Abstract
BACKGROUND:
Retrospective studies suggest that watch-and-wait is a safe alternative to total mesorectal excision in selected patients with a clinical complete response after chemoradiotherapy.
OBJECTIVE:
Determine the proportion of rectal cancer patients who may benefit from watch-and-wait.
DESIGN:
Retrospective analysis of data from prospectively maintained databases.
SETTING:
Comprehensive cancer center.
PATIENTS:
Consecutive patients with stage II or III rectal adenocarcinoma treated with TNT using induction chemotherapy between 2012 and 2019 under the care of the same surgeon.
INTERVENTION:
Induction-type total neoadjuvant therapy consisted of eight cycles of leucovorin-fluorouracil-oxaliplatin or five cycles of capecitabine-oxaliplatin before chemoradiotherapy. Patients with a clinical complete response were offered watch-and-wait, and patients with residual tumor were offered total mesorectal excision.
MAIN OUTCOMES AND MEASURES:
Tumor response was assessed with a digital rectal exam, endoscopy, and MRI. Patient characteristics and recurrence-free survival were compared between the watch-and-wait group and the total mesorectal excision group.
RESULTS:
A total of 88 patients were included in the analysis. One (1%) died during neoadjuvant therapy. Fifty-five patients (62.5%) had an incomplete clinical response and underwent surgery; 10 (18%) of the 55 developed distant metastasis, and 3 (5%) developed local recurrence. The remaining 32 patients (36.3%) had a cCR and underwent watch-and-wait. On average, patients in the watch-and-wait group were older and had smaller, more distal tumors compared with the surgery group. The median radiation dose, number of chemotherapy cycles, rate of adverse events, and length of follow-up did not differ substantively between the total mesorectal excision group and the watch-and-wait group. In the watch-and-wait group, 2 (6%) patients developed tumor regrowth, and one of them had distant metastasis. Recurrence-free survival was significantly higher in the watch-and-wait group.
LIMITATIONS:
Generalizability, sample size, follow-up duration.
CONCLUSIONS:
Approximately one-third of patients with stage II or III rectal cancer can benefit from a watch-and-wait approach with the aim of preserving the rectum if treated with induction-type total neoadjuvant therapy and followed by an experienced multidisciplinary team.
Keywords: Clinical complete response, Chemoradiotherapy, Organ preservation, Rectal cancer, Total neoadjuvant therapy, Watch-and-wait
Abstract
ANTECEDENTES:
Estudios retrospectivos sugieren que observar y esperar es una alternativa segura a la escisión mesorrectal total en pacientes seleccionados con una respuesta clínica completa después de la quimiorradioterapia.
OBJETIVO:
Determinar la proporción de pacientes con cáncer de recto que pueden beneficiarse de observar y esperar.
DISEÑO:
Análisis retrospectivo de datos de bases de datos mantenidas de forma prospectiva.
ESCENARIO:
Centro Oncológico Integral.
PACIENTES:
Pacientes consecutivos con adenocarcinoma de recto en estadio II o III tratados con TNT utilizando quimioterapia de inducción entre 2012 y 2019 bajo el cuidado del mismo cirujano.
INTERVENCIÓN:
La terapia neoadyuvante total de tipo inducción consistió en ocho ciclos de leucovorín-fluorouracilo-oxaliplatino o cinco ciclos de capecitabina-oxaliplatino antes de la quimiorradioterapia. A los pacientes con una respuesta clínica completa se les ofreció observar y esperar, y a los pacientes con tumor residual se les ofreció la escisión mesorrectal total.
PRINCIPALES RESULTADOS Y MEDIDAS:
La respuesta del tumor se evaluó con un tacto rectal, endoscopia y resonancia magnética. Se compararon las características de los pacientes y la supervivencia libre de recurrencia entre el grupo de observación y espera y el grupo de escisión mesorrectal total.
RESULTADOS:
Se incluyó en el análisis a un total de 88 pacientes. Uno (1%) murió durante la terapia neoadyuvante. Cincuenta y cinco pacientes (62.5%) tuvieron una respuesta clínica incompleta y se sometieron a cirugía; 10 (18%) de los 55 desarrollaron metástasis a distancia y 3 (5%) desarrollaron recidiva local. Los 32 pacientes restantes (36.3%) tuvieron una cCR (respuesta clínica completa) y se sometieron a observar y esperar. En promedio, los pacientes del grupo de observación y espera eran mayores y tenían tumores más pequeños y distales en comparación con el grupo de cirugía. La dosis mediana de radiación, el número de ciclos de quimioterapia, la tasa de eventos adversos y la duración del seguimiento no difirieron sustancialmente entre el grupo de escisión mesorrectal total y el grupo de observación y espera. En el grupo de observación y espera, 2 (6%) pacientes desarrollaron recrecimiento del tumor y uno de ellos tuvo metástasis a distancia. La supervivencia libre de recurrencia fue significativamente mayor en el grupo de observación y espera.
LIMITACIONES:
Generalizabilidad, tamaño de la muestra, duración del seguimiento.
CONCLUSIONES:
Aproximadamente un tercio de los pacientes con cáncer de recto en estadio II o III pueden beneficiarse de un abordaje de observación y espera con el objetivo de preservar el recto si se tratan con terapia neoadyuvante total de tipo inducción y son seguidos por un equipo multidisciplinario experimentado.
INTRODUCTION
Standard treatment of locally advanced rectal cancer consists of neoadjuvant chemoradiotherapy (CRT), total mesorectal excision (TME), and postoperative adjuvant chemotherapy.1 This intensive treatment leads to excellent local tumor control and patient survival, but is associated with short- and long-term morbidity that impairs each patient’s quality of life permanently.2,3 While CRT and adjuvant chemotherapy are associated with specific toxicity and may compound surgery-related morbidity, most of the side effects of multimodal treatment that impair the patient’s quality of life are attributable to TME.4 Even with the technological advances of robotic and transanal TME, some patients with distal rectal cancer will still require a permanent colostomy.5 In addition, patients who undergo a sphincter-saving procedure develop a combination of defecatory symptoms known as low anterior resection syndrome.6 These symptoms are associated with significant impairment of patients’ quality of life. With the age-adjusted incidence of rectal cancer increasing steadily in young patients,7 alternatives to TME are needed.
Some patients with locally advanced rectal cancer have a pathological complete response (pCR) to neoadjuvant CRT. As patients with pCR have excellent prognosis,8 surgeons question the added value of TME for patients with a clinical complete response (cCR) to CRT. Several institutional case series have reported that a watch-and-wait (WW) strategy can result in sustained organ preservation in patients with a cCR to CRT. Up to 30% of patients entered in WW protocols eventually experienced tumor regrowth, but most of the cases were surgically salvageable.9 In some series, the survival rate in patients with cCR entered in a WW protocol was equivalent to that in patients found to have a pCR after TME. Most of these series, recently published together as an international multicenter registry study,10 are heterogeneous in terms of tumor stages, radiation dosage, sensitizing chemotherapy, the criteria and timing for assessment of response, and surveillance follow-up protocols. As these series included only selected patients entered in the WW protocol without reporting the total number of patients with similar-stage rectal tumors treated with neoadjuvant therapy during the study period, the possibility of selection bias cannot be excluded. Without a reference denominator, the number of patients who would have potentially benefited from organ preservation using a WW strategy is unknown.10-12
The guidelines of the National Comprehensive Cancer Network for treatment of rectal cancer1 include total neoadjuvant therapy (TNT)—systemic chemotherapy before rather than after TME—which was developed in part as a strategy to increase the rate of tumor response.13,14 The impact of TNT on the potential for organ preservation through avoidance of surgery is unknown. Our study was therefore aimed at determining the proportion of patients with MRI-staged locally advanced rectal cancer who could benefit from an organ preservation strategy involving TNT, using predefined clinical and radiological criteria of tumor response and a standard follow-up protocol.
MATERIALS AND METHODS
Patients
Using data from prospectively maintained databases, we retrospectively identified all consecutive patients diagnosed with rectal adenocarcinoma and treated with TNT with induction from November 1, 2012, to April 10, 2019, at a tertiary cancer center by a single surgeon with an interest in WW. The study includes rectal adenocarcinoma located within 13 cm from the anal verge, staged with phased-array MRI. We excluded patients who started neoadjuvant therapy prior to being seen by the treating physician, patients with T4 tumors requiring pelvic exenteration or “en bloc” resections, patients requiring a stoma prior to starting neoadjuvant therapy, patients who received only systemic chemotherapy or CRT as neoadjuvant therapy, patients who received systemic chemotherapy after CRT (consolidation chemotherapy), patients included in the OPRA trial, and patients lost to follow-up. The data on demographics, tumor characteristics, treatment details, tumor response, and short-term oncological outcomes that were obtained from the databases were complemented by data from electronic medical records when needed. The study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center, and a waiver of informed consent was obtained.
In addition to a full colonoscopy, all patients had a digital rectal exam and flexible sigmoidoscopy with imaging documentation of the tumor at the initial visit. All patients had an MRI with a rectal cancer protocol and a CT scan of the chest, abdomen, and pelvis.
Treatment and Follow-up
The neoadjuvant chemotherapy regimen consisted of mFOLFOX6 (fluorouracil, leucovorin, oxaliplatin; 8 cycles) or CapeOx (capecitabine, oxaliplatin; 5 cycles) before CRT.13 The CRT regimen consisted of a planned dose of 5600 cGy in 28 fractions over a 5-6 weeks period and sensitizing fluorouracil or capecitabine for the duration of the radiotherapy.
Tumor response to the neoadjuvant therapy was assessed between 8 and 12 weeks after completion of TNT by digital rectal exam; flexible sigmoidoscopy; rectal MRI; and a CT of the chest, abdomen, and pelvis. Clinical response was classified as complete, near complete, or incomplete according to previously defined criteria15 developed by consensus under the leadership of the treating surgeon. Patients with incomplete response were recommended to undergo TME and patients with a cCR were offered WW regardless of age or comorbidities. Patients with near complete response were offered reassessment of response after an additional 6 to 8 weeks. If response improved over time, observation continued; otherwise, TME was recommended. The final treatment decision was reached by agreement between the patient and the surgeon after discussion about the oncologic and functional outcomes of TME, the lack of evidence supporting WW, and the unknown risk of potential tumor regrowth.
All patients entered in the WW protocol underwent a digital rectal exam (regardless of whether the tumor was reachable by the examiner’s finger at baseline) and flexible sigmoidoscopy every 4 months for 2 years and then every 6 months for 3 years. They underwent rectal MRI twice a year for 2 years and yearly thereafter.15 They also underwent CT of the chest, abdomen, and pelvis once a year and surveillance colonoscopy. Patients who underwent TME were followed according to the guidelines of the National Comprehensive Cancer Network.1
In patients with an initial clinical response, tumor regrowth was usually suspected if a digital rectal exam, endoscopy, or MRI identified changes suggesting tumor progression relative to the previous exam. Tumor regrowth was confirmed histologically in all patients. Patients with signs of tumor regrowth were recommended additional treatment; in most cases, the recommended treatment was TME. Treatment outcomes for patients with tumor regrowth were recorded. A diagnosis of local recurrence required histologic evidence of a tumor at the primary site after an R0 or R1 TME. Systemic recurrence was defined as any distant metastasis confirmed by imaging and/or biopsy.
Statistics
Continuous and categorical variables in the WW group were compared to those in the surgery group using the Wilcoxon rank-sum test or Pearson’s χ2 test, unless otherwise noted. For recurrence-free survival and overall survival, Kaplan-Meier estimates were calculated and comparisons were made using the log-rank test. P values < .05 were considered statistically significant.
RESULTS
A total of 88 consecutive patients met the selection criteria and started TNT (Figure 1) with induction chemotherapy between November 1, 2012, and April 10, 2019. One patient died during CRT from mesenteric ischemia. A total of 55 patients (62.5%) had an incomplete clinical response and were recommended surgery. One patient was treated with local excision. The remaining 54 patients underwent either low anterior resection (n = 45) or abdominoperineal resection (n = 9). None of the patients in the Surgery group died in the perioperative period. Most of the patients who underwent surgery had residual cancer in the bowel wall (4 ypT1, 17 ypT2, 26 ypT3, and 2 yT4) and/or mesorectal lymph nodes (19 ypN+), but 6 patients (11%) had a pCR (ypT0N0) (Table 1). None of the patients had a positive circumferential margin or a positive distal resection margin.
Figure 1.
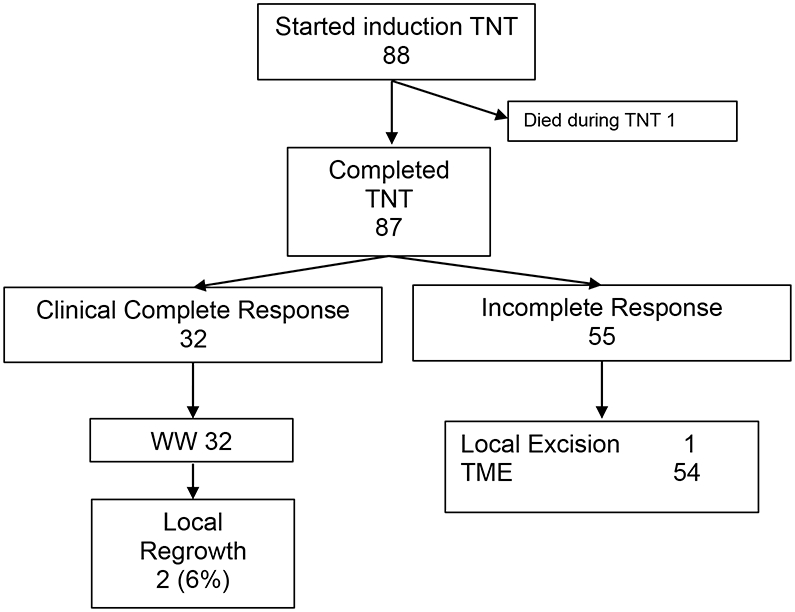
Patient groups.
Table 1.
Patient and Tumor Characteristics
| Characteristic | WW (n = 32) |
Surgery (n = 55) |
P |
|---|---|---|---|
| Median age in years (range) | 62 (38–84) | 55 (30–78) | .03 |
| No. (%) female | 16 (50) | 17 (31) | .08 |
| Tumor distance (cm) from anal verge, median (range) | 5 (0–13) | 6 (2–12) | .06 |
| Body mass index, median (range) | 24 (17–32) | 25 (16–50) | .20 |
| No. (%) of patients with clinical AJCCa stage: | |||
| II | 8 (25) | 11 (20) | .59 |
| III | 24 (75) | 44 (80) | |
| No. (%) of patients with clinical classification: | |||
| cT2 | 1 (3) | 2 (4) | .15 |
| cT3 | 31 (97) | 47 (85) | |
| cT4 | 0 (0) | 6 (11) | |
| Tumor length in mm, median (range)b | 38 (25–85) | 48 (20–103) | .003 |
| No. (%) of patients with ypT stage: | |||
| T0 | 6 (11) | ||
| T1 | 4 (7) | ||
| T2 | 17 (31) | ||
| T3 | 26 (47) | ||
| T4 | 2 (3) | ||
| No. (%) of patients with pathological stage: | |||
| 0 | 6 (11) | ||
| I | 16 (29) | ||
| II | 14 (25) | ||
| III | 18 (33) | ||
| IV | 1 (2) |
AJCC, American Joint Committee on Cancer.
Based on MRI.
A total of 32 (36.4%) patients had a cCR and were offered WW. Patient and tumor characteristics for the WW and Surgery groups are listed in Table 1. Patients in the WW group were older on average than patients in the Surgery group (mean, 62 and 55 years, respectively; P = .03). Clinical T and N classifications were assessed by MRI, and no difference was observed between the groups, but WW patients had smaller tumors compared to the TME group. Treatment variables for the two groups are listed in Table 2. The total dose of radiation and number of cycles of sensitizing chemotherapy were substantively similar in the two groups. The proportion of patients receiving neoadjuvant FOLFOX versus CapeOx and the number of cycles of systemic chemotherapy were also substantively similar in the surgery and WW groups. The groups did not differ in the proportion of patients who had adverse events during neoadjuvant therapy (25% vs 35%, p = 0.35).
Table 2.
Treatment Characteristics
| Characteristic | No. of Patients (%) | ||
|---|---|---|---|
| WW (n = 32) | Surgery (n = 55) | P | |
| Induction chemotherapy | |||
| FOLFOX | 32 (100) | 54 (98) | |
| CapeOx | 0 | 1 (2) | - |
| No. of cycles of FOLFOX, median (range) | 8 (6–12) | 8 (4–10) | 0.55 |
| Radiation dose in Gy, median (range) | 50 (39–56) | 50 (45–62.5) | 0.76 |
| Sensitizing chemotherapy | |||
| Capecitabine | 0 | 3 (5) | |
| 5-FU | 32 (100) | 52 (95) | - |
| Toxicity during TNT (any) | 8 (25) | 19 (35) | 0.35 |
| Postoperative complicationsa | 8 (14) | - | |
| Ileus | 3 (1.6) | ||
| Anemia requiring transfusion | 2 (1.1) | ||
| Urinary infection | 1 (0.5) | ||
| Urinary retention | 1 (0.5) | ||
| SSI | 1 (0.5) | ||
Clavien-Dindo grade II.
Clinical and oncological outcomes are listed in Table 3. Median follow-up from completion of TNT was similar for the two groups: 42 months in the WW group and 45 months in the surgery group. Two (3.6%) patients in the Surgery group developed local recurrence alone, 9 (16.3%) had distant metastasis alone, and 1 (1.8%) had both local and systemic recurrence (Table 3). With median follow-up of 45 (3–70.5) months from the end of TNT, 40 patients in the Surgery group remained alive and free of disease. Of the 32 patients in the WW group, 2 (6%) patients developed tumor regrowth. One of the two patients had local regrowth alone; this patient underwent local excision of a villous adenoma 20 months after completion of TNT and was alive without evidence of disease. The other patient with local regrowth had a salvage abdominoperineal resection 17 months after TNT. He developed liver and lung metastases 42 months after completion of TNT and eventually died of the disease.
Table 3.
Clinical Outcomes
| Outcome | No. of Patients (%) | |
|---|---|---|
| WW (n = 32) | Surgery (n = 55) | |
| Local regrowth | ||
| Total | 2 (6) | |
| With systemic recurrence | 1 (3) | |
| Local recurrence | ||
| Total | 0 | 3 (5) |
| With systemic recurrence | 1 (2)a | |
| Systemic recurrence (total) | 1 (3) | 10 (18) |
| Death | 1 (3) | 8 (14) |
| Months of follow-up, median (range) | 42 (5.4–69.1) | 45 (3–70.5) |
Included in the 10 patients with systemic recurrence below.
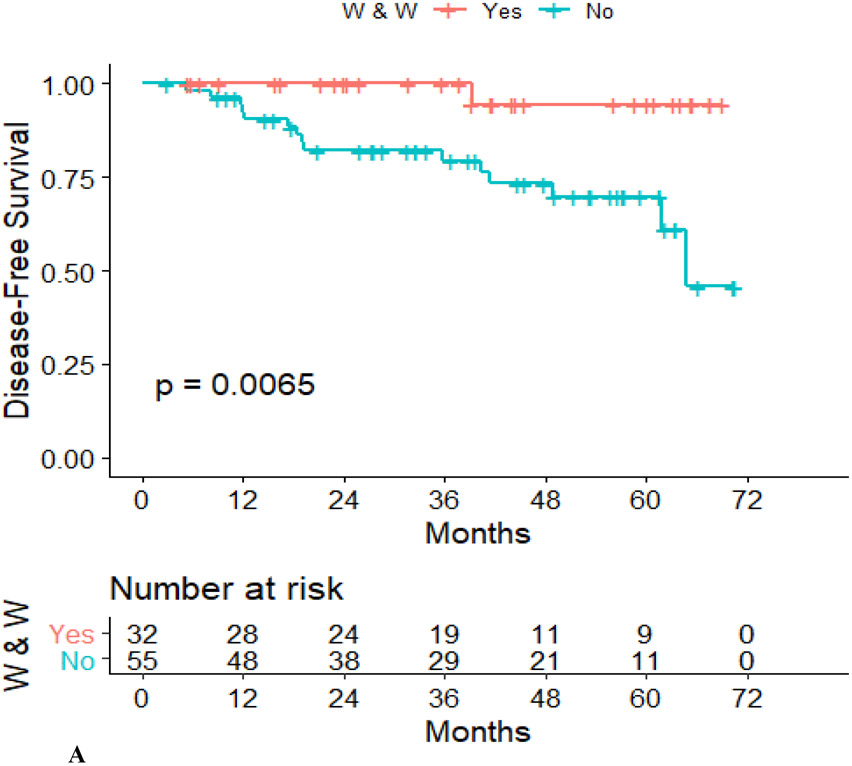
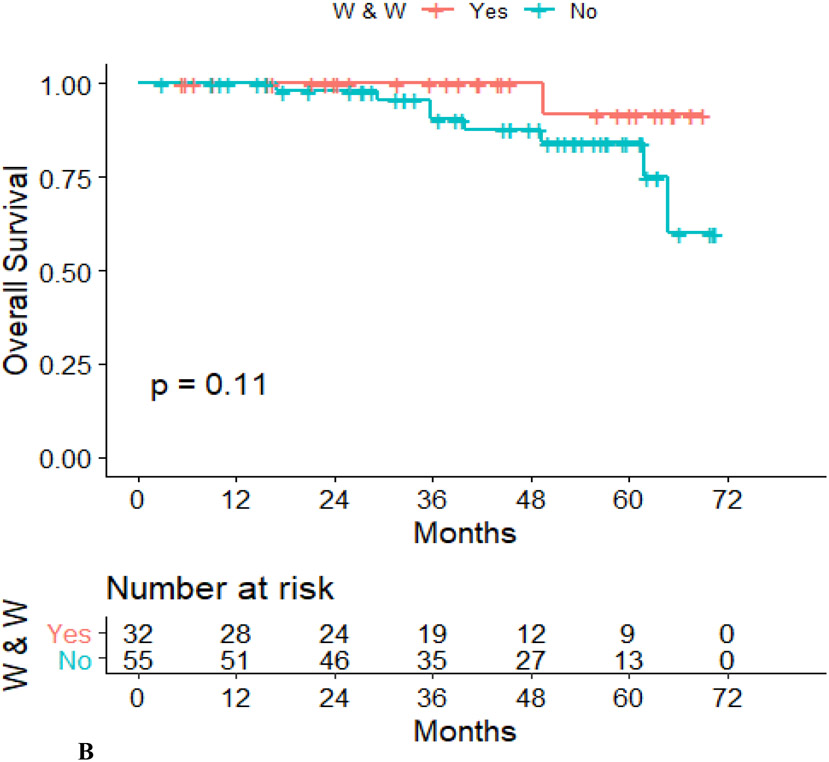
Disease-free survival was lower in the Surgery group than in the WW group (hazard ratio, 0.101; 95% confidence interval, 0.13–0.768; p = .007) (Figure 2A), although overall survival did not differ between the two groups (hazard ratio, 0.212; 95% confidence interval, 0.26–1.71; p = .11) (Figure 2B).
Figure 2.
Recurrence-free survival (A) and overall survival (B) in the WW group and the TME group.
DISCUSSION
This study shows that more than one-third of patients with locally advanced rectal cancer treated with TNT using induction chemotherapy can achieve rectal preservation by undergoing a WW management strategy based on predefined response criteria. It also shows a low rate of tumor regrowth and distant metastasis when compared to our TME group.
The current literature12 on WW consists of retrospective institutional cohort series of selected patients with a cCR after neoadjuvant therapy, but without information on the entire pool of patients treated during the study period. Without that information, the proportion of patients that can potentially benefit from WW cannot be determined. Estimates are currently based on the expected pCR rate for a given patient population treated with CRT. In our study, more than 43% of patients with stage II and III rectal cancer had a cCR or pCR after TNT, which is consistent with the pCR rates reported for TNT.13,14 Only one study16 has reported WW outcomes for patients treated with an extended neoadjuvant protocol. In that study, patients received 54 Gy of radiation concurrently with three cycles of infusional fluorouracil-leucovorin, followed by three additional cycles of fluorouracil-leucovorin after radiation for a total of 9 weeks. Response was assessed after all treatment had been delivered and within 10 weeks of completion of radiotherapy. The authors reported a cCR rate of 68%, a tumor regrowth rate of 25%, and an organ preservation rate of 51% (median follow-up, 26 months), which is considerably higher than the rate of organ preservation in our study. However, 24% of patients in that study had tumors of clinical stage I, which have a greater response rate compared to stage II or stage III patients.17
The 2-year cumulative incidence of regrowth of 6% in our study is significantly lower than the 25% rate reported in the International Watch & Wait database, a pooled analysis of retrospective case series (www.iwwd.org).10 This difference can probably be attributed to our use of predefined clinical and radiological criteria for tumor response. However, even with predefined response criteria, the identification of complete responders is challenging. Two (6%) of the 32 patients offered WW experienced regrowth, and 6 (10%) of the 55 patients who underwent surgery were found to have a pCR, reflecting the persistent challenge of identifying all sustained responders as well as incomplete responders using current criteria. Thus, further work is needed to redefine current or new endoscopic and radiologic features that can help to improve our ability to identify true responders versus nonresponders. This will facilitate reductions in the risk of tumor regrowth in patients entered in WW protocols and in the proportion of patients who undergo unnecessary surgery.
An important finding of our study is the difference in average age between patients initially recommended WW and patients initially recommended surgery. This difference most likely represents treatment selection bias favoring organ preservation in older, more frail patients and more aggressive treatment in pursuit of better oncologic outcomes in younger patients. In addition, tumors in the WW group were closer to the anal verge, suggesting a possible bias for organ preservation in patients with more distal tumors, which are less likely to be treated with a sphincter-saving procedure. The difference between groups in mean tumor distance from the anal verge was 1.2 cm, which while not statistically significant, may be clinically relevant. The possibility of observer bias in the interpretation of the digital rectal exam finding, the endoscopic or MRI images when assessing tumor response cannot be excluded. Finally, we cannot exclude the possibility that this difference reflects differences in the rate of response between younger and older patients.
The strengths of the study include the following factors minimizing potential confounding. First, the study included all consecutive patients with stage II or III rectal adenocarcinoma evaluated at baseline, staged by MRI, and treated by a single surgeon with extensive experience in WW management. Second, all patients received TNT with induction therapy, and response was assessed using standardized criteria at least 8 weeks after completion of TNT. Third, the follow-up protocol was also standardized.
The study’s potential weaknesses include limited generalizability, given that all the patients in the study were treated by the same surgeon. Because of the lack of randomization, the relative benefits of induction chemotherapy with respect to organ preservation could not be fully ascertained. In addition, the possibility remains that, since the number of patients was relatively small and follow-up was relatively short, the proportion of patients benefiting from organ preservation may be overestimated. Finally, this series includes only patients treated with induction chemotherapy followed by chemoradiation which has been the preferred treatment approach for patients with locally advanced rectal cancer at our institution for years.13 Therefore, these findings cannot be extrapolated to patients treated with CRT followed by consolidation chemotherapy.
Ideal proof of the safety of a WW strategy would come from a prospective study in which patients with cCR would be randomized to WW or TME. Unfortunately, it is unlikely that patients with a cCR will accept randomization to such different treatment plans. Alternative study designs such as initial patient assignment based on individual preference to one of two treatment strategies—one offering WW to patients with cCR and another mandating TME for all patients independent of response—is now under consideration by a national cooperative. Independently of the type of design, the sample size calculation of any prospective trial will require accurate knowledge of the rate of response after neoadjuvant therapy. This study indicates that close to 40% of patients with stage II and III rectal cancer treated with TNT could potentially undergo organ preservation. This information can be used to design future trials.
CONCLUSION
In conclusion, our study suggests that when induction-type TNT is used to maximize response and defined criteria are used in a rigorous manner to assess response, 30–40% of patients with stage II or III rectal cancer can undergo organ-preserving treatment. This information will be important for counseling patients interested in WW strategies and for the design of multi-institutional prospective studies.
Funding/Support:
NCI grant P30 CA008748.
Footnotes
Financial Disclaimers: Dr. Garcia-Aguilar holds equity in Intuitive Surgical and has received honoraria from Medtronic and Johnson & Johnson. Dr. Smith has served as an advisor for Guardant Health.
REFERENCES
- 1.National Comprehensive Cancer Network. NCCN guidelines: rectal cancer. Available from: www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed January 11, 2019.
- 2.Bregendahl S, Emmertsen KJ, Lous J, Laurberg S. Bowel dysfunction after low anterior resection with and without neoadjuvant therapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis. 2013;15:1130–1139. [DOI] [PubMed] [Google Scholar]
- 3.Emmertsen KJ, Laurberg S; Rectal Cancer Function Study Group. Impact of bowel dysfunction on quality of life after sphincter-preserving resection for rectal cancer. Br J Surg. 2013;100:1377–1387. [DOI] [PubMed] [Google Scholar]
- 4.Peeters KCMJ, van de Velde CJH, Leer JWH, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients--a Dutch colorectal cancer group study. J Clin Oncol. 2005;23:6199–6206. [DOI] [PubMed] [Google Scholar]
- 5.Celerier B, Denost Q, Van Geluwe B, Pontallier A, Rullier E. The risk of definitive stoma formation at 10 years after low and ultralow anterior resection for rectal cancer. Colorectal Dis. 2016;18:59–66. [DOI] [PubMed] [Google Scholar]
- 6.Ridolfi TJ, Berger N, Ludwig KA. Low anterior resection syndrome: current management and future directions. Clin Colon Rectal Surg. 2016;29:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey CE, Hu C-Y, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30:1770–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith JJ, Strombom P, Chow OS, et al. Assessment of a watch-and-wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy. JAMA Oncol. 2019;5:e185896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Valk MJM, Hilling DE, Bastiaannet E, et al. ; IWWD Consortium. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391:2537–2545. [DOI] [PubMed] [Google Scholar]
- 11.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:501–513. [DOI] [PubMed] [Google Scholar]
- 13.Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018;4:e180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Aguilar J, Chow OS, Smith DD, et al. ; Timing of Rectal Cancer Response to Chemoradiation Consortium. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16:957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith JJ, Chow OS, Gollub MJ, et al. ; Rectal Cancer Consortium. Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habr-Gama A, Sabbaga J, Gama-Rodrigues J, et al. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum. 2013;56:1109–1117. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Aguilar J, Shi Q, Thomas CR Jr, et al. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol. 2012;19:384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]