Abstract
Intimate partner violence (IPV) increases risk of traumatic brain injury (TBI). Physical assaults increase in frequency and intensity during pregnancy. The consequences of TBI during pregnancy (gravida TBI; gTBI) on offspring development is unknown, for which stress and inflammation during pregnancy worsen fetal developmental outcomes. We hypothesized that gTBI would lead to increased anxiety- and depression-related behavior, altered inflammatory responses and gut pathology, and distorted brain circuitry in mixed-sex offspring compared to mice born to control mothers. Pregnant dams received either diffuse TBI or sham injury (control) 12 days post-coitum. We found that male gTBI offspring were principal drivers of the gTBI effects on health, physiology, and behavior. For example, male, but not female, gTBI offspring weighed significantly less at weaning compared to male control offspring. At post-natal day (PND) 28, gTBI offspring had significantly weaker intralaminar connectivity onto layer 5 pre-frontal pyramidal neurons compared to control offspring. Neurological performance on anxiety-like behaviors was decreased, with only marginal differences in depressive-like behaviors, for gTBI offspring compared to control offspring. At PND42 and PND58, circulating neutrophil and monocyte populations were significantly smaller in gTBI male offspring than control male offspring. In response to a subsequent inflammatory challenge at PND75, gTBI offspring had significantly smaller circulating neutrophil populations than control offspring. Anxiety-like behaviors persisted during the immune challenge in gTBI offspring. However, spleen immune response and gut histology showed no significant differences between groups. The results compel further studies to determine the full extent of gTBI on fetal and maternal outcomes.
Keywords: circuit connectivity, fetal development, gestational injury, immune response, intimate partner violence, maternal brain injury
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability and can occur in any population, regardless of age, sex, or socioeconomic background. However, health disparities can increase the risk and worsen the outcomes of a TBI.1,2 Males and populations under the age of 4, between 15 and 19, and over the age of 65 are reported to have the highest prevalence of TBI.1 By the early 2000s, females comprised 40% of TBI-related emergency room visits, and yet TBIs that go unreported are not able to be counted, particularly those associated with domestic or intimate partner violence (IPV).3–5 One in three females will experience IPV in some form during their lifetime, but only 21% of victims seek medical treatment for physical assault in spite of the fact that 60–90% of IPV co-occurs with TBI5,6 because assault is more likely to be directed toward the head, neck, and face. Further, IPV is the leading cause of death in women of childbearing age, with pregnancy increasing the risk and severity of IPV in younger women and minority groups.7,8
To our knowledge, no studies have determined the long-term consequences of IPV or an isolated TBI during pregnancy (gravida TBI; gTBI) on fetal development and quality of life, and particularly in pre-clinical models. With the alarming rate of IPV, upward of a 90% co-occurrence of IPV and TBI, and the significant lack of research on long-term outcomes on gTBI, there is a critical need for translational research to identify the risks and consequences of gTBI as they apply to vulnerable populations.5,6 This translational study aims to elucidate potential detrimental outcomes associated with gTBI to better inform advocacy and potential outcomes for vulnerable mothers and their children.
The gestational environment can significantly influence fetal development and fetal outcomes, particularly those related to systemic inflammation.9–17 For example, gravida systemic inflammation increases the risk of neurodevelopmental issues, such as cognitive and behavioral disorders, in humans and animal models.10,18 Epidemiologically, long-lasting infections in pregnant mothers have been associated with increased risk of autism spectrum disorder (ASD) and schizophrenia.13,19 With the COVID-19 pandemic, and previous connection between ZIKA virus and birth defects, understanding the role of gravida inflammation and injury is essential.20,21 Stress can also increase gravida systemic inflammation and is associated with worsened fetal outcomes.22 When stressors occur during pregnancy, offspring are at risk for low birth weights, lifelong alterations in stress hormones, and increased susceptibility to neuropsychiatric disorders.12,23
Maternal alterations in gut microbiome and gut health can also alter immunity and gut health in offspring.10,24 Given that an isolated TBI can alter systemic inflammation,25–27 hormones,28,29 and gut health,30–32 the possibility exists for offspring to develop gut health issues. Yet, there is limited information available on how gTBI affects fetal development. One epidemiological study found that pregnant mothers involved in a motor vehicle collision with a severe TBI were 38% more likely to have children with cerebral palsy if born premature.33 Clinical risk studies are informative, but far from causative; most cases of TBI are less severe and require animal modeling to better understand neurodevelopmental consequences.
Though stress and infections can span several months and cross gestational periods, the timing of infection, inflammation, or injury may play a key role on fetal development. For example, inflammation during the second trimester has been associated with neuropsychiatric conditions in offspring.34 The second trimester or mid-gestational periods (10–20 days post-coitum [d.p.c.]) are important for neurodevelopment, such that infections or stress at this time can increase risk for neurodevelopmental changes in offspring.34–36 In the laboratory, maternal infection by lipopolysaccharide (LPS) injected at 9.5 d.p.c. resulted in offspring with autistic-like behaviors and disturbances in dopaminergic neurons in the striatum.15,17,37 LPS administered at 16 d.p.c. increased fetal inflammation and worsened fetal outcomes, including increased pre-term birth, fetal mortality at time of birth, and fetal mortality, within 1 week of birth.38
Though rodent models exist for early-life stress,39 gravida stress,40–42 gestational diabetes,43,44 and infections during pregnancy,10,18 models for gTBI are nonexistent. An isolated TBI to a pregnant female mouse may therefore result in a unique pathophysiology that increases inflammation, disrupting fetal development in terms of cognitive and behavioral disorders, changes in immunity, and cortical development of the offspring.10,24,41,45 To date, the long-term physiological and behavioral effects on the fetus from TBI in pregnant mothers have not been investigated. In this study, we hypothesized that gTBI would lead to increased anxiety- and depression-related behavior, altered inflammatory response and gut pathology, and distorted brain circuitry in offspring.
Methods
Rigor
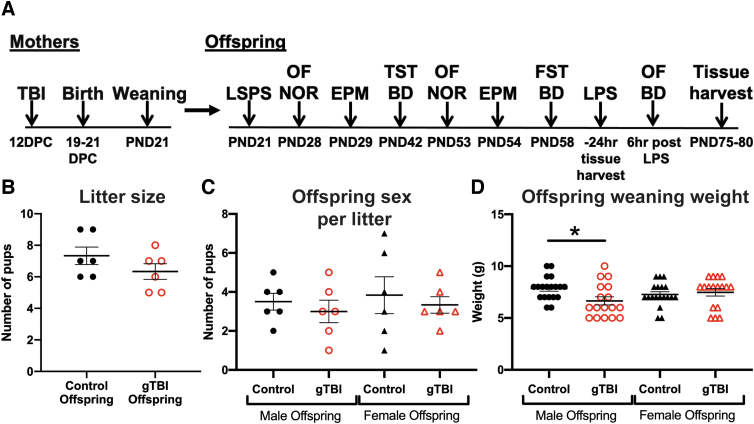
Animal studies were conducted in accordance with the guidelines established by the internal Institutional Animal Care and Use Committee and the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals. The ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines were followed while conducting and reporting this study.46 Surgeries were performed on a total of 18 pregnant dams. A total of 4 pregnant dams died from the injury. No animals were excluded from this study for technical failures (e.g., dura damage during surgery, loose hubs, etc.) or for falling outside of the pre-determined brain-injury inclusion criteria (righting reflex times, 4–8 min). One TBI and 1 sham dam did not give birth to full litters or experienced a spontaneous abortion. A total of 12 moms gave birth naturally, and a total of 80 mixed-sex offspring were weaned and used for the study. Of these, 72 were used for behavior and 8 were used for cortical mapping.
All mice had repeated blood draws using the submandibular vein within 15 min of forced swim and tail suspension testing. A subset of 40 mice were used to measure corticosterone levels after the behavioral stressors. Mice were injected (intraperitoneally; i.p.) with 1 mg/kg of lipopolysaccharide (LPS) 24 h before euthanasia. Flow cytometry outcomes were analyzed from 35 mice. Gut histology was analyzed from 14 male mice. Last, at time of euthanasia, all mice were euthanized with Euthasol® (0.002 mL/g; 07-805-9296; Patterson Veterinary, Greeley, CO).
Animals
Dams were ordered from The Jackson Laboratory (Bar Harbor, ME) at 5 d.p.c., gravida 1, and acclimated for a week before any procedures took place. All pregnant mice were housed individually in a 14-h light/10-h red light (Light Gard Light Tubes; Solar Graphics, Clearwater, FL) cycle at a constant temperature (23°C ± 2°C) and humidity (50% ± 10%). White-light intensity at cage level was 48 lumens, and red-light intensity was 4 lumens. Food (Teklad 2919 irradiated; Envigo, Placentia, CA) and water (Innovive pre-filled acidified water; Innovive, San Diego, CA) were available ad libitum. All cages contained a single water bottle, feed hopper, corncob bedding, and a nesting square, in accordance with standard procedures for the vivarium. Surgeries and injuries were performed at 12 d.p.c. for all dams. All mice that received surgeries and injuries received a welfare examination for the first 3 days after surgery. At birth, offspring were counted and sexed at weaning on post-natal day (PND) 21. Offspring were weaned into new cages with a maximum of 5 same-sex animals in one cage with no animals singly housed.
Maternal gravida midline fluid percussion injury
Because of the potential effect of anesthesia on fetal development, all dams were subjected to the minimum amount of inhaled isoflurane to permit midline fluid percussion injury (mFPI) consistent with methods previously described.27,47 Briefly, the dam was anesthetized using 5% isoflurane in 100% oxygen for 2 min. The dam was then placed in a stereotaxic frame with continuously delivered isoflurane at 2.5% by a nose cone. Body temperature was maintained using a Deltaphase isothermal heating pad (Braintree Scientific Inc., Braintree, MA). A midline incision was made exposing the skull, and a 3-mm midline craniectomy was performed between the bregma and lambda using a trephining tool. A modified Luer lock hub was glued directly over the exposed intact dura and reinforced with cyanoacrylate gel and methylmethacrylate (Hygenic Corp., Akron, OH). The hub was filled with saline to ensure a complete seal. A cap made from a modified syringe tip was placed in the hub to prevent debris and air exposure. All surgeries were completed within 8 min. Animals were placed in a heated recovery cage and monitored until ambulatory before they were returned to their individual home cage (∼10 min).
After 2 h, dams were reanesthetized with 5% isoflurane delivered for 2 min to prepare for TBI induction. The cap was removed, and the hub was visually inspected for debris and intact sinus and dura. The hub was then refilled with normal saline to create a single continuous line of fluid from the injury device to the intact dura. The dam was attached to the fluid percussion device (Custom Design and Fabrication; Virginia Commonwealth University, Richmond, VA) using extension tubing (#2C5643; Baxter, Deerfield, IL). Mice were positioned under the device, and a toe-pinch response was observed before the pendulum on the injury device was dropped from a pre-determined height. A fluid pressure pulse was delivered directly onto the dura to induce a diffuse brain injury. Hubs were removed immediately, and mice were placed on their side on a heating pad. Apnea and seizure-like activity were recorded. The brain was inspected for uniform herniation, bleeding, formation of hematoma, and dura damage. Animals were monitored for righting reflex recovery times as a metric of injury severity.27,47 Righting reflex time was defined as the total time from the initial impact until the animal spontaneously righted itself from a lateral position.
TBI mice in this study had a suppression of righting reflex time of 4–8 min; sham mice were treated in the same manner without delivery of the injury. Suppression of the righting reflex in this time frame is associated with significant increases in central and peripheral inflammation, peripheral pathologies (e.g., lungs), and changes in acute sleep behavior and cognitive behavior.27,48,49 Sham animals spontaneously righted within 20 sec; no shams were excluded for not righting within this time.
Whole-cell patch clamp recording and laser scanning photostimulation cortical circuit mapping
To investigate the effects of gTBI on pre-frontal cortical neurons, ex vivo sagittal brain slices were prepared from offspring as previously described50–52 to quantify synaptic function and circuit connectivity. Mixed-sex mice were killed under isoflurane anesthesia. Brains were collected and sectioned in ice-cold artificial cerebrospinal fluid (aCSF) containing (in mM): 126 NaCl, 2.5 KCl, 26 NaHCO3, 2 CaCl2, 1 MgCl2, 1.25 NaH2PO4, and 10 glucose and saturated with 95% O2 and 5% CO2. Slices were incubated at 32°C in aCSF for 30 min, then kept at room temperature. Slices were transferred to a recording chamber and perfused with room temperature aCSF with 0.2 mM of MNI-Caged glutamate (Tocris-Cookson, Bristol, UK). Neurons were visualized with a 60 × water immersion objective (numerical aperture [NA], 0.9; Olympus Corporation, Tokyo, Japan) and targeted for whole-cell patch clamp recordings. The glass patch electrode contained an internal solution (in mM): 130 K-gluconate, 4 KCl, 2 NaCl, 10 HEPES, 4 ATP-Mg, 0.3 GTP-Na, 1 ethylene glycol tetraacetic acid, and 14 phosphocreatine (pH 7.2, 295 mOsm). Upon obtaining whole-cell configuration, neurons were voltage clamped at −70 mV.
Laser scanning photostimulation (LSPS) mapping combined with glutamate uncaging was performed with a 4 × objective lens (NA 0.16; Olympus) and 20-mW, 1-ms UV laser (355 nm; DPSS Lasers, Santa Clara, CA) pulses. UV laser pulses were delivered onto slices in a 16 × 16 stimulation grid with 100-μm spacing. The stimulation grid was aligned with the pial surface. Laser timing and power were controlled by an optic shutter (model 3050; Conoptics, Danbury, CT), a mechanical shutter (Unibliz® VCM-D1; Vincent Associates, Rochester, NY), and a neutral density filter (Edmund Optics, Barrington, NJ). Digital images were acquired using a charged-coupled device camera (Retiga 2000DC; Teledyne QImaging, Surrey, BC, Canada). Electrophysiological signals were amplified with a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA) and acquired with 6259 data acquisition boards (National Instruments, Austin, TX) under control of open-source Ephus software. Signals were filtered at 2–4 KHz and digitized at 10 kHz. Neuronal responses to laser uncaging were analyzed offline with MATLAB scripts (The MathWorks, Inc., Natick, MA). For analysis, n = 2–5 cells per animal were used.
Behavior assessment in offspring
Open field
The open field (OF) consisted of a square arena (70 × 70 cm) enclosed by white walls measuring 46 cm in height. On the day of OF testing, mice were transported to the testing room and placed in individual cages to acclimate to the room. After 1 h of acclimation, mice were placed in the OF and allowed to explore freely for 5 min. Their movement was tracked by an overhead camera, and distance traveled, time spent in the center, and frequency were automatically calculated using EthoVision XT 10 Software (Noldus Information Technology, Leesburg, VA). One female control offspring, 1 male control offspring, and 1 female gTBI offspring were excluded for improper handling (unexpected challenges in handling juvenile mice) that were then associated with significant outliers in testing.
Novel object recognition
Cognitive impairment was tested using the novel object recognition (NOR) task. The test consisted of three phases: habituation, training, and testing. On training day, mice were habituated to the OF, as described above, which also served as a measure of anxiety and locomotion. After OF, mice were removed from the arena, and two identical objects were placed equidistant from two corners diagonal to each other in the individual testing cages. Mice were then placed in the testing cages and allowed to explore the objects for 5 min. After 5 min, mice were removed from the arena and returned to their home cage for 3 min while the objects were thoroughly cleaned using quatricide®. During this time, one object from the previous trial was replaced with a novel object of similar size, but distinctly different in color and shape. Then, mice were reintroduced to the testing cage and were allowed to explore the objects for 5 min.
For the training and testing trials, time spent exploring each object was quantified, with exploration consisting of mice sniffing, touching, or directing their head toward the objects. During training and testing trials, mice were required to spend a minimum of 10 sec of exploring both objects combined; if this time was not met, the mouse was excluded from analysis. Animals excluded for analysis at PND28 NOR testing are as follows: 2 female control offspring, 1 male control offspring, 3 female gTBI offspring, and 1 male gTBI offspring. Animals excluded for analysis at PND53 NOR testing are as follows: 1 female control offspring, 2 male control offspring. Time spent exploring the objects was calculated by detecting the nose of the animal in the defined sniffing zones adjacent to the objects using EthoVision XT 10 Software (Noldus Information Technology). Discrimination index bounded between 1 and −1 was calculated as follows:
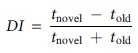
Elevated plus maze
The elevated plus maze (EPM) consisted of four elevated (50 cm) perpendicular arms (50 cm long, 10 cm wide) with two opposing arms containing 30-cm-high opaque walls. EPM testing occurred in the same room as OF and NOR. Two EPMs were spaced 30 cm apart and positioned so that the open arms of one EPM faced the closed arms of the other EPM, so that mice tested concurrently were not visible to each other. On the days of EPM testing, mice were transported on a cart from the housing room and set outside of the testing room until 15 min before they were tested. Mice were brought into the testing room one cage at a time. Each mouse was placed in a closed arm, facing the center of the platform, and given 5 min to explore the EPM. After 5 min elapsed, mice were returned to their home cage. The EPM was cleaned thoroughly between each mouse with quatricide to eliminate odors. EPM performance was recorded and scored using EthoVision XT Software (Noldus Information Technology). One female control offspring, 1 male control offspring, and 1 female gTBI offspring were excluded for improper handling that were then associated with significant outliers in testing at both time points. Additionally, 1 male gTBI offspring was excluded from EPM analysis at PND54.
Tail suspension test
All offspring mice were tested on the tail suspension test (TST) between PND41 and PND42 to assess depressive-like behavior. The tail of an individual mouse was affixed with laboratory tape to a horizontal beam suspended 20 cm from the surface of a table. The beam was divided at 15-cm intervals with a large black Plexiglas divider to block the sight of other mice. Once a mouse was affixed to the beam, video recording was started, and behavior was recorded for 5 min. The amount of time spent immobile was scored by two independent experimenters blind to the treatment conditions. The first minute was excluded from all analyses as an acclimation phase.
Forced swim test
The forced swim test (FST) procedure for the mice was similar to that previously described.53,54 Mice were placed in one of four cylindrical tanks (3000-mL beakers, 30 × 20 cm) filled with room-temperature (23°C–25°C) water at a depth of 15 cm. The water was deep enough so that a mouse could not touch the bottom of the container with either its hindlimbs or tail. Four mice were tested simultaneously, and the four cylindrical tanks were separated by opaque dividers, which made mice not visible to each other. One swim session was conducted for each mouse between PND55 and PND56. A mouse was placed in the water for 5 min and recorded using EthoVision XT Software (Noldus Information Technology). Videos were scored for the time spent swimming and time spent immobile by two independent experimenters who were blinded to treatment conditions.
Blood collection and corticosterone levels
All blood was collected by submandibular vein in BD Microtainer® MAP Microtubes coated with ethylenediaminetetraacetic acid (BD Biosciences, San Jose, CA). Blood draws occurred within 15 min of TST and FST. Collected blood was separated to have 50–75 μL for plasma corticosterone levels and 100 μL for flow cytometry. Blood was spun at 2400 rpm for 10 min at 4°C to separate plasma. Plasma corticosterone levels were determined by enzyme-linked immunosorbent assay (ELISA; ADI-900-097; Enzo Life Sciences, Farmingdale, NY) in triplicate. Plasma samples were warmed to room temperature (23°C) for 30 min and diluted 1:40 with ELISA buffer. Plates were read at a 405-nm wavelength on a plate reader (EPOCH Microplate Spectrophotometer with Gen5 software; Biotek Instruments, Winooski, VT). Mean optical density of blank wells from the plate was subtracted from all readings and averaged between triplicate values. This average level for each animal was used for analysis. Insufficient plasma was collected from 1 female control and therefore excluded from corticosterone measurement.
Flow cytometry
For flow cytometry, 100 μL of fresh, whole blood was immediately blocked with Fc block (1:500) for 10 min and then incubated in the following antibodies: cluster of differentiation 45 (CD45; code, 103112; 1:200; BioLegend, San Diego, CA); cluster of differentiation 11b (CD11b; code, 101208; 1:200; BioLegend); lysine 6C (Ly6C; code, 128033; 1:100; BioLegend); and lysine 6G (Ly6G; code, 127624; 1:200; BioLegend). Blood was then lysed and washed with fluorescence-activated cell sorting (FACS) buffer (1 × Hanks balanced buffer solution treated with 0.5 g of bovine serum albumin, 25 mM of HEPES, and 0.05% sodium azide). 7-Aminoactinomycin D was used as a viability dye. At tissue harvest, spleens were processed through a 70-μm filter with 10 mL of FACS buffer; 100 μL was then collected, blocked, stained, and lysed as described for whole blood. All samples were run immediately after processing to insure a cell viability rate of >90%.
BD FACSCanto II (Becton-Dickinson, Franklin Lakes, NJ) was used to run and analyze all blood and spleen samples. Examples of the gating strategy for blood are shown in Figures 5I and 6E and spleen in Figure 7A. Briefly, 80,000 CD45+ events were collected, and all dead cells were gated out. Then, CD45+ events were gated on CD11b+ events as innate immune cells. Cells were gated on Ly6C and Ly6G to identify monocyte and neutrophil populations, respectively. Events of interest are reported as percent of the population for each cell type. One female control offspring was excluded from terminal spleen and blood analysis as a technical failure.
FIG. 5.
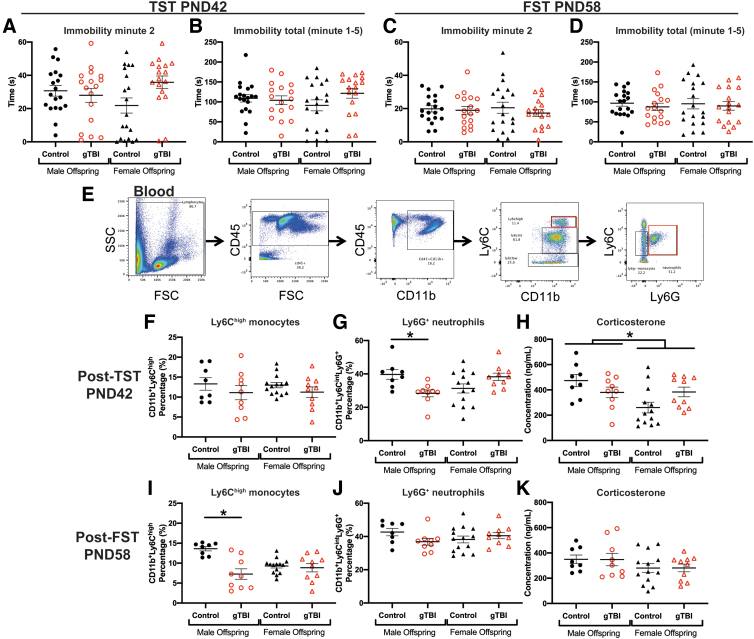
Depressive-like behaviors, immune responses (blood immune cells), and physiological stress (plasma corticosterone) were measured at post-natal day (PND) 42 and PND58. For the tail suspension test (TST) at PND42, (A) immobility during minute 2 and (B) total inactivity showed no effects of gTBI or sex (n = 19 for male control, n = 17 for male gTBI, n = 19 for female control, and n = 17 for female gTBI). For the forced swim test (FST) at PND58, (C) immobility during minute 2 and (D) total immobility time were recorded (n = 19 for male control, n = 17 for male gTBI, n = 19 for female control, and n = 17 female gTBI). To evaluate the physiological immune response to depressive-like behavior testing, flow cytometry was used to quantify blood monocytes and neutrophils as shown in the (E) gating strategy measured by flow. (F) For post-TST measurements, Ly6Chigh monocyte populations were equivalent among groups, whereas (G) Ly6G+ neutrophils were significantly lower in male gTBI offspring compared to control after TST. As the physiological stress response to TST, (H) plasma corticosterone levels showed a significant main effect of sex (n = 8 for male control, n = 9 for male gTBI, n = 13 for female control, and n = 10 for female gTBI). (I) After FST, Ly6Chigh monocytes were significantly lower in male gTBI offspring compared to control after FST, without differences detected in (J) Ly6G+ neutrophils (n = 8 for male control, n = 9 for male gTBI, n = 14 for female control, and n = 10 for female gTBI). (K) Corticosterone levels after FST showed no effects among groups (n = 8 for male control, n = 9 for male gTBI, n = 13 for female control, and n = 10 for female gTBI; two-way ANOVA; +indicates a significant interaction; *p < 0.05). ANOVA, analysis of variance; CD11b, cluster of differentiation 11b; CD45, cluster of differentiation 45; FSC, forward scatter; gTBI, gravida traumatic brain injury; Ly6C, lysine 6C; Ly6G, lysine 6G; SSC, side scatter. Color image is available online.
FIG. 6.
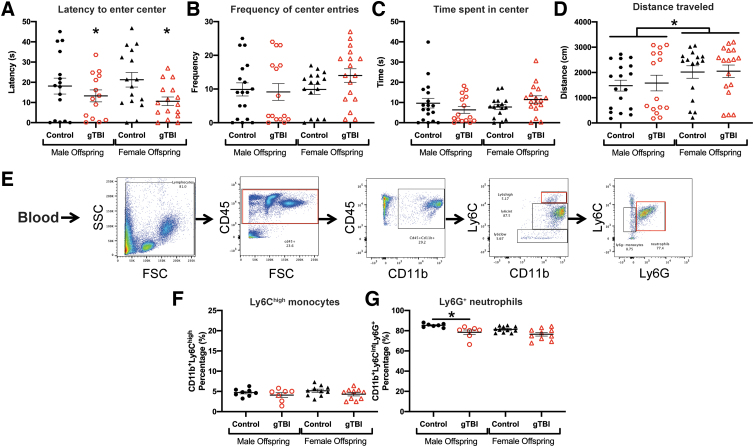
Open field (OF) behavior and immune cell populations were assessed 6 h after an immune challenge with lipopolysaccharide (LPS). (A) Latency to enter the center, (B) number of center entries, and (C) time spent in center were equivalent between groups (n = 18 for male control, n = 15 for male gTBI, n = 16 for female control, and n = 17 female for gTBI). (D) Distance traveled in OF was significantly greater in female offspring than male offspring. (E) Flow cytometry gating strategy for blood monocytes and neutrophils. (F) Ly6Chigh monocyte populations were unchanged among groups. (G) Ly6G+ neutrophil populations in blood were significantly smaller in male gTBI offspring compared to male control offspring (n = 7 for male control, n = 8 for male gTBI, n = 11 for female control, and n = 10 female gTBI; two-way ANOVA; *p < 0.05). ANOVA, analysis of variance; CD11b, cluster of differentiation 11b; CD45, cluster of differentiation 45; FSC, forward scatter; gTBI, gravida traumatic brain injury; Ly6C, lysine 6C; Ly6G, lysine 6G; SSC, side scatter. Color image is available online.
FIG. 7.
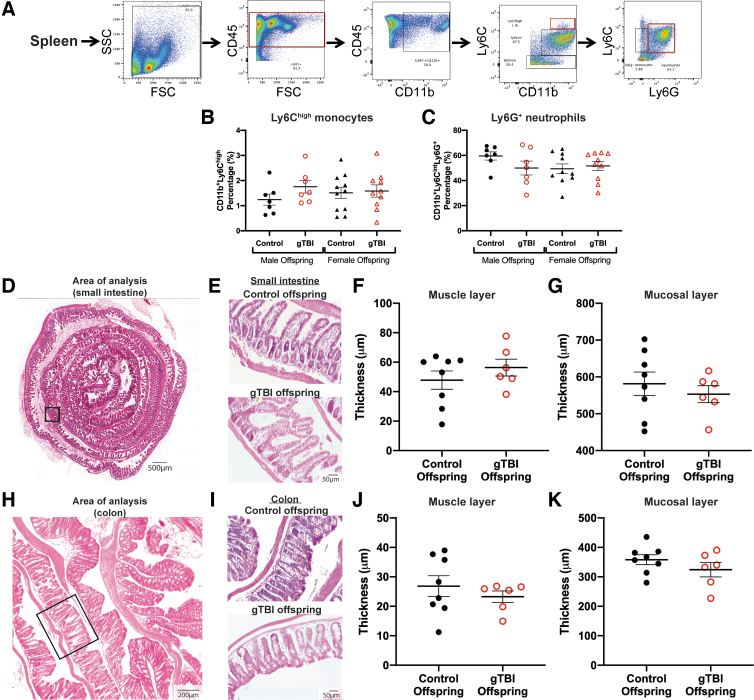
Post-mortem splenic immune cells and gut cytoarchitecture were assessed 24 h after lipopolysaccharide injection. (A) The flow cytometry gating strategy is presented for Ly6Chigh monocytes and Ly6G+ neutrophils in the spleen. (B,C) Neither the populations of monocytes nor neutrophils differed among groups in the spleen (n = 7 for male control, n = 7 for male gTBI, n = 11 for female control, and n = 10 female gTBI). (D) Representative image of the male offspring small intestine as a rolled structure for histological analysis. The box indicates the location of higher magnification images of the small intestine from control and gTBI offspring (E). See Results for a description of the cytoarchitecture. Quantification of (F) muscle layer thickness and (G) mucosal layer thickness for small intestine showed no statistically significant difference between groups. (H) Representative image of the colon of male offspring, with a box to indicate area of analysis (I). Representative images of the colon of male gTBI and control offspring. Quantification of (J) muscle layer thickness (K) and mucosal layer thickness of the colon showed no statistically significant difference between groups (n = 8 for male control, n = 6 for male gTBI). None of the two-way ANOVA results were significant; see Results for details. ANOVA, analysis of variance; CD11b, cluster of differentiation 11b; CD45, cluster of differentiation 45; FSC, forward scatter; gTBI, gravida traumatic brain injury; Ly6C, lysine 6C; Ly6G, lysine 6G; SSC, side scatter. Color image is available online.
Gut histology
Colon sections were prepared as previously described.55 Briefly, the “Swiss rolling” technique was used, and paraffin samples were sectioned at 7 μm. Sections were stained with hematoxylin and eosin for histopathology. Mucosal and muscle thickness were measured (μm) using ImageJ software (NIH, Bethesda, MD). Images were acquired on a Zeiss AXIOImager M2 epifluorescent microscope in bright field (Carl Zeiss Microscopy, Thornwood, NY).
Statistical analysis
A priori power analysis indicated that a sample size of n = 12 per group (sham offspring, gTBI offpsring) was required for a statistical power of 80% for behavioral outcomes. And yet, these power estimation analyses were based on unknown effect sizes for gTBI offspring, which led to an approach to include all mice born to 12 dams. Results from analyses using GraphPad Prism software (version 6; GraphPad Software Inc., La Jolla, CA) are shown as mean ± standard error of the mean, with statistical significance assigned at the 95% confidence level (α < 0.05). Litter sizes and cell outputs for LSPS were analyzed using a two-tailed unpaired t-test. Gut histology and LSPS with glutamate uncaging input were analyzed using a two-way analysis of variance (ANOVA) with Sidak's multiple comparison to compare muscle and mucosal layers and cortical bin depths (1–16), respectively. Sex differences in weaning weight, behavior, and flow cytometry analysis were analyzed using a two-way ANOVA, followed by Tukey's multiple-comparisons test. Resulting test values are included in the Results section, where main effects of TBI and sex and interaction are reported. Between-group significant differences (p < 0.05) after post hoc testing (adjusted p value, q < 0.05) are indicated by an asterisk between groups.
Significant main effects are distinguished by asterisks above gTBI groups (for a main effect of gTBI) and male/female groups (for a main effect of sex) when post hoc tests between individual groups were not significant. Significant interactions with no significant main effects of sex or gTBI are indicated with a plus sign (+) in the figures. Grubb's test with alpha = 0.05 was used to determine outliers, and significant outliers were excluded as follows: 1 female control offspring was excluded from OF at PND28 in the latency and total entries analyses; 1 male gTBI offspring, 1 female control offspring, and 1 female gTBI offspring were excluded from post-LPS OF in the latency analysis.
Data availability statement
Data can be provided upon reasonable request to the corresponding author.
Results
In this study, pregnant females received an mFPI (n = 6) or sham injury as a control (n = 6) at 12 d.p.c. to represent mid-gestational periods. Dams were allowed to give birth naturally before pups were assessed for developmental differences as depicted by the timeline (Fig. 1A). Dams in the gTBI group had a suppression of the righting reflex (294.8 ± 36.9 sec), indicating TBI and is known to include significant increases in peripheral inflammation within the first few hours post-injury.27,48 There were no significant differences in the number of offspring per litter between gTBI and control groups (t(10) = 1.342, p = 0.209; Fig. 1B). There were no significant differences between the number of female and male offspring in either gTBI or control groups (gTBI: F(1, 20) = 0.629, p = 0.437; Sex: F(1, 20) = 0.280, p = 0.603; Interaction: F(1, 20) = 0.000, p = 0.999; Fig. 1C). Mothers were monitored to confirm rearing behavior toward pups and allowed to nurse their own pups to match the clinical presentation of TBI. Surrogacy may introduce additional stresses and confounding factors, which were avoided in the present study. At time of weaning, male gTBI offspring weighed significantly less than male control offspring (gTBI: F(1, 68) = 2.432, p = 0.1235, q = 0.0458) with a significant interaction between gTBI and sex (Interaction: F(1, 68) = 4.905, p = 0.030), but no significant effect of sex alone (Sex: F(1, 68) = 0.149, p = 0.701; Fig. 1D). Overall, gTBI did not disrupt the pregnancy or the birth of live offspring; however, unknown spontaneous abortions may have occurred from the gTBI or anesthesia.
FIG. 1.
Presentation of study design and litter data from gTBI and control offspring. (A) Study design timeline. Briefly, mothers received either diffuse traumatic brain injury (TBI) or sham injury (control) at 12 days post-coitum (d.p.c.) and were allowed to give birth naturally. Offspring were weaned at post-natal day (PND) 21. A subset of offspring was used for laser scanning photostimulation (LSPS) analysis at PND21. Remaining offspring were exposed to behavioral tests over 2 months to cover juvenile and young adult developmental periods, including open field (OF), novel object recognition (NOR), elevated plus maze (EPM), tail suspension test (TST), and forced swim test (FST). Blood draws (BD) were used to quantify immune cell populations by flow cytometry. Plasma corticosterone levels were measured at PND42 and PND58. After 2 months, offspring were given an inflammatory challenge (lipopolysaccharide [LPS]; 1 mg/kg). Mice were tested on OF, and blood was drawn for immune-cell population analysis 6 h post-LPS. Twenty-four hours post-LPS, offspring were euthanatized to collect brain, colon, blood, and spleen. (B) Graph of litter sizes of controls and gTBI groups (n = 6 per group; Studentized t-test showed no significant differences), (C) number of males and females per litter (n = 6 per group), and (D) weaning weights of offspring (n = 19 for control groups and n = 17 for gTBI groups; two-way ANOVA, *p < 0.05). ANOVA, analysis of variance; gTBI, gravida traumatic brain injury. Color image is available online.
Gravida traumatic brain injury resulted in weaker intralaminar connectivity onto pre-frontal pyramidal neurons
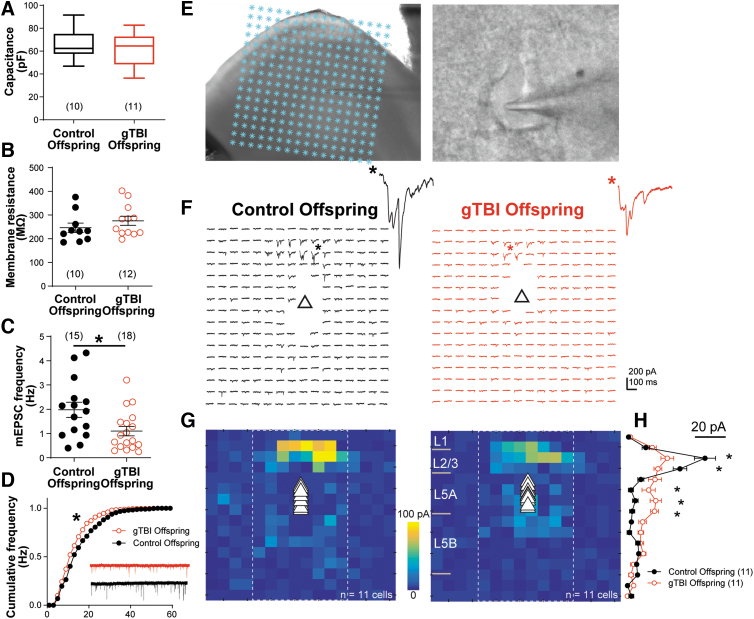
We conducted whole-cell patch clamp recordings to investigate functional changes in layer 5 (L5) projection neurons in the pre-frontal cortex (PFC). We first measured neuronal membrane properties in neurons of gTBI and control offspring. gTBI did not alter L5 PFC neuron membrane capacitance (Control: 65.5 ± 4.2 pF; gTBI: 62.6 ± 4.4 pF; t(19) = 0.48, p = 0.64; Fig. 2A) or input resistance (Control: 246.1 ± 19.7 MΩ; gTBI: 275.5 ± 19.2 MΩ; t(20) = 1.06, p = 0.30; Fig. 2B). The frequency of miniature excitatory post-synaptic currents (mEPSC) was significantly lower in gTBI offspring (1.09 ± 0.19 Hz) than control offspring (1.98 ± 0.31 Hz; t(31) = 2.46, p = 0.019; Fig. 2C). In addition, mEPSC amplitude was significantly reduced in gTBI offspring compared to control offspring (gTBI: D = 0.155, p < 0.05. Fig. 2D). The mEPSC reductions as a result of gTBI prompted us to examine the synaptic connectivity pattern on L5 neurons.
FIG. 2.
gTBI leads to altered synaptic activity and circuit connectivity in pre-frontal cortex (PFC) layer 5 (L5) projection neurons in mixed-sex pups. (A) gTBI does not alter L5 neuron soma capacitance. (B) No statistical difference was found for membrane input resistance. (C) Neurons from gTBI offspring show a significant reduction in miniature excitatory post-synaptic currents (mEPSC) frequency. (D) mEPSC amplitude distribution was significantly reduced in L5 neurons from gTBI offspring (Kolmogorov-Smirnov test on the distribution). (E) Illustration of LSPS combined with glutamate uncaging to map synaptic inputs onto L5 excitatory neurons in the PFC. Asterisks denote laser uncaging locations in a 16 × 16 grid, with a photomicrograph of a patched L5 pyramidal neuron to the right. (F) Representative excitatory response trace maps obtained from an L5 neuron from control (left) and gTBI (right) offspring. Triangle denotes soma location. Synaptic responses not contaminated by the direct soma responses were plotted. (G) Averaged input maps from L5 neurons of control and gTBI offspring (N = 11 neurons for both groups), with soma locations indicated by triangles. (H) Averaged synaptic responses from middle eight column locations (boxed area in G). There is a significant main effect on the group (F(1, 320) = 3.95, two-way ANOVA). Neurons from gTBI offspring showed a marked decrease in L2/L3 inputs (bin 3, p < 0.0001; bin 4, p = 0.002; Sidak's multiple comparison test), with a small yet significant increase in ectopic L5 inputs (bin 6, p < 0.034; bin 7, p = 0.006; bin 8, p = 0.002). *p < 0.05. ANOVA, analysis of variance; gTBI, gravida traumatic brain injury; LSPS, laser scanning photostimulation. Color image is available online.
LSPS combined with glutamate uncaging50,56 was used to map intracortical, excitatory synaptic inputs onto L5 neurons (Fig. 2E). Representative excitatory synaptic response maps to glutamate uncaging in a 16 × 16 cortical location grid from neurons of control and gTBI offspring are shown in Figure 2F. Averaged, pooled response maps are shown in Figure 2G (Control: n = 11 cells; gTBI: n = 11 cells), which indicate that major synaptic inputs were from L2/L3 locations. Notably, synaptic inputs to neurons of gTBI offspring increased from L5 locations, indicating ectopic, disrupted connectivity. Synaptic responses from the middle eight columns were averaged to eliminate negligible inputs from the peripheral eight columns and then plotted based on the binned laminar locations in the cortex (Fig. 2H). Overall, synaptic inputs were significantly different between neurons of control and gTBI offspring (F(1, 320) = 3.954, p = 0.048). Further analysis, using Sidak's multiple comparisons test, indicated that neurons of gTBI offspring had a marked decrease in L2/L3 inputs in bins 3 and 4, with a small yet significant increase in ectopic L5 inputs in bins 6–8. Thus, gTBI altered synaptic activity and connectivity in a major class of projection neurons in the PFC.
Gravida traumatic brain injury spared novel object recognition–associated cognitive performance, but disturbed anxiety-related behavior, as measured in open field
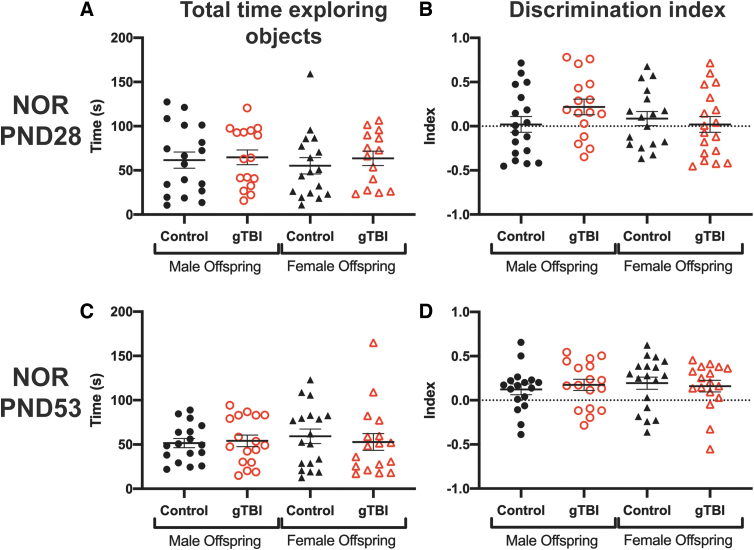
Cognitive performance was evaluated when the offspring were juvenile (PND28) and young adult (PND53) using NOR. For all behaviors, exclusion criteria are listed in the Methods. At PND28, there were no significant differences in total time spent exploring both objects among any groups attributable to gTBI (F(1, 61) = 0.420, p = 0.519) or sex (F(1, 61) = 0.178, p = 0.675) and no significant interaction between gTBI and sex (F(1, 61) = 0.089, p = 0.767; Fig. 3A). Exploration time between objects, calculated as a discrimination index, was not different among any groups because of gTBI (F(1, 61) = 0.168, p = 0.683) or sex (F(1, 61) = 0.850, p = 0.360), and there was no significant interaction (F(1, 61) = 2.544, p = 0.116; Fig. 3B). At PND53, there were no significant differences in total time spent exploring the objects among groups attributable to gTBI (F(1, 65) = 0.070, p = 0.792) or sex (F(1, 65) = 0.176, p = 0.676) and no significant interaction between gTBI and sex (F(1, 65) = 0.348, p = 0.557; Fig. 3C). The discrimination index was not significantly different among groups attributable to gTBI (F(1, 65) = 0.016, p = 0.898) or sex (F(1, 65) = 0.186, p = 0.668), and no significant interaction was found (F(1, 65) = 0.415, p = 0.522; Fig. 3D).
FIG. 3.
Cognitive behavior was assessed using novel object recognition (NOR) tasks. (A) Total time spent exploring both objects and (B) discrimination ratios at post-natal day (PND) 28 are presented (n = 18 for male control, n = 16 for male gTBI, n = 16 for female control, and n = 14 female gTBI). NOR was repeated at PND53, where (C) total time spent exploring both objects and (D) discrimination ratios are presented (n = 17 for male control, n = 17 for male gTBI, n = 18 for female control, and n = 17 for female gTBI). None of the two-way ANOVA results were significant; see Results for details. ANOVA, analysis of variance; gTBI, gravida traumatic brain injury. Color image is available online.
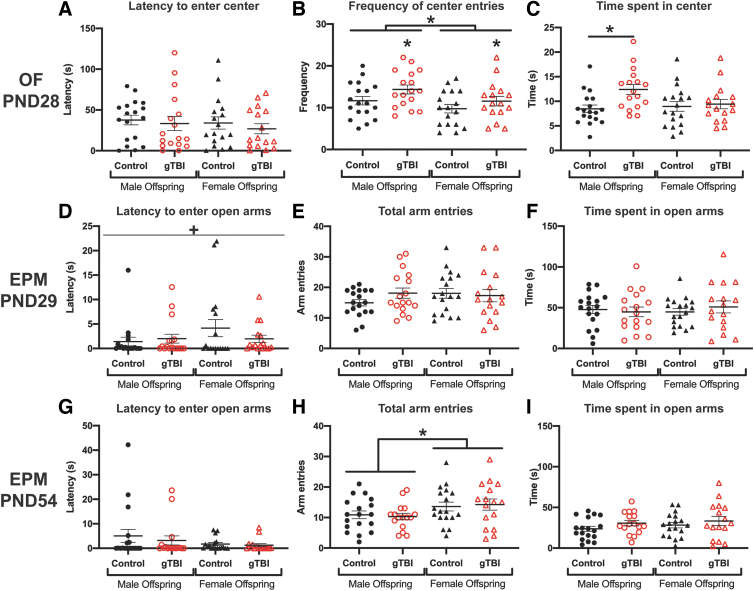
Anxiety-related behavior was assessed by OF and EPM performance. At PND28, latency to enter the center of the OF was not different among any groups attributable to gTBI (F(1, 64) = 0.624, p = 0.433) or sex (F(1, 64) = 0.491, p = 0.486), and no significant interaction between gTBI and sex was detected (F(1, 64) = 0.036, p = 0.850; Fig. 4A). gTBI offspring entered the center of the arena significantly more times than the control offspring (F(1, 64) = 4.465; p = 0.039), and males entered the center more frequently compared to females (F(1, 64) = 4.890, p = 0.031), without a significant interaction between gTBI and sex (F(1, 64) = 0.175, p = 0.677; Fig. 4B). Total time spent in the center of the arena by male gTBI offspring was longer than male control offspring, with an overall effect of gTBI (gTBI: F(1, 65) = 5.570, p = 0.021, q = 0.0195), but no significant differences attributable to sex (F(1, 65) = 1.797, p = 0.185) and no significant interaction (F(1, 65) = 3.420, p = 0.069; Fig. 4C). There were no significant differences in distance traveled in OF attributable to gTBI (F(1, 64) = 0.311, p = 0.579) or sex (F(1, 64) = 3.843, p = 0.054; data not shown).
FIG. 4.
Anxiety-related behaviors were measured in the open field (OF) and elevated plus maze (EPM). (A) Latency to enter center, (B) number of center entries, and (C) total time spent in the center of the OF are presented at post-natal day (PND) 28 (n = 18 for male control, n = 17 for male gTBI, n = 17 for female control, and n = 17 for female gTBI). For the EPM at PND29, (D) latency to enter open arms, (E) total arm entries, and (F) total time spent in open arms showed no gTBI or sex effects (n = 18 for male control, n = 17 for male gTBI, n = 17 for female control, and n = 17 female for gTBI). For the EPM at PND54, (G) latency to enter open arms, (H) total arm entries, and (I) total time spent in open arms showed a significant sex effect for total arm entries as detailed in the Results (n = 18 for male control, n = 16 for male gTBI, n = 17 for female control, and n = 17 for female gTBI; two-way ANOVA; +indicates a significant interaction; *p < 0.05). ANOVA, analysis of variance; gTBI, gravida traumatic brain injury. Color image is available online.
As juveniles (PND29), offspring showed no significant differences in latency to enter an open arm of the EPM attributable to gTBI (F(1, 65) = 0.334, p = 0.565) or sex (F(1, 65) = 2.037, p = 0.158; Fig. 4D); however, a significant interaction between gTBI and sex emerged for latency to enter open arms (F(1, 65) = 4.173, p = 0.045). To confirm that anxiety-related behavioral measurements were not confounded by increased activity, total arm entries were counted. For the total trial, the total number of arm entries (open and closed) were equivalent among groups attributable to gTBI (F(1, 65) = 0.579, p = 0.450) or sex (F(1, 65) = 0.521, p = 0.473), with no significant interaction (F(1, 65) = 1.503, p = 0.225, Fig. 4E). However, the time spent in the open arms were not different among any groups attributable to gTBI (F(1, 65) = 0.082, p = 0.778) or sex (F(1, 65) = 0.085, p = 0.778), with no significant interaction (F(1, 65) = 0.634, p = 0.429; Fig. 4F). As young adults (PND54), offspring showed no significant differences in latency to enter open arms among any groups attributable to gTBI (F(1, 64) = 0.462, p = 0.499) or sex (F(1, 64) = 2.370, p = 0.129), with no significant interaction (F(1, 64) = 0.167, p = 0.684; Fig. 4G). Despite no significant differences in total number of arm entries among groups attributable to gTBI (F(1, 64) = 0.0005, p = 0.983), male offspring had fewer arm entries than female offspring (F(1, 64) = 5.463, p = 0.023).
No significant interaction was found between gTBI and sex for total arm entries (F(1, 64) = 0.182, p = 0.671; Fig. 4H). All offspring spent equivalent amounts of time in the open arms, without significant differences among groups attributable to gTBI (F(1, 64) = 2.381, p = 0.128) or sex (F(1, 64) = 0.868, p = 0.355), with no significant interaction (F(1, 64) = 0.064, p = 0.802; Fig. 4I). Overall, offspring showed differences in anxiety-like behavior, which depended on both gTBI (in OF alone) and sex (in both OF and EPM).
Gravida traumatic brain injury marginally affected depressive-like behaviors in offspring
As young adults, gTBI offspring may harbor affective symptoms related to disturbed, disrupted, or deviated neurodevelopment. TST and FST evaluated depressive-like behaviors. Minute 1 of TST was considered acclimation, and in minute 2 there were no significant differences in time spent immobile among any groups attributable to gTBI (F(1, 68) = 2.048, p = 0.157) or sex F(1, 68) = 0.016, p = 0.901), and with a significant interaction (F(1, 68) = 4.448, p = 0.039; Fig. 5A). However, post hoc tests revealed no significant differences among any groups. Similarly, for the entire testing period, there were no significant differences in immobility among any groups attributable to gTBI (F(1, 68) = 1.138, p = 0.290) or sex (F(1, 68) = 0.0001, p = 0.992), with no significant interaction (F(1, 68) = 2.221, p = 0.141; Fig. 5B). As a behavioral stressor, plasma corticosterone levels were expected to be above the published resting value of 50–150 ng/mL by 15 min after the start of TST.57 Two weeks later, at PND58, the FST evaluated depressive-like behavior.
There were no significant differences in the amount of time spent immobile between gTBI and control offspring during the second minute of the FST (F(1, 68) = 0.6669, p = 0.417), and there was also no effect of sex (F(1, 68) = 0.04357, p = 0.8353) or interaction between gTBI and sex (F(1, 68) = 0.2181, p = 0.6420: Fig. 5C). During the total testing period (minutes 1–5) no significant differences were found among any groups attributable to gTBI (F(1, 68) = 0.4891, p = 0.4867) or sex (F(1, 68) = 0.002, p = 0.9614), with no significant interaction (F(1, 68) = 0.03, p = 0.863; Fig. 5D). Overall, there were marginal differences in depressive-like behaviors between gTBI and control offspring.
Depressive-like behavior was associated with an immune response, but no difference in stress-associated markers; gravida traumatic brain injury influenced blood neutrophil and monocyte populations after the tail suspension test and forced swim test, respectively
We measured the physiological response of inflammation and stress after both TST and FST. Given that even acute stress can increase monocyte recruitment and cytokine expression,58 blood neutrophil and monocyte populations were quantified by flow cytometry after TST and FST to assess behavioral stress-induced shifts in immune cells. The gating strategy for Ly6Chigh monocytes and Ly6G+ neutrophils is shown in Figure 5E. At PND42 after TST, there were no significant differences in monocyte populations among any groups attributable to gTBI (F(1, 37) = 2.333, p = 0.135) or sex (F(1, 37) = 0.005, p = 0.945), with no significant interaction (F(1, 37) = 0.0281, p = 0.868; Fig. 5F). Post hoc tests indicated that male gTBI offspring had significantly smaller populations of neutrophils than male control offspring based on the significant interaction between gTBI and sex (F(1, 37) = 11.830, p = 0.002, q = 0.395, Fig. 5G), without main effects of gTBI (F(1, 37) = 0.655, p = 0.424) or sex (F(1, 37) = 0.086, p = 0.771). As a behavioral stressor, plasma corticosterone levels were expected to be above the published resting value of 50–150 ng/mL by 15 min after the start of TST.57 There were no significant differences in plasma corticosterone levels among any groups attributable to gTBI (F(1, 36) = 0.119, p = 0.732), but males had higher plasma levels of corticosterone than females after TST (F(1, 36) = 5.769, p = 0.022), and there was a significant interaction between gTBI and sex (F(1, 36) = 6.154, p = 0.018; Fig. 5H).
At PND58 after FST, the monocyte populations were significantly smaller in male gTBI offspring compared to male control offspring, with a significant effect of gTBI (F(1, 37) = 14.710, p = 0.001, q = 0.0002) and an interaction between gTBI and sex (F(1, 37) = 11.210, p = 0.002; Fig. 5I) in the absence of a main effect of sex (F(1, 37) = 2.440, p = 0.127). There were no significant differences in neutrophil populations after FST among any groups attributable to gTBI (F(1, 37) = 0.691, p = 0.411) or sex (F(1, 37) = 0.037, p = 0.849), with no significant interaction (F(1, 37) = 3.622, p = 0.065; Fig. 5J). In response to the 5 min of FST stress, there were no significant differences in plasma corticosterone levels among any groups attributable to gTBI (F(1, 36) = 0.0003, p = 0.987) or sex (F(1, 36) = 3.093, p = 0.087), with no significant interaction (F(1, 36) = 0.001, p = 0.978; Fig. 5K).
Gravida traumatic brain injury altered the latency to enter the center of the open field and neutrophil populations in response to immune challenge
Administration of an exogenous immune stressor affected depressive-like behavior and the physiological immune response. An i.p. injection of LPS (0.1 mg/kg) was delivered 24 h before euthanasia. Anxiety and mobility were evaluated in the OF and circulating monocytes and neutrophils quantified in blood. Latency to enter the center of the OF was significantly decreased in gTBI offspring compared to control offspring (F(1, 59) = 4.751, p = 0.033), without a significant main effect of sex (F(1, 59) = 0.015, p = 0.905) or an interaction between gTBI and sex (F(1, 59) = 0.481, p = 0.491; Fig. 6A). The number of entries to the center of the OF showed no main effect of gTBI (F(1, 62) = 0.710, p = 0.403) or sex (F(1, 62) = 1.472, p = 0.230), with no significant interaction (F(1, 62) = 1.489, p = 0.227; Fig. 6B). No significant differences were found in time spent in the center of the OF attributable to gTBI (F(1, 62) = 0.0120, p = 0.913) or sex (F(1, 62) = 0.726, p = 0.398), with no significant interaction (F(1, 62) = 3.400, p = 0.070; Fig. 6C). Females traveled significantly further distances during the OF task than males (F(1, 62) = 3.968, p = 0.051), without a main effect of gTBI (F(1, 62) = 0.070, p = 0.792) or interaction between gTBI and sex (F(1, 62) = 0.019, p = 0.891; Fig. 6D).
After the OF, the peripheral, innate immune response was quantified in offspring at 6 h post-LPS as the percentage of monocytes and neutrophils among myeloid cells. The gating strategy for Ly6Chigh monocytes and Ly6G+ neutrophils from CD45+ myeloid cells is shown in Figure 6E. Though there was a drop in Ly6Chigh monocyte populations in the blood attributable to LPS, we found no significant differences attributable to gTBI (F(1, 31) = 2.708, p = 0.110) or sex (F(1, 31) = 0.562, p = 0.459), with no significant interaction (F(1, 31) = 0.065, p = 0.801; Fig. 6F). Similar to the neutrophil response to TST, neutrophil populations in male gTBI offspring were significantly smaller than male control offspring (F(1, 31) = 14.660, p = 0.001, q = 0.0323). Neutrophil populations were not significantly different among groups attributable to sex (F(1, 31) = 3.714, p = 0.063), and no significant interaction between gTBI and sex was found (F(1, 31) = 0.448, p = 0.509; Fig. 6G). Overall, gTBI offspring had significantly smaller blood neutrophil populations than control offspring.
Post-mortem analysis of the spleen and gut showed no effect of gravida traumatic brain injury
To extend investigations of gTBI and subsequent immune challenge, the spleen and gut mucosa were analyzed. As part of the immune system, the spleen houses several types of immune cells that are released into the circulation during infection.59 Using flow cytometry, splenic immune cells were gated for Ly6Chigh monocytes and Ly6G+ neutrophils using the strategy shown in Figure 7A. In spleen, 24 h post-LPS, populations of Ly6Chigh monocytes showed no main effect attributable to gTBI (F(1, 30) = 1.03, p = 0.318) or sex (F(1, 30) = 0.170, p = 0.684), with no significant interaction (F(1, 30) = 1.243, p = 0.274; Fig. 7B). There were no significant differences in Ly6G+ neutrophils among any groups attributable to gTBI (F(1, 30) = 0.783, p = 0.383) or sex (F(1, 30) = 1.063, p = 0.311), with no significant interaction (F(1, 30) = 2.033, p = 0.164; Fig. 7C).
Given that male gTBI offspring showed behavioral and inflammatory responses that differed from male control offspring, the small intestine and colon from a subset of male mice were analyzed to confirm that behavioral performance and inflammatory responses were not associated with gut cytological disruption. Representative images of the small intestine showed defined smooth muscle layers, no signs of inflammation (e.g., thickness or edema in the muscle), and intact microvilli (e.g., length, structure, no cellular infiltration; Fig. 7D,E). No significant differences were identified between gTBI and control male offspring in the quantification of the thickness of the small intestine muscle layer (t(12) = 0.973, p = 0.350; Fig. 7F) and mucosal layer (t(12) = 0.675, p = 0.513; Fig. 7G). For the colon, representative images show intact cytoarchitecture of the mucosal layer, no infiltration of immune or blood cells, and the absence of edema (Fig. 7H,I). No significant differences were found in thickness of the colon muscle layer (t(12) = 0.806, p = 0.436; Fig. 7J) or mucosal layer (t(12) = 1.181, p = 0.261; Fig. 7K) attributable to gTBI. Thus, gTBI offspring differed from control in the acute inflammatory response phase at 6 h post-LPS during the peak neutrophil response and not 24 h post-LPS when the neutrophil response had resolved.
Discussion
gTBI as a result of IPV, motor vehicle collisions, or falls is grossly neglected in medical research, establishing healthcare disparities among minority and under-represented populations.5,8,60,61 This study is the first to show that an isolated TBI in pregnant mice alters cortical functional connectivity, anxiety-related behaviors, and immunity in offspring spanning juvenile and young adulthood stages. With overarching goals to develop diagnostics, interventions, and therapeutics for gTBI, the approach was to determine 1) whether offspring from diffuse brain-injured mothers were viable and 2) whether chronic neurological and immunological conditions were evident in gTBI offspring.
We found significantly weaker intralaminar connectivity from the L2/L3 locations onto layer 5 pre-frontal cortical neurons in gTBI offspring compared to control offspring. In contrast, inputs from ectopic L5 locations were increased. Synaptic responses and connectivity may be an underlying gTBI-induced circuit pathology not recognized previously. The gTBI offspring had altered immune responses after behavioral stressors and an inflammatory challenge, where gTBI offspring had significantly smaller blood neutrophil populations than control offspring. Decreased anxiety-like and marginal changes in depressive-like behaviors were observed between gTBI and control offspring. Last, no significant differences were noted in small intestine or colon cytoarchitecture between gTBI and control offspring. Thus, gTBI has developmental and chronic consequences for offspring that justify further translational and clinical research to confirm the conditions under which gTBI has adverse outcomes for mothers and their offspring.
In this study, gTBI mice gave birth to viable offspring with comparable numbers to control mice. At weaning, however, male gTBI offspring weighed significantly less than male control offspring. Lower weight at weaning is correlated to birth weight and overall health in mice.62–64 Further, low weaning weight in males leads to subsequent weight gain and possible glucose intolerance.62 Clinically, low birth weights are associated with reduced cognitive performance, educational achievement, and socioeconomic advancement.65,66 It remains to be determined whether low birth and weaning weights would be linked to maternal care and nursing from a mother recovering from TBI or the gestational disruption of TBI. However, if low birth weights were attributable entirely to maternal care, similar differences in weaning weight would have been expected in gTBI female offspring compared to controls. Further, low weaning weights were indicative of the longer-term consequences of gTBI in male offspring observed as anxiety-like behavior and altered immune responses, wherein female gTBI offspring were not significantly different than female control offspring.
Cortical connectivity and synaptic alterations could be the structural etiology for behavioral impairments. A subset of offspring was assessed for functional connectivity in layer 5 cortical neurons. Excitatory projections were reduced distally and increased proximally in neurons of gTBI offspring compared to control offspring at 3 weeks of age. The redistribution of inputs was associated with a reduced number of synaptic contacts as measured by a significant decrease in mESPC frequency.67,68 A similar reduction in functional connectivity in development arises from dendritic spine defects.69,70 In a mouse model of ASD, reduced excitatory synaptic input was reported on to L2/L3 neurons in the medial PFC68 and other ASD models found hyperconnectivity in the neocortex.50
Toxicity during gestation (carbon metabolism dysregulation) reduced excitatory synaptic connections in the hippocampus and elicited schizophrenia-like behavior in offspring.71 Those offspring had deficits in NOR and social interactions along with locomotor hyperactivity.71 Although the present study did not identify significant deficits in NOR, future studies should evaluate social behavior. In post-mortem brains of persons diagnosed with schizophrenia, aberrant connectivity, including reduced synaptic connectivity, was reported in the cortex; however, functional studies still need to determine the contribution of neural circuit dysfunction to the schizophrenia phenotype.72 The gTBI-related change in cortical connectivity, and potentially other unexplored regions, may explain aberrant behavior expression in gTBI offspring.
The blunted functional cortical connectivity in mixed-sex gTBI offspring compared to control led to moderate anxiety-related and marginal depressive-like behavior performance impairments in juvenile and adulthood mice. For anxiety-related behaviors, male gTBI offspring entered the center of the OF often and spent more time there compared to male control offspring, indicative of reduced anxiety or elevated risk-taking. Murine anxiety is associated with increased thigmotaxis and a decreased number of entries to the center and time spent there,73,74 although, in models of ASD and schizophrenia, mice increase exploratory behavior in the OF,75–77 perhaps for similar reasons as male gTBI offspring. Although NOR did not identify cognitive impairment, more sensitive or difficult tests may uncover cognitive dysfunction. Given that cognition was assessed during juvenile and young adult stages, the impact of gTBI may emerge later in life with cognitive aging. At PND54 during EPM testing, male offspring had fewer total arm entries in the EPM than female offspring, without significant differences in other aspects of anxiety-like behavior between gTBI, sex, and time. Therefore, anxiety measures for females may require refinement to account for increased activity. The complexity of neurodevelopment and the coarse behavioral assessments may obscure gTBI effects in subsets of offspring or emerge with gTBI at different gestational stages. The cortical connectivity data, coupled with the behavioral phenotype, indicate a combination of structural and functional consequences of gTBI.
Immune function of offspring is shaped by maternal health and then the environment.12,45 For gTBI offspring, early effects on the immune system could arise from gTBI-induced inflammation and then nursing from a brain-injured mother. Later in life, immune function was assessed in response to behavioral stressors and a secondary immune challenge. Overall, gTBI offspring showed decreased myeloid cell responses compared to control offspring, specifically in males. Acute stressors initiate shifts in blood monocyte and neutrophil populations within minutes to days of the stressor.78,79 For example, acute restraint stress in the mouse increased neutrophil populations 45–75% within minutes to hours of the stressor.79 The immune response to stressors is exposure specific, given that repeated FST did not significantly change monocyte or neutrophil populations, which may discriminate acute from chronic immune responses.79
In this study, neutrophil recruitment was lower in male gTBI offspring compared to male control offspring after TST, indicating a blunted immune response to an acute stressor. And yet, monocytes were lower after FST in male gTBI offspring compared to male control offspring. This blunted response to a chronic stressor may be attributable to either repeated blood draw or potentially from the previous stressor.58,80 In a direct challenge of offspring immune function, myeloid cell populations were quantified at 6 and 24 h after LPS administration.81 Again, the recruited population of neutrophils was lower in male gTBI offspring than control, which may represent chronic immune disease or disorder throughout life.82 Sex differences related to immunity may protect female gTBI offspring and remains for future investigation.
Sex was a biological variable throughout this study, because the timeline transitioned through puberty and immune function is sexually dimorphic. Male gTBI offspring were principal drivers of the observed effects on gTBI on health, physiology, and behavior. Specifically, male gTBI offspring, compared to male control offspring, had lower weaning weights, spent more time in the OF, had less neutrophil and monocyte populations post-TST and post-FST, respectively, and had lower neutrophil populations 6 h post-LPS. Female gTBI offspring were different to female control offspring only in FST immobility and OF latency after LPS administration. In direct comparison, female offspring traveled further distances in the OF after LPS injections compared to male offspring. The observed sex differences add to documented sex differences in neurobehavior, specifically in anxiety-related tasks.83–85 As such, gTBI may selectively affect behavioral performance in male offspring more than female offspring. Physiologically, female offspring had lower corticosterone levels after TST than male offspring regardless of gTBI.
Hormone exposure during pregnancy, which is modified by gestation and TBI, may protect female offspring and harm male offspring and remains to be determined. Clinically, sex differences are evident in disease and psychiatric disorders, with males over-represented in antisocial personalities, ASD, and alcoholism and with females at risk for anxiety and depression diagnoses.86,87 gTBI may be one among many environmental factors that differentially affect the sexes and influence the phenotypic expression of disease and psychiatric disorders, as supported by blunted functional cortical connectivity and immune dysregulation.45,88–90 For future gTBI investigations, sex must remain as a biological variable to discriminate gestational and developmental contributions to disease and psychiatric outcomes.
This study represents the initial analyses of physiological, behavioral, functional, and immunological approaches to uncover the impact of gTBI on offspring. For each litter, the number and combinations of outcome measures are limited. Additional tests that complete the neurological (and psychiatric) profile of gTBI offspring and branch into other organ systems are warranted to uncover the wholistic impact of gTBI. The consequences of gTBI at other gestational stages remain unknown, including gTBI throughout gestation. Early life stressors can increase anxiety symptoms, abnormal hypothalamic-pituitary-adrenocortical (HPA) axis responsivity, and alter brain connectivity and structure in newborn mice.91–93 Future studies can explore gTBI effects on HPA axis function, the consequences for puberty, and the neurological impairments carried into adulthood. Future studies will also determine whether peripheral inflammation and other pathophysiology from the brain-injured pregnant mother can cross the blood–placenta barrier to influence development of all organ systems. For example, gut microbiota diversity may indicate additional lifelong consequences of gTBI given that maternal gut health can influence fetal development.94 The current study provides the scientific rationale to continue investigations into gTBI effects on offspring.
Conclusion
TBI research must be fluid and include vulnerable populations, including pregnant woman affected by IPV, given that they have increased risk for TBI and thereby adverse fetal outcomes. The results of this study begin the conversation to reduce gaps in medical research. In sum, an isolated TBI during pregnancy can disrupt fetal development, as measured by overall health, physiology, behavior, and immune function. gTBI offspring are viable, with subtle behavioral differences, alterations in cortical connectivity, and altered immune function. Thus, in utero exposure and/or rearing by a brain-injured mother may lead to at-risk offspring. Ultimately, further pre-clinical and clinical research may indicate that gTBI affects multiple lives because of the post-injury sequelae.
Acknowledgments
The authors thank the Flow Cytometry Core at the University of Arizona College of Medicine–Phoenix for use of the Canto II. The authors thank Sebastian Tellez for assisting with forced swim and tail suspension scoring, Umar S. Aftab for assisting with tail suspension scoring, and Ahmed Dudic for assisting with gut histology.
Authors' Contributions
Maha Saber was responsible for conceptualization, methodology, study design, leading data collection, data curation, and writing the manuscript. J. Bryce Ortiz was responsible for methodology, study design, data collection and writing and editing the manuscript. Xiaokuang Ma was responsible for assisting in data collection and writing and reviewing the manuscript. Luisa M. Rojas Valencia was responsible for methodology, study design, data collection and writing and reviewing the manuscript. Bret R. Tallent was responsible for study design, methodology, and reviewing the manuscript. P. David Adelson was responsible for funding acquisition and reviewing the manuscript. Rachel K. Rowe was responsible for study design, funding acquisition, and reviewing and editing the manuscript. Shenfeng Qiu was responsible for methodology, data collection, data curation, funding acquisition, and writing, reviewing, and editing the manuscript. Jonathan Lifshitz was responsible for initial conceptualization, study design, data interpretation, writing, reviewing, and editing the manuscript, and funding acquisition.
Funding Information
This research was funded, in part, by Phoenix Children's Hospital mission support, Arizona Alzheimer's Consortium, Fraternal Order of Eagles, and NIH T32-AG044402.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Centers for Disease Control and Prevention (CDC). (2010). Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. Centers for Disease Control and Prevention: Atlanta, GA. [Google Scholar]
- 2. Plancikova, D., Leitgeb, J., Brazinova, A., Melichova, J., Sivco, P., Nemcovska, E., Pekarcikova, J., and Majdan, M. (2020). Characteristics and outcome of severe traumatic brain injuries based on occupational status. Eur. J. Trauma Emerg. Surg. doi: 10.1007/s00068-020-01372-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention (CDC). (2016). Rates of TBI-related emergency department visits by sex—United States, 2001–2010. Centers for Disease Control and Prevention: Atlanta, GA. [Google Scholar]
- 4. Spani, C.B., Braun, D.J., and Van Eldik, L.J. (2018). Sex-related responses after traumatic brain injury: Considerations for preclinical modeling. Front. Neuroendocrinol. 50, 52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zieman, G., Bridwell, A., and Cardenas, J.F. (2017). Traumatic brain injury in domestic violence victims: a retrospective study at the Barrow Neurological Institute. J. Neurotrauma 34, 876–880. [DOI] [PubMed] [Google Scholar]
- 6. Black, M.C., Basile, K.C., Breiding, M.J., Smith, S.G., Walters, M.L., Merrick, M.T., Chen, J., and Stevens, M.R. (2011). The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention: Atlanta, GA. [Google Scholar]
- 7. Bailey, B.A. (2010). Partner violence during pregnancy: prevalence, effects, screening, and management. Int. J. Womens Health 2, 183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lutgendorf, M.A. (2019). Intimate partner violence and women's health. Obstet. Gynecol. 134, 470–480. [DOI] [PubMed] [Google Scholar]
- 9. Liu, Y.Z., Wang, Y.X., and Jiang, C.L. (2017). Inflammation: the common pathway of stress-related diseases. Front. Hum. Neurosci. 11, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hava, G., Vered, L., Yael, M., Mordechai, H., and Mahoud, H. (2006). Alterations in behavior in adult offspring mice following maternal inflammation during pregnancy. Dev. Psychobiol. 48, 162–168. [DOI] [PubMed] [Google Scholar]
- 11. Mazina, V., Gerdts, J., Trinh, S., Ankenman, K., Ward, T., Dennis, M.Y., Girirajan, S., Eichler, E.E., and Bernier, R. (2015). Epigenetics of autism-related impairment: copy number variation and maternal infection. J. Dev. Behav. Pediatr. 36, 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hantsoo, L., Kornfield, S., Anguera, M.C., and Epperson, C.N. (2019). Inflammation: a proposed intermediary between maternal stress and offspring neuropsychiatric risk. Biol. Psychiatry 85, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patterson, P.H. (2011). Maternal infection and immune involvement in autism. Trends Mol. Med. 17, 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zerbo, O., Qian, Y., Yoshida, C., Grether, J.K., Van de Water, J., and Croen, L.A. (2015). Maternal infection during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 45, 4015–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim, S., Kim, H., Yim, Y.S., Ha, S., Atarashi, K., Tan, T.G., Longman, R.S., Honda, K., Littman, D.R., Choi, G.B., and Huh, J.R. (2017). Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549, 528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shin Yim, Y., Park, A., Berrios, J., Lafourcade, M., Pascual, L.M., Soares, N., Yeon Kim, J., Kim, S., Kim, H., Waisman, A., Littman, D.R., Wickersham, I.R., Harnett, M.T., Huh, J.R., and Choi, G.B. (2017). Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature 549, 482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reed, M.D., Yim, Y.S., Wimmer, R.D., Kim, H., Ryu, C., Welch, G.M., Andina, M., King, H.O., Waisman, A., Halassa, M.M., Huh, J.R., and Choi, G.B. (2020). IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature 577, 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caine, E.A., Jagger, B.W., and Diamond, M.S. (2018). Animal models of zika virus infection during pregnancy. Viruses 10, 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khandaker, G.M., Zimbron, J., Lewis, G., and Jones, P.B. (2013). Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol. Med. 43, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwartz, D.A., and Graham, A.L. (2020). Potential maternal and infant outcomes from (Wuhan) Coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses 12, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rice, M.E., Galang, R.R., Roth, N.M., Ellington, S.R., Moore, C.A., Valencia-Prado, M., Ellis, E.M., Tufa, A.J., Taulung, L.A., Alfred, J.M., Pérez-Padilla, J., Delgado-López, C.A., Zaki, S.R., Reagan-Steiner, S., Bhatnagar, J., Nahabedian, J.F. III, Reynolds, M.R., Yeargin-Allsopp, M., Viens, L.J., Olson, S.M., Jones, A.M., Baez-Santiago, M.A., Oppong-Twene, P., VanMaldeghem, K., Simon, E.L., Moore, J.T., Polen, K.D., Hillman, B., Ropeti, R., Nieves-Ferrer, L., Marcano-Huertas, M., Masao, C.A., Anzures, E.J., Hansen, R.L.Jr., Perez-Gonzalez, S.I., Espinet-Crespo, C.P., Luciano-Román, M., Shapiro-Mendoza, C.K., Gilboa, S.M., and Honein, M.A. (2018). Vital signs: zika-associated birth defects and neurodevelopmental abnormalities possibly associated with congenital zika virus infection—U.S. territories and freely associated states, 2018. MMWR Morb. Mortal. Wkly. Rep. 67, 858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coussons-Read, M.E. (2013). Effects of prenatal stress on pregnancy and human development: mechanisms and pathways. Obstet. Med. 6, 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Su, Q., Zhang, H., Zhang, Y., Zhang, H., Ding, D., Zeng, J., Zhu, Z., and Li, H. (2015). Maternal stress in gestation: birth outcomes and stress-related hormone response of the neonates. Pediatr. Neonatol. 56, 376–381. [DOI] [PubMed] [Google Scholar]
- 24. Jasarevic, E., and Bale, T.L. (2019). Prenatal and postnatal contributions of the maternal microbiome on offspring programming. Front. Neuroendocrinol. 55, 100797. [DOI] [PubMed] [Google Scholar]
- 25. Ritzel, R.M., Doran, S.J., Barrett, J.P., Henry, R.J., Ma, E.L., Faden, A.I., and Loane, D.J. (2018). Chronic alterations in systemic immune function after traumatic brain injury. J. Neurotrauma 35, 1419–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schwulst, S.J., Trahanas, D.M., Saber, R., and Perlman, H. (2013). Traumatic brain injury-induced alterations in peripheral immunity. J. Trauma Acute Care Surg. 75, 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saber, M., Giordano, K.R., Hur, Y., Ortiz, J.B., Morrison, H., Godbout, J.P., Murphy, S.M., Lifshitz, J., and Rowe, R.K. (2019). Acute peripheral inflammation and post-traumatic sleep differ between sexes after experimental diffuse brain injury. Eur. J. Neurosci. 52, 2791–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ojo, J.O., Greenberg, M.B., Leary, P., Mouzon, B., Bachmeier, C., Mullan, M., Diamond, D.M., and Crawford, F. (2014). Neurobehavioral, neuropathological and biochemical profiles in a novel mouse model of co-morbid post-traumatic stress disorder and mild traumatic brain injury. Front. Behav. Neurosci. 8, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rowe, R.K., Rumney, B.M., May, H.G., Permana, P., Adelson, P.D., Harman, S.M., Lifshitz, J., and Thomas, T.C. (2016). Diffuse traumatic brain injury affects chronic corticosterone function in the rat. Endocr. Connect. 5, 152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu, C.S., Grandhi, R., Patterson, T.T., and Nicholson, S.E. (2018). A review of traumatic brain injury and the gut microbiome: insights into novel mechanisms of secondary brain injury and promising targets for neuroprotection. Brain Sci. 8, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma, E.L., Smith, A.D., Desai, N., Cheung, L., Hanscom, M., Stoica, B.A., Loane, D.J., Shea-Donohue, T., and Faden, A.I. (2017). Bidirectional brain-gut interactions and chronic pathological changes after traumatic brain injury in mice. Brain Behav. Immun. 66, 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Treangen, T.J., Wagner, J., Burns, M.P., and Villapol, S. (2018). Traumatic brain injury in mice induces acute bacterial dysbiosis within the fecal microbiome. Front. Immunol. 9, 2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Redelmeier, D.A., Naqib, F., Thiruchelvam, D., and Barrett, J.F.R. (2016). Motor vehicle crashes during pregnancy and cerebral palsy during infancy: a longitudinal cohort analysis. BMJ Open 6, e011972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brown, A.S., Hooton, J., Schaefer, C.A., Zhang, H., Petkova, E., Babulas, V., Perrin, M., Gorman, J.M., and Susser, E.S. (2004). Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am. J. Psychiatry 161, 889–895. [DOI] [PubMed] [Google Scholar]
- 35. Ellman, L.M., Schetter, C.D., Hobel, C.J., Chicz-Demet, A., Glynn, L.M., and Sandman, C.A. (2008). Timing of fetal exposure to stress hormones: effects on newborn physical and neuromuscular maturation. Dev. Psychobiol. 50, 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murphy, S.K., Fineberg, A.M., Maxwell, S.D., Alloy, L.B., Zimmermann, L., Krigbaum, N.Y., Cohn, B.A., Drabick, D.A.G., and Ellman, L.M. (2017). Maternal infection and stress during pregnancy and depressive symptoms in adolescent offspring. Psychiatry Res. 257, 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kirsten, T.B., Chaves-Kirsten, G.P., Bernardes, S., Scavone, C., Sarkis, J.E., Bernardi, M.M., and Felicio, L.F. (2015). Lipopolysaccharide exposure induces maternal hypozincemia, and prenatal zinc treatment prevents autistic-like behaviors and disturbances in the striatal dopaminergic and mTOR systems of offspring. PLoS One 10, e0134565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garcia-Flores, V., Romero, R., Miller, D., Xu, Y., Done, B., Veerapaneni, C., Leng, Y., Arenas-Hernandez, M., Khan, N., Panaitescu, B., Hassan, S.S., Alvarez-Salas, L.M., and Gomez-Lopez, N. (2018). Inflammation-induced adverse pregnancy and neonatal outcomes can be improved by the immunomodulatory peptide exendin-4. Front. Immunol. 9, 1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brenhouse, H.C., and Bath, K.G. (2019). Bundling the haystack to find the needle: challenges and opportunities in modeling risk and resilience following early life stress. Front. Neuroendocrinol. 54, 100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baker, S., Chebli, M., Rees, S., Lemarec, N., Godbout, R., and Bielajew, C. (2008). Effects of gestational stress: 1. Evaluation of maternal and juvenile offspring behavior. Brain Res. 1213, 98–110. [DOI] [PubMed] [Google Scholar]
- 41. Champagne, F.A., and Meaney, M.J. (2006). Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol. Psychiatry 59, 1227–1235. [DOI] [PubMed] [Google Scholar]
- 42. Beydoun, H., and Saftlas, A.F. (2008). Physical and mental health outcomes of prenatal maternal stress in human and animal studies: a review of recent evidence. Paediatr. Perinat. Epidemiol. 22, 438–466. [DOI] [PubMed] [Google Scholar]
- 43. Abdul Aziz, S.H., John, C.M., Mohamed Yusof, N.I., Nordin, M., Ramasamy, R., Adam, A., and Mohd Fauzi, F. (2016). Animal model of gestational diabetes mellitus with pathophysiological resemblance to the human condition induced by multiple factors (nutritional, pharmacological, and stress) in rats. Biomed. Res. Int. 2016, 9704607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kiss, A.C., Lima, P.H., Sinzato, Y.K., Takaku, M., Takeno, M.A., Rudge, M.V., and Damasceno, D.C. (2009). Animal models for clinical and gestational diabetes: maternal and fetal outcomes. Diabetol. Metab. Syndr. 1, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bilbo, S.D., Block, C.L., Bolton, J.L., Hanamsagar, R., and Tran, P.K. (2018). Beyond infection—maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp. Neurol. 299, 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kilkenny, C., Browne, W., Cuthill, I.C., Emerson, M., and Altman, D.G.; NC3Rs Reporting Guidelines Working Group. (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br. J. Pharmacol. 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rowe, R.K., Griffiths, D.R., and Lifshitz, J. (2016). Midline (central) fluid percussion model of traumatic brain injury. Methods Mol. Biol. 1462, 211–230. [DOI] [PubMed] [Google Scholar]
- 48. Saber, M., Rice, A.D., Christie, I., Roberts, R.G., Knox, K.S., Nakaji, P., Rowe, R.K., Wang, T., and Lifshitz, J. (2021). Remote ischemic conditioning reduced acute lung injury after traumatic brain injury in the mouse. Shock 55, 256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saber, M., Murphy, S.M., Cho, Y., Lifshitz, J., and Rowe, R.K. (2021). Experimental diffuse brain injury and a model of Alzheimer's disease exhibit disease-specific changes in sleep and incongruous peripheral inflammation. J. Neurosci. Res. 99, 1136–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Qiu, S., Anderson, C.T., Levitt, P., and Shepherd, G.M. (2011). Circuit-specific intracortical hyperconnectivity in mice with deletion of the autism-associated Met receptor tyrosine kinase. J. Neurosci. 31, 5855–5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Suter, B.A., O'Connor, T., Iyer, V., Petreanu, L.T., Hooks, B.M., Kiritani, T., Svoboda, K., and Shepherd, G.M. (2010). Ephus: multipurpose data acquisition software for neuroscience experiments. Front. Neural Circuits 4, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stephany, C.E., Chan, L.L., Parivash, S.N., Dorton, H.M., Piechowicz, M., Qiu, S., and McGee, A.W. (2014). Plasticity of binocularity and visual acuity are differentially limited by nogo receptor. J. Neurosci. 34, 11631–11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Can, A., Dao, D.T., Arad, M., Terrillion, C.E., Piantadosi, S.C., and Gould, T.D. (2012). The mouse forced swim test. J. Vis. Exp. (59), e3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rowe, R.K., Ziebell, J.M., Harrison, J.L., Law, L.M., Adelson, P.D., and Lifshitz, J. (2016). Aging with traumatic brain injury: effects of age at injury on behavioral outcome following diffuse brain injury in rats. Dev. Neurosci. 38, 195–205. [DOI] [PubMed] [Google Scholar]
- 55. Bialkowska, A.B., Ghaleb, A.M., Nandan, M.O., and Yang, V.W. (2016). Improved Swiss-rolling technique for intestinal tissue preparation for immunohistochemical and immunofluorescent analyses. J. Vis. Exp. (113), 54161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shepherd, G.M., and Svoboda, K. (2005). Laminar and columnar organization of ascending excitatory projections to layer 2/3 pyramidal neurons in rat barrel cortex. J. Neurosci. 25, 5670–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gong, S., Miao, Y.L., Jiao, G.Z., Sun, M.J., Li, H., Lin, J., Luo, M.J., and Tan, J.H. (2015). Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One 10, e0117503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wohleb, E.S., McKim, D.B., Sheridan, J.F., and Godbout, J.P. (2014). Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front. Neurosci. 8, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rasouli, J., Lekhraj, R., Ozbalik, M., Lalezari, P., and Casper, D. (2011). Brain-spleen inflammatory coupling: a literature review. Einstein J. Biol. Med. 27, 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ribeiro, M.R., Silva, A.A., Alves, M.T., Batista, R.F., Ribeiro, C.C., Schraiber, L.B., Bettiol, H., and Barbieri, M.A. (2017). Effects of socioeconomic status and social support on violence against pregnant women: a structural equation modeling analysis. PLoS One 12, e0170469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rowe, R.K., Murphy, S.M., Handmaker, H., and Lifshitz, J. (2021). Population-level epidemiology of concussion concurrent with domestic violence in Arizona, USA. J. Neurotrauma. doi: 10.1089/neu.2021.0022. [DOI] [PubMed] [Google Scholar]
- 62. Taylor, J.A., Sommerfeld-Sager, J.M., Meng, C.-X., Nagel, S.C., Shioda, T., and vom Saal, F.S. (2018). Reduced body weight at weaning followed by increased post-weaning growth rate interacts with part-per-trillion fetal serum concentrations of bisphenol A (BPA) to impair glucose tolerance in male mice. PLoS One 13, e0208846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Würbel, H., and Stauffacher, M. (1997). Age and weight at weaning affect corticosterone level and development of stereotypies in ICR-mice. Anim. Behav. 53, 891–900. [Google Scholar]
- 64. Lake, E.T., Staiger, D., Horbar, J., Kenny, M.J., Patrick, T., and Rogowski, J.A. (2015). Disparities in perinatal quality outcomes for very low birth weight infants in neonatal intensive care. Health Serv. Res. 50, 374–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jefferis, B.J., Power, C., and Hertzman, C. (2002). Birth weight, childhood socioeconomic environment, and cognitive development in the 1958 British birth cohort study. BMJ 325, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Torche, F. (2011). The effect of maternal stress on birth outcomes: exploiting a natural experiment. Demography 48, 1473–1491. [DOI] [PubMed] [Google Scholar]
- 67. Greer, P.L., Hanayama, R., Bloodgood, B.L., Mardinly, A.R., Lipton, D.M., Flavell, S.W., Kim, T.K., Griffith, E.C., Waldon, Z., Maehr, R., Ploegh, H.L., Chowdhury, S., Worley, P.F., Steen, J., and Greenberg, M.E. (2010). The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell 140, 704–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lazaro, M.T., Taxidis, J., Shuman, T., Bachmutsky, I., Ikrar, T., Santos, R., Marcello, G.M., Mylavarapu, A., Chandra, S., Foreman, A., Goli, R., Tran, D., Sharma, N., Azhdam, M., Dong, H., Choe, K.Y., Penagarikano, O., Masmanidis, S.C., Racz, B., Xu, X., Geschwind, D.H., and Golshani, P. (2019). Reduced prefrontal synaptic connectivity and disturbed oscillatory population dynamics in the CNTNAP2 model of autism. Cell Rep. 27, 2567–2578.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bureau, I., Shepherd, G.M., and Svoboda, K. (2008). Circuit and plasticity defects in the developing somatosensory cortex of FMR1 knock-out mice. J. Neurosci. 28, 5178–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McKinney, B.C., Grossman, A.W., Elisseou, N.M., and Greenough, W.T. (2005). Dendritic spine abnormalities in the occipital cortex of C57BL/6 Fmr1 knockout mice. Am. J. Med. Genet. B Neuropsychiatr. Genet. 136B, 98–102. [DOI] [PubMed] [Google Scholar]
- 71. Alachkar, A., Wang, L., Yoshimura, R., Hamzeh, A.R., Wang, Z., Sanathara, N., Lee, S.M., Xu, X., Abbott, G.W., and Civelli, O. (2018). Prenatal one-carbon metabolism dysregulation programs schizophrenia-like deficits. Mol. Psychiatry 23, 282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Spencer, K.M. (2009). The functional consequences of cortical circuit abnormalities on gamma oscillations in schizophrenia: insights from computational modeling. Front. Hum. Neurosci. 3, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Simon, P., Dupuis, R., and Costentin, J. (1994). Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav. Brain Res. 61, 59–64. [DOI] [PubMed] [Google Scholar]
- 74. Seibenhener, M.L., and Wooten, M.C. (2015). Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp..(96), e52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kazdoba, T.M., Leach, P.T., Yang, M., Silverman, J.L., Solomon, M., and Crawley, J.N. (2016). Translational mouse models of autism: advancing toward pharmacological therapeutics. Curr. Top. Behav. Neurosci. 28, 1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. van den Buuse, M. (2010). Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr. Bull. 36, 246–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Park, S.J., Lee, J.Y., Kim, S.J., Choi, S.Y., Yune, T.Y., and Ryu, J.H. (2015). Toll-like receptor-2 deficiency induces schizophrenia-like behaviors in mice. Sci. Rep. 5, 8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dhabhar, F.S., Malarkey, W.B., Neri, E., and McEwen, B.S. (2012). Stress-induced redistribution of immune cells—from barracks to boulevards to battlefields: a tale of three hormones—Curt Richter Award winner. Psychoneuroendocrinology 37, 1345–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bowers, S.L., Bilbo, S.D., Dhabhar, F.S., and Nelson, R.J. (2008). Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav. Immun. 22, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pitsillides, C.M., Runnels, J.M., Spencer, J.A., Zhi, L., Wu, M.X., and Lin, C.P. (2011). Cell labeling approaches for fluorescence-based in vivo flow cytometry. Cytom. Part A 79a, 758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Prame Kumar, K., Nicholls, A.J., and Wong, C.H.Y. (2018). Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 371, 551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S., Franceschi, C., Ferrucci, L., Gilroy, D.W., Fasano, A., Miller, G.W., Miller, A.H., Mantovani, A., Weyand, C.M., Barzilai, N., Goronzy, J.J., Rando, T.A., Effros, R.B., Lucia, A., Kleinstreuer, N., and Slavich, G.M. (2019). Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Genn, R.F., Tucci, S.A., Thomas, A., Edwards, J.E., and File, S.E. (2003). Age-associated sex differences in response to food deprivation in two animal tests of anxiety. Neurosci. Biobehav. Rev. 27, 155–161. [DOI] [PubMed] [Google Scholar]
- 84. Tucker, L.B., Burke, J.F., Fu, A.H., and McCabe, J.T. (2017). Neuropsychiatric symptom modeling in male and female C57BL/6J mice after experimental traumatic brain injury. J. Neurotrauma 34, 890–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Johnston, A.L., and File, S.E. (1991). Sex differences in animal tests of anxiety. Physiol. Behav. 49, 245–250. [DOI] [PubMed] [Google Scholar]
- 86. Earls, F. (1987). Sex differences in psychiatric disorders: origins and developmental influences. Psychiatr. Dev. 5, 1–23. [PubMed] [Google Scholar]
- 87. Halladay, A.K., Bishop, S., Constantino, J.N., Daniels, A.M., Koenig, K., Palmer, K., Messinger, D., Pelphrey, K., Sanders, S.J., Singer, A.T., Taylor, J.L., and Szatmari, P. (2015). Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority. Mol. Autism 6, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Brigida, A.L., Schultz, S., Cascone, M., Antonucci, N., and Siniscalco, D. (2017). Endocannabinod signal dysregulation in autism spectrum disorders: a correlation link between inflammatory state and neuro-immune alterations. Int. J. Mol. Sci. 18, 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mahic, M., Che, X., Susser, E., Levin, B., Reichborn-Kjennerud, T., Magnus, P., Stoltenberg, C., Chauhan, L., Briese, T., Bresnahan, M., Suren, P., Hornig, M., Mjaaland, S., and Lipkin, W.I. (2017). Epidemiological and serological investigation into the role of gestational maternal influenza virus infection and autism spectrum disorders. mSphere 2, e00159-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Siniscalco, D., Schultz, S., Brigida, A.L., and Antonucci, N. (2018). Inflammation and neuro-immune dysregulations in autism spectrum disorders. Pharmaceuticals (Basel) 11, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gunnar, M.R., DePasquale, C.E., Reid, B.M., Donzella, B., and Miller, B.S. (2019). Pubertal stress recalibration reverses the effects of early life stress in postinstitutionalized children. Proc. Natl. Acad. Sci. U. S. A. 116, 23984–23988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Faravelli, C., Lo Sauro, C., Godini, L., Lelli, L., Benni, L., Pietrini, F., Lazzeretti, L., Talamba, G.A., Fioravanti, G., and Ricca, V. (2012). Childhood stressful events, HPA axis and anxiety disorders. World J. Psychiatry 2, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mackes, N.K., Golm, D., Sarkar, S., Kumsta, R., Rutter, M., Fairchild, G., Mehta, M.A., and Sonuga-Barke, E.J.S.; ERA Young Adult Follow-up team. (2020). Early childhood deprivation is associated with alterations in adult brain structure despite subsequent environmental enrichment. Proc. Natl. Acad. Sci. U. S. A. 117, 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nyangahu, D.D., Lennard, K.S., Brown, B.P., Darby, M.G., Wendoh, J.M., Havyarimana, E., Smith, P., Butcher, J., Stintzi, A., Mulder, N., Horsnell, W., and Jaspan, H.B. (2018). Disruption of maternal gut microbiota during gestation alters offspring microbiota and immunity. Microbiome 6, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be provided upon reasonable request to the corresponding author.