Abstract
Our bodies most outward facing epithelial barrier, the skin, serves as the frontline defense against myriad environmental assailants. To combat these motley treats, the skin has evolved a sophisticated immunological arsenal. Herein, I provide an overview of the skin’s complex architecture and the distinct micro niches in which immune cells reside and function. I review burgeoning literature on the synchronized immune, stromal, epithelial, and neuronal cell responses in healthy and inflamed skin. Next, I delve into the distinct requirement and mechanisms of long-term immune surveillance and tissue adaptation at the cutaneous frontier. Finally, by discussing the contributions of immune cells in maintaining and restoring tissue integrity, I underscore the constellation of non-canonical functions undertaken by the skin immune system. Just as our skin’s immune system benefits from embracing diverse defense strategies, so too must we in the immunology research community support disparate perspectives and peoples from all walks of life.
Introduction
The skin, our body’s outermost and largest barrier, interfaces with the terrestrial environment and is tasked with protecting our internal organs from external threats. This topologically heterogenous organ not only serves as a physical barrier, but has evolved sophisticated sensory mechanisms to detect extrinsic stimuli that perturb the steady state and mount tailored responses to restore homeostasis(1). The skin’s extraordinary ability to rebound after assaults impinges upon the development and maintenance of diverse physical, chemical, and immunological defense mechanisms that incorporate and adapt to cues from the extrinsic environment(2–4). Over the last decade, technological advances have led to an explosion in our understanding of skin immunology and the remarkable means by which immunity is integrated into skin structure and function(5). Here I discuss the context specific function of the cutaneous immune system and highlight the myriad strategies employed to ensure robust host responses in the face of danger. I conclude by drawing parallels between cutaneous immune heterogeneity and that of researchers engaging in immunological research to emphasize the importance of diversity for the advancement of basic discovery (6, 7).
The skin’s structure
The outermost layer of the skin, the epidermis is comprised of tightly stacked epithelial cells, which form a semipermeable barrier (Figure 1). The epidermis can be subdivided into four distinct layers: stratum basale, stratum spinosum, stratum granulosum and stratum corneum. Proliferative epithelial stem and progenitor cells (ESPCs) reside in the lowermost or basal layer of the epidermis, where they self-renew and differentiate upwards to give rise to the stratified tissue layers(8). Upon exiting the basal layer, epithelial cells lose their mitotic potential and adopt a polygonal shape to intercalate in the spinous layer(9). The next layer is lipid filled barrier made of granules and lamellar bodies and aptly named the granular layer (10). Finally, the lion’s share of the mechanical and chemical protection is provided by the cornified layer(11). Cells anucleate and flatten as they move into this superficial layer. These flat dead cells combined with proteolytic-resistant proteins, hydrophobic lipids, corneodesmosomes, and tight-junctions generate a “Teflon-like” hydrophobic coating to seal the skin. The epidermal layers act in unison to physically restrict the movement of water and noxious agents.
Figure 1: Immune niches in the skin.
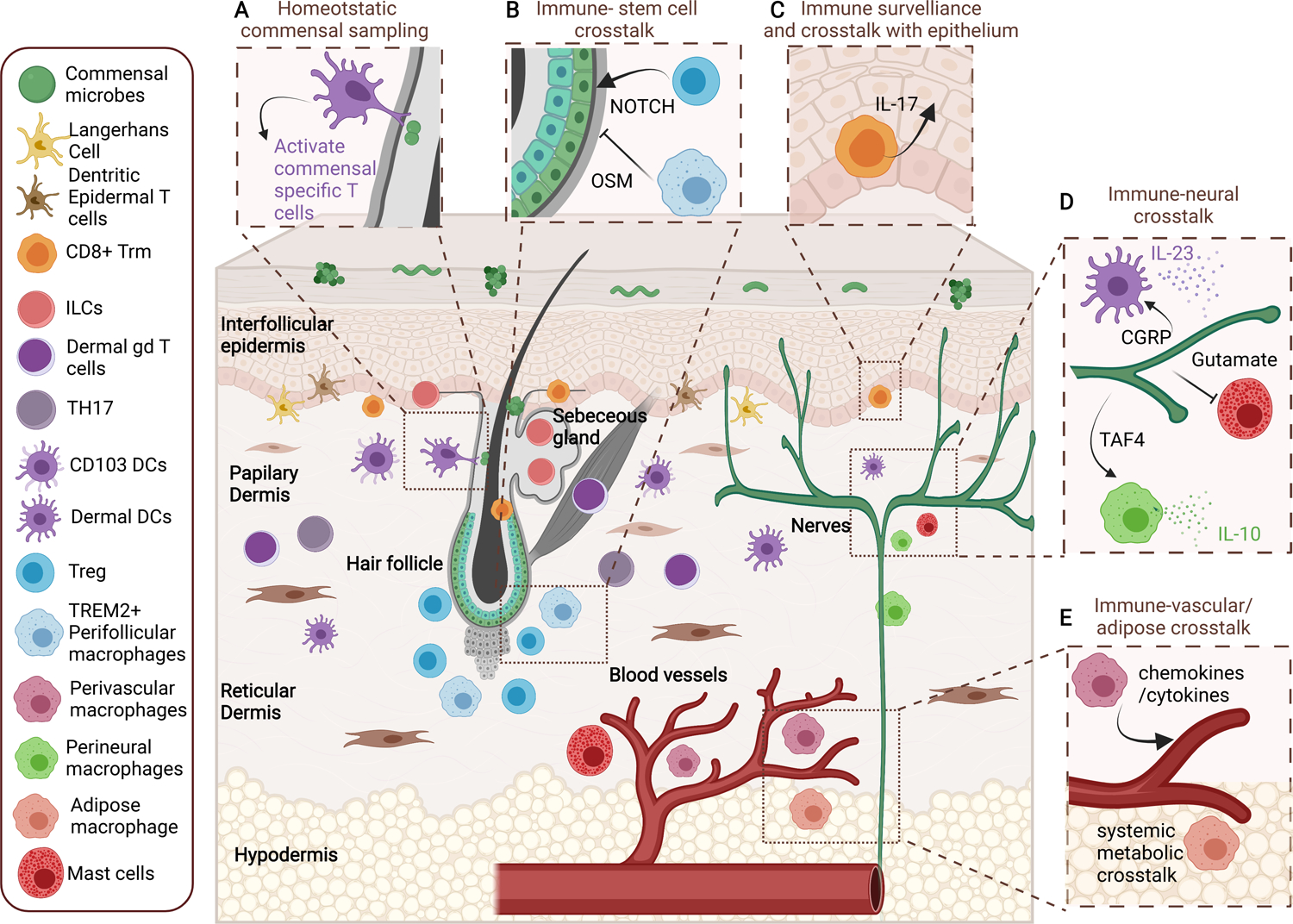
Schematic of distinct immune niches in the skin including: the interfollicular epidermis, pilosebaceous unit (hair follicle and sebaceous gland), papillary, reticular and hypo dermis, vascular and neuronal niches. Each of these locations are home to highly specialized immune populations that crosstalk with adjacent parenchyma to maintain barrier function.
Beneath the epidermis lies the dermis which can be fractioned into three structurally and functionally distinct layers (Figure 1). Directly below the epidermis is the papillary dermis. It is connected to the epidermis via the basement membrane, which provides a supportive niche for epithelial progenitors. Epidermal invaginations such as hair follicles and sebaceous glands (collectively called the pilosebaceous unit) and sweat glands penetrate into the underlying papillary dermis(12–14). These structures are essential for production of surface lipids that act as emollients, thermoregulation by controlling water loss, and sensory and protective functions of hair. The keratinocytes of the hair follicle are contiguous with the epidermis and also form stratified layers with a water tight barrier (15).
The reticular dermis lies underneath and is thicker than the papillary dermis. It is composed of densely packed collagen and elastin fibrils, and extra cellular matrix (ECM) (16). The lowermost layer of the dermis, the hypodermis, is also known as the subcutaneous fat layer and is rich is adipocytes (17). The hypodermis insulates the body from cold and aides in shock absorption. Each of the dermal layers house distinct fibroblast populations with unique functional properties(16, 18). Additionally, the dermis and epidermis are innervated and have both lymphatic and vascular endothelial vessels(19, 20). Below I discuss the distinct immune features of these cutaneous microcosms and highlight the supportive and direct contribution of their parenchyma to immune function.
Local Government
Emerging literature has revealed the remarkable regional segregation of immunity in the skin and the mechanisms by which a unique immune milieu is sustained in each of the skin’s micro niches. Organization of immune networks and maintenance in distinct areas of the skin requires discrete expression of recruitment, retention, survival and activation factors in specific locations to draw in and sustain immune cells with corresponding receptors. In turn, specialized immune cells supply their surrounding parenchyma with context-specific instructive cues that are essential for tissue function.
Epidermal patrolling immune cells
The epidermis is constantly surveyed by Langerhans cells (LCs), CD8+ resident memory T cells (TRM), innate lymphoid cells (ILCs), and in mouse skin, dendritic epidermal γδ T cells (DETCs) (Figure 1) (13, 21, 22). LCs, ILCs and DETCs seed the skin during embryonic development and maintain residence throughout life. Intriguingly, these populations localize to the skin at long before birth and yet their function in development remains largely unexplored. Arising from primitive CSFR1+ yolk sac progenitors and fetal liver monocytes, LC arrive in the epidermis at embryonic day (E) 12.5 (22). Shortly thereafter, around E16, DETCs precursors establish themselves the directly above the basal layer and project their dendrites into the suprabasal epidermal layers where they can sense barrier disruptions(23). TRMS also localize to the basal epidermis and were first identified as persistent epithelial residents following clearance viral infections in mice(24, 25). Thus, their accumulation in the skin was largely thought to be following antigen encounters postnatally(26). However, recent work characterizing prenatal human skin have identified TRMs even before birth and linked their induction and functional responsiveness to microbiota derived signals found in fetal tissue (27, 28). These early studies underscore the role of the fetal microenvironment in establishing skin immunity and raise the tantalizing possibility that maternal health may be a key determinant of early life immunity and susceptibility to childhood skin diseases such as atopic dermatitis(29).
Epidermal resident immune populations rely on local chemokine and cytokine cues for recruitment and survival. For instance, the absence of LCs in fetal dermis and identification of cycling LCs in the epidermis suggests that LC differentiation is largely restricted to the epidermal environment(30, 31). Indeed, keratinocytes robustly express a LC survival factor, interleukin (IL)-34 (32). Similarly, epithelia also express chemokines CCL27 and CCL20 to recruit epidermal lymphocytes and survival factors IL-7 and IL-15 that sustain these cells locally(33–36). Local competition for these factors dictates composition of the epithelial lymphocyte pool. With age and antigen exposure, CD8+ TRMs displace DETCs in the epidermis(37). Thus, the frontline troupes that survey the epidermis for threats, are recruited there even before birth and their homeostatic pool is continually refined throughout life based on microbial and other inflammatory encounters.
Hair follicles are focal centers of immunity
Penetrating into the papillary and reticular dermis, the pilosebaceous unit is a dynamic neighborhood for immunity. This region is enriched in both epidermal and dermal immune cells separated by a basement membrane(36, 38). These cells are recruited and retained in perifollicular region by unique combinations chemokines and cytokines expressed in upper region of the follicle (infundibulum) toward the skin surface(36, 39). Hair follicles (HFs) are also densely colonized with commensal microbes which are known to augment expression of follicular immune modulators(35). While follicle epithelia are contiguous with the intrafollicular epithelium (IFE), the tight junctions are expressed at a lower level than in the IFE likely resulting in a more permeable barrier(40, 41). Dermal antigen presenting cells (APCs), including CD103 dendritic cells (DCs) and dermal DCs and have been observed survey the follicle (42). Indeed, these cells are known to prime homeostatic commensal-specific Tc17 cells following topical microbial colonization(42, 43). Permissive HFs are often considered a portal for allergen and pathogen entry, and rely on commensal signals to maintain robust immune surveillance. Intriguingly, follicular stem cells have evolved unique mechanism to shield themselves from immune mediators by expressing key immune dampening molecules and evade immune detection by lowering their MHCII expression(2, 44). Thus, this mini-organ must weigh active immunity with tissue preservation and the mechanism by which epithelia achieve this balance are actively being investigated.
Endothelial vessels draw immune attention in the reticular and hypodermis
Immune cells in the deep dermis line the vascular endothelium to survey the blood system for internal threats and to efficiently recruit troops during inflammation. Innate immune cells including macrophages and mast cells, are particularly enriched in the perivascular space. (Figure 1)(45). Both macrophages and mast cells seed the skin neonatally from yolk-sac, fetal liver and/or bone marrow progenitors(46). Thus, anticipatory immunity in the fetal skin is not limited to the epidermal skin but also extends to the lower layers of the dermis.
During skin inflammation, perivascular mast cells and macrophages secrete cytokines and chemokines to activate the endothelium to permit entry of neutrophils, monocytes and lymphocytes from circulation(47). The perivascular space is also an epicenter for effector T cell activation as dermal DCs localize to and cluster with effector T cells to mediate contact hypersensitivity (48). Why do leukocytes gather around vessels? Strategically, positioning cutaneous immune cells in this location likely facilitates rapid communication with systemic immunity, while at the same time monitoring organismal nutritional status and health. Indeed, in the hypodermis, fat associated adipose tissue macrophages have with a high endocytic capacity respond to systemic changes in metabolism and infectious challenge(49). Thus, immune surveillance in the skin not only focuses on environmental threats, but also monitors dangers that loom within our body.
What is and isn’t an immune cell?
Immune supportive functions of non-hematopoietic cells, in particular epithelia, fibroblasts and endothelia, have been long appreciated(2). As discussed above these cells produce an array of factors to regulate the distribution and function of immune cells in healthy and inflamed skin. However, more recently, neurons have emerged as key modulators of skin immunity and the neuroimmune interface is increasingly recognized as the next frontier of immunology (19).
Linking inflammatory status to host behavior, such as scratching, allows for physical removal of noxious stimuli in the skin. Indeed, skin innervating nerve fibers robustly express receptors for a number of cytokines including IL-4 receptor, and remarkably inhibiting JAK1 signaling downstream of IL-4ra dramatically improves clinical pruritis(50). The neuroimmune axis is pathologically co-opted pruritis(itch)-associated inflammatory diseases such as atopic dermatitis, psoriasis and chronic spontaneous urticaria(51). Just as immune stimuli provoke neurons, sensory neurons similarly drive inflammation. Blanco et al found that ablating TRPV1+ Nav1.8+ nociceptors (noxious stimuli-sensing neurons) dramatically reduced inflammation in a mouse model of psoriasis(52). These nociceptors interact with dermal DCs and stimulated IL-23 production to fuel IL-17 responses in the skin via the neuropeptide CGRP(53). Thus, communications between neurons and immune cells are mediated by both immune derived cytokine and neuronal peptides and the integration of these two systems links organismal behavior to inflammatory status.
Similar to their immune brethren, neurons are exquisite at sensing noxious, thermal, mechanical and other stressors through G coupled protein surface receptors(19). Neurons also directly sense pathogenic stimuli, such as Staphylococcus aureus, leading to pain stimuli. Strikingly, elimination of nociceptors during infection lead to immune hyperactivation suggesting that pathogens may dampen immune function by modulating neuronal activity(54). Cutaneous neurons also have immunoregulatory functions in the absence of pathogenic infections. Nonpeptidergic neurons in the skin constitutively produce glutamate to suppress mast cell hyperactivation(55). Similarly, neuronally supplied TAF4 modulates macrophage IL-10 production and consequently tissue repair following sunburn-like skin injury(56). These early studies illustrate the dynamic balance between pro and anti-inflammatory function of the cutaneous nervous system. The study of neuroimmune communication has focused on nociceptors, leaving the door open for exploration of other types of neurons for example mechanosensory neurons that innervate the hair follicle or accessory cells such as Schwan cells that activate pain sensing neurons in immune modulation(57, 58).
Concomitant immunity, Inflammatory Memory, and Heterologous Protection
Perhaps one of the most powerful mechanisms of immune mediated-barrier function is the ability to remember and learn from inflammatory encounters. In some cases, immunity is ongoing with chronic antigen persistence such as with chronic infections or microbiota derived antigens (59). These persistent immune responses, occurring in the presence or absence of antigen, are leveraged to initiate rapid and informed responses to subsequent stressors both locally and at distal skin sites.
Concomitant and commensal-driven homeostatic immunity
Concomitant immunity or long-term persistence of pathogens in a host that is also able to maintain strong resistance to reinfection was first described in the context of chronic parasites infections such as Leishmania major (60). This balancing act relies on long-term maintenance of pathogen specific effector-memory and regulatory T cells (Treg) that mitigate tissue pathology but rely on low level pathogen persistence(61, 62). This complex volleying between host and parasite is useful not only to control the pathogen locally, but to maintain resistance to re-infections(63). Thus, the idealized goal of sterile pathogen clearance may not be optimal for coping with chronic and recurring infections that warrant constant immune activity.
Our skin is entrenched in commensals, which are a rich source of antigens throughout our lifetime(64). Commensal signals tune the level of skin immunity by inducing cognate T cell responses and non-specifically by augmenting antimicrobial peptides and other innate immune pathways(35, 42, 65, 66). Within moments of birth, commensals rapidly colonize the skin(67). The neonatal immune system establishes tolerance to these primordial colonizers by inducing commensal specific regulatory T cells (Tregs)(68). Remarkably, our fledgling immune system discriminates colonizing commensals from pathobionts by sensing microbial toxins(69). Early colonization with the α-toxin producing Staphylococcus aureus induces IL-1β production from myeloid cells and inhibits the generation of S. aureus specific Tregs(69). Intriguingly, longitudinal clinical studies uncovered disturbances to commensal microbiota of infants well before the onset of atopic dermis, suggesting that early life commensal-immune interactions may be critical for establishing a healthy barrier and limiting inflammatory diseases(70).
With age and exposure, the composition of the skin microbiota dynamically changes(67). So too, does our immune system’s response to commensals. Engagement of the adult immune system with commensals results in the induction of homeostatic Tc17, Th17, γδ T cell, and MAIT responses. Moreover, while some keystone species are capable of inducing broad immunity, other species and induce unique populations of immune cells. For example, certain strains of Staphylococcus epidermidis uniquely induce a population of Tc17 cells that reside in the epidermis(42). Similarly, Corynebacterium are particularly adept at eliciting Type 17 γδ T cell(71). The details of how the immune system detects and discriminates between these microbes in adulthood to engage unique immune features remains to be seen. Towards this end, DCs, key immune sentinels, have been observed to extend their dendrites through the various epidermal layers to capture antigen and induce commensal specific lymphocytes.(42, 72)
Commensally driven lymphocytes largely reside in the epidermis and upper dermis and constitutively reinforce the barrier by inducing the production of antimicrobial peptides that in turn regulate the microbial ecosystem(42). Indeed, both dysbiosis of skin commensal communities and their translocation to cutaneous lymph nodes have been observed in mice lacking adaptive immunity(73). Thus, the skin barrier uses commensal signals to constitutively tune local immunity. Whether, commensal specific lymphocytes require sustained antigen signal or can form memory in the absence of the inducing microbes remains to be explored.
Inflammatory Memory
Memory of inflammatory encounters endure long after clearance of the initial stimuli or antigen and can be parsed into antigen-specific adaptive immune memory and non-specific innate training(26, 74). Both of these features have been observed in the skin and contribute to local modulation of immunity. More recently, however, we and others have found that non-immune cells also maintain a memory of inflammation(75). Exposure of murine skin to psoriatic-like inflammation results in dramatic and lasting alterations to the chromatin structure of ESPCs that persist for 180 days (~15 human years). Larsen et al. recently found that these retained inflammatory memory domain are induced and maintained by AP-1 family of transcription factors which cooperate with stimulus specific inflammatory transcription factors to index ESPC chromatin(76).
While such memory can be beneficial in boosting barrier immunity, it can also be co-opted to fuel inflammatory disease. Indeed, resident memory lymphocytes have long been thought to drive recurrent psoriatic inflammation(77, 78). However, the resurgence of plaques in the same skin locations suggests that other innate immune and non-immune tissue cells may also retain a memory of disease(79, 80). Accordingly, keratinocytes cultured ex vivo from psoriatic plaques are enriched for progenitor signatures with reduced differentiation signatures compared to non-lesional skin(81). These early studies of patient samples prompt further exploration of tissue memory of inflammation beyond immunity and suggest that achieving long-term clearance of inflammatory disease may require treating both immune dysfunction and broader tissue inflammatory memory.
Heterologous protection
Concomitant immunity and homeostatic commensal-specific responses represent situations in which antigen constitutively stimulates immune activity similar to a “buzzer” whereas, immune memory persists long-after clearance of the initial stimuli much like a “switch”. While these responses can promptly target the initial stimulus, they can also be activated non-specifically via the persistence of local antigen or the production of cytokines and directed towards another secondary stimulus. For instance, co-infection with L. major and Staphylococcus aureus results in augmented immunity against both microbes(82). Similarly, commensal microbes act as natural adjuvants and stimulate both antigen-specific lymphocytes and anti-microbial peptides from keratinocytes to limiting early invasion of Candida albicans and promote clearance of L. major (42, 83).
TRMs and other tissue resident lymphocytes are able to respond to alarmins and other factors released by damaged tissue to respond to non-specific stimuli(84). These cells also play a vital role in early control of transformed cells and restrain tumor growth long-term. For instance, in an epicutaneous melanoma transplant model, CD8+ TRMs limited the outgrowth of tumors but also did not eliminate melanoma cells altogether, maintaining an “equilibrium” with the transformed melanocytes(85). Just as immune cells can non-specifically respond to secondary threats, epithelial memory is also repurposed to boost tissue repair(75). The ability to easily repurpose various immune modules generated at the cutaneous interface may be the most cost-effective means of rapidly coping with recurrent stressors or those that warrant similar responses.
Tissue supportive roles of immunity
The last decade has seen an explosion of studies on the non-canonical functions of immune cells in the skin. These findings extend the role of immune cells well-beyond host defense into the realm of tissue maintenance and repair. In particular, Tregs and macrophages, both enriched in the skin, regulate epithelial and stromal cells function via diverse mechanisms (86).
The hair follicle undergoes cyclical bouts of rest and regeneration impinging upon follicle stem cell (SC) quiescence and activation. For over two decades groups have observed that the composition of immune cells changes with the stage of the follicle cycle, even at steady state(87). However, the reason for this flux and the contribution of perifollicular immune cells to follicle SC behavior and cycle stage was, until recently, unclear. Single nucleotide polymorphisms in genes associated with SC-immune crosstalk are strongly implicated in Alopecia areata, a hair loss disorder, suggesting a functional interaction between immune cells and follicle SCs may be at play(88). Indeed, Rosenblum and colleagues, recently found that Tregs are specifically enriched around the follicle bulge where SCs reside and supply them with activating notch signals to facilitate SC activation and kick-start the hair cycle(38). On the other hand, a dermal TREM2+ macrophages subset secretes oncostatin M to maintain SC quiescence and keep follicles in the resisting phase(89). How these pro-and-anti-quiescence populations are regulated and if follicle SCs are at the reigns or if other tissue signals dictate perifollicular immune dynamics warrants further investigation.
Sebaceous glands, another component of the pilosebaceous unit are responsible for secreting lipid rich sebum to lubricate the skin. Type 3 innate lymphoid cells (ILC3s), residing in the upper hair follicle, control sebaceous hyperplasia and composition of sebum via TNF ligands and notch(13). Consequently, the composition of skin commensals in animals lacking ILC3s was dramatically altered. In contrast to the homeostatic restrain of sebaceous glands, over production of the alarmin TSLP results in migration of T cells into sebaceous glands and hypersecretion of sebum(90). These animals experienced selective depletion of white adipose tissue and dramatic over all weight loss. Thus, regulation of skin appendages by immune cells have consequences for both the local microenvironment and the organismal macroenvironment.
Perhaps the most heavily studied atypical functions of immune cells are in the context of tissue damage and repair(91). Immune derived signals direct repair of epithelial, stromal and neuronal cells following acute tissue damage. Here an interesting bifurcation emerges, whereby crosstalk with damaged epithelium is relegated to patrolling adaptive immune cells and the tissues dermal constituents are engaged by innate immune cells, in particular macrophages.
Following damage, epithelial progenitors at the wounds edge proliferate and migrate to swiftly seal the exposed surface in a process termed re-epithelialization(92). Havran and colleagues published pioneering studies detailing the function of DETCs in epithelial proliferation via the production of various growth factors(93). Since then, IL-17A emanating from homeostatic Tc17 cells, MAIT cells, dermal γδ T cells, and ILC3s and their supplied factor has been identified as a key regulator of re-epithelialization(43, 65, 94, 95). Notably, many of these studies were performed in immunodeficient animals. If IL-17A from distinct cellular sources is required for repair, its temporal dynamics of activation, and the functional redundancy between various homeostatic lymphocytes remain open questions. Moreover, how repair associated lymphocytes are activated following damage requires elucidation. Decoding the mechanisms of lymphocyte-epithelial crosstalk may enable targeting of this interaction in non-healing wounds that fail to re-epithelialize(96). Indeed, aged skin, which often fails to heal, exhibits a severe impairment in the DETC-epithelial crosstalk(97).
During wound repair, the dermis must re-establish its architecture by depositing ECM, promoting revascularization and innervation(98). Following injury, dermal fibroblasts proliferate and differentiate into ECM-producing myofibroblasts. CD301b+ dermal macrophages produce insulin-like growth factor and platelet derived growth factor to directed myofibroblasts differentiation and pro-fibrotic function(99). Loss of CD301b+ macrophages and resultant fibroblast defects leads to a profound delay in repair(100). The macrophage-nervous crosstalk is also central to the repair process. CX3CR1+ macrophages are intimately associated with cutaneous sensory nerves contribute to axonal sprouting following injury(101). Sensory neurons cross regulate macrophages by inducing IL-10 production via the neuropeptide TAF4 to limit fibrosis following UV induced tissue damage(56). Macrophages also orchestrate the dynamics of blood vessel formation and regression following injury, where early in repair macrophages promote angiogenesis and after the wound has healed they direct vessel clearance(102). Thus, highly specialized macrophages populations simultaneously interact with and direct the repair of distinct dermal components(103). If these macrophages are developmentally specified to perform these functions or obtain their unique powers once the occupy distinct niches in the skin (for example perivascular vs. perineuronal) is poorly understood. However, harnessing the pro-repair functions of macrophages may transform regenerative therapies in the skin and beyond.
Conclusion and Perspective
Far from a simple physical barrier, the skin has evolved sophisticated mechanisms to cope with myriad threats, while still maintaining complex microbial communities on the its surface. In addition to classical anti-pathogenic functions, coupling tissue regulatory and repair functions with immunity may be advantages to mitigate immunopathology and collateral tissue damage resulting from inflammation. Another unappreciated dimension of skin immunity is the unique regulation of its resident immune populations relative to other barrier tissues. For instance, homeostatic Th17 cells in the skin are reliant on IL-1 for function while gut Th17 cells are controlled by IL-6(83). Similarly, ILC2s in the skin are uniquely responsive to IL-18 compared to ILC2s from other barrier tissues(104). The aforementioned areas of skin immune complexity, immune-tissue crosstalk, inflammatory memory and tissue repair are being actively investigated and may enable a skin-specific therapeutic modulation of immune function. However, a key principle cemented in field of skin immunity is that one size does not fit all. Highly tailored immune retort is vital to tackle unique challenges and defend the host from diverse onslaughts.
Just as the immune system embraces unique and diverse strategies to safeguard the skin, we immunologists must also seek a range of perspectives to forge new paths to discovery. In fact, I argue that the multifaceted nature of biology demands examination from different angles, and the engine of discovery falters without it. Indeed, a wealth of evidence indicates that diversity positively impacts scientific discovery through improved problem-solving, innovation, prediction, evaluation, and strategic thinking(6, 105, 106). Yet, inclusion of underrepresented and marginalized groups in biomedical research remains a major challenge.
The brutal murders of Ahmaud Arbery, Briana Taylor, George Floyd and countless others further highlighted the paucity of diversity in biomedicine much in the same way that the #metoo movement highlighted the profound gender imbalance and barriers to advancement of women in science. Fortunately, key stakeholders, including research institutions, government agencies, and private foundations, are responding by publicly proclaiming their commitment to diversity, equity, and inclusion (DEI). I am hopeful that our institutional and individual commitments to DEI will allow folks from all walks of life to share the scientific spot light and ultimately transform the process of discovery in the field of immunology.
Acknowledgements
I apologize to my friends and colleagues, due to space constraints I was not able to include all relevant papers published in the exciting area of skin immunology. I want to thank my mentors Drs. Yasmine Belkaid, Julie Segre, and Elaine Fuchs for their inspiring activism in promoting women and diversity in biomedicine.
References
- 1.Pasparakis M, Haase I, and Nestle FO 2014. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol 14: 289–301. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi T, Naik S, and Nagao K 2019. Choreographing Immunity in the Skin Epithelial Barrier. Immunity 50: 552–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xing Y, and Naik S 2020. Under pressure: Stem cell-niche interactions coordinate tissue adaptation to inflammation. Curr Opin Cell Biol 67: 64–70. [DOI] [PubMed] [Google Scholar]
- 4.Chovatiya R, and Medzhitov R 2014. Stress, inflammation, and defense of homeostasis. Mol Cell 54: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konieczny P, and Naik S 2021. Warp Speed Ahead! Technology-Driven Breakthroughs in Skin Immunity and Inflammatory Disease. J Invest Dermatol 141: 15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwasaki A 2019. Why we need to increase diversity in the immunology research community. Nat Immunol 20: 1085–1088. [DOI] [PubMed] [Google Scholar]
- 7.Pepper M 2020. Hey man. Nat Immunol 21: 236. [DOI] [PubMed] [Google Scholar]
- 8.Blanpain C, and Fuchs E 2009. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 10: 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asare A, Levorse J, and Fuchs E 2017. Coupling organelle inheritance with mitosis to balance growth and differentiation. Science 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quiroz FG, Fiore VF, Levorse J, Polak L, Wong E, Pasolli HA, and Fuchs E 2020. Liquid-liquid phase separation drives skin barrier formation. Science 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsui T, and Amagai M 2015. Dissecting the formation, structure and barrier function of the stratum corneum. Int Immunol 27: 269–280. [DOI] [PubMed] [Google Scholar]
- 12.Schneider MR, Schmidt-Ullrich R, and Paus R 2009. The Hair Follicle as a Dynamic Miniorgan. Curr Biol 19: R132–R142. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi T, Voisin B, Kim DY, Kennedy EA, Jo JH, Shih HY, Truong A, Doebel T, Sakamoto K, Cui CY, Schlessinger D, Moro K, Nakae S, Horiuchi K, Zhu J, Leonard WJ, Kong HH, and Nagao K 2019. Homeostatic Control of Sebaceous Glands by Innate Lymphoid Cells Regulates Commensal Bacteria Equilibrium. Cell 176: 982–997 e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joost S, Annusver K, Jacob T, Sun X, Dalessandri T, Sivan U, Sequeira I, Sandberg R, and Kasper M 2020. The Molecular Anatomy of Mouse Skin during Hair Growth and Rest. Cell Stem Cell 26: 441–457 e447. [DOI] [PubMed] [Google Scholar]
- 15.Lay K, Yuan S, Gur-Cohen S, Miao Y, Han T, Naik S, Pasolli HA, Larsen SB, and Fuchs E 2018. Stem cells repurpose proliferation to contain a breach in their niche barrier. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC, and Watt FM 2013. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504: 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shook B, Rivera Gonzalez G, Ebmeier S, Grisotti G, Zwick R, and Horsley V 2016. The Role of Adipocytes in Tissue Regeneration and Stem Cell Niches. Annu Rev Cell Dev Biol 32: 609–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harper RA, and Grove G 1979. Human skin fibroblasts derived from papillary and reticular dermis: differences in growth potential in vitro. Science 204: 526–527. [DOI] [PubMed] [Google Scholar]
- 19.Tamari M, Ver Heul AM, and Kim BS 2021. Immunosensation: Neuroimmune Cross Talk in the Skin. Annu Rev Immunol 39: 369–393. [DOI] [PubMed] [Google Scholar]
- 20.Steele MM, and Lund AW 2021. Afferent Lymphatic Transport and Peripheral Tissue Immunity. J Immunol 206: 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bukhari S, Mertz AF, and Naik S 2019. Eavesdropping on the conversation between immune cells and the skin epithelium. Int Immunol 31: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, Choy SH, Grisotto M, Renia L, Conway SJ, Stanley ER, Chan JK, Ng LG, Samokhvalov IM, Merad M, and Ginhoux F 2012. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med 209: 1167–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Havran WL, and Allison JP 1990. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature 344: 68–70. [DOI] [PubMed] [Google Scholar]
- 24.Masopust D, Vezys V, Marzo AL, and Lefrancois L 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291: 2413–2417. [DOI] [PubMed] [Google Scholar]
- 25.Mueller SN, Zaid A, and Carbone FR 2014. Tissue-resident T cells: dynamic players in skin immunity. Front Immunol 5: 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schenkel JM, and Masopust D 2014. Tissue-resident memory T cells. Immunity 41: 886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds G, Vegh P, Fletcher J, Poyner EFM, Stephenson E, Goh I, Botting RA, Huang N, Olabi B, Dubois A, Dixon D, Green K, Maunder D, Engelbert J, Efremova M, Polanski K, Jardine L, Jones C, Ness T, Horsfall D, McGrath J, Carey C, Popescu DM, Webb S, Wang XN, Sayer B, Park JE, Negri VA, Belokhvostova D, Lynch MD, McDonald D, Filby A, Hagai T, Meyer KB, Husain A, Coxhead J, Vento-Tormo R, Behjati S, Lisgo S, Villani AC, Bacardit J, Jones PH, O’Toole EA, Ogg GS, Rajan N, Reynolds NJ, Teichmann SA, Watt FM, and Haniffa M 2021. Developmental cell programs are co-opted in inflammatory skin disease. Science 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishra A, Lai GC, Yao LJ, Aung TT, Shental N, Rotter-Maskowitz A, Shepherdson E, Singh GSN, Pai R, Shanti A, Wong RMM, Lee A, Khyriem C, Dutertre CA, Chakarov S, Srinivasan KG, Shadan NB, Zhang XM, Khalilnezhad S, Cottier F, Tan ASM, Low G, Chen P, Fan Y, Hor PX, Lee AKM, Choolani M, Vermijlen D, Sharma A, Fuks G, Straussman R, Pavelka N, Malleret B, McGovern N, Albani S, Chan JKY, and Ginhoux F 2021. Microbial exposure during early human development primes fetal immune cells. Cell 184: 3394–3409 e3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaughn AR, Tannhauser P, Sivamani RK, and Shi VY 2017. Mother Nature in Eczema: Maternal Factors Influencing Atopic Dermatitis. Pediatr Dermatol 34: 240–246. [DOI] [PubMed] [Google Scholar]
- 30.Schuster C, Vaculik C, Fiala C, Meindl S, Brandt O, Imhof M, Stingl G, Eppel W, and Elbe-Burger A 2009. HLA-DR+ leukocytes acquire CD1 antigens in embryonic and fetal human skin and contain functional antigen-presenting cells. J Exp Med 206: 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chorro L, Sarde A, Li M, Woollard KJ, Chambon P, Malissen B, Kissenpfennig A, Barbaroux JB, Groves R, and Geissmann F 2009. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J Exp Med 206: 3089–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kundig TM, Frei K, Ginhoux F, Merad M, and Becher B 2012. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 37: 1050–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang V, Lonsdorf AS, Fang L, Kakinuma T, Lee VC, Cha E, Zhang H, Nagao K, Zaleska M, Olszewski WL, and Hwang ST 2008. Cutting edge: rapid accumulation of epidermal CCL27 in skin-draining lymph nodes following topical application of a contact sensitizer recruits CCR10-expressing T cells. J Immunol 180: 6462–6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin Y, Xia M, Sun A, Saylor CM, and Xiong N 2010. CCR10 is important for the development of skin-specific gammadeltaT cells by regulating their migration and location. J Immunol 185: 5723–5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scharschmidt TC, Vasquez KS, Pauli ML, Leitner EG, Chu K, Truong HA, Lowe MM, Sanchez Rodriguez R, Ali N, Laszik ZG, Sonnenburg JL, Millar SE, and Rosenblum MD 2017. Commensal Microbes and Hair Follicle Morphogenesis Coordinately Drive Treg Migration into Neonatal Skin. Cell Host Microbe 21: 467–477 e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adachi T, Kobayashi T, Sugihara E, Yamada T, Ikuta K, Pittaluga S, Saya H, Amagai M, and Nagao K 2015. Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat Med 21: 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaid A, Mackay LK, Rahimpour A, Braun A, Veldhoen M, Carbone FR, Manton JH, Heath WR, and Mueller SN 2014. Persistence of skin-resident memory T cells within an epidermal niche. Proc Natl Acad Sci U S A 111: 5307–5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong HA, Lai K, Ahn R, Corbin K, Lowe MM, Scharschmidt TC, Taravati K, Tan MR, Ricardo-Gonzalez RR, Nosbaum A, Bertolini M, Liao W, Nestle FO, Paus R, Cotsarelis G, Abbas AK, and Rosenblum MD 2017. Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell 169: 1119–1129 e1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagao K, Kobayashi T, Moro K, Ohyama M, Adachi T, Kitashima DY, Ueha S, Horiuchi K, Tanizaki H, Kabashima K, Kubo A, Cho YH, Clausen BE, Matsushima K, Suematsu M, Furtado GC, Lira SA, Farber JM, Udey MC, and Amagai M 2012. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat Immunol 13: 744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zorn-Kruppa M, Vidal YSS, Houdek P, Wladykowski E, Grzybowski S, Gruber R, Gorzelanny C, Harcup J, Schneider SW, Majumdar A, and Brandner JM 2018. Tight Junction barriers in human hair follicles - role of claudin-1. Sci Rep 8: 12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohd F, Todo H, Yoshimoto M, Yusuf E, and Sugibayashi K 2016. Contribution of the Hair Follicular Pathway to Total Skin Permeation of Topically Applied and Exposed Chemicals. Pharmaceutics 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, Quinones M, Brenchley JM, Kong HH, Tussiwand R, Murphy KM, Merad M, Segre JA, and Belkaid Y 2015. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520: 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linehan JL, Harrison OJ, Han SJ, Byrd AL, Vujkovic-Cvijin I, Villarino AV, Sen SK, Shaik J, Smelkinson M, Tamoutounour S, Collins N, Bouladoux N, Dzutsev A, Rosshart SP, Arbuckle JH, Wang CR, Kristie TM, Rehermann B, Trinchieri G, Brenchley JM, O’Shea JJ, and Belkaid Y 2018. Non-classical Immunity Controls Microbiota Impact on Skin Immunity and Tissue Repair. Cell 172: 784–796 e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agudo J, Park ES, Rose SA, Alibo E, Sweeney R, Dhainaut M, Kobayashi KS, Sachidanandam R, Baccarini A, Merad M, and Brown BD 2018. Quiescent Tissue Stem Cells Evade Immune Surveillance. Immunity 48: 271–285 e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunder CA, St John AL, and Abraham SN 2011. Mast cell modulation of the vascular and lymphatic endothelium. Blood 118: 5383–5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Z, Liu S, Xu J, Zhang X, Han D, Liu J, Xia M, Yi L, Shen Q, Xu S, Lu L, and Cao X 2018. Adult Connective Tissue-Resident Mast Cells Originate from Late Erythro-Myeloid Progenitors. Immunity 49: 640–653 e645. [DOI] [PubMed] [Google Scholar]
- 47.Abtin A, Jain R, Mitchell AJ, Roediger B, Brzoska AJ, Tikoo S, Cheng Q, Ng LG, Cavanagh LL, von Andrian UH, Hickey MJ, Firth N, and Weninger W 2014. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat Immunol 15: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Natsuaki Y, Egawa G, Nakamizo S, Ono S, Hanakawa S, Okada T, Kusuba N, Otsuka A, Kitoh A, Honda T, Nakajima S, Tsuchiya S, Sugimoto Y, Ishii KJ, Tsutsui H, Yagita H, Iwakura Y, Kubo M, Ng L, Hashimoto T, Fuentes J, Guttman-Yassky E, Miyachi Y, and Kabashima K 2014. Perivascular leukocyte clusters are essential for efficient activation of effector T cells in the skin. Nat Immunol 15: 1064–1069. [DOI] [PubMed] [Google Scholar]
- 49.Silva HM, Bafica A, Rodrigues-Luiz GF, Chi J, Santos PDA, Reis BS, Hoytema van Konijnenburg DP, Crane A, Arifa RDN, Martin P, Mendes D, Mansur DS, Torres VJ, Cadwell K, Cohen P, Mucida D, and Lafaille JJ 2019. Vasculature-associated fat macrophages readily adapt to inflammatory and metabolic challenges. J Exp Med 216: 786–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, Chen S, Trier AM, Xu AZ, Tripathi SV, Luo J, Gao X, Yang L, Hamilton SL, Wang PL, Brestoff JR, Council ML, Brasington R, Schaffer A, Brombacher F, Hsieh CS, Gereau R. W. t., Miller MJ, Chen ZF, Hu H, Davidson S, Liu Q, and Kim BS 2017. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 171: 217–228 e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruppenstein A, Limberg MM, Loser K, Kremer AE, Homey B, and Raap U 2021. Involvement of Neuro-Immune Interactions in Pruritus With Special Focus on Receptor Expressions. Front Med (Lausanne) 8: 627985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, Paust S, Wood JN, and von Andrian UH 2014. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 510: 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, and Kaplan DH 2015. Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity 43: 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Bubeck Wardenburg J, Hwang SW, Carroll MC, and Woolf CJ 2013. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501: 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang S, Edwards TN, Chaudhri VK, Wu J, Cohen JA, Hirai T, Rittenhouse N, Schmitz EG, Zhou PY, McNeil BD, Yang Y, Koerber HR, Sumpter TL, Poholek AC, Davis BM, Albers KM, Singh H, and Kaplan DH 2021. Nonpeptidergic neurons suppress mast cells via glutamate to maintain skin homeostasis. Cell 184: 2151–2166 e2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoeffel G, Debroas G, Roger A, Rossignol R, Gouilly J, Laprie C, Chasson L, Barbon PV, Balsamo A, Reynders A, Moqrich A, and Ugolini S 2021. Sensory neuron-derived TAFA4 promotes macrophage tissue repair functions. Nature 594: 94–99. [DOI] [PubMed] [Google Scholar]
- 57.Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, Woodbury CJ, and Ginty DD 2011. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 147: 1615–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdo H, Calvo-Enrique L, Lopez JM, Song J, Zhang MD, Usoskin D, El Manira A, Adameyko I, Hjerling-Leffler J, and Ernfors P 2019. Specialized cutaneous Schwann cells initiate pain sensation. Science 365: 695–699. [DOI] [PubMed] [Google Scholar]
- 59.Belkaid Y, and Harrison OJ 2017. Homeostatic Immunity and the Microbiota. Immunity 46: 562–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scott P, and Novais FO 2016. Cutaneous leishmaniasis: immune responses in protection and pathogenesis. Nat Rev Immunol 16: 581–592. [DOI] [PubMed] [Google Scholar]
- 61.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, and Sacks DL 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420: 502–507. [DOI] [PubMed] [Google Scholar]
- 62.Scott P 2020. Long-Lived Skin-Resident Memory T Cells Contribute to Concomitant Immunity in Cutaneous Leishmaniasis. Cold Spring Harb Perspect Biol 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mitchell GF 1991. Co-evolution of parasites and adaptive immune responses. Immunol Today 12: A2–5. [DOI] [PubMed] [Google Scholar]
- 64.Belkaid Y, and Segre JA 2014. Dialogue between skin microbiota and immunity. Science 346: 954–959. [DOI] [PubMed] [Google Scholar]
- 65.Constantinides MG, Link VM, Tamoutounour S, Wong AC, Perez-Chaparro PJ, Han SJ, Chen YE, Li K, Farhat S, Weckel A, Krishnamurthy SR, Vujkovic-Cvijin I, Linehan JL, Bouladoux N, Merrill ED, Roy S, Cua DJ, Adams EJ, Bhandoola A, Scharschmidt TC, Aube J, Fischbach MA, and Belkaid Y 2019. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gallo RL, and Hooper LV 2012. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol 12: 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tamburini S, Shen N, Wu HC, and Clemente JC 2016. The microbiome in early life: implications for health outcomes. Nat Med 22: 713–722. [DOI] [PubMed] [Google Scholar]
- 68.Scharschmidt TC, Vasquez KS, Truong HA, Gearty SV, Pauli ML, Nosbaum A, Gratz IK, Otto M, Moon JJ, Liese J, Abbas AK, Fischbach MA, and Rosenblum MD 2015. A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes. Immunity 43: 1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leech JM, Dhariwala MO, Lowe MM, Chu K, Merana GR, Cornuot C, Weckel A, Ma JM, Leitner EG, Gonzalez JR, Vasquez KS, Diep BA, and Scharschmidt TC 2019. Toxin-Triggered Interleukin-1 Receptor Signaling Enables Early-Life Discrimination of Pathogenic versus Commensal Skin Bacteria. Cell Host Microbe 26: 795–809 e795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kennedy EA, Connolly J, Hourihane JO, Fallon PG, McLean WHI, Murray D, Jo JH, Segre JA, Kong HH, and Irvine AD 2017. Skin microbiome before development of atopic dermatitis: Early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J Allergy Clin Immun 139: 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ridaura VK, Bouladoux N, Claesen J, Chen YE, Byrd AL, Constantinides MG, Merrill ED, Tamoutounour S, Fischbach MA, and Belkaid Y 2018. Contextual control of skin immunity and inflammation by Corynebacterium. J Exp Med 215: 785–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ouchi T, Kubo A, Yokouchi M, Adachi T, Kobayashi T, Kitashima DY, Fujii H, Clausen BE, Koyasu S, Amagai M, and Nagao K 2011. Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome. J Exp Med 208: 2607–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shen W, Li W, Hixon JA, Bouladoux N, Belkaid Y, Dzutzev A, and Durum SK 2014. Adaptive immunity to murine skin commensals. Proc Natl Acad Sci U S A 111: E2977–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Divangahi M, Aaby P, Khader SA, Barreiro LB, Bekkering S, Chavakis T, van Crevel R, Curtis N, DiNardo AR, Dominguez-Andres J, Duivenwoorden R, Fanucchi S, Fayad Z, Fuchs E, Hamon M, Jeffrey KL, Khan N, Joosten LAB, Kaufmann E, Latz E, Matarese G, van der Meer JWM, Mhlanga M, Moorlag S, Mulder WJM, Naik S, Novakovic B, O’Neill L, Ochando J, Ozato K, Riksen NP, Sauerwein R, Sherwood ER, Schlitzer A, Schultze JL, Sieweke MH, Benn CS, Stunnenberg H, Sun J, van de Veerdonk FL, Weis S, Williams DL, Xavier R, and Netea MG 2021. Trained immunity, tolerance, priming and differentiation: distinct immunological processes. Nat Immunol 22: 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naik S, Larsen SB, Gomez NC, Alaverdyan K, Sendoel A, Yuan S, Polak L, Kulukian A, Chai S, and Fuchs E 2017. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 550: 475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larsen SB, Cowley CJ, Sajjath SM, Barrows D, Yang Y, Carroll TS, and Fuchs E 2021. Establishment, maintenance, and recall of inflammatory memory. Cell Stem Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gallais Serezal I, Classon C, Cheuk S, Barrientos-Somarribas M, Wadman E, Martini E, Chang D, Xu Landen N, Ehrstrom M, Nylen S, and Eidsmo L 2018. Resident T Cells in Resolved Psoriasis Steer Tissue Responses that Stratify Clinical Outcome. J Invest Dermatol 138: 1754–1763. [DOI] [PubMed] [Google Scholar]
- 78.Cheuk S, Wiken M, Blomqvist L, Nylen S, Talme T, Stahle M, and Eidsmo L 2014. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J Immunol 192: 3111–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suarez-Farinas M, Fuentes-Duculan J, Lowes MA, and Krueger JG 2011. Resolved psoriasis lesions retain expression of a subset of disease-related genes. J Invest Dermatol 131: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang YW, and Tsai TF 2019. Remission Duration and Long-Term Outcomes in Patients with Moderate-to-Severe Psoriasis Treated by Biologics or Tofacitinib in Controlled Clinical Trials: A 15-Year Single-Center Experience. Dermatol Ther (Heidelb) 9: 553–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swindell WR, Sarkar MK, Liang Y, Xing X, Baliwag J, Elder JT, Johnston A, Ward NL, and Gudjonsson JE 2017. RNA-seq identifies a diminished differentiation gene signature in primary monolayer keratinocytes grown from lesional and uninvolved psoriatic skin. Sci Rep 7: 18045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borbon TY, Scorza BM, Clay GM, Lima Nobre de Queiroz F, Sariol AJ, Bowen JL, Chen Y, Zhanbolat B, Parlet CP, Valadares DG, Cassel SL, Nauseef WM, Horswill AR, Sutterwala FS, and Wilson ME 2019. Coinfection with Leishmania major and Staphylococcus aureus enhances the pathologic responses to both microbes through a pathway involving IL-17A. PLoS Negl Trop Dis 13: e0007247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, Spencer S, Hall JA, Dzutsev A, Kong H, Campbell DJ, Trinchieri G, Segre JA, and Belkaid Y 2012. Compartmentalized control of skin immunity by resident commensals. Science 337: 1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Szabo PA, Miron M, and Farber DL 2019. Location, location, location: Tissue resident memory T cells in mice and humans. Sci Immunol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park SL, Buzzai A, Rautela J, Hor JL, Hochheiser K, Effern M, McBain N, Wagner T, Edwards J, McConville R, Wilmott JS, Scolyer RA, Tuting T, Palendira U, Gyorki D, Mueller SN, Huntington ND, Bedoui S, Holzel M, Mackay LK, Waithman J, and Gebhardt T 2019. Tissue-resident memory CD8(+) T cells promote melanoma-immune equilibrium in skin. Nature 565: 366–371. [DOI] [PubMed] [Google Scholar]
- 86.Naik S, Larsen SB, Cowley CJ, and Fuchs E 2018. Two to Tango: Dialog between Immunity and Stem Cells in Health and Disease. Cell 175: 908–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paus R, van der Veen C, Eichmuller S, Kopp T, Hagen E, Muller-Rover S, and Hofmann U 1998. Generation and cyclic remodeling of the hair follicle immune system in mice. J Invest Dermatol 111: 7–18. [DOI] [PubMed] [Google Scholar]
- 88.Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, Kim H, Singh P, Lee A, Chen WV, Meyer KC, Paus R, Jahoda CA, Amos CI, Gregersen PK, and Christiano AM 2010. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature 466: 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang ECE, Dai Z, Ferrante AW, Drake CG, and Christiano AM 2019. A Subset of TREM2(+) Dermal Macrophages Secretes Oncostatin M to Maintain Hair Follicle Stem Cell Quiescence and Inhibit Hair Growth. Cell Stem Cell 24: 654–669 e656. [DOI] [PubMed] [Google Scholar]
- 90.Choa R, Tohyama J, Wada S, Meng H, Hu J, Okumura M, May RM, Robertson TF, Pai RL, Nace A, Hopkins C, Jacobsen EA, Haldar M, FitzGerald GA, Behrens EM, Minn AJ, Seale P, Cotsarelis G, Kim B, Seykora JT, Li M, Arany Z, and Kambayashi T 2021. Thymic stromal lymphopoietin induces adipose loss through sebum hypersecretion. Science 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mansfield K, and Naik S 2020. Unraveling Immune-Epithelial Interactions in Skin Homeostasis and Injury. Yale J Biol Med 93: 133–143. [PMC free article] [PubMed] [Google Scholar]
- 92.Eming SA, Martin P, and Tomic-Canic M 2014. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 6: 265sr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, and Havran WL 2002. A role for skin gammadelta T cells in wound repair. Science 296: 747–749. [DOI] [PubMed] [Google Scholar]
- 94.Li Z, Hodgkinson T, Gothard EJ, Boroumand S, Lamb R, Cummins I, Narang P, Sawtell A, Coles J, Leonov G, Reboldi A, Buckley CD, Cupedo T, Siebel C, Bayat A, Coles MC, and Ambler CA 2016. Epidermal Notch1 recruits RORgamma(+) group 3 innate lymphoid cells to orchestrate normal skin repair. Nat Commun 7: 11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu L, Chen X, Zhao J, Martin B, Zepp JA, Ko JS, Gu C, Cai G, Ouyang W, Sen G, Stark GR, Su B, Vines CM, Tournier C, Hamilton TA, Vidimos A, Gastman B, Liu C, and Li X 2015. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J Exp Med 212: 1571–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, Patel SB, Khalid L, Isseroff RR, and Tomic-Canic M 2014. Epithelialization in Wound Healing: A Comprehensive Review. Adv Wound Care 3: 445–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Keyes BE, Liu SQ, Asare A, Naik S, Levorse J, Polak L, Lu CP, Nikolova M, Pasolli HA, and Fuchs E 2016. Impaired Epidermal to Dendritic T Cell Signaling Slows Wound Repair in Aged Skin. Cell 167: 1323-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clark RA 1993. Biology of dermal wound repair. Dermatol Clin 11: 647–666. [PubMed] [Google Scholar]
- 99.Shook BA, Wasko RR, Rivera-Gonzalez GC, Salazar-Gatzimas E, Lopez-Giraldez F, Dash BC, Munoz-Rojas AR, Aultman KD, Zwick RK, Lei V, Arbiser JL, Miller-Jensen K, Clark DA, Hsia HC, and Horsley V 2018. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shook B, Xiao E, Kumamoto Y, Iwasaki A, and Horsley V 2016. CD301b+ Macrophages Are Essential for Effective Skin Wound Healing. J Invest Dermatol 136: 1885–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kolter J, Feuerstein R, Zeis P, Hagemeyer N, Paterson N, d’Errico P, Baasch S, Amann L, Masuda T, Losslein A, Gharun K, Meyer-Luehmann M, Waskow C, Franzke CW, Grun D, Lammermann T, Prinz M, and Henneke P 2019. A Subset of Skin Macrophages Contributes to the Surveillance and Regeneration of Local Nerves. Immunity 50: 1482–1497 e1487. [DOI] [PubMed] [Google Scholar]
- 102.Gurevich DB, Severn CE, Twomey C, Greenhough A, Cash J, Toye AM, Mellor H, and Martin P 2018. Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wynn TA, and Vannella KM 2016. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 44: 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ricardo-Gonzalez RR, Van Dyken SJ, Schneider C, Lee J, Nussbaum JC, Liang HE, Vaka D, Eckalbar WL, Molofsky AB, Erle DJ, and Locksley RM 2018. Tissue signals imprint ILC2 identity with anticipatory function. Nat Immunol 19: 1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Valantine HA, Lund PK, and Gammie AE From the NIH: A Systems Approach to Increasing the Diversity of the Biomedical Research Workforce. CBE Life Sci Educ 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gibbs KD, Basson J, Xierali IM, and Broniatowski DA 2016. Decoupling of the minority PhD talent pool and assistant professor hiring in medical school basic science departments in the US. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]


