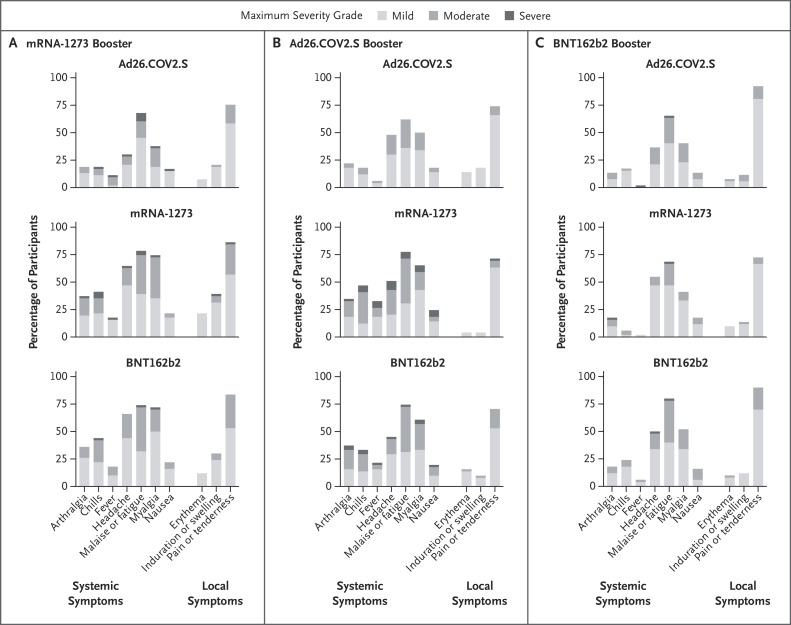
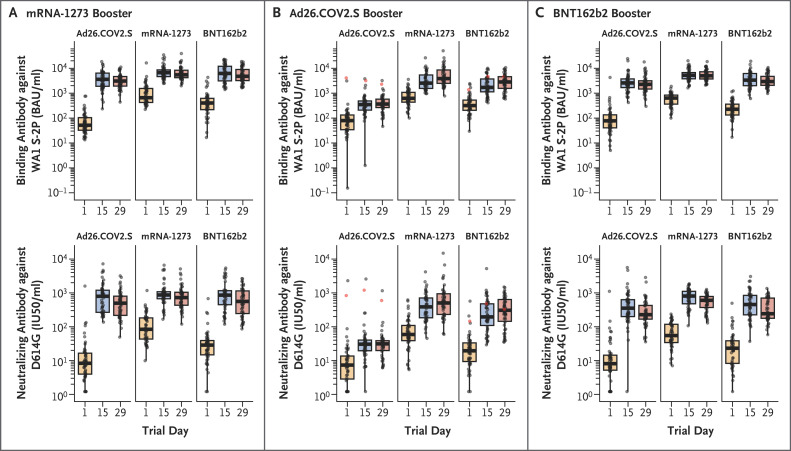
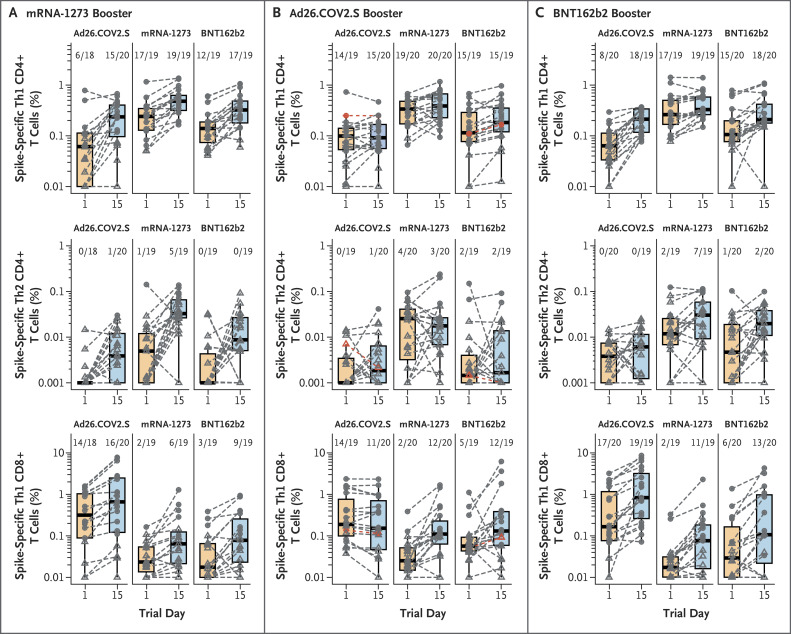
Robert L Atmar
Robert L Atmar, M.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,✉,
Kirsten E Lyke
Kirsten E Lyke, M.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Meagan E Deming
Meagan E Deming, M.D., Ph.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Lisa A Jackson
Lisa A Jackson, M.D., M.P.H.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Angela R Branche
Angela R Branche, M.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Hana M El Sahly
Hana M El Sahly, M.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Christina A Rostad
Christina A Rostad, M.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Judith M Martin
Judith M Martin, M.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Christine Johnston
Christine Johnston, M.D., M.P.H.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Richard E Rupp
Richard E Rupp, M.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Mark J Mulligan
Mark J Mulligan, M.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Rebecca C Brady
Rebecca C Brady, M.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Robert W Frenck Jr
Robert W Frenck Jr, M.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Martín Bäcker
Martín Bäcker, M.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Angelica C Kottkamp
Angelica C Kottkamp, M.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Tara M Babu
Tara M Babu, M.D., M.S.C.I.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Kumaravel Rajakumar
Kumaravel Rajakumar, M.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Srilatha Edupuganti
Srilatha Edupuganti, M.D., M.P.H.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
David Dobrzynski
David Dobrzynski, M.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Rhea N Coler
Rhea N Coler, Ph.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Christine M Posavad
Christine M Posavad, Ph.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Janet I Archer
Janet I Archer, M.Sc.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Sonja Crandon
Sonja Crandon, B.S.N.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Seema U Nayak
Seema U Nayak, M.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Daniel Szydlo
Daniel Szydlo, M.S.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Jillian A Zemanek
Jillian A Zemanek, M.P.H.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Clara P Dominguez Islas
Clara P Dominguez Islas, Ph.D
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Elizabeth R Brown
Elizabeth R Brown, Sc.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Mehul S Suthar
Mehul S Suthar, Ph.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
M Juliana McElrath
M Juliana McElrath, M.D., Ph.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Adrian B McDermott
Adrian B McDermott, Ph.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Sarah E O’Connell
Sarah E O’Connell, M.S.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
David C Montefiori
David C Montefiori, Ph.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Amanda Eaton
Amanda Eaton, M.B.A.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Kathleen M Neuzil
Kathleen M Neuzil, M.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
David S Stephens
David S Stephens, M.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
Paul C Roberts
Paul C Roberts, Ph.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
John H Beigel
John H Beigel, M.D.
1From the Departments of Medicine and Molecular Virology and Microbiology, Baylor College of Medicine, Houston (R.L.A., H.M.E.S.), and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston (R.E.R.); the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore (K.E.L., M.E.D., K.M.N.), and the Division of Microbiology and Infectious Diseases (S.C., S.U.N., P.C.R., J.H.B.) and the Vaccine Research Center (A.B.M., S.E.O.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda; Kaiser Permanente Washington Health Research Institute (L.A.J.), the Departments of Medicine (C.J., T.M.B., M.J. McElrath) and Laboratory Medicine and Pathology (C.J., C.M.P.), University of Washington, the Vaccine and Infectious Disease Division (C.J., C.M.P., C.P.D.I., E.R.B., M.J. McElrath) and the Statistical Center for HIV/AIDS Research and Prevention (D.S., J.A.Z.), Fred Hutchinson Cancer Research Center, and Seattle Children’s Research Institute (R.N.C.) and the Department of Pediatrics (R.N.C.), University of Washington School of Medicine, Seattle; the Department of Medicine, Division of Infectious Diseases, University of Rochester, Rochester (A.R.B., D.D.), NYU Langone Vaccine Center and Division of Infectious Diseases and Immunology, Department of Medicine, NYU Grossman School of Medicine, New York (M.J. Mulligan, A.C.K.), and NYU Langone Hospital–Long Island Vaccine Center Research Clinic and the Division of Infectious Disease, Department of Medicine, NYU Long Island School of Medicine, Mineola (M.B.) — all in New York; the Departments of Pediatrics (C.A.R.), Microbiology and Immunology (M.S.S.), and Medicine (S.E., D.S.S.), the Center for Childhood Infections and Vaccines (C.A.R.), Hope Clinic of Emory Vaccine Center (S.E.), Emory Vaccine Center, and Yerkes National Primate Research Center (M.S.S.), Emory University School of Medicine, Emory University, and Children’s Healthcare of Atlanta (C.A.R.) — all in Atlanta; the Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh (J.M.M., K.R.); Cincinnati Children’s Hospital Medical Center, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati (R.C.B., R.W.F.); and FHI 360 (formerly Family Health International) (J.I.A.) and Duke Human Vaccine Institute (D.C.M.) and the Department of Surgery (D.C.M., A.E.), Duke University Medical Center, Durham, NC.
1,
the DMID 21-0012 Study Group