protected learning and memory in 12‐month‐old APP/PS1 mice. Synapse loss was reduced and synaptic plasticity in the hippocampus was protected in electrophysiology studies, whereas saline‐treated mice showed extensive loss of synapses and impaired synaptic plasticity. The amyloid plaque load was reduced by the GIP analogue, too. The activation of microglia and astrocytes in the chronic inflammation response in the brain was diminished by drug treatment, as were oxidative stress and DNA damage (Duffy & Holscher, 2013; Faivre & Holscher, 2013b). In 19‐month‐old APP/PS1 mice, D‐Ala2GIP was still able to reduce synaptic loss and inflammation in APP/PS1 mice and even in wild‐type control animals. Furthermore, the drug was able to enhance synaptic plasticity in the hippocampus of aged APP/PS1 and wild‐type mice, suggesting that loss of synapses can be reversed by the drug (Faivre & Holscher, 2013a). In addition, oxidative stress and DNA damage were reduced also (Duffy & Holscher, 2013). Direct infusion of native GIP into the brain was found to be effective to prevent memory impairments induced by i.c.v. injection of amyloid (Figueiredo et al., 2010). These and other results demonstrate that GIP receptor agonists have similar protective properties as GLP‐1 receptor agonists and that improving GIP signalling in the brain may be protective in Alzheimer's disease, too (Ji, Xue, Li, et al., 2016).
17. GIP ANALOGUES SHOW NEUROPROTECTIVE EFFECTS IN ANIMAL MODELS OF PARKINSON'S DISEASE
Because GLP‐1 and GIP analogues showed good effects in Alzheimer's disease models and GLP‐1 receptor agonists showed protective effects in Parkinson's disease also, we tested long‐acting GIP analogues in animal models of Parkinson's disease. D‐Ala2‐GIP‐glu‐PAL showed good neuroprotective effects in the MPTP mouse model of Parkinson's disease. Motor coordination and grip strength were normalised by the drug, as was tyrosine hydroxylase expression in dopamine neurons in the SN. Synapse numbers were protected from MPTP toxicity, too. MPTP treatment induced a chronic inflammation response and activated microglia and astrocytes and increased levels of pro‐inflammatory cytokines in the brain. Drug treatment reduced the inflammation response and normalised cAMP/PKA/CREB second messenger signalling in the SN, indicating that growth factor signalling had been restored (Li, Liu, Li, & Holscher, 2016). After chronic MPTP treatment, a model of Parkinson's disease that is considered to be more realistic, D‐Ala2‐GIP‐glu‐PAL, was able to improve motor activity, protected dopamine neurons and additionally reduced the increased α‐synuclein levels in the brain. MPTP treatment leads to a much increased expression of this protein. Moreover, drug treatment reduced the chronic inflammation response in the brain, lowered oxidative stress and lipid peroxidation and increased the levels of BDNF (Li et al., 2017). BDNF can protect synapses in a range of neurodegenerative disorders (Allen et al., 2013; Blurton‐Jones et al., 2009; Nagahara & Tuszynski, 2011). Other research groups found very similar effects of D‐Ala2‐GIP in this mouse model of Parkinson's disease. Again, motor activity and dopamine neurons were protected from MPTP toxicity. The drug effect was blocked by the GIP receptor partial antagonist (Pro3)GIP. D‐Ala2GIP furthermore reduced the levels of oxidative stress in the brain. D‐Ala2‐GIP was able to normalise dopamine levels in the striatum (Verma et al., 2017). Another animal model of Parkinson's disease is the 6‐OHDA lesion rat model. Continuous infusion of GIP by an osmotic minipump reduced the 6‐OHDA toxicity and motor impairments were brought back to normal levels (Yu et al., 2018). These findings show that GIP has similar neuroprotective properties as GLP‐1 has and is a promising research area for developing novel treatments of Parkinson's disease (Ji, Xue, Li, et al., 2016; Verma et al., 2018; Zhang & Holscher, 2020).
18. NOVEL DUAL GLP‐1/GIP RECEPTOR AGONISTS THAT CAN CROSS THE BLOOD–BRAIN BARRIER
As GIP and GLP‐1 both have protective effects and work together on a cell signalling level and on physiological levels in a synergistic fashion, novel GLP‐1 and GIP receptor dual agonists have been developed as drug treatment for type 2 diabetes mellitus (Finan et al., 2013). GIP and GLP‐1 receptors are expressed on the same cells and, when activated, can form receptor dimers that show enhanced second messenger signalling or even activate different second messenger cascades (see Figure 1) (Finan et al., 2016; Wellman & Abizaid, 2015). Studies in animals with type 2 diabetes mellitus have shown an added benefit of GIP analogues when added to GLP‐1 analogues to control blood glucose levels (Gault et al., 2011). Several novel dual agonists have been tested in clinical trials in patients with type 2 diabetes mellitus and some show better effects when compared with single GLP‐1 receptor agonists (Finan et al., 2013; Frias et al., 2017, 2018). We previously tested five different GLP‐1/GIP dual agonists that we have named DA1–DA5 (Hölscher, 2018, 2020a). DA1‐JC (NNC0090‐2746) is a dual agonist that has been acetylated with a C16 fatty acid to enhance the biological half‐life in the blood (Finan et al., 2013; Frias et al., 2017). DA2 is the same peptide that has been PEGylated with a 40‐kDa PEGylation added to increase the biological half‐life (Finan et al., 2013). DA3‐CH is the peptide without any modifications (Panagaki et al., 2018). In addition, we have developed two dual agonist peptides with a CPP modification to enhance blood–brain barrier (BBB) penetration (DA4‐JC and DA5‐CH) (Hölscher, 2018, 2020a).
19. IMPORTANCE OF THE BBB IN TREATING CNS DISEASES
The neuroprotective effects of these peptide drugs correlate directly with their ability to cross the BBB. The basis of the neuroprotective activity of these drugs is that they activate GLP‐1 and GIP receptors on neurons and glia of the CNS. We tested the ability of these drugs to cross the BBB by using fluorescent‐labelled peptides in rodents. When comparing all five dual agonists, DA4‐JC and DA5‐CH were the most effective, followed by DA3‐CH, DA1‐JC and DA2, the PEGylated version, hardly crossed the BBB at all. Lipidated peptides such as liraglutide and DA1‐JC showed lower penetration, whereas exendin‐4 showed better BBB penetration that was on the level of DA3‐CH, the unmodified dual agonist peptide (Li et al., 2020; Zhang et al., 2020). A recent study testing 125I radiolabelled peptides confirmed these results and demonstrated that lipidated peptides such as liraglutide, semaglutide and DA1‐JC crossed the BBB only in limited amounts, just like the PEGylated DA2 peptide. DA3‐CH showed better BBB penetration, but DA4‐JC with a poly‐Lys modification crossed the BBB at the highest level (Salameh et al., 2020). The ability of drugs to protect mice from the effects of MPTP was directly correlated with their ability to cross the BBB. In a direct comparison, DA4‐JC and DA5‐CH were superior to DA3‐CH and DA1‐JC, which in turn were superior to liraglutide (Feng et al., 2018; Zhang et al., 2020).
Unfortunately, there are few studies that measure levels of such drugs in the CSF in humans to estimate the BBB penetrations. In the Phase II trial of Bydureon (exendin‐4), CSF analysis showed that the drug does enter the brain readily (Athauda et al., 2017), confirming the rodent studies. In a study measuring liraglutide levels in the CSF of diabetic patients, only low levels were found, which matches the findings in rodent studies (Christensen et al., 2015). More studies will be needed to be able to make firm statements of how such peptide drugs can cross the BBB.
20. DUAL GLP‐1/GIP RECEPTOR AGONISTS ARE PROTECTIVE IN ANIMAL MODELS OF PARKINSON'S DISEASE
In a direct comparison between GLP‐1 analogues, GIP analogues, oxyntomodulin and DA1‐JC, we found that DA1‐JC was best in protecting SH‐SY5Y cells from rotenone stress (Jalewa et al., 2016). The outcome confirms previous studies that demonstrated that GIP can synergistically add to the protective effects of GLP‐1 receptor activation. DA1‐JC showed protective effects in the MPTP mouse model of Parkinson's disease. The motor impairments were reduced and synapses were protected from stress. Dopamine neurons were protected from MPTP toxicity, too. The chronic inflammation in the brain induced by MPTP and BDNF levels in the brain was improved (Cao et al., 2016; Ji, Xue, Lijun, et al., 2016). However, in a direct comparison with single GIP and GLP‐1 receptor agonists, DA1‐JC did not show improved effects (Li, Liu, Li, & Holscher, 2016; Liu, Jalewa, et al., 2015). We then tested DA1‐JC in the 6‐OHDA rat model of Parkinson's disease. DA1‐JC did improve motor activity, however dopamine neurons in the SN were protected from toxicity to some extent. Dopamine levels in the basal ganglia were found to be improved after 6‐OHDA treatment, but did not reach the levels seen in non‐lesioned rats. The levels of glial‐derived neurotrophic factor (GDNF), a protective growth factor for dopamine neurons (Airaksinen & Saarma, 2002), were improved by DA1‐JC, too. Furthermore, Akt and CREB second messenger cell signalling that is associated with growth factor activity had been improved. Autophagy was also normalised by the DA1‐JC (Jalewa et al., 2017). In a direct comparison, DA3‐CH was more effective than liraglutide in the MPTP mouse model of Parkinson's disease. DA3‐CH was superior in motor tests and in protecting dopamine neurons (Yuan et al., 2017). In a follow‐up experiment, liraglutide, DA1‐JC, DA4‐JC and DA5‐CH had been compared at equal doses in the MPTP model. Importantly, the dual agonists DA4‐JC and DA5‐CH that show enhanced crossing of the BBB offered the best neuroprotection. In rotarod motor tests and grip strength tests, DA5‐CH was best in improving motor impairments. Dopamine neurons in the SN were better protected by DA5‐CH and DA4‐JC than by the other peptide drugs. The levels of pro‐inflammatory cytokines were lowest in the animals treated by DA5‐CH,and the levels of GDNF in the brain were increased the most by DA4‐JC. Synapse protection was highest by DA4‐JC and DA5‐CH treatment, whereas DA1‐JC and liraglutide were not very effective (Figure 2) (Feng et al., 2018). In a follow‐up study, in a direct comparison between DA5‐CH and exendin‐4, DA5 was more effective in the MPTP mouse model. When testing different doses, DA5‐CH was effective in protecting the brain at much lower doses compared with exendin‐4 (Zhang et al., 2020). When comparing DA5‐CH with liraglutide at equal doses in the same Parkinson's disease animal model, DA5‐CH was more effective in reducing lipid peroxidation, in reducing the number of apoptotic neurons in the SN and in normalising autophagy in the SN and striatum. Importantly, mitochondria were protected from mitophagy by reducing the Bax/Bcl‐2 ratio (Zhang et al., 2020). In a follow‐up study, testing DA5‐CH in direct comparison with exendin‐4 in the 6‐OHDA rat model of Parkinson's disease, Da5‐CH was more potent in normalising motor activity, increasing dopamine levels in the striatum, reducing the loss of dopamine neurons in the SN pars compacta, reducing the chronic inflammation response and levels of pro‐inflammatory cytokines in the brain, improving mitogenesis and autophagy, normalising insulin signalling and reducing the amount of α‐synuclein (Zhang et al., 2021). As exendin‐4 has already shown good neuroprotective effects in patients with Parkinson's disease (Cheong et al., 2020), the results are most encouraging and suggest that DA5‐CH may be more effective in the clinic.
FIGURE 2.
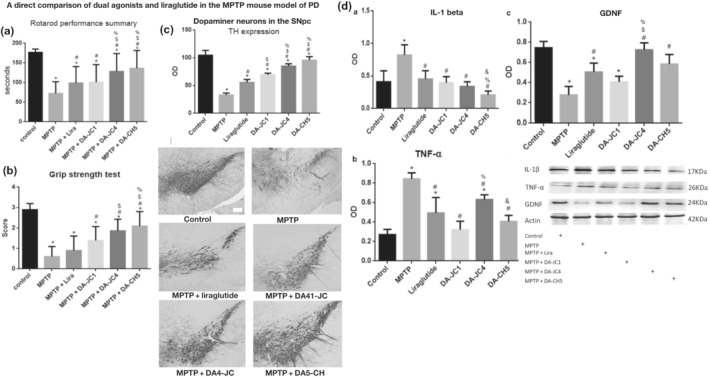
A direct comparison between three dual agonists and liraglutide in the MPTP mouse model of Parkinson's disease (PD). (a) Motor performance in the rotarod test. The dual agonists DA4‐JC and DA5‐CH that can penetrate the BBB best were most effective. *P < .05 compared with controls; # P < .05 compared with the MPTP group; $ P < .05 compared with liraglutide + MPTP group. % P < .05 compared with DA‐JC1 + MPTP group, % P < .05 compared with DA1‐JC + MPTP group. (b): The grip strength test showed the same outcome. *P < .05 compared with controls; # P < .05 compared with MPTP group; $ P < .05 compared to the liraglutide + MPTP group. % P < .05 compared with DA1‐JC + MPTP group. (c) Protection of dopamine neurons in the substantria nigra (SN). Quantification of TH‐positive neurons in the SN shows that the dual agonists DA4‐JC and DA5‐CH were again the most effective. *P < .05 compared with control group; # P < .05 compared to MPTP group; $ P < .05 compared with the Liraglutide group; $ P < .05 compared to the liraglutide group; % P < .05 compared with the DA‐JC1 group; % P < .05 compared with the DA‐JC1 group. Shown are representative images, scale bar = 200 μm. (d) Western blot quantification of pro‐inflammatory cytokines and of the growth factor glial cell derived neurotrophic factor (GDNF) in the brain. (a,b) All peptides tested reduced the pro‐inflammatory cytokines. (c) DA4‐JC was best in normalising GDNF expression in the brain. Adapted from (Feng et al., 2018)
21. DUAL GLP‐1/GIP RECEPTOR AGONISTS ARE PROTECTIVE IN ALZHEIMER'S DISEASE ANIMAL MODELS
In preclinical studies, DA3‐CH showed neuroprotective effects in the APP/PS1 mouse model of Alzheimer's disease. DA3‐CH improved learning and memory of water maze tasks and reduced the amyloid plaque load in the brain. Endoplasmic reticulum stress and autophagy biomarker levels were improved by DA3‐CH, too (Panagaki et al., 2018). In a direct comparison between liraglutide and DA4‐JC, the dual agonist was superior in improving memory formation and long‐term potentiation of synaptic plasticity in the hippocampus of APP/PS1 mice. Furthermore, DA4‐JC was more potent in reducing amyloid plaque levels. Chronic inflammation as shown in microglia activation and levels of pro‐inflammatory cytokines was more potently reduced by DA4‐JC (Figure 3) (Maskery et al., 2020). DA5‐CH also showed good protective effects in the APP/PS1 mouse model. DA5‐CH improved working and spatial memory and lowered the amyloid plaque and phosphorylated tau protein levels in the brain. In electrophysiology recordings, DA5‐CH was able to reverse the impairment of synaptic plasticity (LTP) in the hippocampus. Additionally, DA5‐CH normalised insulin signalling and PI3K and AKT second messenger signalling (Cao et al., 2018). The i.c.v. streptozotocin (STZ) rat model of insulin desensitisation in the brain is considered to be a model of sporadic Alzheimer's disease (Lester‐Coll et al., 2006; Moloney et al., 2010; Steen et al., 2005; Talbot et al., 2012). Enhancing levels of GLP‐1 was effective in reversing insulin desensitisation in this model (Knezovic et al., 2018). When testing DA4‐JC in the streptozotocin model, drug treatment improved memory formation and decreased the levels of phosphorylated tau in the brain. DA4‐JC also reduced the chronic inflammation response. Apoptosis and mitophagy were reduced by DA4‐JC and insulin signalling was re‐sensitised as shown by reduced levels of phospho‐IRS1Ser1101 levels and elevated phospho‐AktSer473 levels in the brain (Shi et al., 2017). In a separate study, DA5‐CH showed good neuroprotective effects in the streptozotocin rat model. Tau phosphorylation in the brain was reduced, insulin signalling normalised and inflammation markers were reduced. In EEG recordings, streptozotocin i.c.v. injection reduced theta rhythm and treatment with DA5‐CH reversed this impairment (Li et al., 2020).
FIGURE 3.
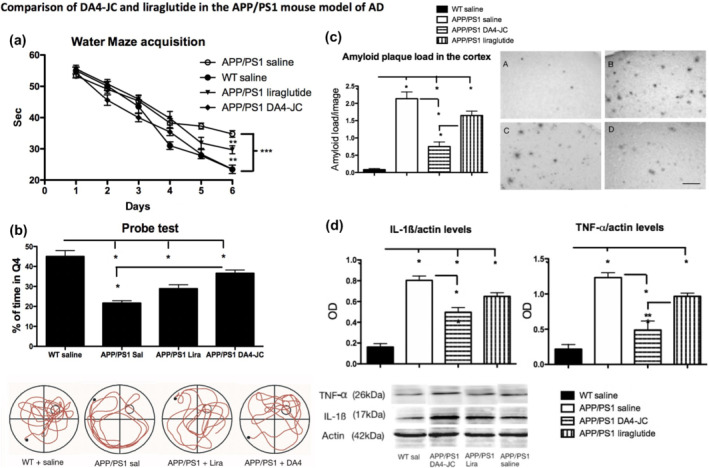
Comparing the effects of the dual agonist DA4‐JC with those of liraglutide side by side in the APP/PS1 model of Alzheimer's disease (AD). (a) Acquisition times for water maze training. The dual agonist improved learning times better than liraglutide. (b) Probe test percentage of target quadrant swim times. In this recall test, DA4‐JC was superior in memory consolidation and recall than liraglutide. Sample swimming tracks are shown below. (c) Quantification of beta‐amyloid plaque loads in the neocortex. DA4‐JC was more effective in reducing the amyloid load compared with liraglutide sample micrographs are shown: A = WT; B = APP/PS1 Sal; C = APP/PS1 lira; D = APP/PS1 DA4‐JC. Scale bar = 50 μm. (d) Quantification of pro‐inflammatory cytokines in the brain. DA4‐JC was more effective in reducing the chronic inflammation response. *P < .05; Sample bands are shown. Adapted from (Maskery et al., 2020)
As liraglutide has already shown protective effects in a Phase II clinical trial in Alzheimer's disease patients, the results reported here suggest that DA4‐JC and DA5‐CH may have superior effects in slowing down disease progression.
22. DUAL GLP‐1/GIP RECEPTOR AGONISTS ARE PROTECTIVE IN STROKE AND EPILEPSY ANIMAL MODELS
We tested the dual agonist DA1‐JC in the MCAO rat reperfusion model and compared it with the GLP‐1 analogue Val(8)‐GLP‐1(glu‐PAL). Drug‐treated groups showed reduced scores of neurological dysfunction, cerebral infarction size and percentage of apoptotic neurons in the brain. In addition, levels of mitophagy marker Bax and the inflammation marker iNOS were reduced, whereas levels of the mitogenesis marker Bcl‐2 was significantly increased. DA1‐JC was more effective in protecting against neurodegeneration than Val(8)‐GLP‐1(glu‐PAL), as measures of neurological dysfunction, cerebral infarction size and expression of Bcl‐2 were improved, whereas the percentage of apoptotic neurons and the levels of Bax and iNOS were lower in the DA1‐JC group (Han et al., 2016).
In the pilocarpine‐induced epileptogenesis rat model, DA3‐CH reduced the activation of microglia and astrocytes and the associated release of the pro‐inflammatory cytokines in the brain. Furthermore, DA3‐CH reduced the levels of the mitochondrial pro‐apoptotic protein Bax while increasing the levels of the anti‐apoptotic protein Bcl‐2. DA3‐CH protected neurons from neurotoxicity in the hippocampus area CA1 as evaluated by quantification of neuronal numbers. These findings in two different models of neurodegenerative disorders demonstrate the dual GLP‐1/GIP agonists have generic neuroprotective properties that suggest that these drugs may be protective in treating these conditions.
23. CONCLUSION
There is good preclinical evidence that GLP‐1 and GIP receptor agonists are neuroprotective in a range of neurodegenerative disorders. The observation that they reduce the chronic inflammation response, normalise insulin and other growth factor signalling, enhance energy utilisation and protect mitochondria can explain why these drugs have protective effects in a diverse range of neurodegenerative disorders that all share these pathological features. Dual GLP‐1/GIP receptor agonists can be more effective than the single receptor agonists and the ability of crossing the BBB is a key parameter that determines their potency. First results from clinical trials in patients with Alzheimer's disease or Parkinson's disease testing GLP‐1 receptor agonists show clear protective effects and are a proof of concept that this research strategy is viable. Improved drugs that are designed to treat CNS diseases and can cross the BBB at a better rate than GLP‐1 receptor agonists that have been developed to remain in the bloodstream for longer times to treat type 2 diabetes mellitus hold promise to be more effective in treating Alzheimer's disease or Parkinson's disease. Dual receptor agonists that are designed to cross the BBB are currently the best available candidates for novel treatments for such neurodegenerative disorders.
23.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY http://www.guidetopharmacology.org and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos, et al., 2019; Alexander, Fabbro, et al., 2019; Alexander, Mathie, et al., 2019).
CONFLICT OF INTEREST
The author is a named inventor on patents and patent application that cover the use of GLP‐1, GIP and dual GLP‐1/GIP receptor agonists as treatments for neurodegenerative disorders. The patents are owned by Ulster University and Lancaster University, UK. He is the CSO of the company Kariya Pharmaceuticals.
24.
Hölscher, C. (2022). Protective properties of GLP‐1 and associated peptide hormones in neurodegenerative disorders. British Journal of Pharmacology, 179(4), 695–714. 10.1111/bph.15508
DATA AVAILABILITY STATEMENT
This is a review and all data included in this paper have been published elsewhere.
REFERENCES
- Abdel‐Latif, R. G. , Heeba, G. H. , Taye, A. , & Khalifa, M. M. A. (2018). Lixisenatide, a novel GLP‐1 analog, protects against cerebral ischemia/reperfusion injury in diabetic rats. Naunyn‐Schmiedeberg's Archives of Pharmacology, 391(7), 705–717. 10.1007/s00210-018-1497-1 [DOI] [PubMed] [Google Scholar]
- Airaksinen, M. S. , & Saarma, M. (2002). The GDNF family: Signalling, biological functions and therapeutic value. Nature Reviews Neuroscience, 3(5), 383–394. 10.1038/nrn812 [DOI] [PubMed] [Google Scholar]
- Aisen, P. S. (2002). The potential of anti‐inflammatory drugs for the treatment of Alzheimer's disease. Lancet Neurology, 1(5), 279–284. 10.1016/S1474-4422(02)00133-3 [DOI] [PubMed] [Google Scholar]
- Akimoto, H. , Negishi, A. , Oshima, S. , Wakiyama, H. , Okita, M. , Horii, N. , Inoue, N. , Ohshima, S. , & Kobayashi, D. (2020). Antidiabetic drugs for the risk of Alzheimer disease in patients with type 2 DM using FAERS. American Journal of Alzheimer's Disease and Other Dementias, 35, 1533317519899546. 10.1177/1533317519899546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama, H. , Barger, S. , Barnum, S. , Bradt, B. , Bauer, J. , Cole, G. M. , Cooper, N. R. , Eikelenboom, P. , Emmerling, M. , Fiebich, B. L. , Finch, C. E. , Frautschy, S. , Griffin, W. S. , Hampel, H. , Hull, M. , Landreth, G. , Lue, L. , Mrak, R. , Mackenzie, I. R. , … Wyss‐Coray, T. (2000). Inflammation and Alzheimer's disease. Neurobiology of Aging, 21(3), 383–421. 10.1016/S0197-4580(00)00124-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy, D. , Solmaz, V. , Cavusoglu, T. , Meral, A. , Ates, U. , & Erbas, O. (2017). Neuroprotective effects of exenatide in a rotenone‐induced rat model of Parkinson's disease. The American Journal of the Medical Sciences, 354(3), 319–324. 10.1016/j.amjms.2017.05.002 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , & CGTP collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176(Suppl 1), S21–S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , & CGTP collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Catalytic receptors. British Journal of Pharmacology, 176(Suppl 1), S247–S296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , & CGTP collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176(Suppl 1), S142–S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, S. J. , Watson, J. J. , Shoemark, D. K. , Barua, N. U. , & Patel, N. K. (2013). GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacology & Therapeutics, 138(2), 155–175. 10.1016/j.pharmthera.2013.01.004 [DOI] [PubMed] [Google Scholar]
- An, Y. , Varma, V. R. , Varma, S. , Casanova, R. , Dammer, E. , Pletnikova, O. , Chia, C. W. , Egan, J. M. , Ferrucci, L. , Troncoso, J. , Levey, A. I. , Lah, J. , Seyfried, N. T. , Legido‐Quigley, C. , O'Brien, R. , & Thambisetty, M. (2018). Evidence for brain glucose dysregulation in Alzheimer's disease. Alzheimer's & Dementia, 14(3), 318–329. 10.1016/j.jalz.2017.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa, M. , Mita, T. , Azuma, K. , Ebato, C. , Goto, H. , Nomiyama, T. , Fujitani, Y. , Hirose, T. , Kawamori, R. , & Watada, H. (2010). Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon‐like peptide‐1 receptor agonist, exendin‐4. Diabetes, 59(4), 1030–1037. 10.2337/db09-1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon, R. , & Aharoni, R. (2009). Neuroprotection and neurogeneration in MS and its animal model EAE effected by glatiramer acetate. Journal of Neural Transmission, 116(11), 1443–1449. 10.1007/s00702-009-0272-3 [DOI] [PubMed] [Google Scholar]
- Athauda, D. , & Foltynie, T. (2016). Insulin resistance and Parkinson's disease: A new target for disease modification? Progress in Neurobiology, 145, 98–120. [DOI] [PubMed] [Google Scholar]
- Athauda, D. , Gulyani, S. , Karnati, H. , Li, Y. , Tweedie, D. , Mustapic, M. , Chawla, S. , Chowdhury, K. , Skene, S. S. , Greig, N. H. , Kapogiannis, D. , & Foltynie, T. (2019). Utility of neuronal‐derived exosomes to examine molecular mechanisms that affect motor function in patients with Parkinson disease: A secondary analysis of the Exenatide‐PD trial. JAMA Neurology, 76(4), 420–429. 10.1001/jamaneurol.2018.4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athauda, D. , Maclagan, K. , Skene, S. S. , Bajwa‐Joseph, M. , Letchford, D. , Chowdhury, K. , Hibbert, S. , Budnik, N. , Zampedri, L. , Dickson, J. , Li, Y. , Aviles‐Olmos, I. , Warner, T. T. , Limousin, P. , Lees, A. J. , Greig, N. H. , Tebbs, S. , & Foltynie, T. (2017). Exenatide once weekly versus placebo in Parkinson's disease: A randomised, double‐blind, placebo‐controlled trial. The Lancet, 390(10103), 1664–1675. 10.1016/S0140-6736(17)31585-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviles‐Olmos, I. , Dickson, J. , Kefalopoulou, Z. , Djamshidian, A. , Ell, P. , Soderlund, T. , Whitton, P. , Wyse, R. , Isaacs, T. , Lees, A. , Limousin, P. , & Foltynie, T. (2013). Exenatide and the treatment of patients with Parkinson's disease. The Journal of Clinical Investigation, 123(6), 2730–2736. 10.1172/JCI68295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviles‐Olmos, I. , Dickson, J. , Kefalopoulou, Z. , Djamshidian, A. , Kahan, J. , Ell, P. , Whitton, P. , Wyse, R. , Isaacs, T. , Lees, A. , Limousin, P. , & Foltynie, T. (2014). Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson's disease. Journal of Parkinson's Disease, 4(3), 337–344. 10.3233/JPD-140364 [DOI] [PubMed] [Google Scholar]
- Ayasolla, K. , Khan, M. , Singh, A. K. , & Singh, I. (2004). Inflammatory mediator and beta‐amyloid (25‐35)‐induced ceramide generation and iNOS expression are inhibited by vitamin E. Free Radical Biology & Medicine, 37(3), 325–338. 10.1016/j.freeradbiomed.2004.04.007 [DOI] [PubMed] [Google Scholar]
- Bae, C. S. , & Song, J. (2017). The role of glucagon‐like peptide 1 (GLP1) in type 3 diabetes: GLP‐1 controls insulin resistance, neuroinflammation and neurogenesis in the brain. International Journal of Molecular Sciences, 18, E2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio, L. L. , & Drucker, D. J. (2007). Biology of incretins: GLP‐1 and GIP. Gastroenterology, 132(6), 2131–2157. 10.1053/j.gastro.2007.03.054 [DOI] [PubMed] [Google Scholar]
- Barreto‐Vianna, A. R. C. , Aguila, M. B. , & Mandarim‐de‐Lacerda, C. A. (2017). Beneficial effects of liraglutide (GLP1 analog) in the hippocampal inflammation. Metabolic Brain Disease, 32(5), 1735–1745. 10.1007/s11011-017-0059-4 [DOI] [PubMed] [Google Scholar]
- Basalay, M. V. , Davidson, S. M. , & Yellon, D. M. (2019). Neuroprotection in rats following Ischaemia‐reperfusion injury by GLP‐1 analogues‐Liraglutide and Semaglutide. Cardiovascular Drugs and Therapy, 333, 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista, A. F. , Forny‐Germano, L. , Clarke, J. R. , Lyra e Silva, N. M. , Brito‐Moreira, J. , Boehnke, S. E. , Winterborn, A. , Coe, B. C. , Lablans, A. , Vital, J. F. , Marques, S. A. , Martinez, A. M. B. , Gralle, M. , Holscher, C. , Klein, W. L. , Houzel, J. C. , Ferreira, S. T. , Munoz, D. P. , & de Felice, F. G. (2018). The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non‐human primate model of Alzheimer's disease. The Journal of Pathology, 245(1), 85–100. 10.1002/path.5056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertilsson, G. , Patrone, C. , Zachrisson, O. , Andersson, A. , Dannaeus, K. , Heidrich, J. , Kortesmaa, J. , Mercer, A. , Nielsen, E. , Rönnholm, H. , & Wikström, L. (2008). Peptide hormone exendin‐4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson's disease. Journal of Neuroscience Research, 86(2), 326–338. 10.1002/jnr.21483 [DOI] [PubMed] [Google Scholar]
- Blurton‐Jones, M. , Kitazawa, M. , Martinez‐Coria, H. , Castello, N. A. , Muller, F. J. , Loring, J. F. , Yamasaki, T. R. , Poon, W. W. , Green, K. N. , & LaFerla, F. M. (2009). Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America, 106(32), 13594–13599. 10.1073/pnas.0901402106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlken, J. , Jacob, L. , & Kostev, K. (2018). Association between the use of antihyperglycemic drugs and dementia risk: A case‐control study. Journal of Alzheimer's Disease, 66(2), 725–732. 10.3233/JAD-180808 [DOI] [PubMed] [Google Scholar]
- Cai, H. Y. , Hölscher, C. , Yue, X. H. , Zhang, S. X. , Wang, X. H. , Qiao, F. , Yang, W. , & Qi, J. S. (2014). Lixisenatide rescues spatial memory and synaptic plasticity from amyloid beta protein‐induced impairments in rats. Neuroscience, 277, 6–13. 10.1016/j.neuroscience.2014.02.022 [DOI] [PubMed] [Google Scholar]
- Campbell, J. E. , & Drucker, D. J. (2013). Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metabolism, 17(6), 819–837. 10.1016/j.cmet.2013.04.008 [DOI] [PubMed] [Google Scholar]
- Candeias, E. M. , Sebastião, I. C. , Cardoso, S. M. , Correia, S. C. , Carvalho, C. I. , Plácido, A. I. , Santos, M. S. , Oliveira, C. R. , Moreira, P. I. , & Duarte, A. I. (2015). Gut‐brain connection: The neuroprotective effects of the anti‐diabetic drug liraglutide. World Journal of Diabetes, 6(6), 807–827. 10.4239/wjd.v6.i6.807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, L. , Li, D. , Feng, P. , Li, L. , Xue, G. , Li, G. , & Hölscher, C. (2016). A novel dual GLP‐1 and GIP incretin receptor agonist is neuroprotective in a mouse model of Parkinson's disease by reducing chronic inflammation in the brain. NeuroReport, 27(6), 384–391. 10.1097/WNR.0000000000000548 [DOI] [PubMed] [Google Scholar]
- Cao, Y. , Hölscher, C. , Hu, M. M. , Wang, T. , Zhao, F. , Bai, Y. , Zhang, J. , Wu, M. N. , & Qi, J. S. (2018). DA5‐CH, a novel GLP‐1/GIP dual agonist, effectively ameliorates the cognitive impairments and pathology in the APP/PS1 mouse model of Alzheimer's disease. European Journal of Pharmacology, 827, 215–226. 10.1016/j.ejphar.2018.03.024 [DOI] [PubMed] [Google Scholar]
- Chaudhuri, A. , Ghanim, H. , Vora, M. , Sia, C. L. , Korzeniewski, K. , Dhindsa, S. , Makdissi, A. , & Dandona, P. (2012). Exenatide exerts a potent antiinflammatory effect. The Journal of Clinical Endocrinology and Metabolism, 97(1), 198–207. 10.1210/jc.2011-1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, F. , Wang, W. , Ding, H. , Yang, Q. , Dong, Q. , & Cui, M. (2016). The glucagon‐like peptide‐1 receptor agonist exendin‐4 ameliorates warfarin‐associated hemorrhagic transformation after cerebral ischemia. Journal of Neuroinflammation, 13(1), 204. 10.1186/s12974-016-0661-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. , Sun, J. , Zhao, G. , Guo, A. , Chen, Y. , Fu, R. , & Deng, Y. (2017). Liraglutide improves water maze learning and memory performance while reduces hyperphosphorylation of tau and neurofilaments in APP/PS1/tau triple transgenic mice. Neurochemical Research, 42(8), 2326–2335. 10.1007/s11064-017-2250-8 [DOI] [PubMed] [Google Scholar]
- Cheong, J. L. Y. , de Pablo‐Fernandez, E. , Foltynie, T. , & Noyce, A. J. (2020). The association between type 2 diabetes mellitus and Parkinson's disease. Journal of Parkinson's Disease, 10(3), 775–789. 10.3233/JPD-191900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chohan, H. , Senkevich, K. , Patel, R. K. , Bestwick, J. P. , Jacobs, B. M. , Bandres Ciga, S. , Gan‐Or, Z. , & Noyce, A. J. (2021). Type 2 diabetes as a determinant of Parkinson's disease risk and progression. Movement Disorders. 10.1002/mds.28551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowen, J. A. , de Fonseca, F. R. , Alvarez, E. , Navarro, M. , García‐Segura, L. M. , & Blázquez, E. (1999). Increased glucagon‐like peptide‐1 receptor expression in glia after mechanical lesion of the rat brain. Neuropeptides, 33(3), 212–215. 10.1054/npep.1999.0757 [DOI] [PubMed] [Google Scholar]
- Christensen, M. , Sparre‐Ulrich, A. H. , Hartmann, B. , Grevstad, U. , Rosenkilde, M. M. , Holst, J. J. , Vilsbøll, T. , & Knop, F. K. (2015). Transfer of liraglutide from blood to cerebrospinal fluid is minimal in patients with type 2 diabetes. International Journal of Obesity, 39(11), 1651–1654. 10.1038/ijo.2015.136 [DOI] [PubMed] [Google Scholar]
- Citraro, R. , Iannone, M. , Leo, A. , de Caro, C. , Nesci, V. , Tallarico, M. , Abdalla, K. , Palma, E. , Arturi, F. , de Sarro, G. , Constanti, A. , & Russo, E. (2019). Evaluation of the effects of liraglutide on the development of epilepsy and behavioural alterations in two animal models of epileptogenesis. Brain Research Bulletin, 153, 133–142. 10.1016/j.brainresbull.2019.08.001 [DOI] [PubMed] [Google Scholar]
- Clark, I. , Atwood, C. , Bowen, R. , Paz‐Filho, G. , & Vissel, B. (2012). Tumor necrosis factor‐induced cerebral insulin resistance in Alzheimer's disease links numerous treatment rationales. Pharmacological Reviews, 64(4), 1004–1026. 10.1124/pr.112.005850 [DOI] [PubMed] [Google Scholar]
- Clark, I. A. , & Vissel, B. (2014). Inflammation‐sleep interface in brain disease: TNF, insulin, orexin. Journal of Neuroinflammation, 11(1), 51. 10.1186/1742-2094-11-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, I. A. , & Vissel, B. (2018). Therapeutic implications of how TNF links apolipoprotein E, phosphorylated tau, alpha‐synuclein, amyloid‐beta and insulin resistance in neurodegenerative diseases. British Journal of Pharmacology, 175(20), 3859–3875. 10.1111/bph.14471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton, A. , Baker, L. D. , Hanson, A. , Trittschuh, E. H. , Cholerton, B. , Morgan, A. , Callaghan, M. , Arbuckle, M. , Behl, C. , & Craft, S. (2015). Long‐acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early‐stage Alzheimer's disease dementia. Journal of Alzheimer's Disease: JAD, 44(3), 897–906. 10.3233/JAD-141791 [DOI] [PubMed] [Google Scholar]
- Cole, G. M. , Morihara, T. , Lim, G. P. , Yang, F. , Begum, A. , & Frautschy, S. A. (2004). NSAID and antioxidant prevention of Alzheimer's disease: Lessons from in vitro and animal models. Annals of the New York Academy of Sciences, 1035(1), 68–84. 10.1196/annals.1332.005 [DOI] [PubMed] [Google Scholar]
- Cork, S. C. , Richards, J. E. , Holt, M. K. , Gribble, F. M. , Reimann, F. , & Trapp, S. (2015). Distribution and characterisation of glucagon‐like peptide‐1 receptor expressing cells in the mouse brain. Molecular Metabolism, 4(10), 718–731. 10.1016/j.molmet.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courrèges, J. P. , Vilsbøll, T. , Zdravkovic, M. , le‐Thi, T. , Krarup, T. , Schmitz, O. , Verhoeven, R. , Bugáñová, I. , & Madsbad, S. (2008). Beneficial effects of once‐daily liraglutide, a human glucagon‐like peptide‐1 analogue, on cardiovascular risk biomarkers in patients with type 2 diabetes. Diabetic Medicine, 25(9), 1129–1131. 10.1111/j.1464-5491.2008.02484.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft, S. (2005). Insulin resistance syndrome and Alzheimer's disease: Age‐ and obesity‐related effects on memory, amyloid, and inflammation. Neurobiology of Aging, 26(Suppl 1), 65–69. 10.1016/j.neurobiolaging.2005.08.021 [DOI] [PubMed] [Google Scholar]
- Craft, S. , Baker, L. D. , Montine, T. J. , Minoshima, S. , Watson, G. S. , Claxton, A. , Arbuckle, M. , Callaghan, M. , Tsai, E. , Plymate, S. R. , Green, P. S. , Leverenz, J. , Cross, D. , & Gerton, B. (2012). Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Archives of Neurology, 69(1), 29–38. 10.1001/archneurol.2011.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman‐Yaffe, T. , Gerstein, H. C. , Colhoun, H. M. , Diaz, R. , García‐Pérez, L. E. , Lakshmanan, M. , Bethel, A. , Xavier, D. , Probstfield, J. , Riddle, M. C. , Rydén, L. , Atisso, C. M. , Hall, S. , Rao‐Melacini, P. , Basile, J. , Cushman, W. C. , Franek, E. , Keltai, M. , Lanas, F. , … Temelkova‐Kurktschiev, T. (2020). Effect of dulaglutide on cognitive impairment in type 2 diabetes: An exploratory analysis of the REWIND trial. Lancet Neurology, 19(7), 582–590. 10.1016/S1474-4422(20)30173-3 [DOI] [PubMed] [Google Scholar]
- Dailey, G. E. (2007). Using prandial insulin to achieve glycemic control in type 2 diabetes. The Journal of Family Practice, 56(9), 735–742. [PubMed] [Google Scholar]
- Darsalia, V. , Hua, S. , Larsson, M. , Mallard, C. , Nathanson, D. , Nyström, T. , Sjöholm, Å. , Johansson, M. E. , & Patrone, C. (2014). Exendin‐4 reduces ischemic brain injury in normal and aged type 2 diabetic mice and promotes microglial M2 polarization. PLoS One, 9(8), e103114. 10.1371/journal.pone.0103114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsalia, V. , Klein, T. , Nyström, T. , & Patrone, C. (2018). Glucagon‐like receptor 1 agonists and DPP‐4 inhibitors: Anti‐diabetic drugs with anti‐stroke potential. Neuropharmacology, 136, 280–286. 10.1016/j.neuropharm.2017.08.022 [DOI] [PubMed] [Google Scholar]
- Darsalia, V. , Mansouri, S. , Ortsäter, H. , Olverling, A. , Nozadze, N. , Kappe, C. , Iverfeldt, K. , Tracy, L. M. , Grankvist, N. , Sjöholm, Å. , & Patrone, C. (2012). Glucagon‐like peptide‐1 receptor activation reduces ischaemic brain damage following stroke in type 2 diabetic rats. Clinical Science (London, England), 122(10), 473–483. 10.1042/CS20110374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsalia, V. , Ortsater, H. , Olverling, A. , Darlof, E. , Wolbert, P. , Nystrom, T. , Klein, T. , Sjoholm, A. , & Patrone, C. (2013). The DPP‐4 inhibitor linagliptin counteracts stroke in the normal and diabetic mouse brain: A comparison with glimepiride. Diabetes, 62(4), 1289–1296. 10.2337/db12-0988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Manoel, A. L. , & Macdonald, R. L. (2018). Neuroinflammation as a target for intervention in subarachnoid hemorrhage. Frontiers in Neurology, 9, 292. 10.3389/fneur.2018.00292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza, A. G. , Chaves Filho, A. J. M. , Souza Oliveira, J. V. , de Souza, D. A. A. , Lopes, I. S. , de Carvalho, M. A. J. , de Lima, K. A. , Florenço Sousa, F. C. , Mendes Vasconcelos, S. M. , Macedo, D. , & de França Fonteles, M. M. (2019). Prevention of pentylenetetrazole‐induced kindling and behavioral comorbidities in mice by levetiracetam combined with the GLP‐1 agonist liraglutide: Involvement of brain antioxidant and BDNF upregulating properties. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 109, 429–439. 10.1016/j.biopha.2018.10.066 [DOI] [PubMed] [Google Scholar]
- Dewachter, I. , van Dorpe, J. , Spittaels, K. , Tesseur, I. , van den Haute, C. , Moechars, D. , & van Leuven, F. (2000). Modeling Alzheimer's disease in transgenic mice: Effect of age and of presenilin1 on amyloid biochemistry and pathology in APP/London mice. Experimental Gerontology, 35(6–7), 831–841. 10.1016/S0531-5565(00)00149-2 [DOI] [PubMed] [Google Scholar]
- Dhillon, S. (2018). Semaglutide: First global approval. Drugs, 78(2), 275–284. 10.1007/s40265-018-0871-0 [DOI] [PubMed] [Google Scholar]
- Doyle, M. E. , & Egan, J. M. (2007). Mechanisms of action of glucagon‐like peptide 1 in the pancreas. Pharmacology & Therapeutics, 113(3), 546–593. 10.1016/j.pharmthera.2006.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier, K. C. , Cureton, E. L. , Kwan, R. O. , Curran, B. , Sadjadi, J. , & Victorino, G. P. (2009). Glucagon‐like peptide‐1 protects mesenteric endothelium from injury during inflammation. Peptides, 30(9), 1735–1741. 10.1016/j.peptides.2009.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, A. M. , & Holscher, C. (2013). The incretin analogue D‐Ala(2)GIP reduces plaque load, astrogliosis and oxidative stress in an APP/PS1 mouse model of Alzheimer's disease. Neuroscience, 228, 294–300. 10.1016/j.neuroscience.2012.10.045 [DOI] [PubMed] [Google Scholar]
- During, M. J. , Cao, L. , Zuzga, D. S. , Francis, J. S. , Fitzsimons, H. L. , Jiao, X. , Bland, R. J. , Klugmann, M. , Banks, W. A. , Drucker, D. J. , & Haile, C. N. (2003). Glucagon‐like peptide‐1 receptor is involved in learning and neuroprotection. Nature Medicine, 9(9), 1173–1179. 10.1038/nm919 [DOI] [PubMed] [Google Scholar]
- Edison, P. , Femminella, G. , Frangou, E. , Love, S. , Busza, G. , Holmes, C. , Ritchie, C. , Lawrence, R. , McFarlane, B. , Tadros, G. , Ridha, B. H. , & Bannister, C. (2020). Evaluating the effects of the novel GLP‐1 analogue liraglutide in Alzheimer's disease (ELAD study). CTAD conference 2020, abstract OC30.
- Egefjord, L. , Gejl, M. , Moller, A. , Braendgaard, H. , Gottrup, H. , Antropova, O. , Møller, N. , Poulsen, H. E. , Gjedde, A. , Brock, B. , & Rungby, J. (2012). Effects of liraglutide on neurodegeneration, blood flow and cognition in Alzheimer s disease ‐ protocol for a controlled, randomized double‐blinded trial. Danish Medical Journal, 59, A4519. [PubMed] [Google Scholar]
- Endres, M. , Engelhardt, B. , Koistinaho, J. , Lindvall, O. , Meairs, S. , Mohr, J. P. , Planas, A. , Rothwell, N. , Schwaninger, M. , Schwab, M. E. , Vivien, D. , Wieloch, T. , & Dirnagl, U. (2008). Improving outcome after stroke: Overcoming the translational roadblock. Cerebrovascular Diseases, 25(3), 268–278. 10.1159/000118039 [DOI] [PubMed] [Google Scholar]
- Erbil, D. , Eren, C. Y. , Demirel, C. , Kucuker, M. U. , Solaroglu, I. , & Eser, H. Y. (2019). GLP‐1's role in neuroprotection: A systematic review. Brain Injury, 33(6), 734–819. 10.1080/02699052.2019.1587000 [DOI] [PubMed] [Google Scholar]
- Faivre, E. , & Holscher, C. (2013a). D‐Ala2GIP facilitated synaptic plasticity and reduces plaque load in aged wild type mice and in an Alzheimer's disease mouse model. Journal of Alzheimer's Disease, 35(2), 267–283. 10.3233/JAD-121888 [DOI] [PubMed] [Google Scholar]
- Faivre, E. , & Holscher, C. (2013b). Neuroprotective effects of D‐Ala2GIP on Alzheimer's disease biomarkers in an APP/PS1 mouse model. Alzheimer's Research & Therapy, 5(2), 20–28. 10.1186/alzrt174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femminella, G. D. , Frangou, E. , Love, S. B. , Busza, G. , Holmes, C. , Ritchie, C. , Lawrence, R. , McFarlane, B. , Tadros, G. , Ridha, B. H. , Bannister, C. , Walker, Z. , Archer, H. , Coulthard, E. , Underwood, B. R. , Prasanna, A. , Koranteng, P. , Karim, S. , Junaid, K. , … Edison, P. (2019). Evaluating the effects of the novel GLP‐1 analogue liraglutide in Alzheimer's disease: Study protocol for a randomised controlled trial (ELAD study). Trials, 20(1), 191. 10.1186/s13063-019-3259-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, P. , Zhang, X. , Li, D. , Ji, C. , Yuan, Z. , Wang, R. , Xue, G. , Li, G. , & Hölscher, C. (2018). Two novel dual GLP‐1/GIP receptor agonists are neuroprotective in the MPTP mouse model of Parkinson's disease. Neuropharmacology, 133, 385–394. 10.1016/j.neuropharm.2018.02.012 [DOI] [PubMed] [Google Scholar]
- Ferrari, C. C. , & Tarelli, R. (2011). Parkinson's disease and systemic inflammation. Parkinson's Disease, 2011, 436813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo, C. P. , Pamplona, F. A. , Mazzuco, T. L. , Aguiar, A. S. Jr. , Walz, R. , & Prediger, R. D. (2010). Role of the glucose‐dependent insulinotropic polypeptide and its receptor in the central nervous system: Therapeutic potential in neurological diseases. Behavioural Pharmacology, 21(5–6), 394–408. 10.1097/FBP.0b013e32833c8544 [DOI] [PubMed] [Google Scholar]
- Filchenko, I. , Simanenkova, A. , Chefu, S. , Kolapkova, M. , & Vlasov, T. (2018). Neuroprotective effect of glucagon‐like peptide‐1 receptor agonist is independent of glycaemia normalization in type two diabetic rats. Diabetes & Vascular Disease Research, 15(6), 567–570. 10.1177/1479164118788079 [DOI] [PubMed] [Google Scholar]
- Finan, B. , Ma, T. , Ottaway, N. , Muller, T. D. , Habegger, K. M. , Heppner, K. M. , Kirchner, H. , Holland, J. , Hembree, J. , Raver, C. , Lockie, S. H. , Smiley, D. L. , Gelfanov, V. , Yang, B. , Hofmann, S. , Bruemmer, D. , Drucker, D. J. , Pfluger, P. T. , Perez‐Tilve, D. , … Tschop, M. H. (2013). Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Science Translational Medicine, 5(209), 209ra151. 10.1126/scitranslmed.3007218 [DOI] [PubMed] [Google Scholar]
- Finan, B. , Muller, T. D. , Clemmensen, C. , Perez‐Tilve, D. , DiMarchi, R. D. , & Tschop, M. H. (2016). Reappraisal of GIP pharmacology for metabolic diseases. Trends in Molecular Medicine, 22(5), 359–376. 10.1016/j.molmed.2016.03.005 [DOI] [PubMed] [Google Scholar]
- Freiherr, J. , Hallschmid, M. , Frey, W. H. 2nd , Brünner, Y. F. , Chapman, C. D. , Hölscher, C. , Craft, S. , de Felice, F. G. , & Benedict, C. (2013). Intranasal insulin as a treatment for Alzheimer's disease: A review of basic research and clinical evidence. CNS Drugs, 27(7), 505–514. 10.1007/s40263-013-0076-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias, J. P. , Bastyr, E. J. 3rd , Vignati, L. , Tschöp, M. H. , Schmitt, C. , Owen, K. , Christensen, R. H. , & DiMarchi, R. D. (2017). The sustained effects of a dual GIP/GLP‐1 receptor agonist, NNC0090‐2746, in patients with type 2 diabetes. Cell Metabolism, 26, 343, e342–352. 10.1016/j.cmet.2017.07.011 [DOI] [PubMed] [Google Scholar]
- Frias, J. P. , Nauck, M. A. , van, J. , Kutner, M. E. , Cui, X. , Benson, C. , Urva, S. , Gimeno, R. E. , Milicevic, Z. , Robins, D. , & Haupt, A. (2018). Efficacy and safety of LY3298176, a novel dual GIP and GLP‐1 receptor agonist, in patients with type 2 diabetes: A randomised, placebo‐controlled and active comparator‐controlled phase 2 trial. Lancet, 392(10160), 2180–2193. 10.1016/S0140-6736(18)32260-8 [DOI] [PubMed] [Google Scholar]
- Frolich, L. , Blum‐Degen, D. , Riederer, P. , & Hoyer, S. (1999). A disturbance in the neuronal insulin receptor signal transduction in sporadic Alzheimer's disease. Annals of the New York Academy of Sciences, 893, 290–293. 10.1111/j.1749-6632.1999.tb07839.x [DOI] [PubMed] [Google Scholar]
- Gault, V. A. , Kerr, B. D. , Harriott, P. , & Flatt, P. R. (2011). Administration of an acylated GLP‐1 and GIP preparation provides added beneficial glucose‐lowering and insulinotropic actions over single incretins in mice with type 2 diabetes and obesity. Clinical Science (London, England), 121(3), 107–117. 10.1042/CS20110006 [DOI] [PubMed] [Google Scholar]
- Gejl, M. , Gjedde, A. , Egefjord, L. , Møller, A. , Hansen, S. B. , Vang, K. , Rodell, A. , Brændgaard, H. , Gottrup, H. , Schacht, A. , Møller, N. , Brock, B. , & Rungby, J. (2016). In Alzheimer's disease, six‐month treatment with GLP‐1 analogue prevents decline of brain glucose metabolism: Randomized, placebo‐controlled, double‐blind clinical trial. Frontiers in Aging Neuroscience, 8, 1–10. 10.3389/fnagi.2016.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi, R. , Dargahi, L. , Haeri, A. , Moosavi, M. , Mohamed, Z. , & Ahmadiani, A. (2013). Brain insulin dysregulation: Implication for neurological and neuropsychiatric disorders. Molecular Neurobiology, 47(3), 1045–1065. 10.1007/s12035-013-8404-z [DOI] [PubMed] [Google Scholar]
- Ghasemi, R. , Haeri, A. , Dargahi, L. , Mohamed, Z. , & Ahmadiani, A. (2013). Insulin in the brain: Sources, localization and functions. Molecular Neurobiology, 47(1), 145–171. 10.1007/s12035-012-8339-9 [DOI] [PubMed] [Google Scholar]
- Graham, D. L. , Durai, H. H. , Trammell, T. S. , Noble, B. L. , Mortlock, D. P. , Galli, A. , & Stanwood, G. D. (2020). A novel mouse model of glucagon‐like peptide‐1 receptor expression: A look at the brain. The Journal of Comparative Neurology, 528(14), 2445–2470. 10.1002/cne.24905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, W. S. (2008). Perispinal etanercept: Potential as an Alzheimer therapeutic. Journal of Neuroinflammation, 5(1), 3. 10.1186/1742-2094-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneveld, O. N. , Kappelle, L. J. , & Biessels, G. J. (2016). Potentials of incretin‐based therapies in dementia and stroke in type 2 diabetes mellitus. Journal of Diabetes Investigation, 7(1), 5–16. 10.1111/jdi.12420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, A. , & Holscher, C. (2009). Receptors for the insulin‐like peptide GLP‐1 are expressed on neurons in the CNS. NeuroReport, 20(13), 1161–1166. 10.1097/WNR.0b013e32832fbf14 [DOI] [PubMed] [Google Scholar]
- Hamilton, A. , Patterson, S. , Porter, D. , Gault, V. A. , & Holscher, C. (2011). Novel GLP‐1 mimetics developed to treat type 2 diabetes promote progenitor cell proliferation in the brain. Journal of Neuroscience Research, 89(4), 481–489. 10.1002/jnr.22565 [DOI] [PubMed] [Google Scholar]
- Han, L. , Holscher, C. , Xue, G. F. , Li, G. , & Li, D. (2016). A novel dual‐glucagon‐like peptide‐1 and glucose‐dependent insulinotropic polypeptide receptor agonist is neuroprotective in transient focal cerebral ischemia in the rat. NeuroReport, 27(1), 23–32. 10.1097/WNR.0000000000000490 [DOI] [PubMed] [Google Scholar]
- Hansen, H. H. , Barkholt, P. , Fabricius, K. , Jelsing, J. , Terwel, D. , Pyke, C. , Knudsen, L. B. , & Vrang, N. (2015). The GLP‐1 receptor agonist liraglutide reduces pathology‐specific tau phosphorylation and improves motor function in a transgenic hTauP301L mouse model of tauopathy. Brain Research, 1634, 158–170. [DOI] [PubMed] [Google Scholar]
- Hansen, H. H. , Fabricius, K. , Barkholt, P. , Kongsbak‐Wismann, P. , Schlumberger, C. , Jelsing, J. , Terwel, D. , Termont, A. , Pyke, C. , Knudsen, L. B. , & Vrang, N. (2016). Long‐term treatment with Liraglutide, a glucagon‐like peptide‐1 (GLP‐1) receptor agonist, has no effect on beta‐amyloid plaque load in two transgenic APP/PS1 mouse models of Alzheimer's disease. PLoS One, 11(7), e0158205. 10.1371/journal.pone.0158205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, H. H. , Fabricius, K. , Barkholt, P. , Niehoff, M. L. , Morley, J. E. , Jelsing, J. , Pyke, C. , Knudsen, L. B. , Farr, S. A. , & Vrang, N. (2015). The GLP‐1 receptor agonist liraglutide improves memory function and increases hippocampal CA1 neuronal numbers in a senescence‐accelerated mouse model of Alzheimer's disease. Journal of Alzheimer's Disease, 46(4), 877–888. 10.3233/JAD-143090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkavyi, A. , Abuirmeileh, A. , Lever, R. , Kingsbury, A. E. , Biggs, C. S. , & Whitton, P. S. (2008). Glucagon‐like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson's disease. Journal of Neuroinflammation, 5(1), 19. 10.1186/1742-2094-5-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch, E. C. , & Hunot, S. (2009). Neuroinflammation in Parkinson's disease: A target for neuroprotection? Lancet Neurology, 8(4), 382–397. 10.1016/S1474-4422(09)70062-6 [DOI] [PubMed] [Google Scholar]
- Hölscher, C. (2011). Diabetes as a risk factor for Alzheimer's disease: Insulin signalling impairment in the brain as an alternative model of Alzheimer's disease. Biochemical Society Transactions, 39(4), 891–897. 10.1042/BST0390891 [DOI] [PubMed] [Google Scholar]
- Hölscher, C. (2014). Insulin, incretins and other growth factors as potential novel treatments for Alzheimer's and Parkinson's diseases. Biochemical Society Transactions, 42(2), 593–599. 10.1042/BST20140016 [DOI] [PubMed] [Google Scholar]
- Hölscher, C. (2018). Novel dual GLP‐1/GIP receptor agonists show neuroprotective effects in Alzheimer's and Parkinson's disease models. Neuropharmacology, 136, 251–259. 10.1016/j.neuropharm.2018.01.040 [DOI] [PubMed] [Google Scholar]
- Hölscher, C. (2019). Insulin signaling impairment in the brain as a risk factor in Alzheimer's disease. Frontiers in Aging Neuroscience, 11, 88. 10.3389/fnagi.2019.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölscher, C. (2020a). Brain insulin resistance: Role in neurodegenerative disease and potential for targeting. Expert Opinion on Investigational Drugs, 29(4), 333–348. 10.1080/13543784.2020.1738383 [DOI] [PubMed] [Google Scholar]
- Hölscher, C. (2020b). Evidence for pathophysiological commonalities between metabolic and neurodegenerative disorders. In Söderbom G., Esterline R., Oscarsson J., & Mattson M. P. (Eds.), International review of neurobiology: Metabolic drivers and bioenergetic components of neurodegenerative disease (pp. 65–89). Elsevier. [DOI] [PubMed] [Google Scholar]
- Holubova, M. , Hruba, L. , Popelova, A. , Bencze, M. , Prazienkova, V. , Gengler, S. , Kratochvílová, H. , Haluzík, M. , Železná, B. , Kuneš, J. , & Hölscher, C. (2018). Liraglutide and a lipidized analog of prolactin‐releasing peptide show neuroprotective effects in a mouse model of beta‐amyloid pathology. Neuropharmacology, 144, 377–387. [DOI] [PubMed] [Google Scholar]
- Hoyer, S. (1998). Risk factors for Alzheimer's disease during aging. Impacts of glucose/energy metabolism. Journal of Neural Transmission. Supplementum, 54, 187–194. 10.1007/978-3-7091-7508-8_18 [DOI] [PubMed] [Google Scholar]
- Hoyer, S. (2004). Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. European Journal of Pharmacology, 490(1–3), 115–125. 10.1016/j.ejphar.2004.02.049 [DOI] [PubMed] [Google Scholar]
- Hoyer, S. , Oesterreich, K. , & Wagner, O. (1988). Glucose metabolism as the site of the primary abnormality in early‐onset dementia of Alzheimer type? Journal of Neurology, 235(3), 143–148. 10.1007/BF00314304 [DOI] [PubMed] [Google Scholar]
- Hu, G. , Jousilahti, P. , Bidel, S. , Antikainen, R. , & Tuomilehto, J. (2007). Type 2 diabetes and the risk of Parkinson's disease. Diabetes Care, 30(4), 842–847. 10.2337/dc06-2011 [DOI] [PubMed] [Google Scholar]
- Hunter, K. , & Holscher, C. (2012). Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neuroscience, 13(1), 33–38. 10.1186/1471-2202-13-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai, T. , Ito, S. , Tanimitsu, K. , Udagawa, S. , & Oka, J. (2006). Glucagon‐like peptide‐1 inhibits LPS‐induced IL‐1beta production in cultured rat astrocytes. Neuroscience Research, 55(4), 352–360. 10.1016/j.neures.2006.04.008 [DOI] [PubMed] [Google Scholar]
- Jalewa, J. , Sharma, M. , Gengler, S. , & Hölscher, C. (2017). A novel GLP‐1/GIP dual receptor agonist protects from 6‐OHDA lesion in a rat model of Parkinson's disease. Neuropharmacology, 117, 238–248. 10.1016/j.neuropharm.2017.02.013 [DOI] [PubMed] [Google Scholar]
- Jalewa, J. , Sharma, M. K. , & Holscher, C. (2016). Novel incretin analogues improve autophagy and protect from mitochondrial stress induced by rotenone in SH‐SY5Y cells. Journal of Neurochemistry, 139(1), 55–67. 10.1111/jnc.13736 [DOI] [PubMed] [Google Scholar]
- Ji, C. , Xue, G. F. , Li, G. , Li, D. , & Holscher, C. (2016). Neuroprotective effects of glucose‐dependent insulinotropic polypeptide in Alzheimer's disease. Reviews in the Neurosciences, 27(1), 61–70. 10.1515/revneuro-2015-0021 [DOI] [PubMed] [Google Scholar]
- Ji, C. , Xue, G. F. , Lijun, C. , Feng, P. , Li, D. , Li, L. , Li, G. , & Hölscher, C. (2016). A novel dual GLP‐1 and GIP receptor agonist is neuroprotective in the MPTP mouse model of Parkinson's disease by increasing expression of BDNF. Brain Research, 1634, 1–11. 10.1016/j.brainres.2015.09.035 [DOI] [PubMed] [Google Scholar]
- Jiang, D. , Wang, Y. , Zang, Y. , Liu, X. , Zhao, L. , Wang, Q. , Liu, C. , Feng, W. , Yin, X. , & Fang, Y. (2016). Neuroprotective effects of rhGLP‐1 in diabetic rats with cerebral ischemia/reperfusion injury. Drug Development Research, 77(3), 124–133. 10.1002/ddr.21297 [DOI] [PubMed] [Google Scholar]
- Kaplan, A. M. , & Vigna, S. R. (1994). Gastric inhibitory polypeptide (GIP) binding sites in rat brain. Peptides, 15(2), 297–302. 10.1016/0196-9781(94)90016-7 [DOI] [PubMed] [Google Scholar]
- Kappe, C. , Tracy, L. M. , Patrone, C. , Iverfeldt, K. , & Sjoholm, A. (2012). GLP‐1 secretion by microglial cells and decreased CNS expression in obesity. Journal of Neuroinflammation, 9, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellar, D. , & Craft, S. (2020). Brain insulin resistance in Alzheimer's disease and related disorders: Mechanisms and therapeutic approaches. Lancet Neurology, 19(9), 758–766. 10.1016/S1474-4422(20)30231-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. , Moon, M. , & Park, S. (2009). Exendin‐4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase‐3 expression in an animal model of Parkinson's disease. The Journal of Endocrinology, 202(3), 431–439. 10.1677/JOE-09-0132 [DOI] [PubMed] [Google Scholar]
- Knezovic, A. , Osmanovic Barilar, J. , Babic, A. , Bagaric, R. , Farkas, V. , Riederer, P. , & Salkovic‐Petrisic, M. (2018). Glucagon‐like peptide‐1 mediates effects of oral galactose in streptozotocin‐induced rat model of sporadic Alzheimer's disease. Neuropharmacology, 135, 48–62. 10.1016/j.neuropharm.2018.02.027 [DOI] [PubMed] [Google Scholar]
- Koshal, P. , Jamwal, S. , & Kumar, P. (2018). Glucagon‐like peptide‐1 (GLP‐1) and neurotransmitters signaling in epilepsy: An insight review. Neuropharmacology, 136, 271–279. 10.1016/j.neuropharm.2017.11.015 [DOI] [PubMed] [Google Scholar]
- Koshal, P. , & Kumar, P. (2016a). Effect of liraglutide on corneal kindling epilepsy induced depression and cognitive impairment in mice. Neurochemical Research, 41(7), 1741–1750. 10.1007/s11064-016-1890-4 [DOI] [PubMed] [Google Scholar]
- Koshal, P. , & Kumar, P. (2016b). Neurochemical modulation involved in the beneficial effect of liraglutide, GLP‐1 agonist on PTZ kindling epilepsy‐induced comorbidities in mice. Molecular and Cellular Biochemistry, 415(1–2), 77–87. 10.1007/s11010-016-2678-1 [DOI] [PubMed] [Google Scholar]
- Kuan, Y. C. , Huang, K. W. , Lin, C. L. , Hu, C. J. , & Kao, C. H. (2017). Effects of metformin exposure on neurodegenerative diseases in elderly patients with type 2 diabetes mellitus. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 79, 77–83. 10.1016/j.pnpbp.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Lee, C. H. , Yan, B. , Yoo, K. Y. , Choi, J. H. , Kwon, S. H. , Her, S. , Sohn, Y. , Hwang, I. K. , Cho, J. H. , Kim, Y. M. , & Won, M. H. (2011). Ischemia‐induced changes in glucagon‐like peptide‐1 receptor and neuroprotective effect of its agonist, exendin‐4, in experimental transient cerebral ischemia. Journal of Neuroscience Research, 89(7), 1103–1113. 10.1002/jnr.22596 [DOI] [PubMed] [Google Scholar]
- Lee, Y. J. , Han, S. B. , Nam, S. Y. , Oh, K. W. , & Hong, J. T. (2010). Inflammation and Alzheimer's disease. Archives of Pharmacal Research, 33(10), 1539–1556. 10.1007/s12272-010-1006-7 [DOI] [PubMed] [Google Scholar]
- Lester‐Coll, N. , Rivera, E. J. , Soscia, S. J. , Doiron, K. , Wands, J. R. , & de la Monte, S. M. (2006). Intracerebral streptozotocin model of type 3 diabetes: Relevance to sporadic Alzheimer's disease. Journal of Alzheimer's Disease, 9(1), 13–33. 10.3233/JAD-2006-9102 [DOI] [PubMed] [Google Scholar]
- Li, C. , Liu, W. , Li, X. , Zhang, Z. , Qi, H. , Liu, S. , Yan, N. , Xing, Y. , Hölscher, C. , & Wang, Z. (2020). The novel GLP‐1/GIP analogue DA5‐CH reduces tau phosphorylation and normalizes theta rhythm in the icv. STZ rat model of AD. Brain and Behavior: A Cognitive Neuroscience Perspective, 10, e01505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, P. C. , Liu, L. F. , Jou, M. J. , & Wang, H. K. (2016). The GLP‐1 receptor agonists exendin‐4 and liraglutide alleviate oxidative stress and cognitive and micturition deficits induced by middle cerebral artery occlusion in diabetic mice. BMC Neuroscience, 17(1), 37. 10.1186/s12868-016-0272-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Duffy, K. , Ottinger, M. , Ray, B. , Bailey, J. , Holloway, H. , Tweedie, D. , Perry, T. A. , Mattson, M. P. , Kapogiannis, D. , Sambamurti, K. , Lahiri, D. K. , & Greig, N. H. (2010). GLP‐1 receptor stimulation reduces amyloid‐beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. Journal of Alzheimer's Disease, 19(4), 1205–1219. 10.3233/JAD-2010-1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Liu, W. , Li, L. , & Holscher, C. (2016). Neuroprotective effects of a GIP analogue in the MPTP Parkinson's disease mouse model. Neuropharmacology, 101, 255–263. 10.1016/j.neuropharm.2015.10.002 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Liu, W. , Li, L. , & Holscher, C. (2017). D‐Ala2‐GIP‐glu‐PAL is neuroprotective in a chronic Parkinson's disease mouse model and increases BNDF expression while reducing neuroinflammation and lipid peroxidation. European Journal of Pharmacology, 797, 162–172. 10.1016/j.ejphar.2016.11.050 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Perry, T. , Kindy, M. S. , Harvey, B. K. , Tweedie, D. , Holloway, H. W. , Powers, K. , Shen, H. , Egan, J. M. , Sambamurti, K. , Brossi, A. , Lahiri, D. K. , Mattson, M. P. , Hoffer, B. J. , Wang, Y. , & Greig, N. H. (2009). GLP‐1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and parkinsonism. Proceedings of the National Academy of Sciences of the United States of America, 106(4), 1285–1290. 10.1073/pnas.0806720106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C. C. , Hu, J. , Tsai, C. W. , Yue, M. , Melrose, H. L. , Kanekiyo, T. , & Bu, G. (2015). Neuronal LRP1 regulates glucose metabolism and insulin signaling in the brain. The Journal of Neuroscience, 35(14), 5851–5859. 10.1523/JNEUROSCI.5180-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S. , Jin, Z. , Zhang, Y. , Rong, S. , He, W. , Sun, K. , Wan, D. , Huo, J. , Xiao, L. , Li, X. , Ding, N. , Wang, F. , & Sun, T. (2020). The glucagon‐like peptide‐1 analogue liraglutide reduces seizures susceptibility, cognition dysfunction and neuronal apoptosis in a mouse model of Dravet syndrome. Frontiers in Pharmacology, 11, 136. 10.3389/fphar.2020.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W. , Jalewa, J. , Sharma, M. , Li, G. , Li, L. , & Hölscher, C. (2015). Neuroprotective effects of lixisenatide and liraglutide in the MPTP mouse model of Parkinson's disease. Neuroscience, 303, 42–50. 10.1016/j.neuroscience.2015.06.054 [DOI] [PubMed] [Google Scholar]
- Long‐Smith, C. M. , Manning, S. , McClean, P. L. , Coakley, M. F. , O'Halloran, D. J. , Holscher, C. , & O'Neill, C. (2013). The diabetes drug liraglutide ameliorates aberrant insulin receptor localisation and signalling in parallel with decreasing both amyloid‐beta plaque and glial pathology in a mouse model of Alzheimer's disease. Neuromolecular Medicine, 15(1), 102–114. 10.1007/s12017-012-8199-5 [DOI] [PubMed] [Google Scholar]
- Lourenco, M. V. , Clarke, J. R. , Frozza, R. L. , Bomfim, T. R. , Forny‐Germano, L. , Batista, A. F. , Sathler, L. B. , Brito‐Moreira, J. , Amaral, O. B. , Silva, C. A. , Freitas‐Correa, L. , Espírito‐Santo, S. , Campello‐Costa, P. , Houzel, J. C. , Klein, W. L. , Holscher, C. , Carvalheira, J. B. , Silva, A. M. , Velloso, L. A. , … de Felice, F. G. (2013). TNF‐alpha mediates PKR‐dependent memory impairment and brain IRS‐1 inhibition induced by Alzheimer's beta‐amyloid oligomers in mice and monkeys. Cell Metabolism, 18(6), 831–843. 10.1016/j.cmet.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Luchsinger, J. A. , Perez, T. , Chang, H. , Mehta, P. , Steffener, J. , Pradabhan, G. , Ichise, M. , Manly, J. , Devanand, D. P. , & Bagiella, E. (2016). Metformin in amnestic mild cognitive impairment: Results of a pilot randomized placebo controlled clinical trial. Journal of Alzheimer's Disease, 51(2), 501–514. 10.3233/JAD-150493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw, W. J. , & Bazan, N. G. (2000). Neuroinflammatory signaling upregulation in Alzheimer's disease. Neurochemical Research, 25(9/10), 1173–1184. 10.1023/A:1007627725251 [DOI] [PubMed] [Google Scholar]
- Madsbad, S. , Kielgast, U. , Asmar, M. , Deacon, C. F. , Torekov, S. S. , & Holst, J. J. (2011). An overview of once‐weekly glucagon‐like peptide‐1 receptor agonists‐‐available efficacy and safety data and perspectives for the future. Diabetes, Obesity & Metabolism, 13(5), 394–407. 10.1111/j.1463-1326.2011.01357.x [DOI] [PubMed] [Google Scholar]
- Marso, S. P. , Daniels, G. H. , Brown‐Frandsen, K. , Kristensen, P. , Mann, J. F. , Nauck, M. A. , Nissen, S. E. , Pocock, S. , Poulter, N. R. , Ravn, L. S. , Steinberg, W. M. , Stockner, M. , Zinman, B. , Bergenstal, R. M. , & Buse, J. B. (2016). Liraglutide and cardiovascular outcomes in type 2 diabetes. The New England Journal of Medicine, 375(4), 311–322. 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskery, M. , Goulding, E. M. , Gengler, S. , Melchiorsen, J. U. , Rosenkilde, M. M. , & Holscher, C. (2020). The dual GLP‐1/GIP receptor agonist DA4‐JC shows superior protective properties compared to the GLP‐1 analogue liraglutide in the APP/PS1 mouse model of Alzheimer's disease. American Journal of Alzheimer's Disease and Other Dementias, 35, 153331752095304. 10.1177/1533317520953041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskery, M. P. , Holscher, C. , Jones, S. P. , Price, C. I. , Strain, W. D. , Watkins, C. L. , Werring, D. J. , & Emsley, H. C. A. (2021). Glucagon‐like peptide‐1 receptor agonists as neuroprotective agents for ischemic stroke: A systematic scoping review. Journal of Cerebral Blood Flow and Metabolism, 41(1), 14–30. 10.1177/0271678X20952011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean, P. , Parthsarathy, V. , Faivre, E. , & Holscher, C. (2011). The diabetes drug Liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. The Journal of Neuroscience, 31(17), 6587–6594. 10.1523/JNEUROSCI.0529-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean, P. L. , & Holscher, C. (2014a). Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer's disease. Neuropharmacology, 76, 57–67. 10.1016/j.neuropharm.2013.08.005 [DOI] [PubMed] [Google Scholar]
- McClean, P. L. , & Holscher, C. (2014b). Lixisenatide, a drug developed to treat type 2 diabetes, shows neuroprotective effects in a mouse model of Alzheimer's disease. Neuropharmacology, 86, 241–258. 10.1016/j.neuropharm.2014.07.015 [DOI] [PubMed] [Google Scholar]
- McClean, P. L. , Jalewa, J. , & Holscher, C. (2015). Prophylactic liraglutide treatment prevents amyloid plaque deposition, chronic inflammation and memory impairment in APP/PS1 mice. Behavioural Brain Research, 293, 96–106. 10.1016/j.bbr.2015.07.024 [DOI] [PubMed] [Google Scholar]
- Merchenthaler, I. , Lane, M. , & Shughrue, P. (1999). Distribution of pre‐pro‐glucagon and glucagon‐like peptide‐1 receptor messenger RNAs in the rat central nervous system. The Journal of Comparative Neurology, 403(2), 261–280. [DOI] [PubMed] [Google Scholar]
- Moloney, A. M. , Griffin, R. J. , Timmons, S. , O'Connor, R. , Ravid, R. , & O'Neill, C. (2010). Defects in IGF‐1 receptor, insulin receptor and IRS‐1/2 in Alzheimer's disease indicate possible resistance to IGF‐1 and insulin signalling. Neurobiology of Aging, 31(2), 224–243. 10.1016/j.neurobiolaging.2008.04.002 [DOI] [PubMed] [Google Scholar]
- Mora, F. , Exposito, I. , Sanz, B. , & Blazquez, E. (1992). Selective release of glutamine and glutamic acid produced by perfusion of GLP‐1 (7‐36) amide in the basal ganglia of the conscious rat. Brain Research Bulletin, 29, 359–361. 10.1016/0361-9230(92)90068-9 [DOI] [PubMed] [Google Scholar]
- Moroo, I. , Yamada, T. , Makino, H. , Tooyama, I. , McGeer, P. L. , McGeer, E. G. , & Hirayama, K. (1994). Loss of insulin receptor immunoreactivity from the substantia nigra pars compacta neurons in Parkinson's disease. Acta Neuropathologica (Berl), 87(4), 343–348. 10.1007/BF00313602 [DOI] [PubMed] [Google Scholar]
- Müller, T. D. , Finan, B. , Bloom, S. R. , D'Alessio, D. , Drucker, D. J. , Flatt, P. R. , Fritsche, A. , Gribble, F. , Grill, H. J. , Habener, J. F. , Holst, J. J. , Langhans, W. , Meier, J. J. , Nauck, M. A. , Perez‐Tilve, D. , Pocai, A. , Reimann, F. , Sandoval, D. A. , Schwartz, T. W. , … Tschöp, M. H. (2019). Glucagon‐like peptide 1 (GLP‐1). Molecular Metabolism, 30, 72–130. 10.1016/j.molmet.2019.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustapic, M. , Tran, J. , Craft, S. , & Kapogiannis, D. (2019). Extracellular vesicle biomarkers track cognitive changes following intranasal insulin in Alzheimer's disease. Journal of Alzheimer's Disease, 69(2), 489–498. 10.3233/JAD-180578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara, A. H. , & Tuszynski, M. H. (2011). Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nature Reviews Drug Discovery, 10(3), 209–219. 10.1038/nrd3366 [DOI] [PubMed] [Google Scholar]
- Novak, P. , Pimentel Maldonado, D. A. , & Novak, V. (2019). Safety and preliminary efficacy of intranasal insulin for cognitive impairment in Parkinson disease and multiple system atrophy: A double‐blinded placebo‐controlled pilot study. PLoS One, 14(4), e0214364. 10.1371/journal.pone.0214364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg, J. , Anderson, M. F. , Meister, B. , Alborn, A. M. , Ström, A. K. , Brederlau, A. , Illerskog, A. C. , Nilsson, O. , Kieffer, T. J. , Hietala, M. A. , Ricksten, A. , & Eriksson, P. S. (2005). Glucose‐dependent insulinotropic polypeptide is expressed in adult hippocampus and induces progenitor cell proliferation. The Journal of Neuroscience, 25(7), 1816–1825. 10.1523/JNEUROSCI.4920-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg, J. , Jacobsson, C. , Anderson, M. F. , & Eriksson, P. S. (2007). Immunohistochemical distribution of glucose‐dependent insulinotropic polypeptide in the adult rat brain. Journal of Neuroscience Research, 85(10), 2099–2119. 10.1002/jnr.21349 [DOI] [PubMed] [Google Scholar]
- Ohshima, R. , Hotsumi, K. , Holscher, C. , & Seki, K. (2015). Age‐related decrease in glucagon‐like peptide‐1 in mouse prefrontal cortex but not in hippocampus despite the preservation of its receptor. American Journal of BioScience, 1, 11–27. [Google Scholar]
- Paladugu, L. , Gharaibeh, A. , Kolli, N. , Learman, C. , Hall, T. C. , Li, L. , Rossignol, J. , Maiti, P. , & Dunbar, G. L. (2021). Liraglutide has anti‐inflammatory and anti‐amyloid properties in streptozotocin‐induced and 5xFAD mouse models of Alzheimer's disease. International Journal of Molecular Sciences, 22(2), 860. 10.3390/ijms22020860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagaki, T. , Gengler, S. , & Holscher, C. (2018). The novel DA3‐CH dual Incretin restores endoplasmic reticulum stress and autophagy impairments to attenuate Alzheimer‐like pathology and cognitive decrements in the APPSWE/PS1DeltaE9 mouse model. Journal of Alzheimer's Disease, 66(1), 195–218. 10.3233/JAD-180584 [DOI] [PubMed] [Google Scholar]
- Panagaki, T. , Michael, M. , & Hölscher, C. (2017). Liraglutide restores chronic ER stress, autophagy impairments and apoptotic signalling in SH‐SY5Y cells. Scientific Reports, 7(1), 16158. 10.1038/s41598-017-16488-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, C. R. , Moon, M. J. , Park, S. , Kim, D. K. , Cho, E. B. , Millar, R. P. , Hwang, J. I. , & Seong, J. Y. (2013). A novel glucagon‐related peptide (GCRP) and its receptor GCRPR account for coevolution of their family members in vertebrates. PLoS One, 8(6), e65420. 10.1371/journal.pone.0065420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthsarathy, V. , & Holscher, C. (2013a). Chronic treatment with the GLP1 analogue liraglutide increases cell proliferation and differentiation into neurons in an AD mouse model. PLoS One, 8(3), e58784. 10.1371/journal.pone.0058784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthsarathy, V. , & Holscher, C. (2013b). The type 2 diabetes drug liraglutide reduces chronic inflammation induced by irradiation in the mouse brain. European Journal of Pharmacology, 700(1–3), 42–50. 10.1016/j.ejphar.2012.12.012 [DOI] [PubMed] [Google Scholar]
- Porter, D. W. , Irwin, N. , Flatt, P. R. , Holscher, C. , & Gault, V. A. (2010). Prolonged GIP receptor activation improves cognitive function, hippocampal synaptic plasticity and glucose homeostasis in high‐fat fed mice. European Journal of Pharmacology, 650, 688–693. [DOI] [PubMed] [Google Scholar]
- Porter, D. W. , Kerr, B. D. , Flatt, P. R. , Holscher, C. , & Gault, V. A. (2010). Four weeks administration of Liraglutide improves memory and learning as well as glycaemic control in mice with high fat dietary‐induced obesity and insulin resistance. Diabetes, Obesity & Metabolism, 12(10), 891–899. 10.1111/j.1463-1326.2010.01259.x [DOI] [PubMed] [Google Scholar]
- Qi, L. , Ke, L. , Liu, X. , Liao, L. , Ke, S. , Liu, X. , Wang, Y. , Lin, X. , Zhou, Y. , Wu, L. , Chen, Z. , & Liu, L. (2016). Subcutaneous administration of liraglutide ameliorates learning and memory impairment by modulating tau hyperphosphorylation via the glycogen synthase kinase‐3beta pathway in an amyloid beta protein induced alzheimer disease mouse model. European Journal of Pharmacology, 783, 23–32. 10.1016/j.ejphar.2016.04.052 [DOI] [PubMed] [Google Scholar]
- Rhee, S. Y. , Han, K. D. , Kwon, H. , Park, S. E. , Park, Y. G. , Kim, Y. H. , Yoo, S. J. , Rhee, E. J. , & Lee, W. Y. (2020). Association between glycemic status and the risk of Parkinson disease: A nationwide population‐based study. Diabetes Care, 43(9), 2169–2175. 10.2337/dc19-0760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell, N. J. , & Hopkins, S. J. (1995). Cytokines and the nervous system II: Actions and mechanisms of action. Trends in Neurosciences, 18(3), 130–136. 10.1016/0166-2236(95)93890-A [DOI] [PubMed] [Google Scholar]
- Salameh, T. S. , Rhea, E. M. , Talbot, K. , & Banks, W. A. (2020). Brain uptake pharmacokinetics of incretin receptor agonists showing promise as Alzheimer's and Parkinson's disease therapeutics. Biochemical Pharmacology, 180, 114187. 10.1016/j.bcp.2020.114187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles, G. N. , Calio, M. L. , Holscher, C. , Pacheco‐Soares, C. , Porcionatto, M. , & Lobo, A. O. (2020). Neuroprotective and restorative properties of the GLP‐1/GIP dual agonist DA‐JC1 compared with a GLP‐1 single agonist in Alzheimer's disease. Neuropharmacology, 162, 107813. 10.1016/j.neuropharm.2019.107813 [DOI] [PubMed] [Google Scholar]
- Santos, L. E. , & Ferreira, S. T. (2018). Crosstalk between endoplasmic reticulum stress and brain inflammation in Alzheimer's disease. Neuropharmacology, 136, 350–360. 10.1016/j.neuropharm.2017.11.016 [DOI] [PubMed] [Google Scholar]
- Schmidt, L. J. , Habacher, W. , Augustin, T. , Krahulec, E. , & Semlitsch, T. (2014). A systematic review and meta‐analysis of the efficacy of lixisenatide in the treatment of patients with type 2 diabetes. Diabetes, Obesity & Metabolism, 16(9), 769–779. 10.1111/dom.12269 [DOI] [PubMed] [Google Scholar]
- Schubert, M. , Brazil, D. P. , Burks, D. J. , Kushner, J. A. , Ye, J. , Flint, C. L. , Farhang‐Fallah, J. , Dikkes, P. , Warot, X. M. , Rio, C. , Corfas, G. , & White, M. F. (2003). Insulin receptor substrate‐2 deficiency impairs brain growth and promotes tau phosphorylation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(18), 7084–7092. 10.1523/JNEUROSCI.23-18-07084.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergi, D. , Renaud, J. , Simola, N. , & Martinoli, M. G. (2019). Diabetes, a contemporary risk for Parkinson's disease: Epidemiological and cellular evidences. Frontiers in Aging Neuroscience, 11, 302. 10.3389/fnagi.2019.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, M. , Jalewa, J. , & Holscher, C. (2013). Neuroprotective and anti‐apoptotic effects of liraglutide on SH‐SY5Y cells exposed to methylglyoxal stress. Journal of Neurochemistry, 128, 459–471. [DOI] [PubMed] [Google Scholar]
- Shi, L. J. , Zhang, Z. , Li, L. , & Hölscher, C. (2017). A novel dual GLP‐1/GIP receptor agonist alleviates cognitive decline by re‐sensitizing insulin signaling in the Alzheimer icv. STZ Rat Model. Behavioural Brain Research, 237, 65–74. [DOI] [PubMed] [Google Scholar]
- Steen, E. , Terry, B. M. , J. Rivera, E. , Cannon, J. L. , Neely, T. R. , Tavares, R. , Xu, X. J. , Wands, J. R. , & de la Monte, S. M. (2005). Impaired insulin and insulin‐like growth factor expression and signaling mechanisms in Alzheimer's disease ‐ is this type 3 diabetes? Journal of Alzheimer's Disease, 7(1), 63–80. 10.3233/JAD-2005-7107 [DOI] [PubMed] [Google Scholar]
- Svenningsson, P. , Wirdefeldt, K. , Yin, L. , Fang, F. , Markaki, I. , Efendic, S. , & Ludvigsson, J. F. (2016). Reduced incidence of Parkinson's disease after dipeptidyl peptidase‐4 inhibitors‐a nationwide case‐control study. Movement Disorders, 31(9), 1422–1423. 10.1002/mds.26734 [DOI] [PubMed] [Google Scholar]
- Talbot, K. (2014). Brain insulin resistance in Alzheimer's disease and its potential treatment with GLP‐1 analogs. Neurodegenerative Disease Management, 4(1), 31–40. 10.2217/nmt.13.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot, K. (2021). Direct demonstration of brain insulin resistance in Alzheimer's and Parkinson's disease dementia and its alleviation with incretin receptor agonists. AD/PD 2021 conference, Boston USA
- Talbot, K. , Wang, H. Y. , Kazi, H. , Han, L. Y. , Bakshi, K. P. , Stucky, A. , Fuino, R. L. , Kawaguchi, K. R. , Samoyedny, A. J. , Wilson, R. S. , Arvanitakis, Z. , Schneider, J. A. , Wolf, B. A. , Bennett, D. A. , Trojanowski, J. Q. , & Arnold, S. E. (2012). Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF‐1 resistance, IRS‐1 dysregulation, and cognitive decline. The Journal of Clinical Investigation, 122(4), 1316–1338. 10.1172/JCI59903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey, M. G. , & Goldberg, M. S. (2010). Neuroinflammation in Parkinson's disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiology of Disease, 37(3), 510–518. 10.1016/j.nbd.2009.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto, S. , Miyamoto, N. , Yatomi, K. , Tanaka, Y. , Oishi, H. , Arai, H. , Hattori, N. , & Urabe, T. (2011). Exendin‐4, a glucagon‐like peptide‐1 receptor agonist, provides neuroprotection in mice transient focal cerebral ischemia. Journal of Cerebral Blood Flow and Metabolism, 31(8), 1696–1705. 10.1038/jcbfm.2011.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usdin, T. B. , Mezey, E. , Button, D. C. , Brownstein, M. J. , & Bonner, T. I. (1993). Gastric inhibitory polypeptide receptor, a member of the secretin‐vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology, 133(6), 2861–2870. 10.1210/endo.133.6.8243312 [DOI] [PubMed] [Google Scholar]
- Varvel, N. H. , Neher, J. J. , Bosch, A. , Wang, W. , Ransohoff, R. M. , Miller, R. J. , & Dingledine, R. (2016). Infiltrating monocytes promote brain inflammation and exacerbate neuronal damage after status epilepticus. Proceedings of the National Academy of Sciences of the United States of America, 113(38), E5665–E5674. 10.1073/pnas.1604263113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma, M. K. , Goel, R. , Krishnadas, N. , & Nemmani, K. V. S. (2018). Targeting glucose‐dependent insulinotropic polypeptide receptor for neurodegenerative disorders. Expert Opinion on Therapeutic Targets, 22(7), 615–628. 10.1080/14728222.2018.1487952 [DOI] [PubMed] [Google Scholar]
- Verma, M. K. , Goel, R. , Nandakumar, K. , & Nemmani, K. V. (2017). Effect of D‐Ala2GIP, a stable GIP receptor agonist on MPTP‐induced neuronal impairments in mice. European Journal of Pharmacology, 804, 38–45. 10.1016/j.ejphar.2017.03.059 [DOI] [PubMed] [Google Scholar]
- Wadden, T. , Hollander, P. , Klein, S. , Niswender, K. , Woo, V. , Hale, P. M. , & Aronne, L. (2013). Weight maintenance and additional weight loss with liraglutide after low‐calorie diet‐induced weight loss: The SCALE maintenance randomized study. International Journal of Obesity, 37(11), 1443–1451. 10.1038/ijo.2013.120 [DOI] [PubMed] [Google Scholar]
- Wang, R. F. , Xue, G. F. , Hölscher, C. , Tian, M. J. , Feng, P. , Zheng, J. Y. , & Li, D. F. (2018). Post‐treatment with the GLP‐1 analogue liraglutide alleviate chronic inflammation and mitochondrial stress induced by status epilepticus. Epilepsy Research, 142, 45–52. 10.1016/j.eplepsyres.2018.03.009 [DOI] [PubMed] [Google Scholar]
- Watson, K. T. , Wroolie, T. E. , Tong, G. , Foland‐Ross, L. C. , Frangou, S. , Singh, M. , McIntyre, R. S. , Roat‐Shumway, S. , Myoraku, A. , Reiss, A. L. , & Rasgon, N. L. (2019). Neural correlates of liraglutide effects in persons at risk for Alzheimer's disease. Behavioural Brain Research, 356, 271–278. 10.1016/j.bbr.2018.08.006 [DOI] [PubMed] [Google Scholar]
- Wellman, M. , & Abizaid, A. (2015). Growth hormone secretagogue receptor dimers: A new pharmacological target. eNeuro, 2, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiciński, M. , Socha, M. , Malinowski, B. , Wódkiewicz, E. , Walczak, M. , Górski, K. , Słupski, M. , & Pawlak‐Osińska, K. (2019). Liraglutide and its neuroprotective properties‐focus on possible biochemical mechanisms in Alzheimer's disease and cerebral ischemic events. International Journal of Molecular Sciences, 20(5), 1050. 10.3390/ijms20051050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. , Feng, P. , Zhang, X. , Li, D. , Wang, R. , Ji, C. , Li, G. , & Hölscher, C. (2019). The diabetes drug semaglutide reduces infarct size, inflammation, and apoptosis, and normalizes neurogenesis in a rat model of stroke. Neuropharmacology, 158, 107748. 10.1016/j.neuropharm.2019.107748 [DOI] [PubMed] [Google Scholar]
- Yu, Y. W. , Hsueh, S. C. , Lai, J. H. , Chen, Y. H. , Kang, S. J. , Chen, K. Y. , Hsieh, T. H. , Hoffer, B. , Li, Y. , Greig, N. , & Chiang, Y. H. (2018). Glucose‐dependent insulinotropic polypeptide mitigates 6‐OHDA‐induced behavioral impairments in parkinsonian rats. International Journal of Molecular Sciences, 19(4), 1153. 10.3390/ijms19041153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, Z. , Li, L. , Feng, P. , Xue, G. , Ji, C. , Li, G. , & Hölscher, C. (2017). A novel GLP‐1/GIP dual agonist is more effective than liraglutide in reducing inflammation and enhancing GDNF release in the MPTP mouse model of Parkinson's disease. European Journal of Pharmacology, 812, 82–90. 10.1016/j.ejphar.2017.06.029 [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Zhang, L. , Li, L. , & Holscher, C. (2018). Neuroprotective effects of the novel GLP‐1 long acting analogue semaglutide in the MPTP Parkinson's disease mouse model. Neuropeptides, 71, 70–80. 10.1016/j.npep.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Zhang, L. , Li, L. , & Holscher, C. (2019). Semaglutide is neuroprotective and reduces alpha‐synuclein levels in the chronic MPTP mouse model of Parkinson's disease. Journal of Parkinson's Disease, 9(1), 157–171. 10.3233/JPD-181503 [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Zhang, L. , Li, Y. , Li, L. , Melchiorsen, J. , Rosenkilde, M. , & Hölscher, C. (2020). The novel dual GLP‐1/GIP receptor agonist DA‐CH5 is superior to single GLP‐1 receptor agonists in the MPTP model of Parkinson's disease. Journal of Parkinson's Disease, 10(2), 523–542. 10.3233/JPD-191768 [DOI] [PubMed] [Google Scholar]
- Zhang, L. Y. , Jin, Q. , Hölscher, C. , & Li, L. (2021). Glucagon‐like peptide‐1/glucose‐dependent insulinotropic polypeptide dual receptor agonist DACH5 is superior to exendin‐4 in protecting neurons in the 6‐hydroxydopamine rat Parkinson model. Neural Regeneration Research, 16(8), 1660–1670. 10.4103/1673-5374.303045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. F. , Chen, Y. M. , Li, L. , & Hölscher, C. (2015). Neuroprotective effects of (Val8)GLP‐1‐Glu‐PAL in the MPTP Parkinson's disease mouse model. Behavioural Brain Research, 293, 107–113. 10.1016/j.bbr.2015.07.021 [DOI] [PubMed] [Google Scholar]
- Zhang, Z. Q. , & Holscher, C. (2020). GIP has neuroprotective effects in Alzheimer and Parkinson's disease models. Peptides, 125, 170184. 10.1016/j.peptides.2019.170184 [DOI] [PubMed] [Google Scholar]
- Zhou, M. , Chen, S. , Peng, P. , Gu, Z. , Yu, J. , Zhao, G. , & Deng, Y. (2019). Dulaglutide ameliorates STZ induced AD‐like impairment of learning and memory ability by modulating hyperphosphorylation of tau and NFs through GSK3beta. Biochemical and Biophysical Research Communications, 511(1), 154–160. 10.1016/j.bbrc.2019.01.103 [DOI] [PubMed] [Google Scholar]
- Zhu, H. , Zhang, Y. , Shi, Z. , Lu, D. , Li, T. , Ding, Y. , Ruan, Y. , & Xu, A. (2016). The neuroprotection of Liraglutide against Ischaemia‐induced apoptosis through the activation of the PI3K/AKT and MAPK pathways. Scientific Reports, 6(1), 26859. 10.1038/srep26859 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review and all data included in this paper have been published elsewhere.


