Abstract
Objective:
Primary objective: to determine if transfusion of short-storage red blood cells (RBCs) compared to standard-issue RBCs reduced risk of delirium/coma in critically ill children. Secondary objective: to assess if RBC transfusion was independently associated with delirium/coma.
Design:
This study was performed in two stages. First we compared patients receiving either short-storage or standard RBCs in a multi-institutional prospective randomized controlled trial (RCT). Then we compared all transfused patients in the RCT to a single-center cohort of non-transfused patients matched for confounders of delirium/coma.
Setting:
Twenty academic pediatric intensive care units who participated in the Age of Transfused Blood in Critically Ill Children (ABC-PICU) trial.
Patients:
Children 3-days to 16-years of age who were transfused RBCs within the first seven days of admission.
Interventions:
Subjects were randomized to either short-storage RBC study arm (defined as RBCs stored for up to seven days) or standard-issue RBC study arm. In addition, subjects were screened for delirium prior to transfusion and every 12-hours after transfusion for up to three days.
Measurements and Main Results:
Primary outcome measure was development of delirium/coma within three days of initial transfusion. Additional outcome measures were dose-response relationship between volume of RBCs transfused and delirium/coma, and comparison of delirium/coma rates between transfused patients and individually-matched non-transfused patients. We included 146 subjects in the stage I analysis; 69 were randomized to short-storage RBCs and 77 to standard-issue. There was no significant difference in delirium/coma development between study arms (79.5% vs 70.1%, p=0.184). In the stage II analysis, adjusted odds for delirium in the transfused cohort was more than 8-fold higher than in the non-transfused matched cohort, even after controlling for hemoglobin (aOR 8.9; CI 2.8–28.4, p<0.001).
Conclusions:
RBC transfusions (and not anemia) are independently associated with increased odds of subsequent delirium/coma. However, storage age of RBCs does not affect delirium risk.
Keywords: delirium, pediatric critical care, red blood cell (RBC) transfusions, age of blood, Cornell Assessment of Pediatric Delirium (CAPD)
Tweet:
#Delirium rates are significantly higher in #RBC-transfused #pedsICU. However, RBC storage age does not affect risk.
Introduction
Delirium occurs frequently in the pediatric intensive care unit (PICU)[1]. It is a behavioral syndrome defined as an acute and fluctuating change in awareness and cognition, that occurs as a direct result of the underlying medical condition or as a side effect of treatment[2]. Pediatric delirium is strongly associated with substantial morbidity[3–7].
Delirium can be conceptualized as evidence of acute brain dysfunction, an important component of multiple organ dysfunction syndrome (MODS) in children with critical illness[8,9]. With the recent development of bedside tools designed to screen children for delirium on a daily basis, clinical guidelines call for the inclusion of delirium as a criterion for neurologic dysfunction when capturing MODS in critical illness[10].
The Age of Transfused Blood in Critically Ill Children (ABC-PICU) trial was designed to determine if transfusion of short-storage red blood cells (RBCs) reduced development of MODS, as compared to transfusion of standard RBCs. In this international randomized controlled trial, the primary outcome measure was development of new or progressive MODS[11]. ABC-PICU began enrolling children in February 2014, and focused on MODS as defined in 1996 by Proulx, et al[9]. At that time, validated pediatric delirium screening tools were not yet widely available, and delirium was not included as evidence of neurologic dysfunction. Therefore, an ancillary study for the ABC-PICU trial, Transfusion Associated Delirium (TAD-ABC-PICU), was developed. Enrollment in this ancillary study began in June 2016.
The primary objective of TAD-ABC-PICU was to determine if storage duration of RBCs affected the development or duration of delirium and/or coma. The rationale for this study was based upon data indicating that RBC transfusion in critically ill patients stimulates an inflammatory response, and literature suggesting that systemic inflammation can lead to delirium/coma in children[1,12,13]. Secondary objectives were to: (i) determine if RBC transfusion dose affected development of delirium/coma, and (ii) determine if RBC transfusion is independently associated with development of delirium/coma, by comparing to a retrospective matched cohort of non-transfused children. We hypothesized that short-storage RBC would be associated with a decreased incidence of post-transfusion delirium/coma when compared to standard-issue RBC, that development of delirium/coma would be dose-dependent, and that RBC transfusion would be independently associated with subsequent delirium/coma.
Materials and Methods
Study Design
The ABC-PICU study was a multi-institutional, double-blinded, randomized controlled trial comparing transfusion of short-storage RBCs (defined as RBCs stored for up to seven days) with standard-issue RBCs (defined as delivery of the oldest available in hospital inventory at time of transfusion order). Eligible patients were between 3-days and 16-years of age, projected to remain in PICU for at least 24-hours and were transfused RBCs within the first seven days of PICU admission. Patients were excluded if they had received a prior RBC transfusion within 28-days. Each clinical site’s institutional review board approved this study, with informed consent obtained from all subjects/guardians and assent obtained when appropriate. Details of the clinical trial protocol, including full exclusion criteria, and results of the ABC-PICU study, have been published previously[11,14].
The decision to transfuse a patient was made independent of the study, at the discretion of the critical care team. The ABC-PICU protocol suggested that the guidelines for restrictive transfusions should be followed (i.e.: restrict transfusions if hemoglobin >7 in a hemodynamically stable non-cardiac surgery patient). Study arm allocation occurred in the blood bank; collection date of the RBC unit was concealed prior to PICU delivery. Computer-generated randomization was stratified by patient age-group/study site. All RBC units were leukocyte-reduced prior to storage. The primary outcome was development of new or progressive MODS, as defined by Proulx, et al[9].
For this ancillary study, in addition to the ABC-PICU protocol described above, all participating sites screened each enrolled subject for delirium. The Cornell Assessment of Pediatric Delirium (CAPD), used as the delirium screening tool for this study, is well-validated and easy to use in children of all ages[15] (Supplemental Figure 1). Delirium as defined by the CAPD tool has been shown to correlate with important clinical outcomes, and use of the tool has been recommended for critically ill children by national and international societies[3,4,7,10,16–18].
This ancillary study was performed in two stages.
Stage I: Prospective Delirium Screening
Each participating site’s principal investigator (PI) and research coordinator (RC) completed delirium training with an online tutorial and quiz. The PI and/or RC then provided personal training to the bedside nurse of each enrolled subject. Prior to transfusion, the bedside nurse screened the child for delirium using the CAPD. The CAPD was scored twice a day (once each 12-hour shift) for the first 3-days after the transfusion (or until PICU discharge or death, whichever occurred first).
Each subject was assigned a cognitive status for each assessment period which was classified as delirium, coma, or delirium-free/coma-free (DFCF). Delirium was defined as a CAPD score of nine or higher, consistent with the pediatric delirium literature [15]. Coma was defined as unarousable to verbal stimulation for the entire 12-hour assessment period. DFCF was defined as the absence of delirium and coma.
The primary outcome measure was presence of delirium and/or coma at any time during the three days after transfusion. We chose the composite outcome of delirium/coma as the primary endpoint, as delirium cannot be assessed in the setting of coma, and delirium and coma are described as progressive levels of neurologic dysfunction. This approach is consistent with the majority of delirium literature which shows that patients who are delirium- and coma-free (i.e.: patients with “normal” brain function) have more favorable outcomes[19,20].
Data Collection
Clinical data regarding delirium scores and medication exposures were entered into a secure Research Electronic Data Capture (REDCap) database that supplemented the primary ABC-PICU data set, which captured demographic, clinical, and transfusion-related data (including RBC dose, study-arm allocation, and RBC storage time). In addition, a Pediatric Logistic Organ Dysfunction-2 score (PELOD-2) was captured for each subject on the day of randomization which indicated the severity of existing MODS (including need for invasive mechanical ventilation) prior to transfusion[21].
Stage II: Matched Cohort
After completion of the prospective study, each enrolled patient was individually matched with two non-transfused subjects from an existing delirium study database. The dataset used for matching included 1547 subjects who were screened for delirium twice daily at a single clinical site between September 2014-August 2015. This dataset was used in a previously published study describing the epidemiology and outcomes of delirium in critically ill children[3]. Patients were matched for clinical characteristics at ‘time zero’, defined as day of RBC transfusion in the TAD cohort. Each transfused child was individually matched 2:1 for the following predetermined variables (all of which have been described as independently related to development of pediatric delirium): age category (exact match), PELOD-2 score (± 2 points), ICU day (± 2 days), opiate exposure (yes/no) on the day of transfusion, and benzodiazepine exposure (yes/no) on the day of transfusion[3]. Transfused and matched subjects were followed for 3 days from time zero, with twice-daily delirium screening. The outcome of interest was presence of delirium and/or coma at any time within the three days after time zero.
Statistical Analyses
Baseline and post-randomization patient characteristics were assessed using frequency distributions, univariate descriptive statistics (means ± standard deviation or medians/interquartile ranges), and percentages for categorical data. The principal analysis (influence of short-storage versus standard-issue) on the primary outcome (delirium/coma), was conducted using relative risk and Chi-Square test to compare proportions.
Logistic regression assessed effect of RBC storage time and known prognostic factors on delirium/coma, by calculating unadjusted relative risk, confidence intervals, and p-values. Poisson regression with robust standard errors was used to calculate adjusted relative risks, adjusting (based on clinical and statistical rationale) for site, age, PELOD-2, opiate and benzodiazepine exposure, and cognitive status prior to transfusion. An exchangeable correlation was used to account for clustering by center in the multivariable model.
Mean delirium duration was compared between short-storage/standard-issue using independent t-test. Effect of RBC dose on subsequent delirium/coma was assessed by comparing mean dose (mL/kg) between patients who did and did not have delirium/coma using two-sided t-test. Logistic regression was used to calculate relative risk of delirium/coma for each one unit increase in RBC dose. To explore the effect of anemia severity (as measured by hemoglobin prior to transfusion) and risk of delirium, a Wilcoxon rank-sum test was used.
With respect to the matched cohort, Chi-square/Mann-Whitney U tests were used to look at the differences between the transfused group and control group for all matching variables. To compare delirium/coma development within three days between transfused subjects and non-transfused controls, a conditional logistic regression model generated an odds ratio accounting for the matched pairs, and additionally controlling for hemoglobin, gender, developmental disability, PELOD score, and cognitive status (including deep-sedation) at time zero.
All p-values are two-sided with significance set at p<0.05. Analyses were performed using Statistical Analysis System, Version 9.3 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Twenty PICUs in the United States participated in the TAD–ABC–PICU study. One-hundred forty-six subjects were enrolled and screened for delirium/coma between June 9, 2016--May 17, 2018. Site enrollment ranged from 1–17 subjects, with median of 6 subjects/site. Average age was 5.3 (± 5.0) years, and 46% of subjects were male. Patients were in ICU for a mean of 2.16 days, and had a median of one organ dysfunction present, at time of transfusion. Fifty-one percent of subjects received benzodiazepines and 67% received opiates on day of transfusion. Sixty-nine subjects (47%) were randomly allocated to the short-storage arm, and 77 subjects (53%) to the usual care arm. Baseline characteristics of patients allocated to short-storage vs standard-issue RBC arms are shown in Table 1.
Table 1.
Comparison of Baseline Characteristics Between Study Arms (N = 146)
| Characteristics | Short-storage RBC arm | Standard-issue RBC arm | p-value |
|---|---|---|---|
| N = 69 | N = 77 | ||
| Age at admission (years) (mean, SD) | 5.2 ± 5.2 | 5.4 ± 4.9 | 0.81 |
| Hemoglobin prior to transfusion (g/dl) (mean,SD) | 6.9 ± 0.9 | 7.0 ± 1.4 | 0.27 |
| Sex (n,%) | 0.62 | ||
| Female | 39 (56.5) | 40 (51.9) | |
| Male | 30 (43.5) | 37 (48.1) | |
| Type of ICU admission (n,%) | 0.56 | ||
| General medical | 55 (79.7) | 56 (72.7) | |
| Cardiac | 0 | 3 (3.9) | |
| Surgical | 7 (10.1) | 12 (15.6) | |
| Trauma | 7 (10.1) | 6 (7.8) | |
| Time from admission to transfusion (days) (mean, SD) | 2.16 (1.5) | 2.17 (1.6) |
0.97 |
| PELOD-2 Score at randomization (mean, SD) | 5.7 (2.9) | 4.8 (2.9) | 0.06 |
| Opiate exposure (n,%) | 52 (75.4%) | 46 (59.7%) | 0.04 |
| Benzodiazepine exposure (n,%) | 42 (60.9%) | 32 (41.6%) | 0.02 |
SD: standard deviation; IQR: interquartile range; MODS: multiple organ dysfunction syndrome; PELOD-2: Pediatric Logistic Organ Dysfunction-2 score; RBC: red blood cell.
The first RBC transfusion was given 2.0 ± 1.5 days after PICU admission. The mean RBC storage age for the short-storage arm was 5.38 days, as compared to 21.96 days for the standard-issue arm. The mean pre-transfusion hemoglobin was 6.9 for the short-storage arm, and 7.0 for the standard-issue arm (p=0.27).
Of the 146 subjects, 109 (74.7%) developed delirium and/or coma within three days following RBC transfusion. When compared by treatment arm, 55 subjects (79.5%) who received short-storage RBCs developed delirium/coma, as compared to 54 subjects (70.1%) who received standard-issue RBCs (p=0.18). Of note, patients in the short-storage arm were more likely to receive benzodiazepines (60.9% vs 41.6%, p=0.02) and opiates (75.4% vs 59.7%, p = 0.045) on day of transfusion.
In univariate analysis, relative risk of delirium/coma was 1.14 (0.94–1.37, p=0.18) for children in the short-storage arm. (Results did not meaningfully differ when coma was excluded: the relative risk of delirium was 1.05 (0.85–1.31) for children in the short-storage arm). In addition, duration of delirium/coma did not differ between study arms (median 3, IQR 2–4, days in both groups, p=0.59). However, other relevant clinical factors were found to affect relative risk for delirium/coma in univariate analyses. For example, exposure to benzodiazepines and opiates prior to transfusion conferred an increased risk for delirium/coma (OR 1.68 and 1.83 respectively, p<0.001 for both). In addition, presence of delirium/coma prior to transfusion was associated with a relative risk of 2.6 (1.82–3.71, p<0.001) for subsequent delirium/coma, as compared to children who were DFCF at time of transfusion (Table 2).
Table 2.
RBC Storage Time and Clinical Characteristics: Relative Risk of Delirium/Coma within 3 days of RBC Transfusion
| Characteristic | Univariate Analyses | |||||
|---|---|---|---|---|---|---|
| N | Primary Outcome N (%) | Relative Risk | LCL | UCL | P-value | |
| Age of blood | ||||||
| Short-storage RBCs | 69 | 55 (79.7) | 1.14 | 0.94 | 1.37 | 0.184 |
| Standard-issue RBCs | 77 | 54 (70.1) | Ref. | |||
| Patients Age (Days)* | 146 | 0.996 | 0.99 | 1.002 | 0.2108 | |
| PELOD-2 on randomization* | 146 | 1.08 | 1.02 | 1.15 | 0.0118 | |
| Opiates pre-transfusion | ||||||
| Yes | 98 | 86 (87.8) | 1.83 | 1.35 | 2.48 | <.0001 |
| No | 48 | 23 (47.9) | Ref. | |||
| Benzodiazepines pre-transfusion | ||||||
| Yes | 74 | 69 (93.2) | 1.68 | 1.35 | 2.08 | <.0001 |
| No | 72 | 40 (55.6) | Ref. | |||
| Cognitive status pre-transfusion** | ||||||
| Delirium/Coma | 88 | 87 (98.9) | 2.6 | 1.82 | 3.71 | <.0001 |
| Delirium-Free/Coma-Free | 50 | 19 (38.0) | Ref. | |||
Relative Risk was calculated per 100 days of increase in age, and per 1-unit increase in PELOD-2 score, respectively.
Cognitive status prior to transfusion was missing for eight subjects
[LCL: lower confidence level; UCL: upper confidence level; PELOD-2: Pediatric Logistic Organ Dysfunction-2 score (scores range from 0–33, with higher score reflecting greater severity of MODS prior to red blood cell transfusion)]
The multivariable model included 138 subjects (cognitive status prior to transfusion was not available for eight subjects). The model included RBC storage time, patient age, PELOD-2 score, opiate exposure, benzodiazepine exposure, and cognitive status (all prior to transfusion). While PELOD-2 score, opiate and benzodiazepine exposure, and cognitive status prior to transfusion were all significantly related to delirium/coma in the 72 hours after transfusion, RBC storage time was not significant (OR 0.95 [0.83–1.08], p=0.38, for short-storage arm) (Figure 1).
Figure 1. Multivariable analysis of clinical factors related to delirium/coma development within 3 days following red blood cell transfusion.
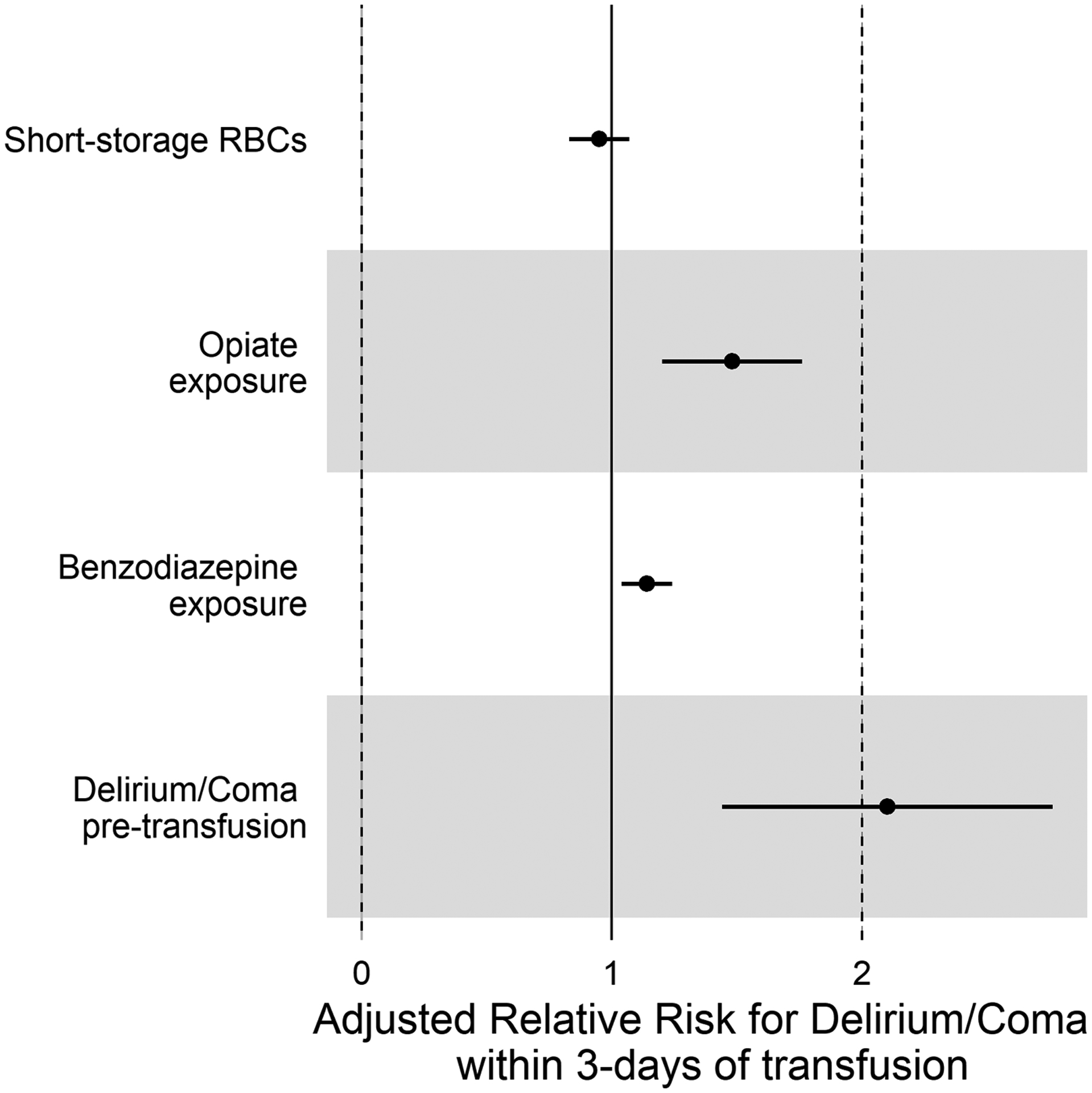
This Forest plot demonstrates increased risk for delirium/coma in transfused patients who had altered cognitive status (as measured by existing delirium or coma at time of transfusion), and/or exposure to opiates and benzodiazepines. However, there was no independent relationship between the storage-age of the transfused blood and subsequent delirium/coma. Analyses were adjusted for patient age and existing organ dysfunction (as measured by the PELOD-2) at time of transfusion.
[PELOD: pediatric logistic organ dysfunction; RBC: red blood cells].
No association was found between dose intensity and delirium/coma. Mean volume of transfusion in the group of children with subsequent delirium/coma was 12.9 ± 5.62 mL/kg, as compared to 11.8 ± 4.63 mL/kg in the group of children who were DFCF (p = 0.28). Relative risk of delirium was not dependent on dose (RR 1.01 [0.99 – 1.03] for every 1 mL/kg increase in RBC exposure).
Finally, there was no association between anemia severity (as measured by hemoglobin prior to transfusion) and risk of delirium. In univariate analysis, mean pre-transfusion hemoglobin was 6.4 in patients who did not develop delirium/coma within 3 days, as compared to 7.2 in patients who developed delirium/coma (p=0.006). A multivariable regression (accounting for variables shown to be associated with delirium rates in our cohort) showed that for each 1-point increase in hemoglobin prior to transfusion, the aOR for delirium/coma was 1.62 (0.99–3.10, p=0.056).
In stage II, after completion of data collection for the TAD-ABC–PICU study, enrolled subjects were individually matched 2:1 with non-transfused patients from a comprehensive single-center delirium study database. Matching criteria included age, PELOD-2 score (which includes mechanical ventilation, and was used as a time-specific surrogate for severity of illness at time of transfusion), ICU day, opiate exposure, and benzodiazepine exposure (all on the day of transfusion). Seven patients had no appropriate match, and were excluded from further analyses. For 11 patients, only one appropriate match was found. For 29 of the 267 matched controls, no hemoglobin was available. This resulted in 139 transfused patients (mean hemoglobin 7.0, SD 1.2) and 238 non-transfused controls (mean hemoglobin 9.4, SD 2.2) available for multivariable analysis. (As we are unable to match transfused and non-transfused cohorts for hemoglobin at time 0, hemoglobin levels were included in the conditional logistic regression). Chi-square and Mann-Whitney U tests showed appropriate matching between groups (Table 3). As described above, outcome was defined as delirium/coma within 3-days.
Table 3.
Comparison of demographic and clinical characteristics between TAD-subjects and Non-transfused controls*
| Characteristic | Transfused cohort (n=139) | Controls (n=267) | p-value |
|---|---|---|---|
| Age category (years) (n, %) | 0.99 | ||
| 0–2 | 36 (25.9%) | 69 (25.8%) | |
| >2–5 | 29 (20.9%) | 55 (20.6%) | |
| >5–13 | 21 (15.1%) | 41 (15.4%) | |
| >13 | 53 (38.1%) | 102 (38.2%) | |
| PELOD-2 | |||
| Mean (SD) | 4.9 (2.7) | 4.5 (2.5) | 0.15 |
| ICU day | |||
| Mean (SD) | 2.1 (1.6) | 2.0 (1.4) | 0.53 |
| Opiate exposure (n, %) | 0.97 | ||
| yes | 94 (67.6%) | 181 (67.8%) | |
| no | 45 (32.4%) | 86 (32.2%) | |
| Benzodiazepine exposure (n, %) | 0.98 | ||
| yes | 71 (51.1%) | 136 (50.9%) | |
| no | 68 (48.9%) | 131 (49.1%) | |
| Outcome: Cognitive status within three days (n, %) | <0.001 | ||
| delirium/coma | 103 (74.1%) | 115 (43.1%) | |
| DFCF | 36 (25.9%) | 152 (56.9%) | |
matched for day of randomization in TAD-subjects
[DFCF: delirium-free/coma-free; PELOD-2: Pediatric Logistic Organ Dysfunction-2 score]
Delirium/coma rate in the transfused cohort was 74.1%, as compared to 43.1% in matched non-transfused patients (p<0.001). The adjusted odds ratio for delirium/coma in children who were transfused RBCs, after controlling for hemoglobin, gender, developmental disability, and altered cognitive status (as measured by existing delirium or coma at time of transfusion), was 8.9, when compared to the matched cohort of children who did not receive RBC transfusions (95% CI 2.8–28.4, p<0.001) (Table 4).
Table 4.
Conditional logistic regression model, accounting for matched pairs, describing odds for delirium/coma development in transfused vs. non-transfused patients
| Characteristic | Adjusted Odds Ratio | Confidence Interval | p-value |
|---|---|---|---|
|
Receipt of RBC transfusion
(Ref: No transfusion) |
8.94 | 2.8–28.4 | <0.001 |
|
Delirium/Coma at time 0
(Ref: DFCF) |
5.08 | 1.4–18.4 | 0.013 |
| Developmental disability | 4.02 | 1.2–13.6 | 0.026 |
| PELOD-2 score | 1.41 | 0.8–2.6 | 0.259 |
| Male gender | 1.17 | 0.4–3.2 | 0.755 |
| Hemoglobin* | 0.94 | 0.7–1.2 | 0.597 |
Odds ratio was calculated per 1 unit increase in hemoglobin (in mg/dL) at time zero.
[DFCF: Delirium-free and Coma-free; PELOD-2: Pediatric Logistic Organ Dysfunction-2 score (scores range from 0–33, with higher score reflecting greater severity of MODS at time 0)]
Discussion
More than 200,000 children are admitted to PICUs in the United States each year, and approximately one-fifth of these children receive RBC transfusions[22]. Research suggests that RBC transfusion and storage time may be linked to delirium/coma development and duration in adults[23,24]. This is an area that had not yet been studied in children.
Storage Duration of RBCs is not associated with Pediatric Delirium
When designing this study, we hypothesized that RBC storage time would be positively associated with delirium development. Although the etiology of transfusion-associated delirium is not completely understood, it is theorized that inefficient oxygen delivery (related to poor oxygen unloading by stored RBCs) may contribute. In addition, many experts in pediatric delirium posit that neuro-inflammation, accentuated by transfusion-related inefficient perfusion and oxidative stress, play important roles in the pathologic pathways[13]. Since existing transfusion literature proposes that transfusion of RBCs with longer storage duration might cause more inflammation, oxidative stress, and hypoperfusion, we theorized that short-storage RBCs would pose less of a delirium risk in children[12]. However, in this study, storage time of RBCs did not affect the development or duration of delirium. This is consistent with findings from the primary ABC-PICU study, where the use of short-storage RBCs did not affect development of new or progressive MODS[14]. It is also consistent with a large body of recent transfusion literature, where six separate studies and a recent meta-analysis all showed no significant differences in important outcome measures with transfusion of short-storage RBCs, and point estimates often favored standard-issue RBCs[25–32].
RBC Transfusions are Independently Associated with Pediatric Delirium
This is the first multi-institutional pediatric study to describe an independent association between RBC transfusion and subsequent delirium/coma. After carefully controlling for other predictors, delirium odds were more than 7 times higher in transfused patients. These findings are consistent with prior delirium literature. Preliminary data from a single academic PICU showed a strong and independent association between RBC transfusion and delirium[33]. Also consistent with our cohort, this prior study showed no association between hemoglobin prior to transfusion and development of subsequent delirium[33].
To further support this lack of causal relationship between anemia and delirium, there was no association between hemoglobin and risk for delirium development in our matched cohort. It is noteworthy that the mean pre-transfusion hemoglobin in the ABC-PICU cohort was statistically higher in the children who developed delirium (p=0.006). In multivariable analysis, the adjusted odds ratio for delirium/coma was 1.62 for each 1-point increase in hemoglobin prior to transfusion(p=0.056). Although this just missed statistical significance, it is consistent with our hypothesis that RBC transfusions in-and-of-themselves -rather than anemia-- may increase delirium risk.
Study Strengths and Limitations
Study strengths included a large multi-institutional group, prospective study design, and randomization and blinding that prevented ascertainment bias. Another important strength of this study was CAPD training of study staff prior to delirium screening. In addition, there was careful determination of cognitive status at time of transfusion, as pre-existing delirium/coma is the single biggest factor related to subsequent delirium/coma. Many delirium studies in adults were not able to include this very important temporal variable. Hence, our findings regarding the lack of association between RBC storage time and delirium development are likely widely applicable to academic PICUs across the United States.
A study limitation is that, despite randomization, this cohort of 146 patients from the ABC PICU study included more children in the short-storage arm who were exposed to opiates and benzodiazepines prior to transfusion. As these medications are known to be associated with delirium development, it is possible this led to increased delirium risk in the short-storage arm and may have confounded the effect of RBC storage time on subsequent delirium. Another limitation is the inability to match for hemoglobin level between the non-transfused and transfused cohorts. To mitigate against these possibilities, we included opiate and benzodiazepine exposure in our multivariable model for the randomized controlled trial, and hemoglobin as a variable in the conditional logistic regression for the matched comparisons.
Finally, the control group used for matching came from a single institution with an established focus on delirium prevention. It’s possible that delirium rates in this cohort are lower than delirium rates in other PICUs, and may have falsely accentuated the contribution of RBC transfusion to delirium development. However, even within this single PICU, retrospective multivariable analyses showed that delirium rates amongst transfused patients were more than double the rates in non-transfused patients [33].
Conclusions
In this cohort, storage time of RBCs did not affect development of delirium/coma in critically ill children. Overall, delirium rates were significantly higher in transfused subjects, even after controlling for other predictors of delirium. As there was no relationship between severity of anemia and delirium development, RBC transfusion practices may represent an important modifiable risk factor for pediatric delirium. As delirium is closely related to morbidity, restrictive transfusion strategies may decrease delirium rates and lead to improved patient outcomes.
Supplementary Material
Supplemental Figure 1. Cornell Assessment of Pediatric Delirium (CAPD). The CAPD is a bedside delirium screening tool validated for use in children of all ages. A score of nine or higher is consistent with a diagnosis of delirium. Reproduced with permission from Traube et al., 2014 [15].
Acknowledgements:
We gratefully acknowledge the contributions of Elham Sabri, MS, senior statistician at the Ottawa Hospital Research Institute, University of Ottawa, Ontario, Canada. We also thank the ABC-PICU Steering Committee for their vision and leadership. The authors also want to acknowledge the efforts of Dr. Lauren Marsillio, for her help enrolling patients in this study. Dr. Marsillio’s untimely death is mourned by all who knew her.
Financial support:
Funding provide by the NIH/NHLBI, Institute of Circulatory and Respiratory Health of the Canadian Institutes Health Research, Washington University in St Louis, Weill Cornell Medical College, the Programme Hospitalier de Recherche Clinique of the French Ministry of Health, the Quebec Ministry of Health and Social Services, the Groupe Francophone de Réanimation et d’Urgences Pédiatriques (GFRUP), and the Établissement Français du Sang.
Copyright Form Disclosure:
Drs. Traube, Nellis, Avery, McQuillen, Fitzgerald, and Spinella received support for article research from the National Institutes of Health (NIH). Dr. Nellis’ institution received funding from the National Heart, Lung, and Blood Institute. Dr. McQuillen’s institution received funding from the National Institute of Child Health and Human Development. Dr. Fitzgerald’s institution received funding from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Muszynski’s institution received funding from Washington University at St. Louis. Dr. Hanson’s institution received funding from the NIH. Dr. Lacroix’s institution received funding from the Canadian Institutes of Health Research. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Name of the Institutions where the work was performed:
- Advocate Children’s Hospital
- Ann & Robert H. Lurie Children’s Hospital of Chicago
- Children’s Hospital Colorado
- Children’s Hospital Los Angeles
- Children’s Hospital of Orange County
- Children’s Hospital of Philadelphia
- Children’s Medical Center Dallas
- Diamond Children’s Medical Center
- Duke University
- Medical College of Wisconsin
- Nationwide Children’s Hospital
- Riley Hospital for Children
- University of Alabama
- University of California San Francisco
- University of Cincinnati College of Medicine
- University of Florida College of Medicine
- University of Rochester
- Virginia Commonwealth University
- Washington University School of Medicine
- Weill Cornell Medical College
Address for reprints: Reprints will not be ordered.
Contributor Information
Chani Traube, Weill Cornell Medical College, New York, NY.
Marisa Tucci, Department of Pediatrics, CHU Sainte-Justine, University of Montreal, Montreal, Canada.
Marianne E. Nellis, Weill Cornell Medical College, New York, NY.
K. Leslie Avery, Division Chief, Department of Pediatrics, University of Florida College of Medicine, Gainesville, FL.
Patrick S. McQuillen, Department of Pediatrics, University of California San Francisco, San Francisco, CA.
Julie C. Fitzgerald, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
Jennifer A. Muszynski, Nationwide Children’s Hospital, Columbus, OH.
Jill M. Cholette, University of Rochester, Golisano Children’s Hospital, Rochester, NY.
Adam J. Schwarz, CHOC Children’s Hospital, Orange, Ca
Erika L. Stalets, Interim Division Director, Division of Critical Care Medicine, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH.
Maureen A. Quaid, Division Director, Critical Care, Department of Pediatrics, Advocate Children’s Hospital, Park Ridge, IL.
Sheila J. Hanson, Department of Pediatrics and Children’s Wisconsin, Critical Care Section, Medical College of Wisconsin, Milwaukee, WI.
Jacques Lacroix, Division of Pediatric Critical Care, Department of Pediatrics, CHU Sainte-Justine, Université de Montréal, Montréal (QC), Canada.
Ron W. Reeder, Department of Pediatrics, University of Utah, Salt Lake City, UT.
Philip C. Spinella, Washington University School of Medicine, St. Louis, MO.
References
- [1].Traube C, Silver G, Reeder RW, Doyle H, Hegel E, Wolfe HA, et al. Delirium in Critically Ill Children: An International Point Prevalence Study. Crit Care Med 2017;45:584–90. 10.1097/CCM.0000000000002250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Arlington, VA, American Psychiatric Association, 2013. n.d. [Google Scholar]
- [3].Traube C, Silver G, Gerber LM, Kaur S, Mauer EA, Kerson A, et al. Delirium and Mortality in Critically Ill Children: Epidemiology and Outcomes of Pediatric Delirium. Crit Care Med 2017;45:891–8. 10.1097/CCM.0000000000002324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Silver G, Doyle H, Hegel E, Kaur S, Mauer EA, Gerber LM, et al. Association Between Pediatric Delirium and Quality of Life After Discharge. Crit Care Med 2020;48:1829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Siegel EJ, Traube C. Pediatric delirium: epidemiology and outcomes. Curr Opin Pediatr 2020;32:743–9. 10.1097/MOP.0000000000000960. [DOI] [PubMed] [Google Scholar]
- [6].Traube C, Mauer EA, Gerber LM, Kaur S, Joyce C, Kerson A, et al. Cost Associated With Pediatric Delirium in the ICU: Crit Care Med 2016;44:e1175–9. 10.1097/CCM.0000000000002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dervan LA, Di Gennaro JL, Farris RWD, Watson RS. Delirium in a tertiary PICU: Risk factors and outcomes*. Pediatr Crit Care Med 2020:21–32. 10.1097/pcc.0000000000002126. [DOI] [PubMed] [Google Scholar]
- [8].Dechnik A, Traube C. Delirium in hospitalised children. Lancet Child Adolesc Health 2020;4:312–21. 10.1016/S2352-4642(19)30377-3. [DOI] [PubMed] [Google Scholar]
- [9].Proulx F, Fayon M, Ann Farrell C, Lacroix J, Gauthier M. Epidemiology of Sepsis and Multiple Organ Dysfunction Syndrome in Children. Chest 1996;109:1033–7. 10.1378/chest.109.4.1033. [DOI] [PubMed] [Google Scholar]
- [10].Harris J, Ramelet A-S, van Dijk M, Pokorna P, Wielenga J, Tume L, et al. Clinical recommendations for pain, sedation, withdrawal and delirium assessment in critically ill infants and children: an ESPNIC position statement for healthcare professionals. Intensive Care Med 2016;42:972–86. 10.1007/s00134-016-4344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tucci M, Lacroix J, Fergusson D, Doctor A, Hébert P, Berg RA, et al. The age of blood in pediatric intensive care units (ABC PICU): study protocol for a randomized controlled trial. Trials 2018;19. 10.1186/s13063-018-2809-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Spinella PC, Doctor A. Role of Transfused Red Blood Cells for Shock and Coagulopathy Within Remote Damage Control Resuscitation: Shock 2014;41:30–4. 10.1097/SHK.0000000000000089. [DOI] [PubMed] [Google Scholar]
- [13].Maldonado JR. Neuropathogenesis of Delirium: Review of Current Etiologic Theories and Common Pathways. Am J Geriatr Psychiatry 2013;21:1190–222. 10.1016/j.jagp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- [14].Spinella PC, Tucci M, Fergusson DA, Lacroix J, Hébert PC, Leteurtre S, et al. Effect of Fresh vs Standard-issue Red Blood Cell Transfusions on Multiple Organ Dysfunction Syndrome in Critically Ill Pediatric Patients: A Randomized Clinical Trial. JAMA 2019;322:2179. 10.1001/jama.2019.17478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Traube C, Silver G, Kearney J, Patel A, Atkinson TM, Yoon MJ, et al. Cornell Assessment of Pediatric Delirium: A Valid, Rapid, Observational Tool for Screening Delirium in the PICU. Crit Care Med 2014;42:656–63. 10.1097/CCM.0b013e3182a66b76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Meyburg J, Dill M-L, Traube C, Silver G, von Haken R. Patterns of Postoperative Delirium in Children. Pediatr Crit Care Med 2017;18:128–33. 10.1097/PCC.0000000000000993. [DOI] [PubMed] [Google Scholar]
- [17].Patel AK, Biagas KV, Clarke EC, Gerber LM, Mauer E, Silver G, et al. Delirium in Children After Cardiac Bypass Surgery. Pediatr Crit Care Med 2017;18:165–71. 10.1097/PCC.0000000000001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Alvarez RV, Palmer C, Czaja AS, Peyton C, Silver G, Traube C, et al. Delirium is a Common and Early Finding in Patients in the Pediatric Cardiac Intensive Care Unit. J Pediatr 2018;195:206–12. 10.1016/j.jpeds.2017.11.064. [DOI] [PubMed] [Google Scholar]
- [19].Skrobik Y, Leger C, Cossette M, Michaud V, Turgeon J. Factors Predisposing to Coma and Delirium: Fentanyl and Midazolam Exposure; CYP3A5, ABCB1, and ABCG2 Genetic Polymorphisms; and Inflammatory Factors*. Crit Care Med 2013;41:999–1008. 10.1097/CCM.0b013e318275d014. [DOI] [PubMed] [Google Scholar]
- [20].Girard TD, Pandharipande PP, Carson SS, Schmidt GA, Wright PE, Canonico AE, et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: The MIND randomized, placebo-controlled trial*. Crit Care Med 2010;38:428–37. 10.1097/CCM.0b013e3181c58715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F. PELOD-2: An Update of the PEdiatric Logistic Organ Dysfunction Score. Crit Care Med 2013;41:1761–73. 10.1097/CCM.0b013e31828a2bbd. [DOI] [PubMed] [Google Scholar]
- [22].Demaret P, Tucci M, Karam O, Trottier H, Ducruet T, Lacroix J. Clinical Outcomes Associated With RBC Transfusions in Critically Ill Children: A 1-Year Prospective Study*. Pediatr Crit Care Med 2015;16:505–14. 10.1097/PCC.0000000000000423. [DOI] [PubMed] [Google Scholar]
- [23].Zhang Z-Y, Gao D-P, Yang J, Sun X, Zhang H, Hu J, et al. Impact of length of red blood cells transfusion on postoperative delirium in elderly patients undergoing hip fracture surgery: A cohort study. Injury 2016;47:408–12. 10.1016/j.injury.2015.10.009. [DOI] [PubMed] [Google Scholar]
- [24].Behrends M, DePalma G, Sands L, Leung J. Association Between Intraoperative Blood Transfusions and Early Postoperative Delirium in Older Adults. J Am Geriatr Soc 2013;61:365–70. 10.1111/jgs.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fergusson DA, Hébert P, Hogan DL, LeBel L, Rouvinez-Bouali N, Smyth JA, et al. Effect of Fresh Red Blood Cell Transfusions on Clinical Outcomes in Premature, Very Low-Birth-Weight Infants: The ARIPI Randomized Trial. JAMA 2012;308:1443. 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- [26].Dhabangi A, Ainomugisha B, Cserti-Gazdewich C, Ddungu H, Kyeyune D, Musisi E, et al. Effect of Transfusion of Red Blood Cells With Longer vs Shorter Storage Duration on Elevated Blood Lactate Levels in Children With Severe Anemia: The TOTAL Randomized Clinical Trial. JAMA 2015;314:2514. 10.1001/jama.2015.13977. [DOI] [PubMed] [Google Scholar]
- [27].Lacroix J, Hébert PC, Fergusson DA, Tinmouth A, Cook DJ, Marshall JC, et al. Age of Transfused Blood in Critically Ill Adults. N Engl J Med 2015;372:1410–8. 10.1056/NEJMoa1500704. [DOI] [PubMed] [Google Scholar]
- [28].Heddle NM, Cook RJ, Arnold DM, Liu Y, Barty R, Crowther MA, et al. Effect of Short-Term vs. Long-Term Blood Storage on Mortality after Transfusion. N Engl J Med 2016;375:1937–45. 10.1056/NEJMoa1609014. [DOI] [PubMed] [Google Scholar]
- [29].Cooper DJ, McQuilten ZK, Nichol A, Ady B, Aubron C, Bailey M, et al. Age of Red Cells for Transfusion and Outcomes in Critically Ill Adults. N Engl J Med 2017;377:1858–67. 10.1056/NEJMoa1707572. [DOI] [PubMed] [Google Scholar]
- [30].Steiner ME, Ness PM, Assmann SF, Triulzi DJ, Sloan SR, Delaney M, et al. Effects of Red-Cell Storage Duration on Patients Undergoing Cardiac Surgery. N Engl J Med 2015;372:1419–29. 10.1056/NEJMoa1414219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].McQuilten ZK, French CJ, Nichol A, Higgins A, Cooper DJ. Effect of age of red cells for transfusion on patient outcomes: a systematic review and meta-analysis. Transfus Med Rev 2018;32:77–88. 10.1016/j.tmrv.2018.02.002. [DOI] [PubMed] [Google Scholar]
- [32].Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA 2016;316:2025. 10.1001/jama.2016.9185. [DOI] [PubMed] [Google Scholar]
- [33].Nellis ME, Goel R, Feinstein S, Shahbaz S, Kaur S, Traube C. Association Between Transfusion of RBCs and Subsequent Development of Delirium in Critically Ill Children. Pediatr Crit Care Med 2018;19:925–9. 10.1097/PCC.0000000000001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Cornell Assessment of Pediatric Delirium (CAPD). The CAPD is a bedside delirium screening tool validated for use in children of all ages. A score of nine or higher is consistent with a diagnosis of delirium. Reproduced with permission from Traube et al., 2014 [15].


