Key Clinical Points.
Rapid Diagnostic Testing for SARS-CoV-2
Rapid diagnostic tests (RDTs) that are authorized by the Food and Drug Administration to diagnose severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are either nucleic acid amplification tests to detect genes or antigen-based immunoassays to detect proteins of SARS-CoV-2.
RDTs are approved for use in persons with symptoms of coronavirus disease 2019 (Covid-19) and in asymptomatic persons who are close contacts of a person with Covid-19 or who have been in a potential high-risk transmission setting.
Symptomatic persons should undergo testing as soon as possible, quarantine while awaiting test results, and consider retesting if they have a negative RDT, particularly if they have a high pretest probability of infection.
Asymptomatic persons with a known exposure to SARS-CoV-2 should undergo testing 5 to 7 days after exposure, and if the RDT is negative, they should undergo testing again 2 days later.
Persons with a known exposure to SARS-CoV-2 who are not fully vaccinated should quarantine while awaiting test results, and persons who test positive should isolate, contact a health care provider or public health department, and inform close contacts about the infection.
This Journal feature begins with a case vignette highlighting a common clinical problem. Evidence supporting various strategies is then presented, followed by a review of formal guidelines, when they exist. The article ends with the author’s clinical recommendations.
A 38-year-old woman with type 2 diabetes has a telemedicine visit after learning that a person with whom she had close contact at an indoor wedding 3 days earlier has tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The woman reports that she is asymptomatic and received a second vaccination against SARS-CoV-2 approximately 9 months ago but has not received a booster vaccination. She attended the wedding with her husband, who has also received two vaccinations, and her two unvaccinated children, who are 5 and 8 years of age. Her husband had mild nasal congestion and a cough the evening before her appointment. She had previously purchased rapid diagnostic tests that received emergency use authorization from the Food and Drug Administration for home-based SARS-CoV-2 testing and wonders whether using these tests would be appropriate. What would you advise?
The Clinical Problem
Approximately 300 million cases of confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and 5.5 million deaths from coronavirus disease 2019 (Covid-19) have been reported to the World Health Organization (WHO).1 SARS-CoV-2 testing has been critical in identifying cases of Covid-19, reducing transmission, and informing public health infection-control measures.2 However, limited access to diagnostic testing in underserved communities and incomplete reporting of Covid-19 data to the WHO3 mean that official numbers, although staggering, probably represent a fraction of total infections and deaths from the Covid-19 pandemic.4,5
Globally, clinical laboratories have performed approximately 3 billion molecular diagnostic tests for SARS-CoV-2.3 The United States has performed more than 600 million tests (2.0 tests per person), which is more in absolute terms than any other country, although China has not reported complete testing data.3 However, total per capita testing rates have been higher across Europe, including the United Kingdom, Denmark, and Austria (4.8, 8.2, and 12.3 tests per person, respectively).3 Performance of high volumes of nucleic acid amplification tests (NAATs) is technically challenging, labor intensive, reliant on efficient specimen transport and reporting systems, and expensive, all of which contribute to inequitable access to testing.6
During the past several decades, rapid diagnostic tests (RDTs) such as urine tests to detect human chorionic gonadotropin and tests to detect human immunodeficiency virus have been increasingly used across health care settings and in both high- and low-resource environments. These tests have facilitated diagnosis and treatment and reduced reliance on laboratory infrastructure.7 The National Institutes of Health and other funding agencies began supporting the research and development of new diagnostic tests early in the Covid-19 pandemic, and diagnostic companies prioritized the production of both molecular-based and antigen-based RDTs for SARS-CoV-2.
More than 1000 types of molecular and antigen-based immunoassay tests to detect SARS-CoV-2, including at least 400 RDTs, are now commercially available worldwide.8 RDTs are widely available for over-the-counter purchase in Europe, but they have been more difficult to obtain in the United States and in low- and middle-income countries.9 In December 2021, the Biden administration announced plans to purchase 500 million rapid, at-home tests, with the initial delivery starting in January 2022, and to continue using the Defense Production Act to increase both laboratory-based and rapid diagnostic testing.9 Although RDTs for SARS-CoV-2 are currently in short supply, they are expected to become more widely available in clinic-, community-, and home-based settings, and there is a growing need to understand their clinical indications and interpretation.
Strategies and Evidence
Clinical Course and Diagnostic Testing
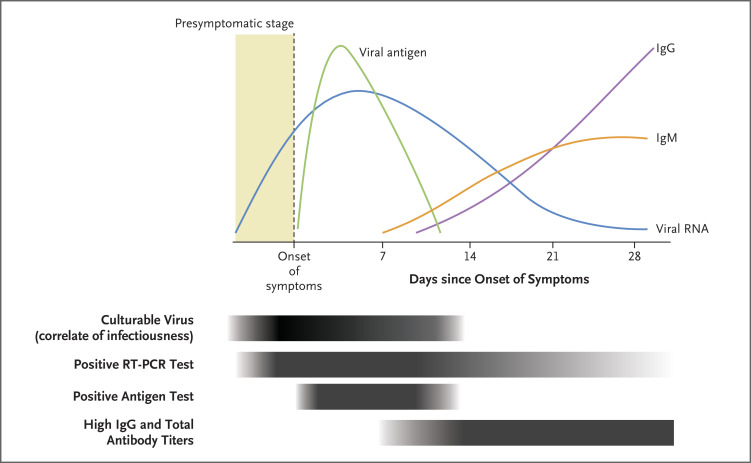
The pathophysiology of acute SARS-CoV-2 infection, the clinical course of Covid-19, and the host immunologic response provide a basis for diagnostic testing strategies (Figure 1).10,11 SARS-CoV-2 is predominantly a respiratory airway pathogen, and transmission occurs largely through inhalation of small droplets and aerosols.12 Novel genomic viral variants, including the B.1.617.2 (delta) variant, have higher transmissibility than the original D614G virus, leading to faster dissemination within populations, but they share the same pathophysiology of infection and disease. The WHO recently named the B.1.1.529 (omicron) variant as the sixth “variant of concern,” and available evidence suggests it is more transmissible but less virulent than previous variants.
Figure 1. Pathophysiology and Timeline of Viremia, Antigenemia, and Immune Response during Acute SARS-CoV-2 Infection.
In some persons, reverse-transcriptase–polymerase-chain-reaction (RT-PCR) tests can remain positive for weeks or months after initial infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), but this positivity rarely indicates replication-competent virus that can result in infection.
Symptoms of Covid-19 (Table 1) appear 2 to 14 days after exposure, with an average onset 5 to 6 days after infection.13,14 Most persons with Covid-19 have mild-to-moderate symptoms and recover at home, but some, particularly older or unvaccinated adults and those with underlying medical conditions or immunocompromise, may have serious illness.13 SARS-CoV-2 infection also occurs without causing symptoms or Covid-19, and asymptomatic persons can contribute to viral transmission.15-17 Humoral immunity wanes after initial vaccination,18 but booster immunizations have been shown to reduce the incidence of adverse outcomes.19 Viral load levels and clearance may be similar among vaccinated and unvaccinated adults,20 and adults who have not received a booster immunization have a higher risk of Covid-19–related hospitalization or death than those who have received one.21
Table 1. Symptoms of Covid-19 and Signs or Symptoms of Severe Covid-19.*.
| Symptoms or Signs |
|---|
| Typical symptoms of Covid-19 |
| Fever or chills |
| Congestion, rhinorrhea |
| Cough |
| Fatigue |
| Loss of taste or smell |
| Nausea, vomiting |
| Less common symptoms of Covid-19 |
| Sore throat |
| Headache |
| Myalgias, arthralgias |
| Diarrhea |
| Rash |
| Red or irritated eyes |
| Signs or symptoms of severe Covid-19 |
| Difficulty breathing |
| Shortness of breath (dyspnea) |
| Persistent chest pain or pressure |
| Confusion |
| Loss of speech or mobility |
| Cyanosis |
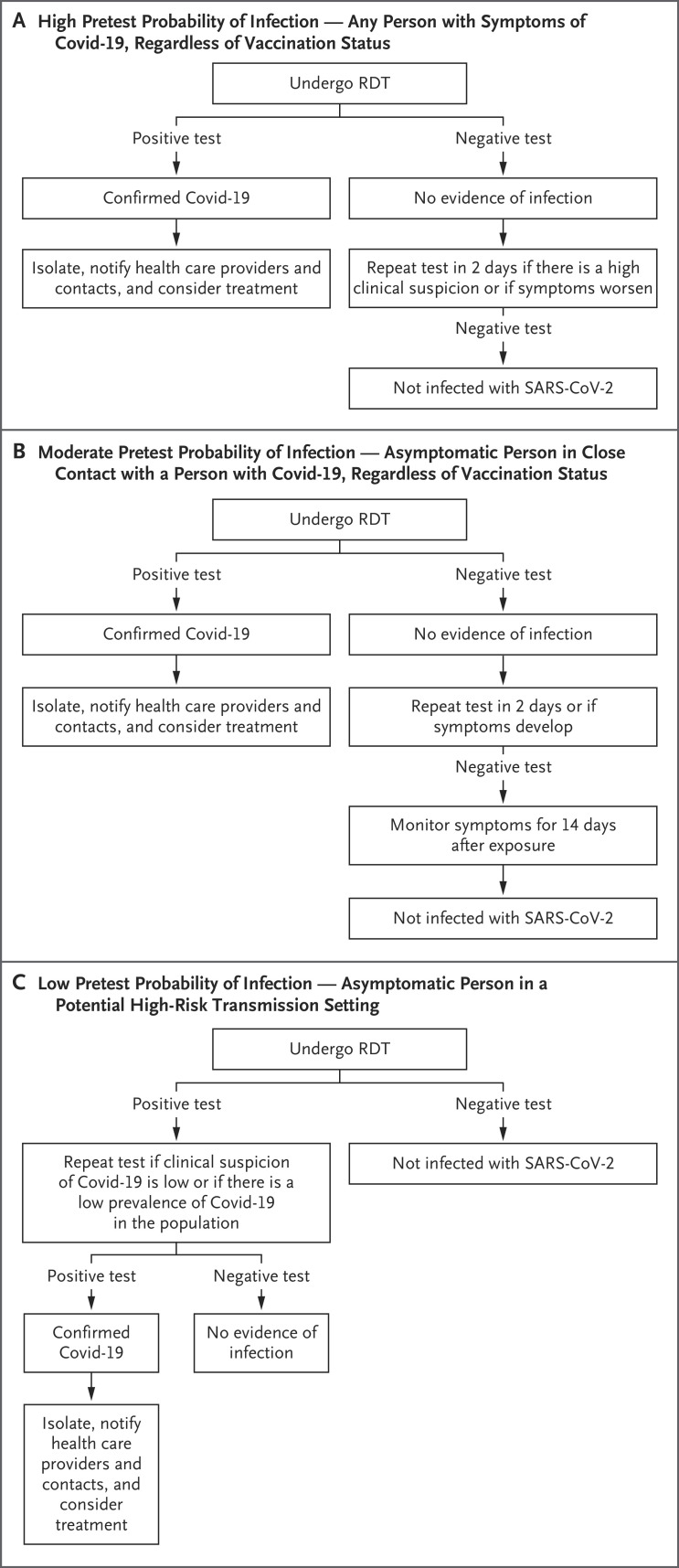
Three common indications for diagnostic SARS-CoV-2 testing, as recommended by the WHO22 and the Centers for Disease Control and Prevention (CDC),23 range from high to low pretest probability of infection (Figure 2). First, anyone with Covid-19 symptoms, regardless of vaccination status, should undergo testing for SARS-CoV-2. Second, asymptomatic persons, regardless of vaccination status, who are close contacts of someone with known or probable SARS-CoV-2 infection should undergo diagnostic testing. Persons who are unvaccinated or who have not received a vaccine booster within the previous 6 months have a higher pretest probability of infection than those who are fully vaccinated, whereas others have a low or moderate pretest probability of infection. Third, testing should be considered in asymptomatic persons who have been in a setting where the risk of transmission is high, such as in an airplane or at a sporting event. Use of an RDT may also be considered in persons who plan to be in a group setting, even though they may have a low pretest probability of infection; this testing should occur as close to the time of the gathering as possible.
Figure 2. Indications and Algorithms for Rapid Diagnostic Tests (RDTs) for SARS-CoV-2.
The Centers for Disease Control and Prevention defines a close contact as a person who was less than 6 feet away for 15 minutes or more over a 24-hour period.13,23 Potential high-risk transmission settings include an airplane, a concert or sporting event, and a crowded or poorly ventilated indoor area.13,22,23 Covid-19 denotes coronavirus disease 2019.
Diagnostic testing for acute SARS-CoV-2 infection can be performed with either molecular NAATs or antigen-based assays, and both are available as RDTs.22,23 Molecular NAATs detect the presence of viral gene targets, including the N, S, and E genes and the open reading frame 1ab (ORF 1ab). Reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assays are the most widely used diagnostic SARS-CoV-2 NAATs worldwide.24 Antigen-based tests, also called immunoassays, detect domains of the surface proteins, including the nucleocapsid, spike, and receptor-binding domains, that are specific to SARS-CoV-2. Although both techniques are highly specific, NAATs are generally more sensitive than antigen-based tests because they amplify target genomic sequences. Tests to detect host IgG or IgM antibodies to SARS-CoV-2 should not be used to diagnose acute infection.
The clinical performance of diagnostic SARS-CoV-2 testing extends beyond pathogen targets such as viral proteins and RNA and includes clinical characteristics (e.g., the patient’s viral load and the time since exposure or symptom onset), operational testing attributes (e.g., the specimen type, swab technique, transport conditions, and laboratory technique), and analytic test properties (e.g., sample preparation and signal amplification).7,25 Although NAATs are highly sensitive and accurate, they can remain positive for weeks to months after infection.26,27 Viral culture studies suggest that SARS-CoV-2 may be capable of replicating only for 10 to 14 days after symptom onset, so NAATs may detect remnant viral RNA well past the time period of recovering replication-competent virus.26,27 Conversely, antigen-based assays remain positive for 5 to 12 days after symptom onset and perform better in persons with a high viral load,28 which correlates with disease severity and death.29 Thus, antigen-based tests may correlate better with replication-competent SARS-CoV-2 than molecular tests and may provide information about potential transmissibility.30
RDTs for Acute SARS-CoV-2 Infection
The Food and Drug Administration (FDA) and WHO have each conducted an expedited review process to accelerate the temporary approval of diagnostic SARS-CoV-2 tests.31,32 As of December 2021, the FDA had granted emergency use authorization (EUA) status to 28 RDTs for the diagnosis of acute SARS-CoV-2 infection, and more FDA-authorized tests are expected.31 In the European Union, more than 140 different companies have had an antigen-based RDT registered on the “common list” for approved use.33 The molecular and antigen-based RDTs with EUA status have various pathogen targets, detection methods, swabbing sites to obtain specimens, indications for use, and performance characteristics (see Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).
In order for an RDT to receive temporary approval by the FDA, WHO, and European Union regulatory agencies, it must have at least 80% sensitivity (positive percent agreement) and 98% specificity (negative percent agreement), as compared with a reference standard of laboratory-based RT-PCR testing, although the WHO has allowed for specificity of 97% or greater.22,31,32,34 Approval by the FDA is also based on a prospective cohort study involving at least 30 persons with SARS-CoV-2 infection and 30 persons without SARS-CoV-2 infection.31 An EUA from the European Union is based on performance data that may be obtained either through a prospective clinical study or through retrospective in vitro laboratory testing.33,34 The regulatory agencies require monitoring and reporting of test performance with respect to viral variants, although these requirements have not been well enforced; they do not require independent verification of clinical validation data provided by each test manufacturer.31,32,34
For several molecular RDTs that are intended for use in low-complexity settings, the FDA has issued EUA status with a Clinical Laboratory Improvement Amendments (CLIA) certificate of waiver (which can be obtained by community health centers, nursing homes, schools, churches, and other gathering places for collecting specimens and performing testing). Some of these RDTs are also approved for home-based use.31 These molecular RDTs, which use RT-PCR, loop-mediated isothermal amplification, or nicking enzyme-assisted amplification to detect the viral RNA of SARS-CoV-2, provide results in 13 to 55 minutes. All molecular RDTs are approved for use in symptomatic persons, and a few also have approval for the screening of asymptomatic persons.
Similarly, many antigen-based RDTs have received FDA EUA status for use in settings that have received a CLIA waiver or for home-based use.31 These antigen-based RDTs are immunoassays that use SARS-CoV-2–specific antibodies to bind viral proteins (mostly nucleocapsid) and generate either a visual or fluorescence signal. Most are lateral-flow assays on a nitrocellulose membrane, whereas others involve the use of thin microfluidic test strips, magnetic beads, or an immunofluorescence readout to enhance protein capture and detection.28 All antigen-based RDTs are approved for use in symptomatic persons and provide results in 10 to 30 minutes. Several have EUA status for screening of asymptomatic persons; most of these tests are intended to be used twice over a period of 3 days, although a small number with high sensitivity for detecting asymptomatic infection are approved for use without serial testing.31,35
Although direct-comparison studies are limited and often retrospective, antigen-based RDTs have a lower sensitivity than molecular RDTs, as compared with a reference standard of laboratory-based RT-PCR tests, particularly among persons who have a low viral load or no replication-competent virus.36-38 However, antigen-based RDTs can detect infection early in the disease course (within 5 to 7 days after symptom onset) when viral loads are high (i.e., a low RT-PCR cycle threshold); these high viral loads account for most transmissions.39-41 Studies have shown varying degrees of clinical accuracy (sensitivity, 36 to 82%; specificity, approximately 98 to 100%) when various antigen-based RDTs are used for screening asymptomatic persons.35,42,43
Although home-based RDTs broaden the use of testing, they have been shown to be more accurate when performed by trained health care providers than by untrained persons.44,45 Persons who perform tests at home should carefully follow test kit instructions.
Interpretation of Results of Testing and Screening
The appropriate interpretation of RDTs for SARS-CoV-2 testing and screening depends on the clinical indication and the pretest probability of infection (Figure 2). Among persons with a moderate-to-high pretest probability, which includes symptomatic persons and asymptomatic persons who have had close contact with a person with Covid-19, a positive RDT indicates a confirmed SARS-CoV-2 infection. However, RDTs may have false negative results, and repeat testing should be considered in cases of high clinical suspicion or worsening symptoms and in the serial screening of asymptomatic persons. A second negative RDT 2 days after the initial test or a negative laboratory-based NAAT would help to rule out SARS-CoV-2 infection. All symptomatic persons and asymptomatic persons who have not been fully vaccinated and who have had exposure to an infected contact should quarantine while awaiting test results. Although the standard CDC definition of “full vaccination” has been 2 weeks after the second dose in a two-dose vaccination series, many experts (including this author) propose that the definition should include a booster vaccination in persons who are eligible to receive one.
In persons with a low pretest probability of infection (e.g., asymptomatic persons without a known SARS-CoV-2 exposure), a single negative RDT provides reassurance that SARS-CoV-2 infection is unlikely. However, given imperfect specificity, a positive RDT may indicate a false positive result. If there is low clinical suspicion or a low prevalence of Covid-19 in the population, then repeat testing should be performed. A second positive RDT or positive laboratory-based NAAT would confirm SARS-CoV-2 infection. All asymptomatic persons (vaccinated or unvaccinated) with potential or known exposure should monitor for symptoms for 14 days.
In persons with exposure to SARS-CoV-2, testing is generally not useful in the first 48 hours after exposure, since the virus will not have achieved a sufficient viral load.13 The most appropriate window for testing is generally considered to be 5 to 7 days after exposure, which is the average peak of symptoms and viral load.13 Therefore, for a single-test strategy, asymptomatic, exposed persons could use an RDT 5 to 7 days after exposure. For a two-test strategy, which is the FDA-approved indication for most RDTs for asymptomatic screening, a second RDT should be performed 2 days after a negative test. All symptomatic persons should be tested at the onset of symptoms and, if test results are negative, repeat testing should be considered if clinical suspicion remains high or symptoms worsen.13 In persons with low pretest probability of infection who have a positive RDT, a confirmatory test should be performed promptly.
Routine serial screening strategies with frequent testing have been proposed and implemented to quickly detect SARS-CoV-2 and reduce transmission.46-50 However, when the population prevalence of SARS-CoV-2 is low, the probability of a false positive RDT increases.51,52
Areas of Uncertainty
The global production, delivery, and implementation of RDTs remain challenging.53 Because RDTs may have the greatest benefit in persons who have limited access to laboratory-based NAATs, diagnostic tests should be made available and affordable for underserved populations. In order to reduce global and national inequalities in access to testing, enhanced global coordination, streamlined regulatory approvals, and increased funding are needed.
Data are lacking from well-designed implementation studies to ascertain the acceptability, accuracy, and effect of community- or home-based testing on SARS-CoV-2 transmission and Covid-19 outcomes. Data are also lacking as to the best way to integrate diagnostic testing into routine medical and surgical care.54 Although some RDTs appear to perform well in the detection of major viral variants,55 most field-based validation studies were conducted before the emergence of the delta and omicron variants. New viral mutations may directly alter the genomic sequence that is detected by molecular RDTs, and the FDA recently issued warnings about two molecular tests that are not expected to detect the omicron variant.56 Since antigen-based RDTs detect epitopes on the surface proteins (mostly nucleocapsid), their performance is more dependent on protein structure and confirmation than on single genomic mutations. Nonetheless, clinical studies are urgently needed to evaluate the performance of molecular and antigen-based RDTs, including saliva-based tests, in detecting emerging variants of concern. Studies are also needed to evaluate the performance of these tests with respect to breakthrough infections among vaccinated persons (with or without booster vaccination).
Guidelines
Guidelines from the WHO, CDC, and European Center for Disease Prevention and Control all endorse and recommend the use of RDTs for diagnosis in persons who have symptoms consistent with Covid-19 and in the screening of asymptomatic persons who are at high risk for acute SARS-CoV-2 infection.22,23,34 The recommendations in this article are generally consistent with these guidelines. On the basis of a very low-to-moderate quality of evidence,57 the Infectious Diseases Society of America (IDSA) recommends either a molecular RT-PCR RDT or a laboratory-based NAAT over antigen-based RDTs both for testing of symptomatic persons and for screening of asymptomatic persons. However, the IDSA acknowledges that antigen-based RDTs may be helpful in areas where molecular testing is neither readily available nor feasible. Recommended indications for testing and use of RDTs in specific situations are summarized in Table 2.
Table 2. Summary of Major Guidelines and Recommendations for RDTs to Detect SARS-CoV-2.*.
| Guideline or Recommendation | WHO | CDC | ECDC | IDSA |
|---|---|---|---|---|
| Endorsement of RDTs | ||||
| Antigen-based RDT | Yes | Yes | Yes | No |
| Molecular RDT | Yes | Yes | Yes | Yes |
| Testing indication | ||||
| Person with symptoms of Covid-19 | Yes | Yes | Yes | Yes, molecular test only |
| Asymptomatic person with high pretest probability of infection | Yes | Yes | Yes | Yes, molecular test only |
| Screening in asymptomatic person with low pretest probability of infection | Yes† | Yes | Yes, if population prevalence ≥10% | Yes, molecular test only |
| Specific situation | ||||
| Repeat serial RDTs after negative test, if high clinical suspicion | Yes† | Yes | Yes | No |
| Confirmatory testing recommended | No | No | Yes‡ | Yes§ |
| Timing for testing an asymptomatic person after an exposure | NC | 5–7 days | 2–7 days | NC |
| Provide support for patient performing swab specimen collection | No | Yes | No | Yes |
| Endorse home-based RDT | No | Yes | NC | NC |
| Case registration, isolation, and contact tracing | Yes | Yes | Yes | NC |
ECDC denotes European Center for Disease Prevention and Control, IDSA Infectious Diseases Society of America, NC no comment in guideline document, RDT rapid diagnostic test, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
The WHO endorses antigen-based RDTs for serial screening strategies when there is a suspected outbreak of Covid-19 in congregate settings, including schools, nursing homes, and health care facilities, and emphasizes that these tests will be most reliable in settings with ongoing transmission, which they define as a test positivity rate of 5% or higher.22
The ECDC recommends confirmation of all antigen-based RDTs with either a laboratory-based nucleic acid amplification test (NAAT) or a second different antigen-based RDT.
The IDSA recommends confirmation of negative antigen-based RDTs with a laboratory-based NAAT in symptomatic patients who have a high clinical pretest probability of infection.
Conclusions and Recommendations
The woman in the vignette and her family are at moderate risk for acquiring SARS-CoV-2 infection owing to their close contact 3 days previously with a person who was found to have confirmed Covid-19. All family members should be tested for SARS-CoV-2 infection. She has type 2 diabetes, which increases her risk of severe illness. If the woman and her children remain asymptomatic, testing is appropriate 5 to 7 days after exposure and can be performed with the use of an RDT that has received FDA EUA status for home-based testing of asymptomatic persons. Quarantine is not currently recommended for asymptomatic persons who have had two vaccinations and are awaiting test results, but given her unboosted vaccination status and emerging data regarding the omicron variant, I would advise her to minimize contact with others.
If the test is negative, it should be repeated in 2 days with another home test or a laboratory-based NAAT (depending on availability). Close monitoring for symptoms is recommended for 2 weeks after exposure, with quarantine and retesting if symptoms develop.
It appears that the woman’s husband may have symptoms of Covid-19. He should quarantine and be tested promptly with any FDA-approved, home-based RDT. If the test is negative, a second RDT or laboratory-based NAAT should be considered, particularly if his condition worsens. The children’s return to day care or school should be guided by local regulations.
Any persons who have a positive RDT should contact a health care provider or public health department to report their infection and to discuss any symptoms as well as therapy, hospitalization, or both. According to CDC recommendations for persons who test positive, persons who are asymptomatic may discontinue isolation 5 days after a positive test, and those who have symptoms that are resolving (and who are afebrile for 24 hours without the use of antipyretic agents) may discontinue isolation 5 days after a positive test or 5 days after the onset of symptoms, whichever is later. In these persons, the use of a well-fitted mask in public is recommended for 5 days after the end of the isolation period. In persons who continue to have symptoms or fever, a 10-day isolation period is recommended. Like many other experts, I recommend that all persons have a negative test in order to safely discontinue isolation before a full 10 days after a positive test. The patient and her husband should be encouraged to receive booster vaccinations and to vaccinate their children.
Attachments
Supplementary Appendix
Disclosure Forms
This article was published on January 7, 2022, at NEJM.org.
Footnotes
Disclosure forms provided by the author are available with the full text of this article at NEJM.org.
References
- 1.World Health Organization. WHO coronavirus (COVID-19) dashboard (https://covid19.who.int).
- 2.Technical specifications for selection of essential in vitro diagnostics for SARS-CoV-2. Geneva: World Health Organization, April 19, 2021. (https://www.who.int/publications/m/item/technical-specifications-for-selection-of-essential-in-vitro-diagnostics-for-sars-cov-2). [Google Scholar]
- 3.Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our World in Data (https://ourworldindata.org/coronavirus).
- 4.Wu SL, Mertens AN, Crider YS, et al. Substantial underestimation of SARS-CoV-2 infection in the United States. Nat Commun 2020;11:4507-4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Institute for Health Metrics and Evaluation. COVID-19 has caused 6.9 million deaths globally, more than double what official reports show. May 6, 2021. (http://www.healthdata.org/news-release/covid-19-has-caused-69-million-deaths-globally-more-double-what-official-reports-show).
- 6.Yadav H, Shah D, Sayed S, Horton S, Schroeder LF. Availability of essential diagnostics in ten low-income and middle-income countries: results from national health facility surveys. Lancet Glob Health 2021;9(11):e1553-e1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drain PK, Hyle EP, Noubary F, et al. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis 2014;14:239-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foundation for Innovative New Diagnostics. Test directory (https://www.finddx.org/test-directory/).
- 9.The White House. Fact sheet: President Biden announces new actions to protect Americans and help communities and hospitals battle omicron. December 21, 2021. (https://www.whitehouse.gov/briefing-room/statements-releases/2021/12/21/fact-sheet-president-biden-announces-new-actions-to-protect-americans-and-help-communities-and-hospitals-battle-omicron/).
- 10.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020;26:672-675. [DOI] [PubMed] [Google Scholar]
- 11.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020;581:465-469. [DOI] [PubMed] [Google Scholar]
- 12.Jones TC, Biele G, Mühlemann B, et al. Estimating infectiousness throughout SARS-CoV-2 infection course. Science 2021;373:eabi5273-eabi5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Symptoms of COVID-19. February 2021. (https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html).
- 14.World Health Organization. Coronavirus disease (COVID-19): symptoms (https://www.who.int/health-topics/coronavirus#tab=tab_3).
- 15.Byambasuren O, Cardona M, Bell K, Clark J, McLaws M-L, Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. Off J Assoc Med Microbiol Infect Dis Can 2020;5:223-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu X, Nergiz AI, Maraolo AE, Bogoch II, Low N, Cevik M. The role of asymptomatic and pre-symptomatic infection in SARS-CoV-2 transmission — a living systematic review. Clin Microbiol Infect 2021;27:511-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buitrago-Garcia D, Egli-Gany D, Counotte MJ, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med 2020;17(9):e1003346-e1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021;385(24):e84-e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med 2021;385:2421-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kissler SM, Fauver JR, Mack C, et al. Viral dynamics of SARS-CoV-2 variants in vaccinated and unvaccinated persons. N Engl J Med 2021;385:2489-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med 2021;385:2413-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Antigen-detection in the diagnosis of SARS-CoV-2 infection. Interim guidance. October 6, 2021. (https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays).
- 23.Centers for Disease Control and Prevention. Testing for COVID-19. October 4, 2021. (https://www.cdc.gov/coronavirus/2019-ncov/testing/index.html).
- 24.European Commission Joint Research Center. COVID-19 in vitro diagnostic medical devices (https://covid-19-diagnostics.jrc.ec.europa.eu/devices?device_id=&manufacturer=&text_name=&marking=Yes&method=&rapid_diag=1&target_type=6&search_method=AND#form_content).
- 25.Mina MJ, Andersen KG. COVID-19 testing: one size does not fit all. Science 2021;371:126-127. [DOI] [PubMed] [Google Scholar]
- 26.Bullard J, Dust K, Funk D, et al. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin Infect Dis 2020;71:2663-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S, Kim T, Lee E, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea. JAMA Intern Med 2020;180:1447-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drain PK, Ampajwala M, Chappel C, et al. A rapid, high-sensitivity SARS-CoV-2 nucleocapsid immunoassay to aid diagnosis of acute COVID-19 at the point of care: a clinical performance study. Infect Dis Ther 2021;10:753-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fajnzylber J, Regan J, Coxen K, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun 2020;11:5493-5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pekosz A, Parvu V, Li M, et al. Antigen-based testing but not real-time polymerase chain reaction correlates with severe acute respiratory syndrome coronavirus 2 viral culture. Clin Infect Dis 2021;73(9):e2861-e2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Food and Drug Administration. Emergency use authorizations for medical devices. 2021. (https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations-medical-devices#covid19ivd).
- 32.World Health Organization. Emergency use listing procedure for in vitro diagnostics. 2020. (https://www.who.int/teams/regulation-prequalification/eul/in-vitro-emergency-use-listing-procedure).
- 33.European Commission Directorate-General for Health and Food Safety. EU health preparedness: a common list of COVID-19 rapid antigen tests; a common standardised set of data to be included in COVID-19 test result certificates; and a common list of COVID-19 laboratory based antigenic assays. December 2021. (https://ec.europa.eu/health/sites/default/files/preparedness_response/docs/covid-19_rat_common-list_en.pdf).
- 34.European Centre for Disease Prevention and Control. Options for the use of rapid antigen tests for COVID-19 in the EU/EEA — first update. October 26, 2021. (https://www.ecdc.europa.eu/en/publications-data/options-use-rapid-antigen-tests-covid-19-eueea-first-update).
- 35.Drain P, Sulaiman R, Hoppers M, Lindner NM, Lawson V, Ellis JE. Performance of the LumiraDx Microfluidic Immunofluorescence Point-of-Care SARS-CoV-2 Antigen Test in asymptomatic adults and children. Am J Clin Pathol 2021. October 20 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corman VM, Haage VC, Bleicker T, et al. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: a single-centre laboratory evaluation study. Lancet Microbe 2021;2(7):e311-e319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jääskeläinen AE, Ahava MJ, Jokela P, et al. Evaluation of three rapid lateral flow antigen detection tests for the diagnosis of SARS-CoV-2 infection. J Clin Virol 2021;137:104785-104785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindner AK, Krüger LJ, Nikolai O, et al. SARS-CoV-2 variant of concern B.1.1.7: diagnostic accuracy of three antigen-detecting rapid tests. June 15, 2021. (https://www.medrxiv.org/content/10.1101/2021.06.15.21258502v1). preprint. [DOI] [PMC free article] [PubMed]
- 39.Brümmer LE, Katzenschlager S, Gaeddert M, et al. Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: a living systematic review and meta-analysis. PLoS Med 2021;18(8):e1003735-e1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dinnes J, Deeks JJ, Berhane S, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev 2021;3:CD013705-CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J, Song J-U, Shim SR. Comparing the diagnostic accuracy of rapid antigen detection tests to real time polymerase chain reaction in the diagnosis of SARS-CoV-2 infection: a systematic review and meta-analysis. J Clin Virol 2021;144:104985-104985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prince-Guerra JL, Almendares O, Nolen LD, et al. Evaluation of Abbott BinaxNOW rapid antigen test for SARS-CoV-2 infection at two community-based testing sites — Pima County, Arizona, November 3–17, 2020. MMWR Morb Mortal Wkly Rep 2021;70:100-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pray IW, Ford L, Cole D, et al. Performance of an antigen-based test for asymptomatic and symptomatic SARS-CoV-2 testing at two university campuses — Wisconsin, September–October 2020. MMWR Morb Mortal Wkly Rep 2021;69:1642-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frediani JK, Levy JM, Rao A, et al. Multidisciplinary assessment of the Abbott BinaxNOW SARS-CoV-2 point-of-care antigen test in the context of emerging viral variants and self-administration. Sci Rep 2021;11:14604-14604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindner AK, Nikolai O, Kausch F, et al. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with self-collected nasal swab versus professional-collected nasopharyngeal swab. Eur Respir J 2021;57:2003961-2003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mina MJ, Parker R, Larremore DB. Rethinking COVID-19 test sensitivity — a strategy for containment. N Engl J Med 2020;383(22):e120-e120. [DOI] [PubMed] [Google Scholar]
- 47.Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv 2021;7:eabd5393-eabd5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Love N, Ready D, Turner C, et al. The acceptability of testing contacts of confirmed COVID-19 cases using serial, self-administered lateral flow devices as an alternative to self-isolation. March 26, 2021. (https://www.medrxiv.org/content/10.1101/2021.03.23.21254168v1). preprint. [DOI] [PubMed]
- 49.Pavelka M, Van-Zandvoort K, Abbott S, et al. The impact of population-wide rapid antigen testing on SARS-CoV-2 prevalence in Slovakia. Science 2021;372:635-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young BC, Eyre DW, Kendrick S, et al. Daily testing for contacts of individuals with SARS-CoV-2 infection and attendance and SARS-CoV-2 transmission in English secondary schools and colleges: an open-label, cluster-randomised trial. Lancet 2021;398:1217-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martín-Sánchez V, Fernández-Villa T, Carvajal Urueña A, et al. Role of rapid antigen testing in population-based SARS-CoV-2 screening. J Clin Med 2021;10:3854-3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ricks S, Kendall EA, Dowdy DW, Sacks JA, Schumacher SG, Arinaminpathy N. Quantifying the potential value of antigen-detection rapid diagnostic tests for COVID-19: a modelling analysis. BMC Med 2021;19:75-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peeling RW, Olliaro PL, Boeras DI, Fongwen N. Scaling up COVID-19 rapid antigen tests: promises and challenges. Lancet Infect Dis 2021;21(9):e290-e295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drain PK, Garrett N. SARS-CoV-2 pandemic expanding in sub-Saharan Africa: considerations for COVID-19 in people living with HIV. EClinicalMedicine 2020;22:100342-100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bekliz M, Adea K, Essaidi-Laziosi M, et al. SARS-CoV-2 rapid diagnostic tests for emerging variants. Lancet Microbe 2021;2(8):e351-e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Food and Drug Administration. SARS-CoV-2 viral mutations: impact on COVID-19 tests. 2021. (https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-viral-mutations-impact-covid-19-tests).
- 57.Hanson KE, Altayar O, Caliendo AM, et al. IDSA guidelines on the diagnosis of COVID-19: antigen testing. Arlington, VA: Infectious Diseases Society of America, May 27, 2021. (www.idsociety.org/COVID19guidelines/Ag). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.