Abstract
Introduction
Obesity is a global health challenge, and pharmacologic options are emerging. Once daily subcutaneous administration of 3 mg liraglutide, a glucagon like peptide-1 analogue, has been shown to induce weight loss in clinical trials, but real-world effectiveness data are scarce.
Methods
It is a single-centre retrospective cohort study of patients who were prescribed liraglutide on top of lifestyle adaptations after multidisciplinary evaluation. In Belgium, liraglutide is only indicated for weight management if the BMI is >30 kg/m<sup>2</sup> or ≥27 kg/m<sup>2</sup> with comorbidities such as dysglycaemia, dyslipidaemia, hypertension, or obstructive sleep apnoea. No indication is covered by the compulsory health care insurance. Liraglutide was started at 0.6 mg/day and uptitrated weekly until 3 mg/day or the maximum tolerated dose. Treatment status and body weight were evaluated at the 4-month routine visit.
Results
Between June 2016 and January 2020, liraglutide was prescribed to 115 patients (77% female), with a median age of 47 (IQR 37.7–54.0) years, a median body weight of 98.4 (IQR 90.0–112.2) kg, a BMI of 34.8 (IQR 32.2–37.4) kg/m<sup>2</sup>, and an HbA1c level of 5.6%. Five (4%) patients did not actually initiate treatment, 9 (8%) stopped treatment, and 8 (7%) were lost to follow-up. At the 4-month visit, the median body weight had decreased significantly by 9.2% to 90.8 (IQR 82.0–103.5) kg (p < 0.001). Patients using 3.0 mg/day (n = 60) had lost 8.0 (IQR 5.8–10.4) kg. The weight loss was similar (p = 0.9622) in patients that used a lower daily dose because of intolerance: 7.4 (IQR 6.2–9.6) kg for 1.2 mg (n = 3), 7.8 (IQR 4.1–7.8) kg for 1.8 mg (n = 16), and 9.0 (IQR 4.8–10.7) kg for 2.4 mg/day (n = 14). Weight loss was minimal if liraglutide treatment was not started or stopped prematurely (median 3.0 [IQR 0.3–4.8] kg, p < 0.001, vs. on treatment). Further analysis showed an additional weight reduction of 1.8 kg in the patients that had started metformin <3 months before the start of liraglutide (p < 0.001). The main reasons for liraglutide discontinuation were gastrointestinal complaints (n = 5/9) and drug cost (n = 2/9).
Conclusion
In this selected group of patients, the majority complied with liraglutide treatment over the initial 4-month period and achieved a significant weight loss, irrespective of the maximally tolerated maintenance dose. Addition of metformin induced a small but significant additional weight loss.
Keywords: Weight reduction, Obesity, Liraglutide, Metformin
Introduction
Obesity is a growing global health challenge with few pharmacologic options. According to the 2018 Belgian Health Survey, 15.9% of the Belgian population was living with obesity (defined as BMI ≥ 30 kg/m2), and the prevalence increased with age [1]. Since 1997, the average BMI of the adult Belgian person has increased from 24.7 to 25.7 kg/m2. Given the plethora of comorbidities of overweight and obesity, such as type 2 diabetes (T2D), hyperlipidaemia, cardiovascular disease, and depression, the high prevalence has a significant impact on quality of life, life expectancy, and health care budget [2].
Despite the high costs related to obesity, the health care expenses for prevention and weight management therapy are limited. First-line therapy for obesity is intensive life style adaptation with reduced caloric intake and increased physical activity [3]. As in many countries, reimbursement for dietary follow-up, psychological consultation, or pharmacotherapy for weight management is minimal in Belgium [4]. Bariatric surgery is currently the only reimbursed treatment option for a select subgroup of patients with severe and complicated obesity [5].
However, evidence is now emerging for a new era of pharmacotherapy to support weight management. Recently, the first results from clinical trial programmes with several molecules including semaglutide [6] and tirzepatide [7] have shown the potential to induce >10% weight loss in the majority of patients and up to 15–20% in the best responders. The European Medicines Agency (EMA) approved the combination naltrexone/bupropion for weight loss in 2015, based on studies showing a 5–6% weight loss in patients with obesity or complicated overweight, with or without T2D [8, 9, 10, 11]. However, market access for Belgium was only obtained in 2020. Before that, orlistat and liraglutide had been the only available options on the Belgian market to support medical weight loss. With orlistat, a 3.0% weight loss benefit was seen compared to placebo, but at the cost of gastrointestinal side effects [12]. In a double-blind clinical trial of 3,731 patients without T2D but a BMI ≥30 or a BMI ≥27 and dyslipidaemia or hypertension, patients using liraglutide 3 mg per day on top of lifestyle advice lost on average 8.0% of their body weight after 56 weeks, which was 5.3% more than the placebo group [13]. Furthermore, the SCALE clinical trial programme showed benefit of liraglutide treatment specifically in patients with T2D [11] or obstructive sleep apnoea [14]. To examine the effectiveness, tolerance, and adherence to liraglutide treatment in the real world, we retrospectively analysed all patients that were advised to start liraglutide treatment after multidisciplinary evaluation.
Methods
Study Population
In this retrospective study, we analysed adult patients who visited the Obesity Clinic UZ Leuven for weight management between 21 June 2016 and 1 January 2020 and who were prescribed liraglutide on top of lifestyle modifications after multidisciplinary evaluation by a dietician, psychologist, and endocrinologist. Liraglutide was prescribed according to the approved label in Europe: BMI ≥30 kg/m2 or BMI ≥27 kg/m2 when associated with weight-related comorbidities as dysglycaemia, hypertension, dyslipidaemia, or obstructive sleep apnoea. None of these indications is covered by the compulsory health care insurance in Belgium. Liraglutide was started at 0.6 mg/day and uptitrated with 0.6 mg every week until 3 mg/day or the maximum tolerated dose. The escalation scheme was explained explicitly during the consultation, and printed instructions were provided. Concurrent metformin was allowed and continued in case of T2D, but also in patients with off-label metformin use in case of significant insulin resistance (defined as HOMA-2-IR ≥1.7) or polycystic ovarian syndrome (PCOS) [15]. T2D was defined by an HbA1c >6.5% in the past and still taking 1 or more antidiabetic drugs. All patients received lifestyle recommendations including individualized advice for healthy food diet and physical activity.
Assessments
Body weight change and treatment status at the 4-month follow-up visit were the primary outcomes. Other parameters that were evaluated were the maximally tolerated liraglutide dose, therapy adherence, reasons of stopping the treatment, and adverse effects. We performed a subgroup analysis evaluating the effect of metformin intake on weight reduction in patients using liraglutide.
Data Management and Ethical Approval
The data from baseline visit and 4-month follow-up were retrieved from the structured electronic medical record and pseudonymized for statistical analysis. All procedures were approved by the local ethics committee (EC UZ Leuven, MP 014935) and the latest version of the Declaration of Helsinki.
Statistics
Categorical variables are presented as n (%). Continuous variables are described by median and interquartile range (IQR). The intergroup differences were tested using Student's t test for normally distributed data and the Mann-Whitney U test for non-normally distributed data. Proportions were compared using χ2 test or Fischer's exact test, where appropriate. A 1-way ANOVA or Kruskal-Wallis test was used to compare multiple groups. All analyses were performed using SPSS (IBM, version 26), and p values <0.05 were considered statistically significant.
Results
Patient Characteristics and Treatment Adherence
Between June 2016 and January 2020, a total of 115 patients were advised to start liraglutide at the UZ Leuven obesity clinic. Patient characteristics are summarized in Table 1. The patients were mostly female (88/115 [77%]), had a median age of 47.0 years (IQR 37.7–54.0), and a median body weight of 98.4 (IQR 90.0–112.2) kg or a BMI of 34.8 (IQR 32.2–37.4) kg/m2. Nine (8%) patients had been diagnosed with T2D, the median HbA1c was 5.6 (IQR 5.3–5.8), and 65 (57%) patients were taking metformin (Table 1). Metformin was used for treatment for T2D (9 patients), but also in patients with significant insulin resistance (43 patients) or PCOS (3 patients).
Table 1.
Patient characteristics at baseline
| Characteristic | Study group n = 115 |
|---|---|
| Age, years | 47.0 (37.7–54.0) |
| Female sex | 88 (77) |
| Weight, kg | 98.4 (90.0–112.2) |
| BMI, kg/m2 | 34.8 (32.2–37.4) |
| HbA1c, % | 5.6 (5.3–5.8) |
| Diabetes type 2 | 9 (8) |
| Metformin intake | 65 (57) |
Values are expressed as median and interquartile range (IQR) deviation for continuous variables or as n (%) for binary variables. HbA1c was available in 102/115 patients. BMI, body mass index.
At the 4-month follow-up visit, 93 (80.9%) patients were still taking liraglutide (Table 2). Five (4.3%) patients had never started the treatment after the prescription, and 9 (7.8%) patients had discontinued the treatment prematurely. The remaining 8 patients (7.0%) were lost to follow-up. The majority of patients (60/93 [65%]) were using the maximal dose of 3.0 mg liraglutide daily, while 14 (15%) patients were using 2.4 mg, 16 (17%) patients 1.8 mg, and 3 (3%) patients remained at a dose of 1.2 mg per day.
Table 2.
Reasons for not reaching maximum liraglutide dose or not starting treatment
| Saxenda submaximal dose | 33/107 | 30 |
| Gastrointestinal complaints | 16 | 15.0 |
| Sufficient effect of current dose | 12 | 11.2 |
| Headache | 2 | 1.9 |
| Palpitations | 2 | 1.9 |
| Injection site complaints | 1 | 0.9 |
| Saxenda stopped | 9/107 | 8.4 |
| No motivation | 1 | 0.9 |
| Pregnancy | 1 | 0.9 |
| Drug cost* | 3 | 2.8 |
| Gastrointestinal complaints* | 5 | 4.7 |
| Insufficient effect | 1 | 0.9 |
| Saxenda not started | 5/107 | 4.7 |
| Drug cost | 1 | 0.9 |
| Did not understand | 1 | 0.9 |
| Unknown | 3 | 2.8 |
Values are expressed as n (%).
One patient stopped liraglutide intake because of a combination of cost and diarrhoea. Eight patients (7.0%) were lost to follow-up.
Body Weight Evaluation
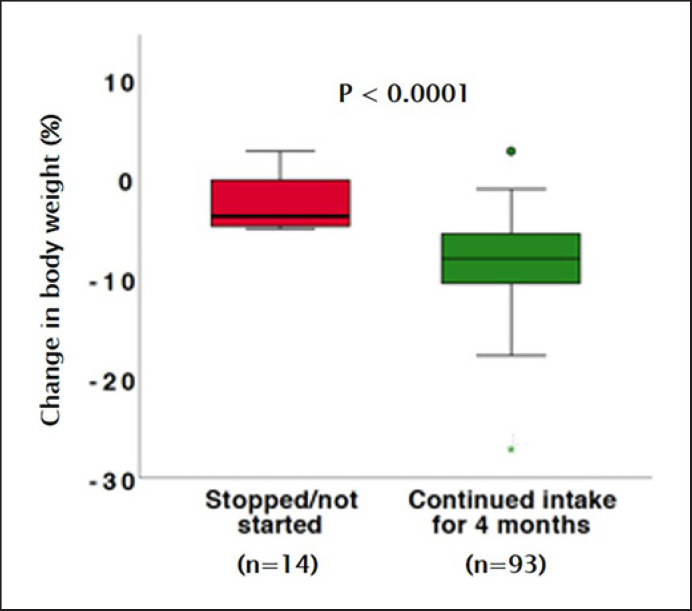
At the 4-month visit, the median body weight had decreased by a significant 9.2%, from 98.4 (IQR 89.9–112.2) kg to 90.8 (IQR 82.0–103.5) kg (p < 0.001) in the total study group (in-trial analysis). This translates to a significant decrease in BMI from 34.7 (IQR 32.2–37.4) kg/m2 to 32.2 (IQR 29.6–34.4) kg/m2. The patients who had not started liraglutide treatment, or had stopped before the follow-up visit, had significantly less weight loss than those on active treatment (median 3.0 [IQR 0.3–4.8] kg weight loss, p < 0.001, vs. on treatment) (Fig. 1).
Fig. 1.
Change in body weight and body mass index, 4 months after the start of liraglutide (green, n = 93) and in patients who stopped or did not start liraglutide (red, n = 10/14 patients). Eight patients were lost to follow-up and are not included in the figure.
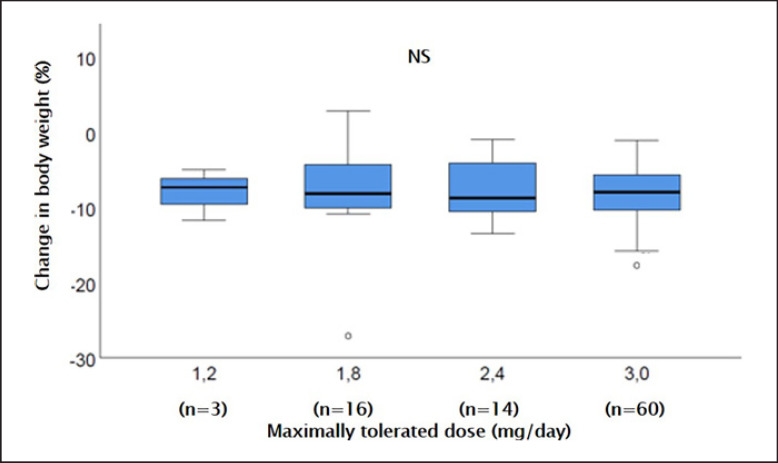
Patients that were using the prescribed dose of 3.0 mg daily (n = 60) had lost 8.0 (IQR 5.8–10.4) kg. The weight loss was similar (Kruskal-Wallis, p = 0.9622) in patients that had reduced their daily liraglutide dose because of intolerance: 7.4 (IQR 6.2–9.6) kg weight loss for 1.2 mg (n = 3), 7.8 (IQR 4.1–7.8) kg for 1.8 mg (n = 16), and 9.0 (IQR 4.8–10.7) kg for 2.4 mg (n = 14) (Fig. 2). In only 12 out of 115 patients, HbA1c was measured at the 4-month visit. In those patients, the median HbA1c had dropped to 5.3 (IQR 5.0–5.7) %.
Fig. 2.
Change in body weight after 4 months of treatment with liraglutide, according to the used liraglutide maintenance dose. The maximum dose reached in an individual patient was determined by side effects in the majority of patients (n = 19), but could also be influenced by sufficient experienced weight loss (n = 12) or injection site complaints (n = 1).
Effect of Metformin on Top of Liraglutide Intake
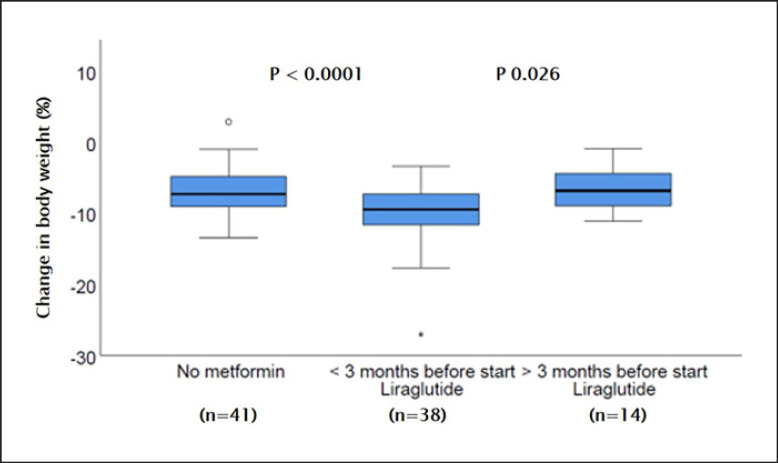
In total, 65/115 (57%) patients were co-treated with metformin. Of the 93 patients who were actively using liraglutide on the 4-month follow-up visit, 38 (41%) patients had been prescribed metformin <3 months before initiation of liraglutide. Fourteen (15%) patients had been taking metformin long term (>3 months) before starting liraglutide. The weight reduction was highest in the subgroup started on metformin <3 months before start of liraglutide (median 9.2 [IQR 7.1–11.7] kg), which was significantly more than in patients without metformin (median 7.4 [IQR 4.8–9.2] kg, p < 0.001) and patients who were already on metformin for >3 months before start of liraglutide (median 6.9 [IQR 4.3–9.4] kg, p < 0.026) (Fig. 3).
Fig. 3.
Change in body weight after 4 months of treatment with liraglutide, according to intake of metformin.
Treatment Tolerance and Adverse Events
Thirty-three patients did not support the prescribed dose of 3.0 mg liraglutide daily, mainly because of side effects. 16/107 (15%) patients complained about gastrointestinal issues such as nausea, vomiting, and diarrhoea, 2 (2%) patients reported headaches under higher doses of liraglutide, 2 (2%) patients reported palpitations, and 1 patient had complications at the place of injection. Twelve (12%) patients already achieved satisfactory results at lower dose and did not further increase. Gastrointestinal discomfort was the main reason for discontinuation of liraglutide (5/9 patients that stopped), followed by treatment cost (3 patients), pregnancy (1 patient), and insufficient effect or lack of motivation (2 patients). The 5 patients that did not initiate treatment reported cost (1 patient), unclear instructions (1 patients), or unknown reasons (3 patients) (Table 2).
Discussion
This study confirms the clinical effectiveness of liraglutide for weight reduction in patients with obesity in combination with lifestyle modifications in a specialized obesity centre in Belgium. The majority of patients (81%) complied with liraglutide treatment over the initial 4-month period of follow-up. Although only 60 out of the 93 patients (64.5%) were able to uptitrate to the proposed maintenance dose of 3.0 mg of liraglutide per day, patients at lower doses achieved similar weight loss. Addition of metformin had a small but significant additional effect during this short-term follow-up. Overall, these real-world data confirm the effectiveness and value of liraglutide for weight management.
Liraglutide is a GLP-1 analogue that has been used successfully for glycaemic control in T2D with a daily subcutaneous injection of 1.2–1.8 mg [16]. The higher dose of 3.0 mg liraglutide daily for 56 weeks resulted in 6.0% weight loss among patients with T2D and overweight or obesity [11], which is probably mediated by reduced appetite and energy intake [17]. In a large randomized controlled trial of patients with obesity or prediabetes and an average BMI of 38.3 kg/m2, 3.0 mg liraglutide daily induced an 8.0% weight loss after 1 year when given on top of lifestyle modifications [13]. While no statistical analysis of 4-month treatment was presented in that study, the graphs show that the weight loss was in the range of 6–8% from baseline after 16 weeks. The population in this study was very similar to the SCALE obesity and prediabetes trial in terms of age (45 years), sex (77% female), and starting body weight (BMI 34.8 kg/m2). As such, the average weight loss of 7% in the current real-life study was very similar to the clinical trials, similar to real-life data from Canada [18], and better than a recent real-life study from Korea where liraglutide without intensive lifestyle intervention only yielded 3.5% weight loss [19]. With 81% continuation of the drug at 4 months, the attrition was low in our study compared to the Canadian (67%) and Korean (56%) studies [18, 19], but higher than in the clinical trial setting [13].
In contrast to the clinical trials where the dose of liraglutide was uptitrated to 3.0 mg daily, in this study, we evaluated the effects of liraglutide by the maximally tolerated dose. Interestingly, we observed that the total weight loss was comparable in the individuals that used submaximal doses, which is not what we expected based on earlier dose-response observations [20]. The retrospective nature of the study does not allow to interpret the underlying mechanism for this observation. It could indicate an individual sensitivity to GLP-1 analogues as has been proposed [21], but it could also be influenced by financial considerations or tolerability as 12 (11%) patients were on a submaximal dose because of sufficient effect and further increasing the dose of liraglutide comes with increasing cost and 1 patient (0.9%) because of injection site complaints.
Indeed, previous studies have stipulated that GLP-1 associated side effects are usually transient, but dose dependent and hence the uptitration scheme for therapy initiation [22]. In our study, liraglutide was generally well tolerated, but the proposed dose of 3.0 mg per day was reached in only 65% of patients. The main reasons to stop liraglutide were gastrointestinal complaints such as nausea, vomiting, and diarrhoea in 5% of patients, comparable to other studies where 6% of patients stopped the treatment with liraglutide because of these complaints [13].
Another major reason not to start liraglutide or stop prematurely with this treatment was the drug cost. In Belgium, liraglutide was not covered by the compulsory health care insurance (throughout the study period). One month of liraglutide 3.0 mg cost about 250 EUR, which is considered a lot by most people living in Belgium.
While our study shows the real-life effectiveness of liraglutide for weight management, it is important to consider patient selection and motivation in the current study. First of all, only patients that were assessed by the multidisciplinary obesity clinic team and considered good candidates for medical therapy were included in this study. Therefore, our result might not be transferrable to a practice where there is no dietary and psychological evaluation to complement the physicians' judgement. Furthermore, patients that were considered good candidates for metabolic surgery − with BMI >40 kg/m2 or BMI >35 kg/m2 with comorbidities − might have been offered surgery preferentially given its proven benefit, and the fact that it is covered by the health insurance in those cases. Finally, the patients' preference regarding injectable therapy and financial aspects of the treatment might have biased the study outcomes in a positive way. Nonetheless, the current study shows the potential of medical therapy in that subgroup of patients that needs additional intervention on top of life style adaptations, but does not qualify for metabolic surgery.
In addition, this study suggests that the combination of metformin and liraglutide treatment might further increase the effect on weight reduction. Interestingly, we did only see that benefit in patients who had started metformin in the 3 months prior to the initiation of liraglutide and not in those patients that had been using metformin long term, for T2D, diagnosis of PCOS, etc. Again the retrospective nature of the study does not allow definitive interpretation, but we assume that T2D, prediabetes, and PCOS might have been diagnosed during the referral visit to the obesity clinic, or at least short term before the multidisciplinary consult. Indeed, metformin is known to cause a modest weight reduction in patients with diabetes, prediabetes, and even in patients with obesity without diabetes, but with increased insulin resistance [23]. Furthermore, the effects of GLP-1 therapy on glycaemic control might also have influenced the decision to start liraglutide during the multidisciplinary consult.
This study has its limitations. First, it is a retrospective study, modest in sample size, and no control group was available. However, we observed that the patients who were prescribed liraglutide, but did not initiate the treatment or stopped prematurely, lost about 2.9 kg of body weight, which is comparable to what is reported for placebo in the RCTs [13, 24]. Second, as mentioned above, the positive results from this experience from a single tertiary centre with a multidisciplinary obesity team might not be transferrable to other treatment settings. Finally, patient preference, motivation, and financial capabilities regarding treatment options might have influenced the treatment decision and thus the results.
Conclusion
In a selected group of patients, the addition of liraglutide to lifestyle adaptations is well tolerated, and it effectively reduces body weight regardless of the maximally tolerated dose. Addition of metformin to liraglutide might induce more weight reduction.
Statement of Ethics
The study was approved by the Ethics Committee Research UZ/KU Leuven (Nr. MP014935). This consent protocol was reviewed and the need for written and informed consent was waived by the Ethics Committee Research UZ/KU Leuven.
Conflict of Interest Statement
R.V.G. serves or has served on the speakers bureau for Astra Zeneca, Boehringer Ingelheim, Goodlife Pharma, Mundipharma, Novo Nordisk, and Sanofi. Financial compensation for these activities has been received by KU Leuven. The other authors declare no conflicts of interest relevant to this study.
Funding Sources
S.T. is a beneficiary of a scholarship grant from the Frans Van de Werf Fund, KU Leuven.
Author Contributions
B.V.D.S. and R.V.G. contributed to study concept and design; L.T., F.V.N., and C.M. contributed to data acquisition; L.T., S.T., and R.V.G. contributed to statistical analysis; all authors contributed to interpretation and critical revision of the manuscript; R.V.G. contributed to supervision.
Data Availability Statement
Data can be made available by the corresponding author on reasonable request.
References
- 1.Drieskens S, Gisle L, Charafeddine R, Demarest S, Braekman E, Nguyen D, et al. Gezondheidsenquête 2018: Levensstijl. Samenvatting van de resultaten. In: Sciensano, editor. Sciensano, rapportnum. Brussels, Belgium: Sciensano; 2018. Available from: www.gezondheidsenquete.be. [Google Scholar]
- 2.Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med. 2017 Apr;5((7)):161. doi: 10.21037/atm.2017.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durrer Schutz D, Busetto L, Dicker D, Farpour-Lambert N, Pryke R, Toplak H, et al. European practical and patient-centred guidelines for adult obesity management in primary care. Obes Facts. 2019 Mar;12((1)):40–66. doi: 10.1159/000496183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belgian Association for the Study of Obesity . Consensus BASO 2020 Een praktische gids voor de evaluatie en behandeling van overgewicht en obesitas. 2020. pp. p. 1–234. [Google Scholar]
- 5.Sjöström L. Review of the key results from the Swedish obese subjects (SOS) trial: a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013 Mar;273((3)):219–34. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 6.Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021 Mar;384((11)):989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 7.Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018 Nov;392((10160)):2180–93. doi: 10.1016/S0140-6736(18)32260-8. [DOI] [PubMed] [Google Scholar]
- 8.Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376((9741)):595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 9.Apovian CM, Aronne L, Rubino D, Still C, Wyatt H, Burns C, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity‐related risk factors (COR-II) Obesity. 2013 May;21((5)):935–43. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O'Neil PM, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity. 2011 Jan;19((1)):110–20. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. J Am Med Assoc. 2015 Aug;314((7)):687–99. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 12.Davidson MH, Hauptman J, DiGirolamo M, Foreyt JP, Halsted CH, Heber D, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistata randomized controlled trial. JAMA. 1999 Jan;281((3)):235–42. doi: 10.1001/jama.281.3.235. [DOI] [PubMed] [Google Scholar]
- 13.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015 Jul;373((1)):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 14.Blackman A, Foster GD, Zammit G, Rosenberg R, Aronne L, Wadden T, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the scale sleep apnea randomized clinical trial. Int J Obes. 2016 Aug;40((8)):1310–9. doi: 10.1038/ijo.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004 Jun;27((6)):1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 16.Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes. Diabetes Care. 2009 Jan;32((1)):84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes. 2014;38((6)):784–93. doi: 10.1038/ijo.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wharton S, Liu A, Pakseresht A, Nørtoft E, Haase CL, Mancini J, et al. Real-world clinical effectiveness of liraglutide 3.0 mg for weight management in Canada. Obesity. 2019 Jun;27((6)):917–24. doi: 10.1002/oby.22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JH, Kim JY, Choi JH, Park HS, Shin HY, Lee JM, et al. Effectiveness of liraglutide 3 mg for the treatment of obesity in a real-world setting without intensive lifestyle intervention. Int J Obes. 2021 Apr;((4)):45. doi: 10.1038/s41366-021-00739-z. [DOI] [PubMed] [Google Scholar]
- 20.Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374((9701)):1606–16. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 21.Jensterle M, Pirš B, Goričar K, Dolžan V, Janež A. Genetic variability in GLP-1 receptor is associated with inter-individual differences in weight lowering potential of liraglutide in obese women with PCOS: a pilot study. Eur J Clin Pharmacol. 2015 Jul;71((7)):817–24. doi: 10.1007/s00228-015-1868-1. [DOI] [PubMed] [Google Scholar]
- 22.Lean MEJ, Carraro R, Finer N, Hartvig H, Lindegaard ML, Rössner S, et al. Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int J Obes. 2014;38((5)):689–97. doi: 10.1038/ijo.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bray GA, Edelstein SL, Crandall JP, Aroda VR, Franks PW, Fujimoto W, et al. Long-term safety, tolerability, and weight loss associated with metformin in the diabetes prevention program outcomes study. Diabetes Care. 2012 Apr;35((4)):731–7. doi: 10.2337/dc11-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blackman A, Foster GD, Zammit G, Rosenberg R, Aronne L, Wadden T, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the scale sleep apnea randomized clinical trial. Int J Obes. 2016;40((8)):1310–9. doi: 10.1038/ijo.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be made available by the corresponding author on reasonable request.