Abstract
Objective.
Mebendazole and other anti-parasitic drugs are being used off-prescription based on social media and unofficial accounts of their anti-cancer activity. The purpose of this study was to conduct a controlled evaluation of mebendazole’s therapeutic efficacy in cell culture and in vivo models of ovarian cancer. The majority of ovarian cancers harbor p53 null or missense mutations, therefore the effects of p53 mutations and a mutant p53 reactivator, PRIMA-1MET (APR246) on mebendazole activity were evaluated.
Methods.
Mebendazole was evaluated in cisplatin-resistant high grade serous stage 3C ovarian cancer patient derived xenograft (PDX) models: PDX-0003 (p53 null) and PDX-0030 (p53 positive), and on ovarian cancer cell lines: MES-OV (p53 R282W), ES2 (p53 S241F), A2780 (p53 wild type), SKOV3 parental (p53 null) and isogenic sublines, SKOV3 R273H p53 and SKOV3 R248W p53. Drug synergy and mechanisms were evaluated in cell cultures using isobolograms, clonogenic assays and western blots. Prevention of tumor establishment was studied in a MES-OV orthotopic model.
Results.
Mebendazole inhibited growth of ovarian cancer cell cultures at nanomolar concentrations and PDXs at doses up to 50 mg/kg, and reduced orthotopic tumor establishment at 50 mg/kg. The mechanism of mebendazole was associated with p53-independent induction of p21 and tubule depolymerization. PRIMA-1MET also inhibited tumor establishment and worked synergistically with mebendazole in cell culture to inhibit growth and induce intrinsic apoptosis through a p53- and tubule destabilization-independent mechanism.
Conclusion.
This work demonstrates the therapeutic potential of repurposing mebendazole and supports clinical development of mebendazole for ovarian cancer therapy and maintenance.
Keywords: Mebendazole, Ovarian Cancer, Repurposed, PRIMA-1MET, p53
Graphical Abstract
1. Introduction
Epithelial ovarian cancer is the most lethal gynecologic cancer, largely due to the predilection of advanced stage disease and the inevitable development of resistance to chemotherapy despite initial high response rates. Improvements in front line treatment options have resulted in an 80% rate of remission, but <50% of patients remain alive at 5 years [1]. Maintenance options after completion of chemotherapy include bevacizumab and PARP inhibitor therapy after treatment of newly diagnosed ovarian cancer and platinum sensitive recurrent disease. Although these treatments offer substantial clinical benefit, they have limited or uncertain impact on overall survival and impose considerable toxicity and expense [2–5]. Given the poor survival rates and considerable toxicities that patients encounter with conventional chemotherapy, novel agents and therapeutic methods are critically needed.
Repurposing of drugs currently used or approved for other indications is a promising strategy to reduce the cost and time required to develop new anti-cancer drugs. The anti-parasitic drugs fenbendazole, flubendazole, albendazole, and mebendazole have been used safely to treat pinworm and other helminthic infections in humans and animals for decades. The rationale for developing these drugs as cancer therapeutics is their microtubule destabilizing activities [6,7]. While evidence for the efficacy of benzimidazoles is increasing in the scientific literature [8–13], social media has exploded with accounts of de-wormer reports showing efficacy as an anti-cancer treatment off prescription (Supplemental Tables S1, S2, S3). Clearly, scientifically designed studies are needed to evaluate these drugs as anti-cancer agents and provide information to appropriately design clinical trials and applications.
In addition to the tubulin network destabilizing activity, the mechanism of fenbendazole’s anti-cancer activity in human lung cancer cell lines was shown to occur through increasing the stability of the p53 protein and induction of apoptosis [7,14]. This raised concerns regarding further development of benzimadazoles for epithelial ovarian cancer, because p53 missense gain-of-function mutations that can cause resistance to apoptosis are common in all histologic types of epithelial ovarian cancer [15]. Thus, we proposed the hypothesis that a drug, such as PRIMA-1MET (APR-246), which can reactivate wild type function in a missense mutant p53, would act synergistically with mebendazole to kill ovarian cancer cells with missense gain-of-function p53 mutations. We chose to focus this study on mebendazole because it was shown to be more potent than fenbendazole in cell culture studies [16].
The purposes of this study were to evaluate mebendazole’s effects on growth and survival of ovarian cancer cell lines and xenograft tumors with a range of p53 mutation statuses and to elucidate the mechanism of mebendazole and potential for use in ovarian cancer therapy and maintenance.
2. Methods
2.1. Assessment of off-prescription use of mebendazole and fenbendazole
Facebook.com and mycancerstory.rocks, were monitored from October 25th through December 17th, 2019 using the key words mebendazole, fenbendazole, dewormer and cancer treatment. All information on diagnosis date, cancer, stage, metastases, therapy, date alternate therapy began, alternate therapy, results date, and results were tabulated and de-identified.
2.2. Cell lines and culture conditions
Human ovarian cancer cell lines A2780 (RRID:CVCL_0134) and SKOV3 (RRID:CVCL_0532) were obtained from ATCC. ES2/GFP-luc and MES-OV/GFP-luc (RRID:CVCL_CZ92) cell line, hereafter called ES2 and MES-OV, respectively, were a generous gift from Dr. Francois Moisan, Stanford University. SKOV3 cells stably transfected with mutant p53 (R248W, R273H) or parent vector (pLenti-GIII-CMV-GFP-2A-Puro) were provided by Jeremy Chien, PhD (University of Kansas Medical Center). All cell lines were authenticated by autosomal short tandem repeat (STR) profile determination and comparison with reference databases by the University of Arizona Genetics Core and IDEXX BioAnalytics (Columbia, MO, USA) within three years of receipt and use. A2780 and SKOV3 parental p53 null cell lines, and SKOV3 sublines stably transfected with mutant p53 expression vectors were cultured in RPMI 1640 (Sigma, St. Louis, MO). MESOV, ES2 cells were cultured in DMEM (Sigma, St. Louis, MO). Media were supplemented with 10% FBS (Serum Source International Inc. Charlotte, NC) and 1× antibiotic/antimycotic (EMD Millipore, Burlington, MA). Cells were cultured at 37 °C with 5% CO2. All cell lines were authenticated and confirmed to be mycoplasma free and were tested periodically.
2.3. Synthesis of PRIMA-1MET
The PRIMA-1MET (APR-246) was synthesized for the animal study by G. P. based on a published method [17] using scheme shown below.
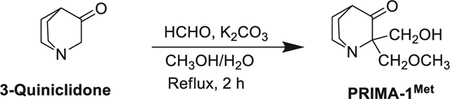
This method is described in Supplementary methods.
2.4. MTT cytotoxicity assay
Cytotoxic effects of mebendazole (Cayman chemicals, Michigan, USA) and PRIMA-1MET (Cayman Chemicals, Michigan, USA) alone and in combination over a range of doses were measured on ovarian cancer cell lines using the MTT assay. This method is described in Supplementary methods.
2.5. Clonogenic assay
Ovarian cancer cells (MES-OV, A2780 and parental SKOV-3) were seeded in 6-well plates in triplicate (1500, 500 and 2500 cells/well, respectively). After 24 h, the cells were treated with mebendazole and incubated in 5% CO2 incubator for 8 h, and then cells were maintained in fresh complete growth medium until cells in control have formed sufficiently large clones. Cells were fixed with 4% paraformaldehyde, then stained with 0.5% crystal violet dye. The visible colonies were photographed and the colonies containing at least 50 cells were counted using Gel Count Colony counter (Oxford Optronix, UK).
2.6. Patient derived xenograft (PDX) model
Immunocompromised mice bearing subcutaneous PDX tumors that had achieved ~ 100 mm3 tumor size were randomized into treatment groups: control for vehicle (sesame oil) or mebendazole at 10 mg/kg, 25 mg/kg and 50 mg/kg doses administered by oral gavage. Treatment was initiated and administered three times per week for three weeks. Observation of animals and measurements of tumor volume and body weight continued until the tumor burden reached approximately 1000 mm3, at which time the mice were euthanized. Mice were monitored at least 3 weeks post-treatment. This method is described in detail in the Supplementary Methods.
2.7. Combination index
Mebendazole and PRIMA-1MET were mixed at a 1:1 ratio of their IC50 concentrations, and a series of 2-fold dilutions were evaluated for combination effects using the Chou and Talalay method [18]. This method is described in detail in the Supplementary Methods.
2.8. Immunoblot analysis
Proteins were extracted from whole-cell lysates of ovarian cancer cells, electrophoresed into SDS gels and transferred to blots which were probed with specific antibodies. The total band intensity was quantified by using Image Lab software (Bio Rad, Hercules, CA, USA) and the protein expression levers were determined by normalizing the band intensity of the protein of interest against the band intensity of the loading control β-actin protein. This method is described in detail in the Supplementary Methods.
2.9. Measurement of soluble and assembled tubulin
Soluble (depolymerized) tubulin and assembled (polymerized) tubulin were extracted and measured in western blots as described previously [19,20]. This method is described in detail in the Supplementary Methods.
2.10. In vivo orthotopic intraperitoneal xenograft model
Immunocompromised mice injected with 2 × 107 MES-OV GFPluc cells were randomized into four treatment groups and treated with either a mixture of sesame oil and PBS (for orally gavage of the control group); mebendazole (50 mg/kg in sesame oil, administered by oral gavage); PRIMA-1MET (10 mg/kg in PBS, administered by i.p. injection); or a combination of mebendazole and PRIMA-1MET. Mice were euthanized 9 weeks after tumor cell injection, and the intraperitoneal tumors and vital organs were collected, weighed and preserved for further study. This method is described in detail in the Supplementary Methods.
2.11. Statistical analysis
In the PDX model, the effects of mebendazole on tumor size over time was evaluated by a One-Way ANOVA and the dose-response relationship was measured by Mixed effect. For the PDX-0003 model, statistical analysis excluded time points past 43 due to termination of the 10 mg/kg mebendazole treatment group resulting in incomplete data. In the PDX-0003 model, the control group consisted of 7 mice throughout the 50 days of the experiment; the 10 mg/kg mebendazole group consisted of 5 mice that decreased to 3 on day 43 and to zero on day 46; the 25 mg/kg mebendazole group consisted of 6 mice that decreased to 5 on day 78 and zero on day 92; and the 50 mg/kg mebendazole group consisted of 7 mice that decreased to 6 on day 50, 5 on day 71, 3 on day 78 and two on day 99. For the PDX-0030 model, there were 10 mice in each group, however on day 36, each of the control, 10 mg/kg and 25 mg/kg mebendazole groups were reduced to 7 mice; while the 50 mg/kg mebendazole group was reduced to 8 mice on day 18 and 5 mice on day 36. Differences in body weights over time between the groups were evaluated using a One-way ANOVA for the normally distributed data and the Friedman test for the nonparametric data. Both of these analysis included multiple comparisons of each treatment group with the control group. p values <0.05 were considered statistically significant. The PDX tumor analyses were performed using Prism 8.0 (GraphPad, San Diego, CA, USA).
Western blot and animal tumor weight results were analyzed by one-way ANOVA followed by Tukeys’ post hoc test (GraphPad Prism version 7.0 or 8.0). A p value of <0.05 denoted statistical significance.
For the orthotopic model of maintenance therapy, there were 6 mice per group with the exception of the PRIMA-1MET group with 12 mice. The mice in control and PRIMA-1MET groups had complete data, while two mice in the mebendazole and three mice in the combination treatment group died early with no tumor, and their tumor weights were subsequently set to be zero. In addition to the tumor weight variable, a binary variable (notumor) was created which equals 1 if the tumor weight is zero and equals 0 otherwise. This variable indicates whether a mouse is tumor-free or not. To assess the additive effects of mebendazole and PRIMA-1MET, a logistic regression model was used to analyze the notumor variable with two factors mebendazole (indicating whether Mebendazole was part of the treatment) and PRIMA-1MET (indicating whether PRIMA-1MET was part of the treatment) and their interaction. Pairwise comparison between the groups were performed on the notumor variable using the Fisher’s exact test. The aforementioned analysis was conducted using SAS 9.4. Body weights at the end of the experiment were compared with the Kruskal-Wallis test with multiple comparisons using Prism 8.0 (GraphPad, San Diego, CA, USA).
3. Results
3.1. Therapeutic efficacy of mebendazole against ovarian cancer PDX models
To assess the potential utility of mebendazole for ovarian cancer therapy, the in vivo efficacy of mebendazole at three different doses on the growth of two PDX models (PDX-0003 and PDX-0030) was evaluated. The tumors used to establish the PDXs were diagnosed as high grade serous ovarian cancers that stained positively for paired box gene-8 (PAX-8) and Wilm’s Tumor-1 (WT-1), and were collected from patients initially diagnosed as stage 3C who developed recurrent platinum resistant (<6 months progression free interval after primary platinum-based chemotherapy). PDX-0003 stained negatively for p53 and had a TP53 gene truncation mutation, while PDX-0030 stained positively for p53, which is considered diagnostic for a missense p53 mutation. Once the PDXs grafted onto immunocompromised mice reached ~100 mm3, the mice were randomized into 4 treatment groups: control (sesame oil) or mebendazole at 10 mg/kg, 25 mg/kg and 50 mg/kg doses administered by oral gavage three times per week for three weeks followed by observation. Mice were terminated when their tumor size reached ~1000mm3. Otherwise, all mice were monitored at least 3 weeks post-treatment before termination. The p53 null PDX-0003 tumor grew faster than the p53 positive PDX-0030 tumor (Fig. 1A). All three doses of mebendazole significantly inhibited tumor growth in both PDX models (PDX-0003: One-way ANOVA, p < 0.0019, multiple comparisons adjusted p = 0.05 for 10 mg/kg, p = 0.005 for 25 mg/kg and p = 0.007 for 50 mg/kg, PDX-0030: One-way ANOVA p < 0.0001, multiple comparisons adjusted p < 0.0001 for 10 mg/kg, p < 0.0001 for 25 mg/kg and p = 0.0001 for 50 mg/kg). Mixed-effects analysis determined that the anti-tumor effects of mebendazole were significantly dose-responsive in both models (Time × Dose F = 4.61, p < 0.0001 for PDX-0003; Time × Dose F = 2.73, p < 0.0001 for PDX-0030).
Fig. 1.
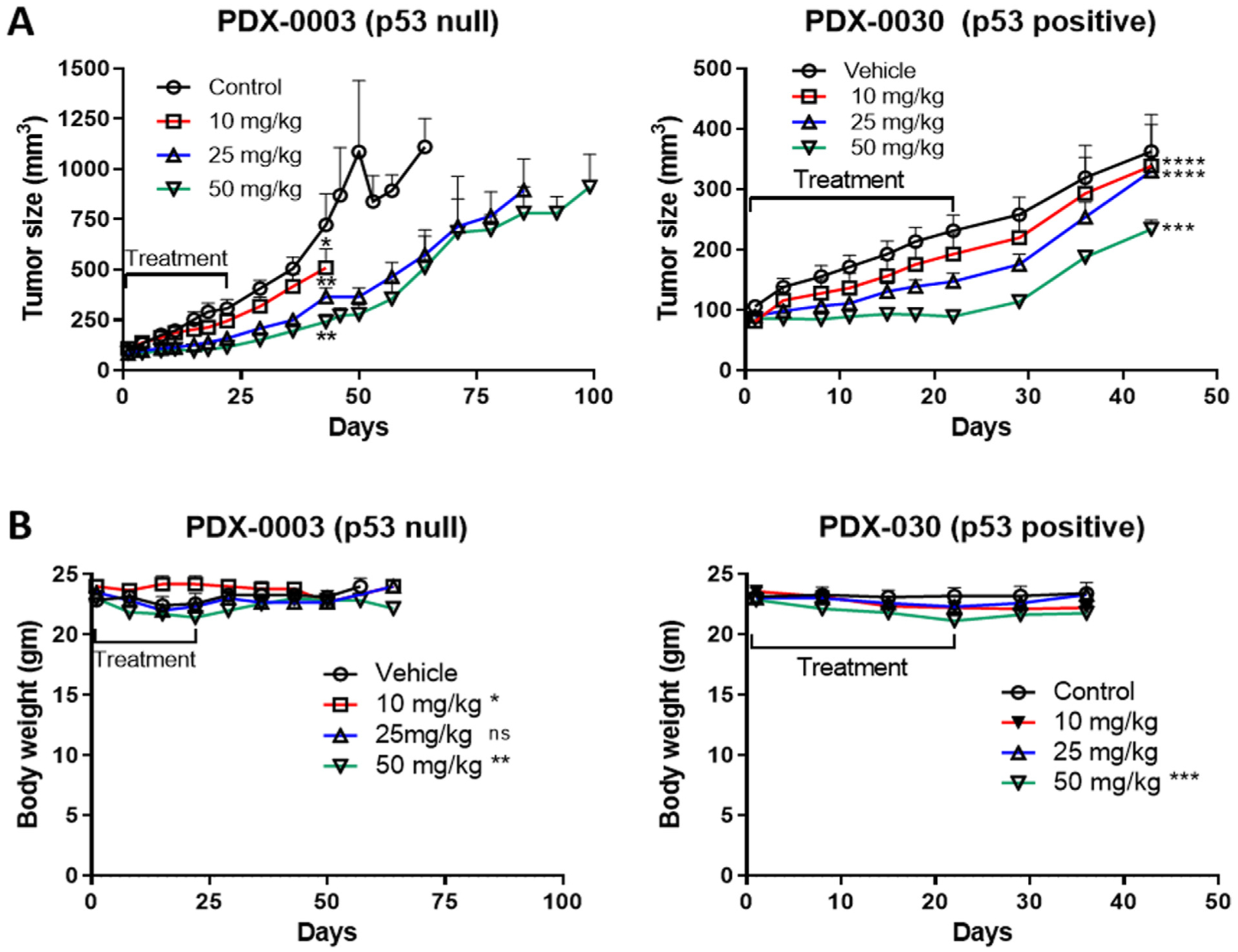
Therapeutic effect of mebendazole on Patient derived xenograft (PDX) model of ovarian cancer. (A) Tumor size reduction in PDX-0003 (p53 null) and PDX-0030 (p53 positive) models treated with either vehicle or mebendazole 10 mg/kg, 25 mg/kg or 50 mg/kg doses. One-way ANOVA, *p ≤ 0.05, ** p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001 (B) Total body weight of PDX-0003 and PDX-0030 mice treated with vehicle or mebendazole 10 mg/kg, 25 mg/kg or 50 mg/kg doses.
During the 3 week treatment period, there were significant differences in the body weights between the treatment groups and control group for both PDX models reflecting differences in toxicity (Friedman test p = 0.012 for PDX-0003 and p = 0.012 for PDX-0030, Fig. 1B). In PDX-0003 however, multiple comparisons found no significant differences between the individual treatment groups and the control group (p > 0.05). In PDX-0030, multiple comparisons revealed a significant decrease in body weight for the highest dose group (50 mg/kg), but not between the lower dose groups and the control group (p = 0.008 for 50 mg/kg, p > 0.05 for 20 and 25 mg/kg). There were also significant differences between the groups when body weights were compared throughout the entire study period (treatment and post-treatment) (Fig. 1B, PDX-0003 Mixed Effect Model p = 0.0001, PDX-0030 Friedman test p = 0.0002). In the PDX-0003 model, multiple comparisons revealed that the significant differences were between only the 10 and 50 mg/kg dose groups and control group (10 mg/kg adjusted p = 0.049, 50 mg/kg adjusted p = 0.005, 25 mg/kg adjusted p > 0.05). The difference in the 10 mg/kg dose was due to a transient elevation in body weight, which did not occur in the PDX-0030 model. Multiple comparisons in the PDX-0030 model, revealed significant differences between only the 50 mg/kg dose group and the control in PDX-0030 (50 mg/kg adjusted p = 0.0004; 10 and 25 mg/kg adjusted p > 0.05). In both models, the difference in the 50 mg/kg dose group was due to decreased body weight. Taken together, these data suggest that the highest dose of mebendazole (50 mg/kg) exerts slight toxicity, however the weight loss was 5%, which is less than the 10% body weight standardly used to define a maximum tolerated dose.
3.2. Association of p53 status with mebendazole sensitivity in ovarian cancer cell lines
Since p53 activity has been reported to be involved in the mechanism of mebendazole, yet our study documented that mebendazole is active in a p53 null PDX model, we compared the mebendazole sensitivities of six human ovarian cancer cell lines with different p53 statuses in cell culture MTT assays (Fig. 2A). The MES-OV and ES2 cell lines have endogenous missense p53 mutations, while the A2780 cell line has wild type p53. To control for missense p53 mutations in an isogenic background, the SKOV3 p53 null parental cell line was compared to established sublines harboring missense p53 mutations. The IC50 values determined ranged from 400 nM to 1.7 μM for mebendazole, with the SKOV3 (p53 null) cell line exhibiting the least sensitivity (Fig. 2A). These doses surround the physiologically-achievable plasma concentration of 590 nM observed in humans over 24 h after taking the marketed formulation of mebendazole [21]. Improved formulations are being developed to increase the bioavailability of mebendazole [21].
Fig. 2.
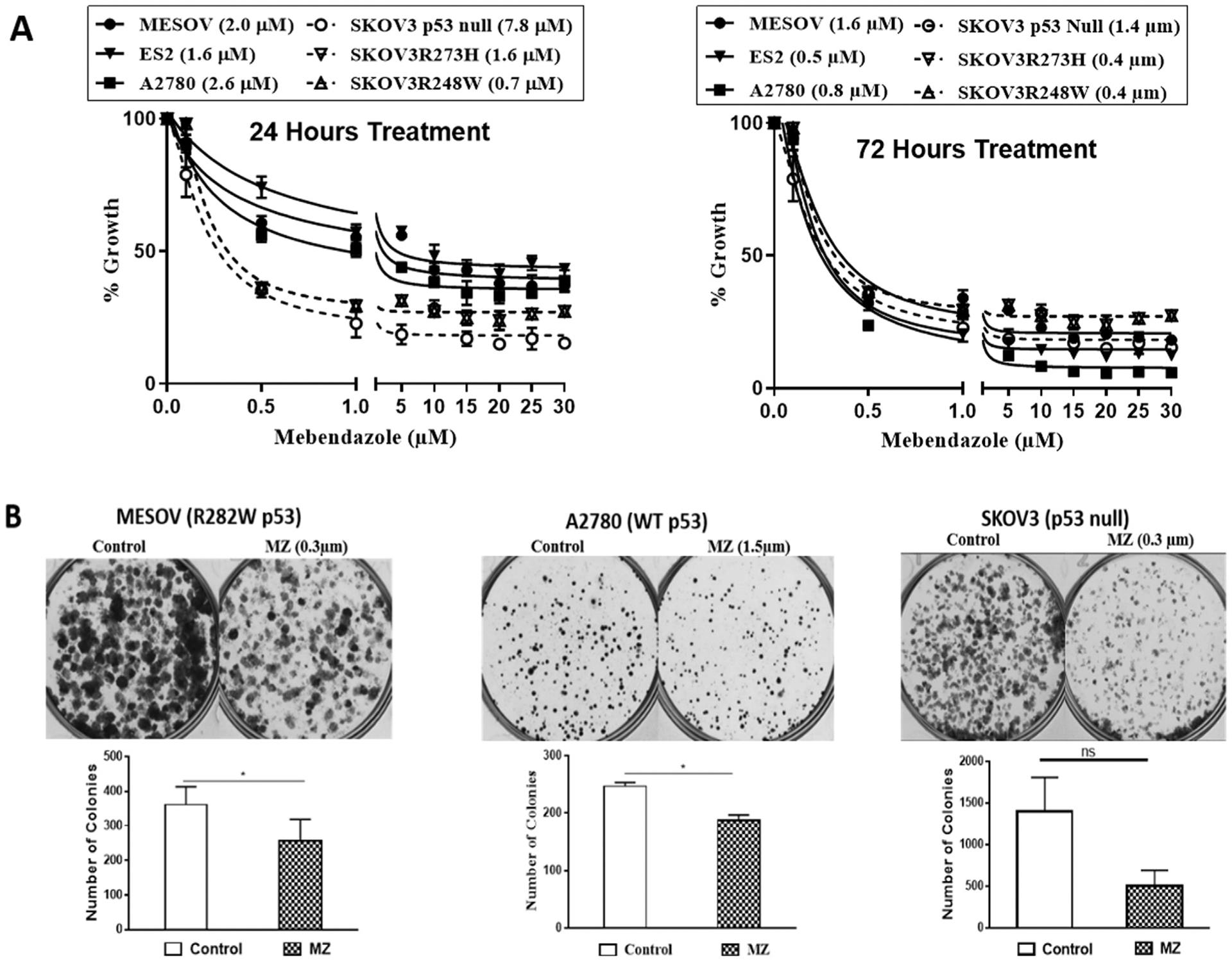
Mebendazole’s inhibitory effects on ovarian cancer cells; (A) MTT assay results of 48 or 72 h mebendazole treatment of MESOV, ES2, A2780, SKOV3 null p53, SKOV3 R248W p53 and SKOV3 R273H p53 cells. (B) Colony formation assay in MES-OV (R282W p53), A2780 (WT) and SKOV3 (p53 null) cells treated with mebendazole for 8 h. Cell colonies were counted after staining with 0.5% crystal violet dye. * p ≤ 0.05.
A clonogenic assay demonstrated that mebendazole significantly reduced cell survival in MES-OV (R282W p53) and A2780 (p53 WT) cell lines (Fig. 2B, C & S1), however the observed reduction in SKOV3 (p53 null) cell colonies did not achieve statistical significance (Fig. 2D & S1).
3.3. Synergistic interaction of mebendazole and PRIMA-1MET
We next determined if the p53 reactivator, PRIMA-1MET, could enhance the activity of mebendazole in a cell line specific manner depending on the p53 mutation status. Isobolograms of mebendazole and PRIMA-1MET combinations demonstrated CI values indicating synergistic interaction of the drugs in all cell lines regardless of their p53 statuses (Fig. 3 and Supplementary table S4). DRI values indicate that either mebendazole or PRIMA-1MET can be used at lower doses in drug combinations to achieve the same efficacy caused by higher doses of the single drug treatments.
Fig. 3.
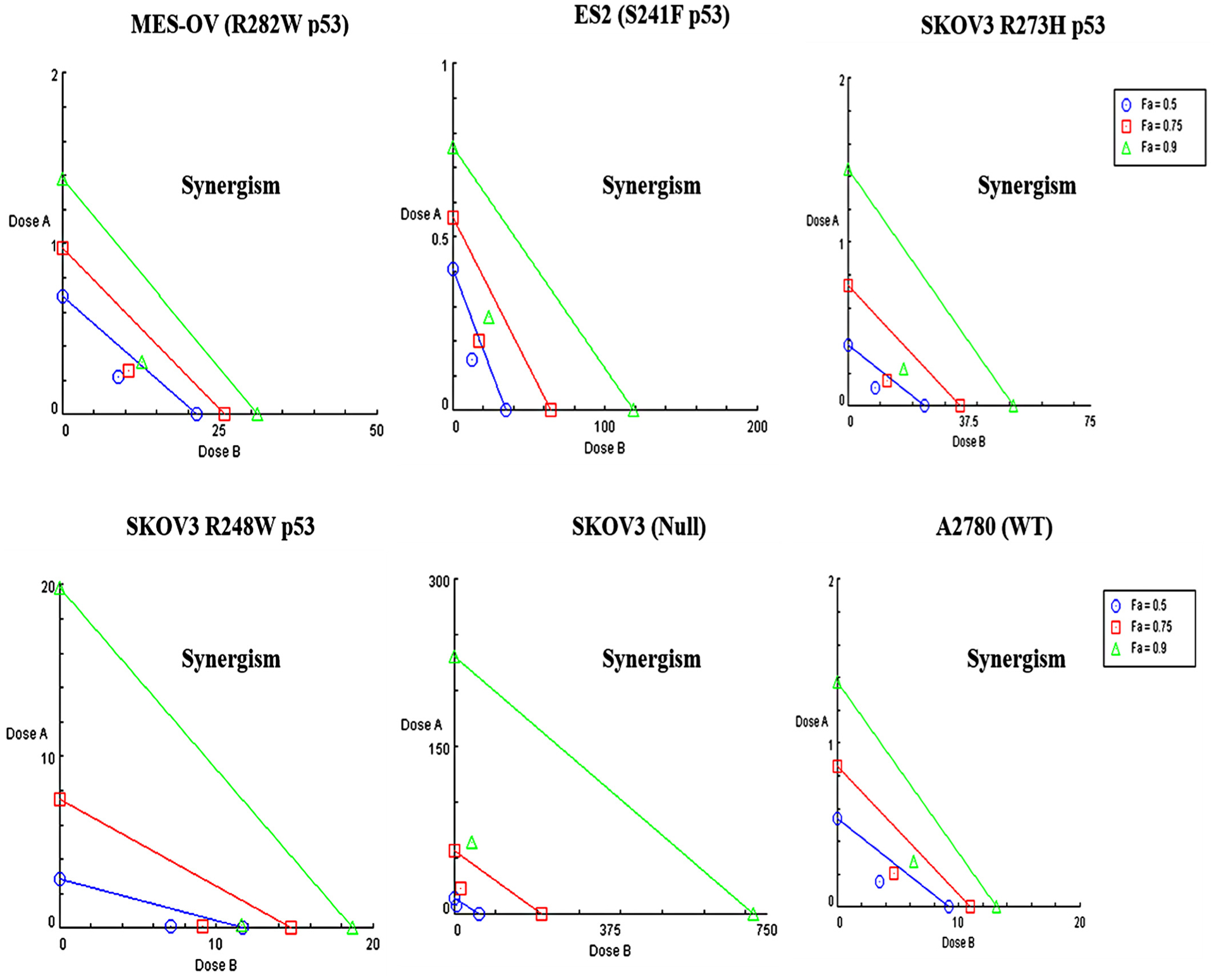
Isobolograms of Mebendazole and PRIMA-1MET Combinations. Isobolograms of Mebendazole and PRIMA-1MET in a different p53 mutant ovarian cancer cells (MES-OV (R282 p53), ES2 (S241F p53), SKOV3 (R273H p53), SKOV3 (R248W p53), SKOV3 (p53 Null) and A2780 (WT)). Dose A: Mebendazole, Dose B: PRIMA-1MET.
3.4. Mechanisms of mebendazole and PRIMA-1MET alone and in combination
To study the mechanism of mebendazole and PRIMA-1MET synergy, drug effects on proteins and microtubules were evaluated by western blot (Fig. 4). Neither drug alone or in combination increased p53 protein expression in any of the cell lines (Fig. 4A, B, S3 & S3.1). The p21 protein expression was also measured as a down-stream readout of p53 transcriptional activity. Mebendazole significantly increased p21 protein expression in all cell lines regardless of p53 status, except for in the ES2 cell line (Fig. 4A, C, S4 & S4.1). PRIMA-1MET did not induce p21 expression when used as a single agent and did not significantly affect the mebendazole induction of p21 (Fig. 4A & 4C). In all cell lines with endogenous or exogenous missense mutant p53, mebendazole decreased tubulin polymerization (Fig. 4D, E, S5, S5.1 & S5.2). PRIMA-1MET had no effect on tubule polymerization when used alone, and prevented mebendazole inhibition of tubule polymerization (Fig. 4D, 4E, S5, S5.1 & S5.2). Taken together these data demonstrate associations of p21 induction and tubule depolymerization with mebendazole activity, with the addition of PRIMA-1MET having no effect on the p21 induction, but with an antagonism of the tubule depolymerization.
Fig. 4.
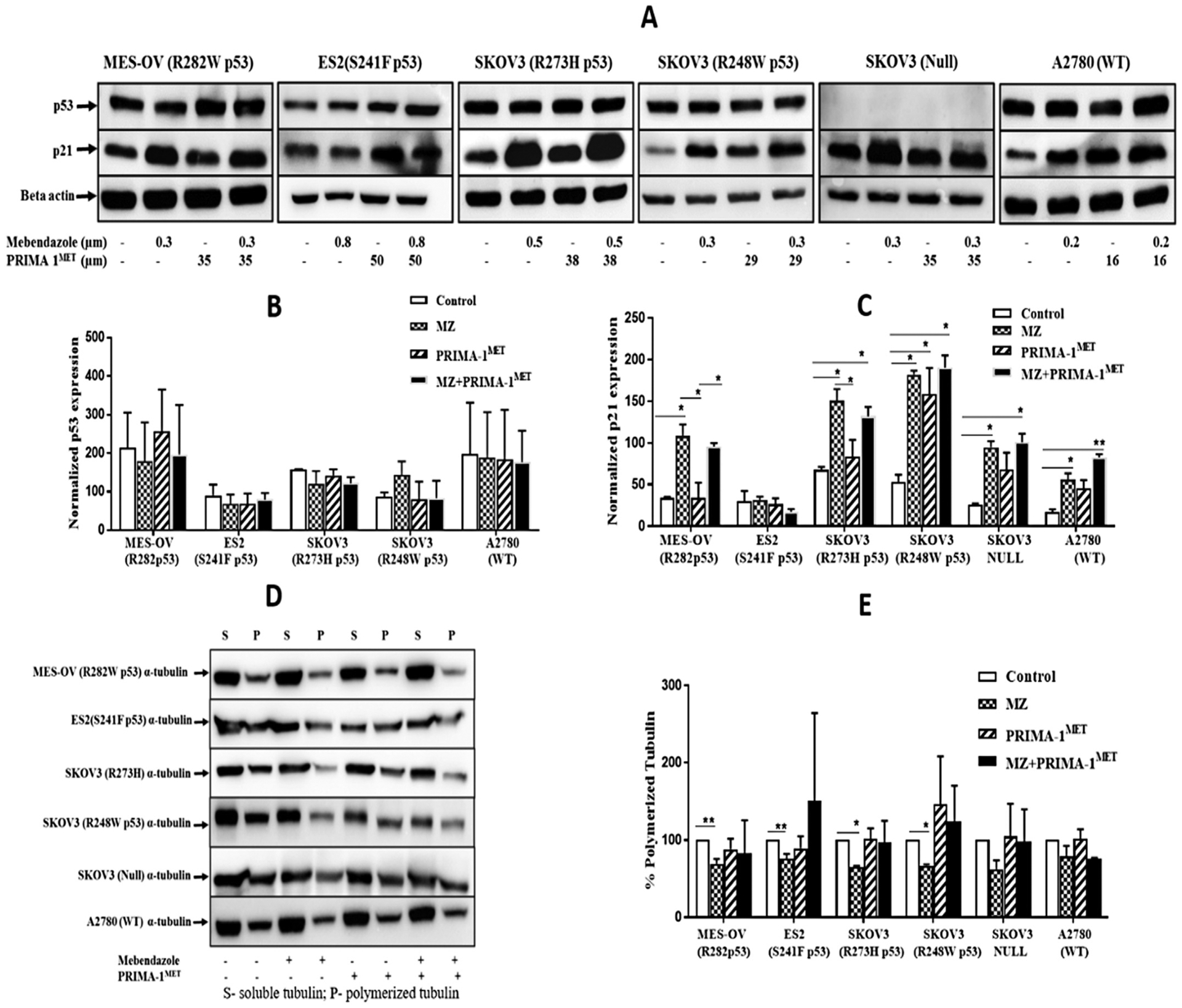
Mebendazole increases p21 independent of p53 in ovarian cancer cell lines. (A) Western blots of p53 & p21 protein expression after treatment with mebendazole and PRIMA-1MET in ovarian cancer cells with different p53 status. Histogram of (B) p53 and (C) p21 protein level were normalized to the β actin levels. (D) Effect of mebendazole in microtubule organization in ovarian cancer cells. Ovarian cancer cells were treated with either mebendazole / PRIMA-1MET alone or combination of both the drugs for 24 h, then lysed and fractionated into soluble (S) and polymerized (P) extracts. The extracts were separated with SDS-PAGE, transferred onto PVDF membranes and probed with anti-α-tubulin antibodies. Representative Immunoblot analysis of alpha tubulin in all ovarian cancer cells. (E) Intensity of each band of the immunoblot was measured by the Image Lab program. The change in percentage of polymerized tubulin (%PT) induced by each treatment was determined using the formula: % PT = PT/(PT + ST) × 100. This experiment was repeated 2 to 5 times. Statistical analysis was performed using one-way ANOVA followed by Tukeys’ post hoc test. * p < 0.05, ** p ≤ 0.01.
To further explore the mechanism of mebendazole and PRIMA-1MET synergy, markers of apoptosis were evaluated in cultures treated with the drugs alone and in combination. When administered as a single agent, mebendazole, but not PRIMA-1MET, induced cleavages of caspases 9 and 3 and PARP-1 (Fig. 5A). The statistical significance of the inductions in replicate experiments varied across the cell lines (Fig. 5B, C & D). The combination treatment significantly induced these cleavages to a greater extent compared to control treatment in all cell lines, except for SKOV3 (R273H p53) for caspase 9 and SKOV3 (R248W P53) for PARP-1 (Fig. 5B–D, S6, S6.1, S7, S7.1, S8 & S8). Overall, these results demonstrate that the combined drug treatment induces greater levels of intrinsic apoptosis compared to the single drug treatments.
Fig. 5.
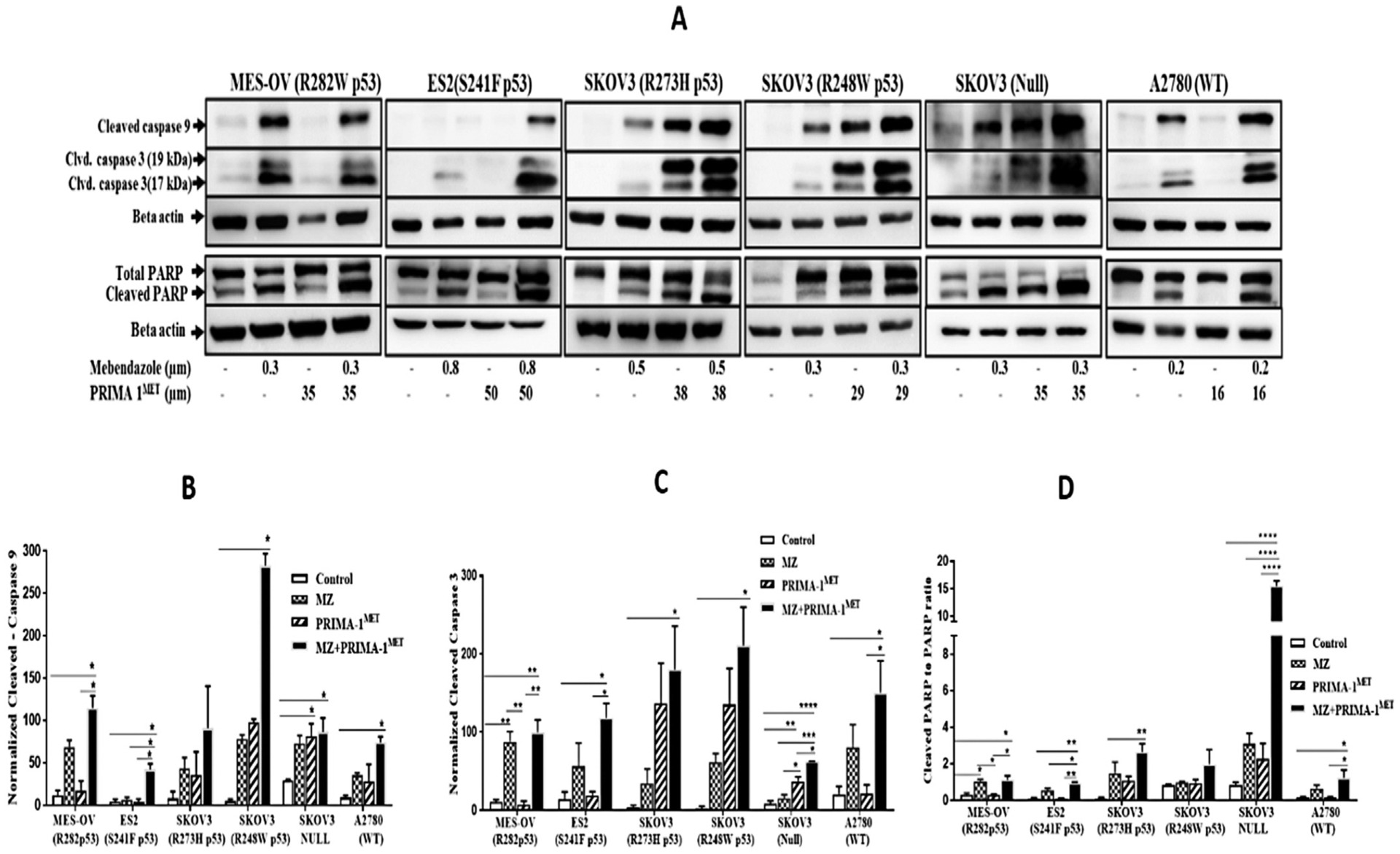
Mebendazole, PRIMA-1MET drug combination induces apoptosis in in vitro. (A) Western blots of cleaved caspase 9, Cleaved Caspase 3 and cleaved PARP protein expression level after treatment with mebendazole and PRIMA-1MET in ovarian cancer cells with different p53 status. Histogram of cleaved (B) caspase 9 and (C) caspase 3 protein level were normalized to the β actin levels. (D) Cleaved PARP to PARP ratio level. Statistical analysis was performed using one-way ANOVA followed by Tukeys’ post hoc test. * p < 0.05, ** p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
3.5. In vivo validation of mebendazole alone or in combination with PRIMA-1MET
The efficacy of mebendazole, PRIMA-1MET and the drug combination in vivo was examined in an orthotopic model of ovarian cancer maintenance therapy using the MES-OV (R282W p53) cell line. To model maintenance therapy, drug treatment was initiated prior to tumor establishment. Mebendazole and PRIMA-1MET were administered alone and in combination. Due to the unexplained death of two mice in the mebendazole treatment group and an average 5% weight loss in the remaining mice during the first week of treatment, the dosing was reduced to every other day, after which the average weight was restored (Fig. 6A). The body weights at the end of the experiment were not significantly different between any of the treatment groups and control group (Kruskal-Wallis test with multiple comparisons p > 0.05). The experiment was terminated after 6 weeks of treatment as planned. Tumor-free rates were 0% in the untreated controls, 25% in the PRIMA-1MET treatment group, 67% in the mebendazole treatment group, and 83% in the combination treatment group (Fig. 6B). An additive logistic regression model indicated that mebendazole (p = 0.005, OR = 27.535, 95% CI: 2.725, 278.265), but not PRIMA-1MET (p = 0.182, OR = 4.998, 95% CI: 0.472, 52.936) is associated with preventing tumor establishment. Histologic analysis of kidney and liver showed no evidence of toxicity (Fig. 6C).
Fig. 6.
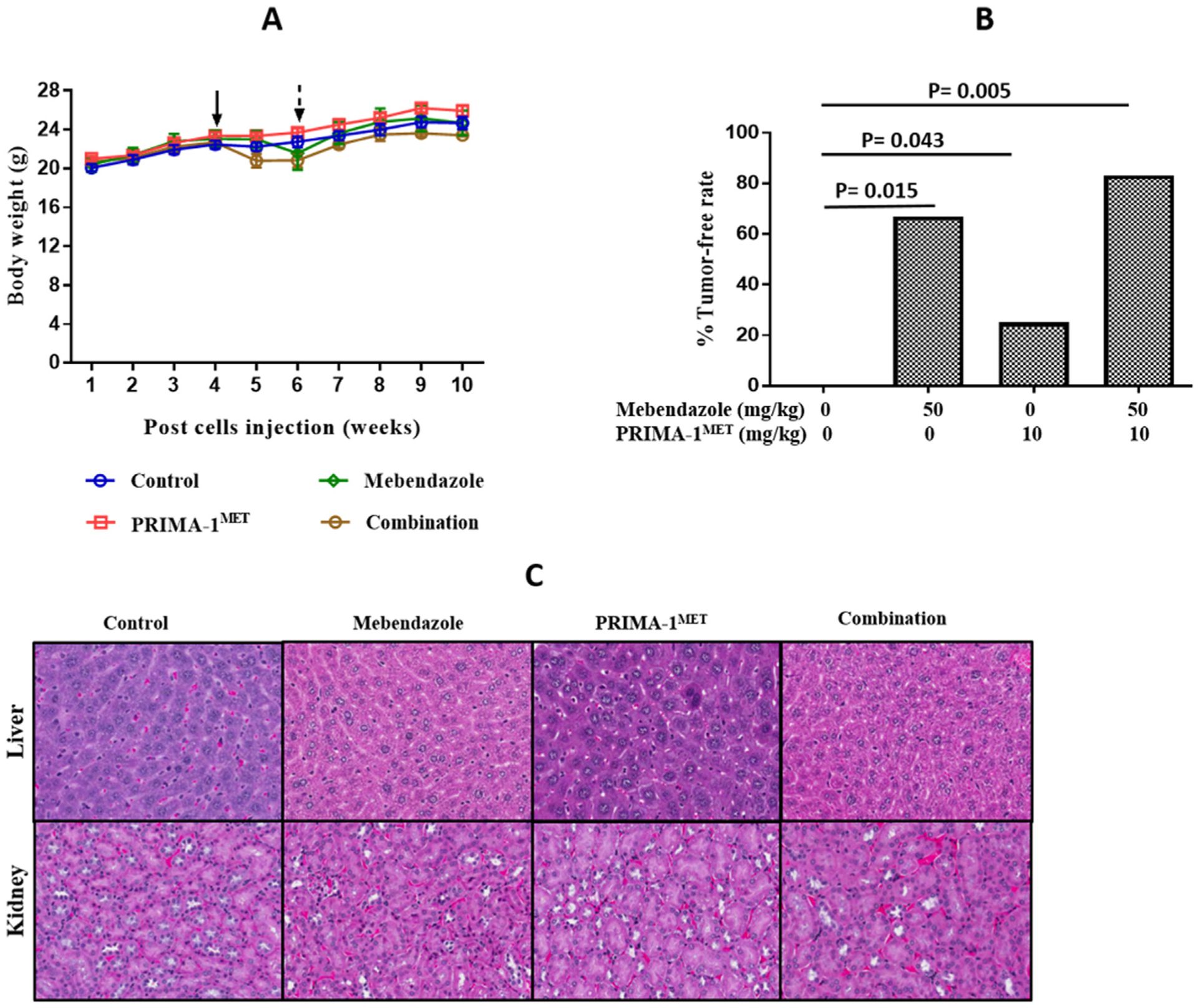
In vivo validation of effect of mebendazole alone or in combination with PRIMA-1MET on ovarian cancer treatment. Tumors were established in 6 week old athymic nude mice by i.p. injection of 2 × 107 MES-OV GFP/LUC cells. Peritoneal tumors were excised from MES-OV injected athymic nude mice after the treatment period. (A) Mouse body weight throughout the experiment. The solid arrow indicates when treatment was started and the dotted arrow indicates when the dosing schedule was reduced. (B) Tumor free rates in the treatment groups, Logistic Regression Model: Mebendazole (p = 0.005, OR = 27.535, 95% CI: 2.725, 278.265) and PRIMA-1MET (p = 0.182, OR = 4.998, 95% CI: 0.472, 52.936) functioned additively in preventing tumor development. # Fisher’s exact test demonstrated that the combination was more effective compared to control p = 0.015 and compared to PRIMA-1METp = 0.043. (C) 20× imaging of H & E staining of liver and kidney specimen of mice tumor model to determine toxicity of drug combination.
4. Discussion
This study demonstrates, for the first time, preclinical efficacy of mebendazole against human ovarian cancer cell lines with confirmation of in vivo therapeutic and maintenance therapy activity in PDX and orthotopic models. Due to the high frequency of p53 mutations in ovarian cancer, the cytotoxic potencies of mebendazole across a series of cell lines with various p53 status were compared. Mebendazole exhibited IC50 values in the nanomolar to micromolar range across all cell lines tested regardless of their p53 statuses, however the p53 null cell line was the least sensitive to mebendazole inhibition of growth and clonogenic survival. Combination treatment of mebendazole with the p53 reactivator, PRIMA-1MET exerted synergistic cytotoxicity for all cell lines regardless of p53 status. Parallel evaluation of the isogenic SKOV3 p53 null parental cell line, SKOV3 R273H subline and SKOV3 R248W subline verified that the p53-independence observed was not due to other heterogeneities present in the non-isogenic cell lines.
Mechanistic studies indicated PRIMA-1MET increased p21 levels only in cell lines that harbored missense mutant p53, which is consistent with the p21 induction by wild type p53 and the p53 reactivation activity of PRIMA-1MET. Mebendazole induced p21 expression and tubule depolymerization, without inducing p53 levels. While PRIMA-1MET did not enhance the p21 induction, it did interfere with the tubule depolymerization activity of mebendazole. Our observation of synergistic inhibition of growth and induction of apoptosis by the drug combination compared to the single drug treatments, suggest that PRIMA-1MET interference with tubule depolymerization is not sufficient to prevent the drug synergy. Therefore, p53-independent off-target activities reported for PRIMA-1MET are likely to contribute to its mechanism of synergy with mebendazole [22–24]. Our previous study also documented that p53-independent mechanisms contribute to the synergy of PRIMA-1MET with a novel small molecule drug, SHetA2 (NSC 726189) in ovarian cancer cell lines [25].
Significant reduction in growth of a p53 null and a p53 positive PDX ovarian cancer model is consistent with the ability of mebendazole to induce p53-independent apoptosis and suggests that ovarian cancer patients with p53 null or p53 positive tumors will respond to mebendazole therapy. The cisplatin resistant status of these two PDX models suggests that mebendazole could be effective in treating platinum-resistant ovarian cancer. We also explored the potential of mebendazole for use ovarian cancer maintenance therapy to prevent tumor recurrence after platinum-based chemotherapy. Both mebendazole and PRIMA-1MET significantly reduced tumor establishment (increased the tumor free rate) in our missense mutant p53 orthotopic model of ovarian cancer maintenance therapy. While no additive or synergistic interaction of the two drugs was observed in this model, additional studies with varied dose combinations are needed to evaluate additive or synergistic effects.
In clinical studies, maintenance therapy with the vascular endothelial growth factor (VEGF)-targeted angiogenesis inhibitor, bevacizumab, prolonged progression free survival for both newly diagnosed and platinum sensitive recurrent disease, and prolonged overall survival by 5 months when used for maintenance therapy after treatment of platinum sensitive recurrent disease [2,5,26]. Although the overall survival benefit is statistically significant, the difference of only 5 months highlights the need for superior or combination maintenance strategies to enable a more clinically meaningful difference.
The prolonged nature of maintenance therapy requires minimal or tolerable toxicity to be feasible. In our study, mebendazole toxicity was observed by a significant reduction in body weight at the highest dose used daily oral administration of 50 mg/kg. This toxicity was controlled by a dose reduction to every other day treatment. The restoration of body growth and the lack of histologic liver or kidney damage in specimens collected at the end of the experiment confirm that the dose-reduction controlled the toxicity. Extensive world-wide use of mebendazole has found this drug to be safe with side effects limited to abdominal pain and discomfort, flatulence, and diarrhea at low dose {Guerini, 2019 #9802}. At higher doses, case reports of rare side effects (neutropenia, marrow aplasia alopecia, allergic reaction, elevations in transaminases) most often occurred in patients with altered drug metabolism or hypersensitivity and were usually reversible with complete recovery after a few days of drug {Colle, 1999 #9804;Fernández-Bañares, 1986 #9803}.
In conclusion, mebendazole exhibits potent anti-cancer activity against ovarian cancer cell lines in cell culture and in PDX and orthotopic mouse models. The p53-independent nature of the mechanism indicates that the high rate of p53 mutation in ovarian cancers will not interfere with mebendazole’s clinical activity. Sensitivities of two cisplatin-resistant PDX models to mebendazole offers promising data to support use of this drug as a second line treatment after failure of platinum-based chemotherapy. A significant increase in the number of tumor free mice in our maintenance therapy model suggests that mebendazole may be an effective maintenance therapy to increase the progression free survival of ovarian cancer patients after platinum-based chemotherapy. Reversal of mebendazole toxicity with dose reduction confirmed that potential toxicity in clinical trials could be controlled with careful monitoring of side effects and dose reduction when relevant. Overall, these results support development of clinical trials evaluating mebendazole for ovarian cancer treatment.
Supplementary Material
HIGHLIGHTS.
Mebendazole inhibits growth of ovarian cancer cell lines and high grade serous ovarian PDX tumors.
Mebendazole inhibits tumor establishment in an orthotopic ovarian cancer model of maintenance therapy.
Mebendazole’s activity is related to p21 elevation and tubule destabilization, and complemented by mutant p53 reactivation.
Acknowledgments
This research was funded by Presbyterian Health Foundation (PHF) Clinician Scientist Development Grant to Dr. Gunderson. We thank the Center for Cancer Prevention and Drug Development (CCPDD) at the Stephenson Cancer Center University of Oklahoma Health Sciences Center for funding the PRIMA-1MET synthesis. Research reported in this publication was supported in part by the National Cancer Institute Cancer Center Support Grant P30CA225520 awarded to the University of Oklahoma Stephenson Cancer Center through use of the Biostatistics and Research Design, Cancer Tissue Pathology and Molecular Imaging Cores. We thank Katlyn D. Benbrook and Sadaf Anya Saeed for assisting in the necropsy for the orthotopic model. The graphical abstract was created with BioRender.com.
Footnotes
Declaration of Competing Interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2020.10.010.
References
- [1].Siegel R, Ma J, Zou Z, Jemal A, Cancer statistics, 2014, CA Cancer J. Clin 64 (2014) 9–29. [DOI] [PubMed] [Google Scholar]
- [2].Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. , Incorporation of bevacizumab in the primary treatment of ovarian cancer, N. Engl. J. Med 365 (2011) 2473–2483. [DOI] [PubMed] [Google Scholar]
- [3].Gonzalez-Martin A, Pothuri B, Vergote I, DePont Christensen R., Graybill W, Mirza MR, et al. , Niraparib in patients with newly diagnosed advanced ovarian cancer, N. Engl. J. Med 381 (2019) 2391–2402. [DOI] [PubMed] [Google Scholar]
- [4].Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. , Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer, N. Engl. J. Med 379 (2018) 2495–2505. [DOI] [PubMed] [Google Scholar]
- [5].Oza AM, Cook AD, Pfisterer J, Embleton A, Ledermann JA, Pujade-Lauraine E, et al. , Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial, Lancet Oncol. 16 (2015) 928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Spagnuolo PA, Hu J, Hurren R, Wang X, Gronda M, Sukhai MA, et al. , The antihelmintic flubendazole inhibits microtubule function through a mechanism distinct from Vinca alkaloids and displays preclinical activity in leukemia and myeloma, Blood. 115 (2010) 4824–4833. [DOI] [PubMed] [Google Scholar]
- [7].Dogra N, Kumar A, Mukhopadhyay T, Fenbendazole acts as a moderate microtubule destabilizing agent and causes cancer cell death by modulating multiple cellular pathways, Sci. Rep 8 (2018) 11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Duan Q, Liu Y, Rockwell S, Fenbendazole as a potential anticancer drug, Anticancer Res. 33 (2013) 355–362. [PMC free article] [PubMed] [Google Scholar]
- [9].Ghasemi F, Black M, Vizeacoumar F, Pinto N, Ruicci KM, Le C, et al. , Repurposing Albendazole: new potential as a chemotherapeutic agent with preferential activity against HPV-negative head and neck squamous cell cancer, Oncotarget. 8 (2017) 71512–71519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hou ZJ, Luo X, Zhang W, Peng F, Cui B, Wu SJ, et al. , Flubendazole, FDA-approved anthelmintic, targets breast cancer stem-like cells, Oncotarget. 6 (2015) 6326–6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lin S, Yang L, Yao Y, Xu L, Xiang Y, Zhao H, et al. , Flubendazole demonstrates valid antitumor effects by inhibiting STAT3 and activating autophagy, J. Exp. Clin. Cancer Res 38 (2019) 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Oh E, Kim YJ, An H, Sung D, Cho TM, Farrand L, et al. , Flubendazole elicits anti-metastatic effects in triple-negative breast cancer via STAT3 inhibition, Int. J. Cancer 143 (2018) 1978–1993. [DOI] [PubMed] [Google Scholar]
- [13].Guerini AE, Triggiani L, Maddalo M, Bonu ML, Frassine F, Baiguini A, et al. , Mebendazole as a candidate for drug repurposing in oncology: an extensive review of current literature, Cancers (Basel). (2019) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dogra N, Mukhopadhyay T, Impairment of the ubiquitin-proteasome pathway by methyl N-(6-phenylsulfanyl-1H-benzimidazol-2-yl)carbamate leads to a potent cytotoxic effect in tumor cells: a novel antiproliferative agent with a potential therapeutic implication, J. Biol. Chem 287 (2012) 30625–30640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rechsteiner M, Zimmermann AK, Wild PJ, Caduff R, von Teichman A, Fink D, et al. , TP53 mutations are common in all subtypes of epithelial ovarian cancer and occur concomitantly with KRAS mutations in the mucinous type, Exp. Mol. Pathol 95 (2013) 235–241. [DOI] [PubMed] [Google Scholar]
- [16].Lai SR, Castello SA, Robinson AC, Koehler JW, In vitro anti-tubulin effects of mebendazole and fenbendazole on canine glioma cells, Vet. Comp. Oncol 15 (2017) 1445–1454. [DOI] [PubMed] [Google Scholar]
- [17].Nielsen AT, Systems with bridgehead nitrogen. β-Ketols of the 1-Azabicyclo [2.2. 2] octane series, J. Organ. Chem 31 (1966) 1053–1059. [Google Scholar]
- [18].Chou T-C, Talalay P, Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors, Adv. Enzym. Regul 22 (1984) 27–55. [DOI] [PubMed] [Google Scholar]
- [19].Poruchynsky MS, Kim J-H, Nogales E, Annable T, Loganzo F, Greenberger LM, et al. , Tumor cells resistant to a microtubule-depolymerizing hemiasterlin analogue, HTI-286, have mutations in α-or β-tubulin and increased microtubule stability, Biochemistry. 43 (2004) 13944–13954. [DOI] [PubMed] [Google Scholar]
- [20].Chu SW, Badar S, Morris DL, Pourgholami MH, Potent inhibition of tubulin polymerisation and proliferation of paclitaxel-resistant 1A9PTX22 human ovarian cancer cells by albendazole, Anticancer Res. 29 (2009) 3791–3796. [PubMed] [Google Scholar]
- [21].Chaudhary S, Garg T, Rath G, Murthy RR, Goyal AK, Enhancing the bioavailability of mebendazole by integrating the principles solid dispersion and nanocrystal techniques, for safe and effective management of human echinococcosis, Artific. Cells Nanomed. Biotechnol 44 (2016) 937–942. [DOI] [PubMed] [Google Scholar]
- [22].Tessoulin B, Descamps G, Moreau P, Maiga S, Lode L, Godon C, et al. , PRIMA-1Met induces myeloma cell death independent of p53 by impairing the GSH/ROS balance, Blood. 124 (2014) 1626–1636. [DOI] [PubMed] [Google Scholar]
- [23].Sobhani M, Abdi J, Manujendra SN, Chen C, Chang H, PRIMA-1Met induces apoptosis in Waldenstrom’s Macroglobulinemia cells independent of p53, Cancer Biol. Ther 16 (2015) 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lu T, Zou Y, Xu G, Potter JA, Taylor GL, Duan Q, et al. , PRIMA-1Met suppresses colorectal cancer independent of p53 by targeting MEK, Oncotarget. 7 (2016) 83017–83030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ramraj SK, Elayapillai SP, Pelikan RC, Zhao YD, Isingizwe ZR, Kennedy AL, et al. , Novel ovarian cancer maintenance therapy targeted at mortalin and mutant p53, Int. J. Cancer 147 (4) (2020) 1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, et al. , Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial, Lancet Oncol. 18 (2017) 779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


