Abstract
The incretin hormone glucagon‐like peptide‐1 (GLP‐1) is inactivated by the enzyme dipeptidyl peptidase‐4 even before it leaves the gut, but it seems to act predominantly via activation of intestinal sensory neurons expressing GLP‐1 receptors. Thus, activation of vagal afferents is probably responsible for its effects on appetite and food intake, gastrointestinal secretion and motility, and pancreatic endocrine secretion. However, GLP‐1 receptors are widely expressed in the gastrointestinal (GI) tract, including epithelial cells in the stomach, and the Brunner glands, in endocrine cells of the gut epithelium, and on mucosal lymphocytes. In this way, GLP‐1 may have important local actions of epithelial protection and endocrine signalling and may interact with the immune system. We review the formation and release of GLP‐1 from the endocrine L cells and its fate after release and describe the localization of its receptor throughout the GI tract and discuss its direct or indirect actions in the GI tract.
LINKED ARTICLES
This article is part of a themed issue on GLP1 receptor ligands (BJP 75th Anniversary). To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v179.4/issuetoc
Abbreviations
- CCK
cholecystokinin
- DPP‐4
dipeptidyl peptidase‐4
- FISH
fluorescent in situ hybridization
- GI
gastrointestinal
- GIP
glucose‐dependent insulinotropic polypeptide
- GRPP
glicentin‐related pancreatic peptide
- IELS
intraepithelial lymphocytes
- IGLE
intraganglionic laminar endings
- IHC
immunohistochemistry
- LPL
lamina propria lymphocytes
- nNOS
neuronal nitric oxide synthase
- SCFA
short chain fatty acids
1. GLP‐1 IN THE GUT
Glucagon‐like peptide‐1 (GLP‐1) is, apart for the expression in some of the neurons in the nucleus of the solitary tract in the brain stem (Larsen et al., 1997), a gut hormone. It was discovered later than the classical gut and pancreatic hormones (secretin, the first hormone to be discovered, in 1902, gastrin in 1905; and insulin, glucagon and cholecystokinin [CCK] in the 1920s), but it was reported in the late 1940s that a possibly glucagon‐like, hyperglycaemic substance might be produced in the stomach (Sutherland & De Duve, 1948). With the advent of the glucagon radioimmunoassay (almost simultaneously with the development of the insulin radioimmunoassay by Berson and Yalow in 1959) and therefore antisera against the glucagon molecule (Unger et al., 1959), it became possible to show that a quite abundant endocrine cell type occurring especially in the distal intestinal epithelium stained with the glucagon antisera (Orci et al., 1968; Unger et al., 1966). A function for these cells was not deduced, but a variety of experimental observations indicated that the responsible immunoreactive molecule was not glucagon itself (Unger et al., 1968). Further experiments in several laboratories led to the conclusion that the responsible protein was likely to be proglucagon (or at least a fragment of this peptide), the assumed precursor to the pancreatic hormone glucagon (Holst, 1983). In the gut, proglucagon would undergo differential processing, leading to the formation of glicentin, a peptide of 69 amino acids, of which the glucagon sequence would occupy residues no 33–61, and oxyntomodulin, corresponding to residues no 33–69, whereas in the pancreas, it would be processed to glucagon, corresponding to residues no 33–61 (Holst, 1983). All of these peptides, including proglucagon 1–30, were also found in the pancreas supporting the common origin of the products (Moody et al., 1981). Although these peptides fully explained the reactivity of the cells with antisera against glucagon, their discovery did not contribute much to reveal the functions of these cells. The cells were called L cells by the electron microscopists who attempted to classify the various gut endocrine cells according to their electron microscopical morphology and assigned a letter to the cells more or less alphabetically (A and B cells in the pancreas and so forth) (Solcia et al., 1981). However, it was clear from cell‐free translation experiments that the proglucagon molecule was considerably larger than the 69 residues of glicentin (Patzelt & Schug, 1981). Eventually, cloning of the glucagon gene revealed that mammalian proglucagon had 160 residues (see Figure 1) and that the larger C‐terminal fragment, the so‐called major proglucagon fragment (MGF), contained two glucagon‐like sequences (GLP‐1, corresponding to proglucagon 72–108 or GLP‐1(1‐37)), and glucagon like‐peptide‐2 (GLP‐2, corresponding to proglucagon 126–160), framed by consensus prohormone cleavage sites (pairs of basic amino acid residues) (Bell et al., 1983). Finally, it was discovered that a truncated form the GLP‐1 sequence, isolated from both pig and human intestine, was a very powerful and potent stimulator of insulin secretion from isolated perfused pancreas (Holst et al., 1987). It was also demonstrated that the secretion of GLP‐1 was stimulated by carbohydrate ingestion (Kreymann et al., 1987; Orskov & Holst, 1987). Thus, the L cell was apparently producing an incretin hormone, GLP‐1, which contributed to the amplification of insulin secretion, which occurs upon ingestion of nutrients including, but not restricted to, carbohydrates. Indeed, so‐called mimicry studies in humans, where GLP‐1 plasma concentrations were mimicked by intravenous infusions of synthetic GLP‐1, confirmed its ability to stimulate insulin secretion, with even greater potency and efficacy than the already discovered incretin hormone, glucose‐dependent insulinotropic polypeptide (GIP) (Kreymann et al., 1987). The relative contribution of these two incretin hormones to postprandial insulin secretion was recently established in human studies involving receptor antagonists for both the GIP receptor and the GLP‐1 receptor, which showed that GIP contributed with 44%, GLP‐1 with 24% and glucose alone with 33% (Gasbjerg et al., 2019).
FIGURE 1.
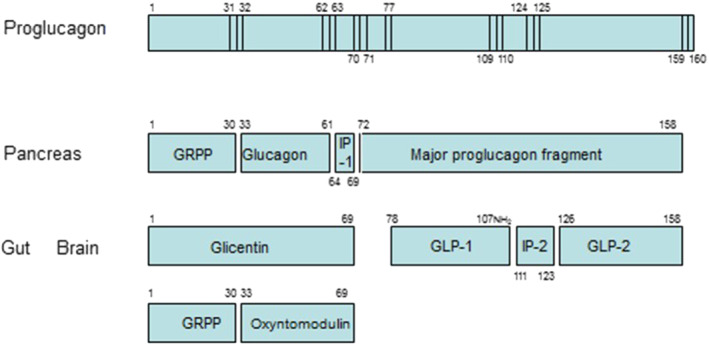
Proglucagon processing. Proglucagon is the precursor of at least six peptides with known biological activity (glucagon, oxyntomodulin, GLP‐1, GLP‐2, the last in both amidated and Gly‐extended forms) and the particular peptides formed vary with the tissue analysed (brain, gut or pancreas). Note that the formation of GLP‐1 is always accompanied by an equal production of GLP‐2. This Figure is based on data reviewed in Holst (2007)
The L cell is thus the origin of several secreted products of proglucagon. Glicentin is secreted in parallel with GLP‐1 and GLP‐2 (Nielsen et al., 2020; Orskov et al., 1986), and part of the glicentin molecule is cleaved to proglucagon 1–30 (also called glicentin‐related pancreatic peptide, GRPP) and oxyntomodulin (Holst, 2007) (see Figure 1). To this date, neither glicentin nor GRPP has been attributed a definitive function. However, oxyntomodulin turned out to be a full agonist of both the glucagon and the GLP‐1 receptors, but with a somewhat lower affinity (100‐fold), which raises doubt about its physiological function (Holst et al., 2018) (whereas in pharmacological amounts, it is a glucagon‐GLP‐1 co‐agonist with interesting properties, Day et al., 2009). Is glucagon also formed in the L cells? This is one of the current research controversies, but it may be secreted from the gut under certain circumstances (Lund et al., 2016). Unlike the pancreas, the gut does not seem to produce and secrete the inactive full‐length GLP‐1 (proglucagon 72–108 or GLP‐1 1–37), and nothing is known about a possible role of the fragment corresponding to proglucagon 72–76. The structure of fully processed GLP‐2 was identified as proglucagon 126–158 (Buhl et al., 1988), and this peptide was later identified as a growth hormone for the gut, acting via a separate receptor, and apparently having a metabolic role completely separate from that of GLP‐1 (Brubaker, 2018), but it should be borne in mind that whenever a molecule of GLP‐1 is secreted, a GLP‐2 molecule is also released (Orskov et al., 1986). The fragment between GLP‐1 and GLP‐2 (sometimes called intervening peptide‐2, proglucagon 111‐123) is probably amidated and is likely to be secreted (unpublished observations in the author's lab), but nothing is known about its possible actions, if any. Thus, the L cell is a classical gut endocrine cell that, when stimulated by nutrient intake, releases two hormones with defined functions, GLP‐1 and GLP‐2 (and a minor amount of the glucagon/GLP‐1 co‐agonist, oxyntomodulin). Today, the L cells are known to also express, in varying amounts, other peptides, most importantly peptide YY (PYY), but expression of GIP, CCK and neurotensin has also been reported. This promiscuous expression is, however, not reflected in a parallel secretion of all these peptides upon a given stimulus (Svendsen et al., 2015). For instance, free fatty acids will still release CCK but not GLP‐1, whereas glucose will release GLP‐1 but not CCK.
Insulin secretion was among the earliest targets investigated for this new peptide, partly because of the similarity to glucagon, which is a known stimulator of insulin secretion, and because GLP‐1 was discovered at a time when there was a huge interest in the incretin function and for identifying the responsible incretin hormones. It was soon discovered that it also inhibits glucagon secretion (Orskov et al., 1988), which turned out to be of particular importance for its future application as a pharmaceutical agent, but otherwise there was no indication that GLP‐1 was anything else than a classical insulinotropic circulating hormone. It was a problem that most of the L cells were located in the distal small intestine and in the colon, contrasting to a function as an incretin hormone released in response to absorption of glucose which would occur in the proximal gut. For GLP‐2, the situation was even more complicated—there was no immediate effect on insulin secretion (it was later found to stimulate glucagon secretion, the functional consequences of which remain unknown) (de Heer et al., 2007). Its receptor was cloned and was found to be expressed mainly in the gut (Munroe et al., 1999), especially in the subepithelial myofibroblasts and in enteric neurons, suggesting that the targets for GLP‐2 action might be local within the gut, questioning the importance of GLP‐2 as a circulating hormone. Recognizing the local actions of GLP‐2 as an intestinal growth hormone, it would be natural to also look for actions of GLP‐1 locally in the gut. Indeed, there is strong evidence that physiologically most of the actions of GLP‐1 are exerted locally via receptors in the gut. The remaining part of this article deals with these local interactions.
2. BIOSYNTHESIS, PROCESSING AND SECRETION OF GLP‐1
The L cell is a classical enteroendocrine cell of the intestinal epithelium of the open type. This means that the cells are flask shaped and have an apical process which reaches the gut lumen (Eissele et al., 1992). The process is equipped with microvilli that apparently express sensing molecules (at least the presence of the sodium–glucose co‐transporter 1 (SGLT1) has been shown rather convincingly to localize to this apical process, Reimann et al., 2008), enabling the cells to react to the various nutrients in the lumen. Basolaterally, it contains relatively large, relatively homogeneous granules known to contain the proglucagon products (Orci et al., 1968; Ravazzola et al., 1979). Secretion thus occurs via exocytosis with emptying of the granular content into the interstitial space, followed by diffusion of the products across the basal lamina and into the lamina propria, where the molecules are taken up by the capillaries and delivered to the circulation. Analysis of mucosal biopsies, immediately frozen and extracted using acid ethanol whereby enzymic activity is thought to be completely inhibited, shows that here GLP‐1 is present in its active, truncated form, that is, corresponding to proglucagon 78–107 (GLP‐1 7–36) (Hansen et al., 1999); see Figure 1. Presumably, intragranular cleavage by prohomone convertase 1/3 occurs at the basic amino acid residues at position 109 and 110 in proglucagon, leaving a C‐terminal structure with arginine in position 109 (Rouille et al., 1995). By the action of another processing enzyme, carboxypeptidase E, the Arg109 is then removed (Friis‐Hansen et al., 2001), exposing Gly108, which is now oxidized by the PAM enzyme complex, whereby the preceding amino acid, no 107 is amidated (Kumar et al., 2016). The amidation efficiency varies between species, but in humans, virtually no Gly‐extended GLP‐1 is found in the intestines, indicating a very high amidation efficiency, whereas in rats and pigs, between a third and half is Gly extended (Kuhre et al., 2014). The amidation is thought to contribute positively to the stability of the hormone but does not otherwise seem to influence its biological activity (Orskov et al., 1993).
Thus, stored GLP‐1 corresponds to amidated proglucagon 78–107 amide, also called truncated GLP‐1 or GLP‐1 (Bang‐Berthelsen et al., 2016; Bell et al., 1983; Billeschou et al., 2021; Billing et al., 2018, 2019; Billings et al., 2018; Brookes et al., 2013; Brubaker, 2018; Bucinskaite et al., 2009; Buckley et al., 2019; Buhl et al., 1988; Canfora et al., 2017; Chen et al., 2017; Christiansen et al., 2018, 2019; Daniel et al., 2002; Day et al., 2009; de Heer et al., 2007; Deacon et al., 1995; Drucker & Yusta, 2014; Eissele et al., 1990, 1992; Fehmann et al., 1995; Friis‐Hansen et al., 2001; Gabery et al., 2020; Gasbjerg et al., 2019; Glass et al., 2017; Gouyer et al., 2010; Hanby et al., 1999) and amide (Hansen et al., 1999). However, studies with isolated perfused pig ileum have indicated that in the venous effluent from this model at least 2/3 of newly released GLP‐1 is already metabolized, that is, on its way from the granules of the cells to the venous drainage (Hansen et al., 1999). Thus, only about 33% and 25% of newly released GLP‐1 survives in the intact form after release from the gut. The metabolite formed in the gut corresponds to the main metabolite formed in the circulation, namely, GLP‐1 (9–36) amide, and studies using specific antagonists have shown that this cleavage is catalysed by the enzyme dipeptidyl‐peptidase‐4 (DPP4), which is also responsible for causing the whole body half‐life of the peptide in the circulation of only about 1–2 min (Deacon et al., 1995; Hansen et al., 1999; Vilsboll et al., 2003). This again corresponds to a plasma clearance that exceeds the cardiac output by a factor of 2–3. Clearly no single organ can be responsible for this, and these data indicate that the peptide is continuously broken down everywhere in the body and that a steady state is never obtained (which also means that the apparent half‐life has no meaning). Further studies searching for the localization of DPP‐4 in the gut showed that, in addition to the presence in the brush border, where it acts as a digestive enzyme processing oligopeptides, it is also expressed on the membranes of the endothelial cells of the gut capillaries (Hansen et al., 1999). Thus, newly released GLP‐1, still in the intact form, diffuses into the lamina propria, and is taken up by the local capillaries, only to be degraded by DPP‐4 sitting on the luminal surface of the endothelium. The fraction of the GLP‐1, which reaches the liver in the intact form (1/3–1/4), is further degraded in the liver, where DPP‐4 is expressed in the sinusoids, whereby half of what arrives to the liver is degraded (Pridal et al., 1996), leaving 12%–15% to reach the systemic circulation. Here, the molecules meet a soluble circulating form of DPP‐4, which is known to cause further degradation (the half‐life of GLP‐1 in isolated plasma is about 20 min at 37°C and even less in whole blood) (Wettergren, Pridal, et al., 1998). This means that very little GLP‐1 actually reaches the pancreas in an intact, active form (Hjollund et al., 2011). This extensive degradation would seem incompatible with a classical endocrine role for GLP‐1 as an incretin hormone secreted from the gut and reaching the pancreas via the circulation. However, it must be considered that the beta cells appear to be the cell type in the body with the highest expression of the GLP‐1 receptor (Wei & Mojsov, 1995), and it is likely that the small fraction of newly released GLP‐1 that actually reaches the pancreas in the active form (around 10%) may stimulate insulin secretion. Experimental support for this assumption will be extremely difficult to provide, given that the experiment would involve elevations of plasma concentrations of GLP‐1 around 2.5 pmol L−1 (with fasting levels usually ranging from 1–10 pmol L−1), which can even be difficult to measure. It may be argued that the importance of the endocrine route might be revealed using the GLP‐1 receptor antagonist, exendin 9–39, which has an appropriate affinity and specificity for this (Schirra et al., 1998), and this has been attempted on numerous occasions, but the approach has an inherent problem: in vivo, glucagon secretion from the pancreas is invariably stimulated by exendin 9–39, resulting in elevated glucose concentrations and as a result insulin secretion may actually increase during exendin 9–39 administration (Gasbjerg et al., 2019). The precise role of círculating GLP‐1 for beta cell function is therefore difficult to gauge using exendin 9–39. However, during administration of DPP‐4 inhibitors, such as sitagliptin or saxagliptin, the plasma concentrations of intact GLP‐1 rise to levels (10–20 pmol L−1) that are likely to directly influence beta cell secretion (Mari et al., 2005) and experiments with co‐administration of exendin 9–39 are consistent with this (Aulinger et al., 2014). Also, individuals with accelerated nutrient passage into the small intestine or with surgical rearrangements exposing the more distal small intestine to nutrients (e.g., gastric bypass operations) may show postprandial concentrations of intact GLP‐1 (Holst, Albrechtsen, et al., 2019) that are sufficient to activate the beta cells directly.
It has been speculated that the lymph drainage of the intestine might serve as a route for delivery of GLP‐1 to the circulation (Lu et al., 2012), but the lymph flow is very limited; there may be species differences, and the amounts delivered to the blood stream are probably very small (Hansen et al., 2015; Jejelava et al., 2018).
3. GASTROINTESTINAL EXPRESSION OF THE GLP‐1 RECEPTOR
One may wonder why this useful metabolic hormone is inactivated so rapidly and extensively after its release. This raises the question whether it might exert local actions after its release. This would require local expression of the GLP‐1 receptor, because we consider any non‐receptor‐mediated effects physiologically irrelevant.
The apparently single GLP‐1 receptor gene was cloned in 1992 (Thorens, 1992), and nothing is known about isoforms generated by differential splicing. There are numerous genetic variants (Michałowska et al., 2021) in humans (more than 50), some of them showing widely differing binding and signalling characteristics (unpublished studies in the authors' lab), but all forms with clearly abnormal signalling are rare and unlikely to explain common pathological traits, such as obesity and Type 2 diabetes.
Accurate mapping of GLP‐1 receptors outside the beta cells of the islets, where expression levels are lower, has turned out be to be difficult. The first attempts at demonstrating a GLP‐1 receptor relied on binding of radioactively labelled GLP‐1 to cells or tissue sections (Fehmann et al., 1995), and after the cloning of the receptor, northern blotting and PCR became possible (Wei & Mojsov, 1996), but generally results obtained with these methods have poor spatial resolution. The early reports included expression in skeletal muscle, white adipose tissue and liver, but better methodology excluded expression in myocytes, adipocytes and hepatocytes (Pyke et al., 2014; Richards et al., 2014). For several years, attempts at visualizing the GLP‐1 receptor with immunohistochemistry (IHC) were plagued by poor antibodies with either low sensitivity or inadequate specificities (McLean et al., 2021; Pyke & Knudsen, 2013), and clearly erroneous localizations were reported with various commercial antisera (McLean et al., 2021). At NovoNordisk, a series of new antibodies was raised against the cloned recombinant and crystalized ectodomain of the receptor, and these antibodies (e.g., 7F282A, Jensen et al., 2018) appear to have the required specificity and, remarkably, show receptor staining on the cell surface as opposed to diffuse cytosolic staining. These antibodies stain receptors transfected into various cells lines, do not show staining in tissue/cells from animals with genetic deletion of the receptor, and label only a single band upon western blotting (no bands in knockout animals), corresponding in size to the cloned receptor. Autoradiographical demonstrations of the receptor have also been greatly improved with the use of labelled exendin‐4, which is a stable, full and equipotent agonist of the receptor and even better with the antagonist exendin 9–39 (Jensen et al., 2015), which is not only stable but also does not get internalized after binding, but stays on the membrane surface. It has previously been shown in vitro that human, rat and mouse GLP‐1 receptors are internalized upon agonist binding (Jorgensen et al., 2007; Widmann et al., 1995), but not upon antagonist binding. It is also possible to treat the animals with the antagonist before injection of the tracer (for in vivo binding) thereby keeping GLP‐1 expression at the cell surface, which enhances the chances of visualization the receptor. This localization technology still does not have the best spatial resolution but, on the other hand, reflects the actual binding of the hormonal ligand, which is, after all, the physiologically relevant parameter (Jensen et al., 2015). In situ hydribization techniques have also been improved, increasing the sensitivity and resolution of the technique (Andersen et al., 2021) but do not reflect presence of the actual receptor protein, which may represent a problem, for example, in studies of neural tissue. In addition, various transgenic mouse models have been developed, with which receptor expression can be followed by fluorescence generated by a co‐expressed fluorescent reporter protein (Richards et al., 2014). If the expression level of the reporter is simultaneously linked to a very active promotor, one may obtain strong signals in any cell, which ever expressed the receptor (Andersen et al., 2021) (which may create the problem that the cell is stained but no longer expresses the receptor). Thus, all of these methods have their advantages and disadvantages, and combinations therefore represent the best approach in a search for receptor expression.
The following account is based on the findings made using transgenic mice in which cells with receptor expression were visualized with the reporter protein tdTomato activated in a receptor promotor specific manner (by Cre‐recombinase), either by fluorescence or after immunostaining for the reporter protein (see Table 1 and Figure 2)(Andersen et al., 2021). We are seeking to confirm these findings in mouse gut, using a combination of methods (fluorescent In situ hybridization (FISH), in situ ligand binding (autoradiography) and immunohistochemistry (IHC).
TABLE 1.
Expression and effects of the GLP‐1 receptor in gastrointestinal tissues and cells
| Mucosa and submucosa | Population size | Submucosal plexuses b | Myenteric plexuses a , b | Physiological effects | |
|---|---|---|---|---|---|
| Gastric: forestomach | ø a , b | ø | ø | ø | |
| Gastric: corpus |
Parietal cells a Chief cells a |
+ ++ ++ |
ø | * |
h,rGastric emptying↓ h,pAcid secretion↓ h,rFood intake↓ rStomach protection↑ rSomatosatin↑ rGastrin↓ |
| Gastric: antrum | Mucous cells a | ++ | ø | * | pMotility↓ |
| Duodenum |
Intraepithelial lymphocytes a , b Lamina propria lymphocytes b |
+++ +++ + |
ø | * |
h,rMotility↓ rIntestinal protection↑ rMucus secretion↑ r5‐HT – rSecretin – rSomatostatin↑ h,rPYY↓ |
| Jejunum |
Enteroendocrine cells a Intraepithelial lymphocytes a Lamina propria lymphocytes b |
++ ++ + |
* | ** |
r5‐HT – rSecretin – rSomatostatin↑ h,rPYY↓ |
| Distal ileum |
Intraepithelial lymphocytes a Lamina propria lymphocytes b |
+ + + |
** | *** | |
| Caecum | ø b | * | * | ||
| Proximal colon |
Enteroendocrine cells b * Intraepithelial lymphocytes b Lamina propria lymphocytes b |
+ + + |
** | *** |
rIntestinal protection↑ rRegulate microbiota rMotility↓ rIntestinal growth↑ |
| Distal colon | ø a , b | ø | ** |
rIntestinal protection↑ rRegulate microbiota rMotility↓ rIntestinal growth↑ |
Note: +, few; ++, moderate number; +++, numerous; ø, no expression; *, minor GLP‐1 receptor expression; ** moderate GLP‐1 receptor expression; ***, strong GLP‐1 receptor expression; superscript h, effects in humans; superscript r, effects in rodents; superscript p, effects in pigs; ↑, increase; ↓, decrease; –, unknown effects.
Anderson et al. (2021).
KV Grundahl et al., submitted
FIGURE 2.
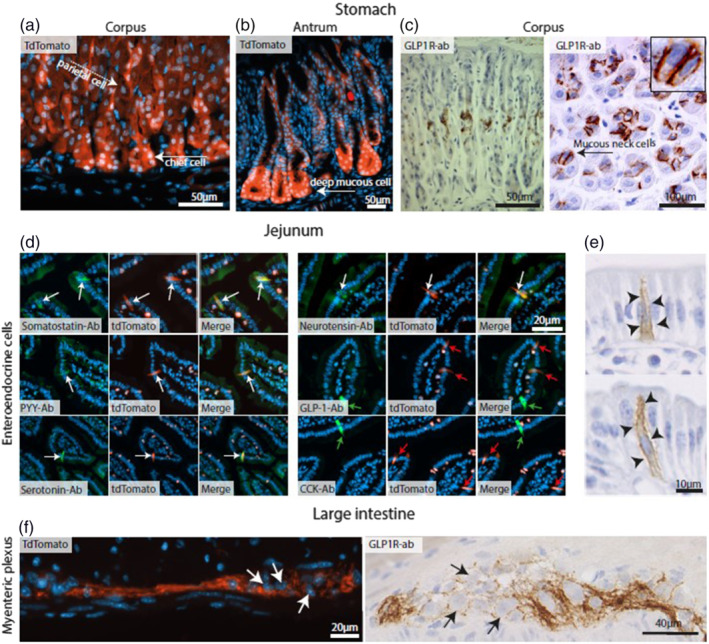
Representative images of gastrointestinal cells expressing GLP‐1 receptors (GLP‐1R) cells. (a) Sections from mouse Glp1r.tdTomato gastric corpus showing Glp‐1r expression in chief cells (white arrow) and parietal cells (white dashed arrow). (b) Sections from mouse Glp1r.tdTomato gastric antrum showing Glp1r‐positive deep mucous cells (white arrow). (c) Sections of rat gastric corpus showing mucous neck cells (black arrow) stained with GLP‐1r antibodies (brown). (d) Glp1r.tdTomato expressing enteroendocrine cells (red cells) in the proximal small intestine co‐localizing (white arrows) with antibodies (green cells) specific for somatostatin, peptide‐YY, 5‐HT (serotonin), and neurotensin, but not GLP‐1 and CCK (green and red arrows) with merged pictures. (e) GLP‐1 receptor antibody staining (brown) in the rat proximal small intestine shows this receptor localizing to the lateral membrane of enteroendocrine cells (arrowheads). (f) Murine large intestine myenteric plexuses stained with Glp1r.tdTomato (red) or GLP‐1 receptor antibodies (brown). Note that expression of GLP‐1 receptors is exclusively found on fibres around the neurons, but not in their soma. Counterstaining performed with either DAPI or haematoxylin
In the stomach, tdTomato‐positive cells were observed primarily around the base of the gastric glands, primarily in corpus chief cells, but also, albeit weak, in parietal cells, as well as in antral mucus‐producing cells. Using autoradiography, but also IHC, clear staining was observed at the mucous neck cells of the gastric corpus. Weak tdTomato was detected towards the pit of the gastric glands, but no Glp‐1r signal was found here by in situ hybridization or antibody staining. Staining with antibody to GLP‐1 receptors confirmed the presence of these receptors in chief cells and parietal cells, whereas in situ hybridization demonstrated expression in Brunner's glands.
Numerous tdTomato‐positive cells were identified in the epithelium of the duodenum and jejunum, whereas fewer positive cells were detected in the distal small intestine, and none in the colon. Notably, receptor expression was never seen in enterocytes, goblet cells or Paneth cells. Immunofluorescence counterstaining identified these proximal receptor‐positive cells to be N cells (neurotensin positive), L cells (peptide YY positive), enterochromaffin cells (5‐HT positive) and delta cells (somatostatin positive). Glp‐1r expression only occurred in a subset of L cells, as GLP‐1 staining showed no co‐localization with tdTomato, whereas PYY staining did. A general finding was staining of nerve fibres in the myenteric plexus throughout the GI tract, while fewer fibres were seen in the submucous plexuses. Positive nerve fibres were not observed in the villi. Glp‐1r was also expressed on intraepithelial lymphocytes (IELs) located not only in villi of the intestinal epithelium but also in the lamina propria (particularly by autoradiography, but not by IHC), whereas there were no tdTomato signals in the lymphocytes in the colon. The main sites of expression are in agreement with those described in previous reports (Heppner et al., 2015; Pyke et al., 2014; Richards et al., 2014), but many more details have been revealed in these recent studies.
It should be mentioned that GLP‐1 receptors are also clearly expressed in the acini of the pancreas with all methods, although the staining intensity is much less that it is in for instance the Brunner glands.
4. FUNCTIONAL SIGNIFICANCE OF GI EXPRESSION OF THE RECEPTOR
4.1. Stomach
The expression of receptors in the stomach raises the question whether there is a local production of GLP‐1 in the stomach. Production of gastric glucagon was suggested already in 1948, when Sutherland and de Duve found a hyperglycaemic substance in gastric extracts (Sutherland & De Duve, 1948). The possibility of extra‐pancreatic production of glucagon has been discussed ever since and, whereas there seems to be little glucagon expression in the human (Holst et al., 1983) and rodent stomach, dogs and cats were reported to harbour A‐like cells and to secrete glucagon from the gastric mucosa (Holst, 1978). From the current point of view, the interest in glucagon is of course that if there is glucagon, then there is also proglucagon and therefore a possibility for GLP‐1 production. Although some production of proglucagon seems possible especially in the fundic regions of the stomach of most species, this is not immediately evident in humans and certainly not in the corpus. The many receptors described in the stomach would therefore be expected to be targets for circulating GLP‐1, and this raises a number of problems as discussed above.
Functional GLP‐1 receptors were described early on in relation to the parietal cells, and the cells were reported to respond to GLP‐1 with acid secretion in a cAMP‐dependent manner (Schmidtler et al., 1994). However, infusion of GLP‐1 in vivo clearly inhibits acid secretion as well as gastric fluid secretion and motility (Wettergren et al., 1993). The inhibitory action was studied in detail by Wettergren et al. in humans, who found that infusions of GLP‐1 giving rise to near physiological levels strongly inhibit both gastric emptying and stimulated acid secretion as well as pancreatic exocrine secretion (Wettergren et al., 1993). A strong inhibitory effect was also observed when secretion was stimulated by sham‐feeding (ingestion of an appetizing meal followed by spitting out during aspirations of gastric secretions), that is, a mechanism exclusively mediated by vagus nerve activity (and completely abolished by vagotomy) (Wettergren et al., 1994). Conversely, the GLP‐1 induced inhibition of stimulated acid secretion was completely lost after truncal vagotomy (Wettergren et al., 1997). Thus, the vagal mechanism completely overrules any effects that might have been exerted on parietal cells by circulating GLP‐1 (and also rules out mechanisms depending on the local release of somatostatin in the stomach, which might inhibit both gastrin and parietal cell secretion, Eissele et al., 1990). In further experiments in chloralose‐sedated pigs subjected to insulin‐induced hypoglycemia (a powerful central stimulus of vagal activity), short infusions of GLP‐1 (2 pmol kg−1 min−1) strongly and immediately suppressed antral motility, gastric and pancreatic secretion, revealing a powerful inhibitory effect of GLP‐1 on efferent vagal activity (Wettergren, Wojdemann, & Holst, 1998). Inhibition of recorded efferent vagal activity after GLP‐1 infusion was also reported by Nakabayashi et al. (Nakabayashi et al., 1996). In further experiments, involving isolated models (stomach, antrum, pancreas) with fully preserved vagal innervation, GLP‐1 did not influence the peripheral transmission of the vagal effects (which were accurately reproducing the effects of centrally induced activity in these models), and it was concluded that the effect of GLP‐1 was exerted centrally (Wettergren, Wojdemann, & Holst, 1998). In experiments in rats, recordings were made from afferent branches of the vagus nerve during brief infusions of GLP‐1, and in these experiments, a dose‐related increase in firing frequency was demonstrated (Bucinskaite et al., 2009), supporting that GLP‐1 would stimulate the activity of sensory afferents of the vagus and suggesting that the effect on efferent vagal activity was the result of inhibition of long, inhibitory vago‐vagal reflexes. Taken together with strong expression of GLP‐1 receptors in numerous cell bodies of the nodose ganglion (the soma of the vagal sensory afferents) (Bucinskaite et al., 2009; Nakagawa et al., 2004), it seemed that GLP‐1 acts to inhibit gastric functions via activation of sensory vagal afferents relaying to vagal motorneurons in the brain stem or in the hypothalamus. This important mechanism and further evidence in support of this mechanism accumulated subsequently will be discussed further in the contribution by Stefan Trapp (2021) in this issue.
4.2. GLP‐1 and mucus producing cells
The dense expression of GLP‐1 receptors in the mucus secreting epithelial cells of the stomach suggests that GLP‐1 could play a role mucosal protection. Similar to the so‐called ‘reparative lineages’ in the gut (Gouyer et al., 2010), the mucous neck cells of the gastric corpus produce mucins (MUC6 and MUC1, Hanby et al., 1999; Gouyer et al., 2010) and growth factors (TFF1, TFF2 and EGF proteins, Gouyer et al., 2010), which are believed to protect the other glandular cells from the acid secreted by neighbouring parietal cells. In accordance, Chen et al. recently showed that administration of the GLP‐1 receptor agonist, exendin‐4, accelerated healing of stomach ulcers in diabetic rats (Chen et al., 2017). The strong tdTomato signal in Brunner's glands, which was confirmed by IHC (Baggio et al., 2018; Hepprich et al., 2020; Pyke et al., 2014) and quantitative polymerase chain reaction (qPCR) (Bang‐Berthelsen et al., 2016), is in accordance with earlier findings in humans and monkeys (Orskov & Poulsen, 1991; Qu et al., 2008), and functional studies in mice have shown that liraglutide causes an upregulation of genes in Brunner's glands associated with protection of the intestinal epithelial layer (Glass et al., 2017). The Brunner's glands secrete bicarbonate, glycoproteins and mucus that coat the duodenal epithelium and thereby protects against the pepsin‐rich and acidic chyme from the stomach (Krause, 2000). In vivo treatment of mice with exendin‐4, the GLP‐1 receptor full agonist led to intracellular translocation of the GLP‐1 receptors in glandular cells of the Brunner glands and was accompanied by mucin emptying from the apical secretory granules (own unpublished studies). Furthermore, Bang‐Bertelsen et al. observed that administration of GLP‐1 receptor agonists led to transcriptional upregulation of the chemokine CCL20, IL‐33 and mucin 5b in the mouse Brunner's glands, as well as improvement in murine colitis models (Bang‐Berthelsen et al., 2016). Taken together, these studies indicate that GLP‐1 may not only induce mucin secretion but also promote release of other important factors that may protect and facilitate healing of the gastrointestinal mucosa.
4.3. Enteroendocrine cells
By co‐staining, the many GLP‐1 receptor‐positive enteroendocrine cells were identified as secretin, 5‐HT, PYY and somatostatin expressing cells in the studies cited above. An account of the difficulties in mapping receptor expression in different cells and tissues based on recent single cell RNA profiling studies was recently given by McLean et al. (2021). In perfused mouse intestine, GLP‐1 has been shown to markedly stimulate somatostatin secretion, and this effect could be blocked with the GLP‐1 receptor antagonist, exendin 9–39 (Jepsen et al., 2019), indicating that the two hormones are locked in a mutual feedback loop. However, with regard to the GLP‐1 receptors on the intestinal PYY and secretin cells, the functional implications remain to be determined. Secretin has been shown to both inhibit gastric emptying (Raybould & Holzer, 1993) and stimulate the exocrine pancreas to release bicarbonate and water into the intestinal lumen thus neutralizing and reducing the acidic chyme coming from the stomach following a meal (Kirk et al., 2017; Kirkegaard et al., 1984). Assuming that GLP‐1 stimulates secretin release, combined with the stimulatory effects on the Brunner glands, GLP‐1 may play an underappreciated role in modulating the intraluminal pH of the small intestine. The finding of receptors in 5‐HT‐secreting enterochromaffin cells in mice suggest that GLP‐1 participates in the regulation of their monoamine secretion (Kirk et al., 2017). In human studies, antagonism of GLP‐1 receptors led to an increase in PYY release that could be explained by an auto‐inhibitory feedback axis of GLP‐1 (Svane et al., 2016) but could also result from prevention of GLP‐1 stimulated somatostatin release.
4.4. Intestinal neuronal structures
The dense network of nerve fibres expressing GLP‐1 receptors throughout the gut strongly support that these fibres might be the targets for GLP‐1, newly released from the L cells. The idea would be that GLP‐1 diffusing from the L cells to the capillaries interacts with sensory nerve fibres before it is broken down and inactivated by DPP‐4 in the capillaries (Hansen et al., 1999). This has important implications for the measurements of GLP‐1. As mentioned above, the extensive degradation of GLP‐1 by DPP‐4 means that most of the circulating GLP‐1 is composed of the inactive metabolite GLP‐1 9–36 amide, whereas only a small fraction corresponds to intact GLP‐1 (7–36) amide (Pedersen et al., 2008). If, however, the major target for newly released GLP‐1 is a receptor expressed on enteric nerve fibres, then the metabolized GLP‐1 probably represents GLP‐1 that already has exerted its activity via the sensory nerve fibres (Holst & Deacon, 2005). This means that the effects of L‐cell secretion is better reflected by the plasma concentration profile of the GLP‐1 metabolite or, more accurately, the sum of intact GLP‐1 and the metabolite, usually designated ‘total GLP‐1’. At any rate, total GLP‐1 is also a better indicator of secretion of GLP‐1 than the profile of intact GLP‐1, which often includes measurements below the detection limit of the assays and does not show clear relation to the stimuli (Holst, Wewer Albrechtsen et al., 2019).
The nature of the GLP‐1 receptor‐positive fibres in the intestinal wall is not clear. The strongest antibody staining for GLP‐1 receptors and the greatest accumulation of 125I‐exendin (Billeschou et al., 2021; Billing et al., 2018, 2019; Billings et al., 2018; Brookes et al., 2013; Brubaker, 2018; Bucinskaite et al., 2009; Buckley et al., 2019; Buhl et al., 1988; Canfora et al., 2017; Chen et al., 2017; Christiansen et al., 2018, 2019; Daniel et al., 2002; Day et al., 2009; de Heer et al., 2007; Deacon et al., 1995; Drucker & Yusta, 2014; Eissele et al., 1990, 1992; Fehmann et al., 1995; Friis‐Hansen et al., 2001; Gabery et al., 2020; Gasbjerg et al., 2019; Glass et al., 2017; Gouyer et al., 2010; Hanby et al., 1999; Hansen et al., 1999, 2015; Hayes et al., 2011) grains were observed in and around myenteric plexuses located in the distal intestine, namely, in the distal ileum and in the proximal and distal large intestine. Myenteric ganglia of the duodenum, jejunum and gastric pylorus also displayed staining for GLP‐1 receptors, whereas the nerve fibres in the gastric corpus and caecum were very weakly stained. In the submucosal plexuses, the strongest GLP‐1 receptor staining was observed in the distal ileum, caecum and proximal large intestine, with weak staining in the jejunum. On the other hand, the submucosal plexuses of the gastric corpus, pylorus, duodenum and distal large intestine were devoid of staining. Interestingly, the GLP‐1 receptor antibody primarily appeared to stain nerve fibres around the somas of the myenteric and submucosal neurons, rather than the actual cell bodies of the enteric neurons. This could be because the GLP‐1 receptor‐producing neurons mainly express ligand receptors on axons and dendrites. However, it has been difficult to find GLP‐1 receptor‐positive nerve cell bodies using FISH, and also the Glp‐1r reporter mice did not reveal such cell bodies. These findings may suggest that at least some of these fibres are extrinsic, for example, vagal.
In the mouse small and large intestine, the majority of the myenteric neurons contain either neuronal nitric oxide synthase (nNOS) or choline acetyl transferase (ChAT), accounting for 95% of myenteric neuron population (Jiang et al., 2017; Qu et al., 2008). ChAT is the synthesizing enzyme for the excitatory neurotransmitter acetylcholine, whereas nNOS is the synthesizing enzyme for the inhibitory neurotransmitter nitric oxide (NO) in motor neurons (Daniel et al., 2002). GLP‐1 receptors co‐localize with nNOS‐positive (i.e., inhibitory) and CHAT‐positive (excitatory) motor neurons (Amato et al., 2010; Richards et al., 2014), but the specificity of the staining was not evaluated. Patch clamp studies on small intestinal primary cultures of GLP‐1 receptor‐fluorescent myenteric neurons derived from the mouse (and therefore likely to represent intrinsic enteric neurons) showed that they were electrically active and that administration of GLP‐1 increased the frequency of their action potentials (Richards et al., 2014). Furthermore, GLP‐1 reduced electrically induced, cholinergic contraction of circular, but not longitudinal, smooth muscle in both duodenum and colon in a concentration‐dependent fashion, an effect that appeared to involve NO signalling (Amato et al., 2010). As the ascending contractions of circular muscle are an important element of peristalsis, this has also been suggested to be the mode of action by which GLP‐1 inhibits peristalsis (Amato et al., 2010). The migrating motor complex (MMC) represents a fasting pattern of electrical activity in the GI tract that elicits strong peristaltic activity, stretching all the way from the stomach to the colon, with intervals of about 1.5 h in humans. Interestingly, GLP‐1 has been shown to inhibit MMC and the associated motor activity in the GI tract (Tolessa et al., 1998a, 1998b), although physiologically there is little secretion of GLP‐1 in the fasting state. As discussed above, GLP‐1 strongly inhibits gastric motor activity and emptying, but this effect seems to depend on inhibition of long vago‐vagal reflexes and is lost after truncal vagotomy (Plamboeck et al., 2013). In addition, GLP‐1 did not appear to inhibit contractions of isolated smooth muscle preparations derived from rat gastric fundus, corpus or duodenum, stimulated with either muscarinic agonists or electric field stimulation (Tolessa et al., 1998a), suggesting that the effect of GLP‐1 on GI motility is mediated via the vagus.
4.5. GLP‐1 receptors on extrinsic afferent nerve endings
The weak or variable staining for GLP‐1 receptors in the somas of the nerve cells suggests that these fibres could be extrinsic afferent or efferent nerve endings. Vagal and rectal afferent nerve endings have been shown to form complex, flattened expansions within the myenteric ganglia known as intraganglionic laminar endings (IGLEs), and these are found throughout the GI tract (Brookes et al., 2013). Williams et al. (2016) demonstrated that a cohort of vagal GLP‐1 receptor‐positive neurons with soma located in the nodose ganglia formed IGLEs in the stomach and proximal duodenum and that they specifically responded to saline‐induced GI distension. Consistent with these findings, GLP‐1 receptors have been suggested to be synthesized in the nodose ganglion neurons and transferred to the periphery (the IGLEs) via axonal transport (Bucinskaite et al., 2009). In further support of these observations, the suppressive effects of intraperitoneal injections of GLP‐1 on food intake and blood glucose levels were attenuated by complete sub‐diaphragmatic vagal de‐afferentation in rats (Hayes et al., 2011), indicating that these systemic effects are mediated in part through activation of GLP‐1 receptors on the vagal afferent nerve terminals. In accordance, intraperitoneal administration of 125I‐exendin (Billeschou et al., 2021; Billing et al., 2018, 2019; Billings et al., 2018; Brookes et al., 2013; Brubaker, 2018; Bucinskaite et al., 2009; Buckley et al., 2019; Buhl et al., 1988; Canfora et al., 2017; Chen et al., 2017; Christiansen et al., 2018; Christiansen et al., 2019; Daniel et al., 2002; Day et al., 2009; de Heer et al., 2007; Deacon et al., 1995; Drucker & Yusta, 2014; Eissele et al., 1990, 1992; Fehmann et al., 1995; Friis‐Hansen et al., 2001; Gabery et al., 2020; Gasbjerg et al., 2019; Glass et al., 2017; Gouyer et al., 2010; Hanby et al., 1999; Hansen et al., 1999, 2015; Hayes et al., 2011) resulted in labelling of myenteric ganglia of the distal ileum, thus supporting the interpretation of experiments involving intraperitoneal administration of GLP‐1 to reflect interactions with nerve fibres within the myenteric plexus (Hayes et al., 2011).
4.6. GLP‐1 receptors on mucosal lymphocytes
Autoradiography and IHC have showed GLP‐1 receptor‐positive IELs and few and scattered lamina propria lymphocytes (LPLs) in the duodenum, distal ileum and proximal colon, whereas caecum and distal colon largely were devoid of mucosal staining. Yusta et al. (2015) also found Glp‐1r mRNA on isolated murine IELs by qPCR, and in vitro studies led the group to conclude that GLP‐1 in the gut may play a role in innate immunity, mucosal integrity and host microbial responses (McLean et al., 2021). Curiously, both the GLP‐1 receptor‐positive IELs and LPLs appear to be scarce or non‐existent in the caecum, proximal or distal large intestine, despite being the primary area where the gut microbiota reside. Deletion of Glp‐1r expression led to an imbalance in the microbiota, as well as increased sensitivity to intestinal injury in mice (Billing et al., 2019), suggesting that interaction with these receptors is involved in a protective role of GLP‐1 for the intestinal mucosa.
4.7. The role of GLP‐2
The sister peptide, GLP‐2, which enhances intestinal mucosa growth and repair, is thought to play an important role in the local maintenance of the intestinal mucosa (Drucker & Yusta, 2014), although the fact that receptor expression is most prominent in the upper small intestine (Munroe et al., 1999), where L‐cell density is less pronounced, and transposition (Thulesen et al., 2001) experiments have pointed to the existence of both local and remote (endocrine) actions of the hormone. The exact GLP‐2 receptor localization has also been a matter of dispute (Pedersen et al., 2015), but there seems to be consensus that it is expressed on enteric neurons and on sub‐epithelial myofibroblasts that produce growth factors that seem to be responsible for the mucosal growth and maintenance effects of GLP‐2 (Yusta et al., 2019). An essential role for GLP‐2 in repair processes after chemotherapy‐induced mucosal damage has been documented in studies involving, for example, GLP‐2 receptor knockout animals (Billeschou et al., 2021). However, there is clear evidence that also GLP‐1 may participate in the repair mechanism (Kissow et al., 2013). The localization of the GLP‐1 receptor responsible for these beneficial actions is not clear, because expression has not been observed in the subepithelial myofibroblasts, but perhaps interactions with the enteric nervous system are involved, as discussed above.
4.8. GLP‐1 in the colon
The role of GLP‐1 in the colon remains un‐defined. The GLP‐1 meal responses are unimpaired by total colectomy in man (Palnaes et al., 1997), suggesting that these responses are independent of colonic GLP‐1 secretion. but of course colonic secretion, at least if elicited by luminal factors, would not be expected to be observable concomitantly with meal ingestion, but to be much delayed. The only reliable stimulus to colonic secretion identified so far appears to be that exerted by malabsorbed bile acids (Adrian et al., 2012; Christiansen et al., 2019), but the timing of such a response in vivo, if existing, is unclear. The gene for angiotensin AT1 receptors, Agtr1a, was expressed in colonic L cells and to stimulate GLP‐1 secretion when activated (Billing et al., 2018, 2019) although the significance of this is unclear. Interestingly, ghrelin was reported to sensitize extrinsic and intrinsic colonic neurons to a GLP‐1 agonist (Buckley et al., 2019) pointing to a possible link between the stomach and the colon (perhaps contributing to the so‐called gastro‐colonic reflex?). In addition, colonic nitrergic and purinergic neurons respond to GLP‐1 (Yan et al., 2019). It is still perplexing that the number of L cells increases towards the distal parts of the gut with a maximum in the distal colon and the rectum (Knudsen et al., 1975). Perhaps this fits best with a local, perhaps mucosal protective role for GLP‐1 (Bang‐Berthelsen et al., 2016). GLP‐1 has been reported to promote growth (and tumorigenesis) in the distal gut (Koehler et al., 2015). Numerous studies of colonic fermentation with formation of short chain fatty acids (SCFAs, mainly acetate, propionate and butyrate) rely on the assumption that the SCFAs stimulate local GLP‐1 secretion with subsequent expedient metabolic effects. But actually, the evidence for this is weak. In studies of isolated perfused colon, SCFAs had no effect on GLP‐1 or PYY secretion unless cAMP levels were enhanced by IBMX addition (Christiansen et al., 2018). In vivo, in humans, documented enhancement of colonic fermentation (as demonstrated by H2‐exhalation) was not associated with measurable release of GLP‐1 (Olesen et al., 1999) and local colonic instillation of acetate resulted in weak stimulation of PYY when instillation occurred in the ascending colon (Canfora et al., 2017; van der Beek et al., 2016). However, these observations do not exclude that a local GLP‐1 release would lead to activation of neurons that could be responsible for both local and central actions.
4.9. The gastrointestinal effects of GLP‐1 receptor agonists
Interestingly, a selective genetic deletion of GLP‐1 receptors in the neurons of the GI tract was not associated with a clear phenotype (McLean et al., 2021; Varin et al., 2019). Perhaps the sensitivity of such an experiment is inappropriate. Nevertheless, the rather dense expression of the receptors here, as outlined above, raises the question whether administration of GLP‐1 receptor agonists, which provide circulating plasma concentrations of the exogenous agonist that are considerably higher than that of the endogenous ligand, have gastrointestinal effects. The most prominent side effects of the GLP‐1 receptor agonists are the so‐called gastrointestinal side effects, that is, nausea, vomiting and diarrhoea. It is often assumed that the nausea is related to the inhibition of gastric emptying, but the association between these effects is weak. The effects on gastric emptying show rapid tachyphylaxis (Nauck et al., 2011), whereas the nausea may remain. In addition it is possible to create a total arrest of gastric emptying without any nausea (Willms et al., 1996). Most likely, these effects are not elicited by the gastrointestinal receptors, but rather by interference of the GLP‐1 receptor agonist with central receptors in the circumventricular organs, particularly in the area postrema, areas that are not normally exposed to such high concentrations of the ligand. The area postrema is known to transmit signals that elicit nausea and vomiting to the brain. One may also ask whether the GLP‐1 receptor agonists act on food intake via the intestinal receptors discussed above or via a similar pathway. Both liraglutide and semaglutide (long‐acting GLP‐1 receptor agonists) are able to reach hypothalamic nuclei associated with the fenestrated regions of the circumventricular organs (Gabery et al., 2020; Secher et al., 2014), and indeed, in experiments with genetic deletion of vagal afferents, the GLP‐1 receptor agonist liraglutide retained full anorectic activity whereas central deletion of GLP‐1 receptors was associated with a complete loss of activity (Sisley et al., 2014).
However, it seems likely that GLP‐1 and the GLP‐1 receptor agonists have prominent direct effects on the GI tract, especially regarding secretion (of mucus) and motility (whereas the effects on gastric emptying is mainly depending on vagal mechanisms). It has also been reported that GLP‐1RAs promote both normal and neoplastic intestinal growth through mechanisms requiring fgf7 (Koehler et al., 2015 ), although the preproglucagon system was found not to play an essential role in normal gut development in mice (Wismann et al., 2017). The clinical use of the GLP‐1 receptor agonists has not been associated with an increase in intestinal neoplasms. There is consensus that the effect on motility is inhibitory as discussed above, and the effects may be pronounced producing extensive relaxation of the intestine, which would explain the tendency to diarrhoea, although there is no evidence that the diarrhoea has a secretory component. At any rate, at least some of the ‘gastrointestinal’ adverse effects show pronounced escape with, for instance, the effects on gastric emptying almost disappearing after a short time (hours) of continuous exposure (Nauck et al., 2011), and the nausea and vomiting may be nearly abolished by slow up titration of the GLP‐1 receptor agonist (Billings et al., 2018). In this way, it is possible to reach even very high concentrations that were previously considered unattainable because of these side effects. Therapy with GLP‐1 receptor agonists was recently reported not to change the composition or amount of the intestinal microbiome (Smits et al., 2021).
5. CONCLUDING REMARKS
The key objective of this review of the actions of GLP‐1 receptor ligands in the gut has been to point out that the gut may be viewed as the primary target for newly released GLP‐1, unlike the general trend in earlier reviews and articles, where the focus is mostly on the brain and the pancreas. The fundamental premise for this is the extremely rapid degradation and inactivation of GLP‐1 in the body, whereby only about 10% or less of what was released in the gut makes it to other targets including the pancreas in the intact, active form. Therefore, the circulating concentrations of intact GLP‐1 are generally very low, in the single digit pmolar range, and it has proven difficult, if not impossible, to demonstrate effects of such low concentrations on any organ system in the body. In conditions with accelerated gastric emptying/intestinal nutrient exposure (e.g., gastric bypass), higher plasma concentrations may be reached, and under these circumstances, the levels of active GLP‐1 may be sufficient to activate receptors in the circumventricular organs of the brains and in the pancreatic islets. Thus, it seems more likely that GLP‐1 physiologically targets receptors situated locally, in the gastrointestinal tract, including those expressed by enteroendocrine epithelial cells, intra‐epithelial and sub‐epithelial lymphocytes and, in particular, numerous intestinal nerve fibres. Activation here may be associated with local effects as well as central transmission of signals via sensory afferents of the vagus. This would be consistent with the view that, physiologically, GLP‐1 mainly acts as an ileal brake hormone, signalling nutritional abundance to the brain and limiting further intake and processing by reducing appetite and gastrointestinal secretions and motility. Synthetic GLP‐1 receptor agonists, which are resistant to DPP‐4 mediated degradation, may reach higher concentrations in the body fluids and reach receptors indiscriminately, resulting in preferential effects on the brain and the pancreas, although side effects related to activation of the gastrointestinal receptors are common.
5.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY (http://www.guidetopharmacology.org) and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos, et al., 2019; Alexander, Fabbro et al., 2019; Alexander, Kelly et al., 2019)
CONFLICT OF INTERESTS
None.
Holst, J. J. , Andersen, D. B. , & Grunddal, K. V. (2022). Actions of glucagon‐like peptide‐1 receptor ligands in the gut. British Journal of Pharmacology, 179(4), 727–742. 10.1111/bph.15611
DATA AVAILABILITY STATEMENT
There are no unpublished data in this article.
REFERENCES
- Adrian, T. E. , Gariballa, S. , Parekh, K. A. , Thomas, S. A. , Saadi, H. , Al, K. J. , Nagelkerke, N. , Gedulin, B. , & Young, A. A. (2012). Rectal taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteers. Diabetologia, 55, 2343–2347. 10.1007/s00125-012-2593-2 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , & CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , & CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , & CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: Transporters. British Journal of Pharmacology, 176, S397–S493. 10.1111/bph.14753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato, A. , Cinci, L. , Rotondo, A. , Serio, R. , Faussone‐Pellegrini, M. S. , Vannucchi, M. G. , & Mule, F. (2010). Peripheral motor action of glucagon‐like peptide‐1 through enteric neuronal receptors. Neurogastroenterology and Motility, 22, 664–e203. 10.1111/j.1365-2982.2010.01476.x [DOI] [PubMed] [Google Scholar]
- Andersen, D. B. , Grunddal, K. V. , Pedersen, J. , Kuhre, R. E. , Lund, M. L. , Holst, J. J. , & Ørskov, C. (2021). Using a reporter mouse to map known and novel sites of GLP‐1 receptor expression in peripheral tissues of male mice. Endocrinology, 162(3), bqaa246. 10.1210/endocr/bqaa246 [DOI] [PubMed] [Google Scholar]
- Aulinger, B. A. , Bedorf, A. , Kutscherauer, G. , de Heer, J. , Holst, J. J. , Goke, B. , & Schirra, J. (2014). Defining the role of GLP‐1 in the enteroinsulinar axis in type 2 diabetes using DPP‐4 inhibition and GLP‐1 receptor blockade. Diabetes, 63, 1079–1092. 10.2337/db13-1455 [DOI] [PubMed] [Google Scholar]
- Baggio, L. L. , Yusta, B. , Mulvihill, E. E. , Cao, X. , Streutker, C. J. , Butany, J. , Cappola, T. P. , Margulies, K. B. , & Drucker, D. J. (2018). GLP‐1 receptor expression within the human heart. Endocrinology, 159, 1570–1584. 10.1210/en.2018-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang‐Berthelsen, C. H. , Holm, T. L. , Pyke, C. , Simonsen, L. , Sokilde, R. , Pociot, F. , Heller, R. S. , Folkersen, L. , Kvist, P. H. , Jackerott, M. , Fleckner, J. , Vilien, M. , Knudsen, L. B. , Heding, A. , & Frederiksen, K. S. (2016). GLP‐1 induces barrier protective expression in Brunner's glands and regulates colonic inflammation. Inflammatory Bowel Diseases, 22, 2078–2097. 10.1097/MIB.0000000000000847 [DOI] [PubMed] [Google Scholar]
- Bell, G. I. , Sanchez‐Pescador, R. , Laybourn, P. J. , & Najarian, R. C. (1983). Exon duplication and divergence in the human preproglucagon gene. Nature, 304, 368–371. 10.1038/304368a0 [DOI] [PubMed] [Google Scholar]
- Billeschou, A. , Hunt, J. E. , Ghimire, A. , Holst, J. J. , & Kissow, H. (2021). Intestinal adaptation upon chemotherapy‐induced intestinal injury in mice depends on GLP‐2 receptor activation. Biomedicines, 9(1), 46. 10.3390/biomedicines9010046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billing, L. J. , Larraufie, P. , Lewis, J. , Leiter, A. , Li, J. , Lam, B. , Yeo, G. S. , Goldspink, D. A. , Kay, R. G. , Gribble, F. M. , & Reimann, F. (2019). Single cell transcriptomic profiling of large intestinal enteroendocrine cells in mice—Identification of selective stimuli for insulin‐like peptide‐5 and glucagon‐like peptide‐1 co‐expressing cells. Molecular Metabolism, 29, 158–169. 10.1016/j.molmet.2019.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billing, L. J. , Smith, C. A. , Larraufie, P. , Goldspink, D. A. , Galvin, S. , Kay, R. G. , Howe, J. D. , Walker, R. , Pruna, M. , Glass, L. , Pais, R. , Gribble, F. M. , & Reimann, F. (2018). Co‐storage and release of insulin‐like peptide‐5, glucagon‐like peptide‐1 and peptideYY from murine and human colonic enteroendocrine cells. Molecular Metabolism, 16, 65–75. 10.1016/j.molmet.2018.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings, L. K. , Doshi, A. , Gouet, D. , Oviedo, A. , Rodbard, H. W. , Tentolouris, N. , Gron, R. , Halladin, N. , & Jodar, E. (2018). Efficacy and safety of IDegLira versus basal‐bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: The DUAL VII randomized clinical trial. Diabetes Care, 41, 1009–1016. 10.2337/dc17-1114 [DOI] [PubMed] [Google Scholar]
- Brookes, S. J. , Spencer, N. J. , Costa, M. , & Zagorodnyuk, V. P. (2013). Extrinsic primary afferent signalling in the gut. Nature Reviews. Gastroenterology & Hepatology, 10, 286–296. 10.1038/nrgastro.2013.29 [DOI] [PubMed] [Google Scholar]
- Brubaker, P. L. (2018). Glucagon‐like peptide‐2 and the regulation of intestinal growth and function. Comprehensive Physiology, 8, 1185–1210. 10.1002/cphy.c170055 [DOI] [PubMed] [Google Scholar]
- Bucinskaite, V. , Tolessa, T. , Pedersen, J. , Rydqvist, B. , Zerihun, L. , Holst, J. J. , & Hellstrom, P. M. (2009). Receptor‐mediated activation of gastric vagal afferents by glucagon‐like peptide‐1 in the rat. Neurogastroenterology and Motility, 21, 978–e78. 10.1111/j.1365-2982.2009.01317.x [DOI] [PubMed] [Google Scholar]
- Buckley, M. M. , O'Brien, R. , Buckley, J. M. , & O'Malley, D. (2019). GHSR‐1 agonist sensitizes rat colonic intrinsic and extrinsic neurons to exendin‐4: A role in the manifestation of postprandial gastrointestinal symptoms in irritable bowel syndrome? Neurogastroenterology and Motility, 31, e13684. [DOI] [PubMed] [Google Scholar]
- Buhl, T. , Thim, L. , Kofod, H. , Orskov, C. , Harling, H. , & Holst, J. J. (1988). Naturally occurring products of proglucagon 111‐160 in the porcine and human small intestine. The Journal of Biological Chemistry, 263, 8621–8624. 10.1016/S0021-9258(18)68350-4 [DOI] [PubMed] [Google Scholar]
- Canfora, E. E. , van der Beek, C. M. , Jocken, J. W. E. , Goossens, G. H. , Holst, J. J. , Olde Damink, S. W. M. , Lenaerts, K. , Dejong, C. H. C. , & Blaak, E. E. (2017). Colonic infusions of short‐chain fatty acid mixtures promote energy metabolism in overweight/obese men: A randomized crossover trial. Scientific Reports, 7(1), 2360. 10.1038/s41598-017-02546-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. C. , Ho, C. C. , Yi, C. H. , Liu, X. Z. , Cheng, T. T. , & Lam, C. F. (2017). Exendin‐4, a glucagon‐like peptide‐1 analogue accelerates healing of chronic gastric ulcer in diabetic rats. PLoS ONE, 12, e0187434. 10.1371/journal.pone.0187434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen, C. B. , Gabe, M. B. N. , Svendsen, B. , Dragsted, L. O. , Rosenkilde, M. M. , & Holst, J. J. (2018). The impact of short‐chain fatty acids on GLP‐1 and PYY secretion from the isolated perfused rat colon. American Journal of Physiology. Gastrointestinal and Liver Physiology, 315, G53–G65. 10.1152/ajpgi.00346.2017 [DOI] [PubMed] [Google Scholar]
- Christiansen, C. B. , Trammell, S. A. J. , Wewer Albrechtsen, N. J. , Schoonjans, K. , Albrechtsen, R. , Gillum, M. P. , Kuhre, R. E. , & Holst, J. J. (2019). Bile acids drive colonic secretion of glucagon‐like‐peptide 1 and peptide‐YY in rodents. American Journal of Physiology. Gastrointestinal and Liver Physiology, 316, G574–G584. 10.1152/ajpgi.00010.2019 [DOI] [PubMed] [Google Scholar]
- Daniel, E. E. , Anvari, M. , Fox‐Threlkeld, J. E. , & McDonald, T. J. (2002). Local, exendin‐(9‐39)‐insensitive, site of action of GLP‐1 in canine ileum. American Journal of Physiology. Gastrointestinal and Liver Physiology, 283, G595–G602. 10.1152/ajpgi.00110.2002 [DOI] [PubMed] [Google Scholar]
- Day, J. W. , Ottaway, N. , Patterson, J. T. , Gelfanov, V. , Smiley, D. , Gidda, J. , Findeisen, H. , Bruemmer, D. , Drucker, D. J. , Chaudhary, N. , Holland, J. , Hembree, J. , Abplanalp, W. , Grant, E. , Ruehl, J. , Wilson, H. , Kirchner, H. , Lockie, S. H. , Hofmann, S. , … Tschop, M. H. (2009). A new glucagon and GLP‐1 co‐agonist eliminates obesity in rodents. Nature Chemical Biology, 5, 749–757. 10.1038/nchembio.209 [DOI] [PubMed] [Google Scholar]
- de Heer, J. , Pedersen, J. , Orskov, C. , & Holst, J. J. (2007). The alpha cell expresses glucagon‐like peptide‐2 receptors and glucagon‐like peptide‐2 stimulates glucagon secretion from the rat pancreas. Diabetologia, 50, 2135–2142. [DOI] [PubMed] [Google Scholar]
- Deacon, C. F. , Johnsen, A. H. , & Holst, J. J. (1995). Degradation of glucagon‐like peptide‐1 by human plasma in vitro yields an N‐terminally truncated peptide that is a major endogenous metabolite in vivo. The Journal of Clinical Endocrinology and Metabolism, 80, 952–957. 10.1210/jcem.80.3.7883856 [DOI] [PubMed] [Google Scholar]
- Drucker, D. J. , & Yusta, B. (2014). Physiology and pharmacology of the enteroendocrine hormone glucagon‐like peptide‐2. Annual Review of Physiology, 76, 561–583. 10.1146/annurev-physiol-021113-170317 [DOI] [PubMed] [Google Scholar]
- Eissele, R. , Goke, R. , Willemer, S. , Harthus, H. P. , Vermeer, H. , Arnold, R. , & Goke, B. (1992). Glucagon‐like peptide‐1 cells in the gastrointestinal tract and pancreas of rat, pig and man. European Journal of Clinical Investigation, 22, 283–291. 10.1111/j.1365-2362.1992.tb01464.x [DOI] [PubMed] [Google Scholar]
- Eissele, R. , Koop, H. , & Arnold, R. (1990). Effect of glucagon‐like peptide‐1 on gastric somatostatin and gastrin secretion in the rat. Scandinavian Journal of Gastroenterology, 25, 449–454. 10.3109/00365529009095514 [DOI] [PubMed] [Google Scholar]
- Fehmann, H. C. , Goke, R. , & Goke, B. (1995). Cell and molecular biology of the incretin hormones glucagon‐ like peptide‐I and glucose‐dependent insulin releasing polypeptide. Endocrine Reviews, 16, 390–410. 10.1210/edrv-16-3-390 [DOI] [PubMed] [Google Scholar]
- Friis‐Hansen, L. , Lacourse, K. A. , Samuelson, L. C. , & Holst, J. J. (2001). Attenuated processing of proglucagon and glucagon‐like peptide‐1 in carboxypeptidase E‐deficient mice. The Journal of Endocrinology, 169, 595–602. 10.1677/joe.0.1690595 [DOI] [PubMed] [Google Scholar]
- Gabery, S. , Salinas, C. G. , Paulsen, S. J. , Ahnfelt‐Ronne, J. , Alanentalo, T. , Baquero, A. F. , Buckley, S. T. , Farkas, E. , Fekete, C. , Frederiksen, K. S. , Helms, H. C. C. , Jeppesen, J. F. , John, L. M. , Pyke, C. , Nohr, J. , Lu, T. T. , Polex‐Wolf, J. , Prevot, V. , Raun, K. , … Knudsen, L. B. (2020). Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight, 5(6), e133429. 10.1172/jci.insight.133429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasbjerg, L. S. , Helsted, M. M. , Hartmann, B. , Jensen, M. H. , Gabe, M. B. N. , Sparre‐Ulrich, A. H. , Veedfald, S. , Stensen, S. , Lanng, A. R. , Bergmann, N. C. , Christensen, M. B. , Vilsboll, T. , Holst, J. J. , Rosenkilde, M. M. , & Knop, F. K. (2019). Separate and combined gluco‐metabolic effects of endogenous glucose‐dependent insulinotropic polypeptide and glucagon‐like peptide‐1 in healthy individuals. Diabetes, 68, 906–917. 10.2337/db18-1123 [DOI] [PubMed] [Google Scholar]
- Glass, L. L. , Calero‐Nieto, F. J. , Jawaid, W. , Larraufie, P. , Kay, R. G. , Gottgens, B. , Reimann, F. , & Gribble, F. M. (2017). Single‐cell RNA‐sequencing reveals a distinct population of proglucagon‐expressing cells specific to the mouse upper small intestine. Molecular Metabolism, 6, 1296–1303. 10.1016/j.molmet.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouyer, V. , Leir, S. H. , Tetaert, D. , Liu, Y. , Gottrand, F. , Harris, A. , & Desseyn, J. L. (2010). The characterization of the first anti‐mouse Muc6 antibody shows an increased expression of the mucin in pancreatic tissue of Cftr‐knockout mice. Histochemistry and Cell Biology, 133, 517–525. 10.1007/s00418-010-0688-8 [DOI] [PubMed] [Google Scholar]
- Hanby, A. M. , Poulsom, R. , Playford, R. J. , & Wright, N. A. (1999). The mucous neck cell in the human gastric corpus: A distinctive, functional cell lineage. The Journal of Pathology, 187, 331–337. [DOI] [PubMed] [Google Scholar]
- Hansen, L. , Deacon, C. F. , Orskov, C. , & Holst, J. J. (1999). Glucagon‐like peptide‐1‐(7‐36)amide is transformed to glucagon‐like peptide‐1‐(9‐36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine [in process citation]. Endocrinology, 140, 5356–5363. 10.1210/endo.140.11.7143 [DOI] [PubMed] [Google Scholar]
- Hansen, M. , Hjollund, K. R. , Hartmann, B. , Plamboeck, A. , Deacon, C. F. , Wewer Albrechtsen, N. J. , & Holst, J. J. (2015). Important species differences regarding lymph contribution to gut hormone responses. Peptides, 71, 28–31. 10.1016/j.peptides.2015.05.008 [DOI] [PubMed] [Google Scholar]
- Hayes, M. R. , Kanoski, S. E. , De Jonghe, B. C. , Leichner, T. M. , Alhadeff, A. L. , Fortin, S. M. , Arnold, M. , Langhans, W. , & Grill, H. J. (2011). The common hepatic branch of the vagus is not required to mediate the glycemic and food intake suppressive effects of glucagon‐like‐peptide‐1. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 301, R1479–R1485. 10.1152/ajpregu.00356.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner, K. M. , Kirigiti, M. , Secher, A. , Paulsen, S. J. , Buckingham, R. , Pyke, C. , Knudsen, L. B. , Vrang, N. , & Grove, K. L. (2015). Expression and distribution of glucagon‐like peptide‐1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology, 156, 255–267. 10.1210/en.2014-1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepprich, M. , Antwi, K. , Waser, B. , Reubi, J. C. , Wild, D. , & Christ, E. R. (2020). Brunner's gland hyperplasia in a patient after Roux‐Y gastric bypass: An important pitfall in GLP‐1 receptor imaging. Case Reports in Endocrinology, 2020, 4510910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjollund, K. R. , Deacon, C. F. , & Holst, J. J. (2011). Dipeptidyl peptidase‐4 inhibition increases portal concentrations of intact glucagon‐like peptide‐1 (GLP‐1) to a greater extent than peripheral concentrations in anaesthetised pigs. Diabetologia, 54, 2206–2208. 10.1007/s00125-011-2168-7 [DOI] [PubMed] [Google Scholar]
- Holst, J. J. (1978). Extrapancreatic glucagons. Digestion, 17, 168–190. 10.1159/000198107 [DOI] [PubMed] [Google Scholar]
- Holst, J. J. (1983). Gut glucagon, enteroglucagon, gut glucagonlike immunoreactivity, glicentin—Current status. Gastroenterology, 84, 1602–1613. 10.1016/0016-5085(83)90388-8 [DOI] [PubMed] [Google Scholar]
- Holst, J. J. (2007). The physiology of glucagon‐like peptide 1. Physiological Reviews, 87, 1409–1439. 10.1152/physrev.00034.2006 [DOI] [PubMed] [Google Scholar]
- Holst, J. J. , Aggestrup, S. , Loud, F. B. , & Olesen, M. (1983). Content and gel filtration profiles of glucagon‐like and somatostatin‐like immunoreactivity in human fundic mucosa. The Journal of Clinical Endocrinology and Metabolism, 56, 729–732. 10.1210/jcem-56-4-729 [DOI] [PubMed] [Google Scholar]
- Holst, J. J. , Albrechtsen, N. J. W. , Gabe, M. B. N. , & Rosenkilde, M. M. (2018). Oxyntomodulin: Actions and role in diabetes. Peptides, 100, 48–53. 10.1016/j.peptides.2017.09.018 [DOI] [PubMed] [Google Scholar]
- Holst, J. J. , Albrechtsen, N. J. W. , Rosenkilde, M. M. , & Deacon, C. F. (2019). Physiology of the incretin hormones, GIP and GLP‐1‐regulation of release and posttranslational modifications. Comprehensive Physiology, 9, 1339–1381. 10.1002/cphy.c180013 [DOI] [PubMed] [Google Scholar]
- Holst, J. J. , & Deacon, C. F. (2005). Glucagon‐like peptide‐1 mediates the therapeutic actions of DPP‐IV inhibitors. Diabetologia, 48, 612–615. 10.1007/s00125-005-1705-7 [DOI] [PubMed] [Google Scholar]
- Holst, J. J. , Orskov, C. , Nielsen, O. V. , & Schwartz, T. W. (1987). Truncated glucagon‐like peptide I, an insulin‐releasing hormone from the distal gut. FEBS Letters, 211, 169–174. 10.1016/0014-5793(87)81430-8 [DOI] [PubMed] [Google Scholar]
- Holst, J. J. , Wewer Albrechtsen, N. J. , Rosenkilde, M. M. , & Deacon, C. F. (2019). Physiology of the incretin hormones, GIP and GLP‐1 ‐ regulation of release and post‐translational modifications. [DOI] [PubMed]
- Jejelava, N. , Kaufman, S. , Krieger, J. P. , Terra, M. M. , Langhans, W. , & Arnold, M. (2018). Intestinal lymph as a readout of meal‐induced GLP‐1 release in an unrestrained rat model. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 314, R724–R733. 10.1152/ajpregu.00120.2017 [DOI] [PubMed] [Google Scholar]
- Jensen, C. B. , Pyke, C. , Rasch, M. G. , Dahl, A. B. , Knudsen, L. B. , & Secher, A. (2018). Characterization of the glucagonlike peptide‐1 receptor in male mouse brain using a novel antibody and in situ hybridization. Endocrinology, 159, 665–675. 10.1210/en.2017-00812 [DOI] [PubMed] [Google Scholar]
- Jensen, E. P. , Poulsen, S. S. , Kissow, H. , Holstein‐Rathlou, N. H. , Deacon, C. F. , Jensen, B. L. , Holst, J. J. , & Sorensen, C. M. (2015). Activation of GLP‐1 receptors on vascular smooth muscle cells reduces the autoregulatory response in afferent arterioles and increases renal blood flow. American Journal of Physiology. Renal Physiology, 308, F867–F877. 10.1152/ajprenal.00527.2014 [DOI] [PubMed] [Google Scholar]
- Jepsen, S. L. , Grunddal, K. V. , Wewer Albrechtsen, N. J. , Engelstoft, M. S. , Gabe, M. B. N. , Jensen, E. P. , Orskov, C. , Poulsen, S. S. , Rosenkilde, M. M. , Pedersen, J. , Gribble, F. M. , Reimann, F. , Deacon, C. F. , Schwartz, T. W. , Christ, A. D. , Martin, R. E. , & Holst, J. J. (2019). Paracrine crosstalk between intestinal L‐ and D‐cells controls secretion of glucagon‐like peptide‐1 in mice. American Journal of Physiology. Endocrinology and Metabolism, 317, E1081–E1093. 10.1152/ajpendo.00239.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y. , Dong, H. , Eckmann, L. , Hanson, E. M. , Ihn, K. C. , & Mittal, R. K. (2017). Visualizing the enteric nervous system using genetically engineered double reporter mice: Comparison with immunofluorescence. PLoS ONE, 12, e0171239. 10.1371/journal.pone.0171239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen, R. , Kubale, V. , Vrecl, M. , Schwartz, T. W. , & Elling, C. E. (2007). Oxyntomodulin differentially affects glucagon‐like peptide‐1 receptor beta‐arrestin recruitment and signaling through Galpha(s). The Journal of Pharmacology and Experimental Therapeutics, 322, 148–154. 10.1124/jpet.107.120006 [DOI] [PubMed] [Google Scholar]
- Kirk, R. K. , Pyke, C. , von Herrath, M. G. , Hasselby, J. P. , Pedersen, L. , Mortensen, P. G. , Knudsen, L. B. , & Coppieters, K. (2017). Immunohistochemical assessment of glucagon‐like peptide 1 receptor (GLP‐1R) expression in the pancreas of patients with type 2 diabetes. Diabetes, Obesity & Metabolism, 19, 705–712. 10.1111/dom.12879 [DOI] [PubMed] [Google Scholar]
- Kirkegaard, P. , Skov Olsen, P. , Seier Poulsen, S. , Holst, J. J. , Schaffalitzky De Muckadell, O. B. , & Christiansen, J. (1984). Effect of secretin and glucagon on Brunner's gland secretion in the rat. Gut, 25, 264–268. 10.1136/gut.25.3.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissow, H. , Hartmann, B. , Holst, J. J. , & Poulsen, S. S. (2013). Glucagon‐like peptide‐1 as a treatment for chemotherapy‐induced mucositis. Gut, 62, 1724–1733. 10.1136/gutjnl-2012-303280 [DOI] [PubMed] [Google Scholar]
- Knudsen, J. B. , Holst, J. J. , Asnaes, S. , & Johansen, A. (1975). Identification of cells with pancreatic‐type and gut‐type glucagon immunoreactivity in the human colon. Acta Pathologica et Microbiologica Scandinavica. Section A, 83, 741–743. 10.1111/j.1699-0463.1975.tb01407.x [DOI] [PubMed] [Google Scholar]
- Koehler, J. A. , Baggio, L. L. , Yusta, B. , Longuet, C. , Rowland, K. J. , Cao, X. , Holland, D. , Brubaker, P. L. , & Drucker, D. J. (2015). GLP‐1R agonists promote normal and neoplastic intestinal growth through mechanisms requiring Fgf7. Cell Metabolism, 21, 379–391. 10.1016/j.cmet.2015.02.005 [DOI] [PubMed] [Google Scholar]
- Krause, W. J. (2000). Brunner's glands: A structural, histochemical and pathological profile. Progress in Histochemistry and Cytochemistry, 35, 259–367. [PubMed] [Google Scholar]
- Kreymann, B. , Williams, G. , Ghatei, M. A. , & Bloom, S. R. (1987). Glucagon‐like peptide‐1 7‐36: A physiological incretin in man. Lancet, 2, 1300–1304. 10.1016/s0140-6736(87)91194-9 [DOI] [PubMed] [Google Scholar]
- Kuhre, R. E. , Albrechtsen, N. W. , Windelov, J. A. , Svendsen, B. , Hartmann, B. , & Holst, J. J. (2014). GLP‐1 amidation efficiency along the length of the intestine in mice, rats and pigs and in GLP‐1 secreting cell lines. Peptides, 55, 52–57. 10.1016/j.peptides.2014.01.020 [DOI] [PubMed] [Google Scholar]
- Kumar, D. , Mains, R. E. , & Eipper, B. A. (2016). 60 years of POMC: From POMC and alpha‐MSH to PAM, molecular oxygen, copper, and vitamin C. Journal of Molecular Endocrinology, 56, T63–T76. 10.1530/JME-15-0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, P. J. , Tang‐Christensen, M. , Holst, J. J. , & Orskov, C. (1997). Distribution of glucagon‐like peptide‐1 and other preproglucagon‐derived peptides in the rat hypothalamus and brainstem. Neuroscience, 77(1), 257–70. 10.1016/s0306-4522(96)00434-4 [DOI] [PubMed] [Google Scholar]
- Lu, W. J. , Yang, Q. , Yang, L. , Lee, D. , D'Alessio, D. , & Tso, P. (2012). Chylomicron formation and secretion is required for lipid‐stimulated release of incretins GLP‐1 and GIP. Lipids, 47, 571–580. 10.1007/s11745-011-3650-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund, A. , Bagger, J. I. , Wewer Albrechtsen, N. J. , Christensen, M. , Grondahl, M. , Hartmann, B. , Mathiesen, E. R. , Hansen, C. P. , Storkholm, J. H. , van Hall, G. , Rehfeld, J. F. , Hornburg, D. , Meissner, F. , Mann, M. , Larsen, S. , Holst, J. J. , Vilsboll, T. , & Knop, F. K. (2016). Evidence of extrapancreatic glucagon secretion in man. Diabetes, 65, 585–597. 10.2337/db15-1541 [DOI] [PubMed] [Google Scholar]
- Mari, A. , Sallas, W. M. , He, Y. L. , Watson, C. , Ligueros‐Saylan, M. , Dunning, B. E. , Deacon, C. F. , Holst, J. J. , & Foley, J. E. (2005). Vildagliptin, a dipeptidyl peptidase‐IV inhibitor, improves model‐assessed β‐cell function in patients with type 2 diabetes. The Journal of Clinical Endocrinology and Metabolism, 90, 4888–4894. 10.1210/jc.2004-2460 [DOI] [PubMed] [Google Scholar]
- McLean, B. A. , Wong, C. K. , Campbell, J. E. , Hodson, D. J. , Trapp, S. , & Drucker, D. J. (2021). Revisiting the complexity of GLP‐1 action‐from sites of synthesis to receptor activation. Endocrine Reviews, 42(2), 101–132. 10.1210/endrev/bnaa032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michałowska, J. , Miller‐Kasprzak, E. , & Bogdański, P. (2021). Incretin hormones in obesity and related Cardiometabolic disorders: The clinical perspective. Nutrients, 13(2), 351. 10.3390/nu13020351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody, A. J. , Holst, J. J. , Thim, L. , & Jensen, S. L. (1981). Relationship of glicentin to proglucagon and glucagon in the porcine pancreas. Nature, 289, 514–516. 10.1038/289514a0 [DOI] [PubMed] [Google Scholar]
- Munroe, D. G. , Gupta, A. K. , Kooshesh, F. , Vyas, T. B. , Rizkalla, G. , Wang, H. , Demchyshyn, L. , Yang, Z. J. , Kamboj, R. K. , Chen, H. , McCallum, K. , Sumner‐Smith, M. , Drucker, D. J. , & Crivici, A. (1999). Prototypic G protein‐coupled receptor for the intestinotrophic factor glucagon‐like peptide 2. Proceedings of the National Academy of Sciences of the United States of America, 96, 1569–1573. 10.1073/pnas.96.4.1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabayashi, H. , Nishizawa, M. , Nakagawa, A. , Takeda, R. , & Niijima, A. (1996). Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP‐1. The American Journal of Physiology, 271, E808–E813. 10.1152/ajpendo.1996.271.5.E808 [DOI] [PubMed] [Google Scholar]
- Nakagawa, A. , Satake, H. , Nakabayashi, H. , Nishizawa, M. , Furuya, K. , Nakano, S. , Kigoshi, T. , Nakayama, K. , & Uchida, K. (2004). Receptor gene expression of glucagon‐like peptide‐1, but not glucose‐dependent insulinotropic polypeptide, in rat nodose ganglion cells. Autonomic Neuroscience, 110, 36–43. 10.1016/j.autneu.2003.11.001 [DOI] [PubMed] [Google Scholar]
- Nauck, M. A. , Kemmeries, G. , Holst, J. J. , & Meier, J. J. (2011). Rapid tachyphylaxis of the glucagon‐like peptide 1‐induced deceleration of gastric emptying in humans. Diabetes, 60, 1561–1565. 10.2337/db10-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, M. S. , Ritz, C. , Wewer Albrechtsen, N. J. , Holst, J. J. , Le Roux, C. W. , & Sjodin, A. (2020). Oxyntomodulin and glicentin may predict the effect of bariatric surgery on food preferences and weight loss. The Journal of Clinical Endocrinology and Metabolism, 105, e1064–e1074. 10.1210/clinem/dgaa061 [DOI] [PubMed] [Google Scholar]
- Olesen, M. , Gudmand‐Hoyer, E. , Holst, J. J. , & Jorgensen, S. (1999). Importance of colonic bacterial fermentation in short bowel patients: Small intestinal malabsorption of easily digestible carbohydrate. Digestive Diseases and Sciences, 44, 1914–1923. 10.1023/A:1018819428678 [DOI] [PubMed] [Google Scholar]
- Orci, L. , Pictet, R. , Forssmann, W. G. , Renold, A. E. , & Rouiller, C. (1968). Structural evidence for glucagon producing cells in the intestinal mucosa of the rat. Diabetologia, 4, 56–67. 10.1007/BF01241034 [DOI] [PubMed] [Google Scholar]
- Orskov, C. , & Holst, J. J. (1987). Radio‐immunoassays for glucagon‐like peptides 1 and 2 (GLP‐1 and GLP‐2). Scandinavian Journal of Clinical and Laboratory Investigation, 47, 165–174. 10.3109/00365518709168885 [DOI] [PubMed] [Google Scholar]
- Orskov, C. , Holst, J. J. , Knuhtsen, S. , Baldissera, F. G. , Poulsen, S. S. , & Nielsen, O. V. (1986). Glucagon‐like peptides GLP‐1 and GLP‐2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology, 119, 1467–1475. 10.1210/endo-119-4-1467 [DOI] [PubMed] [Google Scholar]
- Orskov, C. , Holst, J. J. , & Nielsen, O. V. (1988). Effect of truncated glucagon‐like peptide‐1 [proglucagon‐(78‐107) amide] on endocrine secretion from pig pancreas, antrum, and nonantral stomach. Endocrinology, 123, 2009–2013. 10.1210/endo-123-4-2009 [DOI] [PubMed] [Google Scholar]
- Orskov, C. , & Poulsen, S. S. (1991). Glucagonlike peptide‐I‐(7‐36)‐amide receptors only in islets of Langerhans. Autoradiographic survey of extracerebral tissues in rats. Diabetes, 40, 1292–1296. 10.2337/diab.40.10.1292 [DOI] [PubMed] [Google Scholar]
- Orskov, C. , Wettergren, A. , & Holst, J. J. (1993). Biological effects and metabolic rates of glucagonlike peptide‐1 7‐36 amide and glucagonlike peptide‐1 7‐37 in healthy subjects are indistinguishable. Diabetes, 42, 658–661. 10.2337/diab.42.5.658 [DOI] [PubMed] [Google Scholar]
- Palnaes, H. C. , Andreasen, J. J. , & Holst, J. J. (1997). The release of gastric inhibitory peptide, glucagon‐like peptide‐I, and insulin after oral glucose test in colectomized subjects. Scandinavian Journal of Gastroenterology, 32, 473–477. [DOI] [PubMed] [Google Scholar]
- Patzelt, C. , & Schug, G. (1981). The major proglucagon fragment: An abundant islet protein and secretory product. FEBS Letters, 129, 127–130. 10.1016/0014-5793(81)80772-7 [DOI] [PubMed] [Google Scholar]
- Pedersen, J. , Pedersen, N. B. , Brix, S. W. , Grunddal, K. V. , Rosenkilde, M. M. , Hartmann, B. , Orskov, C. , Poulsen, S. S. , & Holst, J. J. (2015). The glucagon‐like peptide 2 receptor is expressed in enteric neurons and not in the epithelium of the intestine. Peptides, 67, 20–28. 10.1016/j.peptides.2015.02.007 [DOI] [PubMed] [Google Scholar]
- Pedersen, N. B. , Hjollund, K. R. , Johnsen, A. H. , Orskov, C. , Rosenkilde, M. M. , Hartmann, B. , & Holst, J. J. (2008). Porcine glucagon‐like peptide‐2: Structure, signaling, metabolism and effects. Regulatory Peptides, 146, 310–320. 10.1016/j.regpep.2007.11.003 [DOI] [PubMed] [Google Scholar]
- Plamboeck, A. , Veedfald, S. , Deacon, C. F. , Hartmann, B. , Wettergren, A. , Svendsen, L. B. , Meisner, S. , Hovendal, C. , Vilsboll, T. , Knop, F. K. , & Holst, J. J. (2013). The effect of exogenous GLP‐1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. American Journal of Physiology. Gastrointestinal and Liver Physiology, 304, G1117–G1127. 10.1152/ajpgi.00035.2013 [DOI] [PubMed] [Google Scholar]
- Pridal, L. , Deacon, C. F. , Kirk, O. , Christensen, J. V. , Carr, R. D. , & Holst, J. J. (1996). Glucagon‐like peptide‐1(7‐37) has a larger volume of distribution than glucagon‐like peptide‐1(7‐36)amide in dogs and is degraded more quickly in vitro by dog plasma. European Journal of Drug Metabolism and Pharmacokinetics, 21, 51–59. 10.1007/BF03190278 [DOI] [PubMed] [Google Scholar]
- Pyke, C. , Heller, R. S. , Kirk, R. K. , Orskov, C. , Reedtz‐Runge, S. , Kaastrup, P. , Hvelplund, A. , Bardram, L. , Calatayud, D. , & Knudsen, L. B. (2014). GLP‐1 receptor localization in monkey and human tissue: Novel distribution revealed with extensively validated monoclonal antibody. Endocrinology, 155, 1280–1290. 10.1210/en.2013-1934 [DOI] [PubMed] [Google Scholar]
- Pyke, C. , & Knudsen, L. B. (2013). The glucagon‐like peptide‐1 receptor—Or not? Endocrinology, 154, 4–8. 10.1210/en.2012-2124 [DOI] [PubMed] [Google Scholar]
- Qu, Z. D. , Thacker, M. , Castelucci, P. , Bagyanszki, M. , Epstein, M. L. , & Furness, J. B. (2008). Immunohistochemical analysis of neuron types in the mouse small intestine. Cell and Tissue Research, 334, 147–161. 10.1007/s00441-008-0684-7 [DOI] [PubMed] [Google Scholar]
- Ravazzola, M. , Siperstein, A. , Moody, A. J. , Sundby, F. , Jacobsen, H. , & Orci, L. (1979). Glicentin immunoreactive cells: Their relationship to glucagon‐ producing cells. Endocrinology, 105, 499–508. 10.1210/endo-105-2-499 [DOI] [PubMed] [Google Scholar]
- Raybould, H. E. , & Holzer, H. (1993). Secretin inhibits gastric emptying in rats via a capsaicin‐sensitive vagal afferent pathway. European Journal of Pharmacology, 250, 165–167. 10.1016/0014-2999(93)90636-V [DOI] [PubMed] [Google Scholar]
- Reimann, F. , Habib, A. M. , Tolhurst, G. , Parker, H. E. , Rogers, G. J. , & Gribble, F. M. (2008). Glucose sensing in L cells: A primary cell study. Cell Metabolism, 8, 532–539. 10.1016/j.cmet.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, P. , Parker, H. E. , Adriaenssens, A. E. , Hodgson, J. M. , Cork, S. C. , Trapp, S. , Gribble, F. M. , & Reimann, F. (2014). Identification and characterization of GLP‐1 receptor‐expressing cells using a new transgenic mouse model. Diabetes, 63, 1224–1233. 10.2337/db13-1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouille, Y. , Martin, S. , & Steiner, D. F. (1995). Differential processing of proglucagon by the subtilisin‐like prohormone convertases PC2 and PC3 to generate either glucagon or glucagon‐like peptide. The Journal of Biological Chemistry, 270, 26488–26496. 10.1074/jbc.270.44.26488 [DOI] [PubMed] [Google Scholar]
- Schirra, J. , Sturm, K. , Leicht, P. , Arnold, R. , Goke, B. , & Katschinski, M. (1998). Exendin(9‐39)amide is an antagonist of glucagon‐like peptide‐1(7‐36)amide in humans. The Journal of Clinical Investigation, 101, 1421–1430. 10.1172/JCI1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtler, J. , Dehne, K. , Offermanns, S. , Rosenthal, W. , Classen, M. , & Schepp, W. (1994). Stimulation of rat parietal cell function by histamine and GLP‐1‐(7‐36) amide is mediated by Gs alpha. The American Journal of Physiology, 266, G775–G782. 10.1152/ajpgi.1994.266.5.G775 [DOI] [PubMed] [Google Scholar]
- Secher, A. , Jelsing, J. , Baquero, A. F. , Hecksher‐Sorensen, J. , Cowley, M. A. , Dalboge, L. S. , Hansen, G. , Grove, K. L. , Pyke, C. , Raun, K. , Schaffer, L. , Tang‐Christensen, M. , Verma, S. , Witgen, B. M. , Vrang, N. , & Bjerre, K. L. (2014). The arcuate nucleus mediates GLP‐1 receptor agonist liraglutide‐dependent weight loss. The Journal of Clinical Investigation, 124, 4473–4488. 10.1172/JCI75276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisley, S. , Gutierrez‐Aguilar, R. , Scott, M. , D'Alessio, D. A. , Sandoval, D. A. , & Seeley, R. J. (2014). Neuronal GLP1R mediates liraglutide's anorectic but not glucose‐lowering effect. The Journal of Clinical Investigation, 124, 2456–2463. 10.1172/JCI72434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits, M. M. , Fluitman, K. S. , Herrema, H. , Davids, M. , Kramer, M. H. H. , Groen, A. K. , Belzer, C. , de Vos, W. M. , Cahen, D. L. , Nieuwdorp, M. , & van Raalte, D. H. (2021). Liraglutide and sitagliptin have no effect on intestinal microbiota composition: A 12‐week randomized placebo‐controlled trial in adults with type 2 diabetes. Diabetes & Metabolism, 47, 101223. 10.1016/j.diabet.2021.101223 [DOI] [PubMed] [Google Scholar]
- Solcia, E. , Capella, C. , Buffa, R. , Usellini, L. , Fiocca, R. , Frigerio, B. , Tenti, P. , & Sessa, F. (1981). The diffuse endocrine‐paracrine system of the gut in health and disease: Ultrastructural features. Scandinavian Journal of Gastroenterology. Supplement, 70, 25–36. [PubMed] [Google Scholar]
- Sutherland, E. W. , & De Duve, C. (1948). Origin and distribution of the hyperglycemic‐glycogenolytic factor of the pancreas. The Journal of Biological Chemistry, 175, 663–674. 10.1016/S0021-9258(18)57183-0 [DOI] [PubMed] [Google Scholar]
- Svane, M. S. , Jorgensen, N. B. , Bojsen‐Moller, K. N. , Dirksen, C. , Nielsen, S. , Kristiansen, V. B. , Torang, S. , Wewer Albrechtsen, N. J. , Rehfeld, J. F. , Hartmann, B. , Madsbad, S. , & Holst, J. J. (2016). Peptide YY and glucagon‐like peptide‐1 contribute to decreased food intake after Roux‐en‐Y gastric bypass surgery. International Journal of Obesity, 40, 1699–1706. 10.1038/ijo.2016.121 [DOI] [PubMed] [Google Scholar]
- Svendsen, B. , Pedersen, J. , Albrechtsen, N. J. , Hartmann, B. , Torang, S. , Rehfeld, J. F. , Poulsen, S. S. , & Holst, J. J. (2015). An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology, 156, 847–857. 10.1210/en.2014-1710 [DOI] [PubMed] [Google Scholar]
- Thorens, B. (1992). Expression cloning of the pancreatic beta cell receptor for the gluco‐ incretin hormone glucagon‐like peptide 1. Proceedings of the National Academy of Sciences of the United States of America, 89, 8641–8645. 10.1073/pnas.89.18.8641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulesen, J. , Hartmann, B. , Kissow, H. , Jeppesen, P. B. , Orskov, C. , Holst, J. J. , & Poulsen, S. S. (2001). Intestinal growth adaptation and glucagon‐like peptide 2 in rats with ileal–jejunal transposition or small bowel resection. Digestive Diseases and Sciences, 46, 379–388. 10.1023/A:1005572832571 [DOI] [PubMed] [Google Scholar]
- Tolessa, T. , Gutniak, M. , Holst, J. J. , Efendic, S. , & Hellstrom, P. M. (1998a). Glucagon‐like peptide‐1 retards gastric emptying and small bowel transit in the rat: Effect mediated through central or enteric nervous mechanisms. Digestive Diseases and Sciences, 43, 2284–2290. 10.1023/A:1026678925120 [DOI] [PubMed] [Google Scholar]
- Tolessa, T. , Gutniak, M. , Holst, J. J. , Efendic, S. , & Hellstrom, P. M. (1998b). Inhibitory effect of glucagon‐like peptide‐1 on small bowel motility. Fasting but not fed motility inhibited via nitric oxide independently of insulin and somatostatin. The Journal of Clinical Investigation, 102, 764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger, R. H. , Eisentraut, A. M. , McCall, M. S. , Keller, S. , Lanz, H. C. , & Madison, L. L. (1959). Glucagon antibodies and their use for immunoassay for glucagon. Proceedings of the Society for Experimental Biology and Medicine, 102, 621–623. 10.3181/00379727-102-25338 [DOI] [PubMed] [Google Scholar]
- Unger, R. H. , Ketterer, H. , & Eisentraut, A. M. (1966). Distribution of immunoassayable glucagon in gastrointestinal tissues. Metabolism, 15, 865–867. 10.1016/0026-0495(66)90156-9 [DOI] [PubMed] [Google Scholar]
- Unger, R. H. , Ohneda, A. , Valverde, I. , Eisentraut, A. M. , & Exton, J. (1968). Characterization of the responses of circulating glucagon‐like immunoreactivity to intraduodenal and intravenous administration of glucose. The Journal of Clinical Investigation, 47, 48–65. 10.1172/JCI105714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Beek, C. M. , Canfora, E. E. , Lenaerts, K. , Troost, F. J. , Olde Damink, S. W. M. , Holst, J. J. , Masclee, A. A. , Dejong, C. H. , & Blaak, E. E. (2016). Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clinical Science (London, England), 130, 2073–2082. 10.1042/CS20160263 [DOI] [PubMed] [Google Scholar]
- Varin, E. M. , Mulvihill, E. E. , Baggio, L. L. , Koehler, J. A. , Cao, X. , Seeley, R. J. , & Drucker, D. J. (2019). Distinct neural sites of GLP‐1R expression mediate physiological versus pharmacological control of Incretin action. Cell Reports, 27, 3371–3384. 10.1016/j.celrep.2019.05.055 [DOI] [PubMed] [Google Scholar]
- Vilsboll, T. , Agerso, H. , Krarup, T. , & Holst, J. J. (2003). Similar elimination rates of glucagon‐like peptide‐1 in obese type 2 diabetic patients and healthy subjects. The Journal of Clinical Endocrinology and Metabolism, 88, 220–224. 10.1210/jc.2002-021053 [DOI] [PubMed] [Google Scholar]
- Wei, Y. , & Mojsov, S. (1995). Tissue‐specific expression of the human receptor for glucagon‐ like peptide‐I: Brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Letters, 358, 219–224. 10.1016/0014-5793(94)01430-9 [DOI] [PubMed] [Google Scholar]
- Wei, Y. , & Mojsov, S. (1996). Distribution of GLP‐1 and PACAP receptors in human tissues. Acta Physiologica Scandinavica, 157, 355–357. 10.1046/j.1365-201X.1996.42256000.x [DOI] [PubMed] [Google Scholar]
- Wettergren, A. , Petersen, H. , Orskov, C. , Christiansen, J. , Sheikh, S. P. , & Holst, J. J. (1994). Glucagon‐like peptide‐1 7‐36 amide and peptide YY from the L‐cell of the ileal mucosa are potent inhibitors of vagally induced gastric acid secretion in man. Scandinavian Journal of Gastroenterology, 29, 501–505. 10.3109/00365529409092462 [DOI] [PubMed] [Google Scholar]
- Wettergren, A. , Pridal, L. , Wojdemann, M. , & Holst, J. J. (1998). Amidated and non‐amidated glucagon‐like peptide‐1 (GLP‐1): Non‐pancreatic effects (cephalic phase acid secretion) and stability in plasma in humans. Regulatory Peptides, 77, 83–87. 10.1016/S0167-0115(98)00044-5 [DOI] [PubMed] [Google Scholar]
- Wettergren, A. , Schjoldager, B. , Mortensen, P. E. , Myhre, J. , Christiansen, J. , & Holst, J. J. (1993). Truncated GLP‐1 (proglucagon 78‐107‐amide) inhibits gastric and pancreatic functions in man. Digestive Diseases and Sciences, 38, 665–673. 10.1007/BF01316798 [DOI] [PubMed] [Google Scholar]
- Wettergren, A. , Wojdemann, M. , & Holst, J. J. (1998). Glucagon‐like peptide‐1 inhibits gastropancreatic function by inhibiting central parasympathetic outflow. The American Journal of Physiology, 275, G984–G992. 10.1152/ajpgi.1998.275.5.G984 [DOI] [PubMed] [Google Scholar]
- Wettergren, A. , Wojdemann, M. , Meisner, S. , Stadil, F. , & Holst, J. J. (1997). The inhibitory effect of glucagon‐like peptide‐1 (GLP‐1) 7‐36 amide on gastric acid secretion in humans depends on an intact vagal innervation. Gut, 40, 597–601. 10.1136/gut.40.5.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann, C. , Dolci, W. , & Thorens, B. (1995). Agonist‐induced internalization and recycling of the glucagon‐ like peptide‐1 receptor in transfected fibroblasts and in insulinomas. The Biochemical Journal, 310, 203–214. 10.1042/bj3100203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, E. K. , Chang, R. B. , Strochlic, D. E. , Umans, B. D. , Lowell, B. B. , & Liberles, S. D. (2016). Sensory neurons that detect stretch and nutrients in the digestive system. Cell, 166, 209–221. 10.1016/j.cell.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willms, B. , Werner, J. , Holst, J. J. , Orskov, C. , Creutzfeldt, W. , & Nauck, M. A. (1996). Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: Effects of exogenous glucagon‐like peptide‐1 (GLP‐1)‐ (7‐36) amide in type 2 (noninsulin‐dependent) diabetic patients. The Journal of Clinical Endocrinology and Metabolism, 81, 327–332. 10.1210/jcem.81.1.8550773 [DOI] [PubMed] [Google Scholar]
- Wismann, P. , Barkholt, P. , Secher, T. , Vrang, N. , Hansen, H. B. , Jeppesen, P. B. , Baggio, L. L. , Koehler, J. A. , Drucker, D. J. , Sandoval, D. A. , & Jelsing, J. (2017). The endogenous preproglucagon system is not essential for gut growth homeostasis in mice. Molecular Metabolism, 6, 681–692. 10.1016/j.molmet.2017.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, L. , Tang, Q. , Quan, X. , Ren, H. , Chen, W. , Xia, H. , & Luo, H. (2019). Effects of exendin‐4 on colonic motility in rats and its underlying mechanism. Neurogastroenterology and Motility, 31, e13482. 10.1111/nmo.13482 [DOI] [PubMed] [Google Scholar]
- Yusta, B. , Baggio, L. L. , Koehler, J. , Holland, D. , Cao, X. , Pinnell, L. J. , Johnson‐Henry, K. C. , Yeung, W. , Surette, M. G. , Bang, K. W. , Sherman, P. M. , & Drucker, D. J. (2015). GLP‐1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP‐1R. Diabetes, 64, 2537–2549. 10.2337/db14-1577 [DOI] [PubMed] [Google Scholar]
- Yusta, B. , Matthews, D. , Koehler, J. A. , Pujadas, G. , Kaur, K. D. , & Drucker, D. J. (2019). Localization of glucagon‐like peptide‐2 receptor expression in the mouse. Endocrinology, 160, 1950–1963. 10.1210/en.2019-00398 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no unpublished data in this article.


