Abstract
We review progress towards greater mechanistic understanding and clinical translation of a strategy to improve respiratory and non-respiratory motor function in people with neuromuscular disorders, therapeutic acute intermittent hypoxia (tAIH). In 2016 and 2020, workshops to create and update a “road map to clinical translation” were held to help guide future research and development of tAIH to restore movement in people living with chronic, incomplete spinal cord injuries. After briefly discussing the pioneering, non-targeted basic research inspiring this novel therapeutic approach, we then summarize workshop recommendations, emphasizing critical knowledge gaps, priorities for future research effort, and steps needed to accelerate progress as we evaluate the potential of tAIH for routine clinical use. Highlighted areas include: 1) greater mechanistic understanding, particularly in non-respiratory motor systems; 2) optimization of tAIH protocols to maximize benefits; 3) identification of combinatorial treatments that amplify plasticity or remove plasticity constraints, including task-specific training; 4) identification of biomarkers for individuals most/least likely to benefit from tAIH; 5) assessment of long-term tAIH safety; and 6) development of a simple, safe and effective device to administer tAIH in clinical and home settings. Finally, we update ongoing clinical trials and recent investigations of tAIH in SCI and other clinical disorders that compromise motor function, including ALS, multiple sclerosis, and stroke.
Keywords: intermittent hypoxia, plasticity, translation, therapeutic, breathing, walking, arm, hand, spinal cord injury, SCI, amyotrophic lateral sclerosis, ALS, multiple sclerosis, MS, task-specific training, combinatorial treatment, barriers, road map
Introduction
In this review, we update recent advances towards greater mechanistic understanding and clinical translation of a promising new strategy to improve respiratory and non-respiratory motor function in people with neuromuscular clinical disorders, therapeutic acute intermittent hypoxia (tAIH; preferred abbreviation in rehabilitation context). Since this concept is most advanced in the context of restoring breathing and limb function after spinal cord injury (SCI), we emphasize progress towards restoring function in people with chronic, incomplete SCI. After providing background concerning rehabilitation strategies after SCI, we briefly discuss the history of basic research leading to the realization that tAIH could be a viable treatment strategy to improve breathing after SCI, and the surprise realization that it also improves limb motor function.
The history of tAIH research is a prime example of translational research founded in basic science not initially targeting clinical application. Nevertheless, those fundamental discoveries evolved into a state-of-the-art best characterized as dynamical and bi-directional clinical translation, with the intent of improving respiratory and non-respiratory motor function in people living with chronic, incomplete SCI. Although basic research inspired the innovative concept that tAIH-induced neuroplasticity could be translated for therapeutic advantage, subsequent studies in humans with chronic, incomplete SCI raised new questions that informed the need for additional pre-clinical research in rodent models. Thus, although the history of tAIH exemplifies the concept of “bench-to-bedside” research, it is more accurately portrayed as a continuous, recurrent cycle with net forward momentum towards full scale, multisite clinical trials.
After reviewing the history of tAIH in the context of SCI, we identify additional areas in need of additional research or development and explore recent efforts to investigate the potential of tAIH as a therapeutic modality for other clinical disorders that compromise breathing and/or limb function, such as ALS, multiple sclerosis and stroke.
Therapeutic AIH and neurorehabilitation with SCI
Neurorehabilitation strategies for SCI.
Since regeneration of the injured spinal cord has proven to be an elusive goal despite decades of intensive effort, an alternative strategy is to take advantage of spared neural pathways, and their intrinsic capacity for spontaneous and induced neuroplasticity (Krucoff et al., 2016; Mitchell, 2008; Ramer et al., 2000). Although some spontaneous plasticity occurs in spared neural circuits following injury, leading to meaningful gains in motor function, this spontaneous recovery is most prominent in the first 3-6 months post-injury and is ultimately limited (Onifer et al., 2011). Even after augmenting spontaneous recovery with intensive rehabilitation, most survivors have persistent sensorimotor deficits (Sezer et al., 2015). Since there is a pressing need for novel interventions, neurorehabilitation researchers have begun developing neurobiologically informed strategies to enhance recovery via novel treatments that drive plasticity in spared neural pathways (Warraich and Kleim, 2010).
Therapeutic AIH and SCI.
tAIH is a promising therapeutic modality that elicits spinal synaptic plasticity, strengthening descending neural pathways to spinal motor neurons (Christiansen et al., 2021, 2018; Dale et al., 2014; Dale-Nagle et al., 2010; Navarrete-Opazo and Mitchell, 2014; Vinit et al., 2009; Welch et al., 2020). Initial studies demonstrated tAIH-induced plasticity in respiratory motor systems, restoring breathing function after SCI (Fuller et al., 2003; Golder and Mitchell, 2005; Lovett-Barr et al., 2012; Navarrete-Opazo et al., 2015; A. Navarrete-Opazo et al., 2017). However, although not originally anticipated, AIH also elicits plasticity in limb motor systems in both rodent models and humans with chronic incomplete SCI, including forelimb/arm/hand (Lovett-Barr et al., 2012; Prosser-Loose et al., 2015; Sandhu et al., 2021; Trumbower et al., 2017), as well as leg strength and walking ability (Trumbower et al., 2012; Hayes et al., 2014; Lynch et al., 2017; Angela Navarrete-Opazo et al., 2017a, 2017b). Therapeutic AIH-induced benefits in restoring limb function require combination with task-specific training (Hayes et al., 2014; Prosser-Loose et al., 2015; Welch et al., 2020). Similar combinatorial treatment effects on breathing are unknown, but are under active investigation.
This review emphasizes priorities for tAIH translational research identified in a series of workshops held in 2016 and 2020 in Atlantic Beach, Florida, organized by the University of Florida Breathing Research and Therapeutics (BREATHE) Center, and co-sponsored by the Craig H. Neilsen Foundation and Wings for Life. Workshop recommendations included focus on: 1) greater mechanistic understanding, particularly in non-respiratory motor systems; 2) optimization of tAIH protocols to maximize therapeutic benefits; 3) identification of combinatorial treatments that amplify plasticity or remove plasticity constraints, including task specific training; 4) biomarkers to determine those most/least likely to benefit from tAIH; 5) safety of long-term tAIH therapy; and 6) development of a simple, safe and effective device to administer tAIH in clinical and “in-home” settings.
Workshop participants emphasized the need for studies of combinatorial treatments to maximize functional benefits. For example, participants emphasized the need for greater understanding of: 1) mechanisms giving rise to the synergistic relationship between tAIH and task specific training; 2) drugs that may optimize tAIH efficacy, either enhancing its fundamental mechanism or relieving factors the undermine its efficacy; and/or 3) combined effects of tAIH with electrical and/or optogenetic/chemogenetic neural stimulation. There is also a clear need to establish validated tAIH delivery systems that monitor/record performance to facilitate regulatory approval (FDA), and to develop standards and common data elements for seamless integration of tAIH with existing clinical protocols.
An important goal of the workshops was to facilitate an interdisciplinary research culture supported by professional societies, foundations, industry, and government agencies, and to accelerate knowledge transfer between scientists and practicing clinicians as we seek better SCI treatment solutions. This special edition of Experimental Neurology concerning the impact of intermittent hypoxia on the nervous system is a natural extension of these workshops, featuring over 30 novel research contributions in this thriving research field. Discoveries made as we explore the potential of tAIH and climb the “mountain” of translation are illustrated in Figure 1. We have not yet reached the “mountain peak” (ie. large scale clinical trials), and many international laboratories are actively working to overcome remaining challenges.
Figure 1: Overview of key steps towards tAIH translation.
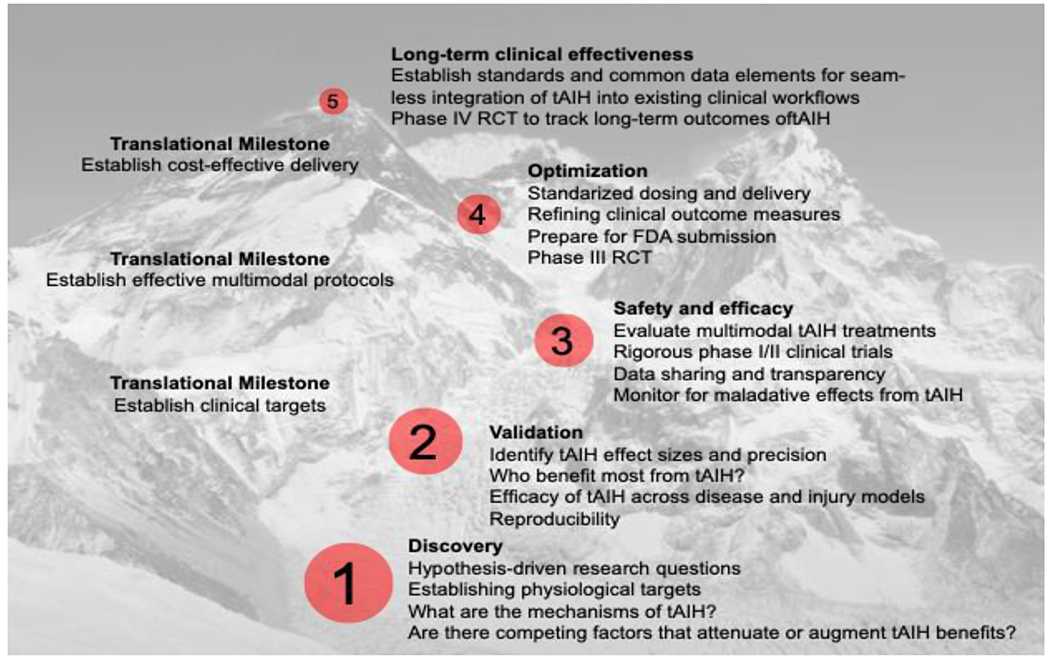
Step 1: Formulate hypothesis driven questions leading to discovery and mechanisms of AIH-induced plasticity. Step 2: Validate tAIH protocols across patients and populations, ensuring reproducibility by identifying those most likely to benefit from tAIH therapy. Step 3: Verify safety and efficacy of tAIH in health and injury or disease. Step 4: Determine optimal tAIH dose and delivery and refine outcome measures to assure safety and detect tAIH effects (and develop adequate device to deliver refined protocols). Step 5: Establish standards for tAIH delivery and practice for randomized control trials (RCT) seeking to track long-term outcomes. Only when all 5 steps have been taken will we be ready to apply tAIH in routine clinical practice.
Translational Roadmap for therapeutic AIH
Therapeutic AIH has considerable potential to improve the efficiency, outcomes and costs of SCI rehabilitation, and promote motor recovery in a range of other clinical neuromuscular disorders. However, there is a clear need for further testing and development of tAIH as a therapeutic modality before it will be appropriate and accepted for widespread clinical use. After a brief presentation of the history of the field, and a synopsis of the current state-of-the-art, we discuss remaining and perceived ‘road blocks’ and performance milestones necessary for broad adoption of tAIH as a therapeutic modality (Figure 1).
The roadmap created by the participants in the 2016 workshop (see footnote) described 4 developmental stages needed for the progression from basic discovery to promising therapeutic: innovation, translation, optimization and application. In the first section, we describe basic science discoveries leading to the realization of tAIH as a viable treatment strategy (innovation); this history will help readers understand the origins of this unique approach and provide a framework for understanding required next steps. In the second section, we discuss the bidirectional translational cycle characterizing tAIH research (translation). In the third, we describe priorities to optimize and validate tAIH for clinical use in humans with chronic SCI (optimization). In the fourth, unanswered questions concerning tAIH are featured (application). Finally, we consider industrial partnerships that (hopefully) will drive progress towards tAIH clinical utilization in home settings, and the potential for applications in other clinical disorders.
Innovation: basic science and early translational tAIH research
Pioneering discoveries in respiratory motor plasticity:
The scientific framework establishing the concept that AIH-induced spinal motor plasticity could be harnessed for therapeutic advantage is based on foundational work concerning AIH-induced plasticity in the respiratory motor system, largely in rats (for reviews see: Dale et al., 2014; Devinney et al., 2013; Feldman et al., 2003; Fields and Mitchell, 2015; Gonzalez-Rothi et al., 2015; Mahamed and Mitchell, 2007; Mitchell et al., 2001; Mitchell and Johnson, 2003). The field was inspired by pioneering experiments in the early 1980s by Millhorn and colleagues at the University of North Carolina (for review, see: Eldridge and Millhorn, 1986). They applied repetitive electrical stimulation to the cut central end of the carotid sinus nerve, which conveys hypoxia-activated carotid chemo-afferent neuron activity to the brainstem; these experiments demonstrated a persistent increase in respiratory-related phrenic nerve activity for hours after the stimulation had ended (Millhorn et al., 1980a), and that this respiratory “memory” requires the neurochemical serotonin (Millhorn et al., 1980b).
The use of AIH (versus electrical stimulation of the carotid sinus nerve) to stimulate carotid chemo-afferent neurons was first elaborated in the early 1990s, with the demonstration that AIH and carotid sinus nerve stimulation elicit similar respiratory motor plasticity in multiple respiratory nerves, including the phrenic, intercostal and hypoglossal nerves (Hayashi et al., 1993; Bach and Mitchell, 1996; Fregosi and Mitchell, 1994). Following AIH, brainstem serotonergic neuron activation and serotonin release near the phrenic motor nucleus is both necessary and sufficient for phrenic motor facilitation (Baker-Herman and Mitchell, 2002; Kinkead et al., 2001; MacFarlane et al., 2009; Figure 2a). In specific, spinal serotonin 2 receptor activation is necessary to initiate, but not to maintain AIH-induced phrenic motor plasticity (Fuller et al., 2001).
Figure 2: Schematic representations of tAIH-Induced motor plasticity.
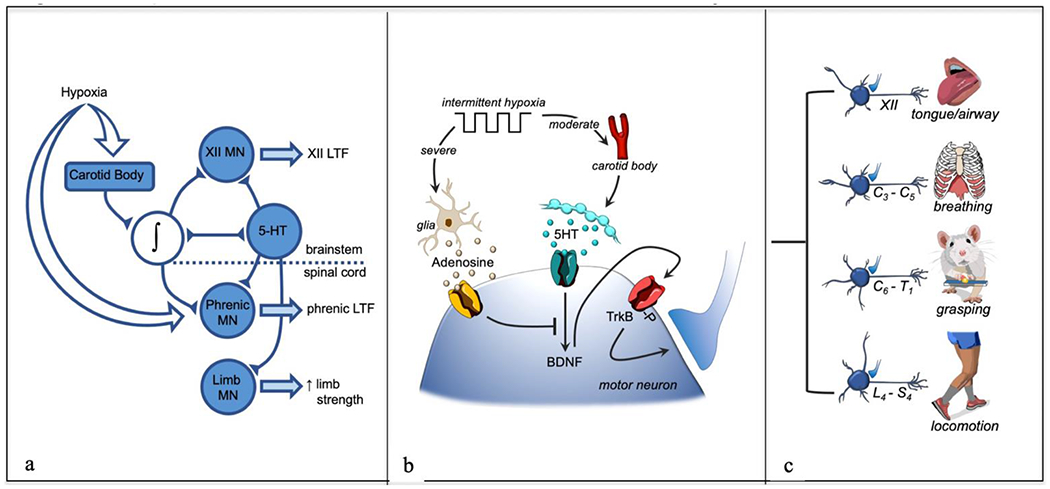
In panel a: Neural network model of tAIH-induced plasticity, including hypoglossal (XII), phrenic and limb motor neurons (MN). In panel b: Working model of cellular mechanisms giving rise to tAIH-induced motor plasticity. Moderate acute intermittent hypoxia (Po2 >40 mmHg) activates carotid body chemo-afferent neurons that then activate raphe serotonergic neurons and trigger serotonin release in widespread motor nuclei. Serotonin activates Gq coupled 5-HT receptors, initiating phrenic motor facilitation via the Q pathway (serotonin-induced, BDNF protein synthesis). Spinal tissue hypoxia also stimulates glia to release of ATP/adenosine into the extracellular space; subsequent binding to Gs coupled adenosine 2A receptors on motor neurons elicits a distinct intracellular signaling cascade that either: 1) elicits a distinct form of phrenic motor plasticity (but only at severe levels of tissue hypoxia), and/or 2) inhibits the Q pathway via cross-talk inhibition. In panel c: tAIH effects are widespread among motor neuron pools, leading to plasticity of many motor systems, including: tongue/upper airway (XII), breathing (C3-C5), grasping (C6-T1) and locomotion (L4-S4). Thus, tAIH elicits global effects on motor neurons that elicit outcomes depending on the target muscle. The impact of AIH is enhanced by combination with task specific training, amplifying BDNF/TrkB effects within the targeted (task specific) motor system.
Another important feature of AIH-induced phrenic motor plasticity is that preconditioning with intermittent hypoxia enhances facilitation following a single AIH presentation (Fields and Mitchell, 2015; Ling et al., 2001; Wilkerson and Mitchell, 2009). Enhanced plasticity (ie. “metaplasticity;” Fields et al., 2015) following intermittent hypoxia preconditioning is clinically relevant since the functional benefits of tAIH are expected to accumulate with repetitive exposures. Mechanisms giving rise to this form of metaplasticity are under active investigation. After decades of basic research, we gained reasonable understanding of cellular/synaptic mechanisms giving rise to AIH-induced phrenic motor plasticity (E. A. Dale-Nagle et al., 2010). Our goal in the present review is to highlight those mechanistic discoveries necessary to inspire and guide clinical translation. A detailed presentation of all known intracellular and intercellular mechanisms giving rise to or regulating AIH-induced phrenic motor plasticity is outside the scope of the present review.
Of particular importance with respect to SCI treatment, episodic spinal serotonin release and serotonin receptor activation within the phrenic motor nucleus are necessary and sufficient for AIH-induced phrenic motor plasticity (Baker-Herman and Mitchell, 2002; Tadjalli and Mitchell, 2019). Based on localization of serotonin effects, it was suggested that AIH-induced plasticity could be harnessed to strengthen spinal respiratory motor output after incomplete cervical SCI, or with other disorders that compromise breathing ability (Fuller et al., 2003; Mitchell, 2008; Fuller and Mitchell, 2017). However, there was some concern that functional benefits would come with a cost, since “high dose” intermittent hypoxia, such as experienced during sleep apnea, elicits pathology (Durán et al., 2001; Mateika and Narwani, 2009; Navarrete-Opazo and Mitchell, 2014; Zhang et al., 2012).
Early translation in respiratory motor system:
In the early 2000s, efforts to harness repetitive AIH as a therapeutic modality to restore breathing function in rat models of chronic, incomplete cervical spinal injury began (E. A. Dale-Nagle et al., 2010; Fuller et al., 2003; Golder and Mitchell, 2005; Vinit et al., 2009). We now know that: “low dose” tAIH restores lost respiratory function in rats with acute (< 2 weeks) cervical spinal injuries (Lovett-Barr et al., 2012; Navarrete-Opazo et al., 2015), but is considerably less effective with chronic SCI (Dougherty et al., 2018; Gonzalez-Rothi et al., 2021; Angela Navarrete-Opazo et al., 2017a). Thus, to improve breathing with chronic spinal cord injury, it is important to understand the mechanistic basis of this limitation, and to optimize tAIH protocols for maximal therapeutic benefit. The limited tAIH benefits observed thus far with chronic SCI may be explained by recent discoveries that cervical SCI causes relative tissue hypoxia below the injury site (ie. the phrenic motor nucleus; Perim et al., 2021) and/or that systemic/neuroinflammation, such as that experienced after spinal cord injury, impairs tAIH-induced plasticity (Huxtable et al., 2013, 2011) (see below).
Surprise translation to other motor systems:
Along the way, a surprise realization was that AIH and repetitive AIH also affect non-respiratory motor systems. This realization was initially inspired by unpublished preliminary data suggesting that a single AIH presentation increases brain-derived neurotrophic factor (BDNF) protein levels in the C7-C8 ventral spinal cord in uninjured rats (Baker-Herman and Mitchell, unpublished), just as it does in more rostral ventral spinal segments encompassing the phrenic motor nucleus (C3-C5; Baker-Herman et al., 2004). In agreement, repetitive AIH increases BDNF immunostaining in phrenic and non-phrenic motor neurons of the C3-C5 spinal cord (Hassan et al., 2018; Lovett-Barr et al., 2012; Satriotomo et al., 2012) as well as in non-respiratory motor neurons of the caudal cervical (C7) and lumbar (L3) spinal cord (Hassan et al., 2018; Lovett-Barr et al., 2012; Satriotomo et al., 2016). We subsequently demonstrated that AIH and/or repetitive AIH improve limb function in spinally injured rats (Lovett-Barr et al., 2012; Prosser-Loose et al., 2015) and humans (Hayes et al., 2014; Trumbower et al., 2012). Thus, we posed the hypothesis that AIH initiates plasticity through similar cellular mechanisms in motor neurons innervating respiratory (phrenic, hypoglossal, intercostal) and non-respiratory motor neurons (Figure 2c; Dale et al., 2014). In this scheme, functional outcomes from the same/similar cellular mechanism vary, depending on muscle targets of the respective motor neuron pools (Figure 2c). These findings opened the door to harness tAIH as a therapeutic modality for diverse motor systems.
The benefits of combining AIH and task specific training:
One critical feature revealed when AIH effects were studied in non-respiratory motor systems is that tAIH effects on limb function are amplified by sequential, combinatorial presentations of AIH and task specific training (Welch et al., 2020). For example, when people with chronic, incomplete SCI are given AIH alone daily for 5 days, walking speed is increased slightly 3 days later (Hayes et al., 2014). When these same subjects practice walking for 30 minutes each day for 5 days, minimal functional benefits are observed. However, when daily AIH is followed by 30 minutes of walking practice for five days, synergistic outcomes are observed 3 days later, expressed as a 38% increase in the distance walked during the 6-minute walk test. Even more dramatically, whereas neither horizontal ladder walking practice nor daily AIH (7 days) alone significantly improved horizontal ladder walking ability in rats, AIH followed 30 minutes later by ladder walking practice elicits nearly complete functional recovery (Lovett-Barr et al., 2012; Prosser-Loose et al., 2015). In the latter study, the requirement for task specificity was demonstrated by the failure of paired daily AIH and treadmill running to improve horizontal ladder walking performance (Prosser-Loose et al., 2015). Thus, with respect to limb function, tAIH acts as a “plasticity primer,” augmenting the effects of task-specific rehabilitation. The mechanistic basis of AIH and task specific training synergy is not known, but we recently published an hypothesis to explain this important interaction (Welch et al., 2020). On the other hand, not all paired synaptic inputs behave in a similar way. For example, whereas either paired pulse facilitation or AIH alone enhance transcranial magnetic stimulation-evoked cortical potentials in the first interosseous muscle of the hand, the effects were additive when both were combined (Christiansen et al., 2021, 2018). Further, in the respiratory motor system, the impact of combined tAIH and task specific training is unknown, but is under active investigation (see ‘ongoing human trials’ section below).
Translation: a “two-way street”
Participants1 at the 2016 tAIH workshop emphasized that the scientific progression and potential of therapeutic AIH as a rehabilitative tool should continue along a “two-way street”: a basic and clinical scientific collaboration of ‘bench to bedside’ and ‘bedside to bench’. Highlighted by the workshop, the pillar of this approach has been prioritization of regular communication and engagement between those conducting mechanistic studies of efficacy or pathogenesis, and those translating findings to humans. Basic, pre-clinical research has uncovered mechanisms of AIH-induced motor plasticity, which then informed human studies; as a result, more than 15 human trials have been published suggesting functional benefits of AIH in people with SCI. Nevertheless, despite the 20+ year history of basic research concerning mechanisms of AIH-induced plasticity, mostly in the phrenic/diaphragm motor system (Mitchell et al., 2001; Feldman et al., 2003; Mitchell, 2008), it is not surprising tAIH translation has been challenging.
For example, initial investigations concerning the impact of AIH on breathing at rest in humans (ie. ventilatory long-term facilitation) produced equivocal results, suggesting: 1) minimal ventilatory long-term facilitation in awake humans (Jordan et al., 2002; McEvoy et al., 1996; White, 2007); 2) expression of AIH-induced ventilatory facilitation only during NREM sleep in humans (Babcock et al., 2003; Babcock and Badr, 1998; Chowdhuri et al., 2008; Pierchala et al., 2008); 3) AIH elicits robust ventilatory long-term facilitation if a background of slight hypercapnia is maintained in awake humans (Harris et al., 2006; Mateika and Sandhu, 2011; Mateika and Syed, 2013) or hypercapnia is added during hypoxic episodes (Vermeulen et al., 2020); 4) ventilatory long-term facilitation varies with time of day (Gerst et al., 2011); and 5) ventilatory long-term facilitation is enhanced in otherwise healthy subjects with obstructive sleep apnea (Khodadadeh et al., 2006; Lee et al., 2009). Thus, a range of biological (eg. sleep, time of day), pathological (eg. sleep apnea) and methodological issues (eg. background hypercapnia or hypercapnic hypoxic episodes) must be considered as we develop tAIH as a therapeutic modality to restore breathing ability in people living with chronic SCI.
The first human study utilizing AIH to increase leg strength in people with chronic, incomplete SCI was published in 2012 (Trumbower et al., 2012). After success in multiple studies investigating limb function (Christiansen et al., 2021, 2018; Hayes et al., 2014; Lynch et al., 2017; Angela Navarrete-Opazo et al., 2017a; Navarrete-Opazo et al., 2015; Tan et al., 2021; Trumbower et al., 2017, 2012), several clinical trials of tAIH and limb or respiratory function in humans living with SCI have been initiated (see below). Further, tAIH is being explored in other clinical disorders that compromise movement (eg. ALS, MS).
Even with such rapid progress, the tAIH workshops in 2016 & 2020 emphasized the need for more basic research to optimize AIH protocols for therapeutic benefit (ie. maximize plasticity), to explore similarities and differences in mechanisms amplifying function in respiratory versus non-respiratory motor systems, and to understand the mechanistic basis for powerful synergies between AIH and task-specific training. Multiple areas requiring additional research and development were highlighted in the workshops, including the need for: 1) greater mechanistic understanding in non-respiratory motor systems; 2) optimization of tAIH protocols to maximize benefits; 3) identification of combinatorial treatments that amplify plasticity, including task specific training; 4) identify biomarkers for individuals most/least likely to benefit from tAIH; 5) assess safety of long-term tAIH; and 6) develop a suitable device to administer tAIH in clinic and home settings. These goals are discussed in the following sections.
Optimizing tAIH protocols through mechanistic understanding
Defining an “optimal protocol” for tAIH is an important but elusive goal since there are so many variables, leading to an unmanageable number of possible protocol permutations. Optimal protocols may involve changes in details of the tAIH protocol, such as the severity and duration of hypoxic episodes, the interval between them, the number and pattern of episodes per day, the number and pattern of daily treatments, implementation of carbon dioxide regulation and the time of day in which tAIH is administered. Optimal protocols may differ among individuals, the nature of their injury (eg. location, severity, chronicity, etc), the motor system requiring treatment (eg. breathing versus limb function, etc.), and biological variables such as age, sex, genetics, and previous experiences (eg. epigenetics). The number of potential combinations and permutations is so great, a firm grasp of mechanisms giving rise to tAIH-induced motor plasticity is essential to guide our efforts.
Investigations of tAIH to date are largely based on relatively short exposures that elicit effects of limited duration. Several lines of evidence suggest that the persistence of functional benefits depends on the duration of tAIH treatment, or other protocol details, such as combination with task specific training. For example, a single AIH presentation lasting 30-120 minutes augments respiratory function for less than one day. In contrast, 7 days of daily AIH augments function for more than one week (Angela Navarrete-Opazo et al., 2017a; Navarrete-Opazo et al., 2015). Combinatorial treatments may extend the duration further since 1 week of paired daily AIH plus horizontal ladder walking practice elicits functional ladder walking benefits lasting at least 3 weeks post-treatment (Lovett-Barr et al., 2012; Prosser-Loose et al., 2015). Although brief tAIH protocols elicit meaningful functional recovery of finite duration, the ultimate potential of tAIH to promote lasting recovery after spinal injury is not yet known. Clearly, certain tAIH protocols have the potential for optimization, maximizing and/or extending the duration of functional benefits. This is another area that requires additional research.
Are functional outcomes sensitive to details of the AIH protocol?
AIH-induced motor plasticity was originally demonstrated in the phrenic motor system of anesthetized rats, with AIH episodes consisting of 3 to 5, moderate hypoxic episodes lasting 2 to 5 minutes each (arterial Po2 >35 mmHg), separated by 5-minute intervals (Hayashi et al., 1994; Bach and Mitchell, 1996; Baker and Mitchell, 2000; Mitchell et al., 2001). In only a few studies have other AIH protocols been used, largely because of a tendency to standardize investigations (Mitchell and Terada, 2011). However, when other protocols were used in the same experimental preparation, there are hints that functional outcomes can be enhanced; for example, 3 to 6, 25 second ventilator apneas with 5-minute intervals appear to elicit greater phrenic long-term facilitation than 3, 5 minute hypoxic episodes with 5 minute intervals (Mahamed and Mitchell, 2008). On the other hand, with severe hypoxic episodes (arterial Po2 < 25-30 mmHg), apparently similar phenotypic plasticity arises from a completely distinct mechanism (Nichols et al., 2012). Even more remarkable, AIH consisting of episodes of an intermediate severity of hypoxia (Po2 ~ 30-35 mmHg) fail to elicit meaningful plasticity (Perim and Mitchell, 2019). Thus, the severity and duration of hypoxic episodes exert powerful influences on functional outcomes, demonstrating that tAIH protocol optimization is essential.
Our hypothesis is that protocol specific features are an emergent property, reflecting the compromise between competing mechanisms of phrenic motor plasticity (Devinney et al., 2016, 2013; Perim and Mitchell, 2019). Specifically, we hypothesize the serotonergic pathway to phrenic motor facilitation (known as the Q pathway) dominates with mild or moderate hypoxia, whereas an adenosine-dependent pathway (known as the S pathway) dominates with severe hypoxia. These serotonergic and adenosinergic mechanisms interact via powerful cross-talk inhibition (Dale et al., 2010; Devinney et al., 2013, Perim and Mitchell, 2019). Since the adenosinergic mechanism becomes more powerful as hypoxemia within episodes becomes progressively more severe, there is a gradual shift from serotonin dominance with an adenosine constraint during mild/moderate AIH (Hoffman et al., 2010), to adenosine dominance with a serotonin constraint during severe AIH (Nichols et al., 2012; Perim et al., 2018). With intermediate hypoxemia (Perim and Mitchell, 2019), or when the duration of hypoxia increases (Devinney et al., 2016), co-equal serotonergic and adenosinergic pathway activation cancels plasticity due to balanced (and mutual) cross-talk inhibition. With this complex, interactive system in mind, a strong rationale for tAIH protocol optimization emerges: the most effective and clinically relevant protocols will maximize serotonin-dependent motor facilitation, while minimizing the constraining influence of adenosine. Thus, AIH protocols with shorter, moderate hypoxia episodes may be optimal since short episodes minimize the tissue hypoxia that triggers spinal adenosine accumulation, while still activating the rapid and robust peripheral chemoreflex response triggering serotonin release within motor nuclei (see Figure 2a). The only currently published evidence for this hypothesis is the greater phrenic LTF elicited by 3, 25 second ventilator apneas versus the standard 3, 5-minute hypoxic episodes (Mahamed and Mitchell, 2008). A direct test of this hypothesis is warranted since ventilator apneas also lead to hypercapnic versus the isocapnic hypoxia used in the “standard protocol.”
There is additional evidence for protocol-specificity in humans. For example, whereas a “standard protocol” consisting of 10, 5 minute hypoxic episodes with 5-minute intervals does not elicit long term facilitation of tidal volume in humans while awake (Diep et al., 2007; Jordan et al., 2002; McEvoy et al., 1996; White, 2007) or asleep (Babcock and Badr, 1998; Chowdhuri et al., 2008; Harris et al., 2006; Wadhwa et al., 2008), a protocol consisting of 15, 45 second hypoxic episodes with 45 second intervals does (Pierchala et al., 2008). The study by Pierchala and colleagues (2008) provided the rationale for selecting 15, 1-minute hypoxic episodes with 1-2 minute intervals in subsequent human trials on limb function (Christiansen et al., 2020, 2018; Hayes et al., 2014; Lynch et al., 2017; Angela Navarrete-Opazo et al., 2017a; Sandhu et al., 2021, 2019; Trumbower et al., 2012). However, the impact of protocol differences on limb function have not been directly investigated to date. Further, no data are available demonstrating competition between serotonergic vs. adenosinergic mechanisms in non-respiratory motor pools. Finally, no data demonstrate that refined AIH protocols are more effective at accumulating benefits with repetitive tAIH. These issues are priorities for future research.
Does hypercapnia augment the functional impact of tAIH?
Studies of tAIH effects on locomotor or limb function in humans to date all used poikilocapnic AIH, where arterial Pco2 levels are not controlled during or after hypoxic exposures. Under these conditions, ventilatory long-term facilitation is difficult to detect in normal, awake humans (Diep et al., 2007; Jordan et al., 2002; McEvoy et al., 1996), although AIH-induced ventilatory long-term facilitation is manifested during sleep (Babcock and Badr, 1998), or when CO2 levels are raised slightly above normal (Harris et al., 2006; Wadhwa et al., 2008). It has been proposed that careful CO2 control during and following AIH is a critical feature of LTF induction and/or maintenance (Mateika et al., 2018). Several lines of evidence support this conclusion. First, since CO2 directly activates raphe neurons (Veasey et al., 1995), combined hypoxia and hypercapnia may be synergistic, yielding greater serotonin release and subsequent downstream signaling (a.k.a. phrenic LTF). Further, combined hypoxia and hypercapnia activate peripheral chemoreceptors more than the sum of their individual effects, disproportionately increasing brainstem raphe serotonergic neuron activity. Consequently, hypercapnic hypoxia also elicits ventilatory long-term facilitation in awake humans (Vermeulen et al., 2020). Finally, if long-term facilitation is evoked, it will reduce baseline arterial Pco2, inhibiting chemoreceptors and obscuring LTF expression; in this condition, experimentally restoring arterial Pco2 to pre-AIH baseline values increases the apparent ventilatory LTF in rats (Olson et al., 2001). Thus, it is essential to evaluate the impact of CO2 in optimizing tAIH efficacy. Unfortunately, information available to date is limited to ventilatory LTF, and it is not known if CO2 impacts tAIH effects on non-respiratory motor pools where chemoreceptors may be less influential. The possibility of differential AIH effects on limb versus respiratory motor systems is supported by the observation that identical, poikilocapnic AIH protocols increase transcranial magnetic stimulation evoked motor potentials in finger (Christiansen et al., 2018), but not diaphragm muscle (Welch et al., 2021). Ultimately, it remains to be tested if mild-moderate hypercapnia can enhance the therapeutic efficacy of tAIH in either respiratory or non-respiratory motor pools.
What is the appropriate number of hypoxic episodes and tAIH treatments?
Intermittent hypoxia can be therapeutic or pathogenic, depending on “dose” (Almendros et al., 2014; Dale et al., 2014; Mateika et al., 2015; Navarrete-Opazo and Mitchell, 2014; Zhang et al., 2012). Thus, it is essential to maximize therapeutic benefits, while minimizing pathology by defining the optimal number of hypoxic episodes per day, and the number of treatment days. The dose-dependent outcome following intermittent hypoxia is related to the: a) severity of hypoxia within episodes, b) duration of hypoxic episodes, c) number of hypoxic episodes per day, and d) total duration of exposure (number of days). Accordingly, AIH protocols that impart beneficial plasticity without detectable pathogenesis utilize relatively brief episodes of mild or moderate hypoxia (eg. 1 minute episodes of 9% inspired oxygen; arterial oxygen saturation > 75%), with a low cycle number per day (eg. < 15 cycles per day). In contrast, pathogenic chronic intermittent hypoxia protocols simulating elements of sleep apnea are characterized by greater number or severity of hypoxic episodes (80-800 episodes per day), lasting months to years. Available evidence from independent laboratories suggest that mild to moderate intensity intermittent hypoxia protocols elicit beneficial effects without adverse impact in humans, including: arterial blood pressure (Ainslie et al., 2007; Foster et al., 2005; Fu et al., 2007; Gerst et al., 2011; Tamisier et al., 2005); heart rate or heart rate variability (Foster et al., 2005; Fu et al., 2007; Gerst et al., 2011); cardiac output (Fu et al., 2007); and cognitive function (Navarrete-Opazo et al., 2016; Weiss et al., 2009). The “tipping point” where pathogenesis offsets the functional benefits of tAIH is not well defined (Dale et al., 2014; Navarrete-Opazo and Mitchell, 2014); thus, additional research to optimize tAIH benefits while minimizing pathogenesis is warranted.
Can we prolong tAIH functional benefits?
An important element in the ultimate success of tAIH concerns the duration of post-treatment therapeutic effects. Due to practical considerations, most published studies to date report only short-term effects, ranging from hours to weeks post-treatment. For example, if tAIH effects last only 3 weeks, pragmatic concerns may limit its utility as a therapeutic modality since it is not clear if individuals with chronic SCI would continue daily or even weekly tAIH treatments unless functional benefits are large. Unfortunately, there is little available information concerning the persistence of tAIH effects beyond 3-4 weeks. Although one week of daily AIH can have effects that last one week or more in breathing ability (Fuller et al., 2001; Angela Navarrete-Opazo et al., 2017a; Navarrete-Opazo et al., 2015), one week of dAIH combined with task specific training last at least 3 weeks in forelimb function (Arnold et al., 2021; Lovett-Barr et al., 2012; Prosser-Loose et al., 2015). Strategies to enhance persistence of tAIH effects (similar to long term memory consolidation; Abraham, 2003; Nicoll, 2017) would be of major benefit to the field and the potential impact of tAIH as a therapeutic modality.
Combinatorial treatments to enhance functional outcomes (further optimization)
It is widely accepted that no single treatment is likely to “cure” spinal cord injury. Functional improvements from combinatorial therapies will likely be necessary to truly optimize functional outcomes. Combinatorial treatments may rely on drug treatments that optimize tAIH efficacy by removing the influence of factors that undermine AIH-induced motor plasticity (eg. adenosine, inflammation), or add treatments that synergize with tAIH (eg. task specific training, ampakines), or may consist of distinct rehabilitation or neuromodulatory strategies. Potential combinatorial treatments inspired by advances in mechanistic understanding or technological developments are discussed below. However, it is also crucial to avoid combinations that unintentionally undermine efficacy by interfering with key elements in mechanisms of tAIH-induced plasticity.
Potential drug Treatments to optimize AIH efficacy:
Adenosine receptor inhibition to relieve cross-talk inhibition.
Mutually inhibitory cellular mechanisms compete for dominance of AIH-induced motor plasticity (E. A. Dale-Nagle et al., 2010a; Erica A. Dale-Nagle et al., 2010b; Devinney et al., 2013; Gonzalez-Rothi et al., 2015). In a safe, clinically relevant range, mild to moderate hypoxic episodes rapidly activate raphe serotonergic neurons, initiating spinal, serotonin-dependent phrenic motor facilitation (Mitchell et al., 2001; Baker-Herman and Mitchell, 2002; Dale-Nagle et al., 2010; MacFarlane et al., 2009; Devinney et al., 2013). The relevant serotonin receptors in the phrenic motor nucleus are Gq protein coupled 5-HT2A and 5-HT2B receptors (MacFarlane et al., 2011; Tadjalli and Mitchell, 2019). Spinal tissue hypoxia develops more slowly, triggering glial ATP release and increasing extracellular adenosine concentrations. Adenosine activates Gs protein coupled adenosine 2A (A2A receptors that increase cyclic AMP (cAMP) levels within phrenic motor neurons (Seven et al., 2018). Low cAMP levels activate protein kinase A, which mediates cross-talk inhibition of the Q pathway to phrenic motor facilitation and constrains moderate AIH-induced pLTF (Fields and Mitchell, 2017; Hoffman et al., 2010; Hoffman and Mitchell, 2013). With more severe hypoxic episodes, greater adenosine accumulation and receptor activation drives sufficient cAMP formation to activate the higher threshold kinase, exchange protein activated by cAMP (EPAC), initiating the S pathway to phrenic motor facilitation (Fields et al., 2015; Fields and Mitchell, 2017). Thus, with mild hypoxic episodes, A2A receptor inhibition releases the adenosine constraint on the Q pathway (Hoffman et al., 2010), but suppresses plasticity with severe hypoxic episodes (Nichols et al., 2012). This complex adenosine-serotonin interaction suggests that pretreatment with an A2A receptor inhibitor is a viable strategy to amplify (mild or moderate) tAIH-induced plasticity.
Indeed, A2A receptor antagonist administration enhances phrenic and diaphragm LTF in uninjured rats (Hoffman et al., 2010; Navarrete-Opazo et al., 2014). In rats with chronic SCI (8 weeks post-injury), a combination of A2A receptor inhibition followed by daily AIH for 7 days greatly enhanced functional gains in breathing ability (Navarrete-Opazo et al., 2017a). In striking contrast, with acute SCI (2 weeks post-injury), A2A receptor inhibition abolishes daily AIH enhanced diaphragm function (Navarrete-Opazo et al., 2015); thus, with inadequate serotonergic innervation, A2A receptor-induced phrenic plasticity dominates functional recovery. Collectively, available evidence suggest that A2A receptor inhibition may be an important adjunct, enhancing moderate tAIH-induced functional recovery in humans with chronic SCI. Trials are underway to explore use of non-specific adenosine receptor inhibitors, such as caffeine, to enhance tAIH-induced functional recovery in humans living with chronic, incomplete SCI.
Ampakines to enhance AIH-induced motor facilitation.
AMPA receptor-mediated glutamate neurotransmission provides excitatory drive to respiratory motor neurons (Funk et al., 1995). Ampakines are positive allosteric modulators of AMPA receptors that enhance AMPA-mediated glutamatergic neurotransmission (Lynch, 2006). AIH strengthens glutamatergic synapses onto phrenic motoneurons by increasing NMDA receptor currents (Ling, 2008; McGuire et al., 2008, 2005; Mitchell et al., 2001). Systemic ampakine administration prior to AIH enhances moderate AIH-induced phrenic and hypoglossal long-term facilitation in mice (ElMallah et al., 2015; Turner et al., 2016; Wollman et al., 2020a), reduces the number of hypoxic episodes needed for phrenic LTF in rats (Thakre et al., 2021), and improves breathing in a rat model of cervical SCI (Thakre et al., 2021; Wollman et al., 2020b). Thus, ampakines enhance AIH-induced LTF via glutamatergic AMPA receptor/channel enhancement (Suppiramaniam et al., 2001). Although ampakines have been approved for human use to reverse respiratory depression (Boyle et al., 2012; Dahan et al., 2010), no human clinical trials have yet explored combined AIH and Ampakines to augment functional outcomes in humans with SCI.
Anti-inflammatory drugs.
tAIH-induced phrenic motor facilitation is undermined by systemic inflammation (Huxtable et al., 2015, 2013, 2011; Vinit et al., 2011), which is highly prevalent in persons with SCI (da Silva Alves et al., 2013; Hayes et al., 2002; Wang et al., 2007). Mechanistic studies in rats show that endotoxins impair AIH-induced motor facilitation by: 1) activating p38 MAP kinase (Huxtable et al., 2015); & 2) okadaic-sensitive protein phosphatases (Tadjalli et al., 2021); thereby 3) inhibiting molecules necessary for the Q pathway to phrenic motor facilitation, such as ERK MAP kinases (Dale-Nagle et al., 2010; Hoffman et al., 2012; Tadjalli et al., 2021); and 4) preventing new BDNF protein synthesis (Agosto-Marlin et al., 2018). High doses of the non-steroidal anti-inflammatory drug, ketoprofen, restore moderate AIH-induced phrenic LTF in the face of pro-inflammatory conditions (Huxtable et al., 2013; Huxtable et al., 2015), although these effects are not mediated by COX-2 enzymatic activity/prostaglandin synthesis (Huxtable et al., 2018). Thus, NSAIDs are unlikely to be useful for restoring/enhancing AIH-induced motor plasticity in humans since doses necessary to inhibit NfKB or other pro-inflammatory transcription factors are poorly tolerated by humans. Anti-inflammatory drugs that inhibit pro-inflammatory transcription factors and/or p38/protein phosphatase activity will be necessary to relieve the adverse consequences of inflammation on tAIH-induced plasticity.
In support of this concept, two recently published human trials combining an NSAID (ibuprofen) or an anti-inflammatory corticosteroid (prednisolone) with AIH yielded the predicted, discrepant results. Whereas ibuprofen at typical doses in humans had no impact on AIH-enhanced ankle strength in people with chronic SCI (Lynch et al., 2017), prednisolone enhanced AIH effects on plantar flexion torque (Sandhu et al., 2019). Although p38 MAP kinase inhibitors reverse the adverse effect of inflammation on phrenic LTF (Huxtable et al., 2015), human trials have not been conducted to test the ability of p38 MAP kinase inhibitors to enhance AIH induced motor facilitation. Such novel approaches are worthy of investigation since currently available anti-inflammatory drugs (eg. prednisolone) are not appropriate for this purpose.
Combined tAIH and Cortical or Spinal Stimulation:
Paired corticospinal-motoneuronal stimulation augments voluntary motor control in humans with chronic SCI (Bunday et al., 2018; Bunday and Perez, 2012; Jo and Perez, 2020). Paired corticospinal-motoneuronal stimulation consists of primary motor cortex stimulation via transcranial magnetic stimulation, paired with supramaximal stimulation of peripheral nerve axons. Paired stimulation augments synaptic transmission and induces NMDA receptor- dependent spike timing-dependent plasticity (Dongés et al., 2018). Paired corticospinal-motoneuronal stimulation combined with AIH is a promising approach to augment voluntary motor control in people with chronic SCI (Christiansen et al., 2018, 2021). In these studies, the authors suggested that AIH-induced BDNF expression facilitates NMDA receptor-dependent synaptic plasticity elicited by paired corticospinal-motoneuron stimulation (Crozier et al., 2008). Alternately, AIH-induced serotonin release could augment spike-timing-dependent plasticity since Gq-coupled metabotropic receptors amplify NMDA receptor-dependent calcium influx (Gu, 2002). However, paired corticospinal-motoneuron stimulation and AIH appear to be additive (not synergistic) in amplifying evoked corticospinal responses (Christiansen et al., 2018, 2021).
Another candidate for combination with AIH is epidural electrical stimulation. Accumulating evidence indicates that both epidural and transcutaneous spinal stimulation restore upper (Gad et al., 2018; Inanici et al., 2018; Lu et al., 2016) and lower limb function (Angeli et al., 2018; Gad et al., 2017, 2015; Gill et al., 2018; Grahn et al., 2017; Harkema et al., 2011; Wagner et al., 2018), and improve autonomic function (Aslan et al., 2018; Darrow et al., 2019; Gad et al., 2018; Harkema et al., 2011). Although mechanisms of electrical spinal stimulation remain unclear, it may modulate the excitability of spinal networks that enable movement (Edgerton and Harkema, 2011), or activate sensory afferent axons entering the spinal dorsal roots (Greiner et al., 2021). In rodent models, electrical stimulation of the nervous system increases BDNF and TrkB expression, thereby promoting neuroplasticity (Al-Majed et al., 2004; Ghorbani et al., 2020). Increased BDNF expression following electrical stimulation may arise via calcium influx through voltage-gated calcium channels, activating calcium-sensitive kinases that initiate BDNF protein synthesis (Vermehren-Schmaedick et al., 2015; Wenjin et al., 2011). Combined BDNF elevations from AIH and electrical stimulation may disproportionately amplify plasticity and its functional benefits (see Welch et al., 2020).
AIH and Task Specific Training
We have come to realize that AIH effects are greatly enhanced when paired with task-specific training (TST; Welch et al., 2020). Synergy exists between these treatment strategies such that their combined effects are greater than the sum of individual treatments (Welch et al. 2020; Figure 3). For example, in a rat model of forelimb function after cervical SCI, AIH or TST (horizontal ladder walking) were ineffective when applied separately, yet profound functional benefits were observed when applied together (Prosser-Loose et al., 2015). In this same study, non-task-specific treadmill training failed to improve ladder walking performance, even when combined with AIH. In humans with chronic, incomplete SCI, 5 consecutive days of AIH marginally improved walking speed, and daily walking practice had minimal impact (Hayes et al., 2014). On the other hand, when AIH was presented 30 min before daily overground walking practice, functional benefits in walking endurance were vastly improved (Hayes et al., 2014). Thus, it is essential to investigate mechanisms giving rise to the combined benefits of tAIH with appropriate, task-specific training (Welch et al., 2020).
Figure 3: Synergy between Acute Intermittent Hypoxia and Task-Specific Training.
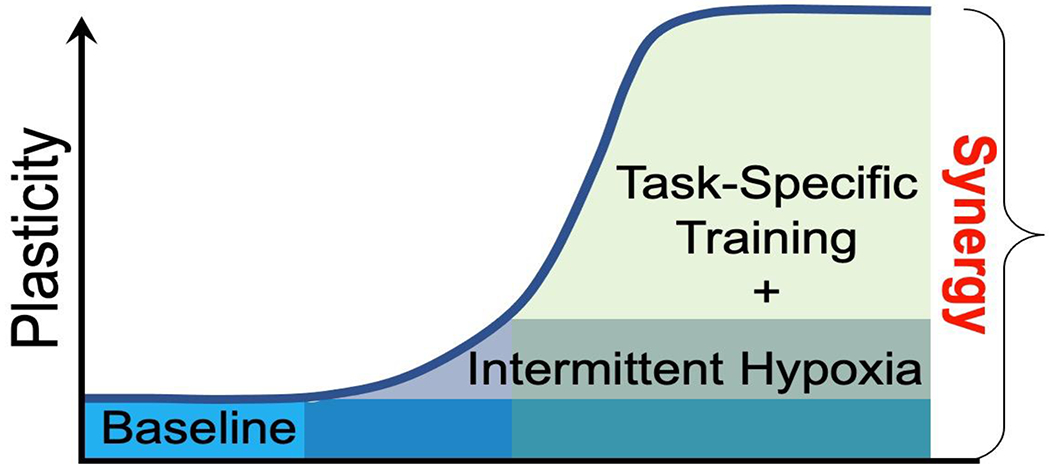
Combinatorial treatments (eg. intermittent hypoxia and task-specific training) elicit greater motor plasticity versus intermittent hypoxia or task specific training alone, largely due to unique effects on motor neurons that are only elicited when both AIH and task specific training are combined. In specific, we hypothesize that greater BDNF accumulation in motor neurons exposed to AIH and then activated during task-specific training elicits greater dose-dependent plasticity (ie. motor behavior vs. motor neuron BDNF expression; Welch et al., 2020). In this scheme, task specific training alone (teal) elicits minimal plasticity; in contrast, intermittent hypoxia alone (darker green) elicits some plasticity. However, only when AIH is paired with task specific training is synergy revealed (light green).
While these initial studies are promising, only two studies of paired AIH and task specific training have been reported. Nevertheless, they were sufficiently compelling to stimulate 4 ongoing clinical trials based on AIH combined with some form of TST. These ongoing studies aim to corroborate prior investigations, and further test the potential benefits of AIH-TST synergy. In a previous report, we proposed that AIH primes the nervous system for plasticity by elevating BDNF/TrkB signaling in diverse motor neuron pools, whereas TST selectively activates specific neural circuits, increasing BDNF only in task-relevant neuronal circuits via activity (calcium) dependent mechanisms (Welch et al., 2020). In this scheme, the AIH-TST convergence selectively boosts BDNF levels in targeted neurons, supplementing that already produced in response to AIH, and augmenting BDNF/TrkB induced plasticity uniquely in that circuit (not motor systems that aren’t engaged in the trained task). This hypothesis requires direct testing.
Biomarkers: distinguishing those who will benefit most/least from tAIH
Though successful on average, human SCI trials exhibit considerable inter-individual variability in AIH-induced motor facilitation. We pooled the incidence of low responders to tAIH from previously published human SCI trials (see Table 1). Since individual raw values were not reported in the cited manuscripts, the meta-analysis of responders vs non responders presented in Table 1 represents the relative change from baseline depicted graphically for each individual.
Table 1.
Low-Responders to Therapeutic Acute Intermittent Hypoxia in Human SCI Trials
| Author and Year | Therapy | Outcome | Low Responders/Total Subjects |
|---|---|---|---|
| Trumbower et al., 2012 | 1-day tAIH | Plantar flexion torque | 4/10 |
| Tester et al., 2014 | 10-days tAIH | Breathing | 4/8 |
| Hayes et al., 2015 | 5-days tAIH | Walking endurance | 3/9 |
| 5-days tAIH+ Walking | Walking endurance | 4/10 | |
| Sankari et al., 2015 | 1-day tAIH | Breathing | 3/6 |
| Navarrette-Opazo et al., 2016 | 5-days tAIH+ Walking | Walking endurance | 7/17 |
| Lynch et al., 2017 | 1-days tAIH | Plantar flexion torque | 7/18 |
| Trumbower et al., 2017 | 5-days tAIH+ Hand Exercise | Hand Opening | 2/6 |
| Sandhu et al., 2019 | Single tAIH+ Placebo | Plantar flexion torque | 12/28 |
| Sutor et al., 2021 | Single tAIH | Breathing | 5/17 |
| Pooled Total of Low-Responders | 51/129 (40%) | ||
In addition, as data from different functional outcome measures (ie., ankle strength, walking endurance and breathing) were compared, relative change from baseline (%) was chosen as a criterion to differentiate responders from non-responders. Individual subjects with changes ≤ 25% of their baseline value were arbitrarily classified as minimal tAIH responders. Despite its limitations, reporting percent change from baseline gives randomized trial results in clinically relevant terms that are understandable by patients and clinicians alike. Based on this criterion, in 9 published SCI studies, ~40% of SCI participants are minimal responders.
This meta-analysis of published human trials suggests that a high priority should be placed on understanding physiological and/or genetic factors that modify tAIH-induced plasticity (and its potential functional benefits), and identifying biomarkers to differentiate between individuals most/least likely to benefit from treatment. Ultimately, genetic and/or functional biomarkers will be key to successful design of full-scale clinical trials, and may help identify factors that must be overcome before tAIH will prove useful in minimal responders.
Age and sex influences on AIH-induced plasticity
Estrogen has potent and diverse effects on neural circuits associated with phrenic motor plasticity (Behan et al., 2002; Nelson et al., 2011). For example, estrogen regulates key intracellular and extracellular mechanisms that in turn regulating BDNF (Sohrabji et al., 1995) and serotonin synthesis (Hiroi et al., 2006), as well as serotonin receptor density (Biegon and McEwen, 1982; Sumner and Fink, 1998). Since testosterone is the essential precursor of estrogen, diminished levels of estrogen or testosterone could decrease serotonin or BDNF/TrkB and, thus, AIH-induced motor plasticity. It is therefore essential to consider factors that alter sex hormones when evaluating the potential therapeutic efficacy of tAIH. Relevant factors include sex, stage of the estrus cycle, advancing age and SCI itself (Otzel et al., 2018).
AIH-induced spinal motor plasticity exhibits a profound age-dependent sexual dimorphism in rats. For example, moderate AIH-induced phrenic LTF decreases from young adult (~3 months) to middle-aged male (~1 year) rats (Zabka et al., 2001a), but increases in middle aged female rats (Zabka et al., 2003, 2001b). Both effects are associated with differential, age-dependent changes in CNS estrogen levels (Behan et al., 2002; Behan and Thomas, 2005; Dougherty et al., 2017; Zabka et al., 2006). For instance, gonadectomy in young adult male rats abolishes AIH-induced phrenic LTF, an effect reversed by supplemental testosterone but not forms of testosterone that cannot be converted to estrogen or in the presence of aromatase inhibitors (enzyme that converts testosterone to estrogen; Zabka et al., 2006). Thus, even in male rats, the relevant sex hormone is estrogen versus testosterone per se (Zabka et al., 2006). Similarly, in ovariectomized female rats, AIH-induced phrenic LTF is abolished, but can be restored within minutes after spinal estradiol administration (Dougherty et al., 2017). Since supplemental testosterone restores phrenic LTF in middle-aged male rats (Nelson et al., 2011), declining CNS testosterone/estrogen levels account for reduced phrenic LTF with age in males. Conversely, since estrogen levels increase from young adult to middle age in female rats, estrogen likely mediates age-dependent increases in AIH-induced phrenic LTF.
The rodent estrous cycle is rapid (~5 days) and gives rise to variations in AIH-dependent LTF (Zabka et al., 2001b). In young female rats, AIH-induced phrenic LTF is minimal in estrus, but increases in diestrus, when estrogen levels are high (Zabka et al., 2001b; Zabka et al., 2003). As female rats reach 1 year old, AIH induced phrenic LTF peaks during diestrus (Zabka et al., 2001b). After peak expression in middle-age, phrenic LTF declines in geriatric females (Zabka et al., 2003), consistent with the idea that estradiol levels are a key regulator of AIH-induced phrenic LTF. Unfortunately, we know little concerning sex differences in the distinct mechanisms of phrenic motor plasticity or their interactions.
No human trials have adequately investigated AIH responses as a function of age or sex in humans. Based on rodent studies discussed above, younger subjects may exhibit greater capacity for AIH-induced plasticity and, therefore, greater therapeutic benefit from tAIH. However, this possibility has not been tested. Age-related sexual dimorphism in AIH-induced spinal motor facilitation is particularly relevant in the SCI population, which disproportionately affects men (84%), with an average age over 40 (National Spinal Cord Injury Statistical, 2020). In addition to the effects of advancing age, SCI per se decreases testosterone levels in men (Peterson et al., 2018), potentially further undermining the potential for robust LTF. Although the average age of participants in the trials summarized in Table 1 was 46 ±13 years, no relationship between age and LTF magnitude was reported. In 8 AIH trials on people with SCI (Table 2), only 16% of all subjects studied were women, and no studies reported phase of the menstrual cycle or menopausal status. Thus, it is important in future human trials to report individual responses and participant’s age, sex and phase of the menstrual cycle to adequately enable future meta-analyses of age-related sexual dimorphism in response to tAIH.
Table 2.
Demographics of SCI Participants Enrolled in AIH Clinical Trials
| Author and Year | Sex | Age | Level of injury | ISNSCI | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| M | F | Mean±SD | A | B | C | D | ||
| Trumbower et al., 2012 | 12 | 1 | 46±10.5 | C | 0 | 0 | 5 | 8 |
| Tester et al., 2014 | 4 | 4 | 53.1±12.23 | C & T | 1 | 0 | 2 | 5 |
| Hayes et al., 2014 | 16 | 3 | 43.42±14.99 | C & T | 0 | 0 | 2 | 17 |
| Sankari et al., 2015 | 11 | 5 | 40.7±15 | C & T | 14 | 0 | 1 | 1 |
| Navarrate-Opazo et al., 2017 a,b | 31 | 4 | 40.6±16.5 | C, T, & L | 0 | 0 | 13 | 22 |
| Lynch et al., 2017 | 9 | 1 | 52.1±13.1 | C & T | 0 | 0 | 0 | 10 |
| Trumbower et al., 2017 | 6 | 0 | 43±5 | C5 | 0 | 0 | 3 | 3 |
| Sandhu et al., 2019 | 13 | 1 | 46.28±9.5 | C & T | 0 | 0 | 5 | 9 |
| Sutor et al., 2021 | 13 | 4 | 34.1±14.5 | C & T | 6 | 7 | 3 | 1 |
|
| ||||||||
| Total % | 95.04% | 19.01% | 17.36% | 5.79% | 28.10% | 62.81% | ||
Since reduced sex hormone levels with age and/or spinal cord injury are associated with reduced ability to express AIH-induced motor plasticity in rodents, the use of sex hormone replacement therapy might be explored as a potential therapeutic to improve outcomes. Although systemic and intrathecal 17β-estradiol restores AIH-induced phrenic motor facilitation in ovariectomized female rats (Dougherty et al., 2017), no studies have yet investigated the impact of hormone replacement therapy on tAIH induced motor facilitation in humans.
Level of spinal injury & injury severity
There is limited evidence to suggest an association between the level & severity of SCI on AIH-induced motor plasticity. Since AIH is a non-invasive approach to strengthen spared neural pathways to spinal motoneurons, most SCI participants enrolled in AIH trials had incomplete injuries, with the most common ASIA impairment scores of D (62%) or C (26%) (See Table 2). Three studies (Sankari et al., 2015; Sutor et al., 2021; Tester et al., 2014) enrolled participants with ASIA scores of A (17%). Trumbower and colleagues (2012) found no relationship between injury severity, as measured by lower extremity motor score, and AIH-induced facilitation of ankle plantar flexion strength. On the other hand, Sankari and colleagues (2015) studied participants classified as ASIA A (14 of 16 participants), and report a clear relationship between the level of SCI and ventilatory LTF; greater responses were observed in individuals with cervical versus thoracic SCI. These authors also demonstrated increased peripheral chemoreflex sensitivity in individuals with cervical SCI and suggested that increased chemoreflexes could secondarily enhance AIH-induced ventilatory LTF. More studies on individuals scored as ASIA A or B are needed to determine treatment efficacy in this population, particularly in motor systems above the site of injury.
Time post-injury
Most human studies of AIH-induced motor facilitation in people with SCI were conducted in participants with chronic SCI, long after spontaneous recovery is likely (Goshgarian, 2003). Available data from rodent studies suggest serotonin-dependent recovery of respiratory motor function is limited with acute spinal cord injury due to disrupted spinal serotonergic projections, but increases with time post-injury as spared serotonergic neurons re-innervate motor nuclei below the injury site (Ciesla et al., 2021; Golder et al., 2003; Golder and Mitchell, 2005).
Following SCI, systemic hypotension and pericyte constriction of spinal capillaries decreases spinal oxygen delivery, reducing spinal tissue oxygen concentration at any given level of arterial Po2 (Li et al., 2017; Partida et al., 2016; Perim et al., 2021). Reduced spinal oxygen tensions at given inspired or arterial Po2 levels increase spinal adenosine accumulation and A2A receptor activation relative to raphe neuron activation (which seems more linked to Po2 levels), thereby constraining serotonin-dependent motor facilitation (Hoffman et al., 2010; Dougherty et al., 2018; Perim and Mitchell, 2019). With acute injuries, compromised serotonergic innervation and greater tissue hypoxia are expected to shift the balance in favor of adenosine-dominant plasticity (Navarrete-Opazo et al., 2015; Dougherty et al., 2018). Thus, although one-week of daily AIH beginning 1-week post-cervical hemisection nearly restores the ability to increase tidal volume at 2-weeks post-injury (Lovett-Barr et al., 2012; Navarrete-Opazo et al., 2015), functional recovery results from adenosine versus serotonin dependent mechanisms (Navarrete-Opazo et al., 2015). In striking contrast, daily AIH initiated 8-weeks post-cervical hemisection (ie. chronic) elicits minimal functional recovery despite near restoration of serotonergic innervation within the phrenic motor nucleus (Navarrete-Opazo et al., 2017; Ciesla et al., 2021). Equivalent functional recovery could only be established if the rats were pre-treated with an A2A receptor inhibitor prior to AIH on each treatment day (Navarrete-Opazo et al., 2017). In contrast, recovery of reach-to-grasp function occurs with tAIH and TST initiated 8 weeks post-cervical hemisection, even without A2A receptor inhibition (Arnold et al., 2021).
Thus, time post-injury has a profound impact on the potential for daily AIH to elicit meaning functional recovery. The dramatic difference in response to pre-treatment with an A2A receptor inhibitor highlights the importance of time post-injury (ie. abolishes plasticity in acute, reveals plasticity in chronically injured rats). To date, we are not aware of any studies explicitly exploring the impact of time post-injury on functional outcomes following tAIH treatment in any motor system in humans. Future research should consider the potential to optimize tAIH protocols as a function of time post-injury (acute vs. chronic) since underlying mechanisms may differ.
Peripheral Chemoreceptor Responsiveness
Increased peripheral chemoreflex sensitivity may contribute to differential tAIH effects in healthy individuals versus people with SCI. For example, a recent study by Vermeulen and colleagues (2020) demonstrated that hyperoxia following acute intermittent hypercapnic hypoxia depressed breathing more than during pre-AIH baseline, consistent with increased peripheral chemoreflex drive to breathe. Furthermore, Sankari and colleagues (2015) found that people with cervical SCI exhibit a greater reduction in ventilation during transient hyperoxia post-AIH versus those with thoracic SCI or in able-bodied controls. Thus, carotid chemoreflex plasticity may drive, at least part, increased breathing in those with cervical SCI. On the other hand, greater chemoreflex sensitivity may also trigger more raphe neuron activation and serotonin release on/near motor neurons, enhancing tAIH-induced spinal plasticity. Collectively, these data suggest that chemoreflex testing may shed light on tAIH responders versus non-responders.
Sleep-disordered breathing in SCI
Sleep disorders are highly prevalent in individuals with cervical (93%) and thoracic (55%) SCI (Chiodo et al., 2016; Sankari et al., 2014). While many people living with SCI exhibit obstructive sleep apneas due to neuromuscular weakness in upper airway muscles, the prevalence of central sleep apnea is very frequent in those with cervical SCI (Sankari et al., 2014). Regardless mechanisms giving rise to sleep apnea (obstructive vs. central), most individuals with cervical and thoracic SCI experience intermittent hypoxia on a nightly basis. Thus, it is essential to understand the impact of sleep apnea (including associated CIH) on AIH-induced respiratory and non-respiratory motor plasticity.
In rats, even one day of intermittent hypoxia simulating that experienced during moderate obstructive sleep apnea (15 episodes per hour; 8 hours) abolishes moderate AIH-induced phrenic LTF due to systemic and neuroinflammation (Huxtable et al., 2015). On the other hand, mild chronic intermittent hypoxia protocols (6 episodes per hour, 12 hours per day, 7 days) enhance AIH-induced phrenic LTF (Ling et al., 2001; Fields and Mitchell, 2015). Thus, outcomes following intense or prolonged intermittent hypoxia preconditioning depend on details of the exposure, at least in rats.
In otherwise healthy humans, AIH-induced ventilatory and genioglossus long-term facilitation is enhanced in awake individuals with obstructive sleep apnea versus those without (Gerst et al., 2011). In this situation, individuals have likely experienced chronic intermittent hypoxia (and other sleep apnea associated factors; Mateika, 2019) for extended periods (years), and likely have multiple co-morbidities. In otherwise healthy individuals, genioglossus muscle facilitation reduces upper airway resistance and/or stiffens upper airways, minimizing obstructive apneas (Rowley et al., 2007). Genioglossus facilitation will have less impact on central apneas.
In individuals with cervical SCI, the high prevalence of central apneas during sleep likely results from increased “loop gain,” which causes ventilatory instability expressed as alternating cycles of apnea or hypopnea with hyperventilation (Asyali et al., 2002; Khoo, 2001; Mateika and Narwani, 2009). Although sleep apnea is generally regarded as pathogenic (Navarrete-Opazo and Mitchell, 2014), increased “loop gain” and breathing instability could potentially be viewed as beneficial for individuals with SCI. Recurrent apneas and intermittent hypoxia may be viewed as a form of “self-medication,” where modest intermittent hypoxia improves motor recovery following injury; this benefit may be sufficient “reward” to offset risk of morbidity. With modest sleep apnea, there may be a net benefit to injured individuals (Dale et al., 2014; Navarrete-Opazo and Mitchell, 2014). From another perspective, increased “loop gain” as with cervical SCI reflects augmented chemoreceptor activation and, potentially, greater brainstem neural network activation, leading to enhanced spinal motor plasticity. In agreement with these ideas, a meta-analysis of available literature led to the conclusion that individuals with moderate sleep apnea demonstrate greater functional benefit in response to tAIH (Vivodtzev et al., 2020).
Genetic Variations
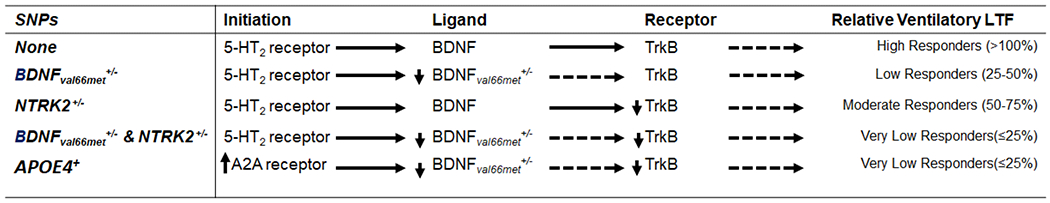
Although there are genetic and/or epigenetic factors that influence AIH-induced phrenic motor plasticity in rodents (Golder et al., 2005; Baker-Herman et al., 2010; Fuller et al., 2000, 2001), the role of genetics/epigenetics in variable inter-individual human tAIH responses has not been investigated. In principle, any gene that encodes molecules involved in serotonin (synthesis, release, clearance and/or downstream signaling) or BDNF/TrkB function could impact tAIH-induced motor plasticity. Based on the most common dysfunctional genes reported in the US population, the ~40% of participants that exhibit minimal tAIH response could, in principle, be explained by known single nucleotide polymorphisms (SNPs), including: BDNFval66met (prevalence ~30%), NTRK2 (prevalence ~50%) and/or APOE4 (prevalence ~14%), a molecule known to impair other forms of neuroplasticity.
Dysfunctional BDNF gene.
About 30 to 50% of the US population carries the BDNFval66met mutation (SNP id: rs6235) (Petryshen et al., 2010; Shen et al., 2018; Verhagen et al., 2010). Although no human trials have explored the role of dysfunctional BDNF on tAIH-induced motor plasticity, the role of a dysfunctional BDNFval66met single nucleotide polymorphism on exercise-induced neuroplasticity has been studied post-SCI and following traumatic brain injury (Finan et al., 2018; Leech and Hornby, 2017). In people with SCI, acute high-intensity exercise did not increase serum BDNF levels in BDNFval66met carriers (Leech and Hornby, 2017). After traumatic brain injury, individuals carrying the BDNFval66met mutation demonstrated poor motor learning in driving tasks (McHughen et al., 2010). Since BDNF is important in tAIH-induced motor plasticity, carriers of at least 1 BDNFval66met allele could have blunted tAIH-induced motor plasticity (Table 3).
Table 3.
Proposed Mechanism of Impairment in Long Term Motor Facilitation due to Single Nucleotide Polymorphism.
|
Dysfunctional NTRK2 gene.
BDNF promotes neuroplasticity by activation of its high affinity receptor, TrkB, encoded by neurotrophic receptor tyrosine kinase 2 gene (NTRK2). About 50% of the US population has a C to T nucleotide SNP at the NTRK2 promoter region (SNP ID: rs1212171) which decreases TrkB mRNA stability. In individuals carrying the dysfunctional NTRK2 allele, TrkB mRNA degradation lowers TrkB receptor density. The dysfunctional NTRK2 allele is suspected to alter microRNA function, inhibiting TrkB mRNA translation (Maussion et al., 2012). Little is known about functional implications of the dysfunctional NTRK2 SNP, but a positive association between carriers and increased incidence of depression has been reported (Avdoshina et al., 2013; Shen et al., 2018; Zhou et al., 2015). Since phrenic motor neuron TrkB is necessary for AIH-induced phrenic LTF (Dale et al., 2017), reduced TrkB receptor density may impair tAIH-induced plasticity (Table 3). Since gene-gene interactions between BDNF and NTRK2 have been implicated in major depression and cognitive dysfunction (Lin et al., 2009), individuals carrying both BDNFval66met+/− and NTRK2+/− SNPs may have more severely attenuated tAIH-induced motor plasticity.
APOE4 gene.
A significant negative association between the ApoE4 isoform and BDNF expression has been reported in humans (Allard et al., 2017; Alvarez et al., 2014; Chhibber and Zhao, 2017; Strattan et al., 2021), and ApoE4 is reported to impair AIH induced phrenic LTF in mice (Strattan et al., 2021). APOE4 isoforms are associated with greater microglial activation states (Shi et al., 2019), possibly increasing adenosine release and A2A receptor mediated inhibition of the Q pathway to phrenic motor facilitation (Hoffman et al., 2010). Future studies investigating these genetic factors in humans with chronic SCI are warranted.
Screening for tAIH responsiveness: functional biomarkers:
To optimize potential for success in clinical trials, it is important to identify individuals most likely to benefit from tAIH a priori. One reasonable criterion for inclusion in long-term clinical trials is screening to identify individuals most/least likely to respond via genetic biomarkers, plasma hormone levels and/or incidence of sleep apnea, as described above. On the other hand, functional biomarkers should also be considered, such as: 1) elevated peripheral chemoreflex sensitivity, and/or 2) increased ankle torque and/or grip strength following a single AIH presentation. Validated functional biomarkers are of considerable interest since it is reasonable to limit participation in long-term clinical trials to those responding briskly to a single AIH exposure. For example, chemoreflex sensitivity can be assessed in humans via transient alterations in the inspired oxygen concentration, often known as a “Dejours Test” (Zapata et al., 2012; Pfoh et al., 2015; Cunha-Guimaraes et al., 2020; Li et al., 2021). For example, with five breaths of 100% inspired oxygen, peripheral chemoreceptors are transiently silenced; the corresponding decrease in breathing can be used as an index of baseline carotid chemoreceptor drive to breathing. Conversely, up to 5 breaths of inspired nitrogen transiently increase chemoreceptor activity and breathing, providing an index of chemoreflex sensitivity. These methods represent a simple and safe means of estimating chemoreflex sensitivity and, thus, predicted AIH response for that individual.
With respect to limb function, Trumbower and colleagues (2012) reported that participants with chronic incomplete SCI increase maximum voluntary ankle plantarflexion torque by ~80% following a single, 30-minute AIH session, whereas another study (Lynch et al., 2014) reported ~30% enhancement using the same protocol. Variations in effect size could be explained by the fact that participants of the former study (2012) were significantly weaker in baseline conditions (~12Nm peak torque) versus the latter (~60Nm). Thus, these studies are consistent with the interpretation that participants with low baseline torque generation exhibit greater have effect size versus those with higher baseline strength, suggesting a possible “ceiling effect.” Consequently, maximum voluntary ankle plantarflexion torque in response to a single day of AIH holds promise as a functional biomarker for participant selection in tAIH clinical trials. On the other hand, some rats with chronic cervical hemisections improved quickly with the onset of daily AIH plus task specific training, whereas others lagged a few days in their response (Arnold et al., 2021); thus, response to a single day of AIH may not faithfully predict longer term outcomes in response to tAIH. Additional work is needed to validate this potential functional biomarker in humans.
tAIH Safety
Despite evidence that “high dose,” chronic intermittent hypoxia simulating that experienced during moderate or severe sleep apnea elicits considerable morbidity (eg. hypertension, hippocampal cell death, cognitive deficits, metabolic syndrome, and others), pathology has not been reported with “low dose” tAIH (Navarrete-Opazo and Mitchell, 2014). In fact, tAIH is most often associated with beneficial (vs. pathogenic) effects (Dale et al., 2014; Navarette-Opazo et al., 2014; Almendros et al., 2014; Mateika et al., 2015), exemplifying a hormetic dose-response curve (Calabrese and Baldwin, 2002). In a recent, comprehensive study, we analyzed multiple tissues in rats for evidence of pathology after extended tAIH (10, 5 minute episodes per day, 4x per week, 12 weeks); no evidence was found for tAIH-linked pathology in the brain, spinal cord, heart, aorta, kidney, liver, bone or muscle, nor were there adverse effects on systemic blood pressure (Gonzalez-Rothi et al., 2021). Collectively, studies conducted at the Shirley Ryan Ability Lab (Chicago; Trumbower et al., 2012), Emory University (Atlanta; Hayes et al., 2014), Teleton Rehabilitation Institute (Santiago, Chile; Navarrete-Opazo et al., 2017a), Wayne State (Detroit; Sankari et al., 2015) and University of Florida (Gainesville/Jacksonville; Tester et al., 2014; Sutor et al., 2021) all show that AIH can be safely delivered to humans with spinal cord injury, with little evidence of any adverse side-effects (Hayes et al., 2014, Navarrete-Opazo et al., 2017a, Sankari et al., 2015, Tester et al., 2014, Trumbower et al., 2012).
Device development and commercialization
To ensure that tAIH is properly vetted through clinical trials and becomes available for clinical use, it is important to develop a standardized AIH-delivery device. A suitable device should allow for individualized protocol customization, be safe and easy to use, and enable data logging. Optimal tAIH protocols may vary among individuals with respect to: 1) inspired oxygen concentration during hypoxic episodes; 2) episode duration; 3) the duty cycle (ratio of hypoxic to total cycle duration); and 4) total number and pattern of hypoxic episodes per day. The device must enable rapid transition between gas mixtures.
Most AIH studies to date utilized either modified commercially available generators (eg. Hypoxico Inc., USA) or pressurized gas cylinders feeding into programmable mechanical valves. Although pressurized-gas cylinder systems offer good calibration and flexibility suitable for laboratory applications, they are not ideal for clinic or in-home use due to tank storage and replacement schedules. In contrast, commercially available hypoxia generators utilize a pressured swing absorption (PSA)-based method to generate hypoxic gas mixtures from ambient air. These systems provide continuous gas flows with low (9–10% oxygen) or normal oxygen concentrations (21% inspired oxygen). In many current protocols, personnel switch gases delivered to the participant by manually connecting tubing from the generator to the facemask at selected intervals. These systems are suitable for clinic use due to their low-space requirements and maintenance costs. Although PSA-based systems can be more convenient than gas cylinder systems in delivering AIH, the burden of manually switching gas mixtures is a major limitation. Two recent papers pointed out that manual switching can lead to variations in AIH-treatment protocols and, potentially, participant responses (Tan et al., 2020; Vivodtzev et al., 2020). Several design characteristics make PSA-based delivery systems susceptible to inconsistencies in timing, flow rate and inspired oxygen levels; further, available PSA generators are sufficiently limited in their flow rates that reservoir bags are often required to ensure adequate gas available during each breath (Tan et al., 2020). Although this arrangement helps prevent occlusion when an individual’s inspiratory flow exceeds PSA generator capacity (eg. deep breathing, sighs), reservoir bags may also transiently limit gas flow and compromise effective inspired oxygen switching. To overcome these shortcomings, Tan and colleagues developed an automated AIH-delivery system circumventing the need for manual switching between hypoxia and normoxia (Tan et al., 2021). This system incorporates a customized and programmable digital controller to ensure precise, automatic intervals of hypoxia/normoxia, thus maintaining temporal consistency in AIH protocols. It also: 1) provides continuous positive pressure to account for variations in individual breathing characteristics; 2) has a stand-alone graphical user interface to log physiological responses; and 3) has a unique safety feature to prevent large fluctuations in oxygen delivery and improving consistency and accuracy of hypoxic episodes. Comprehensive details of this device, mechanical testing and comparisons with the Hypoxico PSA-device [HYP123; Hypoxico Inc.] are available (Tan et al., 2020).
Briefly, the system utilizes two PSA generators [Hypoxico Inc., USA] connected to a common gas-mixing chamber, which ensures rapid transitions between normoxia (21% oxygen) and hypoxia (Fio2 = 0.9 ± 0.02; ie. 9% O2). An air-blower provides room air with equivalent positive-pressure for SHAM sessions. The mixing chamber and blower connect to a breathing mask worn by the participant via a continuous positive airway pressure tube. The mask contains separate one-way valves for inspiration and expiration to minimize dead space and prevent backflow, with padding along the mask’s borders to provide a secure seal on the face. Two neoprene sleeves with Velcro straps fasten the mask around the participant’s head to minimize leaks.
The facemask inspiratory valve opens during inspiration or when external positive pressure is applied via an electrochemical sensor, moving gas from the blower into to the participant’s mask. The expiratory valve exhausts expired gas into the room. An electrochemical detector continuously monitors mask Fo2 in 15-second intervals, while a physiological recording system [MASIMO rainbow SET, Irvine, CA] monitors heart rate, SpO2 (every second breath), and blood pressure (every fifth breath). If a user’s oxygen saturation (SpO2) drops below 75%, it causes the system to immediately switch from hypoxia to normoxia until SpO2 levels are >90%. Collectively, these system features ensure that the AIH protocol is automated, consistent and safe, with sufficient flexibility to individualize AIH protocols.
For future clinical use, a device is required with a straight-forward user interface enabling quick and intuitive AIH protocol customization, safety and efficacy data logging, and with sufficient capacity to accommodate individuals varying in size and breathing characteristics. It is essential to collect sufficient data to justify FDA approval, thus ensuring availability for medical use. Finally, a smaller transportable device suitable for in-home use will be needed as we implement longer tAIH treatments and translate this approach to patient populations unable to routinely visit a local clinic to receive treatment; for example, with advanced ALS, patients may not be able to visit clinics on a daily basis.
Update: Ongoing Human Trials
A growing cadre of research laboratories is engaging in various aspects of human tAIH research, either as a single AIH intervention or in combination with other approaches. Ongoing clinical trials highlight the interplay between basic and clinical research needed to investigate the potential of tAIH as a treatment modality. Current trials emphasize: 1) breathing; 2) walking ability/leg function; or 3) arm/hand function in SCI (described below). Each trial currently ongoing features tAIH combined with task specific training. However, as recommended during the AIH workshops (2016 & 2020), pilot studies have begun with other neurological disorders (eg. ALS, MS, stroke) and combined treatments (adenosine 2A receptor inhibition). Future work should consider other motor systems, such as swallowing, cough/airway defense and/or trunk muscle control/balance that may benefit from AIH-induced neuroplasticity, or other outcomes such as somato-sensation, pain, autonomic regulation, cognition and other critical functions.
Respiratory function after SCI
Based on decades of studies in rodent models and recent publications demonstrating that AIH elicits spinal neuroplasticity and restores limb function in humans with chronic incomplete SCI (Dale et al., 2014; Fuller et al., 2003; Golder & Mitchell, 2005; Lovett-Barr et al., 2012; Navarrete-Opazo et al., 2015; Navarrete-Opazo et al., 2017; Sankari et al., 2015; Tester et al., 2014), researchers at The University of Florida and Brooks Rehabilitation are conducting a DoD funded clinical trial to determine if tAIH improves respiratory function post-injury. Specifically, since AIH is most effective at restoring limb function when paired with task-specific training (Hayes, 2014; Prosser-Loose, 2015), this phase 2 clinical trial is designed to test the hypothesis that combined AIH and respiratory strength training is more effective at restoring breathing ability than either treatment alone.
Restoring arm and leg function after SCI
Adenosine 2A receptor activation (Hoffman et al., 2010) undermines AIH-induced respiratory motor plasticity, and adenosine 2A receptor inhibition amplifies the impact of daily AIH on breathing ability in rats with cervical spinal cord injuries (Navarrete-Opazo et al., 2017). Based on this work, researchers at Spaulding Rehabilitation Hospital and Harvard Medical School are exploring the impact of combined caffeine and AIH on overground walking ability in people with chronic injuries. In another phase 2 clinical trial, this team is also investigating safety and efficacy of combined tAIH and task-specific gait training on overground walking function in ambulatory and non-ambulatory people with sub-acute (< 12months) spinal injuries. The hypothesis guiding this study is that combined AIH and walking practice elicit greater functional recovery with subacute versus chronic SCI (Naidu et al., 2020). This team is also testing the hypothesis that combined AIH and transcutaneous spinal cord stimulation enhance hand strength/function in people with chronic SCI.
Researchers at The Shirley Ryan Ability Lab are undertaking a multi-center clinical trial evaluating the effectiveness of AIH combined with massed practice training via an exoskeleton Rapael glove to improve upper extremity function in injured individuals. At this site, trials are underway to explore AIH plus training with an upper limb robotic rehabilitation device. Finally, investigators at the Shirley Ryan Ability lab are investigating the potential of combined AIH, transcutaneous spinal cord stimulation and gait training.
Although increased phase 2 clinical trial activity will advance our understanding concerning the therapeutic potential of tAIH, there are many additional treatment combinations and functional outcomes to be explored.
Restoring function in other neuromuscular disorders
Although this review focused on motor recovery after SCI, there is considerable potential to harness tAIH as a therapeutic modality for other neurological disorders that compromise breathing, airway defense or limb function (see Mitchell, 2008 for conceptual review; Dale et al., 2014; Gonzalez-Rothi et al., 2016). In any neuromuscular disorder that would benefit from increased motor neuron strength, tAIH has potential as a treatment strategy. For example, ALS and other motor neuron diseases are characterized by progressive motor neuron loss. Despite substantial respiratory motor neuron loss in rodent models of ALS, the animals retain breathing ability until late in disease progression (Nichols et al., 2013; Seven et al., 2018), reflecting impressive spontaneous compensatory plasticity (Johnson and Mitchell, 2013). Nevertheless, breathing eventually fails (Tankersley et al., 2007). As disease progresses in rodent models, deficits in specific respiratory nerve activity are observed (eg. phrenic nerve; Nichols et al., 2013; Nichols and Mitchell, 2016). However, when exposed to AIH, ALS rats retain the capacity for phrenic LTF (Nichols et al., 2015; Nichols et al., 2017); in fact, phrenic LTF is enhanced at disease end-stage (Nichols et al., 2017), restoring the ability to generate phrenic nerve activity. Thus, in principle, tAIH-induced respiratory motor neuron plasticity could preserve breathing capacity later in disease progression, prolonging survival and quality of life at a time when virtually no treatment options are available (Mitchell, 2008; Nichols et al., 2013). On this basis, pilot studies were initiated to evaluate safety and efficacy of a single tAIH session in humans with ALS. In this limited study, AIH increased collective respiratory muscle EMG activity in a small, heterogeneous population of ALS patients with varied degrees of respiratory impairment (Sajjadi, Wymer, Mitchell and Smith, ibid). Further investigations are warranted.
Although unique elements of motor control are involved in multiple sclerosis (MS) and stroke, pilot studies have been initiated to explore the potential therapeutic efficacy of tAIH to restore limb function in humans with MS (Sandhu and Rymer, Shirley Ryan Ability Lab) and motor impairment due to stroke (Sandhu and Rymer; Shirley Ryan Ability Lab). Results from these studies are not yet available, but preliminary indications suggest that tAIH to treat respiratory and non-respiratory motor impairment is worthy of further investigation in these clinical disorders. tAIH trials may be warranted in other neuromuscular disorders, such as Parkinson’s Disease, Duchenne Muscular Dystrophy (DMD), Pompe Disease and others.
As the translational fly-wheel advances fundamental knowledge and clinical application of tAIH to benefit varied motor systems in a range of clinical disorders, focus on collaboration and communication among groups is essential. To accelerate progress, investigations will benefit from shared science (failures as well as successes) and open communication focused on development of AIH for therapeutic benefit. Coordination across laboratories and consistency in clinical trials may be important to standardize tAIH delivery and outcomes to realize its therapeutic potential in complex patient populations. Given the expense and time required by phase 2 and 3 clinical trials, optimizing our efforts through communication and coordination are critical to avoid futile clinical trials, lost time, and lost opportunity to improve and extend lives.
Conclusions
Despite growing evidence that tAIH-based treatments are safe, simple and effective at improving non-respiratory motor function in persons with chronic, incomplete SCI, several challenges remain before this promising treatment modality will be ready for full scale clinical trials. Validation of clinical utility with respect to dosing, safety and efficacy is still needed to bring tAIH a step closer to being a recognized rehabilitation therapy for SCI (and/or other clinical disorders). There is still uncertainty pertaining to optimal tAIH protocols depending on the type of injury (acute versus chronic; complete versus incomplete; site of injury, etc.), motor system (respiratory, upper airway, limb), combinatorial therapies to optimize outcome (eg., drugs, task-specific training, electrical stimulation, etc.). Remaining uncertainty is complicated by the fact that beneficial responses documented to date are variable, with some individuals responding better than others. Thus, biomarkers (age/sex, genetics, functional biomarkers) are needed to identify those most likely to benefit from tAIH.
As we address these challenges, our success in finding answers will depend on effective communication between those doing mechanistic studies in animal models and those translating findings to humans living with SCI or other clinical disorders. To date, communication within this small field has been effective; ideas have flowed from basic to translational researchers before publication, and the reverse, accelerating the cycle of translation. Although we still do not have final answers, this field has served as an example of a “translational flywheel” or “bench-to-bedside-and-back” research. There is much work to be done before we are ready to undertake a full scale, multi-site clinical trial, but the field is approaching that goal.
Sources of Funding:
This work received support from the National Institutes of Health R01HL147554, R01HD081274; McKnight Brain Institute (BSCIRTF); Department of Defense (CDMRP W81XWH1810718; W81XWH-15-2-0045); US Army Medical Research and Material Command (MRMC, SCI130298); Saskatchewan Health Research Foundation (SHRF, 3695), Wings for Life Foundation (WFL-US-026) and the Craig H. Neilsen Foundation.
1. Participants in the 2016 IH Workshop:
A “Road Map” to clinical translation: therapeutic intermittent hypoxia and recovery of motor function with chronic incomplete SCI.
tAIH Researchers:
Gordon Mitchell, PhD; Professor and Director, University of Florida
W. Zev Rymer, MD; Professor, Rehabilitation Institute of Chicago
Randy Trumbower, DPT/PhD; Assistant Professor; Emory University
Gillian Muir, DVM/PhD; Professor and Dean, University of Saskatchewan
Milap Sandhu, PT/PhD; Rehabilitation Institute of Chicago
Brendan Dougherty, PT/PhD; Assistant Scientist, University of Wisconsin
David Fuller, PhD; Professor, University of Florida
Elisa Gonzalez-Rothi, PT/PhD; Research Assistant Professor, University of Florida
Arun Jayaraman, PT/PhD; Associate Professor; Rehabilitation Institute of Chicago
Anatole (Danny) Martin, PT/PhD; Professor, University of Florida
Barbara Smith, PT/PhD; Research Assistant Professor, University of Florida
Emily Fox, DPT/PhD; Research Assistant Professor, University of Florida/Brooks Rehabilitation
Event Coordonator:
Andrea Zabka Mitchell, DVM/PhD; UF BREATHE Coordinator
Pulmonary Physicians:
Safwan Badr, MD; Professor, Wayne State University
David Gozal, MD; Professor, University of Chicago
Clinical Trials Representatives:
Steven Wolf, PT/PhD; Professor, Emory University
Andrew Blight, PhD; CSO, Acorda Life Science
Steve Kirshblum, MD; Director, SCI Rehabilitation, Kessler Institute for Rehabilitation
Dan Lammertse, MD; Medical Director of Research, Craig Rehabilitation Hospital
Engineers & Industry Representatives:
Neil Euliano, PhD; CTO at OBMedco & Pres. Convergent Engineering, Florida
Ivan Bustamante; former senior manager at Philips; director of clinical research at Respironics
Eric Gjerde; President & CEO, Airon Corporation
Jack Judy, PhD; Professor & Director, University of Florida
Trainees Working on Human tAIH Research:
Lasse Christensen, PhD; Postdoctoral Fellow, University of Miami
Tommy Sutor; PhD student, University of Florida
Shakeel Ahmed, PT; PhD student, University of Florida
Andrew Tan, PhD; Postdoctoral Fellow, Emory University
Michael Urban, DPT/PhD; Postdoctoral Fellow, University of Miami
Alison D’Alessandro, DPT; Neurorehabilitation Clinical Specialist, Brooks Rehabilitation
Representatives of funding agencies:
Linda Jones, PT, MS (Program Officer; Craig H. Neilsen Foundation)
Naomi Kleitman, PhD; VP of Grants and Research, Craig H. Neilsen Foundation
Jane Hsieh, MS; Coordinator of Clinical Trials, Wings for Life
Lyn Jakeman, PhD; Program Director, National Institute for Neurological Disorders and Stroke
Mark Bacon, PhD; Director of Research, International Spinal Research Trust
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare no competing financial interests.
References
- Abraham WC, 2003. How long will long-term potentiation last? Philos Trans R Soc Lond B Biol Sci 358, 735–744. 10.1098/rstb.2002.1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosto-Marlin IM, Nichols NL, Mitchell GS, 2018. Systemic inflammation inhibits serotonin receptor 2-induced phrenic motor facilitation upstream from BDNF/TrkB signaling. J Neurophysiol 119, 2176–2185. 10.1152/jn.00378.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie PN, Barach A, Cummings KJ, Murrell C, Hamlin M, Hellemans J, 2007. Cardiorespiratory and cerebrovascular responses to acute poikilocapnic hypoxia following intermittent and continuous exposure to hypoxia in humans. J Appl Physiol (1985) 102, 1953–1961. 10.1152/japplphysiol.01338.2006 [DOI] [PubMed] [Google Scholar]
- Allard JS, Ntekim O, Johnson SP, Ngwa JS, Bond V, Pinder D, Gillum RF, Fungwe TV, Kwagyan J, Obisesan TO, 2017. APOEε4 impacts up-regulation of brain-derived neurotrophic factor after a six-month stretch and aerobic exercise intervention in mild cognitively impaired elderly African Americans: A pilot study. Exp Gerontol 87, 129–136. 10.1016/j.exger.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Majed AA, Tam SL, Gordon T, 2004. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons. Cell Mol Neurobiol 24, 379–402. 10.1023/b:cemn.0000022770.66463.f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almendros I, Wang Y, Gozal D, 2014. The polymorphic and contradictory aspects of intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol 307, L129–40. 10.1152/ajplung.00089.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez A, Aleixandre M, Linares C, Masliah E, Moessler H, 2014. Apathy and APOE4 are associated with reduced BDNF levels in Alzheimer’s disease. J Alzheimers Dis 42, 1347–1355. 10.3233/JAD-140849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, Ferreira CK, Harkema SJ, 2018. Recovery of Over-Ground Walking after Chronic Motor Complete Spinal Cord Injury. The New England journal of medicine 379, 1244–1250. 10.1056/NEJMoa1803588 [DOI] [PubMed] [Google Scholar]
- Arnold BM, Toosi BM, Caine S, Mitchell GS, Muir GD, 2021. Prolonged acute intermittent hypoxia improves forelimb reach-to-grasp function in a rat model of chronic cervical spinal cord injury. Experimental Neurology 113672. 10.1016/j.expneurol.2021.113672 [DOI] [PubMed] [Google Scholar]
- Aslan SC, Legg Ditterline BE, Park MC, Angeli CA, Rejc E, Chen Y, Ovechkin AV, Krassioukov A, Harkema SJ, 2018. Epidural Spinal Cord Stimulation of Lumbosacral Networks Modulates Arterial Blood Pressure in Individuals With Spinal Cord Injury-Induced Cardiovascular Deficits. Frontiers in physiology 9, 565. 10.3389/fphys.2018.00565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asyali MH, Berry RB, Khoo MC, 2002. Assessment of closed-loop ventilatory stability in obstructive sleep apnea. IEEE Trans Biomed Eng 49, 206–16. 10.1109/10.983454 [DOI] [PubMed] [Google Scholar]
- Avdoshina V, Mocchetti I, Liu C, Young MA, Anastos K, Cohen M, Crystal H, Pearce CL, Golub ET, Tractenberg RE, 2013. Single-nucleotide polymorphisms in TrkB and risk for depression: findings from the women’s interagency HIV study. J Acquir Immune Defic Syndr 64, 138–141. 10.1097/QAI.0b013e3182a468e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock M, Shkoukani M, Aboubakr SE, Badr MS, 2003. Determinants of long-term facilitation in humans during NREM sleep. J Appl Physiol (1985) 94, 53–59. 10.1152/japplphysiol.00476.2002 [DOI] [PubMed] [Google Scholar]
- Babcock MA, Badr MS, 1998. Long-term facilitation of ventilation in humans during NREM sleep. Sleep 21, 709–716. [PubMed] [Google Scholar]
- Bach KB, Mitchell GS, 1996. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104, 251–260. 10.1016/0034-5687(96)00017-5 [DOI] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS, 2000. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol 529, 215–219. 10.1111/j.1469-7793.2000.00215.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS, 2002. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22, 6239–6246. https://doi.org/20026595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Thomas CF, 2005. Sex hormone receptors are expressed in identified respiratory motoneurons in male and female rats. Neuroscience 130, 725–734. 10.1016/j.neuroscience.2004.09.058 [DOI] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Mitchell GS, 2002. Age and gender effects on serotonin-dependent plasticity in respiratory motor control. Respir Physiol Neurobiol 131, 65–77. 10.1016/s1569-9048(02)00038-1 [DOI] [PubMed] [Google Scholar]
- Biegon A, McEwen BS, 1982. Modulation by estradiol of serotonin receptors in brain. J. Neurosci 2, 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J, Stanley N, James LM, Wright N, Johnsen S, Arbon EL, Dijk DJ, 2012. Acute sleep deprivation: the effects of the AMPAKINE compound CX717 on human cognitive performance, alertness and recovery sleep. J Psychopharmacol 26, 1047–57. 10.1177/0269881111405353 [DOI] [PubMed] [Google Scholar]
- Bunday KL, Perez MA, 2012. Motor recovery after spinal cord injury enhanced by strengthening corticospinal synaptic transmission. Current biology: CB 22, 2355–61. 10.1016/j.cub.2012.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunday KL, Urbin MA, Perez MA, 2018. Potentiating paired corticospinal-motoneuronal plasticity after spinal cord injury. Brain Stimul 11, 1083–1092. 10.1016/j.brs.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Chhibber A, Zhao L, 2017. ERbeta and ApoE isoforms interact to regulate BDNF-5-HT2A signaling and synaptic function in the female brain. Alzheimers Res Ther 9, 79. 10.1186/s13195-017-0305-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo AE, Sitrin RG, Bauman KA, 2016. Sleep disordered breathing in spinal cord injury: A systematic review. J Spinal Cord Med 39, 374–382. 10.1080/10790268.2015.1126449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhuri S, Pierchala L, Aboubakr SE, Shkoukani M, Badr MS, 2008. Long-term facilitation of genioglossus activity is present in normal humans during NREM sleep. Respir Physiol Neurobiol 160, 65–75. 10.1016/j.resp.2007.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen L, Chen B, Lei Y, Urbin MA, Richardson MSA, Oudega M, Sandhu M, Rymer WZ, Trumbower RD, Mitchell GS, Perez MA, 2021. Acute intermittent hypoxia boosts spinal plasticity in humans with tetraplegia. Exp Neurol 335, 113483. 10.1016/j.expneurol.2020.113483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen L, Chen B, Lei Y, Urbin MA, Richardson MSA, Oudega M, Sandhu M, Rymer WZ, Trumbower RD, Mitchell GS, Perez MA, 2020. Acute intermittent hypoxia boosts spinal plasticity in humans with tetraplegia. Exp Neurol 335, 113483. 10.1016/j.expneurol.2020.113483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen L, Urbin M, Mitchell GS, Perez MA, 2018. Acute intermittent hypoxia enhances corticospinal synaptic plasticity in humans. eLife 7, e34304. 10.7554/eLife.34304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesla MC, Seven YB, Allen LL, Smith KN, Asa ZA, Simon AK, Holland AE, Santiago JV, Stefan K, Ross A, Gonzalez-Rothi EJ, Mitchell GS, 2021. Serotonergic innervation of respiratory motor nuclei after cervical spinal injury: Impact of intermittent hypoxia. Exp Neurol 338, 113609. 10.1016/j.expneurol.2021.113609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier RA, Bi C, Han YR, Plummer MR, 2008. BDNF Modulation of NMDA Receptors Is Activity Dependent. Journal of Neurophysiology 100, 3264–3274. 10.1152/jn.90418.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Alves E, de Aquino Lemos V, Ruiz da Silva F, Lira FS, dos Santos RVT, Rosa JPP, Caperuto E, Tufik S, de Mello MT, 2013. Low-Grade Inflammation and Spinal Cord Injury: Exercise as Therapy? Mediators of Inflammation 2013, 971841. 10.1155/2013/971841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Aarts L, Smith TW, 2010. Incidence, Reversal, and Prevention of Opioid-induced Respiratory Depression. Anesthesiology 112, 226–38. 10.1097/ALN.0b013e3181c38c25 [DOI] [PubMed] [Google Scholar]
- Dale EA, Ben Mabrouk F, Mitchell GS, 2014. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology (Bethesda) 29, 39–48. 10.1152/physiol.00012.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale EA, Fields DP, Devinney MJ, Mitchell GS, 2017. Phrenic motor neuron TrkB expression is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. Experimental Neurology 287, 130–136. 10.1016/j.expneurol.2016.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle Erica A., Hoffman MS, MacFarlane PM, Mitchell GS, 2010. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol 669, 225–230. 10.1007/978-1-4419-5692-7_45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Satriotomo I, Lovett-Barr MR, Vinit S, Mitchell GS, 2010. Spinal plasticity following intermittent hypoxia: implications for spinal injury. Ann N Y Acad Sci 1198, 252–9. 10.1111/j.1749-6632.2010.05499.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow D, Balser D, Netoff TI, Krassioukov A, Phillips A, Parr A, Samadani U, 2019. Epidural Spinal Cord Stimulation Facilitates Immediate Restoration of Dormant Motor and Autonomic Supraspinal Pathways after Chronic Neurologically Complete Spinal Cord Injury. J Neurotrauma 36, 2325–2336. 10.1089/neu.2018.6006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS, 2013. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann N Y Acad Sci 1279. 10.1111/nyas.12085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Nichols NL, Mitchell GS, 2016. Sustained Hypoxia Elicits Competing Spinal Mechanisms of Phrenic Motor Facilitation. J Neurosci 36, 7877–85. 10.1523/jneurosci.4122-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep TT, Khan TR, Zhang R, Duffin J, 2007. Long-term facilitation of breathing is absent after episodes of hypercapnic hypoxia in awake humans. Respir Physiol Neurobiol 156, 132–136. 10.1016/j.resp.2006.08.011 [DOI] [PubMed] [Google Scholar]
- Dongés SC, D’Amico JM, Butler JE, Taylor JL, 2018. Involvement of N-methyl-d-aspartate receptors in plasticity induced by paired corticospinal-motoneuronal stimulation in humans. Journal of Neurophysiology 119, 652–661. 10.1152/jn.00457.2017 [DOI] [PubMed] [Google Scholar]
- Dougherty BJ, Kopp ES, Watters JJ, 2017. Nongenomic Actions of 17-beta Estradiol Restore Respiratory Neuroplasticity in Young Ovariectomized Female Rats. J Neurosci 37, 6648–6660. 10.1523/jneurosci.0433-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty BJ, Terada J, Springborn SR, Vinit S, MacFarlane PM, Mitchell GS, 2018. Daily acute intermittent hypoxia improves breathing function with acute and chronic spinal injury via distinct mechanisms. Respir Physiol Neurobiol 256, 50–57. 10.1016/j.resp.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán J, Esnaola S, Rubio R, Iztueta A, 2001. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med 163, 685–689. 10.1164/ajrccm.163.3.2005065 [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Harkema S, 2011. Epidural stimulation of the spinal cord in spinal cord injury: current status and future challenges. Expert Rev Neurother 11, 1351–3. 10.1586/ern.11.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge and Millhorn F, 1986. Oscillation, gating, and memory in the respiratory control system. Handbook of Physiology: The Respiratory System, Vol 2, Amer Phys Soc, Bethesda: 93–114. [Google Scholar]
- ElMallah MK, Pagliardini S, Turner SM, Cerreta AJ, Falk DJ, Byrne BJ, Greer JJ, Fuller DD, 2015. Stimulation of Respiratory Motor Output and Ventilation in a Murine Model of Pompe Disease by Ampakines. Am J Respir Cell Mol Biol 53, 326–35. 10.1165/rcmb.2014-03740C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE, 2003. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26, 239–266. 10.1146/annurev.neuro.26.041002.131103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields DP, Mitchell GS, 2017. Divergent cAMP signaling differentially regulates serotonin-induced spinal motor plasticity. Neuropharmacology 113, 82–88. 10.1016/j.neuropharm.2016.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields DP, Mitchell GS, 2015. Spinal metaplasticity in respiratory motor control. Front Neural Circuits 9, 2. 10.3389/fncir.2015.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields DP, Springborn SR, Mitchell GS, 2015. Spinal 5-HT7 receptors induce phrenic motor facilitation via EPAC-mTORC1 signaling. J Neurophysiol 114, 2015–22. 10.1152/jn.00374.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan JD, Udani SV, Patel V, Bailes JE, 2018. The Influence of the Val66Met Polymorphism of Brain-Derived Neurotrophic Factor on Neurological Function after Traumatic Brain Injury. J Alzheimers Dis 65, 1055–1064. 10.3233/jad-180585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GE, McKenzie DC, Milsom WK, Sheel AW, 2005. Effects of two protocols of intermittent hypoxia on human ventilatory, cardiovascular and cerebral responses to hypoxia. J Physiol 567, 689–699. 10.1113/jphysiol.2005.091462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregosi RF, Mitchell GS, 1994. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol 477 (Pt 3), 469–479. 10.1113/jphysiol.1994.sp020208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Townsend NE, Shiller SM, Martini ER, Okazaki K, Shibata S, Truijens MJ, Rodríguez FA, Gore CJ, Stray-Gundersen J, Levine BD, 2007. Intermittent hypobaric hypoxia exposure does not cause sustained alterations in autonomic control of blood pressure in young athletes. Am J Physiol Regul Integr Comp Physiol 292, R1977–1984. 10.1152/ajpregu.00622.2006 [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB Jr., Mitchell GS, 2003. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci 23, 2993–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Wang ZY, Ling L, Olson EB, Bisgard GE, Mitchell GS, 2001. Induced recovery of hypoxic phrenic responses in adult rats exposed to hyperoxia for the first month of life. J Physiol 536, 917–926. 10.1111/j.1469-7793.2001.00917.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL, 1995. Modulation of neural network activity in vitro by cyclothiazide, a drug that blocks desensitization of AMPA receptors. J. Neurosci 15, 4046–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad PN, Gerasimenko Y, Zdunowski S, Turner A, Sayenko D, Lu DC, Edgerton VR, 2017. Weight Bearing Over-ground Stepping in an Exoskeleton with Non-invasive Spinal Cord Neuromodulation after Motor Complete Paraplegia. Frontiers in neuroscience 11, 333. 10.3389/fnins.2017.00333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad PN, Gerasimenko YP, Zdunowski S, Sayenko D, Haakana P, Turner A, Lu D, Roy RR, Edgerton VR, 2015. Iron “ElectriRx” man: Overground stepping in an exoskeleton combined with noninvasive spinal cord stimulation after paralysis. Conference proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference 2015, 1124–7. 10.1109/embc.2015.7318563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad PN, Lee S, Terrafranca N, Zhong H, Turner A, Gerasimenko Y, Edgerton VR, 2018. Non-Invasive Activation of Cervical Spinal Networks after Severe Paralysis. Journal of neurotrauma 35, 2145–2158. 10.1089/neu.2017.5461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerst DG 3rd, Yokhana SS, Carney LM, Lee DS, Badr MS, Qureshi T, Anthouard MN, Mateika JH, 2011. The hypoxic ventilatory response and ventilatory long-term facilitation are altered by time of day and repeated daily exposure to intermittent hypoxia. J Appl Physiol (1985) 110, 15–28. 10.1152/japplphysiol.00524.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbani M, Shahabi P, Karimi P, Soltani-Zangbar H, Morshedi M, Bani S, Jafarzadehgharehziaaddin M, Sadeghzadeh-Oskouei B, Ahmadalipour A, 2020. Impacts of epidural electrical stimulation on Wnt signaling, FAAH, and BDNF following thoracic spinal cord injury in rat. J Cell Physiol. 10.1002/jcp.29793 [DOI] [PubMed] [Google Scholar]
- Gill ML, Grahn PJ, Calvert JS, Linde MB, Lavrov IA, Strommen JA, Beck LA, Sayenko DG, Van Straaten MG, Drubach DI, Veith DD, Thoreson AR, Lopez C, Gerasimenko YP, Edgerton VR, Lee KH, Zhao KD, 2018. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nature medicine, 10.1038/s41591-018-0175-7 [DOI] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC, 2003. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. The Journal of neuroscience: the official journal of the Society for Neuroscience 23, 2494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS, 2005. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci 25, 2925–2932. 10.1523/JNEUROSCI.0148-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rothi EJ, Lee K-Z, Dale EA, Reier PJ, Mitchell GS, Fuller DD, 2015. Intermittent hypoxia and neurorehabilitation. J Appl Physiol (1985) 119, 1455–1465. 10.1152/japplphysiol.00235.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rothi EJ, Tadjalli A, Allen LL, Ciesla MC, Chami ME, Mitchell GS, 2021. Protocol-Specific Effects of Intermittent Hypoxia Pre-Conditioning on Phrenic Motor Plasticity in Rats with Chronic Cervical Spinal Cord Injury. J Neurotrauma 38, 1292–1305. 10.1089/neu.2020.7324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG, 2003. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. Journal of applied physiology (Bethesda, Md.: 1985) 94, 795–810. 10.1152/japplphysiol.00847.2002 [DOI] [PubMed] [Google Scholar]
- Grahn PJ, Lavrov IA, Sayenko DG, Van Straaten MG, Gill ML, Strommen JA, Calvert JS, Drubach DI, Beck LA, Linde MB, Thoreson AR, Lopez C, Mendez AA, Gad PN, Gerasimenko YP, Edgerton VR, Zhao KD, Lee KH, 2017. Enabling Task-Specific Volitional Motor Functions via Spinal Cord Neuromodulation in a Human With Paraplegia. Mayo Clinic proceedings 92, 544–554. 10.1016/j.mayocp.2017.02.014 [DOI] [PubMed] [Google Scholar]
- Greiner N, Barra B, Schiavone G, Lorach H, James N, Conti S, Kaeser M, Fallegger F, Borgognon S, Lacour S, Bloch J, Courtine G, Capogrosso M, 2021. Recruitment of upper-limb motoneurons with epidural electrical stimulation of the cervical spinal cord. Nat Commun 12, 435. 10.1038/s41467-020-20703-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, 2002. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience 111, 815–835. [DOI] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR, 2011. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377, 1938–47. 10.1016/s0140-6736(11)60547-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DP, Balasubramaniam A, Badr MS, Mateika JH, 2006. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol 291, R1111–9. 10.1152/ajpregu.00896.2005 [DOI] [PubMed] [Google Scholar]
- Hassan A, Arnold BM, Caine S, Toosi BM, Verge VMK, Muir GD, 2018. Acute intermittent hypoxia and rehabilitative training following cervical spinal injury alters neuronal hypoxia- and plasticity-associated protein expression. PLoS One 13, e0197486. 10.1371/journal.pone.0197486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR, 1993. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol 265, R811–819. 10.1152/ajpregu.1993.265.4.R811 [DOI] [PubMed] [Google Scholar]
- Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD, 2014. Daily intermittent hypoxia enhances walking after chronic spinal cord injury. Neurology 82, 104–113. 10.1212/01.WNL.0000437416.34298.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes KC, Hull TCL, Delaney GA, Potter PJ, Sequeira KAJ, Campbell K, Popovich PG, 2002. Elevated Serum Titers of Proinflammatory Cytokines and CNS Autoantibodies in Patients with Chronic Spinal Cord Injury. Journal of Neurotrauma 19, 753–761. 10.1089/08977150260139129 [DOI] [PubMed] [Google Scholar]
- Hiroi R, McDevitt RA, Neumaier JF, 2006. Estrogen Selectively Increases Tryptophan Hydroxylase-2 mRNA Expression in Distinct Subregions of Rat Midbrain Raphe Nucleus: Association between Gene Expression and Anxiety Behavior in the Open Field. Biological Psychiatry 60, 288–295. 10.1016/j.biopsych.2005.10.019 [DOI] [PubMed] [Google Scholar]
- Hoffman MS, Golder FJ, Mahamed S, Mitchell GS, 2010. Spinal adenosine A2(A) receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. J Physiol 588, 255–66. 10.1113/jphysiol.2009.180075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Mitchell GS, 2013. Spinal 5-HT7 receptors and protein kinase A constrain intermittent hypoxia-induced phrenic long-term facilitation. Neuroscience 250, 632–43. 10.1016/j.neuroscience.2013.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable AG, Kopp E, Dougherty BJ, Watters JJ, Mitchell GS, 2018. Cyclooxygenase enzyme activity does not impair respiratory motor plasticity after one night of intermittent hypoxia. Respir Physiol Neurobiol 256, 21–28. 10.1016/j.resp.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable AG, Smith SM, Peterson TJ, Watters JJ, Mitchell GS, 2015. Intermittent Hypoxia-Induced Spinal Inflammation Impairs Respiratory Motor Plasticity by a Spinal p38 MAP Kinase-Dependent Mechanism. J Neurosci 35, 6871–80. 10.1523/jneurosci.4539-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable AG, Smith SM, Vinit S, Watters JJ, Mitchell GS, 2013. Systemic LPS induces spinal inflammatory gene expression and impairs phrenic long-term facilitation following acute intermittent hypoxia. Journal of applied physiology (Bethesda, Md.: 1985) 114, 879–87. 10.1152/japplphysiol.01347.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable AG, Vinit S, Windelborn JA, Crader SM, Guenther CH, Watters JJ, Mitchell GS, 2011. Systemic inflammation impairs respiratory chemoreflexes and plasticity. Respiratory physiology & neurobiology 178, 482–9. 10.1016/j.resp.2011.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inanici F, Samejima S, Gad P, Edgerton VR, Hofstetter CP, Moritz CT, 2018. Transcutaneous Electrical Spinal Stimulation Promotes Long-Term Recovery of Upper Extremity Function in Chronic Tetraplegia. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society 26, 1272–1278. 10.1109/tnsre.2018.2834339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Perez MA, 2020. Corticospinal-motor neuronal plasticity promotes exercise-mediated recovery in humans with spinal cord injury. Brain 143, 1368–1382. 10.1093/brain/awaa052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Mitchell GS, 2013. Common mechanisms of compensatory respiratory plasticity in spinal neurological disorders. Respir Physiol Neurobiol 189, 419–428. 10.1016/j.resp.2013.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan AS, Catcheside PG, O’Donoghue FJ, McEvoy RD, 2002. Long-term facilitation of ventilation is not present during wakefulness in healthy men or women. Journal of Applied Physiology 93, 2129–2136. 10.1152/japplphysiol.00135.2002 [DOI] [PubMed] [Google Scholar]
- Khoo MC, 2001. Using loop gain to assess ventilatory control in obstructive sleep apnea. Am J Respir Crit Care Med 163, 1044–5. 10.1164/ajrccm.163.5.ed1101c [DOI] [PubMed] [Google Scholar]
- Kinkead R, Bach KB, Johnson SM, Hodgeman BA, Mitchell GS, 2001. Plasticity in respiratory motor control: intermittent hypoxia and hypercapnia activate opposing serotonergic and noradrenergic modulatory systems. Comp Biochem Physiol A Mol Integr Physiol 130, 207–218. 10.1016/s1095-6433(01)00393-2 [DOI] [PubMed] [Google Scholar]
- Krucoff MO, Rahimpour S, Slutzky MW, Edgerton VR, Turner DA, 2016. Enhancing Nervous System Recovery through Neurobiologics, Neural Interface Training, and Neurorehabilitation. Front. Neurosci 10. 10.3389/fnins.2016.00584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech KA, Hornby TG, 2017. High-Intensity Locomotor Exercise Increases Brain-Derived Neurotrophic Factor in Individuals with Incomplete Spinal Cord Injury. J Neurotrauma 34, 1240–1248. 10.1089/neu.2016.4532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lucas-Osma AM, Black S, Bandet MV, Stephens MJ, Vavrek R, Sanelli L, Fenrich KK, Di Narzo AF, Dracheva S, Winship IR, Fouad K, Bennett DJ, 2017. Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat Med 23, 733–741. 10.1038/nm.4331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, 2008. Serotonin and NMDA receptors in respiratory long-term facilitation. Respir Physiol Neurobiol 164, 233–41. 10.1016/j.resp.2008.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Mitchell GS, 2001. Chronic Intermittent Hypoxia Elicits Serotonin-Dependent Plasticity in the Central Neural Control of Breathing. J Neurosci 21, 5381–5388. 10.1523/JNEUROSCI.21-14-05381.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JER, Hoffman MS, Vinit S, Mitchell GS, 2012. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J. Neurosci 32, 3591–3600. 10.1523/JNEUROSCI.2908-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DC, Edgerton VR, Modaber M, AuYong N, Morikawa E, Zdunowski S, Sarino ME, Sarrafzadeh M, Nuwer MR, Roy RR, Gerasimenko Y, 2016. Engaging Cervical Spinal Cord Networks to Reenable Volitional Control of Hand Function in Tetraplegic Patients. Neurorehabilitation and neural repair 30, 951–962. 10.1177/1545968316644344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, 2006. Glutamate-based therapeutic approaches: ampakines. Curr Opin Pharmacol 6, 82–8. 10.1016/j.coph.2005.09.005 [DOI] [PubMed] [Google Scholar]
- Lynch M, Duffell L, Sandhu M, Srivatsan S, Deatsch K, Kessler A, Mitchell GS, Jayaraman A, Rymer WZ, 2017. Effect of acute intermittent hypoxia on motor function in individuals with chronic spinal cord injury following ibuprofen pretreatment: A pilot study. J Spinal Cord Med 40, 295–303. 10.1080/10790268.2016.1142137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Satriotomo I, Windelborn JA, Mitchell GS, 2009. NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. J Physiol 587, 1931–1942. 10.1113/jphysiol.2008.165597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Vinit S, Mitchell GS, 2011. Serotonin 2A and 2B receptor-induced phrenic motor facilitation: differential requirement for spinal NADPH oxidase activity. Neuroscience 178, 45–55. 10.1016/j.neuroscience.2011.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS, 2008. Simulated apnoeas induce serotonin-dependent respiratory long-term facilitation in rats. J Physiol 586, 2171–2181. 10.1113/jphysiol.2007.149047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS, 2007. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol 92, 27–37. 10.1113/expphysiol.2006.033720 [DOI] [PubMed] [Google Scholar]
- Mateika JH, El-Chami M, Shaheen D, Ivers B, 2015. Intermittent hypoxia: a low-risk research tool with therapeutic value in humans. J Appl Physiol (1985) 118, 520–32. 10.1152/japplphysiol.00564.2014 [DOI] [PubMed] [Google Scholar]
- Mateika JH, Narwani G, 2009. Intermittent hypoxia and respiratory plasticity in humans and other animals: does exposure to intermittent hypoxia promote or mitigate sleep apnoea? Experimental Physiology 94, 279–296. 10.1113/expphysiol.2008.045153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateika JH, Panza G, Alex R, El-Chami M, 2018. The impact of intermittent or sustained carbon dioxide on intermittent hypoxia initiated respiratory plasticity. What is the effect of these combined stimuli on apnea severity? Respir Physiol Neurobiol 256, 58–66. 10.1016/j.resp.2017.10.008 [DOI] [PubMed] [Google Scholar]
- Mateika JH, Sandhu KS, 2011. Experimental protocols and preparations to study respiratory long term facilitation. Respir Physiol Neurobiol 176, 1–11. 10.1016/j.resp.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateika JH, Syed Z, 2013. Intermittent hypoxia, respiratory plasticity and sleep apnea in humans: present knowledge and future investigations. Respir Physiol Neurobiol 188, 289–300. 10.1016/j.resp.2013.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maussion G, Yang J, Yerko V, Barker P, Mechawar N, Ernst C, Turecki G, 2012. Regulation of a Truncated Form of Tropomyosin-Related Kinase B (TrkB) by Hsa-miR-185* in Frontal Cortex of Suicide Completers. PLOS ONE 7, e39301. 10.1371/journal.pone.0039301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy RD, Popovic RM, Saunders NA, White DP, 1996. Effects of sustained and repetitive isocapnic hypoxia on ventilation and genioglossal and diaphragmatic EMGs. Journal of Applied Physiology 81, 866–875. 10.1152/jappl.1996.81.2.866 [DOI] [PubMed] [Google Scholar]
- McGuire M, Liu C, Cao Y, Ling L, 2008. Formation and maintenance of ventilatory long-term facilitation require NMDA but not non-NMDA receptors in awake rats. J Appl Physiol (1985) 105, 942–950. 10.1152/japplphysiol.01274.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L, 2005. Phrenic long-term facilitation requires NMDA receptors in the phrenic motonucleus in rats. J Physiol 567, 599–611. 10.1113/jphysiol.2005.087650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHughen SA, Rodriguez PF, Kleim JA, Kleim ED, Marchal Crespo L, Procaccio V, Cramer SC, 2010. BDNF val66met polymorphism influences motor system function in the human brain. Cereb Cortex 20, 1254–62. 10.1093/cercor/bhp189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG, 1980a. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol 41, 87–103. 10.1016/0034-5687(80)90025-0 [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG, 1980b. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol 42, 171–188. 10.1016/0034-5687(80)90113-9 [DOI] [PubMed] [Google Scholar]
- Mitchell GS, 2008. Respiratory plasticity following intermittent hypoxia: a guide for novel therapeutic approaches to ventilatory control disorders?, in: Genetic Basis for Respiratory Control Disorders. Springer US, Boston, MA, pp. 291–311. 10.1007/978-0-387-70765-5_17 [DOI] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB, 2001. Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol (1985) 90, 2466–2475. 10.1152/jappl.2001.90.6.2466 [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM, 2003. Neuroplasticity in respiratory motor control. J Appl Physiol (1985) 94, 358–374. 10.1152/japplphysiol.00523.2002 [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Terada J, 2011. Should we standardize protocols and preparations used to study respiratory plasticity? Respir Physiol Neurobiol 177, 93–97. 10.1016/j.resp.2011.03.021 [DOI] [PubMed] [Google Scholar]
- Naidu A, Peters DM, Tan AQ, Barth S, Crane A, Link A, Balakrishnan S, Hayes HB, Slocum C, Zafonte RD, Trumbower RD, 2020. Daily acute intermittent hypoxia to improve walking function in persons with subacute spinal cord injury: a randomized clinical trial study protocol. BMC Neurol 20, 273. 10.1186/s12883-020-01851-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical, C., 2020. University of Alabama at Birmingham Spinal Cord Injury Model System Information Network [WWW Document], URL http://www.uab.edu/medicine/sci/
- Navarrete-Opazo A, Alcayaga J, Sepúlveda O, Rojas E, Astudillo C, 2017. Repetitive Intermittent Hypoxia and Locomotor Training Enhances Walking Function in Incomplete Spinal Cord Injury Subjects: A Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. J Neurotrauma 34, 1803–1812. 10.1089/neu.2016.4478 [DOI] [PubMed] [Google Scholar]
- Navarrete-Opazo Angela, Alcayaga J, Sepúlveda O, Rojas E, Astudillo C, 2017a. Repetitive Intermittent Hypoxia and Locomotor Training Enhances Walking Function in Incomplete Spinal Cord Injury Subjects: A Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. J. Neurotrauma 34, 1803–1812. 10.1089/neu.2016.4478 [DOI] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Alcayaga J, Testa D, Quinteros AL, 2016. Intermittent Hypoxia Does not Elicit Memory Impairment in Spinal Cord Injury Patients. Arch Clin Neuropsychol 31, 332–342. 10.1093/arclin/acw012 [DOI] [PubMed] [Google Scholar]
- Navarrete-Opazo Angela, Alcayaga JJ, Sepúlveda O, Varas G, 2017b. Intermittent Hypoxia and Locomotor Training Enhances Dynamic but Not Standing Balance in Patients With Incomplete Spinal Cord Injury. Arch Phys Med Rehabil 98, 415–424. 10.1016/j.apmr.2016.09.114 [DOI] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Mitchell GS, 2014. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol 307, R1181–1197. 10.1152/ajpregu.00208.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Vinit S, Dougherty BJ, Mitchell GS, 2015. Daily acute intermittent hypoxia elicits functional recovery of diaphragm and inspiratory intercostal muscle activity after acute cervical spinal injury. Exp Neurol 266, 1–10. 10.1016/j.expneurol.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson NR, Bird IM, Behan M, 2011. Testosterone restores respiratory long term facilitation in old male rats by an aromatase-dependent mechanism. J Physiol 589, 409–21. 10.1113/jphysiol.2010.198200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Dale EA, Mitchell GS, 2012. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J Appl Physiol (1985) 112, 1678–88. 10.1152/japplphysiol.00060.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Mitchell GS, 2016. QUANTITATIVE ASSESSMENT OF INTEGRATED PHRENIC NERVE ACTIVITY. Respir Physiol Neurobiol 226, 81–86. 10.1016/j.resp.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Van Dyke J, Nashold L, Satriotomo I, Suzuki M, Mitchell GS, 2013. Ventilatory control in ALS. Respir Physiol Neurobiol 189, 429–437. 10.1016/j.resp.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, 2017. A Brief History of Long-Term Potentiation. Neuron 93, 281–290. 10.1016/j.neuron.2016.12.015 [DOI] [PubMed] [Google Scholar]
- Olson EB, Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, Powell FL, Mitchel GS, 2001. Ventilatory long-term facilitation in unanesthetized rats. J Appl Physiol (1985) 91, 709–716. 10.1152/jappl.2001.91.2.709 [DOI] [PubMed] [Google Scholar]
- Onifer SM, Smith GM, Fouad K, 2011. Plasticity after spinal cord injury: relevance to recovery and approaches to facilitate it. Neurotherapeutics 8, 283–293. 10.1007/s13311-011-0034-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otzel DM, Lee J, Ye F, Borst SE, Yarrow JF, 2018. Activity-Based Physical Rehabilitation with Adjuvant Testosterone to Promote Neuromuscular Recovery after Spinal Cord Injury. Int J Mol Sci 19, E1701. 10.3390/ijms19061701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partida E, Mironets E, Hou S, Tom VJ, 2016. Cardiovascular dysfunction following spinal cord injury. Neural Regen Res 11, 189–194. 10.4103/1673-5374.177707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perim RR, El-Chami M, Gonzalez-Rothi EJ, Mitchell GS, 2021. Baseline Arterial CO2 Pressure Regulates Acute Intermittent Hypoxia-Induced Phrenic Long-Term Facilitation in Rats. Front. Physiol 12. 10.3389/fphys.2021.573385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perim RR, Fields DP, Mitchell GS, 2018. Cross-talk inhibition between 5-HT2B and 5-HT7 receptors in phrenic motor facilitation via NADPH oxidase and PKA. Am J Physiol Regul Integr Comp Physiol 314, R709–r715. 10.1152/ajpregu.00393.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perim RR, Mitchell GS, 2019. Circulatory control of phrenic motor plasticity. Respir Physiol Neurobiol 265, 19–23. 10.1016/j.resp.2019.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson MD, Belakovskiy A, McGrath R, Yarrow JF, 2018. Testosterone Deficiency, Weakness, and Multimorbidity in Men. Sci Rep 8, 5897. 10.1038/S41598-018-24347-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryshen TL, Sabeti PC, Aldinger KA, Fry B, Fan JB, Schaffner SF, Waggoner SG, Tahl AR, Sklar P, 2010. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol Psychiatry 15, 810–815. 10.1038/mp.2009.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierchala LA, Mohammed AS, Grullon K, Mateika JH, Badr MS, 2008. Ventilatory long-term facilitation in non-snoring subjects during NREM sleep. Respir Physiol Neurobiol 160, 259–266. 10.1016/j.resp.2007.10.008 [DOI] [PubMed] [Google Scholar]
- Prosser-Loose EJ, Hassan A, Mitchell GS, Muir GD, 2015. Delayed Intervention with Intermittent Hypoxia and Task Training Improves Forelimb Function in a Rat Model of Cervical Spinal Injury. Journal of Neurotrauma 32, 1403–1412. 10.1089/neu.2014.3789 [DOI] [PubMed] [Google Scholar]
- Ramer MS, Harper GP, Bradbury EJ, 2000. Progress in spinal cord research - a refined strategy for the International Spinal Research Trust. Spinal Cord 38, 449–472. 10.1038/sj.sc.3101055 [DOI] [PubMed] [Google Scholar]
- Rowley JA, Deebajah I, Parikh S, Najar A, Saha R, Badr MS, 2007. The influence of episodic hypoxia on upper airway collapsibility in subjects with obstructive sleep apnea. Journal of Applied Physiology 103, 911–916. 10.1152/japplphysiol.01117.2006 [DOI] [PubMed] [Google Scholar]
- Sandhu MS, Gray E, Kocherginsky M, Jayaraman A, Mitchell GS, Rymer WZ, 2019. Prednisolone Pretreatment Enhances Intermittent Hypoxia-Induced Plasticity in Persons With Chronic Incomplete Spinal Cord Injury. Neurorehabil Neural Repair 33, 911–921. 10.1177/1545968319872992 [DOI] [PubMed] [Google Scholar]
- Sandhu MS, Perez MA, Oudega M, Mitchell GS, Rymer WZ, 2021. Efficacy and time course of acute intermittent hypoxia effects in the upper extremities of people with cervical spinal cord injury. Exp Neurol 342, 113722. 10.1016/j.expneurol.2021.113722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankari A, Bascom A, Oomman S, Badr MS, 2014. Sleep disordered breathing in chronic spinal cord injury. J Clin Sleep Med 10, 65–72. 10.5664/jcsm.3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankari A, Bascom AT, Riehani A, Badr MS, 2015. Tetraplegia is associated with enhanced peripheral chemoreflex sensitivity and ventilatory long-term facilitation. J Appl Physiol (1985) 119, 1183–1193. 10.1152/japplphysiol.00088.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satriotomo I, Dale EA, Dahlberg JM, Mitchell GS, 2012. Repetitive acute intermittent hypoxia increases expression of proteins associated with plasticity in the phrenic motor nucleus. Exp Neurol 237, 103–115. 10.1016/j.expneurol.2012.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satriotomo I, Nichols NL, Dale EA, Emery AT, Dahlberg JM, Mitchell GS, 2016. REPETITIVE ACUTE INTERMITTENT HYPOXIA INCREASES GROWTH/NEUROTROPHIC FACTOR EXPRESSION IN NON-RESPIRATORY MOTOR NEURONS. Neuroscience 322, 479–488. 10.1016/j.neuroscience.2016.02.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seven YB, Perim RR, Hobson OR, Simon AK, Tadjalli A, Mitchell GS, 2018. Phrenic motor neuron adenosine 2A receptors elicit phrenic motor facilitation. J Physiol 596, 1501–1512. 10.1113/jp275462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezer N, Akkuş S, Uǧurlu FG, 2015. Chronic complications of spinal cord injury. World J Orthop 6, 24–33. 10.5312/wjo.v6.i1.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T, You Y, Joseph C, Mirzaei M, Klistorner A, Graham SL, Gupta V, 2018. BDNF Polymorphism: A Review of Its Diagnostic and Clinical Relevance in Neurodegenerative Disorders. Aging Dis 9, 523–536. 10.14336/AD.2017.0717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strattan LE, Britsch DRS, Calulot CM, Maggard RSJ, Abner EL, Johnson LA, Alilain WJ, 2021. Novel Influences of Sex and APOE Genotype on Spinal Plasticity and Recovery of Function after Spinal Cord Injury. eNeuro 8. 10.1523/ENEURO.0464-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner BE, Fink G, 1998. Testosterone as well as estrogen increases serotonin2A receptor mRNA and binding site densities in the male rat brain. Brain Res Mol Brain Res 59, 205–214. 10.1016/s0169-328x(98)00148-x [DOI] [PubMed] [Google Scholar]
- Suppiramaniam V, Bahr BA, Sinnarajah S, Owens K, Rogers G, Yilma S, Vodyanoy V, 2001. Member of the Ampakine class of memory enhancers prolongs the single channel open time of reconstituted AMPA receptors. Synapse 40, 154–8. 10.1002/syn.1037 [DOI] [PubMed] [Google Scholar]
- Sutor T, Cavka K, Vose AK, Welch JF, Davenport P, Fuller DD, Mitchell GS, Fox EJ, 2021. Single-session effects of acute intermittent hypoxia on breathing function after human spinal cord injury. Exp Neurol 342, 113735. 10.1016/j.expneurol.2021.113735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadjalli A, Mitchell GS, 2019. Cervical spinal 5-HT(2A) and 5-HT(2B) receptors are both necessary for moderate acute intermittent hypoxia-induced phrenic long-term facilitation. J Appl Physiol (1985) 127, 432–443. 10.1152/japplphysiol.01113.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamisier R, Anand A, Nieto LM, Cunnington D, Weiss JW, 2005. Arterial pressure and muscle sympathetic nerve activity are increased after two hours of sustained but not cyclic hypoxia in healthy humans. J Appl Physiol (1985) 98, 343–349. 10.1152/japplphysiol.00495.2004 [DOI] [PubMed] [Google Scholar]
- Tan AQ, Papadopoulos JM, Corsten AN, Trumbower RD, 2020. An automated pressure-swing absorption system to administer low oxygen therapy for persons with spinal cord injury. Exp Neurol 333, 113408. 10.1016/j.expneurol.2020.113408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AQ, Sohn WJ, Naidu A, Trumbower RD, 2021. Daily acute intermittent hypoxia combined with walking practice enhances walking performance but not intralimb motor coordination in persons with chronic incomplete spinal cord injury. Exp Neurol 340, 113669. 10.1016/j.expneurol.2021.113669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tankersley CG, Haenggeli C, Rothstein JD, 2007. Respiratory impairment in a mouse model of amyotrophic lateral sclerosis. J Appl Physiol (1985) 102, 926–932. 10.1152/japplphysiol.00193.2006 [DOI] [PubMed] [Google Scholar]
- Tester NJ, Fuller DD, Fromm JS, Spiess MR, Behrman AL, Mateika JH, 2014. Long-term facilitation of ventilation in humans with chronic spinal cord injury. Am. J. Respir. Crit. Care Med 189, 57–65. 10.1164/rccm.201305-0848OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakre PP, Sunshine MD, Fuller DD, 2021. Ampakine pretreatment enables a single hypoxic episode to produce phrenic motor facilitation with no added benefit of additional episodes. J Neurophysiol, 10.1152/jn.00307.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbower RD, Hayes HB, Mitchell GS, Wolf SL, Stahl VA, 2017. Effects of acute intermittent hypoxia on hand use after spinal cord trauma: A preliminary study. Neurology 89, 1904–1907. 10.1212/WNL.0000000000004596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ, 2012. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair 26, 163–172. 10.1177/1545968311412055 [DOI] [PubMed] [Google Scholar]
- Turner SM, ElMallah MK, Hoyt AK, Greer JJ, Fuller DD, 2016. Ampakine CX717 potentiates intermittent hypoxia-induced hypoglossal long-term facilitation. J Neurophysiol 116, 1232–1238. 10.1152/jn.00210.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL, 1995. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci 15, 5346–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen M, van der Meij A, van Deurzen PAM, Janzing JGE, Arias-Vásquez A, Buitelaar JK, Franke B, 2010. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Molecular Psychiatry 15, 260–271. 10.1038/mp.2008.109 [DOI] [PubMed] [Google Scholar]
- Vermehren-Schmaedick A, Khanjian RA, Balkowiec A, 2015. Cellular mechanisms of activity-dependent BDNF expression in primary sensory neurons. Neuroscience 310, 665–73. 10.1016/j.neuroscience.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen TD, Benbaruj J, Brown CV, Shafer BM, Floras JS, Foster GE, 2020. Peripheral chemoreflex contribution to ventilatory long-term facilitation induced by acute intermittent hypercapnic hypoxia in males and females. J Physiol 598, 4713–4730. 10.1113/JP280458 [DOI] [PubMed] [Google Scholar]
- Vinit S, Lovett-Barr MR, Mitchell GS, 2009. Intermittent hypoxia induces functional recovery following cervical spinal injury. Respir Physiol Neurobiol 169, 210–217. 10.1016/j.resp.2009.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinit S, Windelborn JA, Mitchell GS, 2011. Lipopolysaccharide attenuates phrenic long-term facilitation following acute intermittent hypoxia. Respir Physiol Neurobiol 176, 130–135. 10.1016/j.resp.2011.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivodtzev I, Tan AQ, Hermann M, Jayaraman A, Stahl V, Rymer WZ, Mitchell GS, Hayes HB, Trumbower RD, 2020. Mild to Moderate Sleep Apnea Is Linked to Hypoxia-induced Motor Recovery after Spinal Cord Injury. Am J Respir Crit Care Med 202, 887–890. 10.1164/rccm.202002-0245LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa H, Gradinaru C, Gates GJ, Badr MS, Mateika JH, 2008. Impact of intermittent hypoxia on long-term facilitation of minute ventilation and heart rate variability in men and women: do sex differences exist? J Appl Physiol (1985) 104, 1625–33. 10.1152/japplphysiol.01273.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FB, Mignardot JB, Le Goff-Mignardot CG, Demesmaeker R, Komi S, Capogrosso M, Rowald A, Seanez I, Caban M, Pirondini E, Vat M, McCracken LA, Heimgartner R, Fodor I, Watrin A, Seguin P, Paoles E, Van Den Keybus K, Eberle G, Schurch B, Pralong E, Becce F, Prior J, Buse N, Buschman R, Neufeld E, Kuster N, Carda S, von Zitzewitz J, Delattre V, Denison T, Lambert H, Minassian K, Bloch J, Courtine G, 2018. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 563, 65–71. 10.1038/s41586-018-0649-2 [DOI] [PubMed] [Google Scholar]
- Wang TD, Wang YH, Huang TS, Su TC, Pan SL, Chen SY, 2007. Circulating levels of markers of inflammation and endothelial activation are increased in men with chronic spinal cord injury. J Formos Med Assoc 106, 919–28. 10.1016/s0929-6646(08)60062-5 [DOI] [PubMed] [Google Scholar]
- Warraich Z, Kleim JA, 2010. Neural plasticity: the biological substrate for neurorehabilitation. PM R 2, S208–219. 10.1016/j.pmrj.2010.10.016 [DOI] [PubMed] [Google Scholar]
- Weiss MD, Tamisier R, Boucher J, Lynch M, Gilmartin G, Weiss JW, Thomas RJ, 2009. A pilot study of sleep, cognition, and respiration under four weeks of intermittent nocturnal hypoxia in adult humans. Sleep Med 10, 739–745. 10.1016/j.sleep.2008.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JF, Perim RR, Argento PJ, Sutor TW, Vose AK, Nair J, Mitchell GS, Fox EJ, 2021. Effect of acute intermittent hypoxia on cortico-diaphragmatic conduction in healthy humans. Exp Neurol 339, 113651. 10.1016/j.expneurol.2021.113651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JF, Sutor TW, Vose AK, Perim RR, Fox EJ, Mitchell GS, 2020. Synergy between Acute Intermittent Hypoxia and Task-Specific Training. Exerc Sport Sci Rev 48, 125–132. 10.1249/JES.0000000000000222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenjin W, Wenchao L, Hao Z, Feng L, Yan W, Wodong S, Xianqun F, Wenlong D, 2011. Electrical stimulation promotes BDNF expression in spinal cord neurons through Ca(2+)- and Erk-dependent signaling pathways. Cell Mol Neurobiol 31, 459–67. 10.1007/s10571-010-9639-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DP, 2007. Long-Term Facilitation (LTF) and Obstructive Sleep Apnea. Respir Physiol Neurobiol 158, 112–113. 10.1016/j.resp.2007.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JE, Mitchell GS, 2009. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol 217, 116–23. 10.1016/j.expneurol.2009.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman LB, Streeter KA, Fuller DD, 2020a. Ampakine pretreatment enables a single brief hypoxic episode to evoke phrenic motor facilitation. J Neurophysiol 123, 993–1003. 10.1152/jn.00708.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman LB, Streeter KA, Fusco AF, Gonzalez-Rothi EJ, Sandhu MS, Greer JJ, Fuller DD, 2020b. Ampakines stimulate phrenic motor output after cervical spinal cord injury. Exp Neurol 334, 113465. 10.1016/j.expneurol.2020.113465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS, 2001a. Long term facilitation of respiratory motor output decreases with age in male rats. J Physiol 531, 509–14. 10.1111/j.1469-7793.2001.0509i.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS, 2001b. Selected Contribution: Time-dependent hypoxic respiratory responses in female rats are influenced by age and by the estrus cycle. Journal of Applied Physiology 91, 2831–2838. 10.1152/jappl.2001.91.6.2831 [DOI] [PubMed] [Google Scholar]
- Zabka AG, Mitchell GS, Behan M, 2006. Conversion from testosterone to oestradiol is required to modulate respiratory long-term facilitation in male rats. J Physiol 576, 903–12. 10.1113/jphysiol.2006.114850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Mitchell GS, Olson EB, Behan M, 2003. Selected Contribution: Chronic intermittent hypoxia enhances respiratory long-term facilitation in geriatric female rats. Journal of Applied Physiology 95, 2614–2623. 10.1152/japplphysiol.00476.2003 [DOI] [PubMed] [Google Scholar]
- Zhang SXL, Wang Y, Gozal D, 2012. Pathological Consequences of Intermittent Hypoxia in the Central Nervous System, in: Comprehensive Physiology. American Cancer Society, pp. 1767–1777. 10.1002/cphy.c100060 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Ding X, Yang Q, Hu J, Shang X, Huang X, Ge L, Zhou T, 2015. Association between Single-Nucleotide Polymorphisms of the Tyrosine Kinase Receptor B (TrkB) and Post-Stroke Depression in China. PLoS One 10, e0144301–e0144301. 10.1371/journal.pone.0144301 [DOI] [PMC free article] [PubMed] [Google Scholar]


