Abstract
Public awareness of traumatic brain injury (TBI) in the military increased recently because of the conflicts in Iraq and Afghanistan where blast injury was the most common mechanism of injury. Besides overt injuries, concerns also exist over the potential adverse consequences of subclinical blast exposures, which are common for many service members. A TBI is a risk factor for the later development of neurodegenerative diseases, including Alzheimer disease (AD)-like disorders. Studies of acute TBI in humans and animals have suggested that increased processing of the amyloid precursor protein (APP) toward the amyloid beta protein (Aβ) may explain the epidemiological associations with AD. In a previous study, however, we found in both rat and mouse models of blast overpressure exposure that rather than increasing, rodent brain Aβ42 levels were decreased after acute blast exposure. Here we subjected APP/presenilin 1 transgenic mice (APP/PS1 Tg) to an extended sequence of repetitive low-level blast exposures (34.5 kPa) administered three times per week over eight weeks. If initiated at 20 weeks of age, these repetitive exposures, which were designed to mimic human subclinical blast exposures, reduced anxiety and improved cognition as well as social interactions in APP/PS1 Tg mice, returning many behavioral parameters in APP/PS1 Tg mice to levels of non-transgenic wild type mice. Repetitive low-level blast exposure was less effective at improving behavioral deficits in APP/PS1 Tg mice when begun at 36 weeks of age. While amyloid plaque loads were unchanged, Aβ 42 levels and Aβ oligomers were reduced in the brain of mice exposed to repetitive low-level blast exposures initiated at 20 weeks of age, although levels did not directly correlate with behavioral parameters in individual animals. These results have implications for understanding the nature of blast effects on the brain and their relationship to human neurodegenerative diseases.
Keywords: amyloid beta protein, Alzheimer disease, blast, transgenic mouse, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a common occurrence in military personnel especially in those deployed to combat zones.1 Public awareness of military-related TBI increased recently because of the conflicts in Iraq and Afghanistan. As in civilian settings, TBI in service members occurs through various mechanisms including motor vehicle accidents and sports injuries. Blast injury because of detonation of high explosives, including improvised explosive devices (IEDs), mortars, and artillery shells, was a major cause of TBI among veterans of the wars in Iraq and Afghanistan.1,2
There are concerns over the potential adverse consequences of subclinical blast exposures, now referred to as military occupational blast exposure,3 in service members both in combat as well as non-combat settings including military breachers who use controlled explosions to gain entry to secured structures.4 Whether repetitive low-level blast exposures may cause later health problems is unknown and is a subject of concern for the United States Department of Defense and Department of Veterans Affairs.3
TBI is a risk factor for the later development of neurodegenerative diseases that may have various underlying pathologies.5–7 Several proteins associated with neurodegenerative diseases accumulate in the brain after TBI, including α-synuclein, tau, the amyloid precursor protein (APP) and its product the amyloid β protein (Aβ).8 In Alzheimer disease (AD), Aβ released into the extracellular space deposits as amyloid plaques composed chiefly of Aβ42.9–11 Many in vitro and in vivo studies show that Aβ42 can be particularly pathogenic.10
In humans, Aβ42 is elevated, and amyloid plaques appear within hours after a severe TBI.7,12–14 Increases in Aβ, APP, and APP processing enzymes also occur after closed impact and direct cortical injuries in wild type mice and rats,15-19 as well as transgenic (Tg) mouse models of AD.20–26 Collectively, these findings suggest that upregulation of APP and its processing enzymes may cause increased Aβ production and explain the epidemiological associations between TBI and AD.7,12
Blast-related TBI in humans probably always involves some element of rotation/acceleration injury, although effects of the primary blast wave likely dominate at the lower pressures associated with the mild TBIs (mTBIs) that were so common in Iraq and Afghanistan.27 Because of its distinctive character, injuries caused by the primary blast wave may differ mechanistically from those caused by higher blast overpressure forces that include tertiary forces.28
Among these differences, we found previously that in both rat and mouse models of blast exposure, rather than being increased, rodent brain Aβ42 levels were decreased in the first week after acute exposure.29 Interestingly, the effect on Aβ42 was most prominent in rats exposed to lower blast overpressures (36.6 kPa and 74.5 kPa), while there were no effects on Aβ42 at a 116.7-kPa-exposure level.29 There were no consistent effects on Aβ40 levels in rats, and Aβ40 was also less proportionately affected in mice.
Thus, in both species, the effect of blast overpressure exposure was much greater on brain Aβ42 than on Aβ40.29 Studies in military personnel have also documented lowered Aβ42 in blood acutely after blast exposure30 along with decreased APP and alteration of the APP signaling network in blood.31
These acute studies raised the intriguing possibility that blast exposure might actually exert beneficial effects on brain Aβ levels. What they did not answer was whether these effects would be sustained and whether lowered brain Aβ levels would be associated with improved functional outcomes. Therefore, in the present study, we subjected a Tg mouse model of AD, which develops elevated brain Aβ42 levels from overexpression of two familial AD (FAD) related mutations, to an extended sequence of repetitive low-level blast exposures mimicking human subclinical blast exposures. We show that repetitive exposures actually improved behavioral deficits and chronically lowered Aβ42 in the brain.
Methods
Animals
The APP/PS1 Tg mice (Tg[APPswe,PSEN1dE9]85Dbo; Stock No. 34829-JAX) were obtained from the Jackson Laboratory on a C57BL/6;C3H genetic background. All studies involving animals were reviewed and approved by the Institutional Animal Care and Use Committees of the Walter Reed Army Institute of Research/Naval Medical Research Center and the James J. Peters VA Medical Center. Studies were conducted in compliance with the Public Health Service policy on the humane care and use of laboratory animals, the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all applicable Federal regulations governing the protection of animals in research.
Blast overpressure exposure
Mice were exposed to overpressure injury using a shock tube, which simulates the effects of air blast exposure under experimental conditions.32 The shock tube has a 12-inch circular diameter and is a 19.5 ft-long steel tube divided into a 2.5 ft compression chamber that is separated from a 17 ft expansion chamber. The compression and expansion chambers are separated by polyethylene MylarTM sheets (Du Pont, Wilmington, DE) that control the peak pressure generated. The peak pressure at the end of the expansion chamber was determined by piezoresistive gauges specifically designed for pressure-time (impulse) measurements (Model 102M152, PCB, Piezotronics, Depew, NY).
Individual mice were anesthetized using an isoflurane gas anesthesia system consisting of a vaporizer, gas lines, and valves and an activated charcoal scavenging system adapted for use with rodents. Mice were placed into a polycarbonate induction chamber, which was closed and immediately flushed with 5% isoflurane in air mixture for 2 min.
To eliminate rotational/accelerational injury during exposure to blast, mice were placed side-by-side along the center (horizontal) axis of the circular (10-inch diameter) rodent constraint device. The rodents were held in place between two layers of fabric that were secured in place between the two rings of the device by four clasps, one at each corner. The constraint device was then secured in place with the animals on their stomachs and facing into the shock tube 10 inches from the end of the shock tube.
Each subject to receive blast exposure was exposed to one 34.5-kPa exposure a day for three days in a row, followed by four days of no exposure, for a total of eight weeks. Sham animals received isoflurane and were placed in the device and the shock tube for the same amount of time as the blast-exposed animals but were not exposed to blast.
Within 10 days after the last blast or sham exposure, animals were transported in a climate-controlled van to the James J. Peters VA Medical Center (Bronx, NY). Animals were shipped in the morning from the Naval Medical Research Center and arrived in the afternoon of the same day at the James J. Peters VA Medical Center, where all other procedures were performed.
Animal housing
Animals were housed at a constant 70–72oF temperature with rooms on a 12:12 h light cycle with lights on at 7 am. All subjects were individually housed in standard clear plastic cages equipped with Bed-O'Cobs laboratory animal bedding (The Andersons, Maumee, Ohio) and EnviroDri nesting paper (Sheppard Specialty Papers, Milford, NJ). Access to food and water was ad libitum. Subjects were housed on racks in random order to prevent rack position effects. Cages were coded to allow maintenance of blinding to groups during behavioral testing.
Behavioral testing
Elevated zero maze
The apparatus consisted of a circular black Plexiglas™ runway 61 cm in diameter and raised 61 cm off the floor (San Diego Instruments, San Diego, CA). The textured runway itself was 5.0 cm across and divided equally into alternating quadrants of open runway enclosed only by a 0.80-cm lip and closed runway with smooth 15.5-cm walls. All subjects received a 5-min trial beginning in a closed arc of the runway. During each trial, subjects were allowed to move freely around the runway, with all movement tracked automatically by a video camera placed on the ceiling directly above the maze.
Data were analyzed by ANYMAZE (San Diego Instruments) yielding measures of total movement time and distance for the entire maze, as well as time spent and distance traveled in each of the individual quadrants. From the quadrant data, measures of total open and closed arc times, latency to enter an open arc, total open arm entries and latency to completely cross an open arc between two closed arcs were calculated. Subject position was determined by centroid location.
Light/dark (L/D) emergence
A L/D emergence task was run in Versamax activity cages with opaque black Plexiglas boxes enclosing the left half of the interiors so that only the right sides were illuminated. Animals began in the dark side and were allowed to freely explore for 10 min with access to the right (light) side through an open doorway located in the center of the monitor. Subject side preference and emergence latencies were tracked by centroid location with all movement automatically tracked and quantified. Light-side emergence latency, time to reach the center of the lighted side (light-side center latency), and percent total light-side duration were calculated from beam breaks. All equipment was wiped clean between tests.
Novel object recognition (NOR)
Mice were habituated to the circular arena (30 cm length × 30 cm width × 40 cm height) for 10 min, 24 h before training. On the training day, two identical objects were placed on opposite ends of the empty arena, and the mouse was allowed to explore the objects freely for 7 min. After 1 h, during which the mouse was held in its home cage, one of the two familiar objects (FOs) was replaced with a novel object (NO), and the mouse was allowed to explore the FO and NO freely for 5 min to assess short-term memory (STM).
After 24 h, during which the mouse was held in its home cage, one of the two FOs was replaced with a NO different from the one used during the STM test. The mouse was allowed to explore the FO and NO freely for 5 min to assess long-term memory (LTM). Raw exploration times for each object were expressed in seconds. Object exploration was defined as sniffing or touching the object with the vibrissae or when the animal's head was oriented toward the object with the nose placed at a distance of <2 cm from the object. All sessions were recorded by video camera (Sentech, Carrollton, TX) and analyzed with ANYMAZE software (San Diego Instruments). In addition, offline analysis by an investigator blind to the treatment status of the animals was performed.
Objects to be discriminated were of different size, shape, and color and were made of Lego plastic material. All objects were wiped with 70% ethanol between trials. A discrimination index (DI) was calculated with the formula: time exploring the NO minus time exploring the FO/total exploration time × 100.
NO localization (NOL)
NOL was assessed using methods described previously.33 At 24 h before training, mice were habituated for 20 min to the same empty arena used for the NOR task. The arena was situated in a well-lit room allowing the animals to see distal visual cues. On the training day, two identical objects were placed in specific locations, and the mouse was allowed to explore the objects freely for 7 min. The test trial was performed after a 1-h delay during which one object was moved to a different location in the arena and the mouse was allowed to explore for 5 min. Time spent investigating the objects in their original or novel locations was recorded. The arena and objects were cleaned before and between trials with 70% ethanol.
Barnes maze
The Barnes maze test was performed using a standard apparatus. The testing was conducted in two phases: training (day 1 to 4) and testing (day 5). Before starting each experiment, mice were acclimated to the testing room for 1 h. Mice were transported from their cage to the center of the platform within a closed starting chamber where they remained for 10 sec before exploring the maze. Mice failing to enter the escape box within 4 min on trials 1–4 were guided to the escape box by the experimenter, and the latency was recorded as 240 sec. Trial 5 was treated as a test trial, and mice were given up to 180 sec to enter the escape box. The platform and the escape box were wiped with 70% ethanol after each trial. Trials were recorded by video camera and analyzed with ANYMAZE software.
Contextual and cued fear conditioning
Sound-attenuated isolation cubicles (Coulbourn Instruments, Holliston, MA) were utilized. Each cubicle was equipped with a grid floor for delivery of the unconditioned stimulus (US) and overhead cameras. All aspects of the test were controlled and monitored by the Freeze Frame conditioning and video tracking system (Actimetrics, Coulbourn Instruments). During training; the chambers were scented with almond extract, lined with white paper towels, had background noise generated by a small fan; chambers were cleaned before and between trials with 70% ethanol.
Each subject was placed inside the conditioning chamber for 2 min before the onset of a conditioned stimulus (CS; an 80 dB, 2 kHz tone), which lasted for 20 sec with a coterminating 2-sec footshock (0.7 mA; US). Three tone/shock pairings were administered with the first/second and second/third separated by 1 min. Each mouse remained in the chamber for an additional 40 sec after the third CS-US pairing before being returned to its home cage.
Freezing was defined as a lack of movement (except for respiration) in each 10-sec interval. Minutes 0–2 during the training session were used to measure baseline freezing. Contextual fear memory testing was performed 24 h after the training session by measuring freezing behavior during a 4-min test in the conditioning chamber under conditions identical to those of the training session with the exception that no footshock or tone (CS or US) was presented. Animals were returned to their home cage for another 24 h at which time cued conditioning was tested.
To create a new context with different properties, the chambers were free of background noise (fan turned off), lined with blue paper towels, scented with lemon extract, and cleaned before and during all trials with isopropanol. Each subject was placed in this novel context for 2 min, and baseline freezing was measured, followed by exposure to the CS (20-sec tone) at 120 and 290 sec.
Social preference test
A three-chamber social preference test was used to assess preference for social versus non-social stimuli. The test was modeled after other published protocols.34,35 The apparatus consisted of a gray opaque polycarbonate rectangle (64 × 41 × 25 cm) that was divided into three chambers using removable partitions. Each divider (41 × 21 cm) had a sliding door of ≈5 × 5 cm to allow free movement of the animal between chambers.
The central chamber served as the starting area while the lateral chambers were used to hold a stimulus. The mouse stimulus was placed in a metallic cage/jail of height 15 cm having a diameter of 7 cm that allowed interactions between the test subject and mouse stimulus but limited aggressive interactions. The protocol comprised three phases that were completed over three days.
On day 1 the test subject was first habituated to the apparatus containing two empty metal cups in the side chambers. The test subject was allowed to explore the three chambers freely for 10 min, and basal activity was recorded. In the pre-test phase on day 2, the subject was allowed to interact with two non-Tg mice of the same age as the test subject (one in each metal cup) for 5 min. During the test phase on the day 3, the test subject was given the choice of interacting with a new mouse (unfamiliar non-Tg) contained in one cup or a novel non-social stimulus (an object) contained in the other cup for 5 min. Movement of the test subject was tracked by ANYMAZE software recording the time in motion, distance moved, entries and exits from the chambers as well as time interacting/sniffing the object or the jailed mouse.
Laboratory evaluations
Aβ enzyme-linked immunosorbent assay (ELISA)
Animals were euthanized by CO2 narcosis, and the brains were quickly removed, frozen, and stored at -80°C until use. The Tris-buffered saline (TBS), Triton X-100, and formic acid fractions from one hemisphere were prepared using a protocol adapted from that described in Kawarabayashi and associates36 and described in more detail by Steele and colleagues.37
The tissues were homogenized with a hand-held homogenizer in 50 mM Tris-HCl buffer, pH 7.4, 150 mM NaCl (TBS) with a protease/phosphatase inhibitors cocktail (ThermoFisher, Waltham, MA) (200 mg tissue/mL), and 0.25 mL were centrifuged at 100,000 g for 1 h at 4°C. The supernatant was saved (TBS fraction), and the pellet homogenized with 1% Triton X-100 in TBS supplemented with protease/phosphatase inhibitor cocktail (ThermoFisher). The supernatant was saved (Triton fraction) and the pellet extracted with ice-cold 70% formic acid and centrifuged as above. The supernatant was saved (formic acid fraction). The Aβ 42 levels in every fraction were determined by ELISA using a commercially available kit that detects human Aβ42 (Wako, Richmond, VA). Data are expressed as pg/mg fresh tissue.
Oligomeric Aβ42 dot blot analysis
Oligomeric Aβ42 was determined by dot blot analysis. Protein concentration was determined with the BCA reagent (ThermoFisher). An aliquot containing 2.5 μg protein was spotted onto a nitrocellulose membrane, and the membrane was air-dried, washed in TBS with 0.1% Tween (TBST), and blocked for 1 h in TBST/5% non-fat dry milk. The membrane was then incubated for 1 h with anti-oligomer antibody A11 (1:1500, #AHB0052, ThermoFisher), washed in TBST, and incubated for 1 h with horseradish peroxidase-conjugated anti-rabbit antibody (1:10,000, #NA-934, Cytiva Lifesciences, Marlborough, MA) diluted in blocking solution.
The immunoreactive signal was visualized with ECL Prime reagent (Cytiva Lifesciences), imaged with an Amersham Image Quant 1200 imaging station, and quantitated by ImageQuantTL software (Cytiva Lifesciences). Data were normalized to sham samples.
Immunohistochemistry
Mice were perfused with 4% paraformaldehyde in phosphate buffered saline (PBS), and the brains dissected and post-fixed overnight in 4% paraformaldehyde. The brains were sectioned into 40 μm-thick coronal sections with a Vibratome (Leica, Wetzlar, Germany). For stereologic analyses, sections that contained the entire hippocampus were selected every 300 μm (interaural 0.72-1.44 mm) from six control and six blast-exposed APP/PS1 Tg animals. Amyloid plaques were identified by immunohistochemical staining with the mouse monoclonal antibody 6E10 (1:1,000, LSBio #LS-C821449, Seattle, WA), which recognizes an epitope in the N-terminal region of both Aβ40 and Aβ42.
Sections were blocked with TBS/0.3% Triton X-100, 5% normal goat serum for 1 h and stained overnight with primary antibodies diluted in blocking solution. The sections were washed in PBS and incubated for 2 h with the appropriate Alexa-fluor-conjugated secondary antibody (1:300, ThermoFisher) in blocking solution. After washing with PBS, the sections were mounted in FluoroGel mounting medium (EMS Science, Hatfield, PA). Total plaque number in the hippocampal region in each section was determined using a Zeiss Axioplan 2 microscope at 40X magnification under ultraviolet illumination.
Thioflavin S staining
Sections were incubated in 1% aqueous Thioflavin S (Sigma-Aldrich, St. Louis, MO) for 8 min at room temperature in the dark. Sections were washed twice for 3 min in 80% ethanol, 3 min with 95% ethanol, rinsed three times with distilled water, and mounted with Fluorogel. Sections were sampled as above, and the total number of Thioflavin S positive plaques in the hippocampal areas was determined.
Statistical analysis
Values are expressed as means ± the standard error of the mean (SEM). The groups and group sizes are indicated in Table 1. Data sets were tested for normality using the D'Agostino-Pearson normality test. Comparisons were performed using repeated-measures analysis of variance (ANOVA), one-way ANOVA, or unpaired t tests. When repeated-measures ANOVA was used, sphericity was assessed using the Mauchly test. If the assumption of sphericity was violated (p < 0.05), significance was determined using the Greenhouse-Geisser correction.
Table 1.
Summary of Behavioral Testing in Blast-Exposed Mice
| Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | |
|---|---|---|---|---|
| Age at time blast exposure was initiated | 20 weeks | 36 weeks | 20 weeks | 20 weeks |
| Groups and group sizes (n) | Tg blast (7) Tg sham (8) | Tg blast (16) Tg sham (16) |
Tg blast (16) Tg sham (16) non-Tg sham (16) |
Tg blast (10) Tg sham (9) non-Tg sham (10) |
| Locomotor activity | Tg blast exhibited increased center time compared with Tg sham | No differences between Tg blast and Tg sham | Not tested | Not tested |
| Elevated zero maze | Tg blast exhibited less anxiety than Tg Sham | Tg blast exhibited less anxiety than Tg sham | Blast rescued anxiety phenotype found in Tg sham mice | Blast rescued anxiety phenotype found in Tg sham mice |
| Light dark escape | Tg blast exhibited less anxiety than Tg sham | No differences between Tg blast and Tg sham | Blast rescued anxiety phenotype found in Tg sham mice | Not tested |
| Novel object recognition (NOR) | Deficits in NOR in Tg sham mice were rescued in Tg blast mice | Deficits in NOR in Tg sham mice were rescued in Tg Blast mice | Deficits in NOR in Tg sham mice were rescued in Tg blast mice | Deficits in NOR in Tg sham mice were rescued in Tg blast mice |
| Novel object localization | Deficits in NOL in Tg sham mice were rescued in Tg blast mice | Not tested | Not tested | Not tested |
| Barnes maze | Tg blast mice showed improved learning curves compared with Tg sham mice | No differences in performance of Tg blast vs. Tg sham | Tg blast exhibited better learning curves than either non-Tg sham or Tg sham mice. | Tg blast exhibited better learning curves than either non-Tg sham or Tg sham mice. |
| Fear conditioning | Tg blast froze more than Tg sham in the cued phase | Neither Tg blast nor Tg sham formed an association between the tone and the shock during the training session | Tg blast mice failed to form an association between the tone and the shock during the training session | No tested |
| Social interaction | Blast improved social interactions in Tg blast vs. Tg sham mice | No differences in social interactions of Tg blast and Tg sham mice | Not tested | Not tested |
Results highlighted in BOLD reflect tests where Tg blast mice performed better than Tg sham.
Between-group comparisons after a significant one-way ANOVA were compared using Fisher Least Significant Difference. For some comparisons, simple linear regressions were performed or Pearson product-moment correlation coefficient, Kendall tau-b, and Spearman rho were calculated. Statistical tests were performed using the programs GraphPad Prism 8.0 (GraphPad Software, San Diego, CA) or SPSS v26 (IBM, Armonk, NY).
Results
Experimental design for blast exposure of APP/PS1 Tg mice
To determine the effects of an extended blast exposure protocol on APP/PS1 Tg mice, we compared APP/PS1 Tg mice exposed to sham or blast conditions. Figure 1 shows the experimental design and timeline of the first two experiments. The groups and group sizes are indicated in Table 1.
FIG. 1.
Timeline of experiments for cohorts 1 and 2. BOP, blast overpressure exposure; NOR, novel object recognition; NOL, novel object localization.
Blast-exposed mice received one 34.5-kPa exposure a day for three days in a row, followed by four days of no exposure, for a total of eight consecutive weeks. Exposures began at 20 weeks of age (cohort 1), an age before APP/PS1 Tg develop substantial plaque loads,38 or 36 weeks (cohort 2), when significant plaque burdens are present.38 Sham-exposed control mice were treated identically to those blast-exposed, including receiving anesthesia and being placed in the blast tube, but did not receive a blast exposure. The timing of the studies for cohorts 1 and 2 is shown in Figure 1.
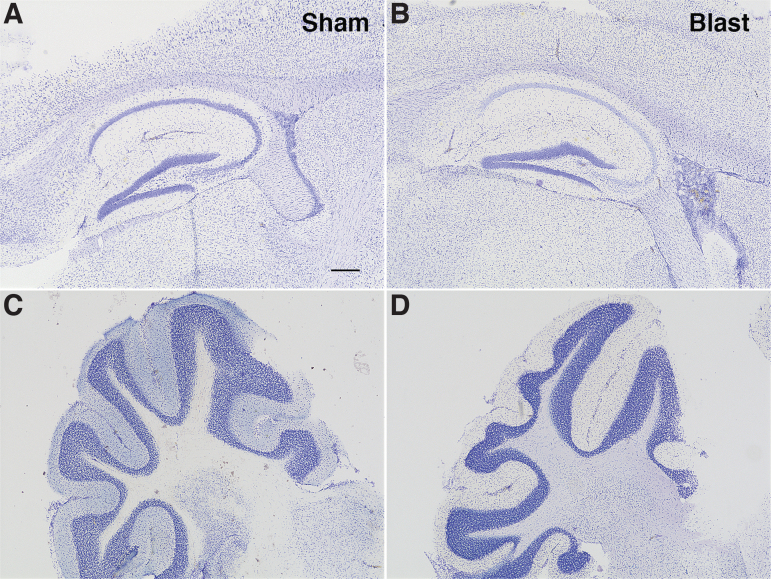
Histopathologic inspection using Nissl staining did not reveal any consistent anatomical abnormalities in blast-exposed animals compared with shams (Fig. 2). Behavioral test results for cohorts 1 and 2 are summarized in Table 1.
FIG. 2.
Histopathology in amyloid precursor protein/presenilin 1 (APP/PS1) transgenic (Tg) mice after blast exposure. Nissl staining in the hippocampus and neocortex (A, B) and cerebellum (C, D) is shown from sham- (A, C) and blast-exposed (B, D) APP/PS1 Tg mice sacrificed at seven weeks after the last blast exposure (35 weeks of age). No significant histological changes were noted. Scale bar = 200 μm.
Repetitive low-level blast exposure reduces anxiety and improves cognition as well as social interactions in APP/PS1 Tg mice when begun at 20 weeks of age
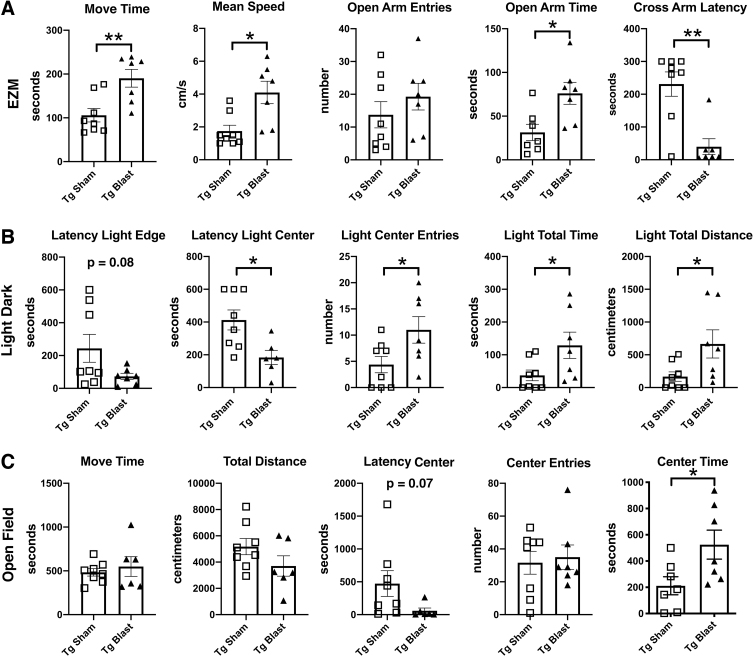
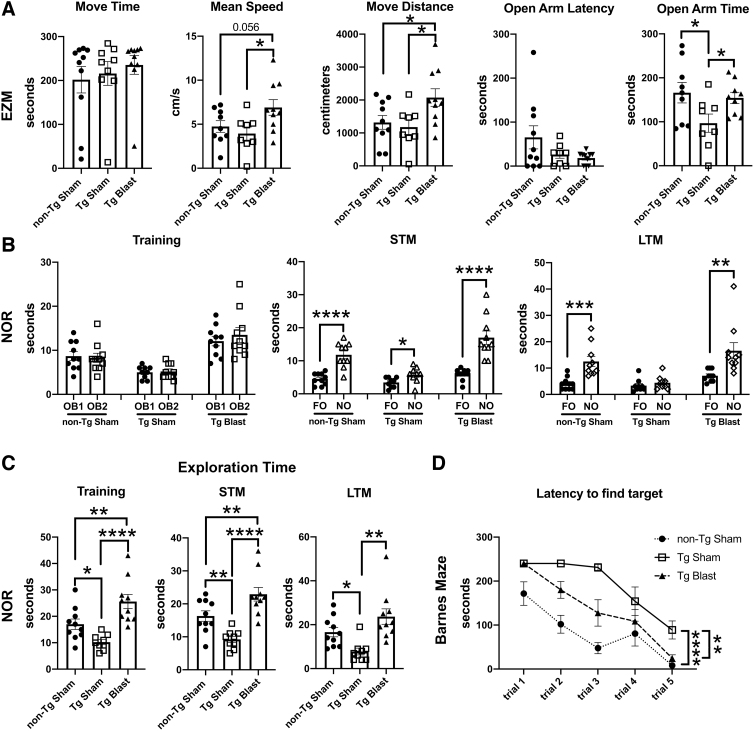
Figure 3 shows testing of sham and blast-exposed APP/PS1 Tg mice from cohort 1 in tests that measure anxiety. In an elevated zero maze (EZM, Fig. 3A), blast-exposed APP/PS1 Tg mice from cohort 1 spent more time in motion and moved faster, as well as spent more time in the open arms and exhibited a shorter latency to cross into the second open arm (cross arm latency). In the L/D escape task (Fig. 3B) blast-exposed APP/PS1 Tg mice exhibited a shorter latency to reach the light center and made more light center entries as well as spent more time and traveled a greater distance on the light side.
FIG. 3.
Elevated zero maze (EZM), light/dark (L/D), and open-field testing of cohort 1. Amyloid precursor protein/presenilin 1 (APP/PS1) transgenic (Tg) mice were exposed to blast (n = 7) or sham (n = 8) conditions beginning at 20 weeks of age and received three blast exposures per week for eight weeks. Behavioral testing was begun at 30 weeks of age (Fig. 1). For the EZM (A), time in motion (Move Time), mean speed, open arm entries, open arm time, and the latency to cross into the second open arm (Cross Arm Latency) area shown. In the L/D task (B), the latency to the light edge, latency to reach the light center, entries into the light center, as well as time total time spent on the light side and total distance traveled on the light side are shown. For the open field (C), time in motion (Move Time), total distance traveled, the latency to the open field center, center entries, and time spent in the center of the open field are shown. Error bars indicate the standard error of the mean. Asterisks indicate values significantly different between groups (*p < 0.05, **p < 0.01, unpaired t tests).
Compared with sham-exposed mice, in an open field test (Fig. 3C), blast-exposed APP/PS1 Tg mice spent more time in the center of the open field. All these results suggest that blast-exposed APP/PS1 Tg mice exhibit less anxiety compared with sham-exposed APP/PS1 Tg mice.
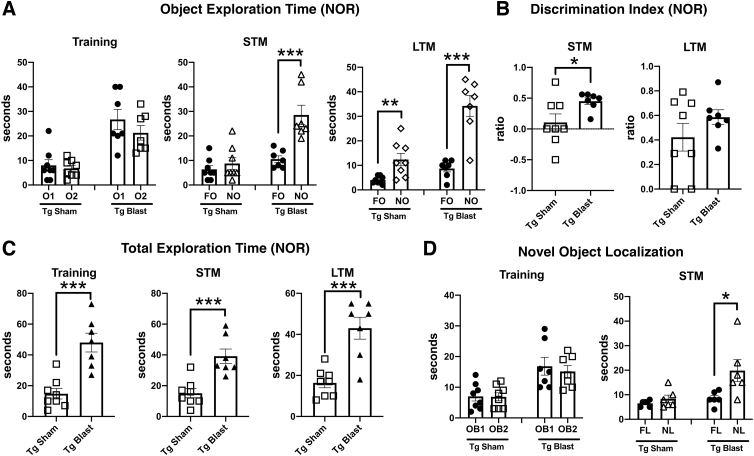
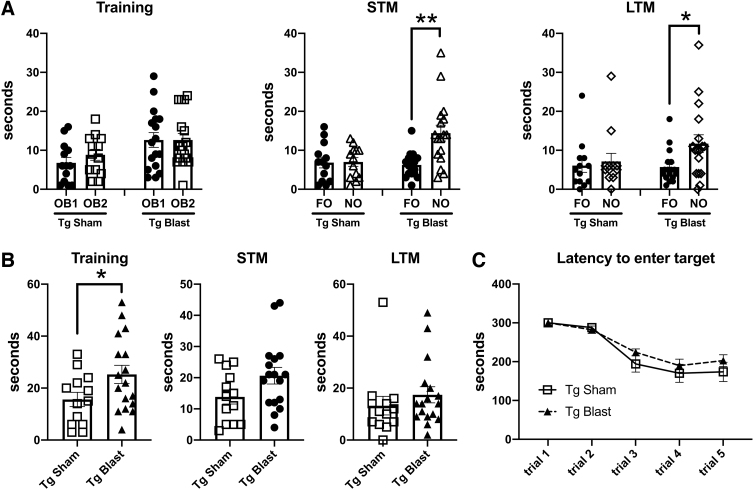
Figure 4 shows testing of mice from cohort 1 in NOR and NOL tasks. In the NOR training session, sham and blast-exposed APP/PS1 Tg mice spent comparable time exploring the two objects that had not been encountered previously (Fig. 4A), although blast-exposed APP/PS1 Tg mice spent more total time exploring the objects (Fig. 4C). In STM testing (Fig. 4A), blast-exposed APP/PS1 Tg spent more time exploring the NO compared with the FO, indicating intact recognition memory, unlike the sham-exposed APP/PS1 Tg who explored the NO no more than the FO, indicating a failure of recognition memory.
FIG. 4.
Novel object recognition (NOR) testing of cohort 1. Blast-exposed (n = 7) and sham-exposed (n = 8) amyloid precursor protein/presenilin 1 (APP/PS1) transgenic (Tg) mice from cohort 1 were tested in novel object recognition (NOR) and novel object localization (NOL) tasks. Panel (A) shows time spent exploring the objects (OB1 and OB2) during the NOR training session as well as exploration of the previously presented familiar object (FO) compared with the novel object (NO) when presented 1 h (short-term memory, STM) or 24 h (long-term memory, LTM) later. Panels (B) and (C) show the discrimination index (B) and total time spent exploring the objects (C) during the indicated NOR sessions. Panel (D) shows time spent exploring the objects (OB1 and OB2) during the NOL training session as well as exploration of the previously presented objects in their familiar location (FL) compared with a novel location (NL) when presented 1 h later (STM). Asterisks indicate values significantly different between groups (*p < 0.05, ***p < 0.001, unpaired t tests).
Blast-exposed APP/PS1 Tg also showed an increased preference for the NO versus FO when a discrimination index was calculated for the STM testing (Fig. 4B), which relates the relative tendency to explore the NO versus FO. In LTM testing, both blast-exposed and sham-exposed APP/PS1 Tg mice preferentially explored the NO versus FO, suggesting that with repeated presentation of the FO, recognition memory improved in the sham-exposed mice. As in the other testing sessions, however, blast-exposed APP/PS1 Tg mice spent more total time exploring the objects in the LTM testing (Fig. 4C).
In a NOL test, when tested 24 h after the training session, blast-exposed APP/PS1 Tg mice explored the object moved to the novel location more, unlike sham-exposed APP/PS1 Tg that explored both objects equally indicating that APP/PS1 Tg mice exposed to blast recognized the change in location of the object while sham exposed APP/PS1 Tg mice did not. Thus, blast-exposed APP/PS1 Tg mice showed intact recognition memory in both NOR and NOL tasks compared with sham-exposed controls that were impaired in both tasks.
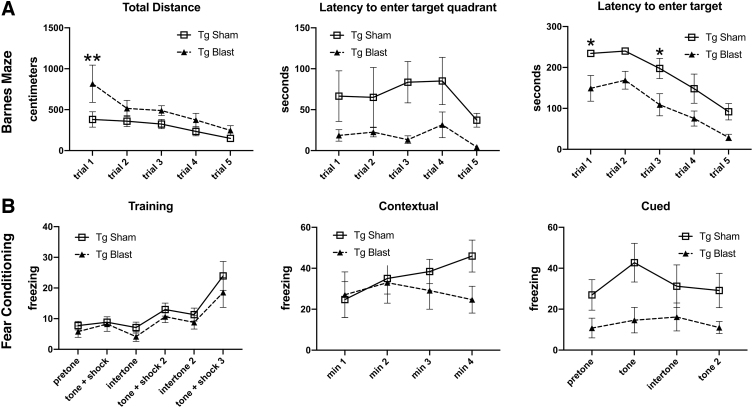
Testing of cohort 1 in a Barnes maze showed that blast-exposed APP/PS1 Tg mice exhibited faster learning curves and shorter latencies to enter the escape hole than sham-exposed APP/PS1 Tg mice (Fig. 5A). Thus, blast-exposed APP/PS1 Tg mice exhibited better cognition than sham exposed APP/PS1 Tg mice across multiple tests.
FIG. 5.
Testing of cohort 1 in the Barnes maze and fear learning. Blast-exposed (n = 7) and control (n = 8) mice from cohort 1 were tested in a Barnes maze or fear conditioning paradigm. For the Barnes maze (A), total distance moved, time to enter the target quadrant, and time to enter the escape hole are shown across the five trials. A repeated measures analysis of variance (ANOVA) revealed a significant within subjects effect by trial (F 2.069, 26.902 = 5.973, p = 0.007) for distance moved but no effect of trial*condition (F 2.069, 26.902 = 1.211, p = 0.315). A test of between subject effects, however, revealed a significant group difference with the transgenic (Tg) blast moving more (F 1, 13 = 6.976, p = 0.020). A repeated measures ANOVA of the time to first enter the target quadrant revealed no significant within subjects effect by trial (F 2.180, 28.339 = 0.906, p = 0.467) or effect of trial*condition (F 2.180, 28.339 = 0.230, p = 0.814). A test of between subject effects, however, revealed a significant group difference with the Tg blast exhibiting shorter latencies (F 1, 13 = 8.973, p = 0.010). A repeated measures ANOVA of the time to enter the target revealed a significant within subjects effect by trial (F 4, 52 = 13.503, p < 0.001) but no effect of trial*condition (F 4, 52 = 0.108, p = 0.979). A test of between subject effects again revealed a significant group difference with the Tg blast exhibiting shorter latencies (F 1, 13 = 38.817, p < 0.001). Asterisks indicate values significantly different between blast- and sham-exposed mice at individual time points (*p < 0.05, **p < 0.01, unpaired t tests). For the fear conditioning paradigm (B), results are shown for the training phase, contextual fear memory, which was tested 24 h after training, and cued fear memory, which was tested another 24 h later. Pre-tone represents freezing before the first presentation of the tone ± shock. A repeated measures ANOVA of freezing during the training sessions revealed a significant within-subjects effect of freezing for baseline versus tone (F 2.813, 36.574 = 10.425, p < 0.001) but no effect of freezing*condition (F 2.813, 36.574 = 0.203, p = 0.883). A test of between-subject effects revealed no significant group differences during the training sessions (F 1, 13 = 0.966, p = 0.344). There were no differences between blast-exposed and control groups in the contextual testing (F 1.742, 19.157 = 2.753; p = 0.095). In the cued phase testing, neither group showed significant freezing after presentation of the tone (F 3, 27 = 0.790, p = 0.510; freezing*condition F 3, 27 = 0.349, p = 0.790). The blast-exposed, however, exhibited increased freezing compared with the controls (F 1, 9 = 8.758, p = 0.016). Error bars in all panels indicate the standard error of the mean.
Figure 5B shows testing of cohort 1 in a fear-learning paradigm. In the training phase, both blast- and sham-exposed APP/PS1 Tg mice exhibited similar learning curves showing increased freezing after repetitive presentation of the tone/shock pairing. There were no differences between blast-exposed and sham groups in the contextual testing. In the cued phase testing, neither group showed significant freezing after presentation of the tone. The blast-exposed APP/PS1 Tg mice, however, exhibited overall increased freezing compared with the controls, indicating that while impaired cued fear learning was present in both groups, in this task, blast exposure altered the general freezing tendency of APP/PS1 Tg mice compared with sham-exposed APP/PS1 Tg mice.
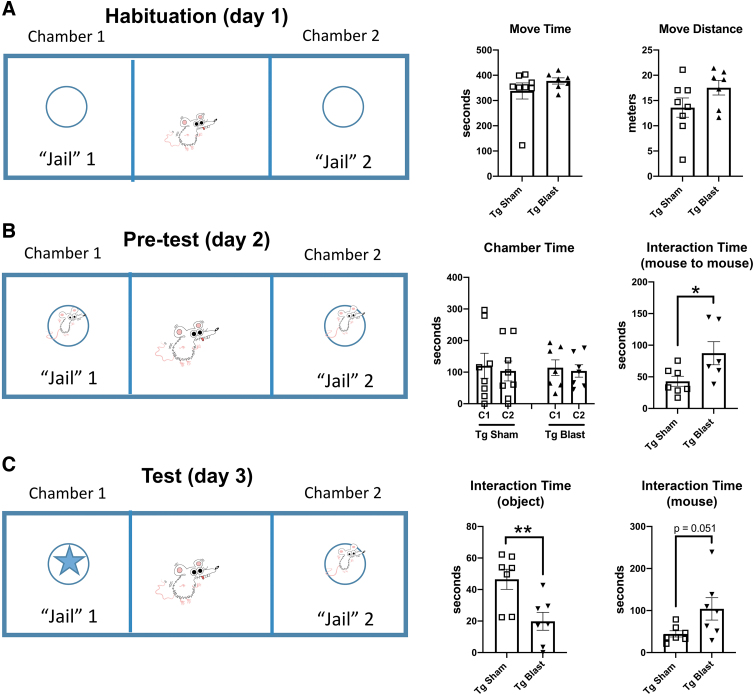
Figure 6 shows social interaction testing of cohort 1. During the habituation phase on day 1, sham- and blast-exposed Tg mice spent an equal amount of time in motion and moved similar distances exploring the empty chambers (Fig. 6A). On day 2, when presented with two unfamiliar test mice in different chambers, sham- and blast-exposed mice spent an equal amount of time in each chamber (Fig. 6B). The blast-exposed mice, however, spent more total time interacting with the test mice (Fig. 6B).
FIG. 6.
Social preference testing of cohort 1. Blast-exposed (n = 7) and control (n = 8) mice from cohort 1 were tested in a social preference test. On day 1 (A), the test subjects were first habituated to the apparatus containing two empty metal cups in the side chambers. Time in motion (Move Time) and distance moved (Move Distance) area shown. The Tg sham and Tg blast mice spent an equal amount of time in motion and moved similar distances. In the pre-test on day 2 (B), subjects were allowed to interact with two non-Tg mice. Time spent in the two chambers (Chamber Time) and total time interacting with the test mice (Interaction TIme) are shown. The Tg sham and Tg blast mice spent an equal amount of time in each chamber (C1 and C2). The Tg blast mice, however, spent more time interacting with the two test mice. Panel (C) shows time interacting with the object and time interacting with the unfamiliar test mouse in the test phase on day 3. Compared with the Tg sham, the Tg blast mice spent less time interacting with the object and more time interacting with the test mouse. Error bars in all panels indicate the standard error of the mean. Asterisks indicate values significantly different between blast- and sham-exposed mice at individual time points (*p < 0.05, **p < 0.01, unpaired t tests).
In the test phase on day 3 (Fig. 6C), when given the choice of exploring an object or unfamiliar test mouse, the Tg blast mice spent less time interacting with the object and more time interacting with the mouse compared with the Tg sham. Thus, repetitive low-level blast exposure improves social interactions in APP/PS1 Tg mice when initiated at 20 weeks of age.
Repetitive low-level blast exposure is less effective at improving behavioral deficits in APP/PS1 Tg mice when begun at 36 weeks of age
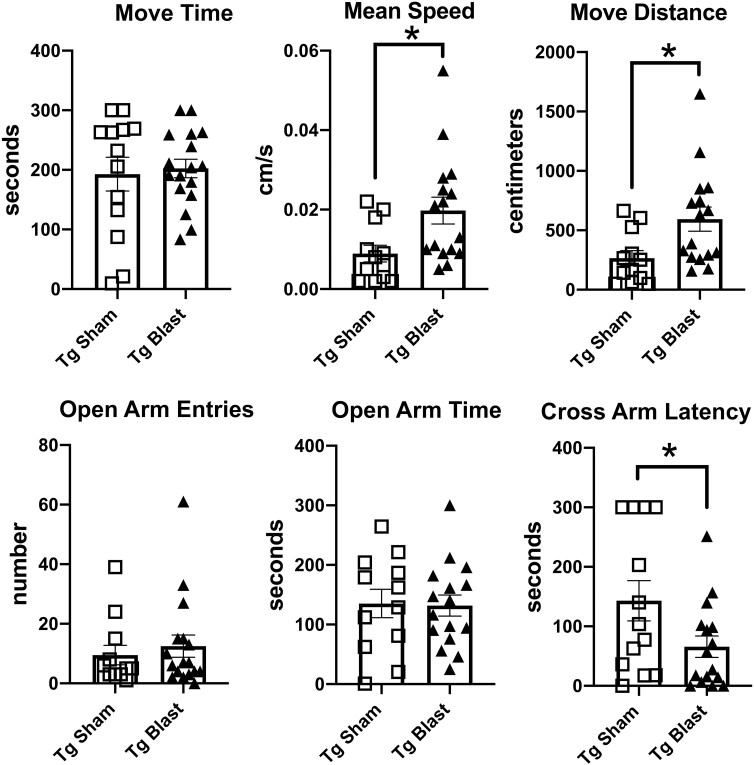
Cohort 2 began blast exposure at 36 weeks of age, a time at which significant plaque burdens are established in APP/PS1 Tg mice.38 When studied between 53 and 62 weeks of age (Fig. 1), there were no differences in locomotor activity between sham- and blast-exposed APP/PS1 Tg mice in an open field. In a L/D escape task, while there was a trend for blast-exposed APP/PS1 Tg mice to make more entries into the light center and spend more time on the light side, these trends did not reach statistical significance (p = 0.06, unpaired t tests in both parameters). In the EZM, as with cohort 1 (Fig. 3), blast-exposed APP/PS1 Tg mice of cohort 2 moved more and exhibited shorter cross arm latencies, although they did not differ from sham-exposed in open arm time (Fig. 7).
FIG. 7.
Elevated zero maze (EZM) testing of cohort 2. Amyloid precursor protein/presenilin 1 (APP/PS1) transgenic (Tg) mice were exposed to blast (n = 16) or sham (n = 16) conditions beginning at 36 weeks of age and received three blast exposures per week for eight weeks. Behavioral testing was begun at 45 weeks of age (Fig. 1). Time in motion (Move Time), mean speed, total distance traveled (Move Distance), open arm entries, open arm time, and the latency to cross into the second open arm (Cross Arm Latency) are displayed. Error bars indicate the standard error of the mean. Asterisks indicate values significantly different (*p < 0.05, unpaired t tests).
In NOR (Fig. 8A), blast-exposed APP/PS1 Tg mice spent more time exploring the NO in both the STM and LTM testing, while the sham exposed APP/PS1 Tg explored the NO and FO a similar amount of time. Blast-exposed APP/PS1 Tg mice also spent more total time exploring the objects during the NOR training session (Fig. 8B). Barnes maze testing (Fig. 8C) revealed that both sham- and blast-exposed APP/PS1 Tg mice showed decreased latencies to enter the target across trials indicating both groups learned the task. There were no differences, however, in the learning curve latencies between the sham- and blast-exposed APP/PS1 Tg mice.
FIG. 8.
Novel object recognition (NOR) and Barnes maze testing of cohort 2. Blast-exposed (n = 16) and sham-exposed (n = 16) amyloid precursor protein/presenilin 1 (APP/PS1) transgenic (Tg) mice from cohort 2 were tested in a NOR and Barnes maze. Panel (A) shows time spent exploring the objects (OB1 and OB2) during the NOR training session as well as exploration of the previously presented familiar object (FO) compared with the novel object (NO) when presented 1 h (short-term memory, STM) or 24 h (long-term memory, LTM) later. Panels (B) shows the total time spend exploring the objects during the indicated NOR sessions. Panel (C) shows the latency to enter the escape hole in the Barnes maze. A repeated measures analysis of variance revealed a significant within subjects effect by trial (F 2.731, 76.456 = 48.668, p < 0.001) but no effect of trial*condition (F 2.731, 76.456 = 1.054, p = 0.370) or between subjects effects (F 1, 28 = 0.971, p = 0.333). Error bars in all panels indicate the standard error of the mean. Asterisks indicate values significantly different (*p < 0.05, **p < 0.01, unpaired t tests).
When fear learning was tested, neither sham- nor blast-exposed APP/PS1 Tg mice showed increased freezing after the presentation of the tone/shock pairings during the training session, suggesting that neither group responded normally to the US. In the cued phase testing, neither sham- nor blast-exposed APP/PS1 Tg mice responded with freezing after presentation of the tone, consistent with neither group having formed an association between the tone with the shock during the training session.
Thus, repetitive low-level blast exposure was less successful at improving behavior in APP/PS1 Tg mice when begun at 36 weeks of age than at 20 weeks of age. Exposure beginning at 36 weeks did not improve Barnes maze performance or rescue fear learning. It also did not improve social interactions. While improving performance in NOR, and partially improving anxiety measures, it appeared less effective at 36 weeks—e.g. not improving open arm time in the EZM (Fig. 7), which was improved at 20 weeks (Fig. 3).
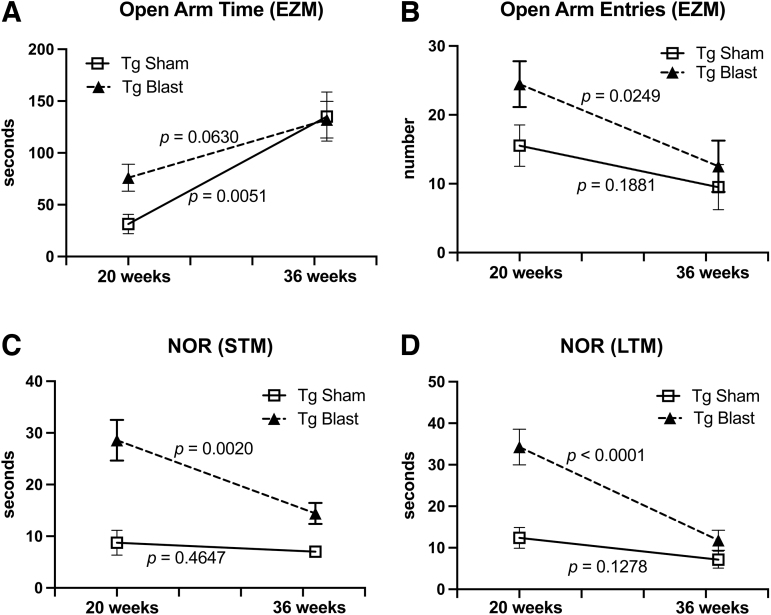
To further explicitly test the effect of age at time of exposure, we performed simple linear regressions comparing behavioral parameters at 20 weeks with 36 weeks. As shown in Figure 9A, although open arm time increased in Tg blast and Tg sham between 20 weeks and 36 weeks, the increase in Tg blast did not reach statistical significance while the increase in Tg sham was statistically significant. By contrast, open arm entries in Tg blast significantly decreased between 20 and 36 weeks but did not change in Tg sham (Fig. 9B).
FIG. 9.
Regression analysis of behavior comparing cohorts 1 and 2. Simple linear regressions were performed comparing cohorts 1 and 2, which were blast exposed beginning at 20 weeks (cohort 1) or 36 weeks (cohort 2) of age. Shown is open arm time (A) or open arm entries (B) in the elevated zero maze (EZM) as well as time spent exploring the novel object in short term memory (STM) (C) or long term memory (LTM) (D) testing of novel object recognition (NOR). The p values indicate whether slopes were significantly non-zero.
In NOR, Tg blast-exposed animals spent less time exploring the NO at 36 weeks compared with 20 weeks in both STM and LTM testing, while NO exploration time was not significantly different in Tg sham (Fig. 9C, 9D). Thus, in both tests, the diminished effect of blast exposure at 36 weeks reflected a worsening of performance in Tg blast rather than a change in performance of Tg sham. This failure may reflect the more advanced amyloid pathology present in APP/PS1 Tg mice at 36 weeks of age,38 which rendered them less responsive to the effects of blast exposure.
Repetitive low-level blast exposure initiated at 20 weeks of age returns many behavioral parameters in APP/PS1 Tg mice to the levels of non-transgenic wild type mice
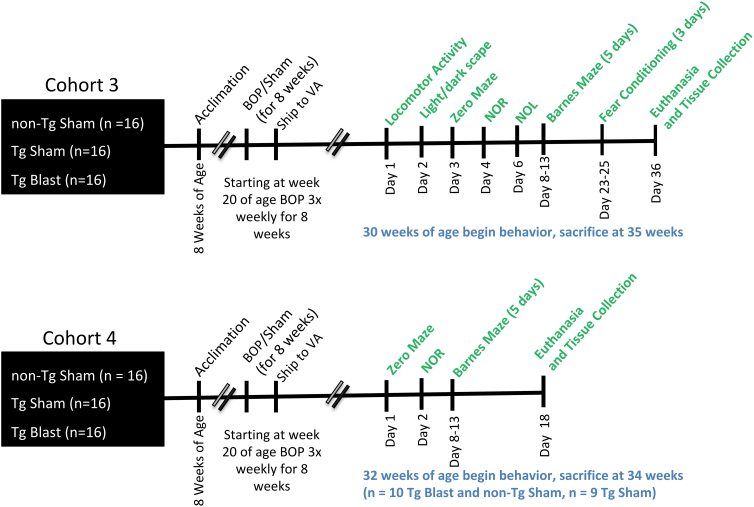
To determine whether repetitive low-level blast exposure could return behavioral parameters in APP/PS1 Tg mice to the levels of non-transgenic wild type mice, we repeated experiments utilizing two additional cohorts of mice (cohorts 3 and 4) that included a control group consisting of sham-exposed non-transgenic (non-Tg) littermates. The three groups (non-Tg sham, Tg sham, and Tg blast) received three blast exposures per week for eight weeks beginning at 20 weeks of age. The groups and group sizes are indicated in Table 1. The timing of behavioral testing and tissue harvesting is shown in Figure 10. Results for behavioral testing of cohorts 3 and 4 are summarized in Table 1.
FIG. 10.
Timeline of experiments for cohorts 3 and 4. BOP, blast overpressure exposure; NOR, novel object recognition; NOL, novel object localization.
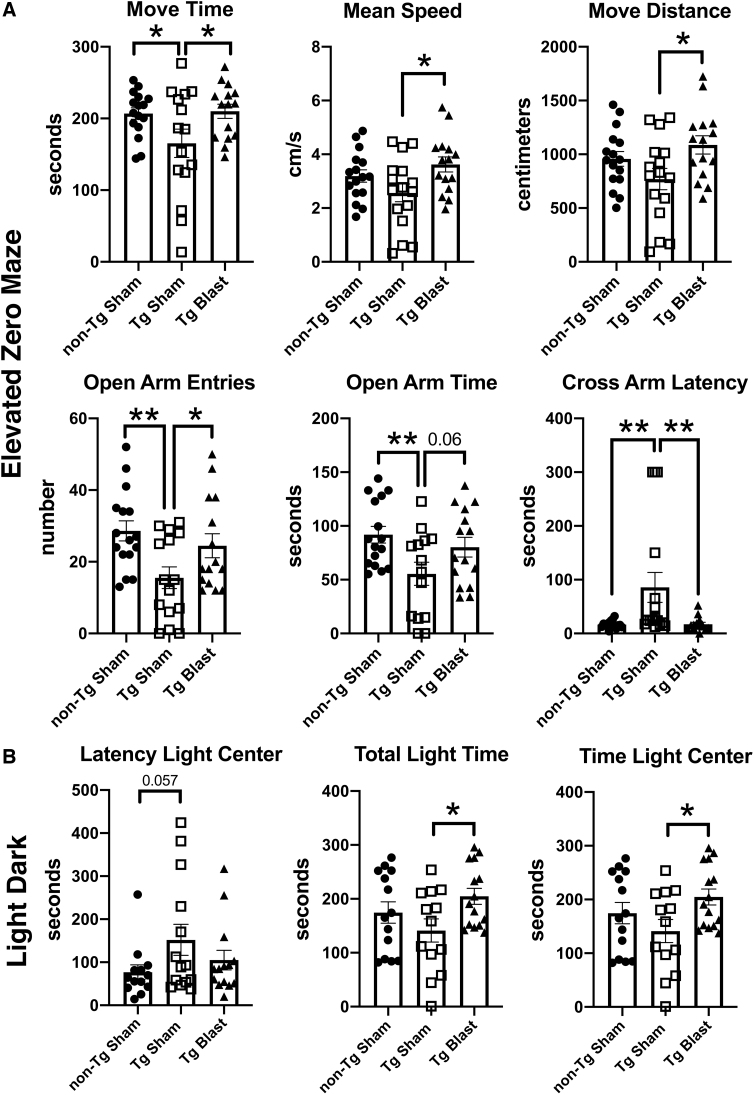
Figure 11 shows testing of cohort 3 in an EZM and a L/D escape task. Comparing Tg sham with non-Tg sham in the EZM (Fig. 11A), Tg sham mice showed evidence of anxiety, moving less and making fewer open arm entries, as well as spending less time in the open arms and exhibiting a prolonged cross-arm latency compared with sham-exposed non-Tg mice. These deficits were rescued in blast-exposed APP/PS1 Tg mice, with all parameters in Tg-blast mice being similar to sham-exposed non-Tg controls.
FIG. 11.
Elevated zero maze (EZM) and light dark (L/D) escape testing of cohort 3. Amyloid precursor protein/presenilin 1 (APP/PS1) transgenic (Tg) mice were exposed to blast (n = 16) or sham (n = 16) conditions. Non-transgenic (non-Tg) littermate controls (n = 16) were exposed to sham conditions. Mice were subjected to blast or sham conditions beginning at 20 weeks of age and received three blast exposures per week for eight weeks. The times for behavioral testing are shown in Figure 8 and Table 1. For the EZM (A), time in motion (Move Time), mean speed, distance moved (Move Distance), open arm entries, time spent in the open arms, and the latency to cross into the second open arm (Cross Arm Latency) are shown. In the L/D escape task (B), the latency to reach the light center as well as total time spent on the light side and time spent in the light center are shown. Error bars indicate the standard error of the mean. Overall group differences were compared using a one-way analysis of variance (ANOVA). Asterisks indicate significant differences between groups after a significant (p < 0.05) one-way ANOVA (*p < 0.05, **p < 0.01, Fisher least significant difference).
Similar trends were found in the L/D escape task (Fig. 11B). While total time spent on the light side and total time in the light center was reduced in Tg sham compared with Tg blast, Tg blast and non-Tg sham did not differ. Thus, repetitive low-level blast exposure rescued the anxiety phenotype found in sham-exposed APP/PS1 Tg mice.
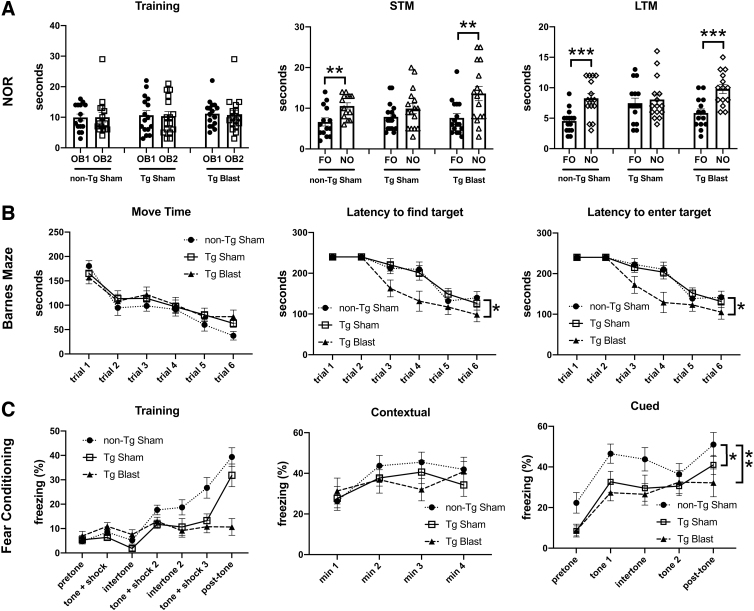
Testing in a NOR task is shown in Fig. 12A. In both STM and LTM testing, sham-exposed Tg mice failed to distinguish the FO and NO. By contrast, sham-exposed non-Tg and blast-exposed Tg mice spent more time exploring the NO than the FO in both STM and LTM testing. Thus, blast exposure rescued recognition memory deficits in APP/PS1 Tg mice. In a Barnes maze, all three groups learned the task, exhibiting progressively shorter latencies across trials to enter the target quadrant or the escape hole (Fig, 12B). Blast-exposed Tg mice, however, exhibited shorter latencies both to enter the target quadrant as well as enter the escape hole compared with either the non-Tg sham or Tg sham groups.
FIG. 12.
Testing of cohort 3 in novel object recognition (NOR), Barnes maze, and fear learning. Amyloid precursor protein/presenilin 1 (APP/PS1) transgenic (Tg) mice were exposed to blast (n = 16) or sham (n = 16) conditions. Non-transgenic (non-Tg) littermate controls (n = 16) were exposed to sham conditions. Panel (A) shows time spent exploring the objects (OB1 and OB2) during the NOR training session as well as exploration of the previously presented familiar object (FO) compared with the novel object (NO) when presented 1 h (short-term memory, STM) or 24 h (long-term memory, LTM) later. Panel (B) shows time in motion (Move Time), the latency to find the target quadrant, and the latency to enter the escape hole in the Barnes maze. For time in motion, a repeated measures analysis of variance (ANOVA) revealed a significant within subjects effect by trial (F 3.697, 162.651 = 25.521, p < 0.001) but no effect of trial*condition (F 7.393, 162.651 = 0.702, p = 0.678) or between subjects effects (F 2, 44 = 1.464, p = 0.242). A repeated measures ANOVA of time to find the target quadrant revealed a significant within subjects effect by trial (F 3.542, 155.062 = 48.808, p < 0.001) but no effect of trial*condition (F 7.048, 155.062 = 1.971, p = 0.062). There were significant between subjects effects (F 2, 44 = 4.314, p = 0.019). Post hoc tests (Fisher least significant difference [LSD]) revealed significant effects for non-Tg sham vs. blast Tg (p = 0.033) and sham Tg vs. blast Tg (p = 0.046) but no difference between non-Tg sham vs. Tg sham (p = 0.981). A repeated measures ANOVA of time to enter the escape hole revealed a significant within-subjects effect by trial (F 3.286, 141.293 = 50.984, p < 0.001) but no effect of trial*condition (F 6.572, 141.293 = 2.064, p = 0.055). There were significant between- subjects effects (F 2, 43 = 4.312, p = 0.020). Post hoc tests (Fisher LSD) revealed significant effects for non-Tg sham vs. blast Tg (p = 0.033) and Tg sham vs. Tg blast (p = 0.043) but no difference between non-Tg sham vs. sham Tg (p = 0.986). For the fear conditioning paradigm (C), results are shown for the training phase, contextual fear memory, which was tested 24 h after training, and cued fear memory, which was tested another 24 h later. Pre-tone represents freezing before the first presentation of the tone ± shock. A repeated measures ANOVA of freezing during the training sessions revealed a significant within-subjects effect of freezing across the training sessions for all groups combined (F 3.353, 147.533 = 33.836, p < 0.001) and a significant interaction effect of freezing*condition (F 6.706, 147.533 = 7.570, p < 0.001). When analyzed alone, however, the Tg blast mice did not show increased freezing across the trials (F 2.468, 37.023 = 1.036; p = 0.378). There were no differences between the groups in the contextual testing (F 2, 43 = 0.473; p = 0.626). In the cued phase testing, a repeated measures ANOVA comparing freezing in the pre-tone to first tone across all groups revealed increased freezing (F 1, 43 = 73.436, p < 0.001) without interaction effects (F 2, 43 = 0.504; p = 0.608). There were significant between-subjects effects (F 2, 43 = 6.108, p = 0.005), however. Post hoc tests revealed significant effects for non-Tg sham vs. Tg blast (p = 0.002) and non-Tg sham vs. Tg sham (p = 0.008) but no difference Tg sham vs. Tg blast (p = 0.594). A repeated measures ANOVA comparing freezing across all groups and all trials revealed increased freezing (F 4, 172 = 20.977, p < 0.001) without interaction effects (F 8, 172 = 0.728; p = 0.666). There were significant between-subjects effects (F 2, 43 = 4.281, p = 0.02), however. Post hoc tests revealed significant effects for non-Tg sham vs. Tg blast (p = 0.008) and non-Tg sham vs. sham Tg (p = 0.032) but no difference between Tg sham vs. Tg blast (p = 0.551). Error bars in all panels indicate the standard error of the mean (*p < 0.05, **p < 0.01, ***p < 0.001, Fisher LSD).
Interpretation of the fear conditioning results for cohort 3 (Fig. 12C) was complicated by the fact that blast-exposed APP/PS1 Tg mice did not show increased freezing across the training trials unlike the wild type non-Tg sham and APP/PS1 Tg sham groups, suggesting that Tg blast mice at baseline exhibited abnormal freezing behavior. In the contextual phase testing, freezing in Tg blast mice was similar to the other two groups, suggesting that Tg blast mice nevertheless had intact memory for the context in which the shocks were presented.
In the cued phase testing, when pre-tone freezing was compared with the first presentation of the tone, all groups showed increased freezing. The Tg sham and Tg blast, however, froze significantly less than non-Tg sham mice (Fig. 12C). Comparing freezing across all trials gave similar results, revealing that Tg sham and Tg blast mice froze significantly less than non-Tg sham mice (Fig. 12C).
Figure 13 shows testing of cohort 4 in EZM and NOR. Comparing Tg sham with non-Tg sham in the EZM (Fig. 13A), Tg sham mice showed evidence of anxiety, moving less distance and spending less time in the open arms compared with sham-exposed non-Tg mice. These deficits were rescued in blast-exposed APP/PS1 Tg mice with parameters being restored to sham-exposed non-Tg controls.
FIG. 13.
Elevated zero maze (EZM), novel object recognition (NOR) and Barnes maze testing of cohort 4. Amyloid precursor protein/presenilin 1 (APP/PS1) transgenic (Tg) mice were exposed to blast (n = 10) or sham (n = 9) conditions. Non-transgenic (non-Tg) littermate controls (n = 10) were exposed to sham conditions. For the EZM (A), time in motion (Move Time), mean speed, distance moved (Move Distance), open arm latency, and time spent in the open arms area are shown. Panel (B) shows time spent exploring the objects (OB1 and OB2) during the novel object recognition (NOR) training session as well as exploration of the previously presented familiar object (FO) compared with the novel object (NO) when presented 1 h (short-term memory, STM) or 24 h (long-term memory, LTM) later. Panel (C) shows the total time spent exploring the objects during the indicated NOR sessions. Error bars in all panels indicate the standard error of the mean. Overall group differences were compared using a one-way analysis of variance (ANOVA). Asterisks indicate significant differences between groups after a significant (p < 0.05) one-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, Fisher least significant difference [LSD]). Panel (D) shows time to enter the target quadrant in the Barnes maze. A repeated measures ANOVA revealed a significant within-subjects effect by trial (F 2.306, 57.641 = 37.499, p < 0.001) but no effect of trial*condition (F 4.611, 57.641 = 2.368, p = 0.055). There were significant between-subjects effects (F 2, 25 = 25.178, p < 0.001). Post hoc tests (Fisher LSD) revealed significant effects for non-Tg sham vs. Tg sham (p < 0.001), non-Tg sham vs. Tg blast (p = 0.003), and Tg blast vs. Tg sham (p = 0.001). A one-way ANOVA of latencies for trial 5 alone revealed significant between-group effects (F 2, 25 = 11.90, p = 0.0002). Post hoc comparisons revealed significant effects for non-Tg vs. Tg sham (p < 0001) and Tg blast vs. Tg sham (p = 0.0013) but no difference between non-Tg and Tg blast (p = 0.35). Error bars in all panels indicate the standard error of the mean (**p < 0.01, ****p < 0.001, Fisher LSD).
Figure 13B shows testing in a NOR task. In LTM testing, sham-exposed Tg mice failed to distinguish the FO and NO. By contrast, sham-exposed non-Tg and blast-exposed Tg mice spent more time exploring the NO than the FO in LTM testing. Sham-exposed Tg mice spent less total time exploring the objects in all three sessions compared with non-Tg sham mice (Fig. 13C). This effect was rescued in blast-exposed Tg mice that spent more time exploring the objects than non-Tg sham mice in training and STM testing. In the Barnes maze (Fig. 13D), non-Tg sham mice and blast-exposed Tg mice learned to find the target significantly faster than Tg sham mice, although the learning curves of the Tg blast mice were not as sharp as those of the non-Tg sham mice.
Thus, blast exposure rescued anxiety and recognition memory deficits in sham-exposed APP/PS1 Tg mice and improved spatial memory compared with sham-exposed APP/PS1 Tg mice. Table 1 summarizes the behavioral testing results in cohorts 3 and 4.
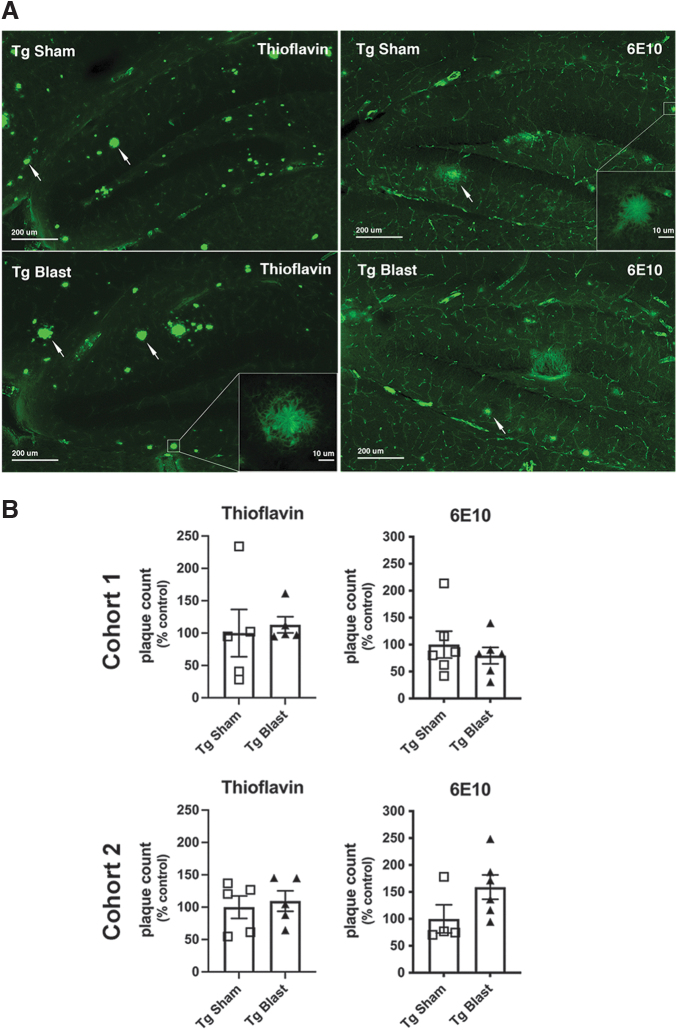
Repetitive low-level blast exposure reduces soluble, insoluble, and oligomeric Aβ levels, but amyloid plaque burden is unchanged by blast exposure
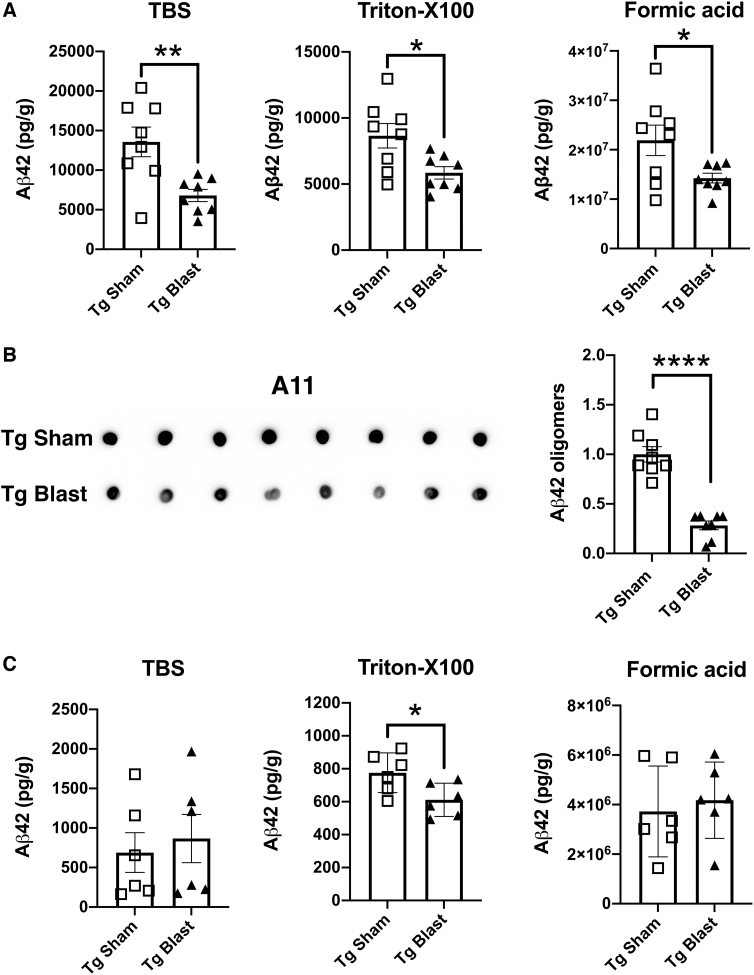
To determine the effects of repetitive low-level blast exposure on plaque load, we measured plaque loads in APP/PS1 Tg mice from cohorts 1 and 2 subjected to blast or sham conditions. Using either thioflavin S staining or immunohistochemical staining with the antibody 6E10, plaque counts were unchanged in these mice (Fig. 14). We next examined Aβ42 levels in the brain of APP/PS1 Tg mice from cohort 3 by ELISA using tissue collected after behavioral testing, which finished when mice were approximately 9 months of age (7 weeks after the last blast exposure; Fig. 8). Aβ42 was decreased in TBS, Triton X-100, and formic acid-extractable fractions in blast- compared with sham-exposed mice (Fig. 15A).
FIG. 14.
Amyloid plaque loads in brains of mice exposed to repetitive low-level blast exposure. Plaque density in the hippocampus was determined in amyloid precursor protein/presenilin 1 (APP/PS1) transgenic (Tg) mice from cohorts 1 and 2 subjected to blast or sham conditions using either thioflavin S staining or immunohistochemical staining with the antibody 6E10. Panel (A) shows representative sections stained with thioflavin S or immunostained with antibody 6E10 from cohort 1. Scale bars = 200 μm; insets = 10 μm. Panel (B) shows quantitative plaque counts expressed as number per hippocampus. Error bars in all panels indicate the standard error of the mean. There were no statistically significant differences between the groups.
FIG. 15.
The Aβ42 levels and Aβ oligomers in the brain of mice exposed to repetitive low-level blast. In panel (A), Aβ42 levels were determined by enzyme-linked immunosorbent assay in blast- or sham-exposed amyloid precursor protein/presenilin 1 (APP/PS1) transgenic (Tg) mice from cohort 3. In panel (B), Aβ oligomers were determined in the Tris-buffered saline (TBS) fraction using the same samples studied in panel (A) with antibody A11. A representative dot blot is shown and is quantified in the bar graph. Panel (C) shows Aβ42 in a group of mice from cohort 4 that were euthanized within one week of the last blast exposure. Error bars indicate the standard error of the mean (*p < 0.05, **p < 0.01, ****p < 0.0001, unpaired t tests).
Levels of oligomeric Aβ were determined in cohort 3 using monoclonal antibody A11. As shown in Figure 15B, oligomeric Aβ in blast-exposed APP/PS1 Tg mice was decreased to about 33% of that in sham-exposed APP/PS1 Tg mice. In addition, we examined Aβ42 in a group of mice from cohort 4 that were euthanized within one week of the last blast exposure. In these mice, which were euthanized at six months of age and thus younger than cohort 3, Aβ 42 was decreased in the Triton X-100 fraction while Aβ 42 in TBS and formic acid-extractable fractions were unchanged (Fig. 15C).
These studies thus show that while repetitive low-level blast exposure does not alter amyloid plaque load, Aβ 42 levels and Aβ oligomers are reduced, and these reductions are sustained for at least 3 months after the last blast exposure.
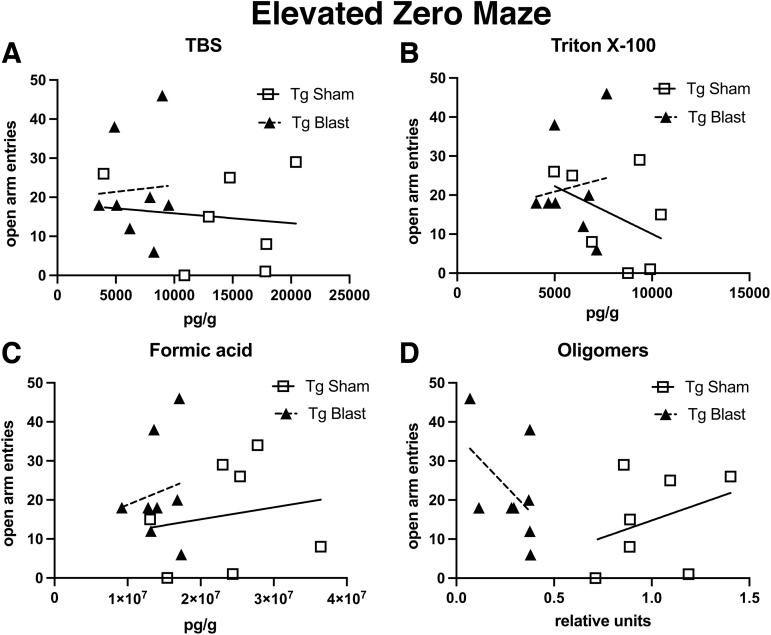
Next, we determined whether levels of soluble, insoluble, or oligomeric Aβ42 could be directly correlated with behavioral parameters in individual animals in cohort 3. Table 2 shows correlation coefficients calculated between Aβ42 levels and open arm entries in the EZM or the DI in NOR. There were no significant correlations when the blast or sham was analyzed separately and only one significant negative correlation between DI and TBS soluble Aβ42 when the sham and blast were pooled (Table 2).
Table 2.
Correlation between Aβ42 Levels and Behavioral Parameters in the Elevated Zero Maze and Novel Object Recognition
| |
Pearson |
Kendall tau-b |
Spearman rho |
|||
|---|---|---|---|---|---|---|
| Correlation coefficient | p | Correlation coefficient | p | Correlation coefficient | p | |
| Open arm entries (EZM) | ||||||
| Sham | ||||||
| TBS | -0.237 | 0.572 | -0.071 | 0.805 | -0.167 | 0.693 |
| Triton X-100 | 0.070 | 0.869 | 0.071 | 0.805 | 0.143 | 0.736 |
| Formic acid | 0.006 | 0.989 | 0.143 | 0.621 | 0.190 | 0.651 |
| Oligomeric | 0.260 | 0.534 | 0.214 | 0.458 | 0.238 | 0.570 |
| Blast | ||||||
| TBS | 0.056 | 0.896 | -0.038 | 0.899 | 0.000 | 1.000 |
| Triton X-100 | 0.132 | 0.756 | 0.038 | 0.899 | 0.098 | 0.818 |
| Formic acid | 0.157 | 0.710 | 0.113 | 0.702 | 0.098 | 0.818 |
| Oligomeric | -0.489 | 0.219 | -0.385 | 0.200 | -0.528 | 0.179 |
| Sham + Blast: | ||||||
| TBS | -0.227 | 0.397 | -0.160 | 0.391 | -0.233 | 0.385 |
| Triton X-100 | -0.042 | 0.877 | -0.127 | 0.498 | -0.147 | 0.586 |
| Formic acid | -0.70 | 0.798 | 0.008 | 0.964 | 0.024 | 0.931 |
| Oligomeric | -0.176 | 0.514 | -0.119 | 0.527 | -0.151 | 0.578 |
| Discrimination index (NOR ) | ||||||
| Sham | ||||||
| TBS | -0.344 | 0.404 | -0.071 | 0.806 | -0.071 | 0.867 |
| Triton X-100 | -0.486 | 0.222 | -0.357 | 0.216 | -0.476 | 0.233 |
| Formic acid | 0.185 | 0.662 | 0.000 | 1.000 | 0.000 | 1.000 |
| Oligomeric | 0.206 | 0.625 | 0.071 | 0.805 | -0.024 | 0.955 |
| Blast | ||||||
| TBS | -0.455 | 0.258 | -0.357 | .0216 | -0.476 | 0.233 |
| Triton X-100 | 0.306 | 0.461 | 0.071 | 0.805 | 0.119 | 0.779 |
| Formic acid | 0.063 | 0.881 | 0.143 | 0.621 | 0.048 | 0.621 |
| Oligomeric | 0.038 | 0.929 | 0.327 | 0.262 | 0.539 | 0.168 |
| Sham + Blast | ||||||
| TBS | -0.530 | 0.035 | -0.460 | 0.013 | -0.648 | 0.007 |
| Triton X-100 | -0.428 | 0.098 | -0.310 | 0.095 | -0.446 | 0.083 |
| Formic acid | -0.127 | 0.639 | -0.059 | 0.752 | -0.116 | 0.668 |
| Oligomeric | -0.345 | 0.190 | -0.185 | 0.321 | -0.268 | 0.316 |
EZM, elevated zero maze; TBS, Tris buffered saline; NOR, novel object recognition.
Aβ42 levels in APP/PS1 Tg mice from cohort 3 in the TBS, Triton X-100 and formic acid.
Fractions as well as levels of A11 reactive Aβ42 oligomers were correlated with open arm entries in the EZM and the discrimination index in NOR. Correlations with p values less than 0.05 are indicated in bold. Data are shown as sham (n = 8) or blast (n = 8) analyzed alone or pooled (n = 16); sham + blast).
Figure 16 shows open arm entries in the EZM correlated with Aβ42 levels. There was a relatively tight clustering of Aβ42 levels in all of the fractions in the Tg blast, although no correlation was apparent between Aβ42 levels and number of open arm entries in individual animals. While data were generally more spread in Tg sham, there was again no correlation between Aβ42 levels and number of open arm entries in individual animals. Relatively similar results were seen when a DI was calculated for cohort 3 in the STM testing of NOR and correlated with levels of Aβ42 in individual animals (Fig. 17). Thus, while soluble, insoluble, and oligomeric Aβ42 correlate with behavioral parameters in the aggregate, they did not correlate with behavioral performance in individual animals.
FIG. 16.
Correlations between soluble, insoluble and oligomeric Aβ42 with behavioral performance in the elevated zero maze (EZM). The Aβ42 in the Tris-buffered saline (TBS) (A), Triton X-100 (B), and formic acid (C) fractions as well as oligomeric Aβ42 (D) in amyloid precursor protein/presenilin 1 (APP/PS1) transgenic (Tg) mice from cohort 3 (Fig. 15) were correlated with open arm entries in the EZM (Fig. 11). There were no significant correlations (Table 2).
FIG. 17.
Behavioral measures in novel object recognition (NOR) correlated with soluble, insoluble, and oligomeric Aβ42. The Aβ42 in the Tris-buffered saline (TBS) (A), Triton X-100 (B), and formic acid (C) fractions as well as oligomeric Abβ42 (D) determined in amyloid precursor protein/presenilin 1 (APP/PS1) transgenic (Tg) mice from cohort 3 (Fig. 15), were correlated with data for the SM testing phase of NOR (Fig. 12). There were no significant correlations (Table 2).
Discussion
TBI is a risk factor for later development of neurodegenerative diseases that may have varied underlying pathologies.5–7 Aβ deposition is a hallmark of AD, and epidemiological studies support an association of severe TBI with later development of AD.7,12 Changes in brain Aβ levels occur rapidly after TBI with increased levels of soluble Aβ and diffuse cortical deposits present in humans as early as 2 h after a severe injury.7,12–14
The Aβ elevations also occur acutely in the brain in many experimental animal models that mimic the type of contusional and rotation/acceleration injuries associated, for example, with motor vehicle accidents or sports injuries.15–26 In these models, there is also increased expression of APP, along with BACE1 (β-site APP cleaving enzyme 1), the principal β-secretase16,39–42 and the γ-secretase complex that together are responsible for generating Aβ. It has been suggested that upregulation of this amyloidogenic APP processing pathway that favors Aβ production over other non-amyloidogenic APP processing pathways43 may help explain the epidemiological associations between TBI and AD.7,12
We were thus surprised in a previous study that, in both rat and mouse models of blast exposure, rather than being increased, rodent brain Aβ42 levels were decreased after acute exposure.29 Here we subjected a transgenic mouse model of AD to an extended sequence of repetitive low-level blast exposures designed to mimic the equivalent of a human subclinical blast exposure of approximately 5 psi that does not present acute symptoms.
Because blast-related brain injury may involve a combination of injuries related to effects of the primary blast wave as well as damage from rotation/acceleration injury,44,45 during the blast overpressure exposures, head motion is restricted to minimize rotation/acceleration forces. Studies using this exposure level (34.5 kPa) in rodents produce no obvious neuropathological effects or acute behavioral deficits. Because multiple subclinical blast exposures are common for many service members in combat as well as non-combat settings,3 we utilized a protocol that involved three exposures per week delivered one exposure per day over an eight-week period. We began exposures at 20 or 36 weeks of age, which respectively represent times before or after this line of APP/PS1 Tg mice develop significant plaque burdens.38
We show that repetitive blast exposures improved behavioral deficits (Table 1) and chronically lowered Aβ42 in the brain. Improved behavioral effects were seen across a range of anxiety related tests (EZM, L/D, open field). Improved cognition was seen in NOR and NOL tasks as well as Barnes maze. Blast exposure also improved social behavior. These effects were most apparent in APP/PS1 Tg mice that received blast exposures beginning at 20 weeks of age. Beneficial effects were not apparent only in fear learning.
Results were less robust in mice when blast exposure began at 36 weeks of age, likely reflecting the greater difficulty of reversing behavioral deficits in mice with more extensive amyloid burden.39 When these experiments were repeated with inclusion of sham exposed non-Tg littermates, repetitive low-level blast exposure returned many behavioral parameters in APP/PS1 Tg mice to the levels of non-Tg wild type mice.
Accompanying improved behavior, soluble, insoluble, and oligomeric Aβ42 levels were reduced in brain of mice exposed to repetitive low-level blast exposure. This was most apparent in the brain of APP/PS1 Tg mice from cohort 3 in which tissue was collected after behavioral testing that finished when mice were about nine months of age. In these mice Aβ42 was decreased in TBS, Triton X-100, and formic acid-extractable fractions in blast-exposed compared with sham-exposed mice. Further, Aβ oligomers in cohort 3 were decreased to approximately 33% of the levels in sham-exposed APP/PS1 Tg mice.
Oligomeric Aβ is generally considered the most toxic Aβ species.46 Its lowering after blast exposure is consistent with this being one mechanism of blast's beneficial effect. While behavior in the aggregate improved in blast-exposed mice, there was no correlation between oligomeric Aβ or Aβ42 levels in any of the fractions measured with behavioral parameters in individual animals suggesting that other factors are influencing behavioral outcomes as well.
Aβ42 was also determined in a group of mice from cohort 4 that were euthanized within one week after the last blast exposure. In these mice, which were euthanized at six months of age and thus younger than those in cohort 3, Aβ42 was decreased in the Triton X-100 fraction, while Aβ42 in TBS and formic acid-extractable fractions were unchanged. Interestingly, despite the changes in Aβ42 levels, amyloid plaque burdens were unchanged in APP/PS1 Tg mice whether the blast exposure protocol began at 20 weeks (cohort 1) or 36 weeks (cohort 2) of age. Therefore, while repetitive low-level blast exposure does not alter amyloid plaque load, Aβ42 levels and Aβ oligomers were reduced, and these reductions are sustained for at least three months after the last blast exposure.
One previous study examined the effect of blast injury on the same APP/PS1 Tg mouse line studied here.48 In this study, which focused primarily on retinal injury, APP/PS1 Tg mice were exposed to a single 20-psi (137.9-kPa) blast exposure at two to three months of age. Two months later, retinal ganglion cell structure and function were impaired in Tg mice compared with non-Tg littermates. No Aβ deposits were detected in retinas of APP/PS1 Tg mice. Increased APP and Aβ immunoreactivity, however, were found in the blast-exposed Tg animals particularly near blood vessels.
In the brain, a statistically non-significant trend for greater cortical Aβ plaque load was seen in transgenic blast versus sham groups.47 This study differs from ours in both the relatively high level of blast exposure and time-course of studies suggesting that differences in blast dose and frequency may engage different targets after injury. Another recent study also found that normally regulated transgenic overexpression of wild type human APP does not contribute to deficits acutely after TBI and may, in fact, be protective.48 Thus, effects may be complex and at least partly related to the presence of the FAD related mutations in the transgene.
Studies in United States military personnel have documented the relevance of these animal findings to humans by showing that during a 10-day training exercise, which involved repeated blast exposure, Aβ42 was lowered in blood at 24 h after blast exposure.30 Transient reductions in APP and alterations of the APP signaling network in blood were also observed during training exercises that involved a moderate blast exposure.31 These studies suggest that as in experimental animals, altered APP processing is an effect of acute blast injury, although one recent study found elevated serum Aβ42 in military personnel who experienced repeated blast exposures from firing 0.50-caliber rifles in training sessions conducted over multiple days.49 Thus, effects in humans may vary with the type and intensity of exposure.
Our current findings do not explain why Aβ is decreased by repetitive low-level blast exposure. Nonetheless, it is notable that Aβ enzymatic production, proteolysis, and transport out of the brain are regulated by multiple, sometimes competing, processing pathways that can be stimulated and/or suppressed by mild traumatic insults to the brain. For example, Aβ can be internalized and degraded by microglia.50 There is evidence that a mild blast stimulates microglia to migrate toward and internalize substances that have aberrantly crossed the blood–brain barrier (BBB),51 presumably a neuroprotective response attempting to restore normal BBB functions.52 The ability of the very mild CNS injuries produced by the low-level subconcussive blasts our animals were exposed to could plausibly be expected to favor activating some neuroprotective pathways that could facilitate reducing Aβ, which in our transgenic mice is otherwise pathogenic.
Similarly, Aβ can be cleared from brain by a number of distinct proteolytic pathways.53 Moreover, there is growing evidence that transport across the BBB as well as by astroglia-mediated interstitial fluid bulk flow through the perivascular glymphatic system conduct substances, including Aβ and tau into the perineural sheaths of cranial and spinal nerves, meningeal lymphatic vessels, and arachnoid granulations.54–58 A pathway that drains along the olfactory nerve through the cribriform plate has also been described.54
In previous studies, we found that as in non-blast models, levels of APP were increased after blast exposure, although there was no evidence of axonal pathology based on APP immunohistochemical staining.29 Unlike findings in non-blast TBI animal models, however, levels of BACE-1 and the γ-secretase component, presenilin-1, were unchanged after blast exposure.29 Thus, lowered enzymatic processing of APP seems unlikely to explain the current results.
Glymphatic flow is reduced before the appearance of substantial amyloid plaque burden in the same APP/PS1 Tg mouse line we used.59 Consistent with a role for glymphatic flow in the amyloid pathology of APP/PS1 Tg mice, deep cervical lymph node ligation has been reported to exacerbate amyloid pathology,60 while treatment with a compound that promotes perivascular Aβ drainage improved cognitive performance as well as reduced parenchymal Aβ levels and plaque deposition.61 Vascular disease, which is prominent after blast-related TBI,62 may also impair glymphatic outflow after TBI.54,63 How glymphatic transport is affected by a low-level repetitive blast exposure and whether more intense blast exposures could affect this brain clearance system differently than low-level blasts is, however, not fully understood.
Conclusion
Future studies are needed to elucidate the mechanism(s) for how repetitive blast exposure improves behavioral performance and reduces Aβ levels. Such investigations will have practical implications for the management of acute blast injury, because blocking Aβ production by pharmacological or genetic means has been reported to reduce tissue damage acutely and improve outcome after controlled cortical impact injuries in mice.16,17,64 The studies reported here, however, together with our previous findings after acute blast exposure,30 suggest that such strategies may not be applicable to management of chronic blast injury if Aβ is already lowered.
Rather, these findings suggest that, paradoxically, low-level repetitive blast exposure might actually be beneficial for AD-related cognitive and behavioral changes. These findings are relevant to understanding the effects of low-level military occupational exposure.3 They also challenge the notion that any blast exposure must be bad or, at best, neutral in its effects, although how to translate these findings into a practical therapy may not be straightforward. This counterintuitive result will need further exploration in experimental animals.
Acknowledgments
The views expressed in this article reflect the results of research conducted by the authors and do not necessarily reflect the official policy or position of the of the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the Department of the Navy, or the Department of Defense (DoD). Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government. The study protocol was reviewed and approved by the Walter Reed Army Institute of Research/Naval Medical Research Center Institutional Animal Care and Use Committee in compliance with all applicable Federal regulations governing the protection of animals in research. The experiments reported herein were conducted in compliance with the Animal Welfare Act and per the principles set forth in the Guide for Care and Use of Laboratory Animals, Institute of Laboratory Animals Resources, National Research Council, National Academy Press, 2011. Some of the authors are military service members or federal/contracted employees of the United States Government. This work was prepared as part of their official duties. Title 17 U.S.C. § 105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. § 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties.
Authors' Contributions
GPG, RDG, AET, RA, UK, MAG, PRH, DGC, SG, GAE and STA designed the research. GPG, RDG, AET, MGS, RA, UK, JKS, SC, ER, TJ, GMP, AOP, and DP conducted research. GPG, RDG, AET, RA, UK, MAG, PRH, DGC, SG, GAE, and STA drafted and revised the manuscript. All authors reviewed the final manuscript.
Funding Information
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service Awards 1I01RX002660 (GE), 1I01RX000684 (SG), and 1I01RX002333 (SG), 1I21RX003459 (MAGS) and 121RX002876 (MAGS), the Department of Veterans Affairs Office of Research and Development Medical Research Service 1I01BX004067 (GE) and 1I01BX002311 (DC), by Defense Health Program (DHP) work unit number 603115HP.3520.001.A1411 from Joint Program Committee 5 (STA), the Alzheimer's Drug Discovery Foundation (SG) and by NIA P50 AG005138 and P30 AG066514 both to Mary Sano (SG, PRH).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Elder, G.A., Ehrlich, M.E., and Gandy, S. (2019). Relationship of traumatic brain injury to chronic mental health problems and dementia in military veterans. Neurosci. Lett. 707, 134294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoge, C.W., McGurk, D., Thomas, J.L., Cox, A.L., Engel, C.C., and Castro, C.A. (2008). Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463. [DOI] [PubMed] [Google Scholar]
- 3. Engel, C., Hoch, E. and Simmons, M. (2019). The Neurological Effects of Repeated Exposure to Military Occupational Blast: Implications for Prevention and Health. Proceedings, Findings, and Expert Recommendations from the Seventh Department of Defense State-of-the-Science Meeting. Arlington, VA: Rand Corporation. [Google Scholar]
- 4. Carr, W., Stone, J.R., Walilko, T., Young, L.A., Snook, T.L., Paggi, M.E., Tsao, J.W., Jankosky, C.J., Parish, R.V., and Ahlers, S.T. (2016). Repeated low-level blast exposure: a descriptive human subjects study. Mil. Med. 181, Suppl. 5, 28–39. [DOI] [PubMed] [Google Scholar]
- 5. Gandy, S., Ikonomovic, M.D., Mitsis, E., Elder, G., Ahlers, S.T., Barth, J., Stone, J.R., and DeKosky, S.T. (2014). Chronic traumatic encephalopathy: clinical-biomarker correlations and current concepts in pathogenesis. Mol. Neurodegener. 9, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeKosky, S.T., Blennow, K., Ikonomovic, M.D., and Gandy, S. (2013). Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nat. Rev. Neurol. 9, 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeKosky, S.T., Ikonomovic, M.D., and Gandy, S. (2010). Traumatic brain injury—football, warfare, and long-term effects. N. Engl. J. Med. 363, 1293–1296. [DOI] [PubMed] [Google Scholar]
- 8. Uryu, K., Chen, X.H., Martinez, D., Browne, K.D., Johnson, V.E., Graham, D.I., Lee, V.M., Trojanowski, J.Q., and Smith, D.H. (2007). Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp. Neurol. 208, 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fukumoto, H., Asami-Odaka, A., Suzuki, N., Shimada, H., Ihara, Y., and Iwatsubo, T. (1996). Amyloid beta protein deposition in normal aging has the same characteristics as that in Alzheimer's disease. Predominance of A beta 42(43) and association of A beta 40 with cored plaques. Am. J. Pathol. 148, 259–265. [PMC free article] [PubMed] [Google Scholar]
- 10. Gandy, S. (2005). The role of cerebral amyloid beta accumulation in common forms of Alzheimer disease. J. Clin. Invest. 115, 1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iwatsubo, T., Mann, D.M., Odaka, A., Suzuki, N., and Ihara, Y. (1995). Amyloid beta protein (A beta) deposition: A beta 42(43) precedes A beta 40 in Down syndrome. Ann. Neurol. 37, 294–299. [DOI] [PubMed] [Google Scholar]
- 12. Johnson, V.E., Stewart, W., and Smith, D.H. (2010). Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer's disease? Nat. Rev. Neurosci. 11, 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ikonomovic, M.D., Uryu, K., Abrahamson, E.E., Ciallella, J.R., Trojanowski, J.Q., Lee, V.M., Clark, R.S., Marion, D.W., Wisniewski, S.R., and DeKosky, S.T. (2004). Alzheimer's pathology in human temporal cortex surgically excised after severe brain injury. Exp. Neurol. 190, 192–203. [DOI] [PubMed] [Google Scholar]
- 14. DeKosky, S.T., Abrahamson, E.E., Ciallella, J.R., Paljug, W.R., Wisniewski, S.R., Clark, R.S., and Ikonomovic, M.D. (2007). Association of increased cortical soluble abeta42 levels with diffuse plaques after severe brain injury in humans. Arch. Neurol. 64, 541–544. [DOI] [PubMed] [Google Scholar]
- 15. Zhang, Q.G., Laird, M.D., Han, D., Nguyen, K., Scott, E., Dong, Y., Dhandapani, K.M., and Brann, D.W. (2012). Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS One 7, e34504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loane, D.J., Pocivavsek, A., Moussa, C.E., Thompson, R., Matsuoka, Y., Faden, A.I., Rebeck, G.W., and Burns, M.P. (2009). Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat. Med. 15, 377–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Loane, D.J., Washington, P.M., Vardanian, L., Pocivavsek, A., Hoe, H.S., Duff, K.E., Cernak, I., Rebeck, G.W., Faden, A.I., and Burns, M.P. (2011). Modulation of ABCA1 by an LXR agonist reduces beta-amyloid levels and improves outcome after traumatic brain injury. J. Neurotrauma 28, 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu, F., Zhang, Y., and Chuang, D.M. (2012). Lithium reduces BACE1 overexpression, beta amyloid accumulation, and spatial learning deficits in mice with traumatic brain injury. J. Neurotrauma 29, 2342–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tian, L., Guo, R., Yue, X., Lv, Q., Ye, X., Wang, Z., Chen, Z., Wu, B., Xu, G., and Liu, X. (2012). Intranasal administration of nerve growth factor ameliorate beta-amyloid deposition after traumatic brain injury in rats. Brain Res. 1440, 47–55. [DOI] [PubMed] [Google Scholar]
- 20. Smith, D.H., Nakamura, M., McIntosh, T.K., Wang, J., Rodriguez, A., Chen, X.H., Raghupathi, R., Saatman, K.E., Clemens, J., Schmidt, M.L., Lee, V.M., and Trojanowski, J.Q. (1998). Brain trauma induces massive hippocampal neuron death linked to a surge in beta-amyloid levels in mice overexpressing mutant amyloid precursor protein. Am. J. Pathol. 153, 1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tajiri, N., Kellogg, S.L., Shimizu, T., Arendash, G.W., and Borlongan, C.V. (2013). Traumatic brain injury precipitates cognitive impairment and extracellular Abeta aggregation in Alzheimer's disease transgenic mice. PLoS One 8, e78851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tran, H.T., LaFerla, F.M., Holtzman, D.M., and Brody, D.L. (2011). Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-beta accumulation and independently accelerates the development of tau abnormalities. J. Neurosci. 31, 9513–9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tran, H.T., Sanchez, L., Esparza, T.J., and Brody, D.L. (2011). Distinct temporal and anatomical distributions of amyloid-beta and tau abnormalities following controlled cortical impact in transgenic mice. PLoS One 6, e25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uryu, K., Laurer, H., McIntosh, T., Pratico, D., Martinez, D., Leight, S., Lee, V.M., and Trojanowski, J.Q. (2002). Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J. Neurosci. 22, 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Washington, P.M., Morffy, N., Parsadanian, M., Zapple, D.N., and Burns, M.P. (2014). Experimental traumatic brain injury induces rapid aggregation and oligomerization of amyloid-beta in an Alzheimer's disease mouse model. J. Neurotrauma 31, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Webster, S.J., Van Eldik, L.J., Watterson, D.M., and Bachstetter, A.D. (2015). Closed head injury in an age-related Alzheimer mouse model leads to an altered neuroinflammatory response and persistent cognitive impairment. J. Neurosci. 35, 6554–6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elder, G.A., Mitsis, E.M., Ahlers, S.T., and Cristian, A. (2010). Blast-induced mild traumatic brain injury. Psychiatr. Clin. North Am. 33, 757–781. [DOI] [PubMed] [Google Scholar]
- 28. Elder, G.A., Stone, J.R., and Ahlers, S.T. (2014). Effects of low-level blast exposure on the nervous system: is there really a controversy? Front. Neurol. 5, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Gasperi, R., Gama Sosa, M.A., Kim, S.H., Steele, J.W., Shaughness, M.C., Maudlin-Jeronimo, E., Hall, A.A., Dekosky, S.T., McCarron, R.M., Nambiar, M.P., Gandy, S., Ahlers, S.T., and Elder, G.A. (2012). Acute blast injury reduces brain Abeta in two rodent species. Front Neurol 3, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Edwards, K.A., Leete, J.J., Tschiffely, A.E., Moore, C.Y., Dell, K.C., Statz, J.K., Carr, W., Walker, P.B., LoPresti, M.L., Ahlers, S.T., Yarnell, A.M., and Gill, J. (2020). Blast exposure results in tau and neurofilament light chain changes in peripheral blood. Brain Inj. 34, 1213–1221. [DOI] [PubMed] [Google Scholar]
- 31. Gill, J., Cashion, A., Osier, N., Arcurio, L., Motamedi, V., Dell, K.C., Carr, W., Kim, H.S., Yun, S., Walker, P., Ahlers, S., LoPresti, M., and Yarnell, A. (2017). Moderate blast exposure alters gene expression and levels of amyloid precursor protein. Neurol. Genet. 3, e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ahlers, S.T., Vasserman-Stokes, E., Shaughness, M.C., Hall, A.A., Shear, D.A., Chavko, M., McCarron, R.M., and Stone, J.R. (2012). Assessment of the effects of acute and repeated exposure to blast overpressure in rodents: toward a greater understanding of blast and the potential ramifications for injury in humans exposed to blast. Front. Neurol. 3, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perez-Garcia, G., Gama Sosa, M.A., De Gasperi, R., Lashof-Sullivan, M., Maudlin-Jeronimo, E., Stone, J.R., Haghighi, F., Ahlers, S.T., and Elder, G.A. (2018). Chronic post-traumatic stress disorder-related traits in a rat model of low-level blast exposure. Behav. Brain Res. 340, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crawley, J.N. (2004). Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment. Retard. Dev. Disabil. Res. Rev. 10, 248–258. [DOI] [PubMed] [Google Scholar]
- 35. Rein, B., Ma, K., and Yan, Z. (2020). A standardized social preference protocol for measuring social deficits in mouse models of autism. Nat. Protoc. 15, 3464–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kawarabayashi, T., Younkin, L.H., Saido, T.C., Shoji, M., Ashe, K.H., and Younkin, S.G. (2001). Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J. Neurosci. 21, 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steele, J.W., Kim, S.H., Cirrito, J.R., Verges, D.K., Restivo, J.L., Westaway, D., Fraser, P., Hyslop, P.S., Sano, M., Bezprozvanny, I., Ehrlich, M.E., Holtzman, D.M., and Gandy, S. (2009). Acute dosing of latrepirdine (Dimebon), a possible Alzheimer therapeutic, elevates extracellular amyloid-beta levels in vitro and in vivo. Mol. Neurodegener. 4, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jankowsky, J.L., Fadale, D.J., Anderson, J., Xu, G.M., Gonzales, V., Jenkins, N.A., Copeland, N.G., Lee, M.K., Younkin, L.H., Wagner, S.L., Younkin, S.G., and Borchelt, D.R. (2004). Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum. Mol. Genet. 13, 159–170. [DOI] [PubMed] [Google Scholar]
- 39. Zohar, O., Lavy, R., Zi, X., Nelson, T.J., Hongpaisan, J., Pick, C.G., and Alkon, D.L. (2011). PKC activator therapeutic for mild traumatic brain injury in mice. Neurobiol. Dis. 41, 329–337. [DOI] [PubMed] [Google Scholar]
- 40. Blasko, I., Beer, R., Bigl, M., Apelt, J., Franz, G., Rudzki, D., Ransmayr, G., Kampfl, A., and Schliebs, R. (2004). Experimental traumatic brain injury in rats stimulates the expression, production and activity of Alzheimer's disease beta-secretase (BACE-1). J. Neural. Transm. 111, 523–536. [DOI] [PubMed] [Google Scholar]
- 41. Nadler, Y., Alexandrovich, A., Grigoriadis, N., Hartmann, T., Rao, K.S., Shohami, E., and Stein, R. (2008). Increased expression of the gamma-secretase components presenilin-1 and nicastrin in activated astrocytes and microglia following traumatic brain injury. Glia 56, 552–567. [DOI] [PubMed] [Google Scholar]
- 42. Chen, X.H., Siman, R., Iwata, A., Meaney, D.F., Trojanowski, J.Q., and Smith, D.H. (2004). Long-term accumulation of amyloid-beta, beta-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma. Am. J. Pathol. 165, 357–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haass, C., Hung, A.Y., Schlossmacher, M.G., Teplow, D.B., and Selkoe, D.J. (1993). beta-Amyloid peptide and a 3-kDa fragment are derived by distinct cellular mechanisms. J. Biol. Chem. 268, 3021–3024. [PubMed] [Google Scholar]
- 44. Cernak, I., Stein, D.G., Elder, G.A., Ahlers, S., Curley, K., DePalma, R.G., Duda, J., Ikonomovic, M., Iverson, G.L., Kobeissy, F., Koliatsos, V.E., Leggieri, M.J.Jr., Pacifico, A.M., Smith, D.H., Swanson, R., Thompson, F.J., and Tortella, F.C. (2017). Preclinical modelling of militarily relevant traumatic brain injuries: challenges and recommendations for future directions. Brain Inj. 31, 1168–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Song, H., Cui, J., Simonyi, A., Johnson, C.E., Hubler, G.K., DePalma, R.G., and Gu, Z. (2018). Linking blast physics to biological outcomes in mild traumatic brain injury: narrative review and preliminary report of an open-field blast model. Behav. Brain Res. 340, 147–158. [DOI] [PubMed] [Google Scholar]
- 46. Kayed, R., and Lasagna-Reeves, C.A. (2013). Molecular mechanisms of amyloid oligomers toxicity. J. Alzheimers Dis 33, Suppl. 1, S67–S78. [DOI] [PubMed] [Google Scholar]
- 47. Harper, M.M., Hedberg-Buenz, A., Herlein, J., Abrahamson, E.E., Anderson, M.G., Kuehn, M.H., Kardon, R.H., Poolman, P., and Ikonomovic, M.D. (2019). Blast-mediated traumatic brain injury exacerbates retinal damage and amyloidosis in the APPswePSENd19e mouse model of Alzheimer's disease. Invest. Ophthalmol. Vis. Sci. 60, 2716–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maigler, K.C., Buhr, T.J., Park, C.S., Miller, S.A., Kozlowski, D.A., and Marr, R.A. (2021). Assessment of the effects of altered amyloid-beta clearance on behavior following repeat closed-head brain injury in amyloid-beta precursor protein humanized mice. J. Neurotrauma 38, 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thangavelu, B., LaValle, C.R., Egnoto, M.J., Nemes, J., Boutte, A.M., and Kamimori, G.H. (2020). Overpressure exposure from .50-caliber rifle training is associated with increased amyloid beta peptides in serum. Front. Neurol. 11, 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zuroff, L., Daley, D., Black, K.L., and Koronyo-Hamaoui, M. (2017). Clearance of cerebral Abeta in Alzheimer's disease: reassessing the role of microglia and monocytes. Cell. Mol. Life Sci. 74, 2167–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huber, B.R., Meabon, J.S., Hoffer, Z.S., Zhang, J., Hoekstra, J.G., Pagulayan, K.F., McMillan, P.J., Mayer, C.L., Banks, W.A., Kraemer, B.C., Raskind, M.A., McGavern, D.B., Peskind, E.R., and Cook, D.G. (2016). Blast exposure causes dynamic microglial/macrophage responses and microdomains of brain microvessel dysfunction. Neuroscience 319, 206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Logsdon, A.F., Meabon, J.S., Cline, M.M., Bullock, K.M., Raskind, M.A., Peskind, E.R., Banks, W.A., and Cook, D.G. (2018). Blast exposure elicits blood-brain barrier disruption and repair mediated by tight junction integrity and nitric oxide dependent processes. Sci. Rep. 8, 11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tanzi, R.E., Moir, R.D., and Wagner, S.L. (2004). Clearance of Alzheimer's Abeta peptide: the many roads to perdition. Neuron 43, 605–608. [DOI] [PubMed] [Google Scholar]
- 54. Rasmussen, M.K., Mestre, H., and Nedergaard, M. (2018). The glymphatic pathway in neurological disorders. Lancet Neurol. 17, 1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tarasoff-Conway, J.M., Carare, R.O., Osorio, R.S., Glodzik, L., Butler, T., Fieremans, E., Axel, L., Rusinek, H., Nicholson, C., Zlokovic, B.V., Frangione, B., Blennow, K., Menard, J., Zetterberg, H., Wisniewski, T., and de Leon, M.J. (2015). Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 11, 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wardlaw, J.M., Benveniste, H., Nedergaard, M., Zlokovic, B.V., Mestre, H., Lee, H., Doubal, F.N., Brown, R., Ramirez, J., MacIntosh, B.J., Tannenbaum, A., Ballerini, L., Rungta, R.L., Boido, D., Sweeney, M., Montagne, A., Charpak, S., Joutel, A., Smith, K.J., Black, S.E., and colleagues from the Fondation Leducq Transatlantic Network of Excellence on the Role of the Perivascular Space in Cerebral Small Vessel Disease. (2020). Perivascular spaces in the brain: anatomy, physiology and pathology. Nat. Rev. Neurol. 16, 137–153. [DOI] [PubMed] [Google Scholar]
- 57. Sullan, M.J., Asken, B.M., Jaffee, M.S., DeKosky, S.T., and Bauer, R.M. (2018). Glymphatic system disruption as a mediator of brain trauma and chronic traumatic encephalopathy. Neurosci. Biobehav. Rev. 84, 316–324. [DOI] [PubMed] [Google Scholar]
- 58. Iliff, J.J., Chen, M.J., Plog, B.A., Zeppenfeld, D.M., Soltero, M., Yang, L., Singh, I., Deane, R., and Nedergaard, M. (2014). Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 34, 16180–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Peng, W., Achariyar, T.M., Li, B., Liao, Y., Mestre, H., Hitomi, E., Regan, S., Kasper, T., Peng, S., Ding, F., Benveniste, H., Nedergaard, M., and Deane, R. (2016). Suppression of glymphatic fluid transport in a mouse model of Alzheimer's disease. Neurobiol. Dis. 93, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang, L., Zhang, Y., Zhao, Y., Marshall, C., Wu, T., and Xiao, M. (2019). Deep cervical lymph node ligation aggravates AD-like pathology of APP/PS1 mice. Brain Pathol. 29, 176–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang, B., Li, W., Zhuo, Y., Xiang, H., Li, W., Liu, H., Xie, L., Gao, Q., and Tan, S. (2020). L-3-n-Butylphthalide effectively improves the glymphatic clearance and reduce amyloid-beta deposition in Alzheimer's transgenic mice. J. Mol. Neurosci. 71, 1266–1274. [DOI] [PubMed] [Google Scholar]
- 62. Elder, G.A., Gama Sosa, M.A., De Gasperi, R., Stone, J.R., Dickstein, D.L., Haghighi, F., Hof, P.R., and Ahlers, S.T. (2015). Vascular and inflammatory factors in the pathophysiology of blast-induced brain injury. Front. Neurol. 6, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Saito, S., and Ihara, M. (2016). Interaction between cerebrovascular disease and Alzheimer pathology. Curr. Opin. Psychiatry 29, 168–173. [DOI] [PubMed] [Google Scholar]
- 64. Abrahamson, E.E., Ikonomovic, M.D., Dixon, C.E., and DeKosky, S.T. (2009). Simvastatin therapy prevents brain trauma-induced increases in beta-amyloid peptide levels. Ann. Neurol. 66, 407–414. [DOI] [PubMed] [Google Scholar]