Abstract
Introduction
Proactive therapy with topical corticosteroids (TCSs) is the standard treatment for chronic inflammatory diseases such as atopic dermatitis; however, skin atrophy as TCS side effect remains a concern.
Methods
This 16-week, evaluator-blinded, within-patient placebo-controlled, randomized study enrolled volunteers with healthy skin conditions. For 12 weeks, their volar forearm and the back of their hand were applied with hydrocortisone acetate 1% cream (HC), methylprednisolone aceponate 0.1% cream (MPA), betamethasone valerate 0.1% cream (BMV), or an active agent-free base cream (Dermatop® Basiscreme) once daily twice weekly, and pimecrolimus 1% cream (PIM) twice daily twice weekly. Epidermal and dermal thickness was measured by optical coherence tomography and high-frequency ultrasound, respectively. Furthermore, skin atrophy and telangiectasia were determined by contact dermatoscopic photography (Dermaphot®).
Results
After 8 and 12 weeks, only BMV led to significant epidermal thinning on both sites. Four weeks after the end of treatment, epidermal thickness returned to baseline. No dermal thinning, atrophy, or telangiectasia was observed.
Conclusions
MPA, HC, and PIM may be more suitable for repeated and prolonged treatment, especially in chronic diseases.
Keywords: Atopic dermatitis, Corticosteroid, Optical coherence tomography, Pimecrolimus, Skin atrophy
Introduction
Atopic dermatitis (AD) is an inflammatory, highly pruritic, chronic, and chronically relapsing skin disease that negatively affects one's quality of life [1, 2]. Application of topical corticosteroids (TCSs) remains to be the mainstay of treatment for AD; however, long-term use of TCSs causes various side effects, such as skin atrophy, striae distensae, telangiectasia, and skin barrier function impairment [3, 4]. Additional effects include increased water loss, decreased hydration level, and increased skin transparency. These side effects depend on the strength, duration, and dose of the treatment and on the morphological properties of the skin in various anatomical areas [3, 4, 5].
Fortunately, an intermittent therapy regimen has been established to reduce or prevent the frequencies of relapses and to decrease the intensity of exacerbations in the long-term management of AD [6, 7, 8]. This proactive approach starts with an intensive topical anti-inflammatory therapy that lasts until the visible lesions are completely cleared, followed by the intermittent application of low-dose anti-inflammatory drugs on the previously affected skin areas [7]. The rationale of this approach is based on the fact that the skin of patients with AD, though appears normal, may have an impaired barrier function and a subclinical inflammatory infiltrate [9, 10]. Unlike TCSs, topical calcineurin inhibitors (TCIs) do not induce skin atrophy [2, 11, 12]. Proactive application of tacrolimus ointment in adults and children with AD also remarkably reduces the frequencies of disease exacerbations and increases the time to first disease exacerbation [13, 14].
Although TCSs and TCIs do not exhibit skin atrophy when applied twice weekly, prolonged application of TCSs to a nonlesional skin might lead to skin thinning [6]. Therefore, this study mainly aimed to investigate the effects of TCS and TCI treatment for 12 weeks on epidermal and dermal thickness by using optical coherence tomography (OCT), ultrasound, and Dermaphot®.
Materials and Methods
Study Design and Volunteers
This 16-week, evaluator-blinded, within-patient placebo-controlled, randomized, and single-center study enrolled 30 adult volunteers with healthy skin conditions. The inclusion criteria were as follows: age between 18 and 55 years, no skin disease, and skin type I–III according to the Fitzpatrick scale [15]. Conversely, the exclusion criteria were the following: participation in a clinical study within the last 30 days prior to screening; systemic or topical treatment within the last 6 months with drugs (e.g., retinoids, glucocorticosteroids, calcineurin inhibitors, and tar) that are suspected or known to influence skin thickness or telangiectasia formation; systemic therapy with immunosuppressants; severe systemic disease; genetic effects of the epidermal barrier (e.g., Netherton syndrome); intensive natural or artificial UV-light exposure within the last 4 weeks or during the study period; known intolerance to drug components used in the study; and drug or alcohol abuse.
The volunteers randomly applied 2 of the following 5 different study drugs: pimecrolimus 1% cream (PIM) (Elidel®, Novartis Pharma GmbH, Germany), hydrocortisone acetate 1% cream (HC) (Hydrogalen® Creme, GALENpharma GmbH, Germany), methylprednisolone aceponate 0.1% cream (MPA) (Advantan® Creme, Intendis GmbH, Germany), betamethasone valerate 0.1% cream (BMV) (Betagalen® Crème, GALENpharma GmbH, Germany), and an active agent-free base cream (DBC) (Dermatop® Basiscreme; Sanofi-Aventis Deutschland GmbH, Germany). These drugs were topically applied on the right or left volar forearm and back of the hand once daily twice weekly. Only PIM was applied twice daily twice weekly. Meanwhile, 5 volunteers did not apply any cream (placebo group). Because of the different frequency of application or nonapplication, complete blinding of the subjects was not given.
Sequence generation was performed using a computer-based random-number generator (STATA 10 for Windows; StatCorp, College Station, TX, USA). To exclude possible bias by the treated sides, we set the same number of volunteers that applied creams with the same ingredients on the right and left sides. As each of the 30 subjects had one site on one volar forearm and one site on the back of the hand treated with an investigational product and one site on the contralateral side treated with a different investigational product, a total of 10 subjects were investigated in each group. They were instructed to use a different finger for each preparation to avoid intermixing the drug components. In addition, the original tubes were labeled with “volar forearm and back of the hand left” and “volar forearm and back of the hand right,” and individual stencils were made for each volunteer to reproduce the application sides easily. Prior to follow-up observation, the skin stayed untreated for 4 weeks. Emollients could be additionally used but only for twice a week; however, those containing active substances were prohibited during the study period. Furthermore, treatment application was not allowed 12 h before the measurement sessions.
Using OCT, Dermaphot®, ultrasound, transepidermal water loss (TEWL), and corneometry, a blinded study nurse measured all 4 treatment areas on days 0 (start of treatment), 7, 28, 56, 84 (end of treatment period), and 112 (end of follow-up period). The outcome assessor and the statistician were also blinded to the treatment. During each visit, the adverse events (AEs) and serious AEs, their severity, and their relationship to the study drugs were documented.
This study was approved by the institutional review board of the Technische Universität Dresden (Approval No: EK 145052012) and the German Federal Institute for Pharmaceuticals and Medical Products (EudaCT-No. 2011-004953-17, Bundesinstitut für Arzneimittel und Medizinprodukte). In addition, this study conformed to the ethical principles of the Declaration of Helsinki and was posted in clinicaltrial.gov. All volunteers gave their written informed consent to participate in the study.
Skin Measurements
After an acclimatization period of 15 min in an air-conditioned room, the skin was measured using the OCT device, followed by TEWL and corneometry. Thereafter, the skin was moistened with water and then examined using Dermaphot® and ultrasound consecutively.
Epidermal thickness was measured using the OCT system DermaSR (DermaRadar 830; Technische Universität, Dresden, Germany), which uses an 830.3-nm superluminescence diode (spectral width: 46.4 nm; optical power: 1 mW), as previously described [16]. To determine epidermal thickness, we calculated the mean difference between the signals of the air-skin transition and the dermal-epidermal junction of the 600 A-scans of the B-scan image [17]. The arithmetic mean was calculated from 3 individual measurements.
For measuring dermal thickness, we used a 22-MHz ultrasound device (DUB20, tpm − taberna pro medicum GmbH, Germany, CE 0482) with a maximum physical resolution of 72 μm and a signal penetration of up to 8 mm. Epidermal thinning and telangiectasia formation were detected by contact dermatoscopic photography with Dermaphot® (Heine Dermaphot Optotechnik GmbH, Herrsching, Germany) and Nikon® Digital Camera D70 modified for contact dermatoscopic use (Nikon Corporation, Tokyo, Japan). The images were evaluated using a modified Frosh Score (Table 1) [18].
Table 1.
Dermaphot® score for the evaluation of skin atrophy and telangiectasia
| Score | Definition |
|---|---|
| Atrophy | |
| 0 | No change |
| 1 | Slight transparency increase |
| 2 | Moderate thinning of the epidermis with moderate increase in transparency |
| 3 | Severe thinning and increase in transparency |
| 4 | Very severe thinning of the epidermis, the vasculature appearing to be directly under the surface |
| Telangiectasia | |
| 0 | Normal vascular pattern |
| 1 | Capillary hyperemia with slight elongation and dilatation of blood vessels not visible to the naked eye |
| 2 | Moderate telangiectasia, just visible with naked eye |
| 3 | Severe telangiectasia |
| 4 | Very severe telangiectasia with large blunt vessels |
Furthermore, TEWL was measured using the open-chamber evaporimeter Tewameter® TM 300 (Courage + Khazaka Electronic GmbH, Köln, Germany), and skin hydration was determined using Corneometer® CM 825 (Courage + Khazaka Electronic GmbH, Köln, Germany). Capacitance was performed 5 times in each test area. The highest and lowest values were deleted, and the mean value of the 3 remaining measurements was calculated; the results are expressed in grams per square meters per hour or arbitrary units (au) accordingly. The measurement of TEWL and skin hydration was in accordance with the established guidelines [19, 20].
Statistical Methods
The statistical analysis was performed on the intent-to-treat population. In this study, the primary end point was the change of epidermal thickness under treatment with PIM, HC, MPA, BMV, and DBC and without treatment (placebo) between days 0 and 84 measured by OCT. Data were analyzed by paired t-test. A 20% decrease in epidermal thickness was assumed to be relevant. Sample size calculation indicated that 8 volunteers per group were required for a study with 5% significance level and 90% power. The secondary end points were the determination of dermal thickness, skin atrophy and telangiectasia formation, skin hydration changes, and water loss.
Results
Patients and Safety
This study included 30 volunteers (22 females and 8 males); all of them completed the trial. The mean age was 40 years (range: 23–54 years). In addition, 22 study participants reported 43 AEs in which 23 were mild, 19 were moderate, and 1 was severe (tooth root resection with radiculodental cyst treatment). These AEs included headache (n = 16), flu-like symptoms (n = 9), rhinitis (n = 4), herpes labialis (n = 2), dental surgery (n = 2), shoulder surgery (n = 1), hallux rigidus operation (n = 1), gastrointestinal disease (n = 1), cough (n = 1), menstrual pain (n = 1), folliculitis (n = 1), vitamin B12 deficiency (n = 1), perlèche (n = 1), hypertensive crisis (n = 1), and calcaneodynia (n = 1). All AEs were unrelated to the study drugs. Furthermore, we found no AE on the treatment sites, no severe AE, and no protocol violation.
OCT Assessment
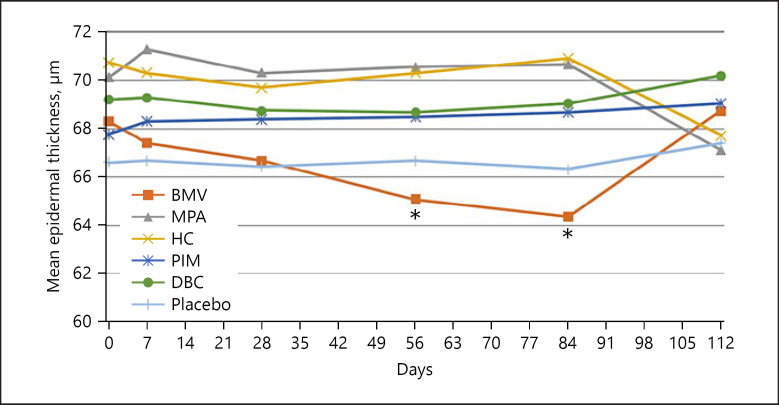
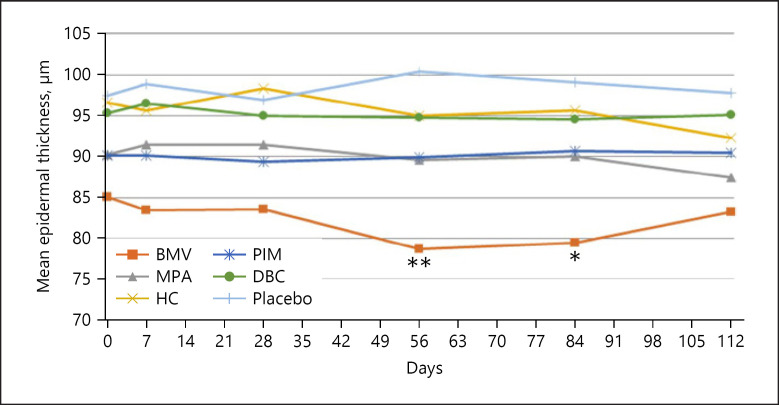
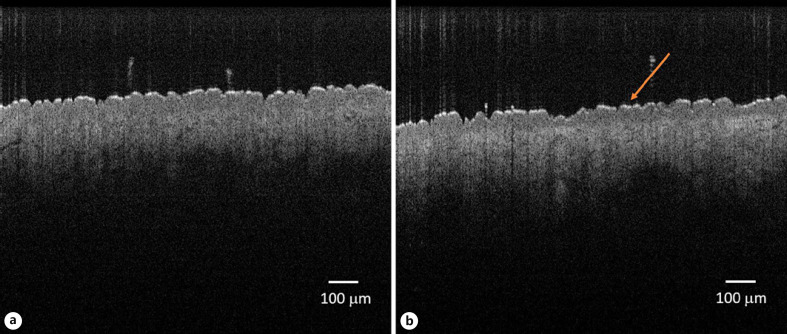
The epidermal thickness decreased continuously on the volar forearm during BMV treatment (shown in Fig. 1), reaching from 68.31 ± 8.09 μm to 64.35 ± 7.96 μm after 84 days. The thinning was −7.96% and statistically significant (p = 0.017). On the back of the hand, the epidermis thinned continuously up to visit 4 (day 56) and remained stable until visit 5 (day 84) (shown in Fig. 2). The epidermal thickness decreased from 85.05 ± 11.42 μm to 79.47 ± 11.99 μm (day 84, −6.56%), indicating a statistical significance (p = 0.025). An example of epidermal thinning under BMV is shown in Figure 3. After treatment completion, the epidermal thickness resumed to baseline on both sites. Meanwhile, treatment with MPA, HC, PIM, and DBC and placebo did not result in significant epidermal thinning on the forearm and the back of the hand (shown in Fig. 1, 2).
Fig. 1.
Mean change of epidermal thickness measured by OCT on the volar forearm (*p < 0.05: significant difference). OCT, optical coherence tomography; BMV, betamethasone valerate; MPA, methylprednisolone aceponate; HC, hydrocortisone acetate; PIM, pimecrolimus; DBC, active agent-free base cream.
Fig. 2.
Mean change of epidermal thickness measured by OCT on the back of the hand (*p < 0.05, **p < 0.01: significant difference). OCT, optical coherence tomography; BMV, betamethasone valerate; MPA, methylprednisolone aceponate; HC, hydrocortisone acetate; PIM, pimecrolimus; DBC, active agent-free base cream.
Fig. 3.
Example of OCT measurement at baseline (a) and epidermal thinning (arrow) after 12 weeks of treatment with BMV on the volar forearm. OCT, optical coherence tomography; BMV, betamethasone valerate.
20-MHz Ultrasound Assessment
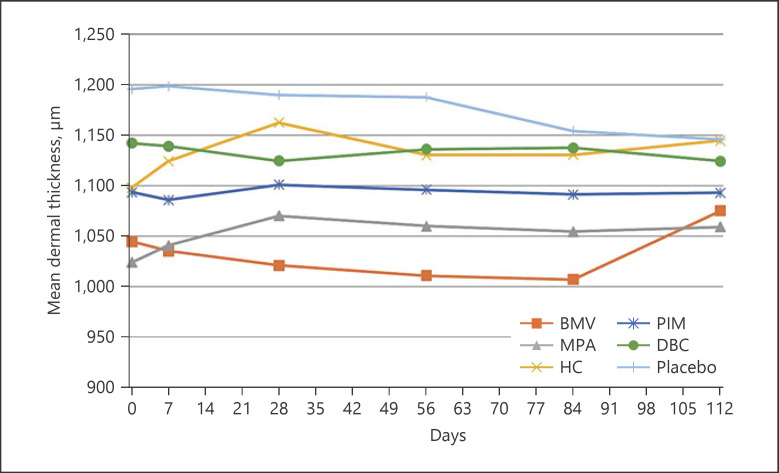
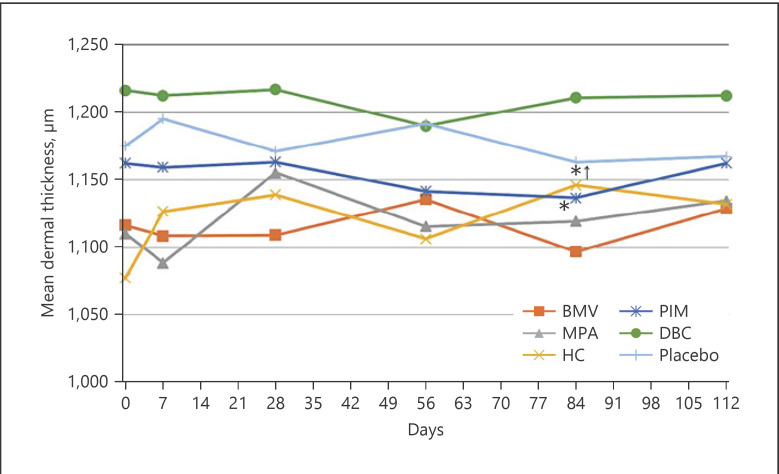
In BMV treatment, dermal thickness decreased continuously from 1044.60 ± 120.91 μm to 1007.00 ± 117.20 μm (−37.6 μm, −3.6%) on the volar forearm and from 1116.40 ± 89.35 μm to 1096.90 ± 67.11 μm (−19.5 μm, −1.75%) on the back of the hand. However, the decrease was statistically not significant. Likewise, treatment with MPA, HC, and DBC and placebo revealed no statistical differences. Conversely, only PIM treatment led to a statistically significant thinning of the dermis, from 1162.50 ± 132.41 μm to 1136.80 ± 126.88 μm (−25.7 μm, −2.2%) on the back of the hand (p = 0.001), whereby no change could be seen up to day 28, and the baseline value was reached after the therapy was completed. Throughout the observation period, the values on the forearm did not change. After 84 days, a reduction of only 2.2 μm (−0.2%) was observed (shown in Fig. 4, 5, 6).
Fig. 4.
Mean change of epidermal thickness measured by ultrasound on the volar forearm (*p < 0.05: significant difference). BMV, betamethasone valerate; MPA, methylprednisolone aceponate; HC, hydrocortisone acetate; PIM, pimecrolimus; DBC, active agent-free base cream.
Fig. 5.
Mean change of epidermal thickness measured by ultrasound on the back of the hand (*p < 0.05: significant difference). BMV, betamethasone valerate; MPA, methylprednisolone aceponate; HC, hydrocortisone acetate; PIM, pimecrolimus; DBC, active agent-free base cream.
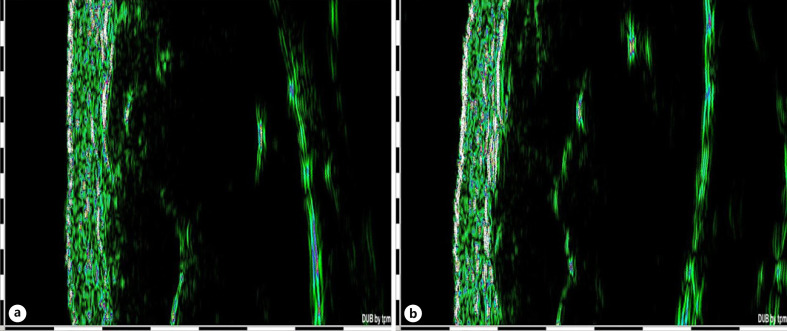
Fig. 6.
Example of ultrasound measurement at baseline (a) and after 12 weeks of treatment with MPA (b). No dermal thinning was assessed. One black and white line on the scale on the left side of the image and at the bottom corresponds to 1 mm. MPA, methylprednisolone aceponate.
Optical Assessment by Dermaphot® and Skin Physiological Parameters
Dermaphot® scores at the volar forearm and back of the hand were not statistically different. In the Wilcoxon signed rank test, no significant changes were observed throughout the observation period. Neither telangiectasia formation nor increased skin transparency was detected.
Regarding TEWL and skin hydration, no significant changes were found in BMV, MPV, HC, PIM, and placebo between days 0 and 84. Only DBC significantly increased skin hydration on the forearm (from 32.42 ± 7.81 au to 36.07 ± 7.93 au, p = 0.029) and on the back of the hand (from 25.86 ± 8.9 au to 31.56 ± 5.23 au, p = 0.016).
Discussion/Conclusion
One of the main side effects of TCSs is skin atrophy, which is characterized by a thin, transparent skin; a cigarette-paper-like surface; increased fragility; and tearing and bruising of the skin [1, 3, 4]. In the epidermis, TCSs may reduce the size of keratinocytes and inhibit their proliferation [3]. Moreover, TCSs may have a negative impact on the intercellular lipid layers of the upper epidermis, contributing to skin barrier dysfunction [21, 22, 23]. Direct and indirect effects of TCS on fibroblasts and the formation of collagen I and III and hyaluronic acid lead to a decrease in dermal thickness [24]. The extent of the skin atrophy depends on the strength of the TCS, frequency and duration of application, anatomical area treated, and the vehicle of the emollient used [3, 24, 25]. Lehmann et al. [26] induced a steroid atrophy of the skin on the forearms by continuous, occlusive application of clobetasol propionate (CP) for 6 weeks. By scanning electron micrographs and histology, they were able to demonstrate a progressive thinning of the epidermis with a reduction of the horny layer to a few wispy layers. The basal cell polarity shifted from columnar to round or cuboidal. The dermal-epidermal interface was flattened, and the dermis showed a great decrease in the ground substance. The compression of the fibers and their realignment parallel to the surface were noticeable. In a comparable experimental setup, the same research group observed an exuberant hyperplasia of the epidermis with a 4-fold maximal increase of viably epidermal thickness within 4 days poststeroid. The dermal restitution was similarly rapid. Fibroblasts appeared very active and within 14 days the ground substance increased continuously to normal levels [27].
Looking at the epidermis, Barnes et al. [24] showed that when used short term on the epidermis, the most atrophogenic TCS was the very potent CP (−26%), followed by BMV (−18%), which is a class III TCS according to Niedner et al. [28] and MPA (−8%), which is a class II TCS. Even the less potent TCS, that is, HC 1%, which was applied twice daily for 4 weeks on the uninvolved forehead skin of patients with AD, demonstrated a 6.1% reduction in epidermal thickness according to the highly sensitive OCT technique, and the thickness recovered within 4 weeks after the end of treatment [11].
Regarding dermal thickness, the results are comparable. Korting et al. [29] investigated the influence of BMV, mometasone furoate, and prednicarbate on total skin thickness over a treatment period of 6 weeks measured by 20-MHz sonography; they found that the decrease in skin thickness was strongest by BMV and least by prednicarbate. Overall, dermal thinning is proportionally less than epidermal thinning [30, 31].
Considering that TCSs have a negative impact on skin integrity and that chronic diseases such as AD require repeated use of anti-inflammatory drugs, a proactive therapy has currently become the standard of care in AD [8]. In clinical randomized controlled trials, fluticasone propionate 0.05% cream, fluticasone propionate 0.005% ointment, and MPA cream used 2 days per week for 16 weeks reduced the risk of relapses of AD and extended the time of remission [32, 33, 34, 35]. Clinically, skin atrophy was not evident in these investigations.
In our study, we applied TCSs of different strengths and therapeutic indexes on the volar forearm and back of the hand of healthy volunteers only once daily twice weekly for 12 weeks and measured the epidermal and dermal thickness by using the highly sensitive OCT and ultrasound, respectively. At baseline, the epidermal thickness on the volar forearm was comparable to the results of Czekalla et al. [36], who measured the epidermal thickness on 5 different body areas using 2-photon microscopy and OCT. In addition to the decrease in epidermal thickness with age, they showed that the thickness of the epidermis on the gluteal region, which is exposed to high mechanical stress, was higher than the epidermal thickness on the dorsal forearm, the volar forearm, the sural region, and the abdomen. The epidermal thickness in sun-expressed skin areas was higher than on those that are sun-protected. Similarly, in our study, the epidermal thickness on the back of the hand, which is also exposed to high mechanical stress and UV radiation, was found to be thicker than the epidermis on the volar forearm. Although BMV was only applied intermittently for 84 days, the epidermal thickness continued to decrease significantly by −6.56% on the back of the hand and −7.96% on the volar forearm. However, the effect was lower than the 20% assumption in sample size calculation; the study was still able to demonstrate this significant difference. We found a continuous decrease of the epidermal thickness for the forearm but not for the back of the hand, where the thickness stayed stable after day 56. Such a plateau was also observed by Jiráková et al. [37] during continuous use of methylprednisolone aceponate for 3 months on the limbs or on the trunk as measured by reflectance confocal microscopy. It is known that highly proliferating cells are seen in the epidermis as early as 2 days after alleviation of TCS, and the viable epidermis is increased in thickness with larger cells of irregular sizes and shapes [27]. Thus, it might be that a balance between atrophogenic effect of TCS and flare-up of epidermal proliferation has been achieved in our study at the back of the hand. Within 4 weeks after the end of the therapy, the epidermal thinning regressed. Meanwhile, the dermal thickness reduced nonsignificantly by −1.75% and −3.6% on the back of the hand and volar forearm, respectively. MPA and HC revealed no changes in skin thickness, particularly epidermal thickness, despite that the epidermis is known to change faster and more strongly than the dermis [38]. Using the skin compression and thickness method, Lubach et al. [39] also demonstrated skin atrophy induced by continuous topical application of CP, which is a highly potent TCS, followed by intermittent application. They found that continuous CP application resulted in −15% dermal thinning. When continued every 5th or 7th day, no any improvement was observed; however, when CP was administered every 10th day, the skin recovered to a more or less normal level.
Topical TCIs are also approved for treating AD and are unlikely to cause skin atrophy. Even when PIM was applied twice daily for 4 weeks on the uninvolved forehead skin of patients with AD, epidermal thinning did not occur according to OCT measurement [11]. Therefore, TCIs are preferred in sensitive locations such as the face, genital area, axilla region, or inguinal folds [1, 23, 40]. They also showed significant superiority over placebo in the proactive treatment of AD. When tacrolimus ointment was applied twice weekly as a proactive approach, disease exacerbations and the number of flares significantly declined, and the quality of life was improved in both adults and children [13, 14]. In our study, as expected, PIM showed no epidermal thinning on the volar forearm or back of the hand. Using ultrasound, we noticed dermal thinning on the back of the hand at −2.2%, whereas that on the volar forearm remained unchanged. Interestingly, the decrease in dermal thinning on the back of the hand was statistically significant, although the standard deviation was approximately 11% and the decrement was less than that of the dermis on the forearm under BMV treatment (−3.6%), which was not significant. In Kyllönen et al. [41] study, the administration of the TCI tacrolimus for over 12 months did not cause skin thinning, as measured by ultrasound. However, the skin thickness increased, as determined by the mean of 8 anatomic locations independently of their AD-presenting status, and was accompanied by an increase in collagen synthesis. The other group treated with conventional steroid-based therapy showed a significant decrease of 8% in skin thickness, supporting our investigation on BMV.
In another study by Hofmann et al. [42], the application of PIM once daily for 3 weeks did not change the skin thickness, as measured by ultrasound; in contrast, mometasone furoate (1 mg/g) and CP 0.05% cream resulted in skin thinning. Hoffman also discovered that signs of skin atrophy could be detected by Dermaphot® only after treatment with a TCS, but not with PIM. In our study, skin thinning was not detected by Dermaphot®. In a study in which PIM was administered under semiocclusion 6 h for 13 consecutive days to skin of domestic pigs, there were no atrophogenic effects seen on skin thickness as measured by ultrasound and histomorphometry [43]. Moreover, PIM did not affect the expression of cytokines, chemokines or adhesion molecules in optimally stimulated human fibroblasts [44]. A decrease of dermal thickness of 2% in our study is most likely not clinically relevant, but interestingly statistically significant. Therefore, PIM does not appear to have a negative effect on complete skin thickness as repeated before.
In the study of Chittock et al. [45] involving patients with quiescent AD treated with Betnovate® 0.1% cream twice per week for 8 weeks, TEWL and capacitance did not change, but treatment with tacrolimus significantly increased skin hydration. In another study, PIM was tremendously effective in reducing TEWL and improving skin hydration in patients with mild-to-moderate AD on the affected skin [46].
In conclusion, the use of the TCSs MPA and HC in the intermittent long-term therapy may be recommended because they did not result in skin atrophy over a period of 3 months. Considering the potential thinning of the epidermis, BMV should rather be avoided. Especially in sensitive regions such as the face and intertrigines, PIM is a good alternative in the proactive therapy because it also does not cause atrophy. Given that epidermal thinning is more pronounced and may occur earlier than dermal atrophy, OCT may be a valuable tool for the early detection of skin atrophy.
However, no statement can be made as to whether TCSs lead to skin atrophy when used over a prolonged period of time. Therefore, further and longer studies, especially in patients with AD, are required.
Statement of Ethics
The study was approved by the institutional review board of the Technische Universität Dresden (Approval No: EK 145052012) and the German Federal Institute for Pharmaceuticals and Medical Products (EudaCT-No. 2011-004953-17, Bundesinstitut für Arzneimittel und Medizinprodukte). The study was conducted in compliance with the ethical principles laid down in the Declaration of Helsinki and posted in clinicaltrial.gov. All volunteers gave their written informed consent to participate in the study.
Conflict of Interest Statement
The authors have declared no conflicting of interests.
Funding Sources
This study was financially supported by the Department of Dermatology, Technische Universität Dresden, Dresden, Germany.
Author Contributions
Roland Aschoff performed the research, designed the research study, and wrote the manuscript. Awena Lang also performed the research, analyzed the data, and drafted parts of the manuscript. Edmund Koch contributed experimental tools. All the authors critically revised and approved the submitted manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author or can be requested via https://katalog.slub-dresden.de/id/0-1551128357.
Acknowledgments
The Department of Clinical Sensoring and Monitoring (Technische Universität Dresden, Dresden, Germany) provided the technical knowledge and the equipment for the testing.
References
- 1.Siegfried EC, Jaworski JC, Kaiser JD, Hebert AA. Systematic review of published trials: long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatr. 2016 Jun;16((16)):75. doi: 10.1186/s12887-016-0607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broeders JA, Ahmed Ali U, Fischer G. Systematic review and meta-analysis of randomized clinical trials (RCTs) comparing topical calcineurin inhibitors with topical corticosteroids for atopic dermatitis: a 15-year experience. J Am Acad Dermatol. 2016 Aug;75((2)):410–9.e3. doi: 10.1016/j.jaad.2016.02.1228. [DOI] [PubMed] [Google Scholar]
- 3.Schoepe S, Schäcke H, May E, Asadullah K. Glucocorticoid therapy-induced skin atrophy. Exp Dermatol. 2006 Jun;15((6)):406–20. doi: 10.1111/j.0906-6705.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 4.Shlivko IL, Kamensky VA, Donchenko EV, Agrba P. Morphological changes in skin of different phototypes under the action of topical corticosteroid therapy and tacrolimus. Skin Res Technol. 2014 May;20((2)):136–40. doi: 10.1111/srt.12095. [DOI] [PubMed] [Google Scholar]
- 5.Luger T, Loske KD, Elsner P, Kapp A, Kerscher M, Korting HC, et al. [Topical skin therapy with glucocorticoids: therapeutic index] J Dtsch Dermatol Ges. 2004 Jul;2((7)):629–34. doi: 10.1046/j.1439-0353.2004.03626.x. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt J, von Kobyletzki L, Svensson A, Apfelbacher C. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2011 Feb;164((2)):415–28. doi: 10.1111/j.1365-2133.2010.10030.x. [DOI] [PubMed] [Google Scholar]
- 7.Wollenberg A, Bieber T. Proactive therapy of atopic dermatitis: an emerging concept. Allergy. 2009 Feb;64((2)):276–8. doi: 10.1111/j.1398-9995.2008.01803.x. [DOI] [PubMed] [Google Scholar]
- 8.Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018 May;32((5)):657–82. doi: 10.1111/jdv.14891. [DOI] [PubMed] [Google Scholar]
- 9.Holm EA, Wulf HC, Thomassen L, Jemec GB. Instrumental assessment of atopic eczema: validation of transepidermal water loss, stratum corneum hydration, erythema, scaling, and edema. J Am Acad Dermatol. 2006 Nov;55((5)):772–80. doi: 10.1016/j.jaad.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 10.Proksch E, Fölster-Holst R, Jensen JM. Skin barrier function, epidermal proliferation and differentiation in eczema. J Dermatol Sci. 2006 Sep;43((3)):159–69. doi: 10.1016/j.jdermsci.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Aschoff R, Schmitt J, Knuschke P, Koch E, Bräutigam M, Meurer M. Evaluation of the atrophogenic potential of hydrocortisone 1% cream and pimecrolimus 1% cream in uninvolved forehead skin of patients with atopic dermatitis using optical coherence tomography. Exp Dermatol. 2011 Oct;20((10)):832–6. doi: 10.1111/j.1600-0625.2011.01335.x. [DOI] [PubMed] [Google Scholar]
- 12.Queille-Roussel C, Graeber M, Thurston M, Lachapelle JM, Decroix J, de Cuyper C, et al. SDZ ASM 981 is the first non-steroid that suppresses established nickel contact dermatitis elicited by allergen challenge. Contact Dermatitis. 2000 Jun;42((6)):349–50. doi: 10.1034/j.1600-0536.2000.042006349.x. [DOI] [PubMed] [Google Scholar]
- 13.Wollenberg A, Reitamo S, Girolomoni G, Lahfa M, Ruzicka T, Healy E, et al. Proactive treatment of atopic dermatitis in adults with 0.1% tacrolimus ointment. Allergy. 2008 Jul;63((7)):742–50. [PubMed] [Google Scholar]
- 14.Thaçi D, Reitamo S, Gonzalez Ensenat MA, Moss C, Boccaletti V, Cainelli T, et al. Proactive disease management with 0.03% tacrolimus ointment for children with atopic dermatitis: results of a randomized, multicentre, comparative study. Br J Dermatol. 2008 Dec;159((6)):1348–56. doi: 10.1111/j.1365-2133.2008.08813.x. [DOI] [PubMed] [Google Scholar]
- 15.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124((6)):869–71. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 16.Koch P, Boller D, Koch E, Welzel J, Hüttmann G. Ultrahigh-resolution FDOCT system for dermatology. Proc SPIE. 2005;5690:24–30. [Google Scholar]
- 17.Popp A, Wendel M, Knels L, Knuschke P, Mehner M, Koch T, et al. Common-path Fourier domain optical coherence tomography of irradiated human skin and ventilated isolated rabbit lungs. Proc SPIE. 2005;5861:145–53. [Google Scholar]
- 18.Frosch PJ, Behrenbeck EM, Frosch K, Macher E. The Duhring chamber assay for corticosteroid atrophy. Br J Dermatol. 1981;104((1)):57–65. doi: 10.1111/j.1365-2133.1981.tb01712.x. [DOI] [PubMed] [Google Scholar]
- 19.Rogiers V, EEMCO Group EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacol Appl Skin Physiol. 2001 Mar–Apr;14((2)):117–28. doi: 10.1159/000056341. [DOI] [PubMed] [Google Scholar]
- 20.Berardesca E. European Group for Efficacy Measurements on Cosmetics and Other Topical Products (EEMCO). EEMCO guidance for the assessment of stratum corneum hydration: electrical methods. Skin Res Technol. 1997 May;3((2)):126–32. doi: 10.1111/j.1600-0846.1997.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 21.Sheu HM, Lee JY, Chai CY, Kuo KW. Depletion of stratum corneum intercellular lipid lamellae and barrier function abnormalities after long-term topical corticosteroids. Br J Dermatol. 1997;136((6)):884–90. [PubMed] [Google Scholar]
- 22.Jensen JM, Pfeiffer S, Witt M, Bräutigam M, Neumann C, Weichenthal M, et al. Different effects of pimecrolimus and betamethasone on the skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol. 2009 Sep;124((33 Suppl 2)):R19–28. doi: 10.1016/j.jaci.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Jensen JM, Weppner M, Dähnhardt-Pfeiffer S, Neumann C, Bräutigam M, Schwarz T, et al. Effects of pimecrolimus compared with triamcinolone acetonide cream on skin barrier structure in atopic dermatitis: a randomized, double-blind, right–left arm trial. Acta Derm Venereol. 2013 Sep 4;93((5)):515–9. doi: 10.2340/00015555-1533. [DOI] [PubMed] [Google Scholar]
- 24.Barnes L, Kaya G, Rollason V. Topical corticosteroid-induced skin atrophy: a comprehensive review. Drug Saf. 2015 May;38((5)):493–509. doi: 10.1007/s40264-015-0287-7. [DOI] [PubMed] [Google Scholar]
- 25.Maubec E, Laouénan C, Deschamps L, Nguyen VT, Scheer-Senyarich I, Wackenheim-Jacobs AC, et al. Topical mineralocorticoid receptor blockade limits glucocorticoid-induced epidermal atrophy in human skin. J Invest Dermatol. 2015 Jul;135((7)):1781–9. doi: 10.1038/jid.2015.44. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann P, Zheng P, Lavker RM, Kligman AM. Corticosteroid atrophy in human skin. A study by light, scanning, and transmission electron microscopy. J Invest Dermatol. 1983;81((2)):169–76. doi: 10.1111/1523-1747.ep12543603. [DOI] [PubMed] [Google Scholar]
- 27.Zheng PS, Lavker RM, Lehmann P, Kligman AM. Morphologic investigations on the rebound phenomenon after corticosteroid-induced atrophy in human skin. J Invest Dermatol. 1984;82((4)):345–52. doi: 10.1111/1523-1747.ep12260665. [DOI] [PubMed] [Google Scholar]
- 28.Niedner R. [Therapy with systemic glucocorticoids] Hautarzt. 2001 Nov;52((11)):1062–4. doi: 10.1007/pl00002604. quiz 1072. [DOI] [PubMed] [Google Scholar]
- 29.Korting HC, Unholzer A, Schäfer-Korting M, Tausch I, Gassmueller J, Nietsch KH. Different skin thinning potential of equipotent medium-strength glucocorticoids. Skin Pharmacol Appl Skin Physiol. 2002;15((2)):85–91. doi: 10.1159/000049394. [DOI] [PubMed] [Google Scholar]
- 30.Josse G, Rouvrais C, Mas A, Haftek M, Delalleau A, Ferraq Y, et al. A multitechnique evaluation of topical corticosteroid treatment. Skin Res Technol. 2009 Feb;15((1)):35–9. doi: 10.1111/j.1600-0846.2008.00326.x. [DOI] [PubMed] [Google Scholar]
- 31.Kolbe L, Kligman AM, Schreiner V, Stoudemayer T. Corticosteroid-induced atrophy and barrier impairment measured by non-invasive methods in human skin. Skin Res Technol. 2001;7((2)):73–7. doi: 10.1034/j.1600-0846.2001.70203.x. [DOI] [PubMed] [Google Scholar]
- 32.Hanifin J, Gupta AK, Rajagopalan R. Intermittent dosing of fluticasone propionate cream for reducing the risk of relapse in atopic dermatitis patients. Br J Dermatol. 2002 Sep;147((3)):528–37. doi: 10.1046/j.1365-2133.2002.05006.x. [DOI] [PubMed] [Google Scholar]
- 33.Berth-Jones J, Damstra RJ, Golsch S, Livden JK, Van Hooteghem O, Allegra F, et al. Twice weekly fluticasone propionate added to emollient maintenance treatment to reduce risk of relapse in atopic dermatitis: randomised, double blind, parallel group study. BMJ. 2003 Jun 21;326((7403)):1367. doi: 10.1136/bmj.326.7403.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Der Meer JB, Glazenburg EJ, Mulder PG, Eggink HF, Coenraads PJ. The management of moderate to severe atopic dermatitis in adults with topical fluticasone propionate. The Netherlands Adult Atopic Dermatitis Study Group. Br J Dermatol. 1999 Jun;140((6)):1114–21. doi: 10.1046/j.1365-2133.1999.02893.x. [DOI] [PubMed] [Google Scholar]
- 35.Peserico A, Städtler G, Sebastian M, Fernandez RS, Vick K, Bieber T. Reduction of relapses of atopic dermatitis with methylprednisolone aceponate cream twice weekly in addition to maintenance treatment with emollient: a multicentre, randomized, double-blind, controlled study. Br J Dermatol. 2008 Apr;158((4)):801–7. doi: 10.1111/j.1365-2133.2008.08436.x. [DOI] [PubMed] [Google Scholar]
- 36.Czekalla C, Schönborn KH, Lademann J, Meinke MC. Noninvasive determination of epidermal and stratum corneum thickness in vivo using two-photon microscopy and optical coherence tomography: impact of body area, age, and gender. Skin Pharmacol Physiol. 2019;32((3)):142–50. doi: 10.1159/000497475. [DOI] [PubMed] [Google Scholar]
- 37.Jiráková A, Rob F, Sečníková Z, Koblová K, Džambová M, Rajská L, et al. Topical corticosteroids but not calcineurin inhibitors induced atrophy after four weeks. Biol Regul Homeost Agens. 2015;29((3)):701–6. [PubMed] [Google Scholar]
- 38.Cossmann M, Welzel J. Evaluation of the atrophogenic potential of different glucocorticoids using optical coherence tomography, 20-MHz ultrasound and profilometry; a double-blind, placebo-controlled trial. Br J Dermatol. 2006 Oct;155((4)):700–6. doi: 10.1111/j.1365-2133.2006.07369.x. [DOI] [PubMed] [Google Scholar]
- 39.Lubach D, Rath J, Kietzmann M. Skin atrophy induced by initial continuous topical application of clobetasol followed by intermittent application. Dermatology. 1995;190((1)):51–5. doi: 10.1159/000246635. [DOI] [PubMed] [Google Scholar]
- 40.Reitamo S, Ortonne JP, Sand C, Cambazard F, Bieber T, Fölster-Holst R, et al. A multicentre, randomized, double-blind, controlled study of long-term treatment with 0.1% tacrolimus ointment in adults with moderate to severe atopic dermatitis. Br J Dermatol. 2005 Jun;152((6)):1282–9. doi: 10.1111/j.1365-2133.2005.06592.x. [DOI] [PubMed] [Google Scholar]
- 41.Kyllönen H, Remitz A, Mandelin JM, Elg P, Reitamo S. Effects of 1-year intermittent treatment with topical tacrolimus monotherapy on skin collagen synthesis in patients with atopic dermatitis. Br J Dermatol. 2004 Jun;150((6)):1174–81. doi: 10.1111/j.1365-2133.2004.06017.x. [DOI] [PubMed] [Google Scholar]
- 42.Hofmann M, Salgo R, Aschoff R, Luger TA, Meurer M, Bräutigam M, et al. Validation of Dermaphot(®) for the assessment of steroid-induced skin atrophy. Arch Dermatol Res. 2013 Apr;305((3)):215–21. doi: 10.1007/s00403-012-1297-2. [DOI] [PubMed] [Google Scholar]
- 43.Meingassner JG, Grassberger M, Fahrngruber H, Moore HD, Schuurman H, Stütz A. A novel anti-inflammatory drug, SDZ ASM 981, for the topical and oral treatment of skin diseases: in vivo pharmacology. Br J Dermatol. 1997;137((4)):568–76. doi: 10.1111/j.1365-2133.1997.tb03788.x. [DOI] [PubMed] [Google Scholar]
- 44.Wolff B, Herzig G, Stuetz A. Pimecrolimus does not affect cytokine and chomekine secretion and adhesion molecule expression in primary human keratinocytes and dermal fibroblasts. J Invest Dermatol. 2003;121((1)):1243. [Google Scholar]
- 45.Chittock J, Brown K, Cork MJ, Danby SG. Comparing the effect of a twice-weekly tacrolimus and betamethasone valerate dose on the subclinical epidermal barrier defect in atopic dermatitis. Acta Derm Venereol. 2015 Jul;95((6)):653–8. doi: 10.2340/00015555-2048. [DOI] [PubMed] [Google Scholar]
- 46.Aschoff R, Schwanebeck U, Bräutigam M, Meurer M. Skin physiological parameters confirm the therapeutic efficacy of pimecrolimus cream 1% in patients with mild-to-moderate atopic dermatitis. Exp Dermatol. 2009;18((1)):24–9. doi: 10.1111/j.1600-0625.2008.00756.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author or can be requested via https://katalog.slub-dresden.de/id/0-1551128357.