Abstract
Introduction:
Potent lifestyle interventions to increase moderate-to-vigorous physical activity (MVPA) are urgently needed for population-level chronic disease prevention. This trial tested the independent and joint effects of a mobile health system automating adaptive goal setting and immediate financial reinforcement for increasing daily walking among insufficiently active adults.
Study design:
Participants were randomized into a 2 (adaptive versus static goal setting) × 2 (immediate versus delayed financial incentive timing) condition factorial trial to increase walking.
Intervention:
Principles of reinforcement and behavioral economics directed intervention design.
Main outcome measures:
Participants wore accelerometers daily that remotely measured MVPA bout-minutes of ≥3 minutes/day for 1 year. Primary outcomes were between-condition differences in: (1) engaging ≥1 bout of MVPA on each day and (2) on days with ≥1 bout, daily total MVPA minutes.
Results:
Participants (N=512 adults, 133,876 day-level observations) were recruited between 2016 and 2018 and were 64.5% female, aged 18–60 years, 18.8% Hispanic, 6.1% African American, and 83% White. Mixed effects hurdle models tested treatment group × phase (time) interactions using an intent-to-treat approach in 2021. Engaging in any ambulatory MVPA was greater for Adaptive versus Static Goal groups (OR=2.34, 95% CI=2.10, 2.60 vs OR=1.66, 95% CI=1.50, 1.84; p<0.001) and for Immediate versus Static Reinforcement groups (OR=2.16 95% CI=1.94, 2.40 vs OR=1.77, 95% CI=1.59, 1.97; p<0.01). The Immediate Reinforcement group increased by 16.54 MVPA minutes/day whereas the Delayed Reinforcement group increased by 9.91 minutes/day (p<0.001). The combined Adaptive Goals + Immediate Reinforcement group increased by 16.52 MVPA minutes/day, significantly more than either Delayed Reinforcement group.
Conclusions:
This study offers automated and scalable behavior change strategies for increasing walking among adults most at risk for chronic diseases attributed to sedentary lifestyles.
Trial registration:
This study is registered at www.clinicaltrials.gov NCT02717663.
INTRODUCTION
Physical activity (PA), and especially moderate-to-vigorous PA (MVPA), are potent chronic disease risk–lowering behaviors.1 Despite technological leaps in wearable activity monitors and growing awareness of numerous benefits of MVPA, the proportion of the U.S. population meeting PA guidelines has not improved substantially over the last decade.2,3 A meta-analysis of individual-level PA interventions demonstrated an intervention effect of only 2.1 minutes/day,4 an improvement unlikely to have a meaningful population impact on meeting guidelines. Disease risk reduction demands potent and scalable interventions to increase MVPA.
Adaptive goal setting is a relatively new approach, where PA goals increase, decrease, or stay the same during a PA intervention in response to day-to-day experience (e.g., illness/injury, schedule changes, travel, motivation).5–8 Typically, scaled goal-setting approaches use either static (e.g., 150 minutes/week of PA) or linearly increasing goals (e.g., increase PA by 50 minutes/week each week) to encourage PA.9,10 These goals typically do not adjust and are insensitive to person-specific change, which is rarely linear. Lack of adaptation may result in lack of attainment. Mobile health (mHealth) technologies enable automated adaptive systems to use frequent assessments of PA (and potentially other inputs) to adjust goals. Short-term studies with daily adaptive goal setting found greater improvements in steps/day over 4–6 months.6,11–14 Riley et al.15,16 posit that intensively adapting interventions delivered in response to dynamic inputs should be more effective than non-adapting interventions, but empirical support has yet to confirm this outcome. Previous adaptive goal-setting studies also targeted steps/day as a measure of PA volume, but are limited by omitting the assessment of PA intensity, which has a stronger relationship to fitness.6,11,13,14,17
Meta-analyses indicate that modest financial incentives with various types, delays, and probabilities were more effective than no-incentive controls at increasing and maintaining PA levels,18 even after incentives were removed.19 mHealth technologies (e.g., texting) provide the capability of reinforcing PA goal attainment with precisely delivered micro-incentives (e.g., $1/goal) in near real time to free-living individuals. Positive reinforcement is most effective when delivered immediately.20–23 None of the interventions included in extant meta-analyses delivered reinforcement in near real time.
This randomized 2 × 2 factorial trial tested for main effects and interactions among goal setting (adaptive versus static) and financial reinforcement (immediate versus delayed non-contingent) on changes in adults’ accelerometer-measured MVPA over 12 months. The study hypothesized independent main effects of goal setting and reinforcement timing for increasing MVPA, such that adaptive goals would outperform static goals and immediate reinforcement would outperform delayed reinforcement for increasing MVPA. Also hypothesized was an interaction between components such that the adaptive goals plus immediate reinforcement would show greater change in MVPA than the other 3 combinations.
METHODS
Study Population
Methodological details of the WalkIT Arizona trial have been published.24 Briefly, 512 inactive adults from Maricopa County, Arizona were enrolled into a 2-year study between 2016 and 2018 with 1 year of intervention and a second year of follow-up. Four neighborhood types were targeted for recruitment: “higher walkable/higher SES,” “higher walkable/lower SES,” “lower walkable/higher SES,” and “lower walkable/lower SES.” As described24 and following Frank and colleagues,25 to arrive at neighborhood classifications, prior to the start of recruitment Maricopa County block groups were ranked and categorized into “lower walkable” (1st–4th deciles) and “higher walkable” (7th–10th deciles) based on GIS-measured walkability components, with the 5th and 6th deciles omitted to minimize the possibility of misclassifying participants near the classification boundaries. Similarly, block groups were ranked from low to high using median household income from the Census’ American Community Survey and then categorized as “lower SES” (1st–5th deciles) or “higher SES” (7th–10th deciles). The 6th decile was excluded to minimize miscategorization. Participants were stratified by neighborhood type and block randomized (block size of 4) by computer into 1 of 4 interventions in the 2 × 2 factorial design: (1) Adaptive Goals + Immediate Reinforcement, (2) Adaptive Goals + Delayed Reinforcement, (3) Static Goals + Immediate Reinforcement, or (4) Static Goals + Delayed Reinforcement. Thus, the treatment factorial design was embedded within an observational study of neighborhood type to efficiently examine multiple research questions related to neighborhood factors on PA maintenance. To control for extreme summer temperatures in the region (i.e., >100 days with temperatures of ≥38° Celsius from June through September), a similar number of participants were randomized across each calendar month. Participants and investigators were blinded during the baseline phase, but not blinded during intervention in this “open label” trial. Participants provided written informed consent and were compensated for completing baseline and 12-month questionnaire measures ($20 and $40, respectively). The protocol was approved by Arizona State University’s IRB and prospectively registered with www.clinicaltrials.gov (NCT02717663).
Participants (aged 18–60 years) were insufficiently active adult men and women who lived in one of the eligible neighborhood quadrants in Maricopa County, Arizona. Facebook marketing ads targeted adults geographically and demographically from eligible block groups. The International Physical Activity Questionnaire short form was used to screen potential participants and activity status was further confirmed by baseline accelerometer measures (i.e.,<150 minutes/week). Adults were excluded with a history of heart failure, type 2 diabetes, myocardial infarction, contraindications to exercise testing, currently or planning to become pregnant in the next 2 years, or currently participating in PA, diet, or weight loss programs. The study required daily access to a mobile phone with text messaging capabilities or iOS or Android smartphone, and willingness to send and receive up to 3 text messages/day and to wear a small accelerometer daily for 1 year. Adults planning trips outside of region for >30 days consecutively or planning to move from their home in the next 2 years were also excluded. Inclusion of participants was not based on race or gender.
Interventions
Participants were informed they would receive a PA intervention that included one-time education materials, daily MVPA goals, feedback on performance, and financial incentives throughout the 12-month intervention phase.
Participants received goals via text messages when they synced their accelerometers with the WalkIT server. Static Goal participants were prescribed 30 minutes of MVPA on ≥5 days/week (e.g., “Goal for 1/15/19 is 30 min.”), aligning with current federal PA guidelines.26,27 Adaptive goal participants goals could increase, decrease, or stay the same. Based on a 9-valid-observation moving window capturing each participant’s recent performance, goals were calculated using a 60th percentile-rank algorithm tested in preliminary studies with overweight adults.6,11,12 If a participant’s MVPA duration on each of the previous 9 observations (ranked from lowest to highest) was 5, 5, 6, 9, 11, 12, 15, 15, and 17 minutes/day, the 60th rank-percentile would yield a goal of 12 minutes/day for the current day (e.g., “Goal for 1/15 is 12 min.”). Adaptive goals ranged from a low of 3 to a high of 60 minutes/day. Static and adaptive goals could be accomplished by accumulating multiple bouts of 3 minutes or longer over a day.
All participants who meet daily goals received positive text messages drawn from a pool of messages (e.g., “Well done, Pat! Goal met! 63 min. Goal for 1/16 is 18 min”). When a daily goal was not met, a simple confirmation of the device sync was sent, avoiding discouraging messages (e.g., “Sync Received. 3 min. Goal for 1/16 is 18 min”).
Participants in both the Immediate and Delayed Reinforcement groups were informed that they could earn $265 during the intervention phase at the onset of the study. Upon syncing their accelerometers, Immediate Reinforcement participants also received notice of points earned for meeting a goal and a running point balance (e.g., “Well done, Pat! Goal met! 63 min. Reward points=100! Balance=300 points. Goal for 1/16 is 18 min”). Participants assigned to receive Immediate Reinforcement (irrespective of goal setting condition) earned points (100 points=$1.00 USD) daily for meeting MVPA goals during the 1-year intervention phase, comprising a sequence of 6 financial reinforcement stages informed by research on reinforcement schedules (details in Adams et al.24). Based on previous research with goals,6,11 most participants would achieve as few as 40% and much as 73% of their static or adaptive goals on average (40%–73% × 365 possible days = 146–265 goals met in total) over 1 year, which was about $146–$265 in incentives. To plan for the possibility that a participant could achieve 100% of their goals over the year, the maximum amount (regardless of goal group) an Immediate Reinforcement participant could earn was $365 over the intervention phase (only 1 did). To encourage engagement, Delayed Reinforcement participants earned up to $265 in financial incentives paid every 60 days on an escalating schedule (i.e., $15 in Month 2, $30 in Month 4, $50 in Month 6, $75 in Month 8, and $95 in Month 10) for wearing and syncing the accelerometer, approximately matching the planned amount of incentives earned by participants in the Immediate Reinforcement condition. Once participants met their reward thresholds—either accumulating 500 points (for $5) in the Immediate Reinforcement group, or syncing at the end of a 60-day interval in the Delayed Reinforcement group—an e-gift card for the participant’s choice from a list of 12 popular retailers was e-mailed immediately using WalkIT’s automated mHealth system. The minimum e-gift card denomination is $5 at most retailers.
Measures
Participants were instructed to wear an accelerometer for at least 10 hours daily for a year. The wrist-worn ActiGraph GT9X Link is a small, water-resistant device, with long battery life and calibration, reliability, and validity data for adults.28–31 Vector magnitude (VM) counts of movements in vertical, antero-posterior, and medio-lateral planes were calculated for 1-minute epochs yielding up to 1,440 VM count values/day. Following the individual calibration method of Barnett et al.,32 all participants in the current study completed a baseline protocol for developing participant-specific accelerometer VM cut points for moderate-intensity or greater walking using indirect calorimetry. Non-wear was defined by the Choi algorithm for adults as ≥90 consecutive minutes with zero VM counts, with an allowance of ≤2 minutes of non-zeros on the vertical axis.33,34 Valid wear days were days when either a PA goal was met or with ≥6 hours of wear. These criteria determined wear adherence and allowed acknowledgement of a participant’s efforts to earn rewards even if the device was worn for a workout only. Participants synced the device through the ActiGraph smartphone app.
Minutes of MVPA were further scored to estimate bout minutes during the intervention in real time.35 The onset of a MVPA bout occurred when 3 non-contiguous minutes of 5 minutes met the criteria. The offset of a bout occurred when 3 consecutive minutes fell below the MVPA threshold. This approach allowed for detection of a minimum MVPA bout of 3 minutes.
At baseline participants reported their gender, age, race/ethnicity, marital status, smoking status, children in household, and educational attainment. BMI was calculated from height and weight measured by stadiometer and digital scale, respectively.
At Month 12, participants were asked: How likely are you to recommend the WalkIT Arizona program to your friends/family? Options ranged from very unlikely (1) to highly likely (10).
Statistical Analysis
To estimate the required sample size, investigators assumed intervention effects (i.e., adaptive versus static goals and immediate versus delayed reinforcement) on baseline versus intervention phase change (i.e., average intervention phase value versus average baseline phase value) in MVPA of 2.1 minutes/day (derived from Conn and colleagues4) and an α of 0.05. In simulations conducted using SAS PROC IML and PROC MIXED36 in SAS, version 9.4, the estimated required complete-case sample size to achieve power ≥0.80 was N=320 participants. This represented a conservative estimate—owing to computational limitations, simulations were based on only 100 repeated observations of MVPA (10 baseline + 90 intervention) per participant, as opposed the 375 observations (10 baseline + 365 intervention) expected by 12 months post-randomization in this study. Assuming a block group–level intraclass correlation for MVPA minutes/day of 0.01, 5 participants per block group, and a projected 30% rate of participant loss, the estimated required baseline sample size was N=471 participants. Because the goal was to have balanced cell sizes across sampling and randomization stages, the target baseline sample size was N=480. This sample size also afforded 0.80 power to detect interaction effects corresponding to a 4.2-minutes/day “difference in differences” in MVPA—for example, a 4.2-minutes/day difference in changes between the Adaptive Goals + Immediate Reinforcement group versus other groups.
Because preliminary analyses revealed that the distribution of daily MVPA values was characterized by a relatively high proportion of zero-minute values and a discontinuity between zero and the lower non-zero value (3 bout-minutes/day), intervention effects on MVPA were tested using intent-to-treat with mixed effects hurdle models (using the GLMMadaptive package in R, version 4.0.2), which simultaneously estimated: (1) the probability of engaging in at least 1 bout of MVPA/day (versus none), via a random-intercept logit model component; and (2) the number of MVPA bout minutes/day, via a random-intercept negative binomial model component. Models drew on all (133,876) daily observations from all (N=512) randomized participants. The authors first examined fixed effect terms for Component (Goal Setting or Reinforcement Timing) × Phase (baseline versus intervention) interactions (Model 1), which captured group differences in the change in the average daily probability of engaging in any MVPA (or average daily number of MVPA minutes), from across the entire 10-day baseline phase, to the average probability of MVPA (or number of MVPA minutes) from across the entirety of the intervention phase. Next (Model 2), interaction terms were introduced to capture group differences in the linear rate of change and differences in the quadratic rate of change during the intervention phase. Missingness in the outcome variables was addressed using maximum likelihood estimation for all models. Significant interactions were probed to obtain level-specific effects (i.e., simple slopes) and SEs.37
Next, the 4 intervention groups were compared on both MVPA outcomes using a parallel modeling approach, with the focal effects instead being interactions of (Model 1) and Linear Time, and Quadratic Time (Model 2) with each of 3 indicator (dummy) vectors coding for group membership, with the Adaptive Goals + Immediate Reinforcement group serving as the reference category. Daily accelerometer wear time and calendar month (dummy coded) were included as covariates in all models. Appendix Tables 1 and 2 provide regression models by group. Appendix Tables 3 and 4 display model-estimated condition- and group-specific predictions of likelihood of any MVPA and mean MVPA minutes, and associated model-estimated CIs, at baseline and at 1, 60, 120, 180, 240, 300, and 360 days post-randomization. Appendix Tables 5 and 6 offer results from zero-inflated negative binomial models, which showed trivial differences compared to hurdle models.
RESULTS
Table 1 displays demographic aspects of the study sample. Figure 1 shows the CONSORT diagram and participant flow. Participants wore the accelerometer on 69.7% (133,876 of 192,000) of planned daily observations and wear time averaged 15.2 and 15.9 hours/day during baseline and intervention phases.
Table 1.
Participant Characteristics by Intervention Condition in 2 × 2 Factorial Randomized Design
| Characteristic | Total (N=512) |
Adaptive goals + immediate reinforcement (n=128) |
Static goals + immediate reinforcement (n=128) |
Adaptive goals + delayed reinforcement (n=128) |
Static goals + delayed reinforcement (n=128) |
|---|---|---|---|---|---|
| Female, n (%) | 330 (64.5) | 82 (64.1) | 80 (62.5) | 81 (63.3) | 87 (68.0) |
| Age, mean (SD) | 45.5 (9.1) | 45.6 (9.5) | 46.0 (8.9) | 46.7 (8.6) | 43.5 (9.3) |
| Racea | |||||
| Caucasian or White, n (%) | 425 (83.0) | 108 (84.3) | 106 (82.8) | 105 (82.0) | 106 (82.8) |
| African American or Black, n (%) | 31 (6.1) | 5 (3.9) | 9 (7.0) | 9 (7.0) | 8 (6.3) |
| American Indian or Alaskan Native, n (%) | 14 (2.7) | 4 (3.1) | 3 (2.3) | 2 (1.6) | 5 (3.9) |
| Asian, n (%) | 12 (2.3) | 4 (3.1) | 3 (2.3) | 3 (2.3) | 2 (1.6) |
| Native Hawaiian or other Pacific Islander, n (%) | 7 (1.4) | 3 (2.3) | 1 (0.8) | 2 (1.6) | 1 (0.8) |
| Prefer not to answer, n (%) | 32 (6.3) | 5 (3.9) | 8 (6.3) | 10 (7.8) | 9 (7.0) |
| Ethnicitya | |||||
| Hispanic or Latino, n (%) | 96 (18.8) | 22 (17.2) | 26 (20.3) | 24 (18.8) | 24 (18.8) |
| Married or living with partner, n (%) | 346 (67.6) | 82 (64.0) | 85 (66.4) | 93 (72.7) | 86 (67.2) |
| BMI, mean (SD) | 33.9 (7.3) | 33.7 (7.3) | 33.8 (7.3) | 33.6 (7.0) | 34.5 (7.5) |
| Current tobacco or e-smoker, n (%) | 36 (7.0) | 5 (3.9) | 13 (10.2) | 6 (4.6) | 12 (9.4) |
| Number of children in household, mean (SD) | 0.97 (1.2) | 0.97 (1.3) | 0.98 (1.3) | 1.05 (1.3) | 0.88 (1.1) |
| Household income, median | $60,000–$79,999 | $60,000–$79,999 | $60,000–$79,999 | $60,000–$79,999 | $60,000–$79,999 |
| Education, median | College graduate | College graduate | College graduate | College graduate | College graduate |
| Neighborhood type | |||||
| High walkable/High income, n (%) | 136 (26.6) | 34 (25.0) | 34 (25.0) | 34 (25.0) | 34 (25.0) |
| High walkable/Low income, n (%) | 132 (25.8) | 33 (25.0) | 33 (25.0) | 33 (25.0) | 33 (25.0) |
| Low walkable/High income, n (%) | 136 (26.6) | 34 (25.0) | 34 (25.0) | 34 (25.0) | 34 (25.0) |
| Low walkable/Low income, n (%) | 108 (21.1) | 27 (25.0) | 27 (25.0) | 27 (25.0) | 27 (25.0) |
| Mean accelerometer wear time (hours/day) | |||||
| Baseline phase, mean (SD) | 15.9 (4.2) | 16.1 (4.2) | 16.0 (4.3) | 15.6 (4.0) | 15.9 (4.3) |
| Intervention phase, mean (SD) | 15.2 (4.9) | 15.1 (5.0) | 15.3 (5.2) | 15.3 (4.7) | 15.1 (4.9) |
Race/Ethnicity cumulative is >100%. Participants were allowed to select all that apply.
Figure 1.
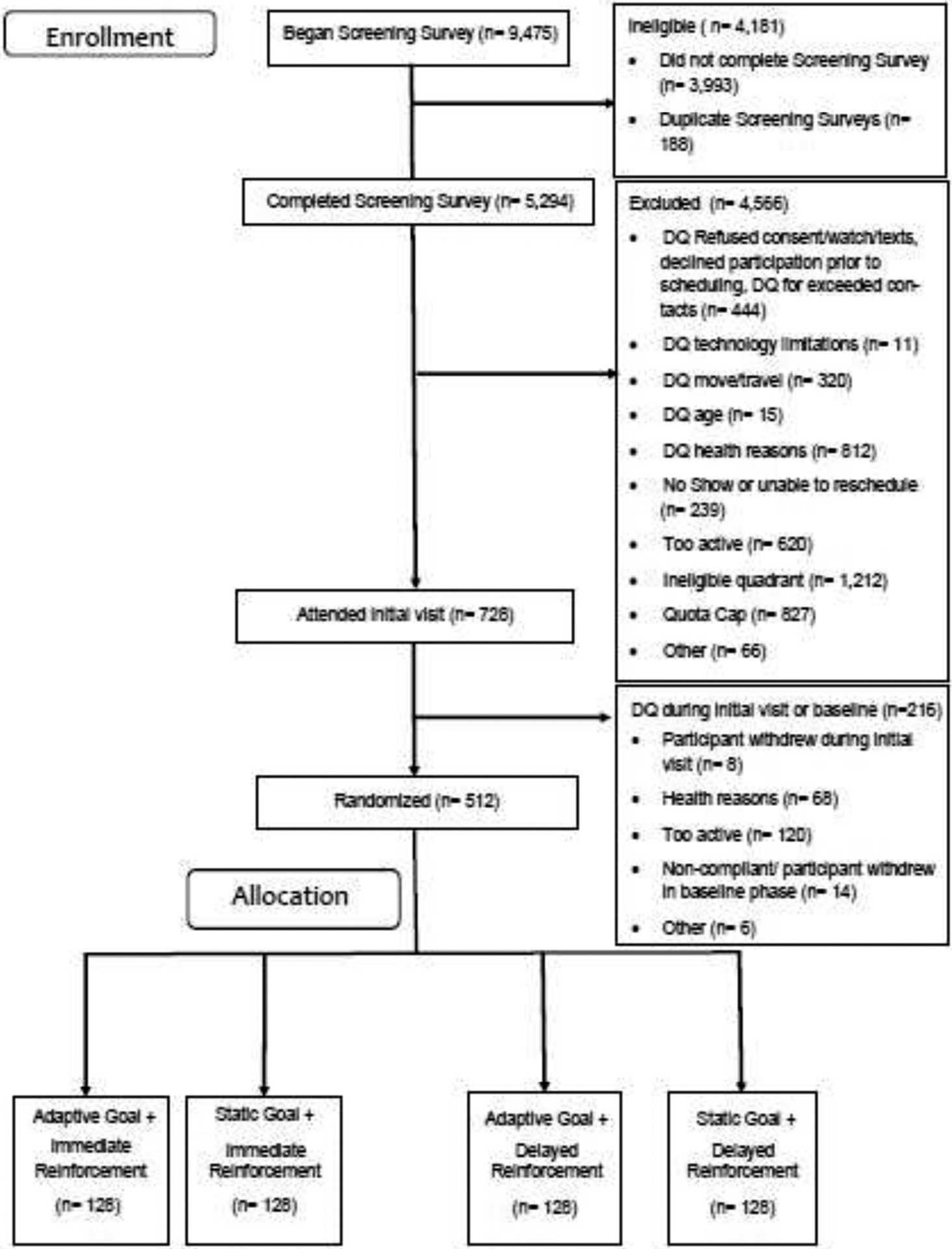
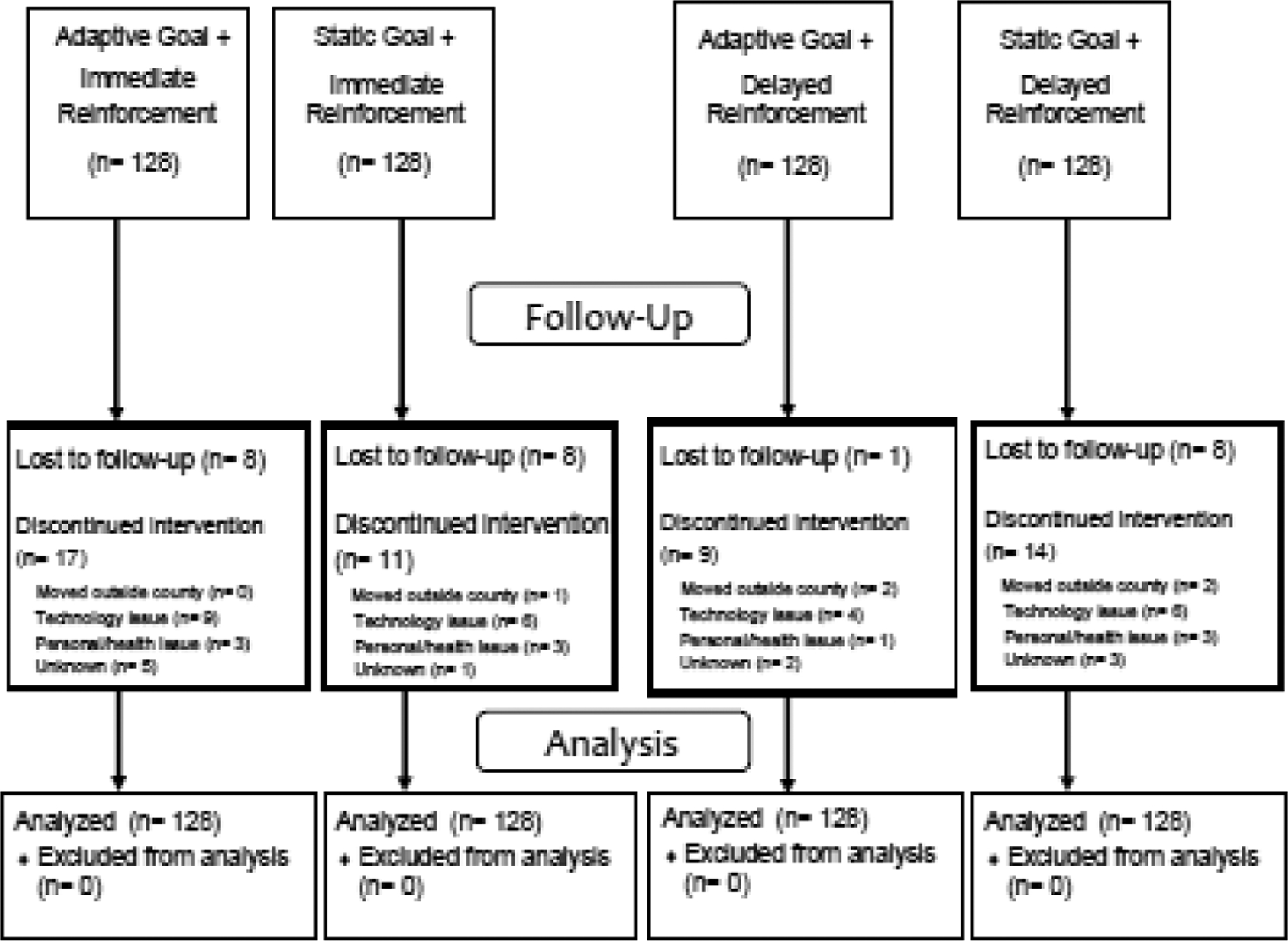
CONSORT diagram of participant flow.
DQ, disqualified.
As shown in Model 1 and Figure 2 (top left), there was a significant Goal Setting × Phase interaction such that the difference between the average daily likelihood of any MVPA during the intervention phase and the average daily likelihood of any MVPA during baseline phase (i.e., the increase in average daily likelihood of any MVPA) was larger for the Adaptive Goals condition (OR=2.34, 95% CI=2.10, 2.60) than for the Static Goals condition (OR=1.66, 95% CI=1.50, 1.84; between-condition p<0.001). The likelihood of any MVPA (versus none) decreased over the course of the intervention phase (Model 2), but as indicated by significant interaction terms, the rate of linear change and patterns of quadratic change differed across conditions. The Adaptive condition showed a pronounced quadratic pattern of change with a relatively steep decline through roughly the first 8 months, followed by a slight increase through the remainder of the intervention, whereas the Static condition showed a monotonically decreasing pattern across the intervention. Both groups sustained MVPA levels higher than baseline levels by end of the year.
Figure 2.
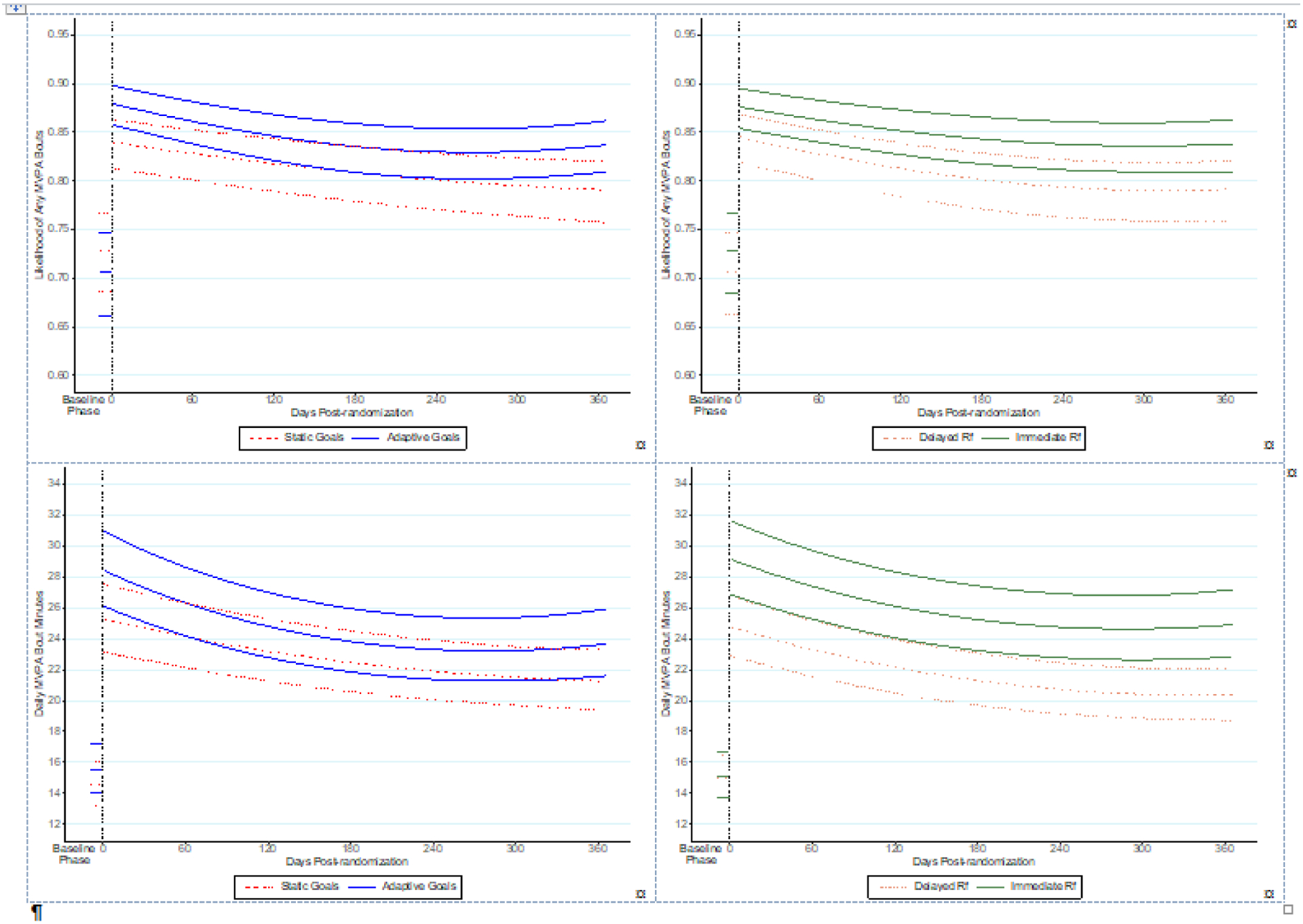
Effects of goal setting (left panels) and reinforcement timing (right panels) on changes to engaging in any MVPA minutes/day (top panels) and on mean MVPA bout minutes/day (bottom panels).a,b,c
aData represent 10-day baseline and 365-day intervention phases.
bVertical dashed lines denote transition between baseline and intervention phases.
cBaseline phase (Days Post Randomization <0) and intervention phase (Days Post Randomization ≥0).
MVPA, moderate-to-vigorous physical activity; Rf, reinforcement.
The average daily MVPA minutes/day during the intervention phase was higher than the average daily MVPA minutes/day during the baseline phase (Model 1 and Figure 2, bottom left panel), but the magnitude of this increase did not differ significantly across Goal Setting conditions (increases of 12.68 vs 13.50 minutes/day and group-specific incidence rate ratio [IRRs]=1.43 and 1.48 in the Static and Adaptive conditions, respectively). However, as indicated by significant Goal Setting × Days post-randomization and Goal Setting × Days post-randomization2 interaction terms (Model 2), rates of linear change and patterns of quadratic change in MVPA minutes/day during the intervention phase did differ across Goal Setting conditions, paralleling the patterns seen for likelihood of any MVPA. MVPA minutes/day in both conditions remained significantly higher than baseline levels by Day 365.
The Reinforcement Timing × Phase interaction (Model 1) was significant such that the difference between the average likelihood of any MVPA during the intervention phase and the average likelihood during the baseline phase (i.e., increase in likelihood) was larger in the Immediate Reinforcement condition (OR=2.16, 95% CI=1.94, 2.40) than in the Delayed Reinforcement condition (OR=1.77, 95% CI=1.59, 1.97; between-condition p<0.01) (Figure 2, top right panel).
There was a significant Reinforcement Timing × Phase interaction. The Immediate Reinforcement condition’s observed increase of 16.54 minutes/day (Model 1: IRR=1.57, 95% CI=1.51, 1.63 for intervention versus baseline difference) was significantly larger than the Delayed condition’s increase of 9.91 minutes/day (IRR=1.34, 95% CI=1.29, 1.39) (Figure 2, bottom left panel). Rates and patterns of change in daily MVPA minutes/day over the intervention phase did not differ across Reinforcement conditions (Model 2).
As shown in Model 1 and Figure 3 (top), the increase in average daily likelihood of any MVPA from the baseline phase to the intervention phase was strongest in the Adaptive Goals + Immediate Reinforcement group (OR=2.55, 95% CI=2.20, 2.97 for intervention versus baseline difference) and significantly different from that observed for the Static Goals + Immediate Reinforcement group (OR=1.89, 95% CI=1.35, 2.62) and for the Static Goals + Delayed Reinforcement group (OR=1.49, 95% CI=1.08, 2.07), but not for that seen in the Adaptive Goals + Delayed Reinforcement group (OR=2.17, 95% CI=1.57, 3.01). Patterns of quadratic change during the intervention phase differed across groups (Model 2), with the Adaptive Goals + Immediate Reinforcement group showing a relatively steep decline over approximately the first 8 months, followed by a slight increase at the end of the intervention. This pattern differed significantly from that observed in the Static Goals + Immediate Reinforcement group, which showed a monotonic decrease over the intervention phase.
Figure 3.
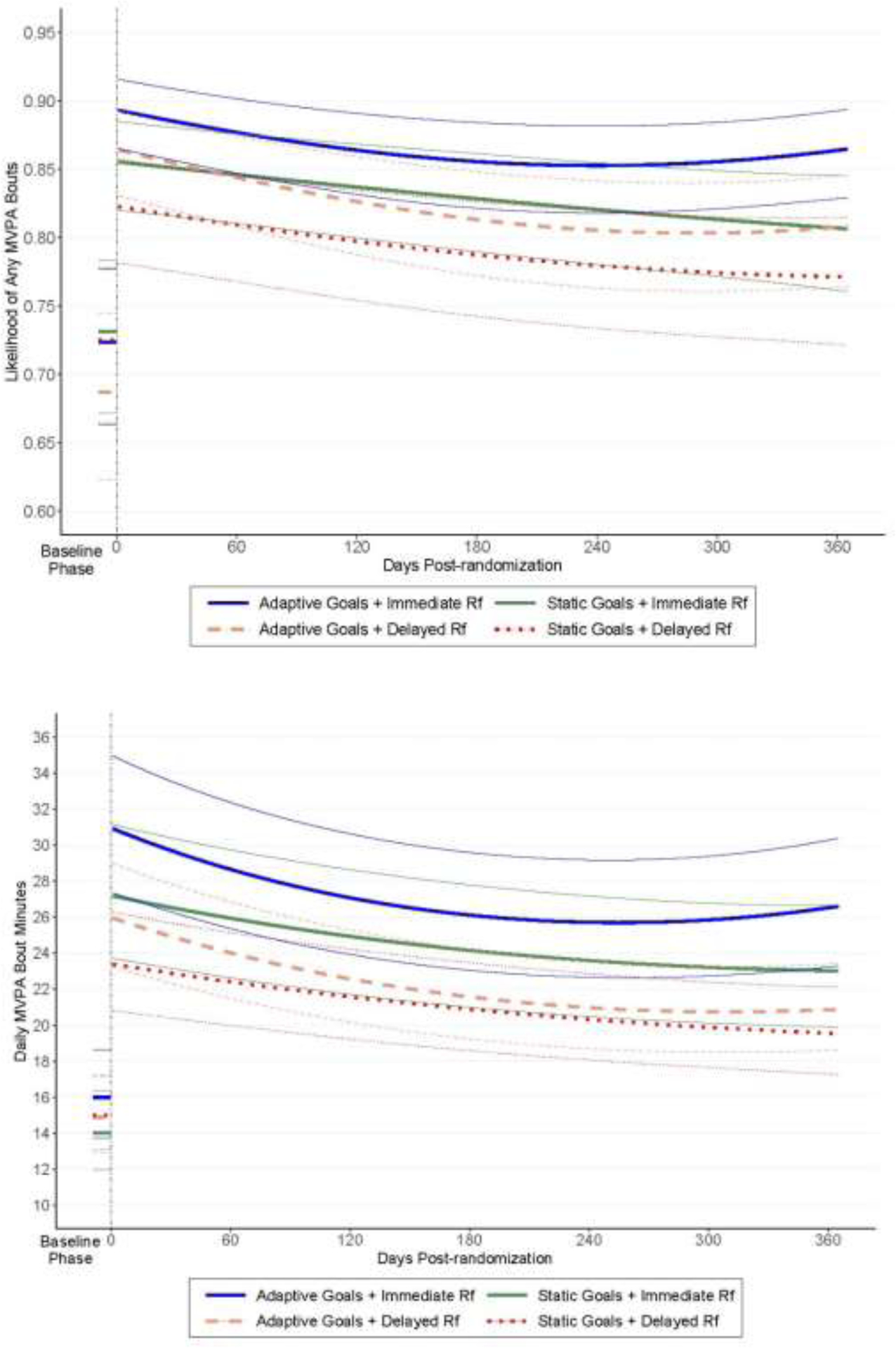
Differences between intervention groups in likelihood of any MVPA (top panel) and mean MVPA bout minutes/day (bottom panel).a,b,c
aData represent 10-day baseline and 365-day intervention phases.
bVertical dashed line denotes transition between baseline and intervention phases.
cBaseline phase (Days Post Randomization <0) and intervention phase (Days Post Randomization ≥0).
MVPA, moderate-to-vigorous physical activity; Rf, reinforcement.
Model 1 and Figure 3 (bottom) show that the increase in average daily MVPA minutes/day from the baseline to the intervention phase differed significantly across groups. The increase of 16.52 minutes/day (IRR=1.54, 95% CI=1.46, 1.62 for intervention versus baseline difference) observed in the Adaptive Goals + Immediate Reinforcement group was not significantly different from the increase of 16.55 minutes/day seen for the Static Goals + Immediate Reinforcement group (IRR=1.64, 95% CI=1.46, 1.83), but was significantly larger than that seen in either of the Delayed Reinforcement groups (10.84 minutes/day in Adaptive + Delayed and 8.87 minutes/day in Static + Delayed; IRR=1.35, 95% CI=1.21, 1.52 and IRR=1.35, 95% CI=1.20, 1.51, respectively).
Participants rated the interventions highly (overall mean=8.2 [SD=2.3] points of 10). No significant differences were observed between the 4 arms (means ranged from 7.97 to 8.29).
DISCUSSION
This study examined independent and joint effects of adaptive goal setting and immediate financial reinforcement on MVPA over 1 year with healthy, inactive adults.6,11,13,17 Investigators tested whether novel intervention components could help participants increase daily initiation and duration of MVPA. Results show that adaptive goal setting and immediate financial reinforcement components independently increased both measures of health-enhancing MVPA over 1 year.
Adaptive goals increased the probability of initiating any MVPA each day by 50% more than static goals, independent of reinforcement timing. Both goal groups showed increased mean MVPA/minutes, but contrary to the hypothesis, the degree of increase did not differ significantly between goal groups. A significant between-group difference in non-linear MVPA trajectories was observed throughout the intervention year. The Adaptive Goal group’s change from a negative to a positive trajectory near Month 8 for both MVPA outcomes, compared with the Static Goal group’s consistent declines, suggests that adaptive goals may combat intervention fatigue.
Static goals entailed a fixed response cost, whereas adaptive goals, which could stay the same, adjust up, or adjust down for each participant, ranged from 3 to 60 minutes/day, depending on daily performance and the adaptive algorithm. This adaptive process approximates an experienced coach adjusting thresholds for success depending on the starting point, motivation, or abilities of a mentee, and dynamically adjusting requirements to shape stronger performance as the mentee improves or struggles. This study and others also suggest that adaptive goals and intervention components may yield more potent behavioral interventions and help maintain improvements.13,16,38,39 Changes reported here were accomplished using an automated approach demonstrating potential for scalability required to address population health.
The Immediate Reinforcement groups also showed an increased likelihood of initiating any MVPA throughout the intervention relative to baseline and mean MVPA minutes/day change throughout the intervention phase was greater by 6.6 minutes/day relative to the Delayed Reinforcement condition. Notably, the between-condition difference in MVPA duration between 2 active interventions represents a 3-fold improvement over meta-analytic findings by Conn et al.4 that compared interventions using a multitude of individual-level behavior change techniques to control groups for increasing PA among healthy adults. The comparative evaluation of active interventions to each other (rather than to passive or measurement only controls) is important for the development of increasingly more potent behavior change interventions. Though other studies have found magnitudes of ≤$1.00 can increase PA,19 even in comparison to delayed incentives,6,11 those studies exclusively set step goals, thus targeting movement volume, rather than activity intensity. The current study shows that small financial rewards can help individuals with goals that have an intensity dimension (i.e., moderate or greater intensity). Results also align to principles of behavior change that emphasize reinforcement immediacy and specificity over magnitude as important considerations with the use of rewards (e.g., smaller, sooner),20–23,40 and that appears to extend to helping inactive adults initiate any and increase MVPA duration.
A commonly asked question is which intervention components produce change in an outcome, or which combination of components produce optimal change,38,41,42 but confounding thwarts post hoc attempts to disentangle unique and joint effects of multicomponent interventions.43,44 The current study was designed to address this question, and the results provide convincing evidence that Adaptive Goals + Immediate Reinforcement produced the strongest joint effect on initiating daily MVPA. Results suggest that a combination with adaptive goals could be used when the purpose of the intervention is to help a subgroup of sedentary adults initiate any activity, while combinations with immediate reinforcement could help increase both any MVPA initiation and MVPA duration among a subgroup who is somewhat, but insufficiently, active daily. The results also suggest new directions such as testing adaptive goals to initiate MVPA among sedentary individuals, followed by immediate reinforcement to increase the duration of MVPA once it occurs reliably.
These results may have important public health implications. Accumulating ≥150 minutes/week (>7.5 MET hours/week) is associated with 6%–32% reductions in the prevalence of myriad chronic diseases45 and can attenuate cardiovascular disease risk in the presence of overweight and obesity by approximately 50%.45–47 In this study, most participants exceeded 7.5 MET hours/week on average at the 1-year timepoint. Finally, these interventions were socially acceptable to participants and well tolerated with no significant adverse events.
Limitations
This study, the first adaptive intervention to target MVPA via actigraphy, observed an immediate effect that persisted throughout the 365-day intervention and achieved 70% average wear compliance, exceeding levels seen with commercial wearables48 and in other RCTs.39,49 Limitations included a sample from a single U.S. region of mainly overweight/obese adults, limiting broader generalizability. However, the sample was purposefully recruited across higher and lower socioeconomic and walkable neighborhood strata with treatment groups balanced on average on confounding via stratified blocked randomization.24 Participants were unaware of the hypotheses and blinded during the baseline, but participants and investigators were not blinded during the intervention. Randomization, passive MVPA measurement at baseline and throughout intervention phases, and intervention component delivery were entirely automated, minimizing potential participant and investigator biases. The impact of varied combinations of incentive design features could be explored in future research among free-living adults.
CONCLUSIONS
This trial found that adaptive goals outperformed static goals for initiating any MVPA, and immediate reinforcement outperformed delayed non-contingent reinforcement for increasing any MVPA and MVPA duration once initiated. Consistent with theoretical expectations, adaptive goals combined with immediate reinforcement produced the strongest change for initiating any and increasing total MVPA minutes over 1 year and mitigated intervention fatigue. These scalable behavior change techniques have implications for chronic disease risk reduction among at-risk adults.1,47,50
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in whole by the National Cancer Institute at NIH (R01CA198915). The funding agency was not involved in any aspect of this study or manuscript. The authors acknowledge the support of Mr. Jeremy Wyatt, Chief Executive Officer, and team at ActiGraph, LLC for their willingness to evolve ActiGraph’s cloud-based system to enable this intervention with free-living individuals; Dr. Meg Bruening for reviewing this manuscript; and special thanks to Ms. Emily Foreman, Ms. Alison Cantley, and Mr. Tsung-Yen (John) Yu for data collection and programming along with many undergraduate and graduate research assistants helping with data collection.
MAA, MT, SS, JCH, VB, CBP, MM, MFH, and SPH declare financial support for this study from the National Cancer Institute of NIH (R01CA198915). The funding agency was not involved in any aspect of this study or manuscript.
No financial disclosures were reported by the authors of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT Author Statement
Marc A. Adams: Conceptualization, Methodology, Software, Investigation, Resources, Data Curation, Writing – Original Draft, Visualization, Supervision, Project administration, Funding acquisition.
Michael Todd: Conceptualization, Methodology, Software, Formal Analysis, Data Curation, Writing – Original Draft, Visualization, Funding acquisition.
Siddhartha S. Angadi: Conceptualization, Methodology, Data Curation, Writing – Review and Editing, Supervision, Project administration, Funding acquisition.
Jane C. Hurley: Conceptualization, Methodology, Investigation, Project administration.
Chad Stecher: Software, Data Curation, Fornal Anaysis, Writing – Review and Editing, Visualization.
Vincent Berardi: Software, Data Curation, Writing – Review and Editing, Visualization.
Christine B. Phillips: Project administration, Data Curation, Writing – Review and Editing.
Mindy McEntee: Project administration, Data Curation, Writing – Review and Editing.
Melbourne F. Hovell: Conceptualization, Methodology, Data Curation, Writing – Review and Editing, Funding acquisition.
Steven P. Hooker: Conceptualization, Methodology, Data Curation, Writing – Review and Editing, Funding acquisition.
REFERENCES
- 1.Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology (Bethesda). 2013;28(5):330–358. 10.1152/physiol.00019.2013. [DOI] [PubMed] [Google Scholar]
- 2.Clarke TC, Norris T, Schiller JS. Early release of selected estimates based on data from the National Health Interview Survey. National Center for Health Statistics; May 2019. [Google Scholar]
- 3.Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S.: adults compliance with the Physical Activity Guidelines for Americans. Am J Prev Med. 2011;40(4):454–461. 10.1016/j.amepre.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Conn VS, Hafdahl AR, Mehr DR. Interventions to increase physical activity among healthy adults: meta-analysis of outcomes. Am J Public Health. 2011;101(4):751–758. 10.2105/ajph.2010.194381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croteau KA. A preliminary study on the impact of a pedometer-based intervention on daily steps. Am J Health Promot. 2004;18(3):217–220. 10.4278/0890-1171-18.3.217. [DOI] [PubMed] [Google Scholar]
- 6.Adams MA, Sallis JF, Norman GJ, Hovell MF, Hekler EB, Perata E. An adaptive physical activity intervention for overweight adults: a randomized controlled trial. PLoS One. 2013;8(12):e82901. 10.1371/journal.pone.0082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dallery J, Cassidy RN, Raiff BR. Single-case experimental designs to evaluate novel technology-based health interventions. J Med Internet Res. 2013;15(2):e22. 10.2196/jmir.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurti AN, Dallery J. Internet-based contingency management increases walking in sedentary adults. J Appl Behav Anal. 2013;46(3):568–581. 10.1002/jaba.58. [DOI] [PubMed] [Google Scholar]
- 9.Patel MS, Small DS, Harrison JD, et al. Effectiveness of behaviorally designed gamification interventions with social incentives for increasing physical activity among overweight and obese adults across the United States: the STEP UP Randomized Clinical Trial. JAMA Intern Med. 2019;179(12):1624–1632. 10.1001/jamainternmed.2019.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakicic JM, Tate DF, Lang W, et al. Objective physical activity and weight loss in adults: the step-up randomized clinical trial. Obesity (Silver Spring). 2014;22(11):2284–2292. 10.1002/oby.20830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams MA, Hurley JC, Todd M, et al. Adaptive goal setting and financial incentives: a 2 × 2 factorial randomized controlled trial to increase adults’ physical activity. BMC Public Health. 2017;17:303. 10.1186/s12889-017-4231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams MA. A Pedometer-based Intervention to Increase Physical Activity: Applying Frequent, Adaptive Goals and a Percentile Schedule of Reinforcement [Dissertation]. San Diego, California: University of California, San Diego; 2009. [Google Scholar]
- 13.Korinek EV, Phatak SS, Martin CA, et al. Adaptive step goals and rewards: a longitudinal growth model of daily steps for a smartphone-based walking intervention. J Behav Med. 2018;41(1):74–86. 10.1007/s10865-017-9878-3. [DOI] [PubMed] [Google Scholar]
- 14.Poirier J, Bennett WL, Jerome GJ, et al. Effectiveness of an activity tracker- and internet-based adaptive walking program for adults: a randomized controlled trial. J Med Internet Res. 2016;18(2):e34. 10.2196/jmir.5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riley WT, Rivera DE, Atienza AA, Nilsen W, Allison SM, Mermelstein R. Health behavior models in the age of mobile interventions: are our theories up to the task? Transl Behav Med. 2011;1(1):53–71. 10.1007/s13142-011-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riley WT, Serrano KJ, Nilsen W, Atienza AA. Mobile and wireless technologies in health behavior and the potential for intensively adaptive interventions. Curr Opin Psychol. 2015;5:67–71. 10.1016/j.copsyc.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phatak SS, Freigoun MT, Martin CA, et al. Modeling individual differences: a case study of the application of system identification for personalizing a physical activity intervention. J Biomed Inform. 2018;79:82–97. 10.1016/j.jbi.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell MS, Goodman JM, Alter DA, et al. Financial incentives for exercise adherence in adults: systematic review and meta-analysis. Am J Prev Med. 2013;45(5):658–667. 10.1016/j.amepre.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell MS, Orstad SL, Biswas A, et al. Financial incentives for physical activity in adults: systematic review and meta-analysis. Br J Sports Med. 2020;54(21):1259–1268. 10.1136/bjsports-2019-100633. [DOI] [PubMed] [Google Scholar]
- 20.Ferster CB, Skinner BF. Schedules of Reinforcement. New York, NY: Appleton-Century-Crofts; 1957. 10.1037/10627-000. [DOI] [Google Scholar]
- 21.Critchfield TS, Kollins SH. Temporal discounting: basic research and the analysis of socially important behavior. J Appl Behav Anal. 2001;34(1):101–122. 10.1901/jaba.2001.34-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull. 2004;130(5):769–792. 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thirumurthy H, Asch DA, Volpp KG. The uncertain effect of financial incentives to improve health behaviors. JAMA. 2019;321(15):1451–1452. 10.1001/jama.2019.2560. [DOI] [PubMed] [Google Scholar]
- 24.Adams MA, Hurley JC, Phillips CB, et al. Rationale, design, and baseline characteristics of WalkIT Arizona: a factorial randomized trial testing adaptive goals and financial reinforcement to increase walking across higher and lower walkable neighborhoods. Contemp Clin Trials. 2019;81:87–101. 10.1016/j.cct.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frank LD, Sallis JF, Saelens BE, et al. The development of a walkability index: application to the Neighborhood Quality of Life Study. Br J Sports Med. 2010;44(13):924–933. 10.1136/bjsm.2009.058701. [DOI] [PubMed] [Google Scholar]
- 26.2008 Physical Activity Guidelines for Americans. HHS; 2008. [Google Scholar]
- 27.Powell KE, King AC, Buchner DM, et al. The Scientific Foundation for the Physical Activity Guidelines for Americans, 2nd Edition. J Phys Act Health. 2019;16(1):1–11. 10.1123/jpah.2018-0618. [DOI] [PubMed] [Google Scholar]
- 28.Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–781. 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Welk GJ, Schaben JA, Morrow JR Jr. Reliability of accelerometry-based activity monitors: a generalizability study. Med Sci Sports Exerc. 2004;36(9):1637–1645. [PubMed] [Google Scholar]
- 30.John D, Freedson P. ActiGraph and Actical physical activity monitors: a peek under the hood. Med Sci Sports Exerc. 2012;44(1 suppl 1):S86–S89. 10.1249/mss.0b013e3182399f5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aadland E, Ylvisaker E. Reliability of the Actigraph GT3X+ accelerometer in adults under free-living conditions. PLoS One. 2015;10(8):e0134606. 10.1371/journal.pone.0134606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnett A, Cerin E, Vandelanotte C, Matsumoto A, Jenkins D. Validity of treadmill- and track-based individual calibration methods for estimating free-living walking speed and VO2 using the Actigraph accelerometer. BMC Sports Sci Med Rehabil. 2015;7:29. 10.1186/s13102-015-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–364. 10.1249/mss.0b013e3181ed61a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi L, Ward SC, Schnelle JF, Buchowski MS. Assessment of wear/nonwear time classification algorithms for triaxial accelerometer. Med Sci Sports Exerc. 2012;44(10):2009–2016. 10.1249/mss.0b013e318258cb36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 36.Psioda M. Random effects simulation for sample size calculations using SAS ®. Paper presented at the annual meeting of Southeast SAS Users Group, Durham, NC, 2012. [Google Scholar]
- 37.Bauer DJ, Curran PJ. Probing interactions in fixed and multilevel regression: inferential and graphical techniques. Multivariate Behav Res. 2005;40(3):373–400. 10.1207/s15327906mbr4003_5. [DOI] [PubMed] [Google Scholar]
- 38.Hekler EB, Rivera DE, Martin CA, et al. Tutorial for using control systems engineering to optimize adaptive mobile health interventions. J Med Internet Res. 2018;20(6):e214. 10.2196/jmir.8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klasnja P, Rosenberg DE, Zhou J, Anau J, Gupta A, Arterburn DE. A quality-improvement optimization pilot of BariFit, a mobile health intervention to promote physical activity after bariatric surgery. Transl Behav Med. 2021;11(2):530–539. 10.1093/tbm/ibaa040. [DOI] [PubMed] [Google Scholar]
- 40.Bickel WK, Vuchinich RE. Reframing health behavior change with behavioral economics. Mahwah, NJ: Lawrence Erlbaum; 2000. 10.4324/9781410605061. [DOI] [Google Scholar]
- 41.Riley WT, Rivera DE. Methodologies for optimizing behavioral interventions: introduction to special section. Transl Behav Med. 2014;4(3):234–237. 10.1007/s13142-014-0281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins LM, Baker TB, Mermelstein RJ, et al. The multiphase optimization strategy for engineering effective tobacco use interventions. Ann Behav Med. 2011;41(2):208–226. 10.1007/s12160-010-9253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins LM, Dziak JJ, Li R. Design of experiments with multiple independent variables: a resource management perspective on complete and reduced factorial designs. Psychol Methods. 2009;14(3):202–224. 10.1037/a0015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins LM, Dziak JJ, Kugler KC, Trail JB. Factorial experiments: efficient tools for evaluation of intervention components. Am J Prev Med. 2014;47(4):498–504. 10.1016/j.amepre.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, Cash RE, Bower JK, Focht BC, Paskett ED. Physical activity and risk of cardiovascular disease by weight status among U.S adults. PLoS One. 2020;15(5):e0232893. 10.1371/journal.pone.0232893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bennie JA, De Cocker K, Teychenne MJ, Brown WJ, Biddle SJH. The epidemiology of aerobic physical activity and muscle-strengthening activity guideline adherence among 383,928 U.S. adults. Int J Behav Nutr Phys Act. 2019;16(1):34. 10.1186/s12966-019-0797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arem H, Moore SC, Patel A, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015;175(6):959–967. 10.1001/jamainternmed.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ledger D, McCaffrey D. Inside wearables: how the science of human behavior change offers the secret to long-term engagement. Endeavour Partners. 2014;200(93):1. [Google Scholar]
- 49.Patel MS, Polsky D, Kennedy EH, et al. Smartphones vs wearable devices for remotely monitoring physical activity after hospital discharge: a secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3(2):e1920677. 10.1001/jamanetworkopen.2019.20677. [DOI] [PubMed] [Google Scholar]
- 50.Moore SC, Lee IM, Weiderpass E, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176(6):816–825. 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


