Abstract
Given previous biological evidence of immunomodulatory effects of coffee, we hypothesized that the association between coffee intake of colorectal cancer patients and survival differs by immune responses. Using a molecular pathological epidemiology database of 4,465 incident colorectal cancer cases, including 1,262 cases with molecular data, in the Nurses’ Health Study and the Health Professionals Follow-up Study, we examined the association between coffee intake of colorectal cancer patients and survival in strata of levels of histopathologic lymphocytic reaction and T-cell infiltrates in tumor tissue. We did not observe a significant association of coffee intake with colorectal cancer-specific mortality [multivariable-adjusted hazard ratio (HR) for one cup increase of coffee intake per day, 0.93; 95% confidence interval (CI), 0.84-1.03]. Although statistical significance was not reached at the stringent level (α=0.005), the association of coffee intake with colorectal cancer-specific mortality differed by Crohn's-like lymphoid reaction (Pinteraction=.007). Coffee intake was associated with lower colorectal cancer-specific mortality in patients with high Crohn's-like reaction (multivariable HR for one cup increase of coffee intake per day, 0.55; 95% CI, 0.37–0.81; Ptrend=.002), but not in patients with intermediate Crohn's-like reaction (the corresponding HR, 1.02; 95% CI, 0.72–1.44) or negative/low Crohn's-like reaction (the corresponding HR, 0.95; 95% CI, 0.83–1.07). The associations of coffee intake with colorectal cancer-specific mortality did not significantly differ by levels of other lymphocytic reaction or any T-cell subset (Pinteraction>.18). There is suggestive evidence for differential prognostic effects of coffee intake by Crohn’s-like lymphoid reaction in colorectal cancer.
Keywords: colorectal neoplasms, beverage, immunofluorescence, tumor microenvironment
INTRODUCTION
The biological importance of the immune system in colorectal cancer is well supported by previous studies.1 For example, a high-level immune response featuring cytotoxic and memory T-cells in colorectal cancer has been associated with better patient survival.2 Evidence also suggests that certain dietary factors influence both the immune system and colorectal cancer mortality.3, 4 Therefore, elucidating the interplay between the immune system and modifying diet in colorectal cancer progression is of considerable importance, potentially contributing to the development of novel cancer treatment strategies.
Among dietary factors, coffee has been hypothesized to provide a positive prognostic effect against colorectal cancer mortality. Several studies recently reported a statistically significant association of higher coffee intake after colorectal cancer diagnosis with lower tumor recurrence and improved survival.5, 6 However, the mechanisms behind these associations remain to be elucidated. Coffee contains a complex mixture of bioactive compounds,7 some of which, including polyphenols and caffeine, have been suggested to influence adaptive immune responses.7-9 Colorectal cancers are heterogeneous tumors with complex interactions between neoplastic and immune cells in the tumor microenvironment,10 potentially influenced by the immunomodulatory effects of coffee. However, no study has yet examined the effect of coffee on colorectal cancer survival according to immune responses in cancer tissue.
We therefore tested our hypothesis that the association between coffee intake of colorectal cancer patients and survival differs by histopathologic lymphocytic reaction and T-cell infiltrates in colorectal cancer tissue. To test our hypothesis, we used a database of 4,465 incident colorectal cancer cases, including 1,262 cases with available data on coffee intake and tumor molecular markers from two large prospective cohort studies in the US.
PATIENTS AND METHODS
Study Population
We utilized data from two large US prospective cohort studies, the Nurses’ Health Study (NHS, 121,701 women aged 30-55 years followed since 1976) and the Health Professionals Follow-up Study (HPFS, 51,529 men aged 40 to 75 years followed since 1986). Study participants were sent follow-up questionnaires every two years to update information on their demographic and lifestyle factors, medical history, and newly diagnosed diseases including colorectal cancer. The follow-up rate has been over 90% for each questionnaire cycle in these two cohorts. The National Death Index was used to confirm deaths of study participants and identify patients with unreported lethal colorectal cancer.
We documented 4,465 colorectal cancer cases in the two cohort studies through 2012, including 1,262 colorectal cancer cases with available data on coffee intake and immune responses. Patients were followed until death or end of follow-up (June 1, 2016 for the NHS; January 1, 2016 for the HPFS), whichever came first. Participating physicians, blinded to exposure data, reviewed medical records of colorectal cancer patients to collect data on tumor characteristics and to identify causes of death for deceased patients.
Informed consent was obtained from all study participants at study enrolment. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health (Boston, MA, USA), and those of participating registries as required.
Assessment of Coffee Intake and Other Covariates
To assess dietary intake, food frequency questionnaires (FFQs) were initially collected in 1980 for NHS and in 1986 for HPFS. For the NHS, a 61-item semi-quantitative FFQ was used at baseline,11 which was expanded to approximately 130 food and beverage items in 1984, 1986, and every four years thereafter. For the HPFS cohort, baseline dietary intake was assessed with a 131-item FFQ that was also used for updates generally every four years thereafter.12 For each item, FFQs prompted participants to report their average intake over the preceding year for a specified serving size of each food and beverage from nine possible responses, ranging from (almost) never to ≥6 times per day. In addition, questionnaires were sent every two years to collect data on lifestyle factors, including smoking status, body weight, and physical activity. Data on coffee intake and other covariates in colorectal cancer patients were obtained from the first questionnaire returned between 6 and 48 months after colorectal cancer diagnosis. Data on prediagnostic coffee intake were obtained from the last questionnaire prior to colorectal cancer diagnosis.
Tumor Tissue Analyses
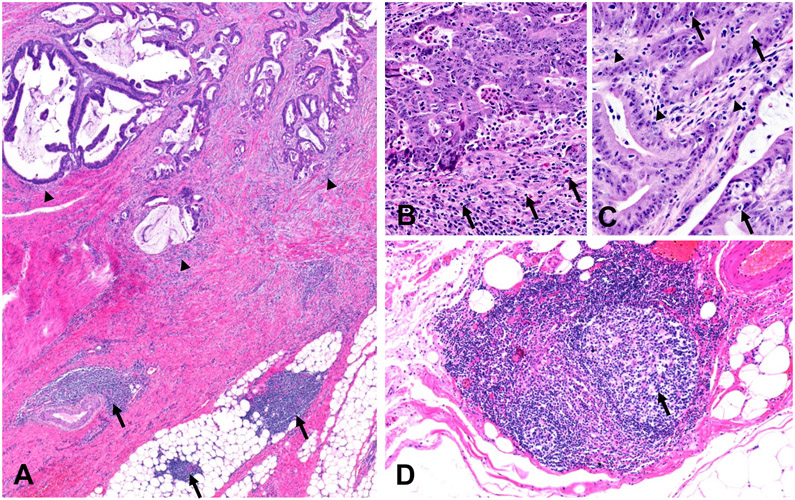
The details of tumor tissue analyses have been described.13-15 Briefly, we obtained formalin-fixed paraffin-embedded tumor tissue samples from hospitals throughout the U.S. where colorectal cancer patients underwent surgical resection. The study pathologist (S. Ogino), who was blinded to other data, reviewed hematoxylin and eosin-stained tissue sections, and recorded histopathological findings, including tumor differentiation and lymphocytic reactions. Each of lymphocytic reaction patterns (Crohn's-like lymphoid reaction, peritumoural lymphocytic reaction, intratumoural periglandular reaction, and tumor-infiltrating lymphocytes) was graded as negative/low, intermediate, and high (Figure).13, 15 The second pathologist (J.N. Glickman), who was unaware of other data, independently re-reviewed 398 selected cases, and a good inter-observer correlation was observed, as previously described.15
Figure.
The four lymphocytic reaction patterns in colorectal cancer. (A) Low-power view showing peritumoral lymphocytic reaction (arrowheads) and Crohn’s-like lymphoid reaction (arrows). (B-C) Higher-power views from the invasive front (B) and tumor center (C) showing peritumoral lymphocytic reaction (B: arrows), intratumoral periglandular lymphocytic reaction (C: arrowheads), and tumor-infiltrating lymphocytes (C: arrows). (D) Higher-power view showing a lymphoid follicle with germinal center (arrow) involved in the Crohn’s-like lymphoid reaction.
We also constructed tissue microarrays, including up to four tumor cores from each case.16 We simultaneously measured the expression of CD3, CD4, CD8, CD45RO (one isoform of PTPRC gene products), and FOXP3 in T cells within tumor epithelial and stromal regions in CRC tissue microarrays by a multiplex immunofluorescence assay,17 following recommendations of standardized protein nomenclature.18 For the Wald test, we used log-transformed T-cell density variables to improve normality.
Statistical Analysis
All statistical analyses were performed using SAS software (version 9.4, SAS Institute, Cary, NC). All P values were two-sided, and a P <.005 was considered statistically significant given multiple hypothesis testing. Our primary hypothesis testing was statistical interaction (using the Wald test on the cross-product) between coffee intake of colorectal cancer patients (cup/day, continuous) and each lymphocytic reaction (ordinal) in the Cox proportional hazards regression model for colorectal cancer-specific mortality analyses. We also assessed a statistical interaction between coffee intake of colorectal cancer patients and each T-cell density (log-transformed, continuous). All other analyses were secondary. We estimated hazard ratios (HRs) for one cup increase of coffee per day in strata of levels of lymphocytic reactions or T-cell subsets using a re-parameterization of the interaction term in a single regression model.19 Survival time was defined as the period from return of the first questionnaire after colorectal cancer diagnosis to death or the end of follow-up whichever came first.
We applied an inverse probability weighting (IPW) method to reduce selection bias due to the availability of questionnaire data after colorectal cancer diagnosis.20 Multivariable Cox proportional hazards models initially included covariates described in the footnote to Table 2. A backward stepwise elimination procedure was performed with a threshold of P = 0.05 to select variables for the final models (Supplementary Table 1). To limit degrees of freedom of the models, the cases with missing data on categorical variables were included in the majority category. For cases with missing data on continuous variables, we substituted the mean value and included a separate indicator variable in the model. It was confirmed that no substantial alteration occurred in results when we excluded cases with missing information in any of the covariates. The proportionality of hazards assumption was generally satisfied using the assessment of the cross-product of coffee intake of colorectal cancer patients and survival time in strata of each lymphocytic reaction component and T cell subset (P >.24).
Table 2.
Colorectal cancer-specific mortality according to coffee intake in strata of lymphocytic reaction patterns and T-cell subsets
| Colorectal cancer-specific mortality HR for one cup increase of coffee per daya |
||||
|---|---|---|---|---|
| No. of Cases | No. of Events | Age-adjusted HR (95% CI) |
Multivariable-adjusted HR (95% CI)b |
|
| All colorectal cancer cases | 1262 | 237 | 1.01 (0.92-1.11) | 0.93 (0.84-1.03) |
| Lymphocytic reaction | ||||
| Crohn's-like lymphoid reaction | ||||
| Negative/low | 757 | 168 | 1.00 (0.89-1.12) | 0.95 (0.83-1.07) |
| Intermediate | 196 | 25 | 1.12 (0.81-1.57) | 1.02 (0.72-1.44) |
| High | 87 | 7 | 0.69 (0.45-1.06) | 0.55 (0.37-0.81) |
| P interaction c | .088 | .007 | ||
| Peritumoral lymphocytic reaction | ||||
| Negative/low | 143 | 45 | 0.91 (0.72-1.15) | 0.92 (0.71-1.18) |
| Intermediate | 880 | 173 | 1.06 (0.95-1.19) | 0.98 (0.87-1.10) |
| High | 222 | 17 | 0.94 (0.67-1.31) | 0.81 (0.54-1.20) |
| P interaction c | .63 | .42 | ||
| Intratumoral periglandular reaction | ||||
| Negative/low | 143 | 41 | 0.94 (0.74-1.20) | 0.87 (0.66-1.13) |
| Intermediate | 933 | 180 | 1.04 (0.93-1.16) | 0.96 (0.86-1.08) |
| High | 184 | 15 | 1.06 (0.79-1.42) | 0.95 (0.68-1.33) |
| P interaction c | .77 | .94 | ||
| Tumor-infiltrating lymphocytes | ||||
| Negative/low | 924 | 196 | 1.00 (0.90-1.11) | 0.94 (0.84-1.04) |
| Intermediate | 197 | 33 | 1.07 (0.80-1.42) | 0.93 (0.70-1.23) |
| High | 138 | 7 | 1.13 (0.80-1.58) | 1.03 (0.71-1.52) |
| P interaction c | .53 | .66 | ||
| T-cell subset | ||||
| CD3+CD4+FOXP3+ cell density | ||||
| C1 (low) | 376 | 86 | 1.00 (0.86-1.18) | 0.94 (0.80-1.11) |
| C2 (Intermediate) | 202 | 33 | 1.11 (0.84-1.48) | 1.03 (0.78-1.35) |
| C3 (high) | 202 | 30 | 0.90 (0.71-1.13) | 0.87 (0.67-1.13) |
| P interaction c | .59 | .72 | ||
| CD3+CD4+CD45RO+ cell density | ||||
| C1 (low) | 260 | 57 | 1.03 (0.84-1.27) | 0.92 (0.75-1.13) |
| C2 (Intermediate) | 260 | 54 | 1.00 (0.80-1.25) | 0.95 (0.77-1.17) |
| C3 (high) | 260 | 38 | 1.00 (0.84-1.19) | 1.05 (0.88-1.25) |
| P interaction c | .63 | .74 | ||
| CD3+CD4+CD45RO− cell density | ||||
| C1 (low) | 264 | 51 | 1.04 (0.84-1.28) | 1.00 (0.80-1.24) |
| C2 (Intermediate) | 258 | 57 | 1.04 (0.86-1.27) | 0.92 (0.77-1.10) |
| C3 (high) | 258 | 41 | 0.91 (0.72-1.15) | 0.91 (0.72-1.16) |
| P interaction c | .67 | .87 | ||
| CD3+CD8+CD45RO+ cell density | ||||
| C1 (low) | 260 | 55 | 1.05 (0.89-1.23) | 1.01 (0.87-1.17) |
| C2 (Intermediate) | 260 | 61 | 1.02 (0.79-1.32) | 0.96 (0.74-1.23) |
| C3 (high) | 260 | 33 | 0.88 (0.65-1.19) | 0.90 (0.69-1.16) |
| P interaction c | .35 | .73 | ||
| CD3+CD8+CD45RO− cell density | ||||
| C1 (low) | 356 | 67 | 1.06 (0.88-1.29) | 1.02 (0.85-1.22) |
| C2 (Intermediate) | 212 | 51 | 1.21 (0.98-1.50) | 1.10 (0.94-1.30) |
| C3 (high) | 212 | 31 | 0.73 (0.52-1.01) | 0.74 (0.54-1.00) |
| P interaction c | .19 | .27 | ||
Coffee intake of colorectal cancer patients was obtained from the first questionnaire returned between 6 and 48 months after diagnosis. The inverse probability weighting method was applied to reduce selection bias due to the availability of questionnaire data after cancer diagnosis.
The multivariable Cox regression model initially included sex, age, year of diagnosis, prediagnostic coffee intake, lifestyle factors after colorectal cancer diagnosis (physical activity, pack-year of smoking, alcohol intake, total fat intake, folate intake, calcium intake, vitamin D intake, and regular use of aspirin), family history of colorectal cancer, tumor location, tumor differentiation, disease stage, microsatellite instability, CpG island methylator phenotype, KRAS, BRAF, and PIK3CA mutations, and long-interspersed nucleotide element-1 methylation level. A backward elimination with a threshold of P = 0.05 was used to select variables for the final models. The variables that remained in the final models are described in Supplementary Table 1.
Pinteraction was calculated using Wald test for the cross-product of coffee intake of colorectal cancer patients (continuous) and each lymphocytic reaction variables (ordinal) or each T-cell density (log-transformed, continuous) in the multivariable Cox regression model.
RESULTS
We included 1,262 colorectal cancer cases with available data on coffee intake and immune responses. Table 1 summarizes the clinical, pathological, and molecular characteristics of colorectal cancer cases according to coffee intake of colorectal cancer patients. During the median follow-up time of 10 years (interquartile range, 5.6 to 16 years), there were 237 colorectal cancer-specific deaths.
Table 1.
Clinical, pathological, and molecular characteristics of colorectal cancer cases according to coffee intake
| Characteristicb | All cases (N=1,262) |
0 cups/day (N=225) |
≦1 cups/day (N=444) |
1-3 cups/day (N=452) |
≧3 cups/day (N=141) |
|
|---|---|---|---|---|---|---|
| Coffee intake of colorectal cancer patients (cup/d) (median, range) | 1.0 (0-10.5) | 0 | 1.0 (0.1-1.0) | 2.5 (1.1-2.9) | 4.5 (3.3-11) | |
| Prediagnostic coffee intake (cup/d) (median, range) | 1.2 (0-10.5) | 0 (0-6.0) | 1.0 (0-7.0) | 2.5 (0-6.0) | 4.5 (0-11) | |
| Sex | Female (NHS) | 720 (57%) | 122 (54%) | 247 (56%) | 268 (59%) | 83 (59%) |
| Male (HPFS) | 542 (43%) | 103 (46%) | 197 (44%) | 184 (41%) | 58 (41%) | |
| Age (years) | 69 ± 9.0 | 69 ± 9.6 | 70 ± 8.9 | 69 ± 8.9 | 68 ± 8.1 | |
| Year of diagnosis | 1995 or before | 434 (34%) | 56 (25%) | 132 (30%) | 170 (37%) | 76 (54%) |
| 1996-2000 | 359 (28%) | 61 (27%) | 134 (30%) | 134 (29%) | 30 (21%) | |
| 2001 or after | 449 (37%) | 108 (48%) | 178 (40%) | 148 (33%) | 35 (24%) | |
| Body mass index (kg/m2) (mean, SD) | 26 ± 4.5 | 27 ± 4.7 | 26 ± 4.5 | 26 ± 4.4 | 26 ± 4.1 | |
| Physical activity (METS-h/week) (median, IQR) | 10 (3.0-28) | 8.6 (2.6-24) | 9.3 (3.3-26) | 12 (3.0-34) | 11 (3.7-21) | |
| Pack-year of smoking (median, IQR) | 6.0 (0-29.0) | 0 (0-15.0) | 2.0 (0-21.0) | 12 (0-33.0) | 20.0 (0-43.0) | |
| Alcohol intake (g/d) (median, IQR) | 2.0 (0-12.0) | 0 (0-3.6) | 2.0 (0-9.9) | 3.0 (0-14.3) | 4.1 (0-15.9) | |
| Regular aspirin use (%) | 463 (36%) | 82 (36%) | 166 (38%) | 165 (37%) | 50 (35%) | |
| Total fat intake (g/d) (median, IQR) | 59 (49-69) | 59 (51-70) | 58 (48-67) | 59 (48-70) | 60 (50-68) | |
| Folate intake (mcg/d) (median, IQR) | 625 (389-866) | 659 (422-853) | 621 (394-930) | 633 (378-826) | 566 (377-817) | |
| Calcium intake (mg/d) (median, IQR) | 994 (714-1,459) | 994 (679-1,563) | 994 (710-1,447) | 998 (716-1,447) | 974 (741-1,379) | |
| Vitamin D intake (IU/d) (median, IQR) | 455 (211-678) | 486 (198-687) | 438 (219-679) | 469 (222-673) | 402 (184-663) | |
| Family history of colorectal cancer in first-degree relative(s) | Absent | 1010 (80%) | 187 (83%) | 351 (79%) | 357 (79%) | 115 (82%) |
| Present | 250 (20%) | 38 (17%) | 93 (21%) | 93 (21%) | 26 (18%) | |
| Tumor location | Cecum | 214 (17%) | 53 (24%) | 79 (18%) | 69 (15%) | 13 (9%) |
| Ascending to transverse | 374 (30%) | 63 (28%) | 125 (28%) | 139 (31%) | 47 (33%) | |
| Descending to sigmoid | 389 (31%) | 61 (27%) | 139 (31%) | 141 (31%) | 48 (34%) | |
| Rectum | 282 (22%) | 48 (21%) | 99 (22%) | 102 (23%) | 33 (23%) | |
| Tumor differentiation | Well to moderate | 1150 (92%) | 204 (91%) | 416 (94%) | 409 (91%) | 121 (88%) |
| Poor | 102 (8.2%) | 20 (9.0%) | 27 (6.1%) | 38 (8.5%) | 17 (12%) | |
| AJCC disease stage | I | 345 (30%) | 61 (30%) | 128 (31%) | 119 (28%) | 37 (28%) |
| II | 415 (36%) | 72 (35%) | 141 (35%) | 152 (37%) | 50 (38%) | |
| III | 327 (28%) | 55 (27%) | 115 (28%) | 120 (29%) | 37 (28%) | |
| IV | 71 (6.1%) | 15 (7.4%) | 25 (6.1%) | 25 (6.0%) | 6 (4.6%) | |
| MSI status | Non-MSI-high | 918 (82%) | 158 (78%) | 321 (84%) | 336 (83%) | 103 (84%) |
| MSI-high | 195 (18%) | 44 (22%) | 63 (16%) | 69 (17%) | 19 (16%) | |
| CIMP status | Low/negative | 875 (82%) | 151 (79%) | 304 (83%) | 323 (83%) | 97 (81%) |
| High | 195 (18%) | 41 (22%) | 64 (17%) | 67 (17%) | 23 (19%) | |
| LINE-1 methylation level (mean, SD) | 64 ± 10 | 65 ± 10 | 64 ± 10 | 64 ± 10 | 63 ± 10 | |
| KRAS mutation | Wild-type | 621 (59%) | 113 (60%) | 221 (61%) | 215 (56%) | 72 (59%) |
| Mutant | 443 (42%) | 78 (41%) | 144 (40%) | 170 (44%) | 51 (42%) | |
| BRAF mutation | Wild-type | 967 (86%) | 171 (82%) | 340 (88%) | 351 (86%) | 105 (84%) |
| Mutant | 157 (14%) | 35 (18%) | 47 (12%) | 55 (14%) | 20 (16%) | |
| PIK3CA mutation | Wild-type | 874 (84%) | 157 (82%) | 303 (83%) | 312 (84%) | 102 (86%) |
| Mutant | 173 (16%) | 35 (18%) | 62 (17%) | 60 (16%) | 16 (14%) | |
| Crohn's-like lymphoid reaction | Negative/low | 757 (73%) | 136 (75%) | 270 (73%) | 265 (71%) | 86 (73%) |
| Intermediate | 196 (19%) | 29 (16%) | 75 (20%) | 71 (19%) | 21 (18%) | |
| High | 87 (8.4%) | 16 (8.8%) | 24 (6.5%) | 35 (9.4%) | 12 (10%) | |
| Peritumoral lymphocytic reaction | Negative/low | 143 (11%) | 31 (14%) | 49 (11%) | 54 (12%) | 9 (6.4%) |
| Intermediate | 890 (71%) | 152 (68%) | 311 (71%) | 325 (72%) | 102 (72%) | |
| High | 222 (18%) | 40 (18%) | 80 (18%) | 72 (16%) | 30 (21%) | |
| Intratumoral periglandular reaction | Negative/low | 143 (11%) | 32 (14%) | 47 (10%) | 55 (12%) | 9 (6.4%) |
| Intermediate | 933 (74%) | 161 (71%) | 330 (74%) | 338 (75%) | 104 (74%) | |
| High | 184 (15%) | 32 (14%) | 66 (15%) | 58 (13%) | 28 (20%) | |
| Tumor-infiltrating lymphocytes | Negative/low | 924 (73%) | 165 (73%) | 332 (75%) | 332 (74%) | 95 (67%) |
| Intermediate | 197 (16%) | 34 (15%) | 65 (15%) | 69 (15%) | 29 (21%) | |
| High | 138 (11%) | 26 (12%) | 46 (10%) | 49 (11%) | 17 (12%) |
Abbreviations: AJCC, American Joint Committee on Cancer: CIMP, CpG island methylator phenotype; HPFS, Health Professionals Follow-up Study; LINE-1, long-interspersed nucleotide element-1; METS, metabolic equivalent task score; MSI, microsatellite instability; NHS, Nurses’ Health Study; SD, standard deviation; IQR, interquartile range.
Continuous variables are shown as mean (SD) or median (IQR or range). Percentage (%) indicates the proportion of cases with a specific clinical or pathological characteristic in all cases or in strata of coffee intake of colorectal cancer patients.
Table 2 shows colorectal cancer survival according to coffee intake in strata of each lymphocytic reaction pattern and T-cell subset. In this molecular pathological epidemiology database, we did not observe a significant association of coffee intake with colorectal cancer-specific mortality [multivariable-adjusted HR for one cup increase of coffee intake per day, 0.93; 95% confidence interval (CI), 0.84-1.03].
In our primary hypothesis testing, although statistical significance was not reached at the stringent level (α=0.005), the association of coffee intake with colorectal cancer-specific mortality differed by Crohn's-like lymphoid reaction (Pinteraction=.007). Coffee intake was associated with lower colorectal cancer-specific mortality in patients with high Crohn's-like reaction (multivariable HR for one cup increase of coffee intake per day, 0.55; 95% CI, 0.37–0.81; Ptrend=.002), but not in patients with intermediate Crohn's-like reaction (the corresponding HR, 1.02; 95% CI, 0.72–1.44) or negative/low Crohn's-like reaction (the corresponding HR, 0.95; 95% CI, 0.83–1.07). The associations of coffee intake with colorectal cancer-specific mortality did not significantly differ by levels of other lymphocytic reaction (peritumoral lymphocytic reaction, intratumoral periglandular reaction, or tumor-infiltrating lymphocytes) or any T-cell subset (Pinteraction>.18).
We also confirmed that results of Cox regression analyses without IPW (Supplementary Table 2) were similar to those with IPW. Lastly, considering possible effects of cancer treatment on coffee intake of colorectal cancer patients, we conducted a sensitivity analysis using questionnaire data between 12 and 48 months after colorectal cancer diagnosis. We confirmed that the results did not change substantially (Supplementary Table 3).
DISCUSSION
We conducted this study to test the hypothesis that the association of coffee intake of colorectal cancer patients and c survival differs by histopathologic lymphocytic reaction and T-cell infiltrates in tumor tissue. Our study has provided suggestive evidence for an interaction of coffee intake and Crohn’s-like lymphoid reaction in the survival analysis. Coffee intake was associated with lower CRC-specific mortality only in CRC with high-level Crohn’s-like reaction.
Current evidence indicates that certain modifiable lifestyle factors may influence the immune system and reduce colorectal cancer mortality.21, 22 Previous epidemiological studies showed that the inverse association of physical activity after colorectal cancer diagnosis with colorectal cancer mortality was stronger for tumors containing a higher density of CD3+ cells,23 and that the association of vitamin D score after colorectal cancer diagnosis with colorectal cancer mortality was stronger for tumors with lower peritumoral lymphocytic reaction.4 Coffee intake has also been suggested to be one of immunomodulatory lifestyle factors.7, 8
Crohn’s-like lymphoid reaction is a specific lymphocytic reaction pattern of transmural lymphoid aggregates exhibited by a subset of colorectal tumors.13, 15 These lymphoid follicles represent important sites for antigen presentation and lymphocyte proliferation and differentiation.24 They have been associated with improved survival in multiple tumor types, including colorectal cancer,13, 24 and have attracted interest as a potential marker for immunotherapy response.25 Our results suggest that coffee intake of colorectal cancer patients may affect the tumor-immune interaction differentially by the degree of Crohn’s-like lymphoid reaction, although our results must be replicated by independent studies. Further efforts are needed to clarify the complex relationships of coffee intake with colorectal cancer mortality classified by various types of immune cells.
The current study has several limitations. First, detailed cancer treatment information was not available. However, treatment strategies would not have been influenced by lymphocytic reactions. Second, cancer recurrence data were unavailable, but colorectal cancer-specific mortality was considered a reasonable endpoint, considering the long follow-up duration of censored cases. Third, we used questionnaire data reported 6-48 months after cancer diagnosis to minimize the effect of active cancer treatment, but we acknowledge that 6-48 months after cancer diagnosis is a broad range of time periods. More specific time periods should be considered in future clinical trials or observational studies. In addition, to address selection bias due to availability of questionnaire data after colorectal cancer diagnosis, we used the inverse probability weighting (IPW) method.20 Fourth, with a sample size of 237 events and 80% power, the minimum detectable HR is 1.44. Therefore, it is possible that we were unable to detect smaller effects.
CONCLUSION
There is suggestive evidence for differential effects of coffee intake on the mortality of colorectal cancer patients subclassified by Crohn’s-like lymphoid reaction. It is possible that coffee intake might be beneficial for colorectal cancer patients with high-level Crohn’s-like reaction. Additional efforts are needed to replicate our findings and elucidate mechanisms of coffee in influencing colorectal cancer outcomes.
Supplementary Material
Acknowledgments
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Funding/Support:
This work was supported by U.S. National Institutes of Health (NIH) grants (P01 CA87969 to M.J. Stampfer; UM1 CA186107 to M.J. Stampfer; P01 CA55075 to W.C. Willett; UM1 CA167552 to W.C. Willett; U01 CA167552 to W.C. Willett and L.A. Mucci; P50 CA127003 to C.S.F.; R01 CA118553 to C.S.F.; R01 CA169141 to C.S.F.; R01 CA137178 to A.T.C.; K24 DK098311 to A.T.C.; R35 CA197735 to S.O.; R01 CA151993 to S.O.; R01 CA248857 to S.O., U. Peters, and A.I. Phipps; R00 CA215314 to M.S.; and K07 CA188126 to X.Z.); by Nodal Award (2016-02) from the Dana-Farber Harvard Cancer Center (to S.O.); by the Stand Up to Cancer Colorectal Cancer Dream Team Translational Research Grant (SU2C-AACR-DT22-17 to C.S.F. and M.G.), administered by the American Association for Cancer Research, a scientific partner of SU2C; by the American Cancer Society Mentored Research Scholar Grant (MRSG-17-220-01 - NEC to M.S.); and by grants from the Project P Fund, The Friends of the Dana-Farber Cancer Institute, Bennett Family Fund, and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. T.U., K.H., and K.F. was supported by fellowship grants from the Uehara Memorial Foundation. K.H. was supported by the Mitsukoshi Health and Welfare Foundation. T.U. was supported by Yasuda Medical Foundation. J.B. was supported by a grant from the Australia Awards-Endeavour Scholarships and Fellowships Program. K.A. and T.U. were supported by a grant from Overseas Research Fellowship (201860083 to K.A.; 201960541 to T.U.) from Japan Society for the Promotion of Science. M.G. was supported by a Conquer Cancer Foundation of ASCO Career Development Award. J.A.M. research is supported by the Douglas Gray Woodruff Chair fund, the Guo Shu Shi Fund, Anonymous Family Fund for Innovations in Colorectal Cancer, Project P fund, and the George Stone Family Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations:
- CI
confidence interval
- FFQ
food frequency questionnaires
- HPFS
Health Professionals Follow-up Study
- HR
hazard ratio
- IPW
inverse probability weighting
- NHS
Nurses’ Health Study
Footnotes
Conflicts of interest: C.S.F. previously served as a consultant for Agios, Bain Capital, Bayer, Celgene, Dicerna, Five Prime Therapeutics, Gilead Sciences, Eli Lilly, Entrinsic Health, Genentech, KEW, Merck, Merrimack Pharmaceuticals, Pfizer Inc, Sanofi, Taiho, and Unum Therapeutics; C.S.F. also serves as a Director for CytomX Therapeutics and owns unexercised stock options for CytomX and Entrinsic Health. M.G. receives research funding from Bristol-Myers Squibb and Merck. J.A.M. received institutional research funding from Boston Biomedical. J.A.M. has also served as an advisor/consultant to Ignyta, Array Pharmaceutical, and Cota. This study was not funded by any of these commercial entities. The other authors declare that they have no conflicts of interest.
Use of Standardized Official Symbols: We use HUGO (Human Genome Organisation)-approved official symbols (or root symbols) for genes and gene products, including CD3, CD4, CD8, FOXP3, PTPRC, all of which are described at www.genenames.org. Gene symbols are italicized, whereas symbols for gene products are not italicized.
References
- 1.Paucek RD, Baltimore D, Li G. The Cellular Immunotherapy Revolution: Arming the Immune System for Precision Therapy. Trends Immunol. 2019;40:292–309. [DOI] [PubMed] [Google Scholar]
- 2.Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. [DOI] [PubMed] [Google Scholar]
- 3.Song M, Zhang X, Meyerhardt JA, et al. Marine omega-3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut. 2017;66:1790–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamada T, Liu L, Nowak JA, et al. Vitamin D status after colorectal cancer diagnosis and patient survival according to immune response to tumour. Eur J Cancer. 2018;103:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guercio BJ, Sato K, Niedzwiecki D, et al. Coffee Intake, Recurrence, and Mortality in Stage III Colon Cancer: Results From CALGB 89803 (Alliance). J Clin Oncol. 2015;33:3598–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Y, Ding M, Yuan C, et al. Association Between Coffee Intake After Diagnosis of Colorectal Cancer and Reduced Mortality. Gastroenterology. 2018;154:916–926 e919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharif K, Watad A, Bragazzi NL, Adawi M, Amital H, Shoenfeld Y. Coffee and autoimmunity: More than a mere hot beverage! Autoimmun Rev. 2017;16:712–721. [DOI] [PubMed] [Google Scholar]
- 8.Horrigan LA, Kelly JP, Connor TJ. Immunomodulatory effects of caffeine: friend or foe? Pharmacol Ther. 2006;111:877–892. [DOI] [PubMed] [Google Scholar]
- 9.Zou T, Yang Y, Xia F, et al. Resveratrol Inhibits CD4+ T cell activation by enhancing the expression and activity of Sirt1. PLoS One. 2013;8:e75139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol. 2017;18:843–850. [DOI] [PubMed] [Google Scholar]
- 11.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 12.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126; discussion 1127-1136. [DOI] [PubMed] [Google Scholar]
- 13.Haruki K, Kosumi K, Li P, et al. An integrated analysis of lymphocytic reaction, tumour molecular characteristics and patient survival in colorectal cancer. Br J Cancer. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujiyoshi K, Vayrynen JP, Borowsky J, et al. Tumour budding, poorly differentiated clusters, and T-cell response in colorectal cancer. EBioMedicine. 2020;57:102860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogino S, Nosho K, Irahara N, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15:6412–6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–2142. [DOI] [PubMed] [Google Scholar]
- 17.Borowsky J, Haruki K, Lau MC, et al. Association of Fusobacterium nucleatum with Specific T-cell Subsets in the Colorectal Carcinoma Microenvironment. Clin Cancer Res. 2021;27:2816–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujiyoshi K, Bruford EA, Mroz P, et al. Opinion: Standardizing gene product nomenclature-a call to action. Proc Natl Acad Sci U S A. 2021;118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nosho K, Irahara N, Shima K, et al. Comprehensive biostatistical analysis of CpG island methylator phenotype in colorectal cancer using a large population-based sample. PLoS One. 2008;3:e3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, Nevo D, Nishihara R, et al. Utility of inverse probability weighting in molecular pathological epidemiology. Eur J Epidemiol. 2018;33:381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogino S, Nowak JA, Hamada T, et al. Integrative analysis of exogenous, endogenous, tumour and immune factors for precision medicine. Gut. 2018;67:1168–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogino S, Nowak JA, Hamada T, Milner DA Jr., Nishihara R. Insights into Pathogenic Interactions Among Environment, Host, and Tumor at the Crossroads of Molecular Pathology and Epidemiology. Annu Rev Pathol. 2019;14:83–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh H, Hamada T, Song M, et al. Physical Activity and Colorectal Cancer Prognosis According to Tumor-Infiltrating T Cells. JNCI Cancer Spectr. 2018;2:pky058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19:307–325. [DOI] [PubMed] [Google Scholar]
- 25.Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.