Abstract
Erectile dysfunction (ED) remains a significant problem in up to 63% of men after robot-assisted radical prostatectomy (RARP). After the discovery of the neurovascular bundle (NVB), additional anatomic description and variation in nerve-sparing (NS) techniques have been described to improve post-RARP ED. However, it remains questionable whether ED rates have improved over time, and this is concerning as competing treatments are introduced that have better ED outcomes. In this review, we describe RARP NS technical modifications that improve erectile function recovery. We focused on reports that included detailed anatomical descriptions as well as video illustrations to disseminate technique. We found that the alternative RARP NS surgical techniques provide better outcomes compared with standard NS RARP. The use of validated quality of life questionnaires is necessary for the appropriate comparison of outcomes. However, the retrospective character and inherent weaknesses of the included studies do not allow one to conclude which is the best NS approach. Overall, there is significant variation in RARP NS techniques and outcomes, and the ideal technical maneuvers to optimize outcomes remains subject to debate. However, there is a consensus on the importance of anatomically dissecting the NVB, minimizing traction and thermal injury as well as preserving the periprostatic fascia. Well-designed randomized controlled trials with videos describing details of different surgical techniques for generalizability are needed to consistently and objectively evaluate sexual function outcomes after RARP to optimize postoperative potency.
Keywords: nerve-sparing, prostatectomy, erectile dysfunction, prostate cancer
Introduction
As initially reported by Walsh 40 years ago, neurovascular bundle (NVB) identification and preservation during radical prostatectomy (RP) attenuates postoperative erectile dysfunction (ED). Since that initial description during open RP, robot-assisted radical prostatectomy (RARP) has largely replaced RP1 because of lower blood loss, postoperative pain,2 and shorter hospital stays. In addition, some claimed that RARP would lead to improved potency because of improved observation and tissue handling3; however, these promises have not been realized. RARP also facilitates video recording that may ease technical dissemination and convergent evolution of the nerve-sparing (NS) approach. However, RARP NS approaches remain varied and ED remains a significant problem. For instance, Vickers and colleagues demonstrated that 12-month potency rates ranged from 8% to 49% at a high-volume academic referral center.4,5 On a population level, the United States Preventative Services Task Force cites a 63% risk of ED when informing men of the risks of PSA screening. Moreover, the majority of men require medical or surgical interventions to obtain erections adequate for intercourse after RP.5
There is a significant need and opportunity to improve RARP erectile function outcomes, particularly as new “non-surgical” treatment options such as stereotactic body radiation therapy and partial gland ablation are marketed to avoid ED outcomes. Although oncologic outcomes for RARP remain the gold standard for comparison, improved health-related quality of life following these new procedures may lead patients to pursue these therapies over the more oncologically proven RARP.6,7 As such, we sought to identify and review technical descriptions and associated outcomes of robotic NS techniques. In summarizing these approaches, our goal is to find consensus or common themes among RARP NS techniques that are associated with improved erectile outcomes. Such a foundation may serve to standardize NS techniques or enable incremental technical advances.
Description of NVB Anatomy and Robotic NS Techniques
We searched peer-reviewed literature on PubMed (https://pubmed.ncbi.nlm.nih.gov) for articles that described novel RARP NS techniques. We focused on articles that included detailed anatomical descriptions as well as video illustrations to disseminate technique (Supplementary Video S1).
Anatomy of the NVB and grading of NS
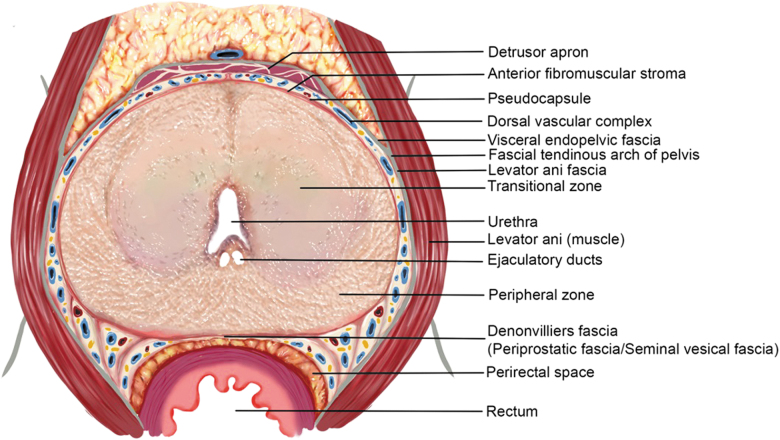
The outer surface of the prostate is covered by layers of connective tissue, known as the periprostatic fascia (PPF). PPF can be divided into three basic components based on its location: the anterior, lateral, and posterior (Denonvilliers') PPF. The anterior PPF covers the anterior prostate including the dorsal venous complex (DVC) and is fused in the midline with the anterior fibromuscular stroma of the prostate. The lateral PPF consists of the levator ani fascia and sometimes a thin inner layer known as prostatic fascia. The latter passes medially to the NVB and laterally to the pseudocapsule of the prostate. Denonvilliers' fascia enfolds the posterior prostatic surface as well as the seminal vesicles and runs from the prostatic apex to the prostatourethral junction (Figs. 1 and 2).8,9
FIG. 1.
Axial section of prostate and PPFs at mid-prostate. Major anatomic landmarks have been annotated. PPF = periprostatic fascia.
FIG. 2.
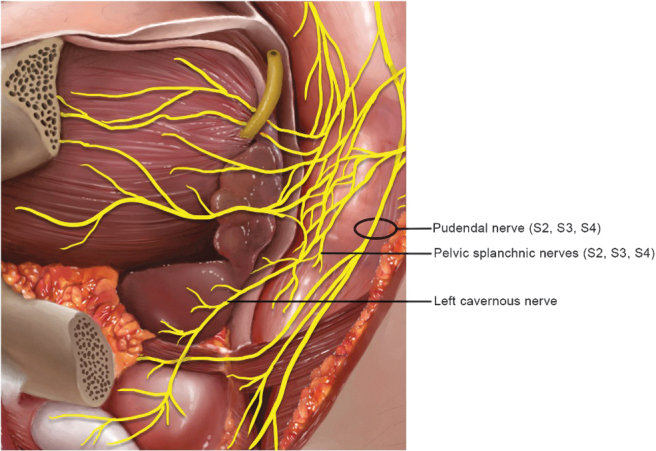
Sagittal left section of the male pelvis demonstrating the course of the cavernosal nerve in the pelvis and at the lateral side of the prostate.
Extent and quality of NS is generally categorized as intrafascial, interfascial, or extrafascial based on the plane of dissection in reference to the PPF.10 Intrafascial NS dissection follows a plane between the prostatic pseudocapsule and the medial aspect of the prostatic fascia. This approach allows total preservation of the NVB and, therefore, offers the best potency outcomes. Interfascial dissection allows for partial NS with dissection between the PPF medially and lateral pelvic fascia laterally. Finally, extrafascial NS involves complete resection of the NVB by carrying the dissection plane completely lateral to the lateral prostatic fascia. This approach offers the most oncologically safe option since it removes as much periprostatic tissue as possible, however also sacrifices erectile function if performed bilaterally. The surgeon determines the appropriate surgical plane based on the patient's anatomy and cancer extent from clinical staging and biopsy characteristics (Fig. 3).8,9
FIG. 3.
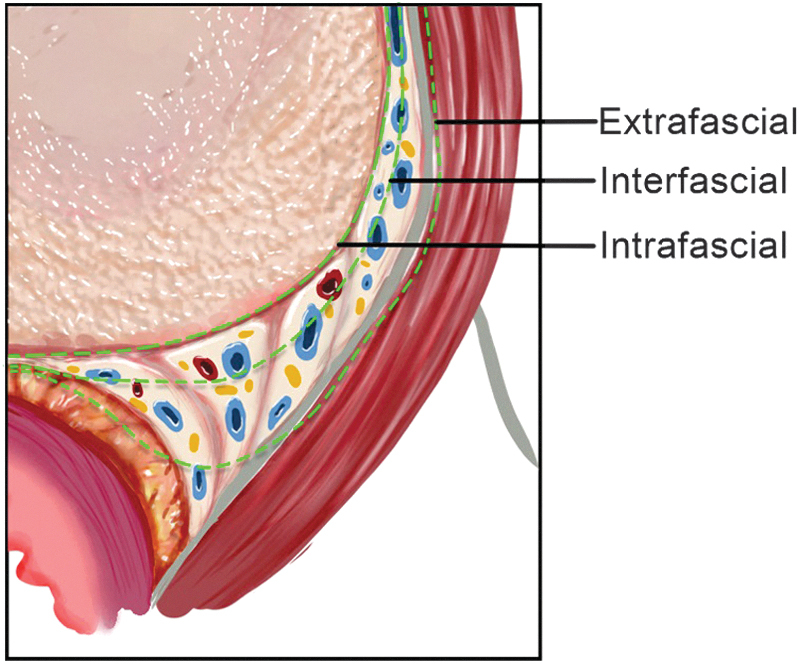
Axial section of the prostatic and PPF at mid-prostate demonstrating the three dissection planes of the NVB (extrafascial, interfascial, and intrafascial). NVB = neurovascular bundle.
Antegrade vs retrograde NS
NS approach can also be categorized as antegrade or retrograde based on the direction of dissection (base to apex vs apex to base). Both approaches start with developing the posterior plane between the prostate and rectum to define the medial border of NVB as well as the posterior prostate contour. During antegrade NS, the NVB is exposed upon entering the triangular space between Denonvilliers' fascia, the lateral pelvic fascia, and the prostate. Reflection of the lateral pelvic fascia off the prostate sets up the interfascial or intrafascial planes for dissection based on the depth of prostatic fascia incision.11 With retrograde NS, the prostate is retracted posteromedially away from the side of interest to expose the ipsilateral levator fascia, which is then sharply opened to expose the NVB at the apex. Dissection between the prostate and the NVB is then performed in an inter- or intrafascial approach until the previously developed posterior plane is reached.11
High anterior release
Menon and associates introduced the high anterior release in which the surgeon develops the intrafascial plane between the prostatic capsule and the prostatic fascia at the base of the seminal vesicles. Dissection is then carried from 1 to 5 o'clock position on the right side and from 11 to 7 o'clock on the left. At the end of the dissection, curtains of periprostatic tissue are suspended from the pubourethral ligament, which Menon described as the “veil of Aphrodite.”12 Further modification extended the dissection anteriorly, preserving the pubovesical ligaments and dorsal vein plexus (“superveil technique”).13
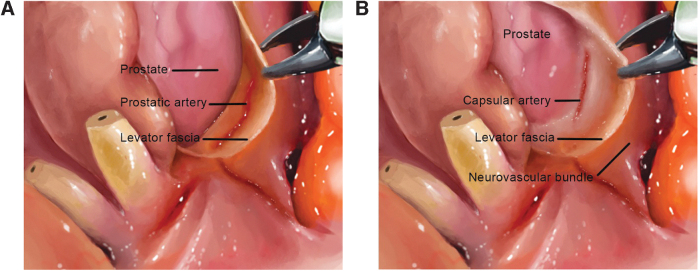
Prostatic vasculature as a landmark
Patel and colleagues described the use of prostatic vasculature as a landmark for NS (Fig. 4).14 The dissection plane is determined by the identification of landmark arteries (prostatic or capsular) running on the posterolateral, posteromedial, and anteromedial border of the prostate. These arteries are recognized during the posterior dissection of the prostate (posterolateral artery) and after opening the levator fascia at the base of the prostate (anteromedial artery) followed by a retrograde lateral dissection (in layers). The author described that the optimal NS is achieved when preserving the triangle formed by these arteries while dissecting the posterior and lateral prostate. The anteromedial prostatic artery is a larger tortuous vessel seen in the medial aspect of the NVB, and it will guide the correct dissection plane according to tumor characteristics and extension on that side. On the contrary, capsular arteries are smaller without tortuosity, which makes them more difficult to observe; they are located more distally relative to the prostatic artery. Complete NS can be attained using a dissection plane that targets the medial aspect of the landmark artery.
FIG. 4.
(A) The prostatic artery can be observed after opening the levator fascia on the base of the prostate. The prostatic artery tends to be tortuous and is seen at the medial aspect of the NVB. (B) The capsular artery can be observed after opening the levator fascia. It is usually found more distally than the prostatic artery, has smaller diameter, and is not tortuous.
Recently, Covas Moschovas and associates described a modification to this approach in which the apical dissection is minimized by preserving the puboprostatic ligaments. The lateral prostatic fascia is also preserved during the lateral dissection of the prostate and NS. In this technique, the DVC is ligated under observation with a delicate running suture after the apical dissection instead of the blind ligation with Vicryl and CT needle at the beginning of the surgery.15 Compared with the traditional approach, this modification led to improved early urinary and sexual function.
Kowalczyk and colleagues also described using capsular veins as a landmark during interfascial NS. These veins run long the medial extent of the NVB and may be split longitudinally in the presence of high-volume cancer on preoperative biopsy and/or MRI.16
Retrograde release of the NVB with preservation of the DVC
de Carvalho and coworkers17 described a technique in which retrograde release of the NVB allows for the preservation of the nervous and vascular structures anterior to the prostate. The technique entails dissection of the anterior bladder neck without entering the endopelvic fascia or ligating the DVC. The posterior bladder neck is incised and the vas deferens and SVs are athermally dissected. The NVB is released anteriorly at the level of the bladder neck, developing an avascular plane underneath the DVC, and subsequently, the dissection continues posterolaterally. Full NS prostatectomy is performed when the NVB is dissected medially to the prostatic artery, merging this plane with the posterior plane previously developed.
Countertraction-free technique to minimize neurapraxia
Kowalczyk and coworkers16 and Alemozaffar and colleagues18 demonstrated a modified antegrade technique that minimizes the countertraction of the NVB to reduce nerve injury and neurapraxia. Early NS techniques and videos often displayed excessive lateral traction on the NVB by the assistant suction tip as well as the robotic Maryland dissector. With meticulous and deliberate avoidance of NVB countertraction by the assistant as well as reduction in the excursion of the robotic scissors during blunt NVB dissection, the authors noted significant improvements in recovery of sexual function and stressed the need for gentle handling of the NVB, regardless of NS approach (Fig. 5A–C).
FIG. 5.
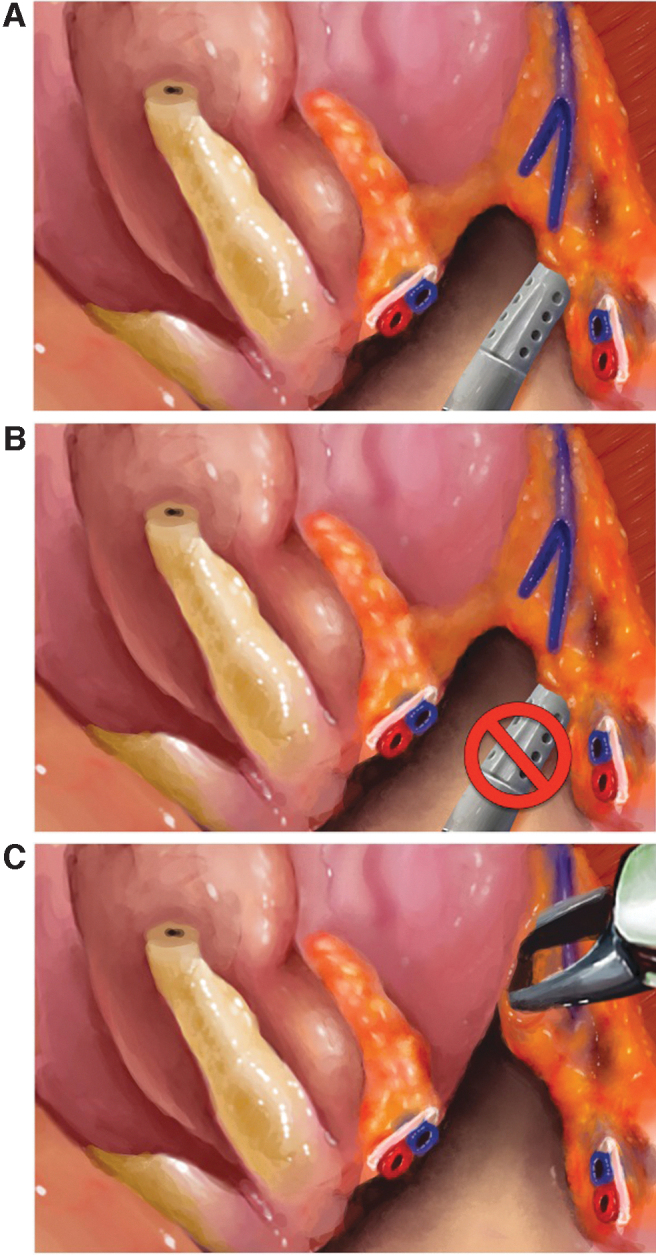
(A) Blunt dissection technique with assistant suction (or robotic instrument) NVB countertraction to facilitate NS dissection. (B) Technique modification to avoid countertraction of the NVB with assistant suction (or robotic instrument). (C) NS dissection with spreading of scissors longitudinally along the medial edge of the NVB. NS, nerve-sparing.
da Vinci SP approach to RARP
Different authors have described the SP approach to RP. However, all studies report a small series of cases performed with different types of trocar placement, surgical techniques, and short-term follow-up. A recent study comparing the SP with the multiport (Xi) robot in patients who underwent RP described similar postoperative pain scores with increased console and operative times for the SP.19 However, the literature still lacks well-designed studies with long-term follow-up comparing the SP approach with conventional RARP in terms of oncologic and functional outcomes.20,21
Summary of Anatomical Landmarks and Potency Outcomes of NS Techniques
Table 1 summarizes the outcomes of the reviewed studies and provides an overview of NS techniques and related sexual function outcomes at approximately 6 and 12 months after RARP.
Table 1.
Robotic Nerve-Sparing Techniques and Potency Outcomes
| Study | NS surgical technique | No. of patients | Mean/median age (years) | Potency definition | Potency 6 months, % | Potency 12 months, % |
|---|---|---|---|---|---|---|
| Menon et al.12 | High anterior release | 1142 (480 standard NS, 285 unilateral Veil, 377 bilateral Veil) | 60.2 | SHIM score ≥21 | Not reported | 40 vs 58 vs 70 |
| Shikanov et al.22 | Extrafascial vs interfascial | 110 vs 703 | 58.5 | Erection firm enough for sexual activity or intercourse | 34 vs 47 | 40 vs 64 |
| Potdevin et al.23 | Interfascial vs athermal intrafascial | 77 vs 70 | 58.6 vs 58.7 | SHIM score ≥20 | 81.8 vs 43.8 | 90.9 vs 66.67 (9 months) |
| Kowalczyk et al.16 | Antegrade; no countertraction vs countertraction | 342 vs 268 | 59.6 vs 57.9 | Erection firm enough for sexual activity or intercourse | 45 vs 28.4 | 50 vs 54.2 |
| Patel et al.25 | Retrograde | 332 | 58.5 | SHIM score ≥21 | 86.1 | 89.7 |
| Patel et al.24 | Retrograde | 404 | 58 | SHIM score ≥21 | 81.7 | 91.5 |
| Patel et al.14 | Prostatic vasculature as a landmark for NS | 133 | 60 | Not assessed | Not assessed | Not assessed |
| Alemozaffar et al.18 | Antegrade | 400 | 59.8 | Erection firm enough for sexual activity or intercourse | 41 (17.82) | 53 (29.1) |
| Ko et al.11 | Antegrade RARP vs retrograde RARP | 172 vs 172 | 57.9 vs 57.2 | Erections firm enough for >50% of attempts of sexual activity or intercourse | 72.1 vs 90.1 | 85.3 vs 92.9 |
| de Carvalho et al.17 | Retrograde release of the NVB with preservation of the DVC | 128 | 63.5 | SHIM score ≥17 | 82.3 | 86.7 |
| Moschovas et al.15 | Modified apical dissection and lateral prostatic fascia preservation vs standard RARP | 103 vs 103 | 58.5 vs 58.4 | Erection firm enough for sexual activity or intercoursea | 66.7 vs 49.3 | 77.9 vs 66.7 |
With or without the use of PDE5 inhibitors.
DVC = deep venous complex; RARP = robot-assisted radical prostatectomy; NS = nerve-sparing; NVB = neurovascular bundle; PDE5 = phosphodiesterase type 5; SHIM = Sexual Health Inventory for Men.
The majority of studies are single-surgeon noncomparative retrospective studies. There are currently no randomized controlled trials that examine the differences between different NS techniques.
Antegrade technical modifications
Menon and colleagues12 published a series of 1142 patients, 33% of which underwent bilateral “Veil” NS prostatectomy. The Veil technique demonstrated a 70% return of potency, defined as the ability to have intercourse, at 12 months in patients with normal preoperative potency compared with 40% undergoing standard NS RARP. However, only 51% of this cohort attained normal baseline function without medication. As one might expect, higher potency rates were noted for patients without preoperative ED, although patients at various levels of ED attained better potency outcomes after a “Veil” NS procedure vs standard NS.
Shikanov and coworkers22 analyzed a total of 110 and 703 cases with bilateral extrafascial and interfascial NS prostatectomy, respectively. Approximately one-third were performed antegrade. Patients with normal preoperative potency had significantly improved potency rates after interfascial (64%) relative to extrafascial (40%) cases at 12 months with a similar trend noted at 6 months.
Kowalczyk and colleagues16 compared NS with (268 cases) vs without (342 cases) NVB countertraction, demonstrating improved 5-month potency rates (defined as erection sufficient for sexual activity) in preoperatively potent men undergoing bilateral intrafascial NS (45.0% vs 28.4%, p = 0.039). No significant differences were observed at 12 months. Adjusted analyses corroborated these results, demonstrating an increased odds of improved 5-month potency in the no countertraction vs countertraction group (odds ratio 1.69; 95% confidence interval 1.01–2.83; p = 0.046) irrespective of older age or lower baseline sexual function.
Utilizing the minimal traction NS technique by Kowalczyk and coworkers,16 Alemozaffar and colleagues18 quantified the learning curve for improving potency outcomes after NS prostatectomy in a retrospective study of 400 consecutive RARPs. The authors demonstrated that the learning curve plateaus after 250–300 cases and also showed that greater surgeon experience as well as minimization of trainee robotic time were associated with better sexual function outcomes at 5 months postoperatively.
Retrograde technical modifications
Potdevin and coworkers23 published a study comparing interfascial vs intrafascial NS in 147 men who undergoing NS RARP. Intrafascial vs interfascial NS significantly improved potency at 6 (81.8% vs 43.8%; p < 0.001) and 12 months (90.9% vs 66.7%; p < 0.01) postoperatively. Patel and associates described retrograde NS RARP outcomes with the “trifecta”24 (incontinence, potency, and biochemical recurrence) and “pentafecta”25 (trifecta plus lack of postoperative complications and negative surgical margins). Potency rate at 12 months was about 90%, although this series included only preoperatively potent men undergoing bilateral intrafascial NS. Recently, de Carvalho and associates17 reported potency outcomes up to 98.4% as early as 9 months postoperatively after retrograde release of the NVB and preservation of the DVC.
Retrograde vs antegrade
Ko and colleagues11 compared 172 antegrade with 172 retrograde NS RARPs, and the retrograde approach facilitated early recovery of potency relative to antegrade (72.1% vs 90.1% at 6 months, and 85.3% vs 92.9% at 12 months).
Discussion
RP remains the most common treatment for the management of clinically significant prostate cancer.26 Post-RP ED has a negative impact on the quality of life and constitutes a financial burden for the patient and the health care system. The prevalence of post-RP ED ranges from 7% to 80%.27 This variation has been attributed to multiple factors, such as differences in definitions of potency, methods of data collection (patient vs physician-reported), patient selection, and surgical technique. Although >90% of RP in the United States are performed robotically,28 there remains no universally accepted surgical technique for NS RARP, and significant variation in outcomes may contribute to the prevalence of population-based ED. Even within high-volume referral centers, there is a significant variation in outcomes. For example, among 2000 prostatectomies performed by 11 highly trained surgeons of a tertiary cancer center, the adjusted probability of erectile function 12 months after prostatectomy ranged from 10% to 50% after adjustment for patient age and baseline erectile function.4 With this in mind, we set out to review RARP technical descriptions with video and associated sexual function outcomes with the goal of identifying factors that may improve outcomes.
First, to effectively spare the cavernosal nerves during prostatectomy, it is crucial to minimize the mechanisms that may cause injury, including transection, traction, and thermal injury. Traction and transection injuries usually occur during excessive bleeding that decreases the operative field observation or as a result of malpositioned surgical instruments. Common examples of the latter include misplaced retractors during open prostatectomy and the traction created by the assistant's suction during laparoscopic and robotic cases. The risk of thermal injury should be eliminated using cautery-free techniques, such as sharp dissection with scissors.29 In this scenario, most surgeons typically use Hem-o-lok clips during the lateral prostatic pedicles dissection and ligation. Furthermore, neuropraxia results from crushing injuries of the NVBs while grasping with instruments or after excessive lateral retraction. It can be minimized with delicate surgical techniques avoiding stretching of the nerves.16 Kowalczyk and associates16 and Alemozaffar and colleagues18 demonstrated that minimizing lateral displacement of the NVB (with the assistant suction and avoiding blunt dissection peeling) was associated with earlier and better recovery of erectile function. Furthermore, greater surgeon experience and minimization of trainee robotic time are associated with better sexual function outcomes.
Second, the preservation of surrounding PPF may also improve postoperative ED outcomes. In 2007, Menon and coworkers12 described the Veil of Aphrodite technique, which was the first to show potency improvements with safe surgical margins compared with conventional NS. Unlike other studies, the authors did not limit the postoperative potency outcomes to baseline potent men but demonstrated improved potency rates even for men with baseline compromised sexual function. Shikanov and colleagues22 compared the extrafascial and interfascial NS approach and confirmed that potency rates after interfascial are better than extrafascial. Multiple authors, including Potdevin and coworkers,23 have also compared the interfascial and intrafascial techniques attempting to demonstrate the benefit of the latter since it preserves more anterolateral nerve fibers than the interfascial approach. A metanalysis by Weng and colleagues30 that included trials of open, laparoscopic, and robotic interfascial and intrafascial NS prostatectomies demonstrated better potency at 6 and 12 months with the intrafascial approach.
Third, patient-specific factors also have a significant impact on erectile function recovery and need to be considered in the assessment of outcomes. Salonia and coworkers31 described that preoperative erectile function was the main predictor of postprostatectomy erectile function recovery. Moreover, cardiovascular risk factors such as dyslipidemia, diabetes mellitus, hypertension, coronary artery disease, and cigarette smoking were identified as independent predictors of ED 2 years after prostatectomy in a cohort of 984 men, irrespective of baseline sexual function and NS status.32
Fourth, studies suggest that retrograde NS approach has better potency outcomes at 12 months post-RARP compared with antegrade. However, the antegrade NS approach may be preferred because dissection from the base toward the apex allows early control of the lateral pedicles. As previously described, neuropraxia or even permanent NVB injury can occur during the handling of the pedicles since they are closely related to the NVB.33 Thus, technical refinements to this approach have been described to minimize NVB traction and facilitate pedicle control.18 Ko and colleagues proposed that the retrograde dissection is less traumatic to the NVB, as it can be performed before controlling the pedicle, minimizing the risk of injury, and improving potency outcomes.11
Although the description of penile rehabilitation approaches and outcomes is beyond the scope of this review, we acknowledge its widespread use postprostatectomy. The overall aim of penile rehabilitation is to improve oxygenation, preserve the endothelium and its function, and minimize fibrosis of the cavernosal tissues.34 In 1997, Montorsi and coworkers performed the first randomized controlled trial demonstrating a benefit in postprostatectomy erectile function with alprostadil intracavernosal injections.35 Since then, multiple trials have been performed to evaluate the role of penile rehabilitation postprostatectomy and the current evidence still lacks to prove irrefutable effectiveness.5,36 More recently, Sari Motlagh et al. performed a systematic review and network meta-analysis and showed that sildenafil 100 mg daily dose is the best penile rehabilitation strategy to improve erectile function recovery rates after RP and that on-demand dose should not be considered.37 However, the authors recognize that the certainty level of these results are moderate and further well-planned randomized trials need to be performed. Other techniques, such as low-intensity extracorporeal shockwave therapy and intraurethral alprostadil, constitute effective alternative approaches but have not been studied as well as PDE5i. Finally, some studies have demonstrated that rehabilitation and treatment in due time postsurgery are better than leaving the erectile tissue to its unassisted postoperative fate.38
Finally, the best assessment of technical medication to improve sexual function would be through a randomized controlled trial. Ideally future randomized controlled trials would be multicenter and multisurgeon trials; however, technical variation and surgeon proficiency must be benchmarked. All men should be potent at baseline and undergo complete bilateral NS procedures. The number of participating patients should be estimated with a power calculation based on the magnitude of difference that the researchers aim to evaluate. For example, for the Expanded Prostate Cancer Index (EPIC)-26 scoring system, Skolarus and colleagues have demonstrated that minimal clinically important differences for urinary and sexual function are 4–6 and 10–12 points, respectively.39 Finally, based on our experience and the published literature, a minimum of a 12-month follow-up period is needed to evaluate sexual function outcomes. Unfortunately, establishing an ideal surgical randomized controlled trial would in reality be extremely difficult. In the absence of an adequate randomized controlled trial, however, prospective studies and meta-analyses are our best tools to assess a technique's impact on sexual function.
Our review needs to be considered in the context of the study design. The absence of standardized methods of reporting outcomes has posed a major challenge for the systematic comparison of NS techniques. The Sexual Health Inventory for Men,40 the EPIC 26-item41 as well as the International Index of Erectile Function (IIEF)42 questionnaire are tools that have been utilized in the assessment of pre- and postoperative sexual function. The use of these different assessment tools and the use of varying cutoffs could potentially introduce variability in potency outcomes, and a lack of consensus on the definition of “potency” makes direct comparisons between techniques virtually impossible. Second, the current literature is derived from single-center retrospective studies rather than randomized controlled trials. Therefore, all inherent limitations of retrospective reports may lead to a considerable difference in terms of potency outcomes when comparing patients from different studies. However, it is challenging to implement randomized surgical trials, as certain surgeons are biased to certain approaches, and multisurgeon trials would be biased by increased heterogeneity in surgical technique. For this particular reason, we believe that it is mandatory documenting the different techniques in videos to compare approaches and associated outcomes. Fourth, this study was limited to articles that included video illustrations of techniques. Therefore, some described techniques in the literature might not have been included in this narrative review.
We believe in the importance of the appropriate surgical video documentation, as emphasized by Dr. Walsh: “intraoperative videos provide objective documentation of surgical technique, and the repeated re-evaluation of outcomes correlated with changes in surgical approach is the best way to reduce morbidity and improve cancer control.” Therefore, the increased access to different surgical approaches reported and illustrated by experts may help surgeons refining existing techniques to ultimately attain better outcomes. To answer the question of which NS approach is the best, randomized controlled trials that incorporate multiple centers and surgeons with standardization of technique through video illustration and feedback are needed to compare or even combine the available techniques systematically.
Conclusions
Although there are significant technical variations in the robotic NS approaches, there is no consensus on key steps or standard technique that leads to the best potency outcomes. Apparently, the most important aspects of the NS approach are the preservation of as much PPF as oncologically appropriate in addition to atraumatic NVB dissection. In addition, comparison of sexual function outcomes between studies is problematic. Future randomized controlled trials with detailed video demonstration with surgical coaching that utilize validated health-related quality of life instruments are needed. High-level evidence will guide consensus on RARP techniques to improve sexual quality of life after RARP.
Supplementary Material
Acknowledgments
Anatomical illustrations and video illustration were made by Vanessa Dudley, MSHS.
Abbreviations Used
- DVC
dorsal venous complex
- ED
erectile dysfunction
- EPIC
Expanded Prostate Cancer Index
- IIEF
International Index of Erectile Function
- MRI
magnetic resonance imaging
- NS
nerve-sparing
- NVB
neurovascular bundle
- PPF
periprostatic fascia
- RARP
robot-assisted radical prostatectomy
- RP
radical prostatectomy
- SHIM
Sexual Health Inventory for Men
Author Disclosure Statement
No competing financial interests exist.
Funding Information
J.C.H. receives research support from the Frederick J. and Theresa Dow Wallace Fund of the New York Community Trust. J.C.H. also receives salary support from NIH R01 CA241758 and PCORI CER-2019C1–15682. J.E.S. is supported by the Damon Runyon Cancer Research Foundation Physician Scientist Training Award and Vinney Scholar Award.
Supplementary Material
References
- 1. https://twitter.com/KeithKow/status/1237406461686435842?s=20. Accessed January 15, 2021.
- 2. Kowalczyk KJ, Weinburg AC, Gu X, et al. . Comparison of outpatient narcotic prescribing patterns after minimally invasive versus retropubic and perineal radical prostatectomy. J Urol 2011;186:1843–1848. [DOI] [PubMed] [Google Scholar]
- 3. Orvieto MA, Coelho RF, Chauhan S, Mathe M, Palmer K, Patel VR. Erectile dysfunction after robot-assisted radical prostatectomy. Expert Rev Anticancer Ther 2010;10:747–754. [DOI] [PubMed] [Google Scholar]
- 4. Vickers A, Savage C, Bianco F, et al. . Cancer control and functional outcomes after radical prostatectomy as markers of surgical quality: Analysis of heterogeneity between surgeons at a single cancer center. Eur Urol 2011;59:317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Capogrosso P, Vertosick EA, Benfante NE, et al. . Are we improving erectile function recovery after radical prostatectomy? Analysis of patients treated over the last decade. Eur Urol 2019;75:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Stam MA, Aaronson NK, Bosch J, et al. . Patient-reported outcomes following treatment of localised prostate cancer and their association with regret about treatment choices. Eur Urol Oncol 2020;3:21–31. [DOI] [PubMed] [Google Scholar]
- 7. Watson V, McCartan N, Krucien N, et al. . Evaluating the trade-offs men with localized prostate cancer make between the risks and benefits of treatments: The COMPARE Study. J Urol 2020;204:273–280. [DOI] [PubMed] [Google Scholar]
- 8. Tavukcu HH, Aytac O, Atug F. Nerve-sparing techniques and results in robot-assisted radical prostatectomy. Investig Clin Urol 2016;57(Suppl 2):S172–S184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walz J, Epstein JI, Ganzer R, et al. . A critical analysis of the current knowledge of surgical anatomy of the prostate related to optimisation of cancer control and preservation of continence and erection in candidates for radical prostatectomy: An update. Eur Urol 2016;70:301–311. [DOI] [PubMed] [Google Scholar]
- 10. Berry A, Korkes F, Hu JC. Landmarks for consistent nerve sparing during robotic-assisted laparoscopic radical prostatectomy. J Endourol 2008;22:1565–1567. [DOI] [PubMed] [Google Scholar]
- 11. Ko YH, Coelho RF, Sivaraman A, et al. . Retrograde versus antegrade nerve sparing during robot-assisted radical prostatectomy: Which is better for achieving early functional recovery? Eur Urol 2013;63:169–177. [DOI] [PubMed] [Google Scholar]
- 12. Menon M, Shrivastava A, Kaul S, et al. . Vattikuti Institute prostatectomy: Contemporary technique and analysis of results. Eur Urol 2007;51:648–657; discussion 57–58. [DOI] [PubMed] [Google Scholar]
- 13. Menon M, Shrivastava A, Bhandari M, Satyanarayana R, Siva S, Agarwal PK. Vattikuti Institute prostatectomy: Technical modifications in 2009. Eur Urol 2009;56:89–96. [DOI] [PubMed] [Google Scholar]
- 14. Patel VR, Schatloff O, Chauhan S, et al. . The role of the prostatic vasculature as a landmark for nerve sparing during robot-assisted radical prostatectomy. Eur Urol 2012;61:571–576. [DOI] [PubMed] [Google Scholar]
- 15. Covas Moschovas M, Bhat S, Onol FF, et al. . Modified apical dissection and lateral prostatic fascia preservation improves early postoperative functional recovery in robotic-assisted laparoscopic radical prostatectomy: Results from a Propensity Score-matched Analysis. Eur Urol 2020;78:875–884. [DOI] [PubMed] [Google Scholar]
- 16. Kowalczyk KJ, Huang AC, Hevelone ND, et al. . Stepwise approach for nerve sparing without countertraction during robot-assisted radical prostatectomy: Technique and outcomes. Eur Urol 2011;60:536–547. [DOI] [PubMed] [Google Scholar]
- 17. de Carvalho PA, Barbosa J, Guglielmetti GB, et al. . Retrograde release of the neurovascular bundle with preservation of dorsal venous complex during robot-assisted radical prostatectomy: Optimizing functional outcomes. Eur Urol 2020;77:628–635. [DOI] [PubMed] [Google Scholar]
- 18. Alemozaffar M, Duclos A, Hevelone ND, et al. . Technical refinement and learning curve for attenuating neurapraxia during robotic-assisted radical prostatectomy to improve sexual function. Eur Urol 2012;61:1222–1228. [DOI] [PubMed] [Google Scholar]
- 19. Moschovas MC, Bhat S, Sandri M, et al. . Comparing the approach to radical prostatectomy using the multiport da Vinci Xi and da Vinci SP Robots: A propensity score analysis of perioperative outcomes. Eur Urol 2021;79:393–404. [DOI] [PubMed] [Google Scholar]
- 20. Covas Moschovas M, Bhat S, Rogers T, et al. . Technical modifications necessary to implement the da Vinci Single-port Robotic System. Eur Urol 2020; 78:415–423. [DOI] [PubMed] [Google Scholar]
- 21. Moschovas MC, Bhat S, Rogers T, et al. . Applications of the da Vinci Single Port (SP) robotic platform in urology: A systematic literature review. Minerva Urol Nefrol 2021;73:6–16. [DOI] [PubMed] [Google Scholar]
- 22. Shikanov S, Woo J, Al-Ahmadie H, et al. . Extrafascial versus interfascial nerve-sparing technique for robotic-assisted laparoscopic prostatectomy: Comparison of functional outcomes and positive surgical margins characteristics. Urology 2009;74:611–616. [DOI] [PubMed] [Google Scholar]
- 23. Potdevin L, Ercolani M, Jeong J, Kim IY. Functional and oncologic outcomes comparing interfascial and intrafascial nerve sparing in robot-assisted laparoscopic radical prostatectomies. J Endourol 2009;23:1479–1484. [DOI] [PubMed] [Google Scholar]
- 24. Patel VR, Coelho RF, Chauhan S, et al. . Continence, potency and oncological outcomes after robotic-assisted radical prostatectomy: Early trifecta results of a high-volume surgeon. BJU Int 2010;106:696–702. [DOI] [PubMed] [Google Scholar]
- 25. Patel VR, Sivaraman A, Coelho RF, et al. . Pentafecta: A new concept for reporting outcomes of robot-assisted laparoscopic radical prostatectomy. Eur Urol 2011;59:702–707. [DOI] [PubMed] [Google Scholar]
- 26. Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol 2010;28:1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alemozaffar M, Regan MM, Cooperberg MR, et al. . Prediction of erectile function following treatment for prostate cancer. JAMA 2011;306:1205–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guru KA, Hussain A, Chandrasekhar R, et al. . Current status of robot-assisted surgery in urology: A multi-national survey of 297 urologic surgeons. Can J Urol 2009;16:4736–4741; discussion 41. [PubMed] [Google Scholar]
- 29. Ahlering TE, Skarecky D, Borin J. Impact of cautery versus cautery-free preservation of neurovascular bundles on early return of potency. J Endourol 2006;20:586–589. [DOI] [PubMed] [Google Scholar]
- 30. Weng H, Zeng XT, Li S, et al. . Intrafascial versus interfascial nerve sparing in radical prostatectomy for localized prostate cancer: A systematic review and meta-analysis. Sci Rep 2017;7:11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salonia A, Burnett AL, Graefen M, et al. . Prevention and management of postprostatectomy sexual dysfunctions. Part 1: Choosing the right patient at the right time for the right surgery. Eur Urol 2012;62:261–272. [DOI] [PubMed] [Google Scholar]
- 32. Teloken PE, Nelson CJ, Karellas M, et al. . Defining the impact of vascular risk factors on erectile function recovery after radical prostatectomy. BJU Int 2013;111:653–657. [DOI] [PubMed] [Google Scholar]
- 33. Pisipati S, Ali A, Mandalapu RS, et al. . Newer concepts in neural anatomy and neurovascular preservation in robotic radical prostatectomy. Indian J Urol 2014;30:399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gandaglia G, Suardi N, Cucchiara V, et al. . Penile rehabilitation after radical prostatectomy: Does it work? Transl Androl Urol 2015;4:110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Montorsi F, Guazzoni G, Strambi LF, et al. . Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: Results of a prospective, randomized trial. J Urol 1997;158:1408–1410. [PubMed] [Google Scholar]
- 36. Clavell-Hernandez J, Wang R. The controversy surrounding penile rehabilitation after radical prostatectomy. Transl Androl Urol 2017;6:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sari Motlagh R, Abufaraj M, Yang L, et al. . Penile rehabilitation strategy after nerve sparing radical prostatectomy: A systematic review and network meta-analysis of randomized trials. J Urol. 2021;205:1018–1030. PubMed PMID: 33443457. Epub 2021/01/15. [DOI] [PubMed] [Google Scholar]
- 38. Jo JK, Jeong SJ, Oh JJ, et al. . Effect of starting penile rehabilitation with sildenafil immediately after robot-assisted laparoscopic radical prostatectomy on erectile function recovery: A prospective randomized trial. J Urol 2018;199:1600–1606. [DOI] [PubMed] [Google Scholar]
- 39. Skolarus TA, Dunn RL, Sanda MG, et al. . Minimally important difference for the Expanded Prostate Cancer Index Composite Short Form. Urology 2015;85:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999;11:319–326. [DOI] [PubMed] [Google Scholar]
- 41. Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 2000;56:899–905. [DOI] [PubMed] [Google Scholar]
- 42. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822–830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.