Abstract
Objective
To assess whether application of a standard algorithm to hospitalizations in the prenatal and postpartum (42 days) periods increases identification of severe maternal morbidity beyond analysis of only the delivery event.
Methods
We performed a retrospective cohort study using data from the Pregnancy to Early Life Longitudinal database, a Massachusetts population-based data system that links records from birth certificates to delivery hospital discharge records and non-birth hospital records for all birthing individuals. We included deliveries from January 1, 2009, to December 31, 2018, distinguishing between ICD-9 and ICD-10 coding. We applied the modified Centers for Disease Control and Prevention (CDC) algorithm for severe maternal morbidity used by the Alliance for Innovation on Maternal Health to hospitalizations across the antenatal period through 42 days postpartum. Morbidity was examined both with and without blood transfusion.
Results
Overall, 594,056 deliveries were included in the analysis, and 3,947 deliveries met criteria for severe maternal morbidity at delivery without transfusion and 9,593 with transfusion for aggregate rates of 150.1 (95% confidence interval (CI): 146.7–153.5) using ICD-9 codes and 196.6 (95% CI: 189.5–203.7) using ICD-10 codes per 10,000 deliveries. Severe maternal morbidity at birth increased steadily across both ICD-9 and ICD-10 from 129.4 in 2009 (95% CI: 126.2–132.6) using ICD-9 to 214.3 per 10,000 (95% CI: 206.9–221.8) in 2018 using ICD-10. Adding prenatal and postpartum hospitalizations increased cases by 21.9% under both ICD-9 and ICD-10, resulting in a 2018 rate of 258.7 per 10,000 (95% CI: 250.5–266.9). The largest increase in detected morbidity in the prenatal or postpartum time period was attributed to sepsis cases.
Conclusion.
Inclusion of prenatal and postpartum hospitalizations in the identification of severe maternal morbidity resulted in increased ascertainment of morbid events. These results suggest a need to ensure surveillance of care quality activities beyond the birth event.
Precis:
Inclusion of prenatal and postpartum hospitalizations in the identification of severe maternal morbidity resulted in a 21.9% increase in ascertainment of morbid events.
Introduction
The societal influence of high rates of maternal mortality in the U.S.1 has been challenging to establish, given the comparatively small number (650–750) of maternal deaths in the U.S. annually compared with other causes of death.2 Since maternal mortality is understood as a valuable, yet somewhat blunt measure of maternal health nationally, research and clinical interest developed in finding a measure to better ascertain a broader range of poor maternal outcomes at birth.3 As such, researchers at the Centers for Disease Control and Prevention (CDC) produced an algorithm based on 25 International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) hospital diagnostic and procedure categories to identify cases of severe maternal morbidity at delivery hospitalization.4 This measure, which can be used with any ICD coded database, has been widely applied in national,5 state,6 commercial insurance7 and hospital8 level analyses. With the adoption of ICD-10 in 2015, a revised algorithm based on 21 codes was developed to allow for the continued assessment of severe maternal morbidity.9,10
The CDC algorithm was initially developed to focus on delivery events, but as the concept of maternal mortality has become more widely understood as encompassing the period of pregnancy through a year postpartum,11 there is potential to broaden our understanding of severe maternal morbidity across the perinatal spectrum. Our objective in this study was to apply the CDC severe ICD code maternal morbidity algorithm to a population-based, longitudinally linked statewide hospital discharge database to examine severe morbidity, not only at the time of the birth hospitalization, but also during pregnancy and the postpartum period within 42 days after birth.
Methods
This retrospective cohort study uses data from the Pregnancy to Early Life Longitudinal (PELL) database. PELL is a Massachusetts population-based data system linking data from live birth and fetal death (≥ 350 grams or ≥ 20 weeks’ gestation) certificates to corresponding delivery hospital discharge records, as well as prior and subsequent deliveries and non-birth hospital utilization records (hospital admissions, observational stays, and emergency room visits) for birthing individuals and infants over time. Data have been linked for 98% of live births and fetal deaths in Massachusetts for birthing individuals and their children since 1998. These records are linked using LinkPro v3.0 (InfoSoft, Inc., Winnipeg, Manitoba, Canada), a SAS-based deterministic and probabilistic matching program. Selected core linkage variables include facility code, medical record number, date of birth or delivery, sex, zip code, and birth weight.
Longitudinal linkage is based on two steps: (1) linkage is initially based on the mother’s unique non-missing encrypted Social Security number; and (2) for records not linked by the above step, we use a unique non-missing combination (concatenated) of hospital number and medical record number. The Massachusetts Department of Public Health (MDPH) and the Center for Health Information and Analysis (CHIA) are the custodians of the PELL data, which are housed at MDPH. The longitudinal link permits examination of any hospital contacts for birthing individuals during the study period. In this analysis, we focus on prenatal, birth and postpartum (within 42 days) hospitalizations. Approval for the study was granted by the Institutional Review Board of the Massachusetts Department of Public Health.
The analysis focused on deliveries from January 1, 2009, to December 31, 2018. Due to differences in the rates of ascertainment of severe maternal morbidity before and after the transition from ICD-9 and ICD-10 codes, we divided our analysis between deliveries and hospitalizations that would have been coded under ICD-9 from those coded under ICD-10. Massachusetts hospitals adopted ICD-10 starting in October 2015 (see Appendix 2, available online at http://links.lww.com/xxx, for respective codes used). We excluded quarter four of 2015 from our analysis given data quality issues during the transition from ICD-9 to ICD-10, resulting from some Massachusetts’ hospitals delaying their adoption of the new codes. To enhance consistency in the analysis of postpartum hospitalizations for severe maternal morbidity under ICD-9, we included deliveries in the initial study period up to June 30, 2015 to ensure postpartum hospitalizations through September 30, 2015 would be coded under ICD-9. Likewise, we began our ICD-10 study period on October 1, 2016 to ensure prenatal hospitalizations would be coded under ICD-10. We also accessed hospitalization data for the first quarter of 2019, which was used to document postpartum hospitalizations for deliveries occurring in the final months of 2018.
The primary outcome was severe maternal morbidity defined by an algorithm developed by the CDC involving multiple diagnosis and procedure codes. As noted, the algorithm itself changed in response to the shift from ICD-9 (25 categories) to ICD-10 (21 categories).10 To enhance comparability over time, we applied a standardized severe maternal morbidity definition based on an algorithm developed as part of an interagency collaboration between the Health Resources and Services Administration (HRSA), CDC, the Agency for Healthcare Research and Quality (AHRQ), and the Alliance for Innovation on Maternal Health (AIM) (Version 07-01-2021). The definition and algorithm rely on the 21 conditions or procedures identified in the CDC revision for ICD-10 and applies them to the period covered by ICD-9, as well.12
Because blood transfusions make up such a large proportion of all severe maternal morbidity cases,13 where appropriate, we present findings both with and without transfusions.
The prenatal period is determined in PELL using the birth date and length of pregnancy based on a variable developed in PELL to maximize the accuracy of the gestational age measurement.14 Postpartum hospital encounters were defined as hospital admissions that occurred at least 1 calendar day after the delivery hospitalization discharge, excluding cases of consecutive transfers from the delivery hospital to another hospital, which were considered a continuation of the delivery hospitalization. The birth certificate or fetal death record delivery date was subtracted from admission date on hospital discharge records to determine the number of days postpartum when the hospital encounter occurred. Encounters were excluded if the difference in the date of delivery and the encounter was less than 1 day or equal to or greater than 42 days. The 42-day period corresponds to the period of greatest rehospitalization of birthing people as identified in prior research.6,7
Since our goal was to identify cases not already ascertained during the delivery event, we used the severe maternal morbidity algorithm to identify those who had a hospitalization with one of the severe maternal morbidity codes during the prenatal period without having a severe maternal morbidity code during the delivery hospitalization. Similarly, we used the same codes to identify hospitalizations in the six weeks postpartum that involved one of the severe maternal morbidity codes among birthing individuals who had not had a hospitalization with one of the codes either during pregnancy or at birth. Absolute and relative differences in rates are presented along with their 95% confidence intervals (CI). The analysis was completed using SAS -STAT 14.3.
Results
In the overall study period, there were a total of 710,371 deliveries (live births and fetal deaths) experienced by 496,463 Massachusetts’ residents in Massachusetts’ hospitals (Appendix 1, available online at http://links.lww.com/xxx). We excluded 9,148 deliveries that were unlinked to hospital discharge records or ectopic or molar pregnancies or pregnancies with abortive outcomes, as well as 86,469 deliveries that occurred during the transition from ICD-9 to ICD-10 (July 1, 2015 to September 30, 2016), and 5,807 deliveries from a hospital with unrealistic reporting of severe maternal morbidity rates after switching to ICD-10 coding. The result was an analytic sample of 594,056 deliveries with 448,953 coded under ICD-9, and 145,103 deliveries under ICD-10.
Of the 594,056 deliveries in our analytic sample, 3,947 met the criteria for severe maternal morbidity at delivery without transfusion (2,697 under ICD-9 and 1,250 under ICD-10) and 9,593 with transfusion (6,740 under ICD-9 and 2,853 under ICD-10) for aggregate rates of 150.1 (ICD-9) (95% CI: 146.7–153.5) and 196.6 (ICD-10) (95% CI: 189.5–203.7) per 10,000 deliveries, respectively. The trend over time (Figure 1) reveals a slight change after the adoption of ICD-10. Under the ICD-9 measure, severe maternal morbidity including transfusions increases by an absolute difference of 48.5 cases per 10,000 (95% CI: 46.6–50.5; relative change, 37.5%) from 2009–2015. Severe maternal morbidity rates with transfusion drop marginally after the switch to ICD-10, but overall rates rise rapidly, by 40.0 per 10,000 (95% CI: 36.8, 43.3; relative change, 23.0%) from 2016–2018 under ICD-10.
Figure 1:
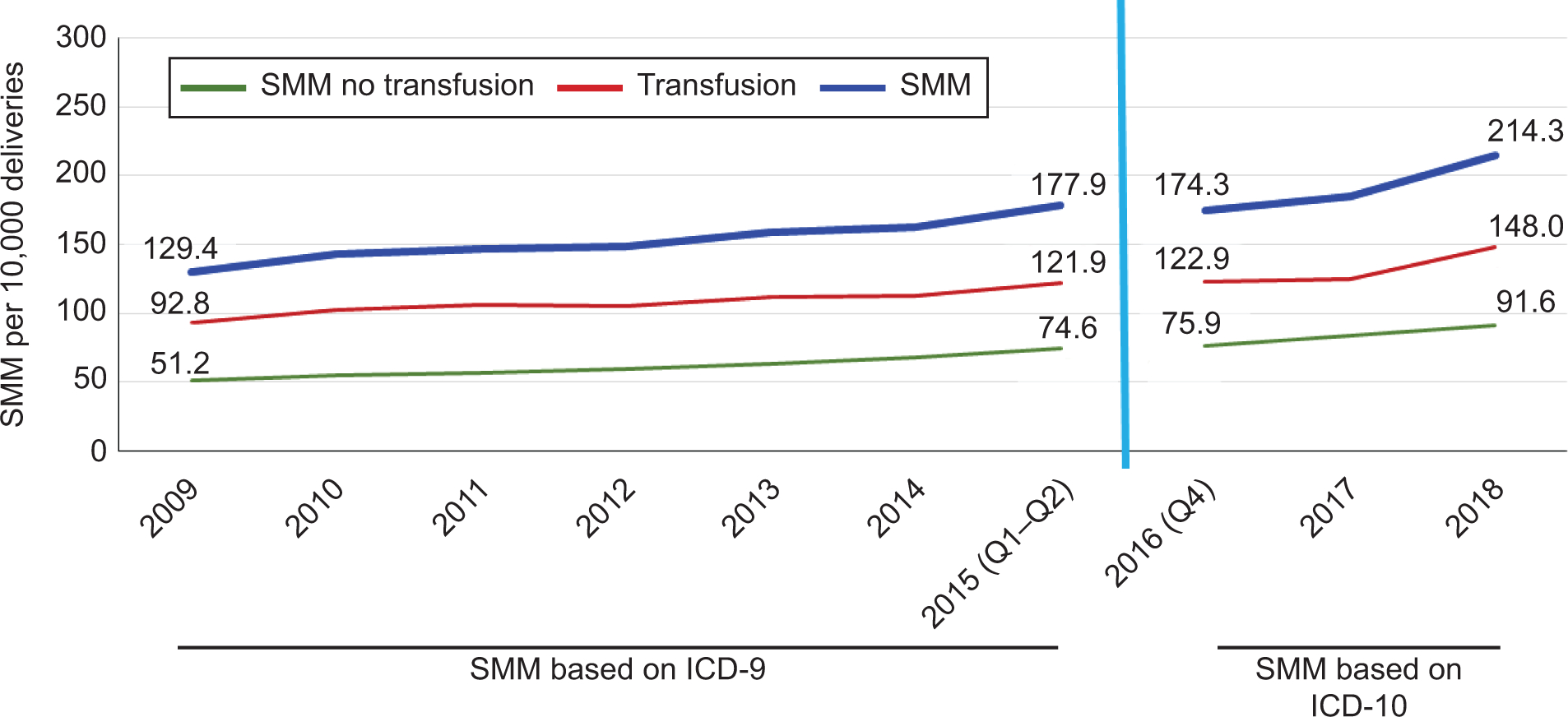
Severe maternal morbidity (SMM) per 10,000 deliveries, standard measures, Massachusetts 2009‒2018. ICD, International Classification of Diseases.
Rates of severe maternal morbidity during pregnancy and postpartum not identified as part of the delivery event are presented in Figure 2. The addition of prenatal and postpartum hospitalizations for severe maternal morbidity from 2009–2015 added 21.9% more cases of morbidity to those identified with the delivery admission, raising the average severe maternal morbidity rate for the period from 150.1 per 10,000 live births and fetal deaths to 182.9 per 10,000 under the ICD-9 measure, a 32.8 per 10,000 absolute difference (95% CI: 31.2–34.4). In the 2016–2018 period under ICD-10, the increase in the severe maternal morbidity rate when including prenatal and postpartum cases was an absolute difference of 43.0 per 10,000 (95% CI: 39.6–46.4; also a 21.9% relative change), raising the average for the period from 196.6 to 239.6 per 10,000. Overall, a similar proportion of additional cases of morbidity came from postpartum hospitalizations (11.5%) compared with prenatal hospitalizations (10.4%).
Figure 2:
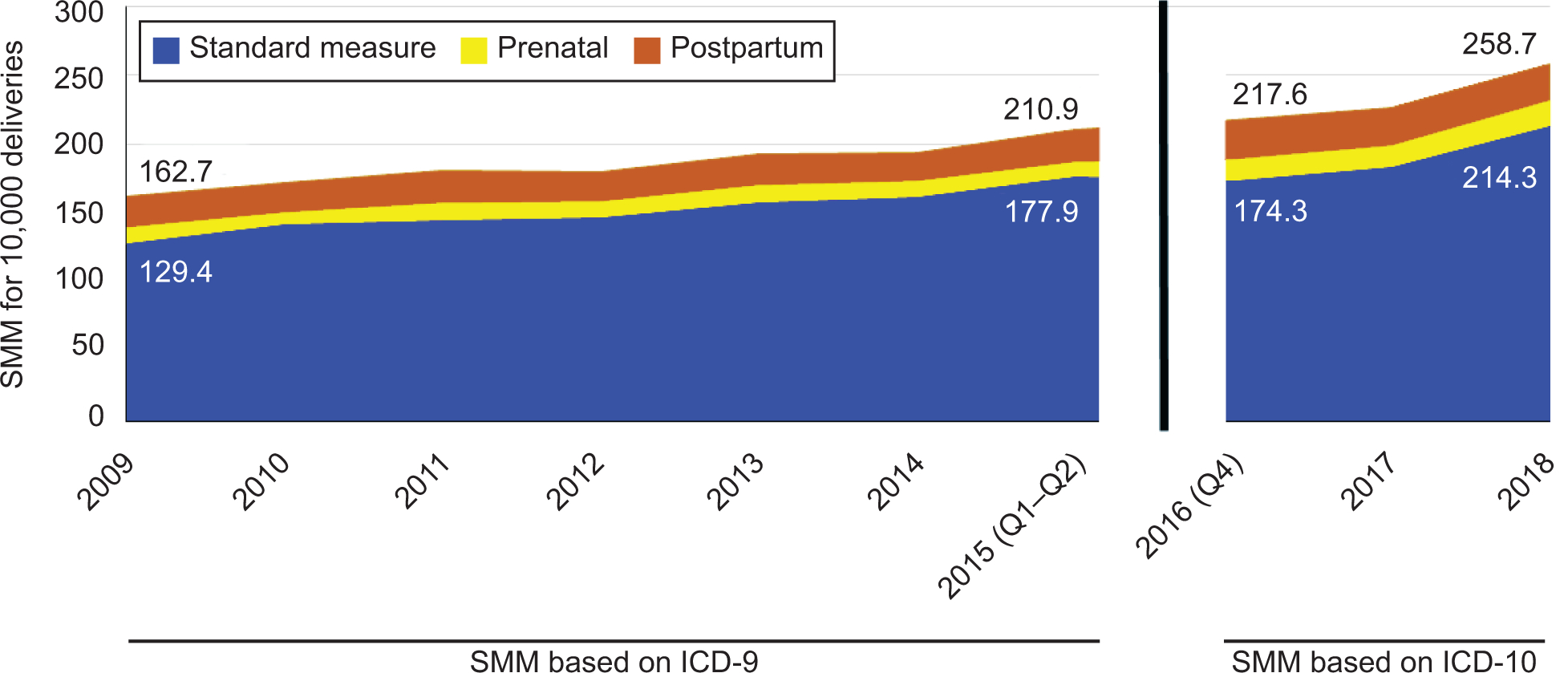
Severe maternal morbidity (SMM) per 10,000 deliveries, by timing, Massachusetts, 2009‒2018. ICD, International Classification of Diseases.
Table 1 presents the distribution of coded diagnoses during severe maternal morbidity hospitalizations during the prenatal, birth and postpartum periods. Under ICD-9 coding, the largest absolute increases in morbidity when including prenatal and postpartum hospitalizations were from transfusion (10.9 cases per 10,000; 95% CI 10.0–11.8), sepsis (6.9; 95% CI 6.2–7.6) and pulmonary edema (4.5; 95% CI 3.9–5.1), with cases of transfusion and pulmonary edema coming primarily from the postpartum period. Since transfusion was relatively common at delivery, the relative increase in cases was limited (10% more cases) with inclusion of prenatal and postpartum hospitalizations, while thrombotic embolism (170%) and sepsis (147%) cases both more than doubled when prenatal and postpartum hospitalizations were included.
Table 1.
Distribution of Coded Diagnoses During Severe Maternal Morbidity Hospitalizations in Prenatal, Birth and Postpartum Periods (per 10,000 deliveries) Massachusetts
| ICD-9 (1/1/2009–6/30/2015 Deliveries) | ICD-10 (9/1/2016–12/31/2018 deliveries) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prenatal* | Delivery | Postpartum* | Cases Added Prenatal & Postpartum (95% CI)+ |
Cases added by non-birth hospitalization | Prenatal* | Delivery | Postpartum* | Cases Added Prenatal & Postpartum (95% CI)+ |
Cases added by non-birth hospitalization | |
| Categories | Rate per 10,000 deliveries | % | Rate per 10,000 deliveries | % | ||||||
| Acute renal failure | 0.9 | 10.0 | 1.5 | 2.4 (2.0–2.8) | 24.3 | 1.2 | 26.1 | 2.1 | 3.3 (2.4–4.2) | 12.7 |
| Cardiac arrest | 0.0 | 0.8 | 0.2 | 0.2 (0.1–0.3) | 23.7 | 0.0 | 1.0 | 0.0 | 0.0 (NA) | 0.0 |
| Heart failure during surgery | 0.1 | 0.9 | 0.1 | 0.2 (0.1–0.3) | 20.0 | 0.0 | 0.0 | 0.0 | 0.0 (NA) | NA |
| Shock | 0.2 | 4.0 | 0.6 | 0.8 (0.6–1.1) | 19.9 | 0.3 | 8.3 | 1.0 | 1.4 (0.8–2.0) | 16.7 |
| Sepsis | 3.0 | 4.7 | 3.9 | 6.9 (6.2–7.6) | 147.4 | 7.3 | 9.3 | 9.0 | 16.3 (14.2–18.4) | 174.8 |
| Disseminated intravascular coagulation | 0.3 | 20.2 | 1.3 | 1.5 (1.2–1.8) | 7.6 | 0.3 | 19.0 | 1.7 | 2.1 (1.4–2.9) | 10.9 |
| Amniotic Fluid Embolism | 0.0 | 0.6 | 0.0 | 0.0 (NA) | 0.0 | 0.0 | 0.8 | 0.1 | 0.1 (−0.1–0.3) | 9.1 |
| Thrombotic Embolism | 1.3 | 2.3 | 2.6 | 3.9 (3.4–4.5) | 169.9 | 1.2 | 4.1 | 3.3 | 4.5 (3.4–5.6) | 110.0 |
| Puerperal cerebrovascular disorders | 0.9 | 4.4 | 2.3 | 3.2 (2.7–3.7) | 72.7 | 0.3 | 5.0 | 2.4 | 2.8 (1.9–3.7) | 54.8 |
| Severe anesthesia complications | 0.1 | 1.9 | 0.0 | 0.1 (0.01–0.2) | 7.0 | 0.0 | 1.1 | 0.0 | 0.0 (NA) | 0.0 |
| Pulmonary edema | 0.4 | 4.7 | 4.1 | 4.5 (3.9–5.1) | 96.2 | 0.5 | 6.5 | 3.4 | 3.9 (2.9–4.9) | 58.9 |
| Adult respiratory distress syndrome | 1.2 | 5.2 | 1.5 | 2.7 (2.2–3.2) | 52.8 | 2.3 | 8.5 | 2.5 | 4.8 (3.7–5.9) | 56.9 |
| Acute myocardial infarct. | 0.0 | 0.2 | 0.2 | 0.2 (0.1–0.3) | 111.1 | 0.1 | 0.3 | 0.3 | 0.4 (0.1–0.7) | 120.0 |
| Eclampsia | 0.0 | 4.2 | 1.4 | 1.5 (1.2–1.8) | 35.1 | 0.1 | 6.8 | 2.1 | 2.1 (1.4–2.9) | 31.6 |
| Sickle cell anemia | 0.2 | 1.0 | 0.0 | 0.2 (0.1–0.3) | 23.9 | 0.4 | 1.2 | 0.0 | 0.4 (0.1–0.7) | 33.3 |
| Aneurysm | 0.0 | 0.4 | 0.0 | 0.0 (NA) | 5.9 | 0.0 | 0.4 | 0.1 | 0.1 (−0.06–0.3) | 33.3 |
| Ventilation | 0.7 | 4.8 | 1.1 | 1.8 (1.4–2.2) | 38.3 | 0.6 | 5.5 | 1.1 | 1.7 (1.0–2.4) | 31.3 |
| Hysterectomy | 0.0 | 8.0 | 0.8 | 0.8 (0.6–1.1) | 10.3 | 0.0 | 11.9 | 0.6 | 0.6 (0.2–1.0) | 4.7 |
| Temporary tracheostomy | 0.1 | 0.1 | 0.1 | 0.2 (0.1–0.3) | 300.0 | 0.0 | 0.0 | 0.0 | 0.0 (NA) | NA |
| Conversion of cardiac rhythm | 0.2 | 0.7 | 0.1 | 0.3 (0.2–0.5) | 37.5 | 0.1 | 1.0 | 0.0 | 0.1 (−0.06–0.3) | 6.7 |
| Transfusion | 3.2 | 106.4 | 7.8 | 10.9 (10.0–11.8) | 10.3 | 3.2 | 134.6 | 6.7 | 9.9 (8.3–11.5) | 7.3 |
Prenatal cases exclusive of delivery; Postpartum cases exclusive of prenatal or delivery
95 percent confidence interval
NA-not applicable
After adoption of ICD-10, the largest absolute increase was seen from sepsis codes, (16.3 per 10,000; 95% CI 14.2–18.4) increasing the total cases identified by 175%. There was also an increase in transfusion cases (9.9 per 10,000; 95% CI 8.3–11.5). Respiratory distress syndrome (4.8; 95% CI 3.7–5.9), thrombotic embolism (4.5; 95% CI 3.4–5.6), and pulmonary edema (3.9; 95% CI 2.9–4.9) codes all contributed to absolute and relative increases in cases with the addition of prenatal and postpartum hospitalizations.
Discussion
By using a population-based, longitudinally linked dataset to examine prenatal and postpartum hospitalizations, we identified an additional 22% of cases of severe maternal morbidity in Massachusetts that would not have been identified by evaluating only the birth admission. Aside from transfusion, prenatally, the diagnoses of sepsis, thrombotic embolism, and adult respiratory distress syndrome were the conditions most associated with prenatal severe maternal morbidity. Postpartum, a majority of additional cases came from pulmonary edema, sepsis, and thrombotic embolism diagnostic codes.
Callaghan et al. examined postpartum cases of severe maternal morbidity from 1998–2009 using the Nationwide Inpatient Sample combining the data into two-year periods. The proportion of cases added in the postpartum period ranged from 18.4% (1998–1999) to 22.5% (2008–2009), increases comparable to those reported here.4 Chen et al. used a commercial and Medicaid database and the ICD-9 algorithm, to examine postpartum hospitalizations for SMM conditions, and our findings share some commonalities, with the top five severe maternal morbidity codes for postpartum hospitalizations (sepsis, thrombotic embolism, respiratory distress, eclampsia and pulmonary edema) largely overlapping.7
Our findings, combined with the knowledge that two-thirds of maternal deaths occur outside the delivery event,11 highlight that an inpatient- or delivery-focused response is insufficient to adequately recognize, prevent or respond to significant morbidity during the antepartum period. Outpatient implementation and surveillance of care quality activities to identify and prevent morbidity from sepsis and embolism, such as standardized, outpatient risk assessment screening for venous thromboembolism and prevention of maternal peripartum infections via screening and vaccinations would appear appropriate.15
The addition of prenatal and postpartum cases raised the rate for severe maternal morbidity in Massachusetts for 2018 to 258.7 per 10,000. If this rate were applied to 2020 U.S. birth totals, that would result in over 93,000 cases of severe maternal morbidity in the U.S., a number that far exceeds current national estimates,5 even in the context of a rapidly declining birth rate in the U.S.16 The higher Massachusetts estimate is likely a function of several factors. First, the Massachusetts SMM rate at the time of delivery was higher than the national rate. The most recent U.S. rate (2014) published by the CDC was 144.0 per 10,000 deliveries,10 while the comparable figure for Massachusetts in 2014 for SMM at birth was 162.6 per 10,000. Also, our data is four years more recent than the latest CDC data, and given that the SMM rate was trending up nationally, the higher current Massachusetts rate may simply represent the continuing rise in SMM cases and the impact of the change to a new algorithm based on ICD-10. Finally, the longitudinally linked birth certificate/hospital discharge data system in Massachusetts allows for better case identification of deliveries, since hospital discharge data do not have date of delivery and rely on ICD diagnosis and procedure codes to identify deliveries.
The expansion of the SMM measure to beyond the birth event has additional implications. As the understanding of maternal mortality has come to include both pregnancy and the postpartum period, and since only about one-third of maternal deaths occur at the time of delivery,11 a broader definition of SMM may need to be developed. Part of that revision may be the need to include conditions (e.g., ectopic pregnancy)17 that can involve SMM but are not linked to the birth as well as the examination of the relationship of societal and community factors (e.g., opioid use, inter-personal violence) to health.18–20
This large population-based study is not without its limitations. Our findings differ from nationally reported data in part because the demographics of our analytic sample of Massachusetts deliveries are not typical of the nation (Appendix 3), with a smaller proportion of births to non-Hispanic Black (10.1% vs 15.0% in U.S.) and Hispanic birthing individuals (18.1% vs 23.5% in U.S.) than the national average. Our sample also has a larger proportion of births to older birthing individuals (57% age 30+) than the U.S. (42% 30+).21 Our data may not be completely comparable to other national studies of SMM that have used the CDC algorithm exclusively,5,7 since we relied on the revisions encompassed by an algorithm produced by a collaboration of CDC, HRSA, AHRQ and AIM.22 As noted, the rates we identified for birth hospitalizations were generally consistent with past studies. For those relying on longitudinally linked data,4,7 the proportion of postpartum cases were also similar.
Given the rarity of maternal death in developed countries, a need for more sensitive measures to capture the experiences of a broader segment of the birthing population led to the creation of a measure of severe maternal morbidity. However, a measure limited in scope to the delivery hospitalization may be insufficient.23 As in the case of maternal mortality, broadening our thinking about severe morbidity to include pregnancy and postpartum will change how we view health during the perinatal period. Instead of primarily a clinical problem at birth, severe maternal morbidity would need to be addressed as a medical and public health challenge over a 21-month period. It would also require the development of consensus measures of conditions during pregnancy and postpartum that are not ascertained by measures centered on the birth event, but which represent significant challenges to health during the perinatal period. The widening development of perinatal linked databases24 has created the opportunity for just such measurement development and related analyses. Further research, particularly at the state level utilizing large, linked, ideally population-based datasets, can help move the discussion from single birth events to where it belongs – the overall examination of the health of birthing individuals in the U.S.
Supplementary Material
Acknowledgement:
The authors thank Judith Jeanty, who assisted with manuscript preparation.
Supported by NIH RO1 MD016026–01.
Footnotes
Financial Disclosure
Elysia Larson disclosed receiving funding from the World Bank and the Harvard T.H. Chan School of Public Health. Audra Meadows disclosed receiving funding from the Institute for Health Care Improvement, Better Maternal Outcomes (consultancy), the Black Birth Equity Community Advisory Board, and the Institute for Perinatal Quality Improvement (consultancy). The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal’s requirements for authorship.
Contributor Information
Eugene R. Declercq, Boston University School of Public Health.
Howard J. Cabral, Boston University School of Public Health.
Xiaohui Cui, Massachusetts Department of Public Health.
Chia-ling Liu, Evalogic Services, Inc..
Ndidiamaka Amutah-Onukagha, Tufts University School of Medicine.
Elysia Larson, Department of Biostatistics, Harvard T. H. Chan School of Public Health.
Audra Meadows, Department of Obstetrics, Gynecology, and Reproductive Sciences, University of California San Diego.
Hafsatou Diop, Massachusetts Department of Public Health.
References
- 1.Weil A, Reichert Ae. Reversing the U.S. Maternal Mortality Crisis: A Report of the Aspen Health Strategy Group. Washington, D.C.2021. [Google Scholar]
- 2.Hoyert D Maternal mortality rates in the United States, 2019. NCHS Health E-Stats. 2021. DOI: 10.15620/cdc:103855. [DOI] [Google Scholar]
- 3.Geller SE, Rosenberg D, Cox SM, Kilpatrick S. Defining a conceptual framework for near-miss maternal morbidity. Journal of the American Medical Women’s Association (1972). 2002;57(3):135–139. [PubMed] [Google Scholar]
- 4.Callaghan WM, Creanga A, Kuklina E. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029–1036. [DOI] [PubMed] [Google Scholar]
- 5.Brown CC, Adams CE, George KE, Moore JE. Associations Between Comorbidities and Severe Maternal Morbidity. Obstet Gynecol. 2020;136(5):892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvey EM, Ahmed S, Manning SE, Diop H, Argani C, Strobino DM. Severe Maternal Morbidity at Delivery and Risk of Hospital Encounters Within 6 Weeks and 1 Year Postpartum. Journal of women’s health. 2017;27(2):140–147. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Cox S, Kuklina EV, Ferre C, Barfield W, Li R. Assessment of Incidence and Factors Associated With Severe Maternal Morbidity After Delivery Discharge Among Women in the US. JAMA Netw Open. 2021;4(2):e2036148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howell E, Egorova N, Janevic T, et al. Race and Ethnicity, Medical Insurance, and Within-Hospital Severe Maternal Morbidity Disparities. Obstet Gynecol. 2020;135(2):285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuklina EV, Goodman DA. Severe Maternal or Near Miss Morbidity: Implications for Public Health Surveillance and Clinical Audit. Clinical obstetrics and gynecology. 2018;61(2):307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC. Severe Maternal Morbidity in the United States. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.html. Accessed October 3, 2021.
- 11.Petersen E, Davis N, Goodman D, et al. Vital Signs: Pregnancy-Related Deaths, United States, 2011–2015, and Strategies for Prevention, 13 States, 2013–2017. Atlanta, GA: Centers for Disease Control and Prevention; May 7, 2019. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alliance for Innovation on Maternal Health. Updates to the AIM Data Collection Plan 2021; https://safehealthcareforeverywoman.org/aim/resources/aim-data-resources/ Accessed August 23, 2021.
- 13.Fingar K, Hambrick M, Heslin K, Moore J. Trends and Disparities in Delivery Hospitalizations Involving Severe Maternal Morbidity, 2006–2015. Rockville, MD: 2018. [PubMed] [Google Scholar]
- 14.Stern JE, Kotelchuck M, Luke B, Declercq E, Cabral H, Diop H. Calculating length of gestation from the Society for Assisted Reproductive Technology Clinic Outcome Reporting System (SART CORS) database versus vital records may alter reported rates of prematurity. Fertil Steril. 2014;101(5):1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahoney J The Alliance for Innovation in Maternal Health Care: A Way Forward. Clinical obstetrics and gynecology. 2018;61(2):400–410. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton B, Martin J, Osterman M. Births: Provisional Data for 2020. Hyattsville, MD: 2021. [Google Scholar]
- 17.Hendriks E, Rosenberg R, Prine L. Ectopic Pregnancy: Diagnosis and Management. Am Fam Physician. 2020;101(10):599–606. [PubMed] [Google Scholar]
- 18.Smid M, Stone N, Baksh L, et al. Pregnancy-Associated Death in Utah: Contribution of Drug-Induced Deaths. Obstet Gynecol. 2019;133(6):1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peeler M, Gupta M, Melvin P, et al. Racial and Ethnic Disparities in Maternal and Infant Outcomes Among Opioid-Exposed Mother-Infant Dyads in Massachusetts (2017–2019). Am J Public Health. 2020;110(12):1828–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman-Mellor S, Margerison CE. Maternal drug-related death and suicide are leading causes of postpartum death in California. American Journal of Obstetrics and Gynecology. 2019;221(5):489.e481–489.e489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. CDC WONDER Online Database. In: United States Department of Health and Human Services CfDCaP, National Center for Health Statistics, Division of Vital Statistics, Natality public-use data 2016–2018., ed. Hyattsville, MD: 2020. [Google Scholar]
- 22.Healthcare Cost and Utilization Project Overview of the National (Nationwide) Inpatient Sample (NIS). 2021; https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed May 3, 2021.
- 23.Main EK, Abreo A, McNulty J, et al. Measuring severe maternal morbidity: validation of potential measures. American Journal of Obstetrics and Gynecology. 2016;214(5):643.e641–643.e610. [DOI] [PubMed] [Google Scholar]
- 24.Cheah SL, Scarf VL, Rossiter C, Thornton C, Homer CSE. Creating the first national linked dataset on perinatal and maternal outcomes in Australia: Methods and challenges. J Biomed Inform. 2019;93:103152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


