Abstract
The incidence rate is directly proportional to the incidence of obesity or overweight and Type 2 diabetes mellitus. Garcinia is a plant that has been proven empirically, preclinically, and clinically to have activities for the avoidance and treatment of metabolic syndrome and on the pathogenesis and pathophysiology caused by the disease. The aim of this study is to create a discussion and summarize information regarding the activity or usefulness of the Garcinia plant. This review article was based on the published journals obtained from Google Scholar, Scopus, and PubMed databases using the keywords Garcinia obesity, Garcinia overweight, and Garcinia metabolic syndrome. Garcinia had many activities related to metabolic syndrome because it was able to reduce body fat mass, blood sugar level, body weight, total cholesterol, and triglyceride level. These activities were mediated by numerous apparatuses of feat together with a reserve of fatty acid synthase, α-amylase, α-glucosidase, and several other enzymes and pathways associated with the metabolic syndrome. Garcinia plant was able to be used as a candidate for a new herbal that had a good effect in treating metabolic syndrome in future.
Keywords: Body fat mass, Garcinia, insulin resistance, metabolic syndrome, obesity
INTRODUCTION
The incidence of metabolic syndrome is directly proportional to the incidence of obesity/overweight and Type 2 diabetes mellitus (T2DM).[1] Obesity is a dangerous aspect leading to several dangerous illnesses, such as diabetes, hypertension, heart syndrome, and stroke.[2,3] The patients with obesity and overweight have 3.6 times the risk of having coronary artery disease,[4] 85% of hypertension was associated with body mass index (BMI) from 25 kg/m2-29.9 kg/m2, and 90% of patients with T2DM have a BMI more than 25 kg/m2.[5]
Nowadays, obesity and overweight are treated by synthetic drugs, which are known to have a lot of side effects and can decrease the quality of life.[6] Considering those limitations, the treatment has been developed using herbal medicines because of their excellent bioactivity and pharmacological properties including anti-obesity and overweight.[7] In addition, herbal medicines have lower side effects than synthetic drugs due to their multicomponent.[8]
Garcinia is one herbal medicine that has been used empirically for treating degenerative diseases such as obesity,[9] diabetic mellitus,[10] and arthritis.[11] Garcinia is known with different names according to its place, namely, Garcinia atroviridis, Garcinia cola, Garcinia cambogia, and Garcinia indica. G. atroviridis is more common on the mainland of Peninsular Malaysia, including Indonesia.[12] G. cambogia has been studied as anti-obesity since 20 years ago.[13] Whereas G. atroviridis and G. cola were effective against obese females with a BMI of 25 kg/m2 in Thailand.[14] The intervention group received hydroxycitric acid (HCA) from G. atroviridis taken before the meals, 3 × 1.15 g/day in 200 ml of water. The results showed that the intervention group experienced a weight loss of 2.8 kg (P = 0.05).[15]
Based on the potential and excellent effects of Garcinia herbs, there are numerous published journals of Garcinia herbs in treating obesity and overweight, which will be more useful if made in one article review. Taken together, the authors believed that this review is concerned with the discussion and summarized the effectivity of Garcinia herbs in treating obesity, overweight, and other metabolic syndrome-related diseases.
PHARMACOLOGICAL ACTIVITY OF GARCINIA AGAINST METABOLIC SYNDROME
The pharmacological content of Garcinia has been shown in Table 1. Garcinol as an antioxidant was able to reduce the BW of mice induced by high-fat diet. In addition, fat, triglycerides (TG), activated protein kinase,[16] and cholesterol were decreased. On the other hand, Akkermansia has increased. Akkermansia could prevent fat absorption to reduce the levels of TG, cholesterol, and body fat.[17]
Table 1.
Garcinia’s effects on Weight Loss and Some Metabolic Syndrome Markers
| No | Nutraceutical | Effects | Ref. |
|---|---|---|---|
| 1 | Garcinia cambogia Camelia sintesis unroasted Coffea Arabica and Lagerstroemiaspeciosa | BW↓Body fat↓ | [17] |
| 2 | Extract of Garcinia Cambogia (HCA-SX) | Appetite↓Weight↓TG↓Glucose↓Insulin↓Insulin resistance↓CRP↓MDA↓IL-6 ↓ | [65] |
| 3 | Extract of Garcinia atroviridis | AgNPs suppressed expression of the CD4+IL17Rhighpopulation. MAPK Pathway did not change in control and AgNP. This Garcinia does’t cause inflammatory effects. |
[66] |
| 4 | Garcinia atroviridis | Group 1 get 1 HCA sachet (@1,15 gram) before eats, 3 times a day for 8 weeks. Fat loss occurs in the skin of the triceps (p<0.05). |
[67] |
| 5 | Garcinia cambogia | Insulin after meals Glycogen synthesis was greater than placebo. GLUT4↓under the placebo group. mRNA [68]/CD36 increased over placebo. HCA increased energy in fat oxidation. |
[69] |
| 6 |
Garcinia indica
Poly-isoprenylated isolated |
BW of the HFD rat↓SGPT↓total holesterol↓TG↓AMPK↓Fat mRNA↓Fat/body↓akkermansia↓ | [70] |
| 7 | Extract of Sphaeranthus indicus flowersand, Garcinia mangostana fruits | BW↓of 1.6% BMI 2.7%, waist circumference 4.6% on 2, 4, in 8 weeks. Cholesterol↓compared to placebo (p=0.016), TG↓ (p=0.012). PPAR-γ, ADRP, CD36 and triglycerides was↓Adiponectin↓ |
[56] |
| 8 | Garcinia mangostana | GMPE could reduce plasma creatinine levels and improved the proximal tubule of the kidney in STZ-induced DM rats. | [63] |
| 9 | Ethanol extract of Garcinia hanburyi | Pro-inflammatory agent↓ | [71] |
| 10 | Ethanol extract of Garcinia brasiliensis | PPR-γ↑ IL-10↓LPL↓FAS↓TNF-α↓ BG↓Alanine aminotransferase↓ | [72] |
| 11 | Extract of Garcinia cambogia 10% hotandcold | Total lipids↓TG↓cholesterol↓LDL↓BG↓Albumin↓Ureum↓ALT↓AST↓and HDL↓ | [73] |
| 12. | Combination green tea (Camellia sinensis) & G. Atroviridis extracts | Cholesterol ↓ TG ↓ LDL ↓ BG ↓ SGOT↓ ↓-amylase↓ ↓-glucose↓ |
[73] |
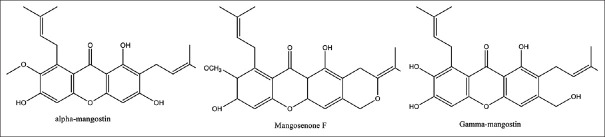
The bioactive content of Garcinia mangostana are α-mangostin, mangosenone F, and γ-mangostin [Figure 1]. G. mangostana extract reduced serum urea levels in renal failure of streptozotocin-induced T2DM rats.[18]
Figure 1.
Chemical structure of Garcinia mangostana fruit
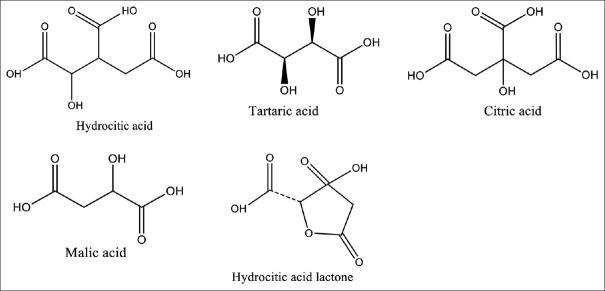
G. cambogia pharmacologically contains HCA, tartaric acid, citric acid, malic acid, and HCA lactone [Figure 2]. The efficacy of G. cambogia, either alone or mixed with other Garcinias such as G. camellia, had the property of reducing body weight, body fat, appetite, TG, blood sugar, insulin after meals, C-reactive protein (CRP), malondialdehyde, glucose transporter Type 4 (GLUT-4), interleukin (IL)-6, TG, low-density lipoprotein (LDL), urea, alanine transaminase (ALT), and aspartate transaminase (AST).[9,10,11,19] The results of several studies indicated that G. atroviridis suppressed the expression of CD4+ IL17R as a pro-inflammatory gene.[20] G. atroviridis could also remove fat under the skin of the triceps for 8 weeks of G. atroviridis administration.[21]
Figure 2.
Chemical of Garcinia cambogia
Garcinia brasiliensis was able to increase peroxisome proliferator-activated receptors γ (PPR-γ), which could inhibit beta-catenin. PPR-γ was bound to the catenin bond domain, namely, pyruvate dehydrogenase kinase, which inhibited the pyruvate dehydrogenase complex in the mitochondria.[22]
PATHOGENESIS AND PATHOPHYSIOLOGY OF METABOLIC SYNDROME
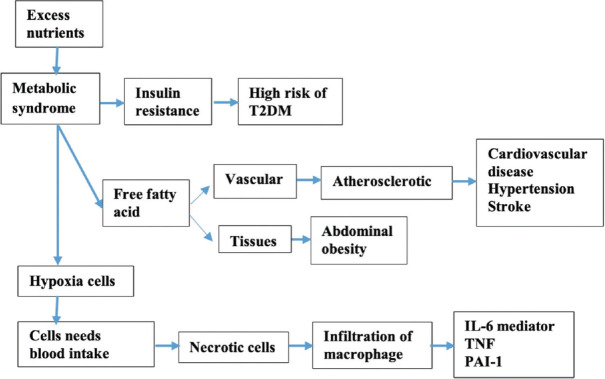
There are four pathogeneses of metabolic syndrome. The first, insulin resistance that is one of the pathological conditions in metabolic syndrome.[23] The second, free fatty acids that are secreted through the encounter of c-AMP during lipolysis. This process is instigated by catecholamines for the period of fasting. This pathway can inhibit insulin's antilipolytic properties that lead to advanced lipolysis.[24] The third, metabolic syndrome that also involves mitochondrial malfunction, increase of fat, and disruption of the antioxidant structure.[25] The last, pathophysiology-related vascular diseases and immune system that considerably escalates the danger of T2DM and atherosclerotic syndrome.[26] Metabolic syndrome is closely related to fat accumulation in tissues that can lead to abdominal obesity.[27] Adipose tissue experiences hyperplasia and hypertrophy in response to excess nutrients. Therefore, cells need blood intake due to induced hypoxia as shown in Figure 3.[28] Hypoxia leads to necrotic cells with macrophage infiltration.[29] Metabolic syndrome is caused by the accumulation of visceral adipose tissue (VAT), which leads to critical VAT threshold (CVATT). CVATT clues metabolic syndromes such as insulin resistance.[30] In VAT accumulation, insulin sensitivity is required. If CVATT occurs, insulin resistance will occur, resulting in the disruption of fat deposits such as in the liver, heart, kidneys, and pancreas.[31]
Figure 3.
Metabolic syndrome's pathophysiology
GARCINIA HERBS
Garcinia described in this review is included in kingdom Plantae, family Clusiaceae, and genus Garcinia.
Bioactivity and pharmacological properties-related metabolic syndrome of Garcinia plants
The activities are a function of the secondary metabolite compounds of Garcinia variants including xanthone derivative compounds, carboxylic acid, HCA, γ-lactone, atroviride, atrovirisidone, atrovirinone, benzophenone, phenolic acid, gambogic acid, isomorellin, isoforbesione, morellic acid, desoxygambogenin, hanburin, and flavonoid compounds.[32]
The activity of Garcinia had been proven pharmacologically to have many bioactivities and pharmacological properties as shown in Figure 3.[33] Its effectiveness in the treatment of obesity had scores of 1.6% in weight loss, 2.7% in reducing BMI, and 4.6% in reducing waist circumference. The decrease in cholesterol level was 26.7 mg/dL and in TG was 56 mg/dL.[34] Garcinia could induce changes in the body's metabolism. Several studies proved that taking Garcinia could reduce body fat composition.[21,35,36,37]
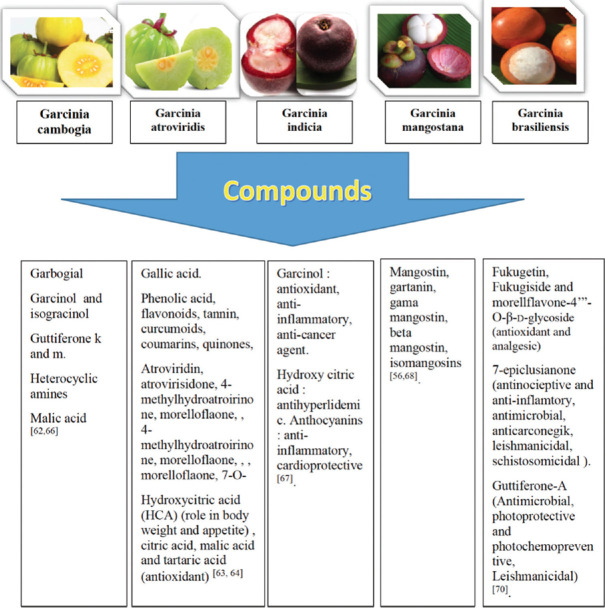
Based on Figure 4, the effects of various Garcinia herbs could be seen to have similar bioactivity and pharmacological properties related to metabolic syndrome disorder. Garcinia herbs had several mechanisms of action reducing body weight such as through increasing FAS, activating the scavenger receptor CD36, and regulating PPR-γ, which could decrease lipid level, cholesterol production, and triglyceride level. Besides, Garcinia herbs had an effect as an antidiabetic agent to reduce GLUT-4, increase glycogenesis, and regulate LPL. These actions decreased insulin resistance and could reduce the blood glucose level. The effectivity of Garcinia herbs in treating metabolic syndrome disorders also had been proven by several studies related to the immune system, which had mentioned that Garcinia herbs could reduce IL-1, IL-6, IL10, and tumor necrosis factor-α (TNF-α). The immune system in metabolic syndrome disorder was usually increased by hypoxia-induced hypertrophy/hyperplasia of adipose tissue, which could lead to necrotic cells with macrophage infiltration. In this case, a decrease of the immune system thereby indicated that the Garcinia herbs had a potential effect on the clinical manifestation of metabolic syndrome. Furthermore, studies showed that the Garcinia herbs could also reduce the alanine aminotransferase, AST, and ALT levels, which indicated hepatic healing. Moreover, the effect of Garcinia might reduce the CRP, an excellent indicator for determining the high/low risk of cardiovascular disease.[12]
Figure 4.
Various Garcinia and their bioactive contents
Roles of Garcinia herbs in metabolic syndrome
Garcinia could induce changes in the body's metabolism. Several studies proved that taking Garcinia could reduce body fat composition, obesity, and overweight.
There were several hypothesis mechanisms for the underlying pathophysiology of metabolic syndrome, and the most widely accepted of these was insulin resistance with fatty acid flux. Other potential mechanisms included low-grade chronic inflammation and oxidative stress.[38,39] Metabolic syndrome began with an excess nutrient intake which caused clinical symptoms of metabolic disorders [Figure 3]. These metabolic disorders included insulin resistance, free fatty acid accumulation, hypoxic cells, dyslipidemia (increased TG, decreased high-density lipoprotein [HDL] levels), central obesity, hypertension, impaired blood sugar tolerance, diabetes mellitus, and high atherosclerotic tendencies.[40] Metabolic syndrome could increase the risk of insulin resistance leading to T2DM disease. The accumulation of free fatty acids also occurred as a result of the metabolic syndrome, which could cause disturbances in blood vessels and tissues. Metabolic disorders in blood vessels could be in the form of atherosclerosis, which had a risk of heart disease, stroke, and hypertension. Disorders caused by metabolic syndrome began with cell hypoxia, cells needing oxygen supply, cell death, and macrophage infiltration in the form of IL-6 mediators, TNF, and PAI-1.[41]
There were several herbal therapies that could improve metabolic syndrome and affect hormones, lipid profile, blood glucose, and inflammatory factors.[42] The lipid profiles improved by herbal medicines contained total cholesterol, LDL, HDL TG,[43] and HDL. The best reduction in TG and total cholesterol was applied G. cambogia with an effective dose of 2,400 mg/day. HDL increased significantly by consuming G. cambogia in the actual dosage of 3,000 mg/day.[44] This proof about the good substance of these herbs for the treatment of metabolic syndrome made Garcinia a candidate for new drugs with good effectiveness in future.
CONCLUSION
The results of the review showed that various types of Garcinia, which were G. cambogia, G. atroviridis, G. indica, Garcinia brasilines, G. mangostana, and Garcinia handbury had bioactivity and pharmacological properties for the treatment of metabolic syndrome including anti-obesity and antihyperlipidemia that worked through various mechanisms and multiple pathways.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hai AA, Iftikhar S, Latif S, Herekar F, Javed S, Patel MJ. Prevalence of metabolic syndrome in overweight and obese patients and their measurement of neck circumference: A cross-sectional study. Cureus. 2019;11:e6114. doi: 10.7759/cureus.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han KM, Kim MS, Kim A, Paik JW, Lee J, Ham BJ. Chronic medical conditions and metabolic syndrome as risk factors for incidence of major depressive disorder: A longitudinal study based on 4.7 million adults in South Korea. J Affect Disord. 2019;257:486–94. doi: 10.1016/j.jad.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. 2017;960:1–17. doi: 10.1007/978-3-319-48382-5_1. [DOI] [PubMed] [Google Scholar]
- 4.Chong PW, Beah ZM, Grube B, Riede L. IQP-GC-101 reduces body weight and body fat mass: A randomized, double-blind, placebo-controlled study. Phytother Res. 2014;28:1520–6. doi: 10.1002/ptr.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puska P, Nishida C, Porter D. World Health Organization. Obesity and Overweight Fact Sheet. 2021. [Last accessed on 2021 Feb 01]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight .
- 6.Kim GW, Lin JE, Blomain ES, Waldman SA. Antiobesity pharmacotherapy: New drugs and emerging targets. Clin Pharmacol Ther. 2014;95:53–66. doi: 10.1038/clpt.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahiya V, Vasudeva N, Sharma S, Kumar A, Rowley D. Lead anti-obesity compounds from nature. Endocr Metab Immune Disord Drug Targets. 2020;20:1637–53. doi: 10.2174/1871530320666200504092012. [DOI] [PubMed] [Google Scholar]
- 8.Fu B, Wang N, Tan HY, Li S, Cheung F, Feng Y. Multi-component herbal products in the prevention and treatment of chemotherapy-associated toxicity and side effects: A review on experimental and clinical evidences. Front Pharmacol. 2018;9:1394. doi: 10.3389/fphar.2018.01394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibuki M, Shoda C, Miwa Y, Ishida A, Tsubota K, Kurihara T. Therapeutic effect of Garcinia cambogia extract and hydroxycitric acid inhibiting hypoxia-inducible factor in a murine model of age-related macular degeneration. Int J Mol Sci. 2019;20:5049. doi: 10.3390/ijms20205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayamizu K, Hirakawa H, Oikawa D, Nakanishi T, Takagi T, Tachibana T, et al. Effect of Garcinia cambogia extract on serum leptin and insulin in mice. Fitoterapia. 2003;74:267–73. doi: 10.1016/s0367-326x(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 11.dos Reis SB, de Oliveira CC, Acedo SC, Miranda DD, Ribeiro ML, Pedrazzoli J, Jr, et al. Attenuation of colitis injury in rats using Garcinia cambogia extract. Phytother Res. 2009;23:324–9. doi: 10.1002/ptr.2626. [DOI] [PubMed] [Google Scholar]
- 12.Espirito Santo BL, Santana LF, Kato Junior WH, de Araújo FO, Bogo D, Freitas KC, et al. Medicinal potential of Garcinia species and their compounds. Molecules. 2020;25:4513. doi: 10.3390/molecules25194513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semwal RB, Semwal DK, Vermaak I, Viljoen A. A comprehensive scientific overview of Garcinia cambogia. Fitoterapia. 2015;102:134–48. doi: 10.1016/j.fitote.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Idris AE, Seke Etet PF, Saeed AA, Farahna M, Satti GM, AlShammari SZ, et al. Evaluation of metabolic, antioxidant and anti-inflammatory effects of Garcinia kola on diabetic rats. Saudi J Biol Sci. 2020;27:3641–6. doi: 10.1016/j.sjbs.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng IS, Huang SW, Lu HC, Wu CL, Chu YC, Lee SD, et al. Oral hydroxycitrate supplementation enhances glycogen synthesis in exercised human skeletal muscle. Br J Nutr. 2012;107:1048–55. doi: 10.1017/S0007114511003862. [DOI] [PubMed] [Google Scholar]
- 16.Lee PS, Teng CY, Kalyanam N, Ho CT, Pan MH. Garcinol reduces obesity in high-fat-diet-fed mice by modulating gut microbiota composition. Mol Nutri Food Res. 2019;63:1800390. doi: 10.1002/mnfr.201800390. [DOI] [PubMed] [Google Scholar]
- 17.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25:1096–103. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansori AN, Susilo R, Hayaza S, Winarni D, Husen SA. Renoprotection by Garcinia mangostana L. pericarp extract in streptozotocin-induced diabetic mice. Iraqi J Veter Sci. 2019;33:13–9. [Google Scholar]
- 19.Al-Askalany SA. Effects of Garcinia cambogia plant extracts on some biochemical parameters of experimental rats blood. Egypt J Agri Res. 2018;96:9. [Google Scholar]
- 20.Yusof NA, Pei Chen L, Kamal NN, Zulkifli NI, Ahmad HN, Wan Qomar WA. Antagonistic effect of biosynthesized AgNPs from Garcinia atroviridis extract on anti-inflammatory properties of CD4+ILRhigh cells from non-obese resistance (NOR) mouse model. Malay J Medic Health Sci. 2018;14(102):1–7. [Google Scholar]
- 21.Lumbantobing CJ, Syukur S, Yerizel E, Purwati E. Benefits of asam gelugur (Garcinia atroviridis) tea as a source of antioxidant compounds on malondialdehyde levels in adults with obesity. AAESRJETS. 2017;34:198–204. [Google Scholar]
- 22.de Castro Moreira ME, Natal DI, Toledo RC, Ramirez NM, Ribeiro SM, Benjamin LD, et al. Bacupari peel extracts (Garcinia brasiliensis) reduce high-fat diet-induced obesity in rats. J Func Foods. 2017;29:143–53. [Google Scholar]
- 23.Das RR, Mangaraj M, Panigrahi SK, Satapathy AK, Mahapatro S, Ray PS. Metabolic syndrome and insulin resistance in schoolchildren from a developing country. Front Nutr. 2020;7:31. doi: 10.3389/fnut.2020.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao J, Wu Y, Rong X, Zheng C, Guo J. Anti-lipolysis induced by insulin in diverse pathophysiologic conditions of adipose tissue. Diabetes Metab Syndr Obes. 2020;13:1575–85. doi: 10.2147/DMSO.S250699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landa-Galvan HV, Rios-Castro E, Romero-Garcia T, Rueda A, Olivares-Reyes JA. Metabolic syndrome diminishes insulin-induced Akt activation and causes a redistribution of Akt-interacting proteins in cardiomyocytes. PLoS One. 2020;15:e0228115. doi: 10.1371/journal.pone.0228115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kintscher U, Foryst-Ludwig A, Haemmerle G, Zechner R. The role of adipose triglyceride lipase and cytosolic lipolysis in cardiac function and heart failure. Cell Rep Med. 2020;1:100001. doi: 10.1016/j.xcrm.2020.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuzawa Y. Establishment of a concept of visceral fat syndrome and discovery of adiponectin. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:131–41. doi: 10.2183/pjab.86.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutkowski JM, Stern JH, Scherer PE. The cell biology of fat expansion. J Cell Biol. 2015;208:501–12. doi: 10.1083/jcb.201409063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egners A, Erdem M, Cramer T. The response of macrophages and neutrophils to hypoxia in the context of cancer and other inflammatory diseases. Mediators Inflamm. 2016;2016:2053646. doi: 10.1155/2016/2053646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freedland ES. Role of a critical visceral adipose tissue threshold (CVATT) in metabolic syndrome: Implications for controlling dietary carbohydrates: A review. Nutr Metab (Lond) 2004;1:12. doi: 10.1186/1743-7075-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17:122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan WN, Khairuddean M, Wong KC, Tong WY, Ibrahim D. Antioxidant compounds from the stem bark of Garcinia atroviridis. J Asian Nat Prod Res. 2016;18:804–11. doi: 10.1080/10286020.2016.1160071. [DOI] [PubMed] [Google Scholar]
- 33.Al-Mansoub MA, Asmawi MZ, Murugaiyah V. Effect of extraction solvents and plant parts used on the antihyperlipidemic and antioxidant effects of Garcinia atroviridis: A comparative study. J Sci Food Agric. 2014;94:1552–8. doi: 10.1002/jsfa.6456. [DOI] [PubMed] [Google Scholar]
- 34.Stern JS, Peerson J, Mishra AT, Sadasiva Rao MV, Rajeswari KP. Efficacy and tolerability of a novel herbal formulation for weight management. Obesity (Silver Spring) 2013;21:921–7. doi: 10.1002/oby.20211. [DOI] [PubMed] [Google Scholar]
- 35.John OD, Mouatt P, Majzoub ME, Thomas T, Panchal SK, Brown L. Physiological and metabolic effects of yellow mangosteen (Garcinia dulcis) rind in rats with diet-induced metabolic syndrome. Int J Mol Sci. 2019;21:272. doi: 10.3390/ijms21010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majeed M, Majeed S, Nagabhushanam K, Lawrence L, Mundkur L. Garcinia indica extract standardized for 20% Garcinol reduces adipogenesis and high fat diet-induced obesity in mice by alleviating endoplasmic reticulum stress. J Func Foods. 2020;67:103863. [Google Scholar]
- 37.Chuah LO, Ho WY, Beh BK, Yeap SK. Updates on antiobesity effect of Garcinia origin (-)-HCA. Evid Based Complement Alternat Med. 2013;2013:751658. doi: 10.1155/2013/751658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCracken E, Monaghan M, Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin Dermatol. 2018;36:14–20. doi: 10.1016/j.clindermatol.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Pan LY, Wang YS, Liu XH, Wang N, Xu W, Xiu YF. Pharmacokinetic comparison of five xanthones in rat plasma after oral administration of crude and processed Garcinia hanburyi extracts. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1126-1127:121737. doi: 10.1016/j.jchromb.2019.121737. [DOI] [PubMed] [Google Scholar]
- 40.Miranda PJ, DeFronzo RA, Califf RM, Guyton JR. Metabolic syndrome: Definition, pathophysiology, and mechanisms. Am Heart J. 2005;149:33–45. doi: 10.1016/j.ahj.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Collins KH, Herzog W, MacDonald GZ, Reimer RA, Rios JL, Smith IC, et al. Obesity, metabolic syndrome, and musculoskeletal disease: Common inflammatory pathways suggest a central role for loss of muscle integrity. Front Physiol. 2018;9:112. doi: 10.3389/fphys.2018.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiu YS, Wu JL, Yeh CT, Yadav VK, Huang HS, Wang LS. γ-Mangostin isolated from Garcinia mangostana L. suppresses inflammation and alleviates symptoms of osteoarthritis via modulating miR-124-3p/IL-6/NF-κB signaling. Aging. 2020;12:6630. doi: 10.18632/aging.103003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiol Dis. 2017;11:215–25. doi: 10.1177/1753944717711379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patti AM, Al-Rasadi K, Giglio RV, Nikolic D, Mannina C, Castellino G, et al. Natural approaches in metabolic syndrome management. Arch Med Sci. 2018;14:422–41. doi: 10.5114/aoms.2017.68717. [DOI] [PMC free article] [PubMed] [Google Scholar]