Abstract
Background and Purpose
Glucagon‐like peptide‐1 (GLP‐1) receptor activation decreases stroke risk in people with Type 2 diabetes (T2D), while animal studies have shown the efficacy of this strategy to counteract stroke‐induced acute brain damage. However, whether GLP‐1 receptor activation also improves recovery in the chronic phase after stroke is unknown. We investigated whether post‐acute, chronic administration of the GLP‐1 receptor agonist, exendin‐4, improves post‐stroke recovery and examined possible underlying mechanisms in T2D and non‐T2D mice.
Experimental Approach
We induced stroke via transient middle cerebral artery occlusion (tMCAO) in T2D/obese mice (8 months of high‐fat diet) and age‐matched controls. Exendin‐4 was administered for 8 weeks from Day 3 post‐tMCAO. We assessed functional recovery by weekly upper‐limb grip strength tests. Insulin sensitivity and glycaemia were evaluated at 4 and 8 weeks post‐tMCAO. Neuronal survival, stroke‐induced neurogenesis, neuroinflammation, atrophy of GABAergic parvalbumin+ interneurons, post‐stroke vascular remodelling and fibrotic scar formation were investigated by immunohistochemistry.
Key Results
Exendin‐4 normalised T2D‐induced impairment of forepaw grip strength recovery in correlation with normalised glycaemia and insulin sensitivity. Moreover, exendin‐4 counteracted T2D‐induced atrophy of parvalbumin+ interneurons and decreased microglia activation. Finally, exendin‐4 normalised density and pericyte coverage of micro‐vessels and restored fibrotic scar formation in T2D mice. In non‐T2D mice, the exendin‐4‐mediated recovery was minor.
Conclusion and Implications
Chronic GLP‐1 receptor activation mediates post‐stroke functional recovery in T2D mice by normalising glucose metabolism and improving neuroplasticity and vascular remodelling in the recovery phase. The results warrant clinical trial of GLP‐1 receptor agonists for rehabilitation after stroke in T2D.
LINKED ARTICLES
This article is part of a themed issue on GLP1 receptor ligands (BJP 75th Anniversary). To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v179.4/issuetoc
Keywords: GLP‐1R agonist, fibrotic scar, neurological recovery, stroke, T2D, vascular remodelling
Abbreviations
- ASCVD
atherosclerotic cardiovascular disease
- BBB
blood–brain barrier
- CR
calretinin
- DCX
doublecortin
- ECA
external carotid artery
- GLP‐1
glucagon‐like peptide 1
- HFD
high‐fat diet
- Iba‐1
ionised calcium‐binding adapter molecule 1
- ICA
internal carotid artery
- IHC
immunohistochemistry
- ITT
insulin tolerance test
- MCA
middle cerebral artery
- NeuN
neuronal nuclei
- NIHSS
National Institute of Health Stroke Scale
- NSC
neural stem cell
- SVZ
subventricular zone
- T2D
type 2 diabetes
- tMCAO
transient middle cerebral artery occlusion
- TTC
3,5‐triphenyltetrazolium chloride
What is already known
Type 2 diabetes (T2D) hampers recovery after stroke but effective therapies are lacking.
GLP‐1 receptor agonists reduce stroke risk and promote acute neuroprotection, but effects on rehabilitation are undetermined.
What does this study add
GLP‐1 receptor activation improves rehabilitation during the post‐stroke chronic phase in T2D mice.
The improved rehabilitation was associated with improved glycaemia and insulin sensitivity and with vascular remodelling.
What is the clinical significance
This study encourages tight glycaemic regulation during the post‐stroke chronic phase to enhance stroke rehabilitation.
This strategy could potentially benefit an large group of patients with T2D and stroke.
1. INTRODUCTION
Glucagon‐like peptide‐1 (GLP‐1) receptor agonists are glucose‐lowering drugs for Type 2 diabetes (T2D) that act in a glucose‐dependent manner, with minimal risks of hypoglycaemia (Gutniak et al., 1992, 1994; Holz Iv et al., 1993). Large randomised clinical trials have shown that these drugs can also reduce the risk of major cardiovascular events (Alfayez et al., 2020; Best et al., 2011; Gerstein et al., 2020; Hernandez et al., 2018; Marso, Bain, et al., 2016; Marso, Daniels, et al., 2016; Paul et al., 2015; Zimmerman et al., 2017). Moreover, several preclinical studies using experimental stroke models indicate that GLP‐1 receptor agonists can reduce stroke‐induced brain damage when given acutely or sub‐acutely before stroke, at stroke onset or immediately after stroke, in both normal or diabetic rodents (Darsalia, Klein, et al., 2018; Darsalia, Larsson, et al., 2018; Dong et al., 2017; Marlet et al., 2018; Yang et al., 2019). What remains to be determined is whether the chronic activation of GLP‐1 receptors in the post‐acute phase of stroke is efficacious to improve functional recovery. This knowledge is crucial since T2D is a strong risk factor for stroke (Benjamin et al., 2018; Rawshani et al., 2018; Zabala et al., 2019) and it is estimated that by 2030 the number of people with diabetes will reach 439 million (Shaw et al., 2010). Therefore, people with T2D constitute an enormous candidate group not only for stroke prevention but also for stroke treatment and care. Moreover, T2D worsens initial stroke outcome and it is also a strong predictor of post‐stroke dependency on supportive care in activities of daily living (Luitse et al., 2012; Megherbi et al., 2003; Pulsinelli et al., 1983; Ullberg et al., 2015). Thus, new and efficacious post‐stroke pharmacological treatments in people with T2D are highly needed.
Acute (Luitse et al., 2013) and persistent (up to 72 h; Baird et al., 2003) hyperglycaemia after stroke is common in both normal and diabetic individuals and is associated with worse outcome. The effective achievement of normoglycaemia is considered to be beneficial in the acute setting, but conclusive evidence is still lacking because aggressive glucose control can be complicated by hypoglycaemic episodes that are detrimental to stroke outcome (Palaiodimou et al., 2019). Importantly, the potential efficacy of glycaemia regulation in the post‐acute, chronic phase after stroke to improve recovery has not been studied clinically. We recently showed the potential efficacy of this strategy by using both dipeptidyl peptidase‐4 (DPP‐4) inhibition and sulfonylurea treatment (Augestad et al., 2020). Whether GLP‐1 analogues also can improve neurological recovery after stroke remains to be addressed.
There are several mechanisms through which delayed GLP‐1 receptor activation after stroke could improve recovery in diabetes. For instance, GLP‐1 improves endothelial function in T2D (Nystrom et al., 2004; Tuttolomondo et al., 2021) and GLP‐1 receptor activation after stroke can stimulate angiogenesis (Chen et al., 2018; Zhao et al., 2015). It has been also shown that the GLP‐1 receptor agonist exendin‐4 can stabilise the blood–brain barrier (BBB) after stroke and reduce inflammation (Chen et al., 2016; Kim et al., 2017; Shan et al., 2019; Yang et al., 2019). Moreover, the modulation of GABAergic activity plays a central role in facilitating functional recovery after stroke (Johnstone et al., 2018) and recently it has been shown that GLP‐1 receptor activation can modulate GABA signalling (Korol et al., 2015) and affects specific populations of GABAergic interneurons (Larsson et al., 2016; Lietzau et al., 2016). Finally, stroke‐induced neurogenesis from the neural stem cells (NSC) in the subventricular zone (SVZ) has been suggested to contribute to stroke recovery (Dillen et al., 2020) and GLP‐1 receptor agonists do enhance this process in rodent stroke models (Darsalia et al., 2012; Yang et al., 2019). Whether these or additional mechanisms triggered by GLP‐1 receptor activation improve recovery in the chronic phase after stroke remains to be determined. It also remains to be investigated whether these positive effects occur through normalisation of glucose metabolism (hyperglycaemia and insulin resistance), as many of the preclinical studies already mentioned have used non‐diabetic rodents.
The first aim of this study was to determine whether a delayed treatment after stroke (starting 3 days post‐stroke) with the specific GLP‐1 receptor agonist exendin‐4 (Goke et al., 1993) improves neurological recovery in a clinically relevant mouse model of obesity‐induced T2D (Augestad et al., 2020; Pintana et al., 2019). We also investigated whether neurological recovery was associated with improved glucose metabolism during the recovery phase. Finally, we investigated whether GLP‐1 receptor activation enhanced stroke‐induced neurogenesis, modulated neuroinflammation and GABAergic interneurons, and impacted on vascular remodelling and fibrotic scar formation in the peri‐infarct/infarct regions. To investigate whether the potential efficacy of GLP‐1 receptor activation in stroke recovery was due to the improvement of diabetic pathology, we performed a parallel study in age‐matched non‐diabetic mice.
2. METHODS
2.1. The T2D animal model and experimental design
All animal care and experimental procedures were in compliance with the 2010/63/EU directive and were approved by the regional ethics committee (approval ID1126, Karolinska Institutet). The study is reported according to the ARRIVE guidelines (Percie du Sert et al., 2020) and the recommendations by the British Journal of Pharmacology (Lilley et al., 2020). Group sizes were determined by a priori power analysis. Based on pilot experiments, anticipated difference between groups in functional recovery was set at 20 ± 10%, with α = 0.05 and a statistical power of 90%. The analyses suggested the sample size of minimum n = 5 per group. However, after taking into consideration the success rate of stroke surgery, mortality and likelihood of statistical outliers, the experimental groups were set at n = 10 each.
2.1.1. Study 1 (diabetic study)
Thirty male C57BL/6JRj mice (Janvier Labs, France RRID:MGI:2670020) were used in this study. All mice were housed in environmentally controlled conditions (25 ± 0.5°C, 12/12 h light/dark cycle with ad libitum access to food and water). Mice were housed under specific pathogen free conditions in type III size cages, with maximum 5 mice per cage. Cages contained wood chip bedding, and cage enrichment was present in the form of plastic red houses and nest material. Animal health and well‐being was evaluated daily. From 4 weeks of age, the mice were fed with either standard laboratory diet (n = 10) or high‐fat diet (HFD: 60% energy from fat) (n = 20) for 8 months. This is a clinically relevant model of T2D, mimicking the gradual development of T2D in humans. To confirm induced T2D after HFD feeding, body weight, fasting blood glucose and insulin sensitivity were measured. Once obesity (over 25% increase in body weight) and hyperglycaemia (fasting glucose over 7 mmol L−1) were established, the mice were subjected to transient middle cerebral artery occlusion (tMCAO) (see Figure 1a).
FIGURE 1.
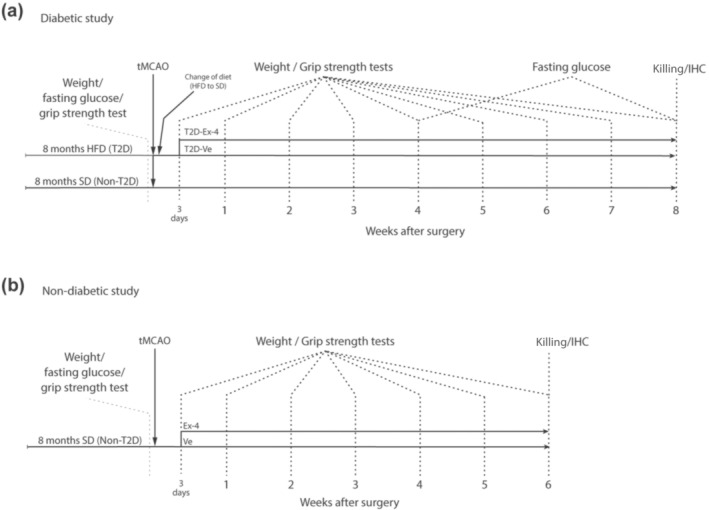
The experimental design. Experimental design of (a) diabetic study and (b) non‐diabetic study
After tMCAO, HFD in the T2D group was replaced with a standard diet to reflect the clinical setting of a balanced post‐stroke diet. Mice fed standard diet from the beginning of the experiment will be referred to as Non‐T2D, whereas mice fed HFD until stroke time will be referred to as T2D.
One mouse from the Non‐T2D group was removed due to unsuccessful tMCAO and 2 mice from the T2D group were removed (euthanised) shortly after surgery as their condition reached a humane endpoint as specified in the ethical approval. The remaining T2D mice were randomised in two groups 3 days after stroke: 1) one group was treated with daily i.p. injections of 0.2 μg kg−1 exendin‐4 (referred to as T2D‐Ex‐4) for 8 weeks, 2) another group received vehicle (PBS) injections (referred to as T2D‐Ve). Two mice from T2D‐Ex‐4 group were later removed since insufficient stroke damage was detected after histological examination. The final number of animals used in this study were as following: Non‐T2D (n = 9), T2D‐Ve (n = 9) and T2D‐Ex‐4 (n = 7). The forelimb sensorimotor function was then measured by assessing upper‐limb grip strength for up to 8 weeks after stroke when the mice were killed (time when the non‐T2D mice have fully recovered). Brains were then collected for histology. See Figure 1a for the plan of the experimental design.
2.1.2. Study 2 (non‐diabetic study)
Twenty 8‐month‐old male C57BL/6JRj mice were used. Six mice were removed after surgery due to either unsuccessful tMCAO or after reaching a humane endpoint. The remaining mice were randomised in two groups: 1) Non‐T2D‐Ve (n = 6) and Non‐T2D‐Ex‐4 (n = 8) and received daily vehicle (PBS) or 0.2 μg kg−1 exendin‐4 injections, respectively, starting from Day 3 after tMCAO until 6 weeks. The forelimb sensorimotor function was tested as for Study 1, but this study was terminated 6 weeks after stroke (time when the non‐T2D‐Ex‐4 mice have fully recovered). Brains were then collected for histology. See Figure 1b for the experimental design.
2.2. Transient middle cerebral artery occlusion (tMCAO)
tMCAO was used to model ischaemic stroke and was induced by the intraluminal filament technique as previously described (Hara et al., 1996). Briefly, mice were anaesthetised with 3% isoflurane and then maintained on 1.5% isoflurane through a snout‐mask throughout the surgery. Body temperature was maintained at 37–38°C using a heated pad. Through a midline incision, the left external (ECA) and internal (ICA) carotid arteries were exposed and a 7–0 monofilament coated with silicone (total diameter 0.17–0.18 mm) was inserted from an incision in ECA into the ICA until it blocked the origin of the MCA. Then the wounds were temporarily closed, and the mice were allowed to wake up. After 25 minutes, the mice were re‐anaesthetised, the wound re‐opened and the occluding filament removed (total occlusion time = 30 min). Stroke induction was considered unsuccessful when the occluding filament could not be advanced within the internal carotid artery beyond 7–8 mm from the carotid bifurcation or if mice lacked neurological impairment symptoms based on the neurological severity score (Bederson et al., 1986). All mice were given analgesic (Carprofen, 5 mg kg−1) and soft food after the surgery.
2.3. Fasting glycaemia and insulin tolerance test
Fasting glycaemia measurement and insulin tolerance test (ITT) were performed before and at 4 and 8 weeks after stroke. Fasting glycaemia was measured after an overnight fasting. For ITT, mice were fasted for 6 h and injected i.p. with human insulin (0.5 unit kg−1) dissolved in saline. Blood glucose was determined before injection of insulin and at pre‐set time points after injection (15, 30, 45, 60, 75 and 90 min). Data are presented as area under the curve (AUC); see Figure 2.
FIGURE 2.
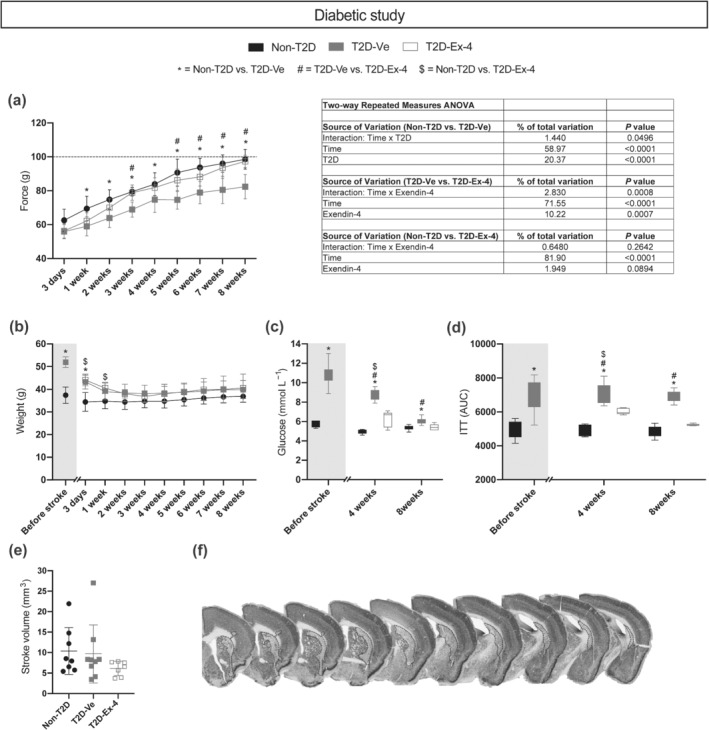
The effect of T2D and exendin‐4 treatment on neurological recovery, metabolic parameters and ischaemic volume after tMCAO. (a) Grip strength recovery and ANOVA table. Dashed line in (a) indicates mean of pre‐stroke grip strength. (b) Body weight. (c) Fasting glucose. (d) Insulin sensitivity (insulin tolerance test, ITT). (e) Ischaemic volume. (f) Representative images of NeuN staining. The dotted lines on images on (f) panel indicate stroke area. Data in (a), (b) and (e) are presented as mean ± SD. Box plots on (c) and (d) show min–max values. Group sizes for (b)–(d): Non‐T2D n = 9, T2D‐Ve n = 9, T2D‐Ex‐4 n = 7. For (a), one statistical outlier was excluded from the T2D‐Ve group, resulting in the following group sizes: Non‐T2D n = 9, T2D‐Ve n = 8, T2D‐Ex‐4 n = 7. For (e), one statistical outlier was excluded from the non‐T2D group, resulting in the following group sizes: Non‐T2D n = 8, T2D‐Ve n = 9, T2D‐Ex‐4 n = 7. Two‐way repeated measures ANOVA with Geisser–Greenhouse's correction followed by Sidak's test was used to analyse grip strength, body weight, fasting glucose and insulin sensitivity (a–d). Kruskal–Wallis with uncorrected Dunn's test was used to analyse ischaemic volume (e). *, # P < 0.05, significantly different from T2D‐Ve; $ P < 0.05, Non‐T2D significantly different from T2D‐Ex‐4
2.4. Assessment of the recovery of forelimb sensorimotor function
The forelimb sensorimotor function was measured by assessing upper‐limb grip strength (Augestad et al., 2020; Pintana et al., 2019) using a grip strength meter (Harvard apparatus, MA, USA) before, at 3 days and at 1–8 weeks after tMCAO. Briefly, mice were firmly held by the body and allowed to grasp the grid with the right forepaw. Mice were gently dragged backward until the grip was released. Ten trials were performed and the highest value was recorded as described previously (Augestad et al., 2020; Pintana et al., 2019). The grip strength test was performed by an experimenter blinded to the treatment groups (Ex‐4 vs. vehicle), but not diet (standard vs. HFD) due to the obvious weight differences during the first weeks after tMCAO.
2.5. Immunohistochemistry and quantitative microscopy
To prepare tissue for immunohistochemistry (IHC), the mice were terminally anaesthetised with an overdose of sodium pentobarbital and transcardially perfused with 4% ice‐cold paraformaldehyde before the brains were removed. After overnight post‐fixation the brains were transferred to a solution of phosphate‐buffered saline (PBS) with 20% sucrose until they sank. The brains were cut in 30‐μm‐thick coronal sections using a sliding microtome and kept in anti‐freeze solution at −20°C.
Immunofluorescence staining was performed using the free‐floating method. Briefly, brain sections were washed in PBS. For DAB staining, sections were incubated with PBS containing 3% H2O2 and 10% methanol for 20 min at room temperature to quench endogenous peroxidases. For both DAB and immunofluorescence stainings, sections were then blocked in PBS containing 3–5% appropriate normal serum and 0.25% Triton‐X100 for 1 h (at room temperature), and incubated overnight in primary antibody solution (at room temperature for the CD31 and CD13 staining and at 4°C for all other staining). The following primary antibodies were used; mouse anti‐NeuN (1:500 dilution, #MAB377, Millipore; RRID:AB_2298772), a neuronal marker; goat anti‐Iba‐1 (1:1000 dilution, #ab5076, Abcam; RRID:AB_2224402), a marker for microglia; rabbit anti‐CD68 (1:2000 dilution, #ab125212, Abcam; RRID:AB_10975465), a marker for phagocytic microglia and macrophages; rabbit anti‐parvalbumin (PV) (1:1000 dilution, #ab11427, Abcam; RRID:AB_298032), a marker of parvalbumin‐expressing interneurons; rat anti‐CD13 (1:200 dilution, #MCA2183, Bio‐RAD; RRID:AB_323691), a marker for pericytes; goat anti‐CD31 (1:200 dilution, #AF3628, R&D Systems; RRID:AB_2161028), a marker for endothelial cells; rabbit anti‐calretinin (CR) (1:400 dilution, #ab92341, Abcam; RRID:AB_2049245), a marker for early post‐mitotic neurons and calretinin‐expressing interneurons; mouse anti‐cFos (1:800 dilution, #ab208942, Abcam; RRID:AB_2747772), a marker for neuronal activation; mouse anti‐doublecortin (DCX) (1:200 dilution, #sc‐271390, Santa Cruz Biotechnology; RRID:AB_10610966), a marker for migrating neuroblasts. After overnight incubation with the primary antibody solution, sections were washed and incubated for 1.5–2 h at RT with the secondary antibody. The following secondary antibodies were used: biotinylated horse anti‐mouse (1:200 dilution, #BA‐2000, Vector Laboratories; RRID:AB_2313581); biotinylated horse anti‐goat (1:200 dilution, #BA‐9500, Vector Laboratories; RRID:AB_2336123); biotinylated horse anti‐rabbit (1:200 dilution, #BA‐1100, Vector Laboratories; RRID:AB_2336201); horse anti‐rabbit DyLight 594 (1:200 dilution, #DI‐1094, Vector Laboratories; RRID:AB_2336414); horse anti‐rabbit DyLight 488 (1:200 dilution, #DI‐1088, Vector Laboratories; RRID:AB_2336403); horse anti‐mouse DyLight 594 (1:200 dilution, #DI‐2594, Vector Laboratories; RRID:AB_2336412); donkey anti‐rat Cy3 (1:500 dilution, #712‐165‐153, Jackson ImmunoResearch; RRID:AB_2340667). For CD31 and cFos, the signal was amplified by incubating sections with a biotinylated secondary antibody, followed by incubation with Alexa fluor‐conjugated streptavidin (#S32357 or #S11223, respectively; both from Invitrogen). For DAB stainings, incubation with biotinylated secondary antibody was followed by incubation with avidin‐biotin complex (1:200 dilution for both reagent A and B, Vectastain Elite ABC kit, Vector Laboratories) for 1 h at RT, and development with DAB. The performed IHC procedures comply with the guidelines of the British Journal of Pharmacology regarding immunoblotting and immunohistochemistry (Alexander et al., 2018).
The Olympus BX51 microscope coupled with computerised set‐up for stereology (Visiopharm, DK or MBF Biosciences, USA) was used for ischaemic volume measurement, Iba‐1+ cell counting and PV+ cell volume measurements. Manual counting of CD68+, PV+, cFOS+, CR+ and DCX+ cells was performed on an Olympus BX40. For cell counting, three consecutive brain sections spaced at 300 μm containing striatum (from Bregma 1 to 0.5 mm) were used. The first section was chosen based on an anatomical location along the rostra‐caudal axis (approximately 1 mm from Bregma). The second and the third sections were 300 and 600 μm caudal from the first section, respectively. Microscopy was performed by experimenters blinded to experimental groups.
In addition to counting of Iba‐1 cells in the entire striatum, Iba‐1 cells were also counted in a specific region of interest (ROI) within the peri‐infarct striatum. This ROI was defined as a 200 μm wide zone, directly adjacent to the infarct region. The number of Iba‐1+ cells within the ROI was counted (in both the ipsilateral and contralateral striatum) and the cell density was expressed as number of cells/mm2.
For analysis of CD31+ vessels and CD13+ pericytes, confocal images were sampled using a LEICA DMi8 confocal microscope. The images of the ipsilateral striatum overlapped both the infarct and peri‐infarct region; on average, images covered 65% infarct region, and 35% peri‐infarct region (determined based on NeuN staining). The collected images were obtained from a z‐stack size of 10 μm at a step size of 1 μm. For maximum image projection, ImageJ software (NIH, USA RRID:SCR_003070) was used for z‐stack image reconstruction. The same acquisition settings were applied for each image. Blood vessel (CD31+) and pericyte (CD13+) density were analysed using the area fraction measurement tool of ImageJ software. The density was expressed as the percentage of the CD31+ or CD13+ area. The coverage of vessels by CD13+ pericytes was assessed by calculating the proportion of CD13+ pericytes covering CD31+ vessels. We used the co‐localization plugin tool of ImageJ (NIH, USA) to define and highlight the covering points of CD13+ with CD31+ in the z‐stack image. The images were reconstructed for a maximum projection and the CD13+/CD31+ coverage area was measured and expressed as a percentage of the total CD31+ area per image. Parenchymal CD13 density was quantified separately by subtracting the density of the perivascular CD13+ cells from the total CD13 density.
2.6. Ischaemic volume measurement
Ischaemic volume was evaluated based on NeuN staining and a Cavalieri estimator probe (MBF Biosciences, USA) on all brain sections with visible stroke. NeuN staining is a consistent method for quantifying neural damage since it exclusively stains neurons. Therefore, it is reliable to evaluate neuronal loss even several weeks post‐stroke unlike live cell markers like TTC (3,5‐triphenyltetrazolium chloride) that accurately identify stroke‐damaged tissue (by lack of positive stain) only within few days after the injury, due to later inflammatory cell infiltration and glial scar formation.
2.7. Mouse insulin ELISA
Serum insulin levels were determined using the Ultra‐Sensitive Mouse Insulin ELISA Kit (CrystalChem, #90080) according to manufacturer's instructions. A total sample volume of 10 μl of serum was used per mouse.
2.8. Data and statistical analysis
All data were analysed by GraphPad Prism Version 8 (GraphPad, USA, RRID:SCR_002798) The data were first checked for statistical outliers by using the ROUT method, and normality by using the Shapiro–Wilk normality test to decide whether to perform parametric or non‐parametric tests. For the diabetic study we compared Non‐T2D versus T2D‐Ve versus T2D‐Ex‐4 mice. For the non‐diabetic study Non‐T2D‐Ve versus Non‐T2D‐Ex‐4 mice were compared.
2.8.1. Parametric tests
Two‐way repeated measures ANOVA with Geisser–Greenhouse's correction followed by Sidak's test was used to analyse grip strength, body weight, fasting glucose and insulin tolerance test (ITT) after stroke. To analyse the volume of PV+ cells, number of cFOS+/PV+ and Iba‐1+ cells, density of CD31+ vessels and CD13+ pericytes, and coverage of CD31+ vessels by CD13+ pericytes, two‐way ANOVA followed by two‐stage linear step‐up procedure of Benjamini, Krieger and Yekutieli test was performed. One‐way ANOVA with Tukey's post hoc test was used to analyse the number of PV+ cells and parenchymal CD13 density in the ipsilateral striatum, in the diabetic study. The number of DCX+ cells in the ipsilateral striatum was analysed using Brown‐Forsythe and Welch ANOVA. In the non‐diabetic study, the ischaemic volume, the number of CD68+ cells and insulin levels were analysed using unpaired t test. Post hoc tests were conducted only if statistical significance was detected by ANOVA.
2.8.2. Non‐parametric tests
For the statistical analysis of ischaemic volume and CD68+ cells in the diabetic study, Kruskal–Wallis with uncorrected Dunn's test was performed.
The group sizes declared above refer to the number of independent values, and statistical analyses were done using these independent values. In a few analyses, concerning the results presented in Figures 2a,e, 5e and 6f–h, some statistical outliers were identified and excluded. Where statistical outliers were excluded, this is indicated in the respective figure legends. The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). All data are expressed as mean ± SD. Values of P < 0.05 were considered statistically significant.
FIGURE 5.
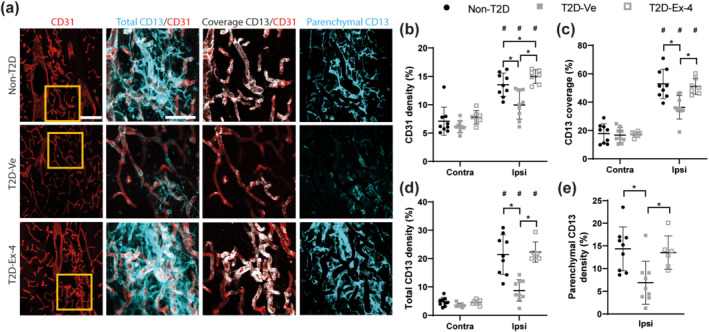
Exendin‐4 restores the density and maturity of vessels in T2D mice after stroke. (a) Representative images of CD31+ (vessels) and CD13+ (pericytes) staining in the ipsilateral striatum. Representative images of the contralateral striatum are included in Supporting Information Figure S2. Scale bar: 100 μm for the left panel (CD31); 50 μm for the two centre and right panels (higher magnification). (b) Density of CD31+ vessels at 8 weeks after stroke. (c) Coverage of vessels by pericytes. (d) Total density of CD13+ pericytes, including both perivascular and parenchymal pericytes. (e) Density of parenchymal CD13+ pericytes. Data presented as mean ± SD. Group sizes for (a)–(d): Non‐T2D n = 9, T2D‐Ve n = 9, T2D‐Ex‐4 n = 7. For (e), one statistical outlier was excluded from the T2D‐Ex‐4 group, resulting in the following group sizes: Non‐T2D n = 9, T2D‐Ve n = 9, T2D‐Ex‐4 n = 6. Two‐way ANOVA followed by two‐stage linear step‐up procedure of Benjamini, Krieger and Yekutieli was used to compare the density of CD31+ vessels, the density of CD13+ pericytes, and coverage of CD31+ vessels by CD13+ pericytes, between non‐T2D and T2D‐Ve and T2D‐Ex‐4, in the contralateral and ipsilateral striatum. One‐way ANOVA with Tukey's post hoc test was used to analyse the density of parenchymal CD13+ pericytes, in the ipsilateral striatum. * P < 0.05, significantly different as indicated; # P < 0.05, contralateral significantly different from ipsilateral, in the same group
FIGURE 6.
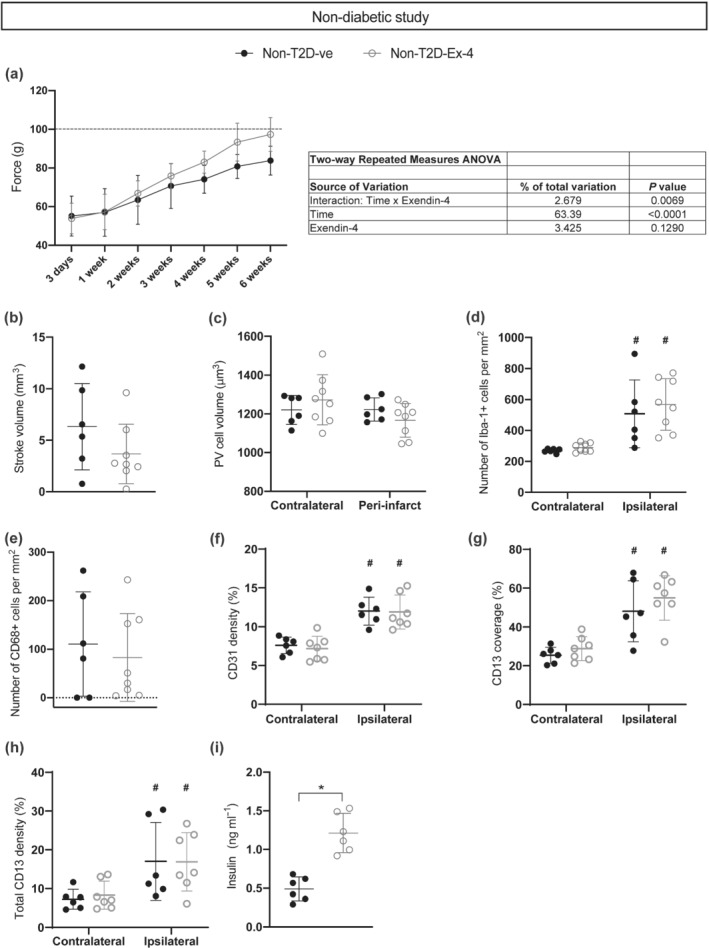
The effect of Exendin‐4 treatment after tMCAO in non‐diabetic mice. (a) Recovery of grip strength and ANOVA table. (b) Ischaemic volume. (c) Average volume of PV+ interneurons. (d) Number of Iba‐1+ cells. (e) Number of CD68+ cells. (f) Density of CD31+ vessels. (g) Coverage of vessels by pericytes. (h) Total density of CD13+ pericytes (including both perivascular and parenchymal pericytes). (i) Serum insulin levels. Data are presented as mean ± SD. Group sizes for (a)–(e): Non‐T2D‐Ve n = 6, non‐T2D‐Ex‐4 n = 8. For (f)–(h), one statistical outlier was excluded from the non‐T2D‐Ex‐4 group, resulting in the following group sizes: Non‐T2D‐Ve n = 6, non‐T2D‐Ex‐4 n = 7. For (i), insulin levels were only measured in n = 6 mice for both groups, resulting in the following group sizes: Non‐T2D‐Ve n = 6, non‐T2D‐Ex‐4 n = 6. Two‐way repeated measures ANOVA with Geisser–Greenhouse's correction followed by Sidak's test was used to compare grip strength between non‐T2D‐Ve and non‐T2D‐Ex‐4. Two‐way ANOVA followed by two‐stage linear step‐up procedure of Benjamini, Krieger and Yekutieli was used to compare the volume of PV+ cells, the number of Iba‐1+ cells, the density of CD31+ vessels, the density of CD13+ pericytes, and coverage of CD31+ vessels by CD13+ pericytes. Unpaired t test was used to analyse ischaemic volume, CD68+ cells and insulin levels. (* P < 0.05, significantly different as indicated; # P < 0.05, contralateral significantly different from ipsilateral, in the same group
2.9. Materials
Exendin‐4 was purchased from Sigma‐Aldrich (#E7144; St. Louis, MO, USA). For ITT, human insulin from Lilly (Indianapolis, IN, USA) was used (Humalog, 100 units ml−1, #VL7516). Details of other materials and suppliers are provided in the specific sections.
2.10. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY (http://www.guidetopharmacology.org) and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
3. RESULTS
3.1. Exendin‐4 normalises the T2D‐induced impairment of neurological recovery after stroke in association with the normalisation of glycaemia and insulin resistance
To detect any potential effects of T2D and exendin‐4 on stroke recovery in T2D mice and potentially correlate this effect with histological outcomes, the experiment was terminated at 8 weeks after tMCAO (time when the Non‐T2D mice fully recovered forepaw grip strength after stroke).
There was no difference between the forepaw grip strength in Non‐T2D and T2D mice before tMCAO (data not shown). At Day 3 post‐tMCAO, stroke decreased forepaw grip strength approximately by 40% (pre‐tMCAO levels are indicated by the dashed line on Figure 2a) in both Non‐T2D and T2D mice (Figure 2a). The two‐way repeated measures ANOVA analysis revealed that during the recovery time (3 days to 8 weeks), forepaw grip strength significantly increased in all mice (main effect–time) (Figure 2a). However, the grip strength was significantly greater in Non‐T2D than in T2D‐Ve mice (main effect–T2D) and a significant interaction between time and T2D was recorded (Figure 2a). These results show that T2D significantly worsened the grip strength recovery. The grip strength in T2D‐Ex‐4 mice was significantly greater versus T2D‐Ve mice (main effect–Ex‐4 treatment) and here too, a significant interaction between time and treatment (Figure 2a) was recorded, indicating that exendin‐4 significantly improved the grip strength in the recovery phase in T2D mice. Later, post hoc statistical tests showed that Non‐T2D mice fully recovered forepaw strength by 8 weeks reaching the pre‐tMCAO levels (Figure 2a), whereas in T2D‐Ve mice the forepaw strength stayed significantly lower (Figure 2a) than in Non‐T2D mice. Remarkably, T2D mice treated with exendin‐4 showed a significant increase in grip strength versus untreated T2D‐Ve mice already from 3 weeks after stroke (Figure 2a) and onwards, and fully recovered their forepaw grip strength by week 8. These differences in grip strength recovery were not determined by the differences in stroke severity (ischaemic volume) (Figure 2e). The stroke injury was generally localised in the dorso‐lateral striatum (Figure 2f).
T2D‐Ve mice significantly and rapidly lost body weight after tMCAO (and the substitution of HFD with standard diet) and from 1–2 weeks after stroke we could not observe any difference in body weight between the groups (Figure 2b). As expected, fasted levels of blood glucose in T2D mice were significantly higher than in Non‐T2D mice before tMCAO (Figure 2c). After tMCAO and the change from HFD to standard diet, blood glucose significantly decreased in the T2D‐Ve group at both 4 (pre‐tMCAO vs. 4 weeks post‐tMCAO) and 8 weeks (4 weeks post‐tMCAO vs. 8 weeks post‐tMCAO) but still remained significantly higher than in Non‐T2D mice both at 4 weeks post‐tMCAO and at 8 weeks, although at this time point the mean glucose levels in T2D‐Ve mice decreased below the diabetic threshold of 7 mmol L−1 (Figure 2c). In T2D mice treated with exendin‐4, fasted blood glucose of ≈ 6 mmol L−1 was reached already at 4 weeks after tMCAO, although still significantly higher than the Non‐T2D mice, and was completely normalised (<5.6 mmol L−1) at 8 weeks after tMCAO (Figure 2c). We also evaluated insulin sensitivity in all groups before stroke and at 4 and 8 weeks post‐tMCAO. Non‐T2D mice maintained normal insulin sensitivity throughout the experiment and T2D‐Ve mice remained insulin resistant (Figure 2d). However, the T2D mice treated with exendin‐4 showed improved insulin sensitivity already at 4 weeks post‐tMCAO compared to T2D‐Ve mice, and then reached the levels of Non‐T2D mice at 8 weeks after tMCAO (Figure 2d).
In summary, these results show that despite normalisation of body weight shortly (2 weeks) after tMCAO, during a large part of the post‐stroke recovery phase (at least for 4 weeks) T2D‐Ve mice remained hyperglycaemic and for the entire recovery phase (8 weeks) they were insulin resistant. Treatment with exendin‐4 in T2D mice normalised hyperglycaemia already 4 weeks after tMCAO and gradually improved insulin sensitivity at 4 and 8 weeks post‐tMCAO. We conclude that the post‐stroke decrease in body weight does not correlate with improvement of recovery in T2D. On the contrary, the impaired post‐stroke neurological recovery in T2D mice is associated with hyperglycaemia and insulin resistance, and exendin‐4 treatment improves stroke recovery by normalising these parameters.
3.2. Improved neurological recovery by exendin‐4 in T2D mice correlates with normalisation of T2D‐induced atrophy of GABAergic parvalbumin+ interneurons, reduced inflammation and improved vascular remodelling and fibrotic scar formation
Stroke similarly decreased (≈60%) the number of surviving PV+ interneurons in the ipsilateral striatum, in all experimental groups (Figure 3a). We have previously shown that shortly (2 weeks) after stroke the soma volume of PV+ interneurons is decreased in the peri‐infarct region of the striatum, and whereas in Non‐T2D mice the soma volume recovers back to normal within 6 weeks after stroke, this atrophy persists in T2D (Pintana et al., 2019). In the present study, potential differences in stroke‐induced atrophy of PV+ interneurons were also assessed by measuring the soma volume of these neurons in the contralateral and ipsilateral peri‐infarct striatum. The results in Figure 3b show a substantial atrophy of PV+ interneuron soma volume in the ipsilateral peri‐infarct striatum of T2D‐Ve mice compared to the corresponding region in Non‐T2D mice and compared to its own contralateral striatum, at 8 weeks after stroke. In T2D mice treated with exendin‐4, no differences were detected in the soma volume of PV+ interneurons in the ipsilateral‐peri‐infarct striatum compared with Non‐T2D mice or compared with its own contralateral striatum (Figure 3b), indicating that the exendin‐4 treatment reversed PV+ interneuron atrophy. To quantify potential differences in basal activation of PV+ interneurons between the groups, we quantified the number of cFos/PV double‐positive neurons in the peri‐infarct striatum. A fraction of PV+ interneurons was activated in both contralateral and ipsilateral striatum, without differences between the groups (Supporting Information Figure S1).
FIGURE 3.
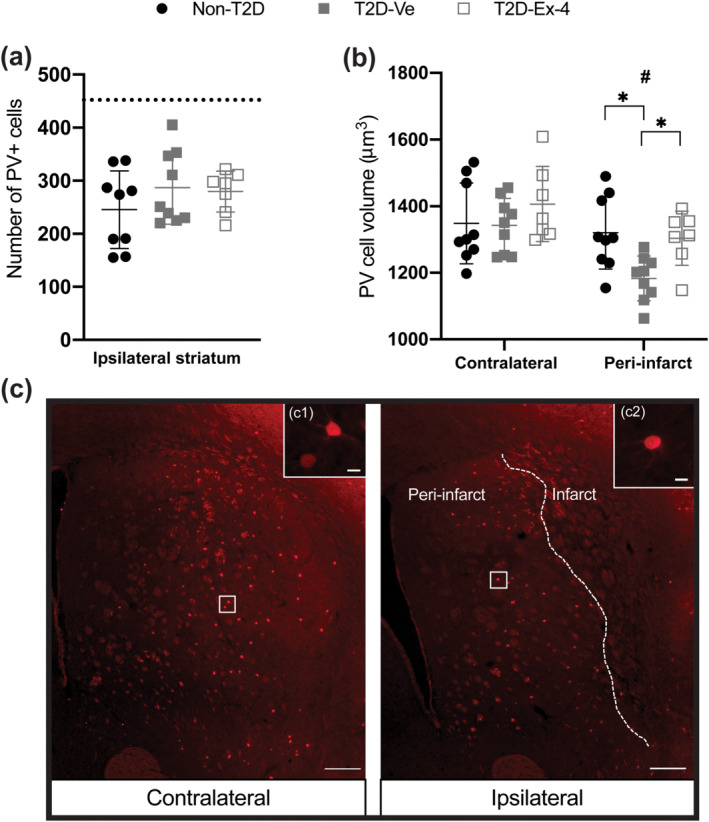
Exendin‐4 counteracts the diet‐induced atrophy of PV+ cells. (a) Number of PV+ cells in the contralateral (represented by dotted line) versus ipsilateral striatum. (b) The average soma volume of PV+ cells. Data presented as mean ± SD. Group sizes for all figures: Non‐T2D n = 9, T2D‐Ve n = 9, T2D‐Ex‐4 n = 7. One‐way ANOVA with Tukey's post hoc test was used to analyse the number of PV+ cells. Two‐way ANOVA followed by two‐stage linear step‐up procedure of Benjamini, Krieger and Yekutieli was used to compare the volume of PV+ cells between non‐T2D and T2D‐Ve and T2D‐Ex‐4. * P < 0.05, significantly different as indicated; # P < 0.05, contralateral significantly different from ipsilateral, in the same group. (c) Representative images of PV+ interneurons in contralateral and ipsilateral striatum, scale bars = 200 and 25 μm for inserts (c1) and (c2)
To assess potential changes in the neuroinflammatory response after stroke, we quantified Iba‐1+ and CD68+ microglia cells in the contralateral and ipsilateral striatum at 8 weeks post‐tMCAO. The total density of Iba‐1+ microglia in the ipsilateral versus respective contralateral striatum was significantly increased after stroke similarly in all groups, indicating persistent neuroinflammation at 8 weeks post‐tMCAO without any additional effects of diabetes or exendin‐4 treatment (Figure 4a). However, when we evaluated density of Iba‐1+ microglia specifically in infarct‐adjacent areas (termed ROI, see Section 2), we recorded a significant effect of exendin‐4 in reducing Iba‐1+ cell density in these regions for non‐T2D compared with T2D‐Ex‐4, with a strong trend for T2D‐Ve compared with T2D‐Ex‐4 (Figure 4b). Microglia/macrophage activation (number of CD68+ cells) was also reduced by exendin‐4 for non‐T2D compared with T2D‐Ex‐4, with a strong trend for T2D‐Ve compared with T2D‐Ex‐4 (Figure 4c).
FIGURE 4.
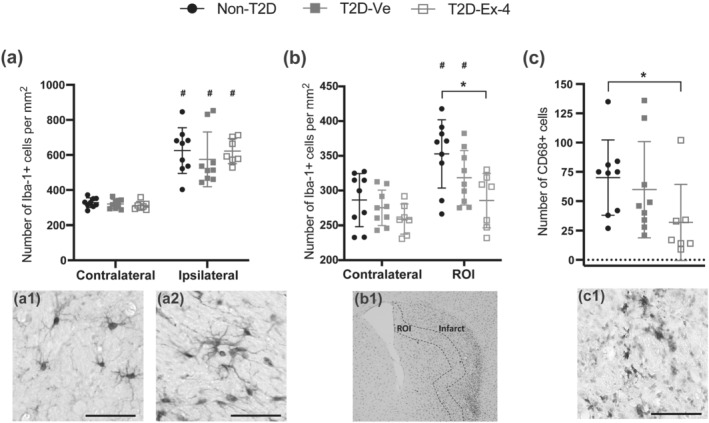
Exendin‐4 decreases neuroinflammation after stroke. (a) Density of Iba‐1+ cells at 8 weeks after stroke. Representative image of Iba‐1+ cells in contralateral (a1) and ipsilateral striatum (a2). (b) Quantification of Iba‐1+ cells in an infarct‐adjacent region of interest (ROI). (b1) Photomicrograph of Iba1 staining, ROI is outlined by dashed line. (c) Number of CD68+ cells in the ipsilateral striatum. (c1) Representative image of CD68+ cells. Data are presented as mean ± SD. Group sizes for all figures: Non‐T2D n = 9, T2D‐Ve n = 9, T2D‐Ex‐4 n = 7. Two‐way ANOVA followed by two‐stage linear step‐up procedure of Benjamini, Krieger and Yekutieli was used to compare the number of Iba‐1+ cells in the contralateral and ipsilateral striatum (in a); and in contralateral versus ROI (in b) between non‐T2D and T2D‐Ve and T2D‐Ex‐4. Kruskal–Wallis with uncorrected Dunn's test was used to compare the number of CD68+ cells between non‐T2D and T2D‐Ve and T2D‐Ex‐4. * P < 0.05, significantly different as indicated; # P < 0.05, contralateral significantly different from ipsilateral, in the same group. Scale bars in (a1), (a2), and (c1) = 50 μm
To assess the effects of T2D and exendin‐4 treatment on vascular remodelling after stroke, CD31+ vessel density and maturity were examined. Striatal vessel density in the infarct area was increased in all groups compared to the respective contralateral striatum (Figure 5b). T2D‐Ve mice, however, had a significantly reduced ipsilateral striatal vessel density compared with non‐T2D mice, which was restored by exendin‐4 treatment (Figure 5b). Notably, exendin‐4 treatment increased vessel density in T2D mice to even slightly above non‐T2D levels (Figure 5b). A similar pattern was found for the coverage of vessels by pericytes, an indicator of vessel maturity. In fact, the coverage of CD31+ vessels by CD13+ pericytes was increased by stroke in all groups, when comparing the ipsilateral versus contralateral striatum (Figure 5c). However, pericyte coverage in the ipsilateral striatum was reduced in T2D mice when comparing T2D‐Ve compared with non‐T2D mice and normalised by exendin‐4 treatment (Figure 5c), indicating improved vascular remodelling and pericyte recruitment.
Similarly, the total pericyte density was increased after stroke in all groups when comparing the ipsilateral compared with contralateral striatum (Figure 5d). The total density of CD13+ pericytes in the ipsilateral striatum was reduced in T2D‐Ve compared with non‐T2D mice and increased by exendin‐4 treatment (Figure 5d). Changes in CD13+ pericyte density between T2D‐Ve compared with non‐T2D mice and the restorative effect of exendin‐4 were observed for both perivascular and parenchymal pericytes, as indicated by the density of parenchymal pericytes in the ipsilateral striatum, an indicator of the fibrotic scar formation after stroke (Figure 5e). There were no differences between groups in the contralateral striatum for all analyses (Figure 5b–d).
We also assessed potential changes in the early phase of stroke‐induced neurogenesis by quantifying neuroblasts (DCX+) and early post‐mitotic neurons (Calretinin+/NeuN‐) (Brandt et al., 2003) in the striatum 8 weeks after tMCAO. The number of DCX+ cells was increased in ipsilateral compared with contralateral striatum in all groups, whereas no differences between ipsilateral compared with contralateral striatum were found for the number of Calretinin+/NeuN− cells (data not shown). We did not record any significant differences between the groups, for both of the assessed cell types (Supporting Information Figure S3a,b).
Overall, these results suggest that T2D impairs recovery of forepaw grip strength during 8 weeks after stroke in association with a substantial atrophy of PV+ interneurons and reduced vascular remodelling and fibrotic scar formation, and that this effect is counteracted by exendin‐4 treatment. Exendin‐4 also reduced neuroinflammation, although we could not correlate this effect with improved neurological recovery, as no differences in Iba‐1+ and CD68+ microglia cells were observed between non‐T2D and T2D‐Ve mice.
3.3. Exendin‐4 marginally improved neurological recovery after stroke in non‐T2D mice
The next step in our study was to investigate the potential efficacy of exendin‐4 to improve neurological recovery after stroke in non‐T2D mice. In order to detect any potential effects of exendin‐4 on stroke recovery in non‐T2D mice and potentially correlate this effect with histological outcomes, the experiment was terminated at 6 weeks after tMCAO (time when the exendin‐4‐treated mice fully recovered forepaw grip strength after stroke). The two‐way repeated measures ANOVA analyses showed a significant time effect (both groups improved grip strength over time) and no significant treatment/ exendin‐4 effect (Figure 6a). However, a significant interaction between time and exendin‐4 treatment was still found (Figure 6a), showing that the difference in grip strength between the groups was increasing over time. This indicates a positive effect of exendin‐4 on neurological recovery that we interpret as minor in comparison with the effect obtained in the T2D study (Figure 2a).
Histological/quantitative assessments of ischaemic volume (Figure 6b), PV+ interneuron volume (Figure 6c), neuroinflammation (Figure 6d,e), CD31+ vessel density (Figure 6f), coverage of vessels by pericytes (Figure 6g) and CD13+ pericyte density (Figure 6h) did not reveal any significant effects of exendin‐4 treatment. However, despite the mice being normoglycaemic, we could detect a significant increase of plasma insulin levels in the exendin‐4‐treated group (Figure 6i).
4. DISCUSSION
Several GLP‐1 receptor agonists have been tested for stroke prevention (Malhotra et al., 2020). However, no clinical studies have investigated the potential efficacy of this class of drugs in post‐stroke recovery and the medical need in this area is very high (Darsalia, Larsson, et al., 2018). We chose to use exendin‐4 in our study because of the well characterised beneficial effects mediated by this drug in the injured brain after stroke (Darsalia, Klein, et al., 2018). Furthermore, it is known that exendin‐4 can cross the blood–brain barrier (Kastin & Akerstrom, 2003).
We show that exendin‐4 administered to diabetic mice after ischaemic stroke leads to a significant improvement of post‐stroke recovery assessed by forepaw grip strength (indicative of neurological functional recovery). This effect was associated with the normalisation of hyperglycaemia and insulin sensitivity, as well as with a reversal of stroke‐induced PV+ interneuron atrophy, and improvement of vascular remodelling as assessed by vascular density, coverage of vessels by pericytes and maintenance of fibrotic scar formation. Additionally, the exendin‐4 treatment reduced stroke‐induced inflammation. In normoglycaemic mice, (in the absence of T2D), exendin‐4 treatment only slightly accelerated neurological recovery after stroke.
The medical need to identify new strategies to improve neurological recovery after stroke in the T2D population is urgent since the global prevalence of T2D is not only markedly increasing but T2D is also one of the strongest risk factors for stroke (see Section 1). This will result in an exceptionally large increase of people with T2D in need of post‐stroke treatment and care. Our results showing that exendin‐4 improved post‐stroke neurological recovery in T2D mice suggest that exendin‐4 could be a valid candidate to meet this medical need. Indeed, exendin‐4 and other GLP‐1 receptor agonists could have several advantages. Already, the 2020 Standards of medical care for diabetes from the American Diabetes Association has recommended the use of GLP‐1 receptor agonists with demonstrated cardiovascular benefit, as an adjunct to metformin in T2D patients with high atherosclerotic cardiovascular disease (ASCVD) risk or established ASCVD for primary or secondary prevention of a cardiovascular event (American Diabetes, 2020). A meta‐analysis of the effect of GLP‐1 receptor agonists including pooled analysis of 56,004 patients encompassing the lixisenatide (ELIXA), liraglutide‐ (LEADER), semaglutide (SUSTAIN‐6 and PIONEER‐6), exenatide (synthetic form of exendin‐4; EXSCEL), albiglutide (HARMONY) and dulaglutide (REWIND) randomised control trials showed a 16% reduction in risk of total stroke, a 15% reduction in risk of nonfatal stroke, and a non‐significant 19% reduction in fatal stroke (Bellastella et al., 2020). Furthermore, there are many published reports that GLP‐1 receptor activation can reduce acute brain damage after stroke in rodents with (Darsalia et al., 2012, 2014) or without T2D (see in Darsalia, Klein, et al., 2018; Maskery et al., 2020). Therefore, if our results are confirmed in other experimental settings (and by using other GLP‐1 receptor agonists), they could reveal that a therapy based on GLP‐1 receptor activation in T2D patients, in addition to preventive and acute/reparative effects, could also present chronic beneficial effects on neurological recovery in T2D patients suffering from stroke. Importantly, this question could also soon find an answer in the clinical setting. Indeed, the Short‐Term Exenatide in Acute ischemic Stroke (STEXAS) study is currently testing the safety of exenatide versus insulin in patients with hyperglycaemia in the acute phase after ischaemic stroke and will include the modified Rankin scale after 3 months in their secondary outcome analysis (Bellastella et al., 2020). In addition, the Treatment with Exenatide in Acute Ischemic Stroke (TEXAIS) PhaseII trial is currently including patients both with and without hyperglycaemia in the acute phase after ischaemic stroke to investigate neurological improvement at 7 days as a primary endpoint and the modified Rankin scale after 90 days as a secondary outcome (Muller et al., 2018). However, it should be noted that these studies will not evaluate the effect of exenatide on a population with a previous T2D diagnosis and thus more studies will be needed in the future (Darsalia, Larsson, et al., 2018).
The mechanisms underlying the improved neurological recovery mediated by exendin‐4 are largely unknown. In our study we demonstrated that neurological recovery by exendin‐4 did not correlate with weight loss during the recovery phase, as both vehicle and exendin‐4‐treated groups achieved normal weight similarly and simultaneously within the first weeks after tMCAO. However, exendin‐4 treatment rapidly (already at 4 weeks) reduced fasting hyperglycaemia and significantly improved insulin sensitivity and both these parameters were entirely normalised 8 weeks post‐tMCAO by exendin‐4. These results suggest the key importance of normalising hyperglycaemia to improve stroke recovery not only acutely after hospitalisation, but also chronically for the entire post‐stroke recovery phase consistent with our previous reports where two other glycaemic strategies (DPP‐4 inhibitors and sulfonylurea) were employed (Augestad et al., 2020). Taken together, the results of the current and the previous study (Augestad et al., 2020) strongly suggest an important contribution from insulin, in order to achieve good rehabilitation after stroke.
Only a few clinical studies have so far investigated the potential detrimental role of insulin resistance on neurological recovery after stroke. A Chinese study of 1245 non‐diabetic patients with mild ischaemic stroke observed a correlation between insulin resistance (measured by HOMA‐IR) in the acute phase with worse functional outcome defined as modified Rankin score 3–6 after 1 year with an adjusted odds ratio 1.42 (1.03–1.95) (Jing et al., 2017). Moreover, Ozkul and colleagues examined the relationships between inflammation, oxidative stress and stroke severity in a small study of 75 acute stroke patients with and without insulin resistance. They found increased IL‐6 and decreased IL‐10 levels in insulin‐resistant patients and a correlation with higher NIHSS scores indicating more severe stroke (Ozkul et al., 2013). Finally, a Japanese registry study by Ago et al. analysed 4655 patients with ischaemic stroke correlated insulin resistance (measured by HOMA‐IR) with worse functional outcome in non‐insulin dependent patients (Ago et al., 2018). Although speculative, the results of these clinical studies support the potential role of exendin‐4 to improve neurological recovery after stroke, through the normalisation of insulin resistance.
The positive effects mediated by exendin‐4 in stroke recovery could also be mediated by indirect (via glycaemia regulation and normalisation of insulin resistance) or direct effects occurring in the brain. Interestingly, we have shown that exendin‐4 treatment reversed T2D‐induced PV+ interneuron atrophy (Pintana et al., 2019). PV+ interneurons play a key role in neuronal plasticity and thus could have significant effects on neurological recovery after stroke (Hattori et al., 2017; Hu et al., 2014). It is difficult to determine whether the efficacy of exendin‐4 in reversing PV+ interneuron atrophy could be linked to metabolic (reduced fasting hyperglycaemia and improved insulin sensitivity) or to direct neuroplasticity effects. Analysing the effects of exendin‐4 on PV+ interneuron atrophy, in non‐diabetic conditions, should have answered this question. However, in the non‐diabetic study, PV+ interneuron volume was already normalised in vehicle group at 6 weeks after stroke when the morphometric analyses were performed. Thus, whether exendin‐4 had any effects on PV+ interneuron volume normalisation at earlier time‐points remains to be investigated. It also remains to be investigated whether the identified effect on PV+ interneurons is specific for these cells or also involves other types of interneurons.
Exendin‐4 treatment reduced stroke‐induced inflammation in T2D, but not in non‐T2D mice. Although the anti‐inflammatory efficacy of exendin‐4 after stroke is well‐established in preclinical studies (Darsalia et al., 2012; Kim et al., 2017), in the present study we could not correlate this effect with improvement in stroke recovery as we could not detect any increase of neuroinflammation in T2D compared with non‐T2D mice at 8 weeks after stroke. However, considering the very many published reports regarding anti‐inflammatory efficacy of GLP‐1 analogues (Marlet et al., 2018), the potential effects of exendin‐4 treatment at earlier time points should not be discounted.
Post‐stroke recovery during the chronic phase requires remodelling of the vasculature (Liu et al., 2014). Remodelling of the vasculature is closely associated with vessel stabilisation by brain pericytes (Ghori et al., 2017; Jean LeBlanc et al., 2018; Kokovay et al., 2006; Zechariah et al., 2013). Impaired post‐stroke vessel density in T2D indicates that diabetic conditions reduce recovery mechanisms under pathological situations, such as stroke (Ergul et al., 2014). Consistent with previous reports, we found that vessel density and coverage of vessels by pericytes were significantly reduced in T2D mice after stroke indicating failure to form new mature micro‐vessels (Cui et al., 2011; Prakash et al., 2013). Interestingly, we show for the first time that this negative impact of T2D on vascular remodelling in the chronic phase of stroke could be completely reversed by exendin‐4 treatment. This novel finding is in line with previously reported beneficial effects of GLP‐1 receptor activation on the vasculature. For example, GLP‐1 has been shown to improve endothelial function in T2D (Nystrom et al., 2004) and GLP‐1 receptor activation after stroke can stimulate angiogenesis (Chen et al., 2018; Zhao et al., 2015), microvascular recruitment and blood flow (Almutairi et al., 2019).
Pericytes play an active role in tissue remodelling after stroke. In particular, it has been suggested that pericytes detach from the vessel wall and migrate into the injured parenchyma where parenchymal pericytes form a dense network contributing to the fibrotic scar after stroke (Fernández‐Klett et al., 2013; Makihara et al., 2015; Roth et al., 2019; Shen et al., 2012). Interestingly, our study shows that the density of parenchymal pericytes is reduced in T2D but is restored to levels, comparable to those in of non‐diabetic animals, when exendin‐4 was administered. Even though the fibrotic scar is still not well studied in stroke, our results suggest that exendin‐4 may play a beneficial role in tissue remodelling during the chronic phase of stroke.
In the non‐diabetic study, we showed that exendin‐4 was only marginally effective to improve neurological recovery after stroke. This was surprising considering the number of reports showing the beneficial effects mediated by GLP‐1 receptor activation in rodent stroke models without T2D (Marlet et al., 2018). Decreased efficacy under non‐diabetic conditions could be explained by the already very robust recovery in vehicle‐treated non‐T2D mice (a ceiling effect). Indeed, these mice recover quickly after stroke and are thus likely to provide little opportunity to further improve recovery after pharmacological treatment. Another important aspect to consider is that in addition to the normalisation of hyperglycaemia and insulin sensitivity, GLP‐1 analogues (and by proxy insulin) also improve or enhance synaptic plasticity and neuroplasticity (Korol et al., 2015; Larsson et al., 2016; Mainardi et al., 2015; McClean et al., 2010). Furthermore several studies have shown the neurotrophic and neuroprotective effects of insulin and insulin‐enhancing drugs in animal models of stroke (Mielke & Wang, 2011; Pomytkin et al., 2018). Thus, the likelihood of the beneficial effects mediated by insulin on stroke recovery should not be discounted. We show that although standard diet‐fed mice (both untreated and treated with exendin‐4) were normoglycaemic, exendin‐4 did induce a significant increase of plasma insulin levels, 6 weeks after tMCAO. Therefore, although speculative, the minor but significant effect of exendin‐4 to improve stroke recovery could be potentially attributed to increased trophic/neuroplasticity mechanisms induced by elevated insulin.
One of the limitations of the study is the use of male mice only. Sex differences are an important issue in stroke research and should not be side stepped. It has been shown that GLP‐1 receptor agonists are more effective in females at reducing the risk of cardiovascular events including stroke (Raparelli et al., 2020), although it is unknown whether the difference in efficacy could also persist during post‐stroke rehabilitation. Nevertheless, this is an important topic that needs to be investigated. We limited our study to male mice, as female mice are resistant to developing the metabolic syndrome after HFD exposure (Pettersson et al., 2012) and thus an addition of females to our study would have introduced an unwanted variable (difference in metabolic state) in this ‘proof of concept’ study and complicated the data interpretation. Other limitations of the study are the lack of histological assessments at intermediate time points and the reliance on a well‐validated (Augestad et al., 2020; Chiazza et al., 2018, 2020; Pintana et al., 2019) but single behavioural readout. Therefore, we acknowledge that our findings will have to be confirmed by additional studies. On the other hand, a strength of our study is represented by the experimental T2D model used here and the clinically relevant study design. Unlike genetic or toxin‐induced T2D models, diet‐induced obesity/T2D more accurately models lifestyle‐induced T2D in humans (Heydemann, 2016). Moreover, this model perfectly replicates the T2D‐induced impairment of functional recovery that is observed in stroke patients (Ullberg et al., 2015). Additionally, by replacing HFD with standard diet after stroke, we have removed the potential confounding effects of different diets during the stroke recovery phase, as well as mimicking the condition of diabetic stroke survivors who experience lifestyle/diet changes after stroke.
In conclusion, our results have demonstrated the effectiveness of a GLP‐1 receptor agonist in improving neurological recovery after stroke in T2D. Our study expands on a previous report showing the significant benefit of glycaemia regulation during the post‐acute, chronic, stroke recovery phase (Augestad et al., 2020) and provides the first experimental evidence for the key role of the normalisation of insulin sensitivity in the improvement of long‐term neurological recovery after stroke. These results strongly motivate additional studies to investigate the use of T2D therapies that, in addition to glycaemia regulation, also improve insulin sensitivity thus promoting better functional recovery after stroke.
AUTHOR CONTRIBUTIONS
I.A. and D. D performed behavioural analysis, immunohistochemistry studies and stereology analysis; acquired and processed images and figures; contributed to discussion; edited the manuscript. D.K. and O.E. acquired and processed images and figures, performed part of the immunohistochemistry, the ELISA experiments, contributed to discussion and edited the manuscript. H.P. performed the ischaemic volume assessment. A. Z and M.L. provided expertise, contributed to discussion and edited the manuscript T.N. provided expertise and resources, contributed to discussion and edited the manuscript. G.P. conceived and provided resources for the vascular part of the study, contributed to discussion and edited the manuscript. V.D. conceived and designed the study, performed the stroke experiments and diabetic tests, contributed to discussion and wrote the manuscript. C.P. conceived, designed and coordinated the research plan, contributed to discussion and wrote the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
T.N. has received unrestricted grants from AstraZeneca and consultancy fees from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Merck and Sanofi‐Aventis. The other authors declare that they have no competing interests.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design and Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1. Activation of PV + cells. Quantification of cFOS+/PV + cells at 8 weeks after stroke in the contralateral vs. peri‐infarct in the ipsilateral striatum showed no stroke effect or any difference between the groups except a trend towards decreased activation of PV + cells in the T2D‐Ve group compared to the non‐T2D group. Data presented as mean±SD. Two‐way ANOVA followed by Two‐stage linear step‐up procedure of Benjamini, Krieger and Yekutieli was used to compare the number of cFOS+/PV + cells between non‐T2D vs. T2D‐Ve vs. T2D‐Ex‐4.
Figure S2. Representative images of CD31 + (vessels) and CD13 + (pericytes) staining in the contralateral striatum. Scale bar: 150 μm.
Figure S3. Neurogenesis. Quantification of DCX + (a) and CR+/NeuN‐ (b) cells 8 weeks after stroke in the ipsilateral striatum showed no difference between the groups. Data presented as mean±SD. Brown‐Forsythe and Welch ANOVA was used to compare the number of DCX + cells and the number of CR+/NeuN‐ cells between non‐T2D vs. T2D‐Ve vs. T2D‐Ex‐4.
ACKNOWLEDGEMENTS
We thank Valentina Gustafsson for help with the IHC analysis, Dr. Fuad Bahram (Södersjukhuset) for technical assistance and Dr. Hans Pettersson for advice on statistical analyses. Financial support was provided by the Swedish Research Council (Vetenskapsrådet), the European Foundation for the Study of Diabetes (EFSD)/Sanofi European Diabetes Research Program in Macrovascular Complications, the Swedish Heart‐Lung Foundation, Diabetesfonden, Ulla Hamberg Angeby och Lennart Angebys Stiftelse, Svensk Förening för Diabetologi, Karolinska Institutet (Foundation for Geriatric Diseases and KI Stiftelser och Fonder), O. E. och Edla Johanssons Stiftelse, Magnus Bergvalls Stiftelse, STROKE Riksförbundet and the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and the Karolinska Institutet.
Augestad, I. L. , Dekens, D. , Karampatsi, D. , Elabi, O. , Zabala, A. , Pintana, H. , Larsson, M. , Nyström, T. , Paul, G. , Darsalia, V. , & Patrone, C. (2022). Normalisation of glucose metabolism by exendin‐4 in the chronic phase after stroke promotes functional recovery in male diabetic mice. British Journal of Pharmacology, 179(4), 677–694. 10.1111/bph.15524
Ingrid Lovise Augestad and Doortje Dekens contributed equally to this study.
Funding information Diabetesfonden; European Foundation for the Study of Diabetes; Hjärt‐Lungfonden; Karolinska Institutet; Magnus Bergvalls Stiftelse; O. E. och Edla Johanssons Stiftelse; STROKE Riksförbundet; Svensk Förening för Diabetologi; The regional agreement on medical training and clinical research (ALF) between Stockholm County Council and the Karolinska Institutet; Ulla Hamberg Angeby och Lennart Angebys Stiftelse; Vetenskapsrådet; Swedish Heart‐Lung Foundation
Contributor Information
Vladimer Darsalia, Email: vladimer.darsalia@ki.se.
Cesare Patrone, Email: cesare.patrone@ki.se.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Ago, T. , Matsuo, R. , Hata, J. , Wakisaka, Y. , Kuroda, J. , Kitazono, T. , Kamouchi, M. , & Fukuoka Stroke Registry, I. (2018). Insulin resistance and clinical outcomes after acute ischemic stroke. Neurology, 90, e1470–e1477. 10.1212/WNL.0000000000005358 [DOI] [PubMed] [Google Scholar]
- Alexander S. P. H., Kelly E., Mathie A., Peters J. A., Veale E. L., Armstrong J. F., Faccenda E., Harding S. D., Pawson A. J., Sharman J. L., Southan C., Buneman O. P., Cidlowski J. A., Christopoulos A., Davenport A. P., Fabbro D., Spedding M., Striessnig J., Davies J. A., … Wong S. S. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Introduction and Other Protein Targets. British Journal of Pharmacology, 176(S1), 10.1111/bph.14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , Cirino, G. , Docherty, J. R. , Giembycz, M. A. , Hoyer, D. , Insel, P. A. , Izzo, A. A. , Ji, Y. , MacEwan, D. J. , Mangum, J. , Wonnacott, S. , & Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology . British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfayez, O. M. , Almohammed, O. A. , Alkhezi, O. S. , Almutairi, A. R. , & Al Yami, M. S. (2020). Indirect comparison of glucagon like peptide‐1 receptor agonists regarding cardiovascular safety and mortality in patients with type 2 diabetes mellitus: Network meta‐analysis. Cardiovascular Diabetology, 19, 96. 10.1186/s12933-020-01070-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almutairi, M. , Al Batran, R. , & Ussher, J. R. (2019). Glucagon‐like peptide‐1 receptor action in the vasculature. Peptides, 111, 26–32. 10.1016/j.peptides.2018.09.002 [DOI] [PubMed] [Google Scholar]
- American Diabetes A . (2020). Standards of medical care in diabetes‐2020 abridged for primary care providers. Clin Diabetes, 38, 10–38. 10.2337/cd20-as01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augestad, I. L. , Pintana, H. , Larsson, M. , Krizhanovskii, C. , Nystrom, T. , Klein, T. , Darsalia, V. , & Patrone, C. (2020). The regulation of glycemia in the recovery phase after stroke counteracts the detrimental effect of obesity‐induced type 2 diabetes on neurological recovery. Diabetes, 69, 1961–1973. 10.2337/db20-0095 [DOI] [PubMed] [Google Scholar]
- Baird, T. A. , Parsons, M. W. , Phan, T. , Butcher, K. S. , Desmond, P. M. , Tress, B. M. , Colman, P. G. , Chambers, B. R. , & Davis, S. M. (2003). Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke, 34, 2208–2214. 10.1161/01.STR.0000085087.41330.FF [DOI] [PubMed] [Google Scholar]
- Bederson, J. B. , Pitts, L. H. , Tsuji, M. , Nishimura, M. C. , Davis, R. L. , & Bartkowski, H. (1986). Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke, 17, 472–476. 10.1161/01.STR.17.3.472 [DOI] [PubMed] [Google Scholar]
- Bellastella, G. , Maiorino, M. I. , Longo, M. , Scappaticcio, L. , Chiodini, P. , Esposito, K. , & Giugliano, D. (2020). Glucagon‐like peptide‐1 receptor agonists and prevention of stroke systematic review of cardiovascular outcome trials with meta‐analysis. Stroke, 51, 666–669. 10.1161/STROKEAHA.119.027557 [DOI] [PubMed] [Google Scholar]
- Benjamin, E. J. , Virani, S. S. , Callaway, C. W. , Chamberlain, A. M. , Chang, A. R. , Cheng, S. , Chiuve, S. E. , Cushman, M. , Delling, F. N. , Deo, R. , de Ferranti, S. D. , Ferguson, J. F. , Fornage, M. , Gillespie, C. , Isasi, C. R. , Jiménez, M. C. , Jordan, L. C. , Judd, S. E. , Lackland, D. , … American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . (2018). Heart disease and stroke statistics‐2018 update: A report from the American Heart Association. Circulation, 137, e67–e492. 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- Best, J. H. , Hoogwerf, B. J. , Herman, W. H. , Pelletier, E. M. , Smith, D. B. , Wenten, M. , & Hussein, M. A. (2011). Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon‐like peptide 1 (GLP‐1) receptor agonist exenatide twice daily or other glucose‐lowering therapies: A retrospective analysis of the LifeLink database. Diabetes Care, 34, 90–95. 10.2337/dc10-1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt, M. D. , Jessberger, S. , Steiner, B. , Kronenberg, G. , Reuter, K. , Bick‐Sander, A. , Behrens, W. , & Kempermann, G. (2003). Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Molecular and Cellular Neurosciences, 24, 603–613. 10.1016/S1044-7431(03)00207-0 [DOI] [PubMed] [Google Scholar]
- Chen, F. , Wang, W. , Ding, H. , Yang, Q. , Dong, Q. , & Cui, M. (2016). The glucagon‐like peptide‐1 receptor agonist exendin‐4 ameliorates warfarin‐associated hemorrhagic transformation after cerebral ischemia. Journal of Neuroinflammation, 13, 204. 10.1186/s12974-016-0661-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Zhang, X. , He, J. , Xie, Y. , & Yang, Y. (2018). Delayed administration of the glucagon‐like peptide 1 analog liraglutide promoting angiogenesis after focal cerebral ischemia in mice. Journal of Stroke and Cerebrovascular Diseases, 27, 1318–1325. 10.1016/j.jstrokecerebrovasdis.2017.12.015 [DOI] [PubMed] [Google Scholar]
- Chiazza, F. , Pintana, H. , Lietzau, G. , Nystrom, T. , Patrone, C. , & Darsalia, V. (2020). The stroke‐induced increase of somatostatin‐expressing neurons is inhibited by diabetes: A potential mechanism at the basis of impaired stroke recovery. Cellular and Molecular Neurobiology, 41, 591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiazza, F. , Tammen, H. , Pintana, H. , Lietzau, G. , Collino, M. , Nystrom, T. , Klein, T. , Darsalia, V. , & Patrone, C. (2018). The effect of DPP‐4 inhibition to improve functional outcome after stroke is mediated by the SDF‐1alpha/CXCR4 pathway. Cardiovascular Diabetology, 17, 60. 10.1186/s12933-018-0702-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, X. , Chopp, M. , Zacharek, A. , Ye, X. , Roberts, C. , & Chen, J. (2011). Angiopoietin/Tie2 pathway mediates type 2 diabetes induced vascular damage after cerebral stroke. Neurobiology of Disease, 43, 285–292. 10.1016/j.nbd.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , Hoyer, D. , Insel, P. A. , Izzo, A. A. , Ji, Y. , MacEwan, D. J. , Sobey, C. G. , Stanford, S. C. , Teixeira, M. M. , Wonnacott, S. , & Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsalia, V. , Hua, S. , Larsson, M. , Mallard, C. , Nathanson, D. , Nystrom, T. , Sjöholm, Å. , Johansson, M. E. , & Patrone, C. (2014). Exendin‐4 reduces ischemic brain injury in normal and aged type 2 diabetic mice and promotes microglial m2 polarization. PLoS ONE, 9, e103114. 10.1371/journal.pone.0103114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsalia, V. , Klein, T. , Nyström, T. , & Patrone, C. (2018). Glucagon‐like receptor 1 agonists and DPP‐4 inhibitors: Anti‐diabetic drugs with anti‐stroke potential. Neuropharmacology, 136, 280–286. 10.1016/j.neuropharm.2017.08.022 [DOI] [PubMed] [Google Scholar]
- Darsalia, V. , Larsson, M. , Klein, T. , & Patrone, C. (2018). The high need for trials assessing functional outcome after stroke rather than stroke prevention with GLP‐1 agonists and DPP‐4 inhibitors. Cardiovascular Diabetology, 17, 32. 10.1186/s12933-018-0674-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsalia, V. , Mansouri, S. , Ortsater, H. , Olverling, A. , Nozadze, N. , Kappe, C. , Iverfeldt, K. , Tracy, L. M. , Grankvist, N. , Sjöholm, Å. , & Patrone, C. (2012). Glucagon‐like peptide‐1 receptor activation reduces ischaemic brain damage following stroke in type 2 diabetic rats. Clinical Science (London, England), 122(10), 473–483. 10.1042/CS20110374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillen, Y. , Kemps, H. , Gervois, P. , Wolfs, E. , & Bronckaers, A. (2020). Adult neurogenesis in the subventricular zone and its regulation after ischemic stroke: Implications for therapeutic approaches. Translational Stroke Research, 11, 60–79. 10.1007/s12975-019-00717-8 [DOI] [PubMed] [Google Scholar]
- Dong, W. , Miao, Y. , Chen, A. , Cheng, M. , Ye, X. , Song, F. , & Zheng, G. (2017). Delayed administration of the GLP‐1 receptor agonist liraglutide improves metabolic and functional recovery after cerebral ischemia in rats. Neuroscience Letters, 641, 1–7. 10.1016/j.neulet.2017.01.045 [DOI] [PubMed] [Google Scholar]
- Ergul, A. , Abdelsaid, M. , Fouda, A. Y. , & Fagan, S. C. (2014). Cerebral neovascularization in diabetes: Implications for stroke recovery and beyond. Journal of Cerebral Blood Flow and Metabolism, 34, 553–563. 10.1038/jcbfm.2014.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐Klett, F. , Potas, J. R. , Hilpert, D. , Blazej, K. , Radke, J. , Huck, J. , Engel, O. , Stenzel, W. , Genové, G. , & Priller, J. (2013). Early loss of pericytes and perivascular stromal cell‐induced scar formation after stroke. Journal of Cerebral Blood Flow and Metabolism, 33, 428–439. 10.1038/jcbfm.2012.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein, H. C. , Hart, R. , Colhoun, H. M. , Diaz, R. , Lakshmanan, M. , Botros, F. T. , Probstfield, J. , Riddle, M. C. , Rydén, L. , Atisso, C. M. , Dyal, L. , Hall, S. , Avezum, A. , Basile, J. , Conget, I. , Cushman, W. C. , Hancu, N. , Hanefeld, M. , Jansky, P. , … Temelkova‐Kurktschiev, T. (2020). The effect of dulaglutide on stroke: An exploratory analysis of the REWIND trial. The Lancet Diabetes & Endocrinology, 8, 106–114. 10.1016/S2213-8587(19)30423-1 [DOI] [PubMed] [Google Scholar]
- Ghori, A. , Freimann, F. B. , Nieminen‐Kelhä, M. , Kremenetskaia, I. , Gertz, K. , Endres, M. , & Vajkoczy, P. (2017). EphrinB2 activation enhances vascular repair mechanisms and reduces brain swelling after mild cerebral ischemia. Arteriosclerosis, Thrombosis, and Vascular Biology, 37, 867–878. 10.1161/ATVBAHA.116.308620 [DOI] [PubMed] [Google Scholar]
- Goke, R. , Fehmann, H. C. , Linn, T. , Schmidt, H. , Krause, M. , Eng, J. , & Goke, B. (1993). Exendin‐4 is a high potency agonist and truncated exendin‐(9‐39)‐amide an antagonist at the glucagon‐like peptide 1‐(7‐36)‐amide receptor of insulin‐secreting beta‐cells. The Journal of Biological Chemistry, 268, 19650–19655. 10.1016/S0021-9258(19)36565-2 [DOI] [PubMed] [Google Scholar]
- Gutniak, M. , Ørkov, C. , Holst, J. J. , Ahrén, B. , & Efendić, S. (1992). Antidiabetogenic effect of glucagon‐like peptide‐1 (7–36)amide in normal subjects and patients with diabetes mellitus. New England Journal of Medicine, 326, 1316–1322. 10.1056/NEJM199205143262003 [DOI] [PubMed] [Google Scholar]
- Gutniak, M. K. , Linde, B. , Holst, J. J. , & Efendic, S. (1994). Subcutaneous injection of the incretin hormone glucagon‐like peptide 1 abolishes postprandial glycemia in NIDDM. Diabetes Care, 17, 1039–1044. 10.2337/diacare.17.9.1039 [DOI] [PubMed] [Google Scholar]
- Hara, H. , Huang, P. L. , Panahian, N. , Fishman, M. C. , & Moskowitz, M. A. (1996). Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient MCA occlusion. Journal of Cerebral Blood Flow and Metabolism, 16, 605–611. 10.1097/00004647-199607000-00010 [DOI] [PubMed] [Google Scholar]
- Hattori, R. , Kuchibhotla, K. V. , Froemke, R. C. , & Komiyama, T. (2017). Functions and dysfunctions of neocortical inhibitory neuron subtypes. Nature Neuroscience, 20, 1199–1208. 10.1038/nn.4619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez, A. F. , Green, J. B. , Janmohamed, S. , D'Agostino, R. B. Sr. , Granger, C. B. , Jones, N. P. , Leiter, L. A. , Rosenberg, A. E. , Sigmon, K. N. , Somerville, M. C. , Thorpe, K. M. , McMurray, J. J. V. , Del Prato, S. , & Harmony Outcomes Committees and Investigators . (2018). Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double‐blind, randomised placebo‐controlled trial. Lancet, 392, 1519–1529. 10.1016/S0140-6736(18)32261-X [DOI] [PubMed] [Google Scholar]
- Heydemann, A. (2016). An overview of murine high fat diet as a model for type 2 diabetes mellitus. Journal Diabetes Research, 2016, 2902351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz Iv, G. G. , Kiihtreiber, W. M. , & Habener, J. F. (1993). Pancreatic beta‐cells are rendered glucose‐competent by the insulinotropic hormone glucagon‐like peptide‐1(7‐37). Nature, 361, 362–365. 10.1038/361362a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, H. , Gan, J. , & Jonas, P. (2014). Interneurons. Fast‐spiking, parvalbumin(+) GABAergic interneurons: From cellular design to microcircuit function. Science, 345, 1255263. 10.1126/science.1255263 [DOI] [PubMed] [Google Scholar]
- Jean LeBlanc, N. , Guruswamy, R. , & ElAli, A. (2018). Vascular endothelial growth factor isoform‐B stimulates neurovascular repair after ischemic Stroke by promoting the function of pericytes via vascular endothelial growth factor receptor‐1. Molecular Neurobiology, 55, 3611–3626. 10.1007/s12035-017-0478-6 [DOI] [PubMed] [Google Scholar]
- Jing, J. , Pan, Y. , Zhao, X. , Zheng, H. , Jia, Q. , Mi, D. , Chen, W. , Li, H. , Liu, L. , Wang, C. , He, Y. , Wang, D. , Wang, Y. , Wang, Y. , & Investigators for ACROSS‐China investigators for A‐C . (2017). Insulin resistance and prognosis of nondiabetic patients with ischemic stroke: The ACROSS‐China study (abnormal glucose regulation in patients with acute stroke across China). Stroke, 48, 887–893. 10.1161/STROKEAHA.116.015613 [DOI] [PubMed] [Google Scholar]
- Johnstone, A. , Levenstein, J. M. , Hinson, E. L. , & Stagg, C. J. (2018). Neurochemical changes underpinning the development of adjunct therapies in recovery after stroke: A role for GABA? Journal of Cerebral Blood Flow and Metabolism, 38, 1564–1583. 10.1177/0271678X17727670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin, A. J. , & Akerstrom, V. (2003). Entry of exendin‐4 into brain is rapid but may be limited at high doses. International Journal of Obesity and Related Metabolic Disorders, 27, 313–318. 10.1038/sj.ijo.0802206 [DOI] [PubMed] [Google Scholar]
- Kim, S. , Jeong, J. , Jung, H. S. , Kim, B. , Kim, Y. E. , Lim, D. S. , Kim, S. D. , & Song, Y. S. (2017). Anti‐inflammatory effect of glucagon like peptide‐1 receptor agonist, exendin‐4, through modulation of IB1/JIP1 expression and JNK signaling in stroke. Experimental Neurobiology, 26, 227–239. 10.5607/en.2017.26.4.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokovay, E. , Li, L. , & Cunningham, L. A. (2006). Angiogenic recruitment of pericytes from bone marrow after stroke. Journal of Cerebral Blood Flow and Metabolism, 26, 545–555. 10.1038/sj.jcbfm.9600214 [DOI] [PubMed] [Google Scholar]
- Korol, S. V. , Jin, Z. , Babateen, O. , & Birnir, B. (2015). GLP‐1 and exendin‐4 transiently enhance GABAA receptor‐mediated synaptic and tonic currents in rat hippocampal CA3 pyramidal neurons. Diabetes, 64, 79–89. 10.2337/db14-0668 [DOI] [PubMed] [Google Scholar]
- Larsson, M. , Lietzau, G. , Nathanson, D. , Ostenson, C. G. , Mallard, C. , Johansson, M. E. , Nyström, T. , Patrone, C. , & Darsalia, V. (2016). Diabetes negatively affects cortical and striatal GABAergic neurons; an effect that is partially counteracted by Exendin‐4. Bioscience Reports, 36(6), 10.1042/BSR20160437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lietzau, G. , Nystrom, T. , Ostenson, C. G. , Darsalia, V. , & Patrone, C. (2016). Type 2 diabetes‐induced neuronal pathology in the piriform cortex of the rat is reversed by the GLP‐1 receptor agonist Exendin‐4. Oncotarget, 7, 5865–5876. 10.18632/oncotarget.6823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley, E. , Stanford, S. C. , Kendall, D. E. , Alexander, S. P. H. , Cirino, G. , Docherty, J. R. , George, C. H. , Insel, P. A. , Izzo, A. A. , Ji, Y. , Panettieri, R. A. , Sobey, C. G. , Stefanska, B. , Stephens, G. , Teixeira, M. , & Ahluwalia, A. (2020). ARRIVE 2.0 and the British Journal of Pharmacology: Updated guidance for 2020. British Journal of Pharmacology, 177, 3611–3616. 10.1111/bph.15178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Wang, Y. , Akamatsu, Y. , Lee, C. C. , Stetler, R. A. , Lawton, M. T. , & Yang, G. Y. (2014). Vascular remodeling after ischemic stroke: Mechanisms and therapeutic potentials. Progress in Neurobiology, 115, 138–156. 10.1016/j.pneurobio.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luitse, M. J. , Biessels, G. J. , Rutten, G. E. , & Kappelle, L. J. (2012). Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurology, 11, 261–271. 10.1016/S1474-4422(12)70005-4 [DOI] [PubMed] [Google Scholar]
- Luitse, M. J. , van Seeters, T. , Horsch, A. D. , Kool, H. A. , Velthuis, B. K. , Kappelle, L. J. , & Biessels, G. J. (2013). Admission hyperglycaemia and cerebral perfusion deficits in acute ischaemic stroke. Cerebrovascular Diseases, 35, 163–167. 10.1159/000346588 [DOI] [PubMed] [Google Scholar]
- Mainardi, M. , Fusco, S. , & Grassi, C. (2015). Modulation of hippocampal neural plasticity by glucose‐related signaling. Neural Plasticity, 2015, 657928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makihara, N. , Arimura, K. , Ago, T. , Tachibana, M. , Nishimura, A. , Nakamura, K. , Matsuo, R. , Wakisaka, Y. , Kuroda, J. , Sugimori, H. , Kamouchi, M. , & Kitazono, T. (2015). Involvement of platelet‐derived growth factor receptor β in fibrosis through extracellular matrix protein production after ischemic stroke. Experimental Neurology, 264, 127–134. 10.1016/j.expneurol.2014.12.007 [DOI] [PubMed] [Google Scholar]
- Malhotra, K. , Katsanos, A. H. , Lambadiari, V. , Goyal, N. , Palaiodimou, L. , Kosmidou, M. , Krogias, C. , Alexandrov, A. V. , & Tsivgoulis, G. (2020). GLP‐1 receptor agonists in diabetes for stroke prevention: A systematic review and meta‐analysis. Journal of Neurology, 267, 2117–2122. 10.1007/s00415-020-09813-4 [DOI] [PubMed] [Google Scholar]
- Marlet, I. R. , Olmestig, J. N. E. , Vilsboll, T. , Rungby, J. , & Kruuse, C. (2018). Neuroprotective mechanisms of glucagon‐like peptide‐1‐based therapies in ischaemic stroke: A systematic review based on pre‐clinical studies. Basic & Clinical Pharmacology & Toxicology, 122, 559–569. 10.1111/bcpt.12974 [DOI] [PubMed] [Google Scholar]
- Marso, S. P. , Bain, S. C. , Consoli, A. , Eliaschewitz, F. G. , Jodar, E. , Leiter, L. A. , Lingvay, I. , Rosenstock, J. , Seufert, J. , Warren, M. L. , Woo, V. , Hansen, O. , Holst, A. G. , Pettersson, J. , Vilsbøll, T. , & SUSTAIN‐6 Investigators . (2016). Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. The New England Journal of Medicine, 375, 1834–1844. 10.1056/NEJMoa1607141 [DOI] [PubMed] [Google Scholar]
- Marso, S. P. , Daniels, G. H. , Brown‐Frandsen, K. , Kristensen, P. , Mann, J. F. , Nauck, M. A. , Nissen, S. E. , Pocock, S. , Poulter, N. R. , Ravn, L. S. , Steinberg, W. M. , Stockner, M. , Zinman, B. , Bergenstal, R. M. , Buse, J. B. , & LEADER steering committee; LEADER Trial investigators collaborators . (2016). Liraglutide and cardiovascular outcomes in type 2 diabetes. The New England Journal of Medicine, 375, 311–322. 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskery, M. P. , Holscher, C. , Jones, S. P. , Price, C. I. , Strain, W. D. , Watkins, C. L. , Werring, D. J. , & Emsley, H. C. A. (2020). Glucagon like peptide‐1 receptor agonists as neuroprotective agents for ischemic stroke: A systematic scoping review. Journal of Cerebral Blood Flow & Metabolism. 41(1), 14–30. 10.1177/0271678X20952011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean, P. L. , Gault, V. A. , Harriott, P. , & Holscher, C. (2010). Glucagon‐like peptide‐1 analogues enhance synaptic plasticity in the brain: A link between diabetes and Alzheimer's disease. European Journal of Pharmacology, 630, 158–162. 10.1016/j.ejphar.2009.12.023 [DOI] [PubMed] [Google Scholar]
- Megherbi, S. E. , Milan, C. , Minier, D. , Couvreur, G. , Osseby, G. V. , Tilling, K. , di Carlo, A. , Inzitari, D. , Wolfe, C. D. , Moreau, T. , Giroud, M. , & European BIOMED Study of Stroke Care Group . (2003). Association between diabetes and stroke subtype on survival and functional outcome 3 months after stroke: Data from the European BIOMED Stroke Project. Stroke, 34, 688–694. 10.1161/01.STR.0000057975.15221.40 [DOI] [PubMed] [Google Scholar]
- Mielke, J. G. , & Wang, Y. T. (2011). Insulin, synaptic function, and opportunities for neuroprotection. Progress in Molecular Biology and Translational Science, 98, 133–186. 10.1016/B978-0-12-385506-0.00004-1 [DOI] [PubMed] [Google Scholar]
- Muller, C. , Cheung, N. W. , Dewey, H. , Churilov, L. , Middleton, S. , Thijs, V. , Ekinci, E. I. , Levi, C. , Lindley, R. , Donnan, G. , Parsons, M. , & Bladin, C. (2018). Treatment with exenatide in acute ischemic stroke trial protocol: A prospective, randomized, open label, blinded end‐point study of exenatide vs. standard care in post stroke hyperglycemia. International Journal of Stroke, 13, 857–862. 10.1177/1747493018784436 [DOI] [PubMed] [Google Scholar]
- Nystrom, T. , Gutniak, M. K. , Zhang, Q. , Zhang, F. , Holst, J. J. , Ahren, B. , & Sjoholm, A. (2004). Effects of glucagon‐like peptide‐1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. American Journal of Physiology Endocrinology and Metabolism, 287, E1209–E1215. 10.1152/ajpendo.00237.2004 [DOI] [PubMed] [Google Scholar]
- Ozkul, A. , Ayhan, M. , Akyol, A. , Turgut, E. T. , Kadikoylu, G. , & Yenisey, C. (2013). The effect of insulin resistance on inflammatory response and oxidative stress in acute cerebral ischemia. Neuro Endocrinology Letters, 34, 52–57. [PubMed] [Google Scholar]
- Palaiodimou, L. , Lioutas, V. A. , Lambadiari, V. , Paraskevas, G. P. , Voumvourakis, K. , & Tsivgoulis, G. (2019). Glycemia management in acute ischemic stroke: Current concepts and novel therapeutic targets. Postgraduate Medicine, 131, 423–437. 10.1080/00325481.2019.1651206 [DOI] [PubMed] [Google Scholar]
- Paul, S. K. , Klein, K. , Maggs, D. , & Best, J. H. (2015). The association of the treatment with glucagon‐like peptide‐1 receptor agonist exenatide or insulin with cardiovascular outcomes in patients with type 2 diabetes: A retrospective observational study. Cardiovascular Diabetology, 14, 10. 10.1186/s12933-015-0178-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percie du Sert, N. , Hurst, V. , Ahluwalia, A. , Alam, S. , Avey, M. T. , Baker, M. , Browne, W. J. , Clark, A. , Cuthill, I. C. , Dirnagl, U. , Emerson, M. , Garner, P. , Holgate, S. T. , Howells, D. W. , Karp, N. A. , Lazic, S. E. , Lidster, K. , MacCallum, C. J. , Macleod, M. , … Würbel, H. (2020). The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. The Journal of Physiology, 598, 3793–3801. 10.1113/JP280389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson, U. S. , Walden, T. B. , Carlsson, P. O. , Jansson, L. , & Phillipson, M. (2012). Female mice are protected against high‐fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS ONE, 7, e46057. 10.1371/journal.pone.0046057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintana, H. , Lietzau, G. , Augestad, I. L. , Chiazza, F. , Nystrom, T. , Patrone, C. , & Darsalia, V. (2019). Obesity‐induced type 2 diabetes impairs neurological recovery after stroke in correlation with decreased neurogenesis and persistent atrophy of parvalbumin‐positive interneurons. Clinical Science (London, England), 133, 1367–1386. 10.1042/CS20190180 [DOI] [PubMed] [Google Scholar]
- Pomytkin, I. , Costa‐Nunes, J. P. , Kasatkin, V. , Veniaminova, E. , Demchenko, A. , Lyundup, A. , Lesch, K. P. , Ponomarev, E. D. , & Strekalova, T. (2018). Insulin receptor in the brain: Mechanisms of activation and the role in the CNS pathology and treatment. CNS Neuroscience & Therapeutics, 24, 763–774. 10.1111/cns.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash, R. , Li, W. , Qu, Z. , Johnson, M. A. , Fagan, S. C. , & Ergul, A. (2013). Vascularization pattern after ischemic stroke is different in control versus diabetic rats: Relevance to stroke recovery. Stroke, 44, 2875–2882. 10.1161/STROKEAHA.113.001660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsinelli, W. A. , Levy, D. E. , Sigsbee, B. , Scherer, P. , & Plum, F. (1983). Increased damage after ischemic stroke in patients with hyperglycemia with or without established diabetes mellitus. The American Journal of Medicine, 74, 540–544. 10.1016/0002-9343(83)91007-0 [DOI] [PubMed] [Google Scholar]
- Raparelli, V. , Elharram, M. , Moura, C. S. , Abrahamowicz, M. , Bernatsky, S. , Behlouli, H. , & Pilote, L. (2020). Sex differences in cardiovascular effectiveness of newer glucose‐lowering drugs added to metformin in type 2 diabetes mellitus. Journal of the American Heart Association, 9, e012940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawshani, A. , Rawshani, A. , Franzen, S. , Sattar, N. , Eliasson, B. , Svensson, A. M. , Zethelius, B. , Miftaraj, M. , McGuire, D. K. , Rosengren, A. , & Gudbjornsdottir, S. (2018). Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. The New England Journal of Medicine, 379, 633–644. 10.1056/NEJMoa1800256 [DOI] [PubMed] [Google Scholar]
- Roth, M. , Gaceb, A. , Enström, A. , Padel, T. , Genové, G. , Özen, I. , & Paul, G. (2019). Regulator of G‐protein signaling 5 regulates the shift from perivascular to parenchymal pericytes in the chronic phase after stroke. The FASEB Journal, 33, 8990–8998. 10.1096/fj.201900153R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan, Y. , Tan, S. , Lin, Y. , Liao, S. , Zhang, B. , Chen, X. , Wang, J. , Deng, Z. , Zeng, Q. , Zhang, L. , Wang, Y. , Hu, X. , Qiu, W. , Peng, L. , & Lu, Z. (2019). The glucagon‐like peptide‐1 receptor agonist reduces inflammation and blood‐brain barrier breakdown in an astrocyte‐dependent manner in experimental stroke. Journal of Neuroinflammation, 16, 242. 10.1186/s12974-019-1638-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, J. E. , Sicree, R. A. , & Zimmet, P. Z. (2010). Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Research and Clinical Practice, 87, 4–14. 10.1016/j.diabres.2009.10.007 [DOI] [PubMed] [Google Scholar]
- Shen, J. , Ishii, Y. , Xu, G. , Dang, T. C. , Hamashima, T. , Matsushima, T. , Yamamoto, S. , Hattori, Y. , Takatsuru, Y. , Nabekura, J. , & Sasahara, M. (2012). PDGFR‐β as a positive regulator of tissue repair in a mouse model of focal cerebral ischemia. Journal of Cerebral Blood Flow and Metabolism, 32, 353–367. 10.1038/jcbfm.2011.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttolomondo, A. , Cirrincione, A. , Casuccio, A. , Del Cuore, A. , Daidone, M. , Di Chiara, T. , Di Raimondo, D. , Corte, V. D. , Maida, C. , Simonetta, I. , Scaglione, S. , & Pinto, A. (2021). Efficacy of dulaglutide on vascular health indexes in subjects with type 2 diabetes: A randomized trial. Cardiovascular Diabetology, 20, 1. 10.1186/s12933-020-01183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullberg, T. , Zia, E. , Petersson, J. , & Norrving, B. (2015). Changes in functional outcome over the first year after stroke: An observational study from the Swedish stroke register. Stroke, 46, 389–394. 10.1161/STROKEAHA.114.006538 [DOI] [PubMed] [Google Scholar]
- Yang, X. , Feng, P. , Zhang, X. , Li, D. , Wang, R. , Ji, C. , Li, G. , & Hölscher, C. (2019). The diabetes drug semaglutide reduces infarct size, inflammation, and apoptosis, and normalizes neurogenesis in a rat model of stroke. Neuropharmacology, 158, 107748. 10.1016/j.neuropharm.2019.107748 [DOI] [PubMed] [Google Scholar]
- Zabala, A. , Darsalia, V. , Holzmann, M. J. , Franzen, S. , Svensson, A. M. , Eliasson, B. , Patrone, C. , Nyström, T. , & Jonsson, M. (2019). Risk of first stroke in people with type 2 diabetes and its relation to glycaemic control: A nationwide observational study. Diabetes, Obesity & Metabolism, 22(2), 182–190. [DOI] [PubMed] [Google Scholar]
- Zechariah, A. , ElAli, A. , Doeppner, T. R. , Jin, F. , Hasan, M. R. , Helfrich, I. , Mies, G. , & Hermann, D. M. (2013). Vascular endothelial growth factor promotes pericyte coverage of brain capillaries, improves cerebral blood flow during subsequent focal cerebral ischemia, and preserves the metabolic penumbra. Stroke, 44, 1690–1697. 10.1161/STROKEAHA.111.000240 [DOI] [PubMed] [Google Scholar]
- Zhao, L. , Xu, J. , Wang, Q. , Qian, Z. , Feng, W. , Yin, X. , & Fang, Y. (2015). Protective effect of rhGLP‐1 (7‐36) on brain ischemia/reperfusion damage in diabetic rats. Brain Research, 1602, 153–159. 10.1016/j.brainres.2015.01.014 [DOI] [PubMed] [Google Scholar]
- Zimmerman, R. S. , Hobbs, T. M. , Wells, B. J. , Kong, S. X. , Kattan, M. W. , Bouchard, J. , Chagin, K. M. , Yu, C. , Sakurada, B. , Milinovich, A. , Weng, W. , Bauman, J. M. , & Pantalone, K. M. (2017). Association of glucagon‐like peptide‐1 receptor agonist use and rates of acute myocardial infarction, stroke and overall mortality in patients with type 2 diabetes mellitus in a large integrated health system. Diabetes, Obesity & Metabolism, 19, 1555–1561. 10.1111/dom.12969 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Activation of PV + cells. Quantification of cFOS+/PV + cells at 8 weeks after stroke in the contralateral vs. peri‐infarct in the ipsilateral striatum showed no stroke effect or any difference between the groups except a trend towards decreased activation of PV + cells in the T2D‐Ve group compared to the non‐T2D group. Data presented as mean±SD. Two‐way ANOVA followed by Two‐stage linear step‐up procedure of Benjamini, Krieger and Yekutieli was used to compare the number of cFOS+/PV + cells between non‐T2D vs. T2D‐Ve vs. T2D‐Ex‐4.
Figure S2. Representative images of CD31 + (vessels) and CD13 + (pericytes) staining in the contralateral striatum. Scale bar: 150 μm.
Figure S3. Neurogenesis. Quantification of DCX + (a) and CR+/NeuN‐ (b) cells 8 weeks after stroke in the ipsilateral striatum showed no difference between the groups. Data presented as mean±SD. Brown‐Forsythe and Welch ANOVA was used to compare the number of DCX + cells and the number of CR+/NeuN‐ cells between non‐T2D vs. T2D‐Ve vs. T2D‐Ex‐4.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


