Abstract
Navigation occurs through a continuum of space and time. The hippocampus is known to encode the immediate position of moving animals. However, active navigation, especially at high speeds, may require representing navigational information beyond the present moment. Using wireless electrophysiological recordings in freely flying bats, we demonstrate that neural activity in area CA1 predominantly encodes nonlocal spatial information up to meters away from the bat’s present position. This spatiotemporal representation extends both forward and backward in time, with an emphasis on future locations, and is found during both random exploration and goal-directed navigation. The representation of position thus extends along a continuum, with each moment containing information about past, present, and future, and may provide a key mechanism for navigating along self-selected and remembered paths.
Bats are renowned for their exceptional navigational abilities and are believed to use map-based navigation to forage in the wild (1–4). The neural circuitry for map-based representations includes the hippocampus, which encodes the immediate spatial position in terrestrial animals (5–7). Consistent with these studies, the basic components for representing the immediate three-dimensional (3D) spatial position in freely flying bats have been identified (8–10). However, is it enough for navigational information to be represented only at the present moment or should such representations extend from the past and into the future during active movement (11–15)? Several mechanisms have been identified in the rodent hippocampus for representing spatial locations extending beyond the immediate position. Theta-phase and theta-sequence coding have been shown to indicate the animal’s past and future positions as well as possible future paths (16–24). Rate modulations based on past or future events have also been observed in a subset of place cells (25–27). However, neurons providing nonlocal positional information are still primarily active when the animal is within the cell’s firing field. Intriguingly, a subset of place cells has been found to be most informative when the neural spiking activity is assigned to the animal’s position a short period of time (~120 ms) into the future (28), suggesting the existence of an additional, nonlocal neural mechanism based on rate-shifted coding. However, because of the movement speed of rodents in small enclosures, an ~120-ms shift corresponds to a position directly under the rat’s nose (29), therefore still representing relatively local position. Here, we investigated rate-shifted coding mechanisms in flying bats, which provide an advantageous model system for studying nonlocal positional coding because of their high-speed (30), ballistic motion through space, which could facilitate identifying neural representations of locations that are spatially distant but still temporally close.
Typically, place coding is thought to be carried out by place cells that encode the immediate spatial position (Fig. 1A, top). We posited that the neural code for 3D spatial navigation in a fast-flying animal may include additional neural populations encoding places that the animal does not currently occupy (nonlocal coding) but that are within the flight path (Fig. 1A, bottom). In this hypothetical spatiotemporal code, a population of neurons is simultaneously active at each spatial position, but only some optimally encode the immediate position, whereas others optimally encode past or future positions that are meters away, leading to a representation of the full flight path in any given moment (Fig. 1A, bottom). This hypothesis predicts that neurons representing distant positions along the path may not exhibit any spatial tuning at all when the neural activity is considered with respect to the bat’s immediate position. For these neurons, the prediction is that spatially selective fields may only emerge when the appropriate lag is applied between the positional and neural information; in other words, their spatial selectivity will be divorced from the present and imbedded in the past or in the future.
Fig. 1. CA1 neurons in flying bats encode nonlocal positions, primarily in the future.
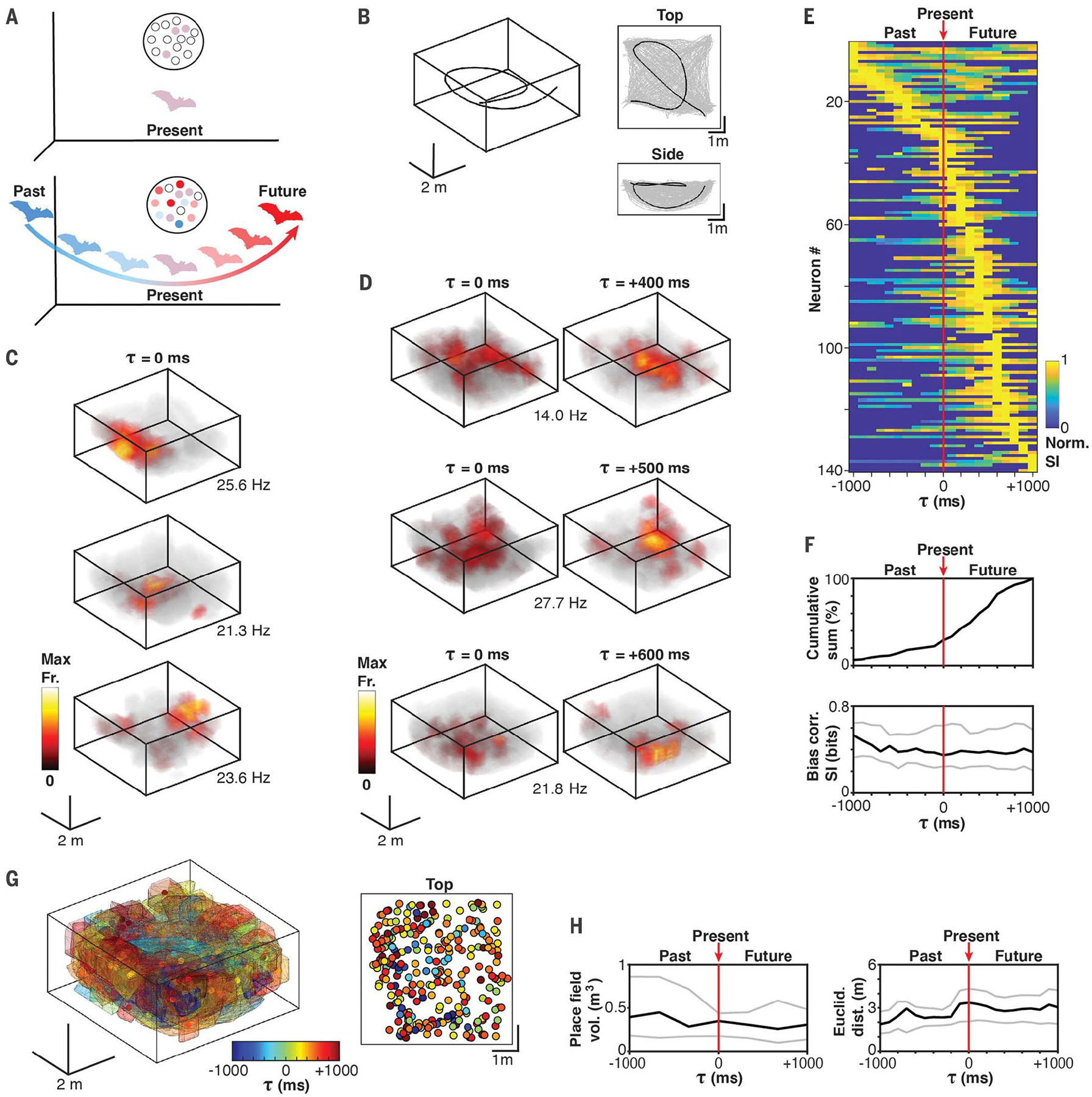
(A) Illustration of nonlocal coding hypothesis. Top: A subset of classical place cells (light purple circles) with place fields overlapping with the immediate spatial position (local). Bottom: Neurons with nonlocal fields encoding past (blue) and future (red) spatial positions. (B) Example of a single flight (left) and the spatial coverage (gray lines) shown from the top and the side for an entire recording session. Black trace corresponds to the example flight trajectory. (C) 3D color-coded firing rate maps for three examples of 3D place cells. Bias-corrected spatial information values for the three cells is as follows: top, 0.81 bits; middle, 0.61 bits; and bottom, 0.61 bits. The peak firing rate is indicated. (D) Same as (C) but for three example neurons exhibiting higher 3D spatial information for future positions. Spatial information values for the example neurons are as follows: top, lag zero = 0.10 bits [not significant (n.s.)] and optimal lag = 0.28 bits; middle: lag zero = 0.11 bits (n.s) and optimal lag = 0.30 bits; and bottom, lag zero = 0.18 bits (n.s.) and optimal lag = 0.58 bits. (E) Normalized spatial information across time lags for each neuron with significant spatial information at one or more time lags (−1000 to 1000 ms in 100-ms steps). Rank order is based on the time lag with maximal spatial information. (F) Cumulative sum of the time lags with maximal spatial information for each neuron (top) and summary of the bias-corrected spatial information values at each lag (one value per neuron) (bottom). The 25th (bottom gray line), 50th (middle black line), and 75th (top gray line) percentiles are shown. (G) Left: Convex hull representation of all significant spatiotemporal field locations across all recorded neurons. Right: top-down view showing location of spatiotemporal field centers. Color code indicates the time lag of maximal spatial information. (H) Field volume across lags (left) and Euclidean distance between all field centers at each lag (right). The 25th (bottom gray line), 50th (middle black line), and 75th (top gray line) percentiles are shown.
We initially performed wireless neurophysiological recordings from the dorsal CA1 region of four Egyptian fruit bats exploring the full 3D volume of a large flight room (Fig. 1B). In this random exploration experiment, a total of 532 well-isolated single units were recorded, of which 304 were sufficiently active during flight and included in subsequent analyses (see the supplementary materials and methods). Bats were encouraged to fly throughout the room to ensure dense spatial coverage (Fig. 1B). The 3D position of the bats was tracked at millimeter spatial resolution (see the supplementary materials and methods).
We used a time-shifting procedure to assess the neuron’s positional coding and to determine whether it is higher at past, present, or future spatial positions (28). We calculated the spatial information after time shifting the spiking activity along flight trajectories (−1000 to +1000 ms in 100-ms steps; see the supplementary materials and methods; fig. S1). In agreement with previous studies (8), 27% (81/304 neurons; P < 0.05, shuffle test with Bonferroni correction) of the neurons were spatially selective at zero lag between spatial position and spikes (τ0) (Fig. 1C and fig. S2). Overall, 46% (140/304 neurons, P < 0.05, shuffle test with Bonferroni correction) of the neurons were spatially selective at one or more time shifts. For most hippocampal neurons, spatial information was highest at nonzero time shifts, with most being optimally informative about future positions (Fig. 1, D and E, and fig. S3). Figure 1D shows three examples of neurons with no significant spatial selectivity at zero lag (τ0) and high spatial selectivity at a future lag. If the hippocampal code were primarily related to the present, then we would expect to see the maximum spatial information concentrated around τ0 (Fig. 1E, red line, and fig. S4). Instead, we saw a continuum, with most neurons being maximally informative at future lags while maintaining a similar amount of spatial information across lags (Fig. 1F). For a large fraction of spatially selective neurons, the spatial information at τ0 was negligible (42%; 59/140 selective neurons), indicating that these neurons would go undetected as being spatially selective using standard procedures, and are thus truly nonlocal. Further, the spatiotemporal firing fields were distributed throughout the entire room and maintained consistent volumes and inter-field distances across lags (Fig. 1, G and H), with typically one (46%) or two (29%) fields per cell (89% had three or fewer fields). Thus, time-lagged firing fields appear to be equivalent to nonlagged fields in terms of their spatial information, spatial distribution, and total volume. Furthermore, we found that spatiotemporal fields were largely stable throughout the session (fig. S5), and the optimal lag time was not related to the mean speed of the bat (fig. S6). A spatial information analysis using distance lags that matched the temporal lags (based on the mean flight speed) produced similar results (fig. S7), and a cross-correlation analysis indicated that the timing relationship of correlated firing is not related to the optimal lag order (fig. S8). Last, nonlocal heading tuning was prominent as well (54%, 142/304), with a high degree of overlap with spatially informative neurons (76%, 107/140), although the distribution of optimal heading tuning was shifted toward the past (fig. S9).
What role can a representation of space that is divorced from the present moment play in a navigating animal? Egyptian fruit bats navigate landscapes spanning hundreds of kilometers to forage for food, flying in highly reproducible paths to and from foraging sites (1). Members of this species also make novel shortcuts between distant foraging sites (3, 4). This suggests that during goal-directed navigation, these bats maintain a detailed spatial memory of both their environment and of paths taken within it. To understand how this nonlocal code may be used for path-based, goal-directed navigation, we engaged animals in a freely paced foraging task in an automated environment [Fig. 2A; (31); see the supplementary materials and methods]. Four reward feeders positioned along one wall of the room fed at different probabilities, with one side feeding at higher probabilities than the other (e.g., 70 and 30%). The probabilities switched after a set number of feeds to induce exploration to all feeders and to keep the animals attentive to the task (see the supplementary materials and methods).
Fig. 2. Nonlocal spatiotemporal coding is present during goal-directed navigation.

(A) Illustration of the foraging task (not drawn to scale; room dimensions: 5.6 m × 5.2 m × 2.5 m). Bats choose between four different feeders. Feeders fed at predetermined probabilities that switched during the session (see the supplementary materials and methods). (B) Left: all the flights on repeatable paths (black lines) are shown for a single recording session. Non-path flights are shown in gray. Right: break out of individual flight paths. Each subplot represents a distinct flight path (see the supplementary materials and methods). (C) Rate maps for three examples neurons with maximum spatial information at future time shifts. Top: all flight data. Middle: parts of flights with heading angles toward the feeders. Bottom: parts of flights with heading angles toward the stands. Maximum firing rate and bias-corrected spatial information values are as follows: left, max fr. = 30.98 Hz, lag zero = 0.65 bits, optimal lag = 1.08 bits; middle, max fr. = 56.11 Hz, lag zero = 0.49 bits, optimal lag = 0.93 bits; and right, max fr. = 9.76 Hz, lag zero = 0 bits (n.s.), optimal lag = 0.37 bits. (D) Cumulative sum of peak spatial information (top) and bias-corrected spatial information across lags. The 25th (bottom gray line), 50th (middle black line), and 75th (top gray line) percentiles are shown. (E) Top-down view showing the locations of spatiotemporal field centers. Color code indicates time lag of maximal spatial information.
We recorded neural activity from three bats engaged in the goal-directed foraging task. One bat performed the task alone and the other two performed the task at the same time (204 of 281 total cells were sufficiently active during behavior; see the supplementary materials and methods). There were no differences in the results between the individual bat and the pair, nor were there any indications of social representations of the other bat under the conditions of this experiment (fig. S10). We therefore combined the data from all three bats in subsequent analyses. Bats formed common paths, and typically only visited the feeders and a few places in the room to rest between feedings (Fig. 2B). Individual bats developed distinct movement patterns (fig. S11), underscoring the self-selected nature and reproducibility of their flight paths during goal-directed navigation (fig. S11B).
To determine whether nonlocal positions are encoded during foraging, we performed the same time-shifting analysis as in the random exploration experiments, focusing on the flights occurring along reproducible paths. Because flight paths were constrained to narrow portions of the room, leading to a heterogeneous sampling of the z axis with respect to the x and y axes, we constrained the analysis to 2D, as was done previously [(32, 33); similar results were found in 3D; fig. S12]. Similar to the random exploration experiment (Fig. 1), neural activity predominantly encoded nonlocal positions, was again shifted toward the future, and maintained a consistent level of spatial information for all temporal lags (Fig. 2, C and D, and fig. S12). Results were robust to multiple shuffle tests designed to account for the flight pattern structure (fig. S13; see the supplementary materials and methods). We found a very high percentage of neurons with significant spatial information (90%, 183/204 neurons) compared with the random exploration experiment, which may be attributed to the increased attentional demand and highly structured behavioral patterns exhibited during the task (34). This high degree of nonlocal spatiotemporal information was exemplified by the ability to decode past, present, and future positions on individual flight trajectories, with higher performance for decoders using the optimal time lag of each neuron (fig. S14; see the supplementary materials and methods). Furthermore, neurons appeared directionally tuned to either the feeders or the stand directions, suggesting that the task environment had a polarizing effect on the neural representations (Fig. 2C). Indeed, 85% (155/183) of spatially informative neurons and 81% (165/204) of total neurons were significantly tuned for heading at one or more time lags (see the supplementary materials and methods). The resulting spatiotemporal fields were distributed throughout the portion of the room used by the bats during foraging (Fig. 2E), with typically one (54%) or two (24%) fields per cell (91% had three or fewer fields).
The results presented thus far suggest that CA1 neurons encode nonlocal navigational information during flight but they do not indicate what might determine the locations of the spatiotemporal fields. We reasoned that spatiotemporal field organization might be linked to the self-organized behavioral structure that bats exhibit during goal-directed navigation. Because we found that neurons were tuned to flight paths headed in the same direction (Fig. 2C), we posited that neurons might also be tuned to other spatial commonalities between paths, specifically the places where paths intersect. To address this hypothesis, we first determined whether there was any overlap between path intersections and the spatiotemporal field locations during the goal-directed foraging task (Fig. 2). We identified the intersections between all pairs of flight paths (Fig. 3A and fig. S15; 10.8 mean ± 8.6 SD path intersections per neuron, for a total of 1931 intersections). We only considered intersections where paths were headed in the same direction (enter and exit angles < |90°|) and only intersected once (see the supplementary materials and methods). Intersections based on this criterion were found for 97% (178/183) of neurons with spatiotemporal fields. We found a high degree of overlap between the nonlocal spatiotemporal fields and intersection locations (Fig. 3B), supporting the hypothesis that temporally shifted firing fields were centered at locations where flight paths intersected. We computed the distance between intersection locations and spatiotemporal field locations and found that 72% (129/178) of the neurons had an intersection within ≤2 m from the neuron’s spatiotemporal field. Moreover, 50% (973/1931) of all the intersections were <2 m from their respective neuron’s spatiotemporal field (Fig. 3C; less dispersed than would be expected by chance, P < 0.01, randomization test; see the supplementary materials and methods). On the basis of the strong directional orientation, it is apparent that neurons are only active along a subset of paths, which could explain why a portion of intersections are far from spatiotemporal fields. Thus, there is a strong correlation between the location of a large subset of intersections and spatiotemporal fields.
Fig. 3. Flight path intersections are aligned with nonlocal spatiotemporal fields.
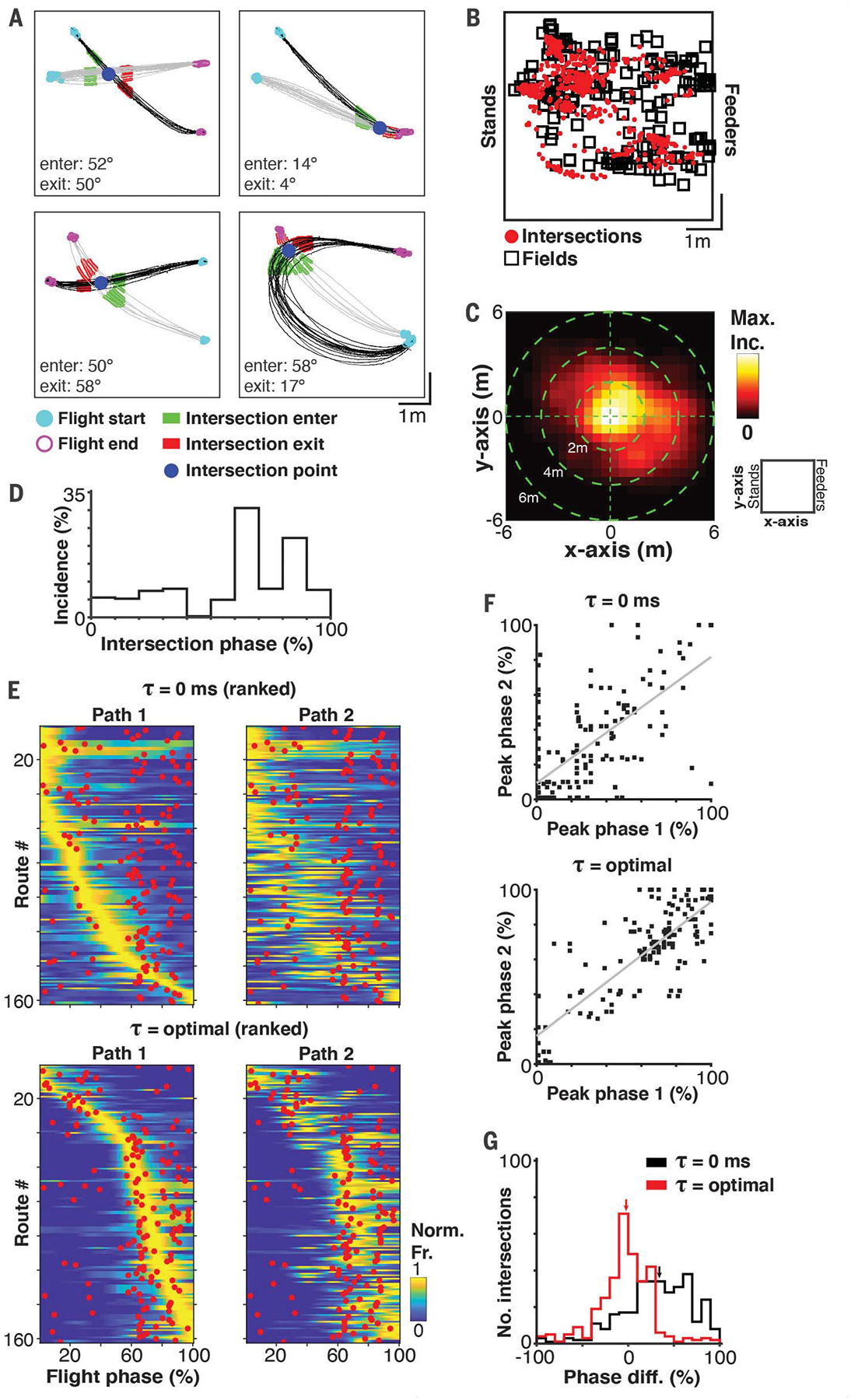
(A) Examples of intersecting flight paths. Each panel shows the flights for two paths (one path in gray and one in black). Blue dot indicates the intersection point. Green (before intersection) and red (after intersection) highlights indicate the portion of the flights used to calculate the intersection angles. Intersection angles (enter and exit) are indicated for each panel. (B) Locations of spatiotemporal fields (black squares; largest field is shown if multiple fields exist per neuron) and intersections (red dots). Note the tight correspondence between the location of firing fields and intersections. (C) 2D heat map of the differences in x axis (stand-feeder axis) and y axis positions between spatiotemporal fields and intersection locations (normalized to maximum incidence). The schematic on the right indicates the x axis and y axis reference frame of the room. (D) Distribution of the intersection phases. For each intersection, there are two phases, one for each path. (E) Firing rate tuning profiles for each pair of paths at lag zero (top) and the optimal lag (bottom). Rank is ordered by the peak firing rates in path #1. Red dots indicate the intersection phase. (F) Scatter plots for the peak phase of path 1 and path 2 at lag zero (top; r = 0.69, P < 0.01) and the optimal lag (bottom; r = 0.81, P < 0.01). Least-squares fit lines are shown in gray. (G) Histograms showing the difference between the peak firing rate phase and the phase of the intersection at lag zero and the optimal lag. Small black and red arrows indicate the median difference for lag zero and the optimal lag, respectively (lag zero median = 34.03%, optimal lag median = −2.27%).
Next, we sought to determine whether spatiotemporal fields could result from the patterns of neural activity along intersecting paths. If the neural activity on each path were temporally advanced or delayed with respect to the intersection, then this could result in a spatiotemporal field at the intersection. For each neuron, we identified pairs of paths with significant spatial information (see the supplementary materials and methods) and an intersection <2 m from the spatiotemporal field of the neuron. We identified 162 path pairs from 29% (53/183) of the spatially informative neurons. We then investigated whether the peak firing rates are aligned to path intersection points when the optimal lag is taken into consideration. We first scaled all the flights from 0 to 100% (flight phase) and then identified the phase of intersections (Fig. 3D) and the peak firing rate phases at lag zero and at the optimal lag (optimal lag is the lag with maximal spatial information for that neuron). Figure 3E shows the intersection phases (red dots) overlaid on the firing rate profiles for each pair of paths at lag zero (top) and the optimal lag (bottom). The peak firing rates for both paths, even though most have little overlap in space, became aligned with the intersection phase at the optimal lag. Furthermore, there was a strong positive correlation between the peak firing rate phases for all pairs of intersecting paths, which increased when accounting for the optimal lag (Fig. 3F). Indeed, simply accounting for the optimal lag substantially shifted the distribution of differences between the peak firing rate phase and the intersection phase toward zero (Fig. 3G; P < 0.01 rank sum test). These results demonstrate a precise alignment between self-organized navigation patterns and neural dynamics, suggesting that spatiotemporal firing fields may form around intersections.
When we recorded from bats flying freely at high speeds during either random exploration or in a goal-directed manner, we found that the neural activity in bat CA1 robustly encodes nonlocal navigational information. Classical place tuning appears to be simply part of a larger continuum representing the bat’s past, present, and future locations. Much of this information would go undetected if past or future positions were not taken into consideration. These findings also complement reports demonstrating that cells with no identified firing fields, i.e., non–place cells, can contribute to the neural code for space at the population level (35, 36), albeit in a different manner. Last, these findings, although functionally similar, are mechanistically distinct from theta-phase and theta-sequence coding and reveal another complementary mechanism by which positional information that extends beyond the animal’s present position can be represented in the hippocampal formation. The diversity of temporal and rate-based coding schemes by which nonlocal positional information can be represented raises important questions for future studies to consider about how simultaneous representations of past, present, and future positions in the hippocampus can be read out effectively by downstream regions.
Our results further indicate that nonlocal representations are anchored near both future and past intersections of self-selected flight paths, which can occur at all phases of flight. This differs from a vectorial tuning to a specific endpoint goal location (32), yet the two could serve as important complements to each other during navigation. These results are also consistent with studies showing evidence for path-invariant representations of spatial positions (29) such as intersections and goal locations. Previous investigations into rate-shifted coding mechanisms in rats showed that neurons optimally encoded a position immediately in front of the animal (28, 29). This could have been because of the slower speeds of movement exhibited by rodents in small experimental environments compared with the high speeds of bat flight, which necessitates planning and rapidly predicting positions far into the future. Combined, these results reveal a positional representation in flying bats that extends along a continuum of space and time and could support a representation of remembered paths.
Spatial coding has been observed in the hippocampus (or analogous structures) across a wide variety of species that have evolved to navigate in very different environments, whether underwater, on the ground, or in the sky (5, 8, 37–39). The way in which a given species negotiates its environment may necessitate weighing the relevance of past and future positions differently. For example, monkeys jumping between tree branches or humans driving a car or skiing downhill at high speeds may require a higher weight placed on future locations where the positional information ahead could be more important for survival than the present position. This notion is consistent with more recent theories highlighting the function of the hippocampus as a flexible predictive map (40). Furthermore, different species may also weigh the importance of specific locations in their environment differently. An Egyptian fruit bat, which naturally forms highly reproducible paths (1) along which it traverses repeatedly at high speeds, may benefit from a nonlocal representation of specific locations along flight paths (e.g., intersection points), yet this may not be the case for an animal exploring the environment in a different manner. Our results further highlight the importance of studying neural circuits during spontaneously emerging behavioral patterns across a diversity of species. Examining neural activity from an ethological perspective that carefully considers how a specific animal moves and operates could help to better inform us of the underlying neural computations that generate behavior (41, 42).
Supplementary Material
ACKNOWLEDGMENTS
We thank O. Tchernichovski for extensive discussion of the results, comments on the manuscript, and help with analysis; D. Foster for discussion of the results and manuscript; J. Widloski, K. Kay, and D. Foster for helpful discussions on a revised version of the manuscript; L. Kang, A. Forli, W. Liberti, T. Schmid, B. Styr, and M. Snyder for careful reading and comments on the manuscript; members of the Yartsev laboratory for discussion and comments; D. Genzel for assistance with the design of the experimental task and training protocols; Y. Minton and L. Loomis for experimental room maintenance and animal care; and G. Lawson and the staff of the Office of Laboratory Animal Care for support with animal husbandry and care.
Funding:
This work was supported by the New York Stem Cell Foundation (NYSCF-RNI40), the Air Force Office of Scientific Research (FA9550-17-1-0412), the Office of Naval Research (N00014-21-1-2063), the Packard Fellowship (2017-66825), the National Institute of Neurological Disorders and Stroke (R01NS118422-01), the Valle Foundation (VS-2020-34), and the Searle Scholars Program (SSP-2016-1412) to M.M.Y.
Footnotes
Competing interests: The authors declare no competing interests.
SUPPLEMENTARY MATERIALS
science.sciencemag.org/content/373/6551/242/suppl/DC1
MDAR Reproducibility Checklist
Data and materials availability:
All data are available in the main text or the supplementary materials.
REFERENCES AND NOTES
- 1.Tsoar A et al. , Proc. Natl. Acad. Sci. U.S.A 108, E718–E724 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geva-Sagiv M, Las L, Yovel Y, Ulanovsky N, Nat. Rev. Neurosci 16, 94–108 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Harten L, Katz A, Goldshtein A, Handel M, Yovel Y, Science 369, 194–197 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Toledo S et al. , Science 369, 188–193 (2020). [DOI] [PubMed] [Google Scholar]
- 5.O’Keefe J, Dostrovsky J, Brain Res 34, 171–175 (1971). [DOI] [PubMed] [Google Scholar]
- 6.Moser EI, Moser MB, McNaughton BL, Nat. Neurosci 20, 1448–1464 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Grieves RM et al. , Nat. Commun 11, 789 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yartsev MM, Ulanovsky N, Science 340, 367–372 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Geva-Sagiv M, Romani S, Las L, Ulanovsky N, Nat. Neurosci 19, 952–958 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Wohlgemuth MJ, Yu C, Moss CF, Front. Cell. Neurosci 12, 270 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nonlocal positional coding has also been observed in awake, stationary animals before and after spatial exploration and at decision points (12–15). [Google Scholar]
- 12.Foster DJ, Wilson MA, Nature 440, 680–683 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Diba K, Buzsáki G, Nat. Neurosci 10, 1241–1242 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson A, Redish AD, J. Neurosci 27, 12176–12189 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redish AD, Nat. Rev. Neurosci 17, 147–159 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Keefe J, Recce ML, Hippocampus 3, 317–330 (1993). [DOI] [PubMed] [Google Scholar]
- 17.Skaggs WE, McNaughton BL, Wilson MA, Barnes CA, Hippocampus 6, 149–172 (1996). [DOI] [PubMed] [Google Scholar]
- 18.Buzsáki G, Neuron 33, 325–340 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Buzsáki G, Hippocampus 15, 827–840 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Buckner RL, Annu. Rev. Psychol 61, 27–48, C1–C8 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Gupta AS, van der Meer MAA, Touretzky DS, Redish AD, Nat. Neurosci 15, 1032–1039 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eliav T et al. , Cell 175, 1119–1130.e15 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Foster DJ, Pfeiffer BE, Science 370, 247–250 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kay K et al. , Cell 180, 552–567.e25 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H, Neuron 27, 623–633 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Frank LM, Brown EN, Wilson M, Neuron 27, 169–178 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Ferbinteanu J, Shapiro ML, Neuron 40, 1227–1239 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Muller RU, Kubie JL, J. Neurosci 9, 4101–4110 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Battaglia FP, Sutherland GR, McNaughton BL, J. Neurosci 24, 4541–4550 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The average flight speed of a bat is ~3 m/s, with peak speeds up to ~6 m/s under laboratory conditions.
- 31.Genzel D, Yartsev MM, Neurosci J. Methods 348, 108970 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarel A, Finkelstein A, Las L, Ulanovsky N, Science 355, 176–180 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Omer DB, Maimon SR, Las L, Ulanovsky N, Science 359, 218–224 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Kentros CG, Agnihotri NT, Streater S, Hawkins RD, Kandel ER, Neuron 42, 283–295 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Meshulam L, Gauthier JL, Brody CD, Tank DW, Bialek W, Neuron 96, 1178–1191.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stefanini F et al. , Neuron 107, 703–716.e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hough GE, Bingman VP, J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol 190, 1047–1062 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Courellis HS et al. , PLOS Biol 17, e3000546 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vinepinsky E et al. , Sci. Rep 10, 14762 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stachenfeld KL, Botvinick MM, Gershman SJ, Nat. Neurosci 20, 1643–1653 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Yartsev MM, Science 358, 466–469 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Datta SR, Anderson DJ, Branson K, Perona P, Leifer A, Neuron 104, 11–24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text or the supplementary materials.


