Abstract
Of the Helicobacter pylori populations from 976 patients, six contained clarithromycin-resistant as well as -susceptible colonies. In each heterogeneous H. pylori population, resistant H. pylori colonies harbored identical 23S ribosomal DNA (rDNA) mutations associated with clarithromycin resistance, while the susceptible H. pylori colonies all had wild-type 23S rDNA. The resistant and susceptible colonies of each of the heterogeneous H. pylori populations had identical randomly amplified polymorphic DNA-PCR genotypes. In conclusion, evaluation of antimicrobial susceptibility can be misinterpreted if only a single colony from the primary H. pylori population is used to test for clarithromycin susceptibility.
Helicobacter pylori infection has been established as an etiologic factor in nonautoimmune gastritis, peptic ulcer disease (PUD), gastric carcinoma, and lymphoma (1, 3, 10). Since curing H. pylori infection prevents peptic ulcer recurrence, the eradication of the organism has become the cornerstone in the treatment of PUD (15). Clarithromycin (CLR) in combination with metronidazole (MTZ) is often used in H. pylori eradication regimens (4). H. pylori resistance to these drugs substantially reduces the success rate of CLR-plus-MTZ eradication regimens (4). The 23S rRNA gene is present in two copies in the H. pylori genome (12, 13). In H. pylori, seven different point mutations (A2115→G, G2141→A, A2142→G, A2142→C, A2143→G, A2143→C, and A2142→T) in the peptidyltransferase region of the V domain of the 23S rRNA gene have been found to be associated with resistance to CLR (5, 9, 11, 12, 18). In a recent study, it was found that among CLR resistant H. pylori, the predominant mutations were A2143→G and A2142→G, while virtually all CLR-susceptible H. pylori had no mutation in their 23S rRNAs (17).
It has been reported that the H. pylori population in a patient can be heterogeneous with respect to MTZ susceptibility (20). Infection by a mixed population of CLR-susceptible and CLR-resistant H. pylori has been reported (6, 7, 8, 16). However, in most of these studies (6, 7, 16), discrimination between mixed H. pylori infection and infection with H. pylori heterozygous for 23S rRNA was not established. The aim of this study was to evaluate the relevance of heterogeneity in susceptibility to CLR in H. pylori populations from patients with gastritis or PUD prior to anti-H. pylori treatment. In this study, a distinction was made between mixed H. pylori infection and infection with H. pylori heterozygous for 23S rRNA.
H. pylori isolates were cultured from gastric biopsy specimens from 976 patients from the Amsterdam area who were referred for upper gastrointestinal tract endoscopies in 1997 and 1998 because of dyspeptic symptoms (14). Briefly, each specimen was smeared on Columbia agar (Oxoid CM 331; Unipath Ltd., Basingstoke, England) plates containing 7% (vol/vol) horse blood. Colonies that exhibited the characteristic morphology were identified as H. pylori if they were urease, catalase, and oxidase positive. The cultures of the antrum and corpus were collected separately with swabs, which were subsequently shaken in 8% glycerol-peptone. These bacterial suspensions were stored at −70°C. CLR susceptibilities were determined by the E-test (AB Biodisk, Solna, Sweden) according to the instructions of the manufacturer (20). Colonies growing within the zone of growth inhibition of the bacterial lawn were subcultured on blood agar for 3 days before assessment of the CLR MIC was performed. Isolates were considered resistant to CLR if the MIC was ≥2 mg of CLR/liter (7). Mutations in the 23S rRNA gene were assessed by a PCR-based reverse hybridization onto a line probe assay (INNO-LiPA) (16). This LiPA allows the simultaneous detection of all of the aforementioned point mutations in the 23S rRNA gene associated with CLR resistance. In addition, the genotype of each H. pylori strain was assessed by randomly amplified polymorphic DNA (RAPD)-PCR using four different primers (14). For analysis, the four profiles were combined.
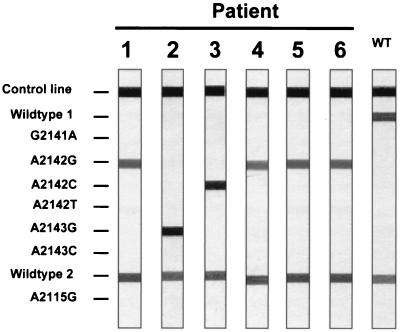
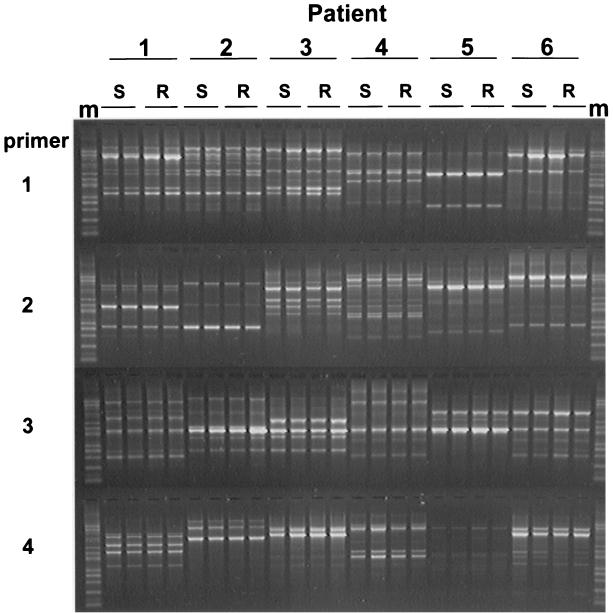
Among the 976 H. pylori populations cultured, 51 (5.2%) were resistant to CLR. Close examination of the E-test plates revealed that six (12%) of these 51 H. pylori populations were heterogeneous regarding CLR susceptibility. In these H. pylori populations, the majority of bacteria were susceptible to CLR, but Helicobacter colonies were also growing within the zones of growth inhibition of the bacterial lawn. For each heterogeneous H. pylori population, the MICs of CLR for two resistant H. pylori colonies and for two susceptible H. pylori colonies were reassessed by E-test, and the DNAs of the colonies were subjected to the 23S ribosomal DNA (rDNA) LiPA (Fig. 1; Table 1). In all heterogeneous H. pylori populations, the MICs of CLR for the two CLR-resistant colonies were high, and the colonies harbored identical 23S rDNA mutations in both copies of rRNA operons, associated with CLR resistance. The MICs for all susceptible H. pylori colonies were low, and the colonies had wild-type 23S rDNA. RAPD-PCR profiles of the resistant and susceptible H. pylori colonies of each of the heterogeneous H. pylori populations were identical, indicating identical genotypes (Fig. 2).
FIG. 1.
LiPA results for clarithromycin-resistant H. pylori isolates from six patients. The results from one of the clarithromycin-resistant H. pylori colonies from the heterogeneous H. pylori population of each of the six patients are presented here. WT, wild type.
TABLE 1.
Comparison of 23S rDNA LiPA results with E-test results for two CLR-resistant and two CLR-susceptible colonies of heterogeneous H. pylori populations
| Patient no. | Colony | First E-test resultsa | Reassessed E-test MIC (μg/ml) | 23S LiPA resultsb |
|---|---|---|---|---|
| 1 | 1 | R | 32 | A2142G |
| 1 | 4 | R | 24 | A2142G |
| 1 | 8 | S | <0.016 | WT |
| 1 | 17 | S | <0.016 | WT |
| 2 | A | R | >256 | A2143G |
| 2 | B | R | >256 | A2143G |
| 2 | D | S | <0.016 | WT |
| 2 | L | S | <0.016 | WT |
| 3 | 102 | S | <0.016 | WT |
| 3 | 103 | S | <0.016 | WT |
| 3 | B | R | >256 | A2142C |
| 3 | E | R | 32 | A2142C |
| 4 | 1 | S | <0.016 | WT |
| 4 | 2 | S | <0.016 | WT |
| 4 | A | R | 32 | A2142G |
| 4 | B | R | 24 | A2142G |
| 5 | 1B | R | >256 | A2142G |
| 5 | C2 | R | >256 | A2142G |
| 5 | C11 | S | <0.016 | WT |
| 5 | C16 | S | <0.016 | WT |
| 6 | 1 | R | 32 | A2142G |
| 6 | 4 | R | 64 | A2142G |
| 6 | 8 | S | <0.016 | WT |
| 6 | 9 | S | <0.016 | WT |
R, CLR resistant; S, CLR susceptible.
WT, wild type.
FIG. 2.
RAPD profiles of CLR-resistant and -susceptible H. pylori isolates from single biopsy specimens. Two CLR-susceptible (S) and two CLR-resistant (R) colonies from the heterogeneous H. pylori populations of six patients were assessed by RAPD-PCR. m, 100-bp molecular size markers.
Our results are in accordance with the results from recent studies by Maeda and coworkers (6), Matsuoka and colleagues (8), and van Doom and collaborators (16) reporting mixed infections with CLR-susceptible and CLR-resistant H. pylori. However, genotyping was not performed in those studies. In addition, in another study, the susceptibility of H. pylori to CLR was assessed by molecular biological techniques directly on biopsy specimens from H. pylori-infected patients (7). Seventeen percent of the H. pylori-positive biopsy specimens yielded 23S rDNA PCR products that hybridyzed with both the wild-type probe and one of the mutant probes. The results were explained by either mixed infection with resistant and susceptible H. pylori or infection by H. pylori heterozygous for the 23S rRNA gene. Our findings favor the first explanation.
Only a limited number of different 23S rDNA point mutations were found among the heterogeneous H. pylori populations. The CLR-resistant H. pylori isolates of four of the six heterogeneous H. pylori populations had the A2142→G point mutation, one had the A2143→G mutation, and another had the A2142→C mutation in their 23S rRNA genes. Possibly, in an environment without CLR, the disadvantage of these point mutations in the 23S rRNA gene in H. pylori is insignificant, resulting in a lack of negative selection of these 23S rRNA mutants. This is supported by the results of in vitro experiments (2, 19). In these experiments, it was found that the growth rates of H. pylori isolates with the A2142→G, A2142→C, or A2143→G mutation did not differ from that of the wild type, but H. pylori isolates with other 23S rDNA mutations grew more slowly (2). In addition, Wang and coworkers showed identical growth rates of wild-type H. pylori and H. pylori with the A2142→G or A2143→G 23S rDNA mutation (19). From the individual growth rates and the patterns of competitive growth, it was concluded that the order of preference of competitive accumulation is A2142→G > A 2143→G >>> A2142→C > A2143→C (A2143→T). The prevalence of the A2142→G, A2143→G, and A2142→C mutations among the heterogeneous H. pylori populations in our study is consistent with this order.
In conclusion, the results show coexistence of CLR-resistant and -susceptible H. pylori isolates with identical genotypes in patients prior to treatment. If only a single colony from the primary H. pylori populations is used to test for CLR susceptibility, the results can be misinterpreted. Assessment of 23S rRNA mutations in H. pylori directly from biopsy specimens by molecular biological techniques, such as the LiPA, has the advantage that infection with a mixed H. pylori population is easily detected. In addition, knowledge of the type of 23S rRNA mutation may be important since CLR MICs are associated with the type of 23S rRNA mutation in H. pylori (17).
REFERENCES
- 1.Blaser M J, Perez-Perez G I, Kleanthous H. Infection with Helicobacter pylori strains possessing cagA associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 2.Debets-Ossenkopp Y J, Brinkman A B, Kuipers E J, Vandenbroucke-Grauls C M J E, Kusters J G. Explaining the bias in the 23S rMA gene mutations associated with clarithromycin resistance in clinical isolates of Helicobacter pylori. Antimicrob Agents Chemother. 1998;42:2749–2751. doi: 10.1128/aac.42.10.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foreman D the Eurogast Study Group. An international association between Helicobacter pylori infection and gastric cancer. Lancet. 1993;341:359–362. [PubMed] [Google Scholar]
- 4.Houben M H, van der Beek D, Hensen E F, Craen A J, Rauws E A J, Tytgat G N J. A systematic review of Helicobacter pylori eradication therapy—the impact of resistance on eradication rates. Aliment Pharmacol Ther. 1999;13:1047–1055. doi: 10.1046/j.1365-2036.1999.00555.x. [DOI] [PubMed] [Google Scholar]
- 5.Hultén K, Gibreel A, Skold O, Engstrand L. Macrolide resistance in Helicobacter pylori: mechanism and stability in strains from clarithromycin-treated patients. Antimicrob Agents Chemother. 1997;41:2550–2553. doi: 10.1128/aac.41.11.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda S, Yoshida H, Matsunaga H, Ogura K, Kawamata O, Shiratori Y, Omata M. Detection of clarithromycin-resistant Helicobacter pylori strains by a preferential homoduplex formation assay. J Clin Microbiol. 2000;38:210–214. doi: 10.1128/jcm.38.1.210-214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marais A, Monteiro L, Occhialini A, Pina M, Lamouliatte H, Megraud F. Direct detection of Helicobacter pylori resistance to macrolides by a polymerase chain reaction/DNA enzyme immunoassay in gastric biopsy specimens. Gut. 1999;44:463–467. doi: 10.1136/gut.44.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuoka M, Yoshida Y, Hayakawa K, Fukuchi S, Sugano K. Simultaneous colonisation of Helicobacter pylori with and without mutations in the 23S rRNA gene in patients with no history of clarithromycin exposure. Gut. 1999;45:503–507. doi: 10.1136/gut.45.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Occhialini A, Urdaci M, Doucet-Populaire F, Bebear C M, Lamouliatte H, Megraud F. Macrolide resistance in Helicobacter pylori: rapid detection of point mutations and assays of macrolide binding to ribosomes. Antimicrob Agents Chemother. 1997;41:2724–2728. doi: 10.1128/aac.41.12.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parsonnet J, Hansen S, Rodriguez L, Gelb A B, Warnke R A, Jellum E, Orentreich N, Vogelman J H, Friedman G D. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 11.Stone G G, Shortridge D, Flamm R K, Versalovic J, Beyer J, Idler K, Zulawinski L, Tanaka S K. Identification of a 23S rRNA gene mutation in clarithromycin-resistant Helicobacter pylori. Helicobacter. 1996;1:227–228. doi: 10.1111/j.1523-5378.1996.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 12.Taylor D E, Ge Z, Purych D, Lo T, Hiratsuka K. Cloning and sequence analysis of two copies of a 23S rRNA gene from Helicobacter pylori and association of clarithromycin resistance with 23S rRNA mutations. Antimicrob Agents Chemother. 1997;41:2621–2628. doi: 10.1128/aac.41.12.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomb J-F, White O, Kervage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, Mckenney K, Fitzgerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 14.van der Ende A, Rauws E A J, Feller M, Mulder C J, Tytgat G N J, Dankert J. Heterogeneous Helicobacter pylori isolates from members of a family with a history of peptic ulcer disease. Gastroenterology. 1996;111:638–647. doi: 10.1053/gast.1996.v111.pm8780568. [DOI] [PubMed] [Google Scholar]
- 15.van der Hulst R W M, Rauws E A J, Köycü B, Keller J J, Tyssen J G P, Bruno M, Tytgat G N J. Prevention of ulcer recurrence after successful eradication of Helicobacter pylori infection: a prospective long term follow-up study. Gastroenterology. 1997;113:1082–1086. doi: 10.1053/gast.1997.v113.pm9322501. [DOI] [PubMed] [Google Scholar]
- 16.van Doorn L-J, Debets-Ossenkopp Y J, Marais A, Sanna R, Mégraud F, Kusters J G, Quint W G V. Rapid detection, by PCR and reverse hybridization, of mutations in the Helicobacter pylori 23S rRNA gene, associated with macrolide resistance. Antimicrob Agents Chemother. 1999;43:1779–1782. doi: 10.1128/aac.43.7.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Doorn L-J, Glupczynski Y, Kusters J G, Mégraud F, Midolo P, Maggi-Solcà N, Queiroz D M M, Nouhan N, Stet E, Quint W G V. Accurate prediction of macrolide resistance in Helicobacter pylori by a PCR line probe assay for detection of mutations in the 23S rRNA gene: multicenter validation study. Antimicrob Agents Chemother. 2001;45:1500–1504. doi: 10.1128/AAC.45.5.1500-1504.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Versalovic J, Osato M S, Spakovsky K, Dore M P, Reddy R, Stone G G, Shortridge D, Flamm R K, Tanaka S K, Graham D Y. Point mutations in the 23S rRNA gene of Helicobacter pylori associated with different levels of clarithromycin resistance. J Antimicrob Chemother. 1996;40:283–286. doi: 10.1093/jac/40.2.283. [DOI] [PubMed] [Google Scholar]
- 19.Wang G, Rahman M S, Humayun M Z, Taylor D E. Multiplex sequence analysis demonstrates the competitive growth advantage of the A-to-G mutants of clarithromycin-resistant Helicobacter pylori. Antimicrob Agents Chemother. 1999;43:683–685. doi: 10.1128/aac.43.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weel J F, van der Hulst R W M, Gerrits Y, Tytgat G N J, van der Ende A, Dankert J. Heterogeneity in susceptibility to metronidazole among Helicobacter pylori isolates from patients with gastritis or peptic ulcer disease. J Clin Microbiol. 1996;34:2158–2162. doi: 10.1128/jcm.34.9.2158-2162.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]