Summary
Rheumatoid arthritis (RA) and its animal model adjuvant arthritis (AA) are inflammatory diseases characterized by chronic inflammation, systemic oxidative stress and disturbed mitochondrial bioenergetics of skeletal muscle. The present study aimed to evaluate the effects of coenzyme Q10 – CoQ10 (100 mg/kg b.w.), omega-3-polyunsaturated fatty acids – ω-3-PUFA (400 mg/kg b.w.) and their combined treatment in AA on impaired skeletal muscle mitochondrial bioenergetics, inflammation and changes in levels CoQ9 and CoQ10 in plasma. Markers of inflammation (C-reactive protein, monocyte-chemotactic protein-1), antioxidant capacity of plasma, respiratory chain parameters of skeletal muscle mitochondria and concentrations of CoQ9 and CoQ10 in plasma and in muscle tissue were estimated. Treatment of the arthritic rats with CoQ10, ω-3-PUFA alone and in combination partially reduced markers of inflammation and increased antioxidant capacity of plasma, significantly increased concentrations of coenzyme Q in mitochondria and improved mitochondrial function in the skeletal muscle. Combined treatment has similar effect on the mitochondrial function as monotherapies; however, it has affected inflammation and antioxidant status more intensively than monotherapies. Long-term supplementary administration of coenzyme Q10 and ω-3-PUFA and especially their combination is able to restore the impaired mitochondrial bioenergetics and antioxidant status in AA.
Keywords: Arthritis, Coenzyme Q10, Omega-3-polyunsaturated fatty acids, Mitochondrial bioenergetics, Skeletal muscle
Introduction
Rheumatoid arthritis (RA) and its animal model – adjuvant arthritis (AA) are inflammatory diseases characterized by chronic inflammation, systemic oxidative stress and joint degeneration. The secondary deterioration of bone quality in RA – are mostly associated with structural changes to collagen, altered bone turnover, increased cortical porosity and damage to the trabecular and cortical microarchitecture (Zofkova and Nemcikova 2018). Alterations of mitochondrial function have an important role in inflammatory diseases. Mitochondria play a central role in ATP formation in the respiratory chain and in maintaining of redox homeostasis. Mitochondria are the major producers, but also targets of reactive oxygen species (ROS) in the cell. Oxidative damage of mitochondria may lead to the dysfunction of the respiratory chain, which further increases ROS formation. Oxidative stress processes are activated under pathological conditions and mitochondria are exposed also to reactive nitrogen species (Kohutiar et al. 2018). Thus, mitochondrial dysfunction can contribute to the development and pathogenesis of inflammatory human diseases (Lopez-Armada et al. 2013). The therapy of RA is an actual problem in clinical rheumatology due to the toxicity and side effects of antirheumatic drugs, therefore new treatment options are being sought (Tawfik 2015). Preservation of mitochondrial function can suppress oxidative stress and may represent a novel therapeutic approach in patients with inflammatory diseases. Weakness and muscle atrophy are the most common symptoms in RA patients (Miro et al. 1996). Ultrastructural alterations including disturbances of myofibrils and mitochondria were observed in muscle bioptic samples from patients affected by RA (De Palma et al. 2000). Due to the unique properties, coenzyme Q10 (CoQ10) can serve as a useful adjuvant in the management of arthritis. CoQ10 is irreplaceable in mitochondrial bioenergetics (Fig. 1), it participates as a cofactor of dehydrogenases in the transport of electrons and protons as well as in ATP production (Crane and Navas 1997) and could be beneficial in treatment of muscle weakness and atrophy.
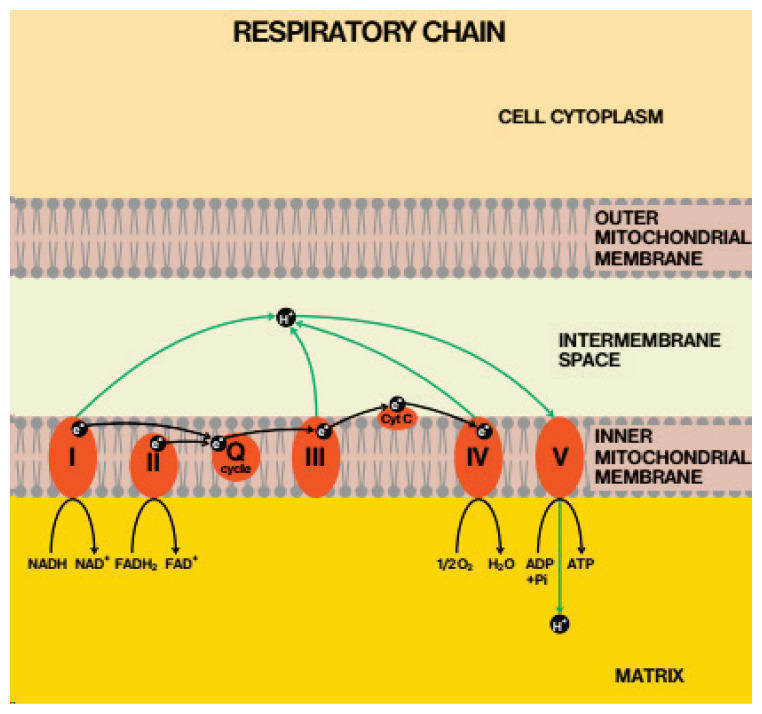
Fig. 1.
Localization of coenzyme Q in mitochondrial respiratory chain. With permission of Gvozdjakova A. (Editor), Mitochondrial Medicine. Mitochondrial metabolism, diagnosis and therapy. Springer, 2008 (Gvozdjakova 2008).
As in Figure 1 is shown, the respiratory chain (RC) located in the inner mitochondrial membrane is organized into five complexes (I, II, III, IV and V). The transport of electrons from NADH and FADH2 and production of electrochemical potential and proton gradients are necessary for synthesis of ATP. Electrons are carried out from complexes I and II to complex III by coenzyme Q (CoQ) which acts as a mobile component of RC. The special bioenergetic role of coenzyme Q action in the protonmotive Q-cycle has been described as a series of oxidation and reduction reactions of ubiquinol – ubiquinone (reduced – oxidized form of CoQ), (Mitchel 1991). Coenzyme Q (CoQ, ubiquinone) is the only lipophilic antioxidant to be biosynthesized, the main form in humans is coenzyme Q10 (CoQ10), but in rat’s coenzyme Q9 (CoQ9). Antiarthritic, antioxidant and antiinflammatory effects of CoQ10 were found in animal studies (Bauerova et al. 2005, Lee et al. 2013, Udhaya Lavinya et al. 2016, Li et al. 2017) and in RA patients (Abdollahzad et al. 2015). In our study with Fatsiphloginum and methotrexate we have shown the therapeutic advantage of combination therapy over monotherapy (Tsiklauri et al. 2019). Beneficial effect of CoQ10 in combination with methotrexate (MTX) was proved in adjuvant-induced arthritis when it suppressed arthritic progression in rats more effectively than MTX alone (Bauerova et al. 2010). Antiinflammatory and immunomodulatory activities for polyunsaturated ω-3 fatty acids (ω-3-PUFA) in RA have been demonstrated (Gioxari et al. 2018). The antiinflammatory properties of ω-3-PUFA are attributed to their interactions with the main inflammatory signaling pathways, suppressive effect on cytokines, prostaglandins and leukotriene formation. The regular dietary intake of ω-3-PUFA may improve the quality of life in patients with RA, however, further investigations are required (Lorente-Cebrian et al. 2015).
The purpose of this study was, to investigate in vivo effects of CoQ10, ω-3-PUFA and their combined treatment on skeletal muscle mitochondrial bioenergetics and antioxidant status in rat adjuvant arthritis, which according to our current knowledge, have not been studied yet. The markers of inflammation C-reactive protein (CRP) and monocyte-chemotactic protein-1 (MCP-1), concentrations of CoQ9, CoQ10 and antioxidant capacity of (AC) plasma were also evaluated.
Materials and Methods
Laboratory animals
Male Lewis rats were obtained from the Breeding Farm Dobra Voda (Slovakia) and housed five per cage under standard conditions with food and water ad libitum and a 12-hour-light/12-hour-dark cycle. The experimental protocol was approved by the Ethics Committee of Centre of Experimental Medicine of The Slovak Academy of Sciences (3144/16-221/3) and by the Slovak State Veterinary and Food Administration in accordance with the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes, and was also in accordance with Slovak legislation.
Induction of adjuvant arthritis
Adjuvant arthritis (AA) was induced to male Lewis rats weighing 160–180 g by a single intradermal injection of 0.1 ml suspension of heat-inactivated Mycobacterium butyricum (Difco Laboratories, Detroit, MI, USA) in incomplete Freund’s adjuvant at the base of the tail. The induction of AA and animal care was performed according to our previously published study (Slovak et al. 2017).
Experimental design and treatments
Rats were randomized into five groups in one experiment: a control group of animals (HC); a group of animals with adjuvant arthritis (AA); a group of animals with AA, which was administered 100 mg/kg CoQ10 (liquid liposomal CoQ10 – LiQSorb®, Tishcon Corp, USA) daily (AA-Q); a group of animals with AA, which was administered 400 mg/kg ω-3-PUFA daily (AA-ω); and a group of animals with AA, which was administered CoQ10 and ω-3-PUFA in the same doses and regimens as in monotherapy (AA-MIX). In each group, eight animals were used. The substances were administered orally by gavage during the whole experiment starting on first experimental day (induction of AA). On day 28, the animals were sacrificed under Zoletil/Xylazine anesthesia and blood for plasma preparations was withdrawn along with the skeletal muscles from each rat. All samples were stored at −80 °C until biochemical analysis.
Isolation of mitochondria
Mitochondria from hind paw skeletal muscle tissue were isolated by means of differential centrifugation according to slightly modified methods (Palmer et al. 1977, Koves et al. 2005). Tissue was minced and homogenized in the isolation medium containing 180 mM KCl, 4 mM EDTA, 20 mM Tris and 0.1 % of bovine serum albumin using a teflon-to-glass homogenizer at 4 °C. Protease type VIII (Sigma-Aldrich) in the dose 2.5 mg/g of the tissue was added into the isolation solution during mixing for 20 min. The homogenate was filtered through double-layered gauze and centrifuged at 700× g for 10 min. The supernatant was decanted and centrifuged twice at 5600× g for 10 min. The mitochondrial pellet was washed in isolation solution without albumin. The resulting pellet was resuspended in the same medium. All procedures were performed at 4 °C. Mitochondrial proteins were estimated by the method of Lowry (1951), the yields were 20–30 mg/1 ml suspension.
Markers of inflammation and antioxidant capacity of plasma
Markers of inflammation, such as C-reactive protein (CRP) and monocyte chemotactic protein-1 (MCP-1) were measured by ELISA. CRP was calculated in μg/ml, MCP-1 in pg/ml. For the determination of MCP-1 concentrations in plasma, an ELISA kit from eBioscience® (Waltham, MA, USA) was used. For the determination of plasmatic concentrations of interleukin CRP, an ELISA kit from R&D Systems Quantikine® (Minneapolis, MN, USA) was used. The assay procedures were applied as described in the product manuals. The results were calculated from the standard calibration curves on internal standards. Antioxidant capacity in plasma was determined using the Randox Total Antioxidant kit with colorimetric detection at 600 nm and calculated in mmol/l according to the manufacturer instructions.
Measurement of mitochondrial function
Respiratory function of mitochondria was determined at 30 °C by amperometric monitoring of oxygen consumption on an oxygraph Gilson 5/6 H (France) equipped with a Clark-type oxygen electrode. The incubation medium was prepared as described by (Rouslin and Millard 1980) with a modification: 122 mM KCl, 3 mM KH2PO4, 0.5 mM EDTA, 12.5 mM HEPES, and 2 % dextrane. NAD-substrate pyruvate/malate (final concentrations 5+5 mM) and FAD-substrate succinate (final concentration 10 mM) with rotenone as complex I inhibitor were used. For assessing stimulated oxygen consumption, 500 nmol of ADP was added. The following parameters of mitochondrial oxidative phosphorylation (OXPHOS) were determined: the rate of oxygen uptake by the mitochondria stimulated with ADP – state 3 (S3) expresses the velocity of oxygen consumption by mitochondria in the presence of ADP and substrate; the rate of basal oxygen uptake by mitochondria without ADP – state 4 (S4) denotes how fast oxygen is used by mitochondria in the presence of substrate only; the oxidative phosphorylation rate (OPR) determines the rate of ATP generation in state 3; coefficient of oxidative phosphorylation (ADP:O) indicates the relationship between ATP synthesis and oxygen consumption; respiratory control ratio (RCR) – ratio of state 3 to state 4, represents the mitochondrial integrity (Estabrook 1967).
Determination of coenzyme Q9 and Q10 in plasma, skeletal muscle mitochondria and skeletal muscle tissue
Concentrations of CoQ9 and CoQ10 were measured by HPLC method, using isocrating pump Alpha 10 and variable wavelength detector Sapphire (both ECOM Ltd, Czech Rep.). Total CoQ9 and CoQ10 concentrations (oxidized + reduced forms) in plasma were measured after oxidation with 1,4-benzoquinone (Merck), according to Mosca (2002). Plasma extraction was performed according to Lang (1986) with some modifications. Five-hundred microliters of plasma was supplemented with 100 μl 1,4-benzoquinone solution (2 mg/ml double distilled water) and vortexed for 10 s. After 10 min 2 ml of the mixture hexane/ethanol (5/2 v/v, Merck) was added, shaken for 5 min and centrifuged. The hexane layer was separated, and extraction procedure was repeated with 1 ml of the extraction mixture. Collected organic layers were evaporated under nitrogen at 50 °C. The residues were taken up in 99.9 % ethanol (Merck) and injected into Separon SGX C18 7 m 3×150 mm column (Tessek). Elution was performed with methanol/acetonitrile/ethanol (6/2/2, v/v/v). Processing of mitochondrial suspension and tissue was similar (Lang et al. 1986). Mitochondrial suspension (50 μl) was extracted with an addition of 1 N hydrochloric acid and extraction mixture. Tissue (approx. 100 mg) was homogenized using an Ultra-Turrax in 1 ml of redistilled water with an addition of 50 μl of t-butylhydroxytoluene (BHT, 10 mg/1 ml of 99.9 % ethanol). Homogenate was extracted by hexane/ethanol mixture (5/2, v/v) with an addition of 1 ml of 0.1 M sodium dodecyl sulphate (SDS). Concentrations of CoQ9 and CoQ10 were detected spectrophotometrically at 275 nm, using external standards (Sigma) with three points calibration curves. Retention time for CoQ9 was about 13 min, for CoQ10 20 min. Data were collected and processed using CSW 32 chromatographic station (DataApex Ltd). Concentrations were calculated: in the plasma in μmol/l, in the mitochondria in nmol/mg of proteins, in the tissue in nmol/g wet weight.
Statistical analyses
Mean and SEM values were calculated for each parameter in each group (eight animals in each experimental group). Statistically significant differences among treated, untreated, and control groups were tested using parametric Analysis of Variance (ANOVA). Post hoc tests (Tukey-Kramer (ANOVA)) were applied in situations where differences among groups were significant at the level of significance α=0.05. After post hoc testing, the following significance levels were specified: extremely significant (p<0.001), very significant (p<0.01), significant (p<0.05), and not significant (p>0.05). The untreated arthritis group was compared with healthy control animals (*), the treated arthritis groups were compared with untreated arthritic animals (#).
Results
Changes during of adjuvant arthritis (AA)
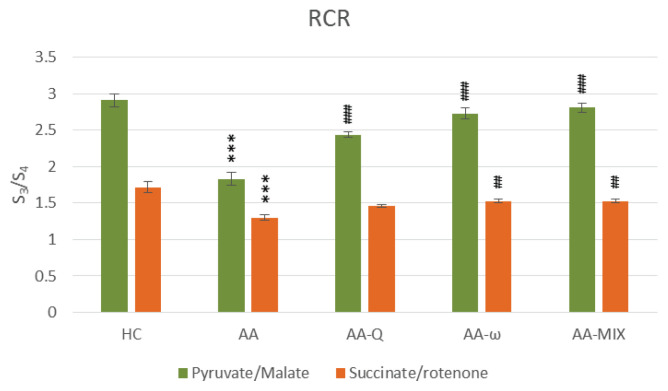
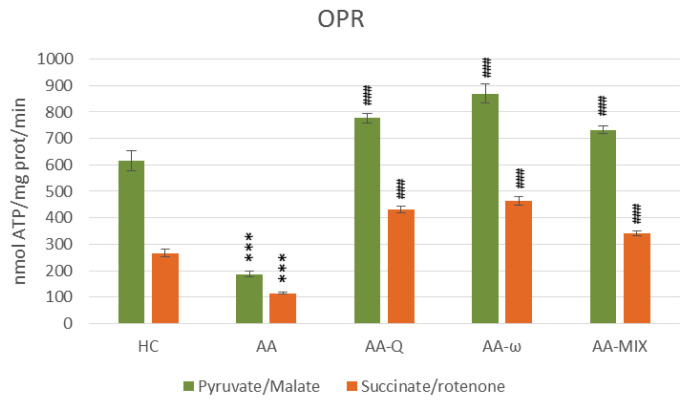
Arthritic rats (AA-group) showed significantly increased markers of inflammation, arthritic score, CRP and MCP-1, and decreased antioxidant capacity (AC), (Table 1). Oxidative phosphorylation parameters of skeletal muscle mitochondria were reduced using both substrates – pyruvate/malate and succinate/rotenone, indicating damage to complexes I and II of mitochondrial respiratory chain (p<0.001), (Figs 2 and 3). Reduced parameters of mitochondrial oxidative phosphorylation were accompanied by decreased concentrations of coenzyme Q9 and coenzyme Q10 in mitochondria (p<0.05). Coenzyme Q concentrations in skeletal muscle tissue were not affected significantly in arthritic rats. Total CoQ9 in plasma was stimulated on day 28 of adjuvant arthritis, CoQ10-TOT concentration was not changed (Table 2).
Table 1.
Markers of inflammation and antioxidant capacity of plasma.
| HC | AA | AA-Q | AA-ω | AA-MIX | |
|---|---|---|---|---|---|
| CRP in plasma (μg/ml) | 457.4±21.3 | 602.2±10.7** | 563.3±14.5 | 618.8±29.1 | 535.7±13.8 |
| MCP-1 in plasma (pg/ml) | 1462±159 | 2925±389** | 2539±144 | 2517±526 | 2481±236 |
| AC in plasma (mmol/l) | 0.673±0.037 | 0.529±0.028* | 0.562±0.033 | 0.565±0.044 | 0.626±0.048 |
| Arthritic score (points) | 8±0 | 22.1±0.824*** | 21.2±0.416 | 22.56±0.412 | 20.11±0.735 |
CRP (C-reactive protein), MCP-1 (monocyte chemotactic protein, AC (antioxidant capacity) and arthritic score. Data are expressed as mean ± standard error of the mean.
p<0.05,
p<0.01 and
p<0.001 vs. HC.
Number of rats in each group was 8. HC: healthy control group, AA: control arthritic animals, AA-Q: arthritic animals administered with CoQ10, AA-ω: arthritic animals administered with ω-3-polyunsaturated fatty acids, AA-mix: arthritic animals administered with CoQ10 and with ω-3-polyunsaturated fatty acids.
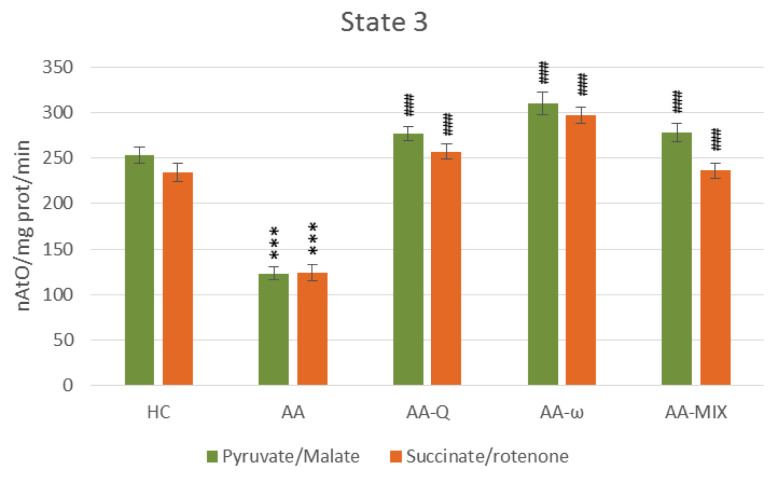
Fig. 2.
State 3 – ADP stimulated respiration of skeletal muscle mitochondria with pyruvate/malate and succinate/rotenone as a substrate. Data are expressed as mean ± standard error of the mean. *** p<0.001 vs. HC, ### p<0.001 vs. AA. Number of rats in each group was 8. HC: healthy control group, AA: control arthritic animals, AA-Q: arthritic animals administered with CoQ10, AA-ω: arthritic animals administered with ω-3-polyunsaturated fatty acids, AA-mix: arthritic animals administered with CoQ10 and with ω-3-polyunsaturated fatty acids.
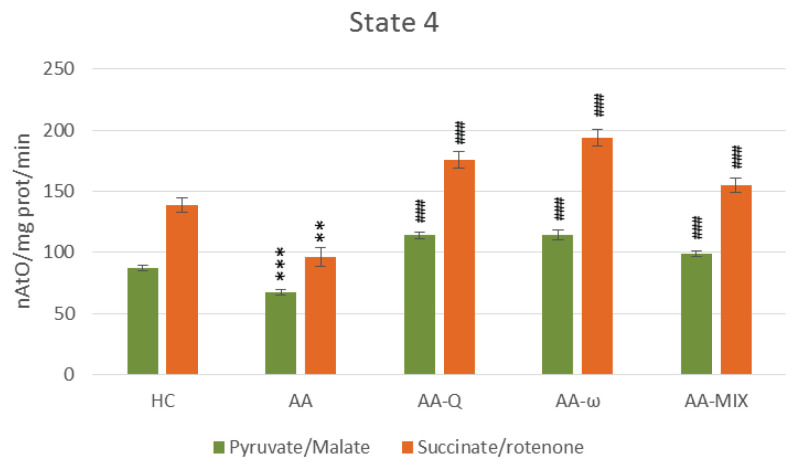
Fig. 3.
State 4 – basal respiration of skeletal muscle mitochondria with pyruvate/malate and succinate/rotenone as a substrate. Data are expressed as mean ± standard error of the mean. ** p<0.01 and *** p<0.001 vs. HC, ### p<0.001 vs. AA. Number of rats in each group was 8. Experimental groups see in Figure 2 legend.
Table 2.
Coenzyme Q9 and Q10 concentrations in plasma, skeletal muscle mitochondria and tissue.
| HC | AA | AA-Q | AA-ω | AA-MIX | |
|---|---|---|---|---|---|
| Plasma | |||||
|
| |||||
| CoQ 9-TOT | 0.328±0.023 | 0.468±0.044* | 0.237±0.016### | 0.336±0.024# | 0.243±0.021### |
| CoQ 10-TOT | 0.031±0.004 | 0.027±0.003 | 0.804±0.069### | 0.030±0.007 | 0.786±0.097### |
|
| |||||
| Mitochondria | |||||
|
| |||||
| CoQ 9-OX | 3.28±0.138 | 2.67±0.128* | 3.16±0.082 | 3.37±0.159## | 3.26±0.121# |
| CoQ 10-OX | 0.144±0.008 | 0.126±0.007* | 0.149±0.004# | 0.156±0.008## | 0.152±0.007## |
|
| |||||
| Tissue | |||||
|
| |||||
| CoQ 9-OX | 43.1±3.01 | 32.7±2.49 | 40.9±4.07 | 40.8±3.80 | 36.0±3.12 |
| CoQ 10-OX | 1.90±0.160 | 1.63±0.187 | 2.43±0.252 | 2.06±0.197 | 1.86±0.190 |
Concentrations of CoQ9 and, CoQ10: plasma (in μmol/l), mitochondria (in nmol/mg of proteins), tissue (in nmol/g wet weight). Data are expressed as mean ± standard error of the mean.
p<0.05, vs. HC,
p<0.05,
p<0.01,
p<0.001 vs. AA.
Number of rats in each group was 8. Experimental groups see in Table 1 legend.
Effects of coenzyme Q10 treatment (AA-Q)
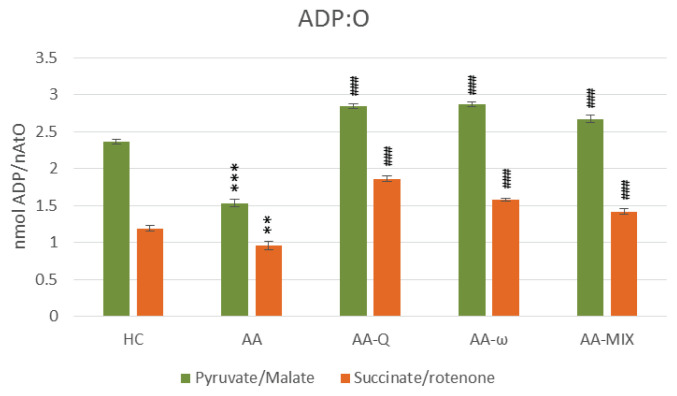
Treatment of arthritic rats with CoQ10 (AA-Q group) for 28 days slightly reduced markers of inflammation and increased AC, although not statistically significant (Table 1). Parameters of OXPHOS – stimulated mitochondrial respiration (State 3 – Fig. 2), basal respiration (State 4 – Fig. 3), rate of ATP generation, efficiency of OXPHOS (ADP:O – Fig. 5) and respiratory control ratio (RCR – Fig. 6) – were improved extremely significantly (p<0.001) in comparison to arthritic rats (AA), the effects were similar using both substrates (Fig. 2–6). Concentration of CoQ10-OX in mitochondria increased significantly, CoQ9-OX not significantly. Treatment with CoQ10 increased its concentrations in plasma extremely significantly (from 0.031 to 0.804 μmol/l, p<0.001), demonstrating the high bioavailability of CoQ10 administered. Elevated concentration of CoQ9-TOT in plasma was corrected (Table 2).
Fig. 5.
ADP:O – coefficient of oxidative phosphorylation of skeletal muscle mitochondria with pyruvate/malate and succinate/rotenone as a substrate. Data are expressed as mean ± standard error of the mean. ** p<0.01 and *** p<0.001 vs. HC, ### p<0.001 vs. AA. The number of rats in each group was 8. Experimental groups see in Figure 2 legend.
Fig. 6.
RCR – respiratory control ratio (S3/S4) of skeletal muscle mitochondria with pyruvate/malate and succinate/rotenone as a substrate. Data are expressed as mean ± standard error of the mean. *** p<0.001 vs. HC, ## p<0.01 and ### p<0.001 vs. AA. Number of rats in each group was 8. Experimental groups see in Figure 2 legend.
Fig. 4.
OPR – oxidative phosphorylation rate of skeletal muscle mitochondria with pyruvate/malate and succinate/rotenone as a substrate. Data are expressed as mean ± standard error of the mean. ***p<0.001 vs. HC, ### p<0.001 vs. AA. Number of rats in each group was 8. Experimental groups see in Figure 2 legend.
Effects of ω-3-PUFA treatment (AA-ω)
Treatment with ω-3-PUFA (AA-ω) for 28 days partially corrected inflammatory markers and increased AC, however, the changes were not statistically significant (Table 1). Mitochondrial respiration and ATP generation in complexes I and II were improved extremely significantly by the treatment (p<0.001), (Fig. 2–6). Mitochondrial CoQ9-OX and CoQ10-OX concentrations were increased (Table 2). Tissue content of CoQ was not affected. Elevated CoQ9-TOT in plasma of AA rats was corrected by ω-3-PUFA treatment, similarly to control rats (Table 2).
Effects of the combined treatment with CoQ10 and ω-3-PUFA (AA-MIX)
Combined treatment with CoQ10 and ω-3-PUFA (AA-MIX) slightly decreased inflammatory markers. AC increased near to healthy controls, to the limit of statistical significance (p=0.121), (Table 1). Similarly, to the administration of substances alone, combination of CoQ10 and ω-3-PUFA stimulated function of skeletal muscle mitochondria in arthritic rats extremely significantly (p<0.001), (Fig. 2–6). Mitochondrial CoQ9-OX and CoQ10-OX concentrations were elevated in comparison to AA rats (p<0.05 and p<0.01, respectively), the values were comparable to controls (Table 2). Content of both forms of coenzyme Q in muscle tissue was not changed significantly in comparison to arthritic as well as to control rats. Bioavailability of CoQ10 was confirmed by its increased concentrations in plasma (from 0.031 to 0.786 μmol/l, p<0.001), comparable to the rats treated with CoQ10 alone. MIX administration corrected CoQ9-TOT in plasma similarly as CoQ10 administration alone (p<0.001), (Table 2).
Discussion
Rheumatoid arthritis (RA) is a systemic inflammatory disease affecting about 1 % of the world’s population. Mitochondria have an important role in proinflammatory signaling, but on the other hand, proinflammatory mediators may alter mitochondrial function. These processes increase mitochondrial oxidative stress (OS) and impair cellular energy metabolism (Lopez-Armada et al. 2013, Balogh et al. 2018, van der Woude et al. 2018) A current study unravels the changes in mitochondria of RA patients. Results depict dysfunction in basic mitochondrial activities, which may be a reason for abrupt functioning of immune cells, leading to autoimmunity in RA patients (Khanna et al. 2017). Similarly, in this experiment we have found significantly decreased ADP stimulated respiration, basal respiration and oxidative phosphorylation rate in AA. These respiratory parameters of skeletal muscle mitochondria were corrected by the treatment with CoQ10 and ω-3-PUFAs. The treatment not only improved the worsened parameters induced by arthritis but even elevated them in comparison to control values. We assume that supplementation with CoQ10 causes its incorporation into the respiratory chain and thus improves oxidative phosphorylation. Supplementation with ω-3-PUFA may contribute to the repair of mitochondrial membranes damaged by oxidative stress.
Deficient antioxidant system contributes to OS that causes damage to lipids and proteins (Phull et al. 2018). Adjuvant arthritis (AA) in rats was manifested with decreased superoxide dismutase (SOD) activity and antioxidant capacity, increased lipid peroxidation in serum and stimulated mitochondrial oxygen species (mtROS) generation in mitochondria, leading to the loss of mitochondrial membrane potential and mitochondrial dysfunction in fibroblasts-like synoviocytes isolated from synovial tissue (Zhang et al. 2016). Our results also showed a reduced total antioxidant status in plasma of arthritic rats (Table 1) in spite of increased concentration of total CoQ9 in plasma (Table 2) which is regarded as a protective response against oxidative damage in the state of increased generation of free radicals associated with arthritis and was found also in experimental model of diabetes mellitus (Kucharska et al. 2000).
In our experiment we showed the ability of CoQ10 and ω-3-PUFA to improve the mitochondrial dysfunction caused by inflammatory processes in AA. Coenzyme Q10 as an essential component of respiratory chain plays an important role in mitochondrial bioenergetics and reducing mitochondrial OS. Previously we found deficit of coenzyme Q in heart and liver mitochondria in rats with streptozotocin-induced diabetes, which is associated with OS and increased lipid peroxidation similarly to inflammatory processes (Kucharska et al. 2000). Another study showed that administration of CoQ10 in combination with ω-3-PUFA prevented the decrease of respiratory chain function in brain mitochondria of streptozotocin-induced diabetic rats (Sumbalova et al. 2005). CoQ10 alone did not improve ATP production in diabetic rats despite its increased concentration in brain mitochondria. Beneficial effect of CoQ10 supplementation on inflammatory cytokines and OS in RA patients was reported (Abdollahzad et al. 2015). Results from human studies suggest that a high content of ω-3-PUFAs in the diet could have a protective role for incidence of RA and could also represent a promising therapeutic option (Navarini et al. 2017). Antiinflammatory and immunomodulatory activities for ω-3-PUFA have been demonstrated in meta-analysis study of 20 randomised controlled trials of RA patients. Consumption of ω-3 fatty acids was found to significantly improve eight disease-activity-related markers (Gioxari et al. 2018). It is well established that ω-3-PUFAs are substrates for synthesis of novel series of lipid mediators (e.g. resolvins, protectins, and maresins) with potent antiinflammatory and proresolving properties, which have been proposed to partly mediate the protective and beneficial actions of ω-3-PUFAs. The regular dietary intake of ω-3-PUFA may improve the quality of life in patients with RA (Lorente-Cebrian et al. 2015). However, there are also fewer studies, in which ω-3-PUFA failed to improve the status of RA patients. In a double-blind, placebo-controlled and randomized study of alpha-linolenic acid administration, during 3 months the patients showed no improvement in assessed markers of RA (Nordstrom et al. 1995). Another double-blind, placebo-controlled study in RA patients did not show superior clinical benefit of daily nutrient supplementation with eicosapentanoic acid, gamma-linoleic acid and micronutrients at the doses tested as compared to placebo (Remans et al. 2004). In our study we found out that ω-3-PUFA treatment stimulated mitochondrial respiration and rapidity of ATP generation in skeletal muscle mitochondria of arthritic rats. The parameters were comparable to controls as well as to the treatment with CoQ10. Mechanisms of the ω-3-PUFA on mitochondrial function can include modulation of the mitochondrial membrane structure as well as metabolic gene regulation, independent of changes in structural membrane organization (Sullivan et al. 2018). Unsaturated fatty acids act as ligands for PPARs, a superfamily of nuclear transcription factors responsible for upregulating genes that influence mitochondria and peroxisome function. Although ω-3-PUFAs can increase ROS production within the inner mitochondrial membrane, they also increase antioxidant capacity and diminish inflammation (Giordano and Visoli 2014). Dietary ω-3-PUFAs induced favorable adaptations within skeletal muscle in older adults, decreased mitochondrial ROS production and increased muscle protein synthesis after exercise (Lalia et al. 2017). Increased mitochondrial coenzyme Q concentration found in our study following the treatment with ω-3-PUFA may result from intracellular antioxidant activities of ω-3-PUFA and positive modulation of mitochondrial membrane structure and function. The effects of combined treatment of arthritic rats with CoQ10 and ω-3-PUFA on the parameters of oxidative phosphorylation did not differ significantly from the effects when administered alone.
After 4 weeks of administration of CoQ10, ω-3-PUFA and their combination, the parameters of mitochondrial bioenergetics were significantly improved. However, inflammatory markers and antioxidant capacity have been corrected only mildly. It is possible that, administration of these compounds few weeks before the disease induction (pre-treatment) will saturate the cells and mitochondrial membranes with CoQ10 and ω-3-PUFA, thus a better therapeutic effect could be achieved.
Conclusions
Antioxidant, antiinflammatory and immunomodulatory properties of the administered substances together with improving bioenergetics of mitochondria may be involved in mechanisms of their beneficial effects in arthritis. We assume that long-term supplementary administration of coenzyme Q10 and ω-3-PUFAs as well as their simultaneous administration to patients with rheumatoid arthritis could be beneficial in terms of influencing the progression of the disease.
Acknowledgements
For technical assistance we thank to Anna Štetková, Jana Urgošová, Danica Mihalová, Karol Švík and Miloslav Zloh. The experiment was supported by the Slovak grant agency: APVV-15-0308, VEGA 2/0136/20 and VEGA 2/0115/19.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- ABDOLLAHZAD H, AGHDASHI MA, ASGHARI JAFARABADI M, ALIPOUR B. Effects of coenzyme Q10 supplementation on inflammatory cytokines (TNF-α, IL-6) and oxidative stress in rheumatoid arthritis patients: A randomized controlled trial. Arch Med Res. 2015;46:527–533. doi: 10.1016/j.arcmed.2015.08.006. [DOI] [PubMed] [Google Scholar]
- BALOGH E, VEALE DJ, McGARRY T, ORR C, SZEKANECZ Z, NG CT, FEARON U, BINIECKA M. Oxidative stress impairs energy metabolism in primary cells and synovial tissue of patients with rheumatoid arthritis. Arthritis Res Ther. 2018;20:95. doi: 10.1186/s13075-018-1592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUEROVA K, KUCHARSKA J, MIHALOVA D, NAVAROVA J, GVOZDJAKOVA A, SUMBALOVA Z. Effect of coenzyme Q (10) supplementation in the rat model of adjuvant arthritis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005;149:501–503. doi: 10.5507/bp.2005.090. [DOI] [PubMed] [Google Scholar]
- BAUEROVA K, PAULOVICOVA E, MIHALOVA D, DRAFI F, STROSOVA M, MASCIA C, BIASI F, ROVENSKY J, KUCHARSKA J, GVOZDJAKOVA A, PONIST S. Combined methotrexate and coenzyme Q10 therapy in adjuvant-induced arthritis evaluated using parameters of inflammation and oxidative stress. Acta Biochemica Polonica. 2010;57:347–354. doi: 10.18388/abp.2010_2415. [DOI] [PubMed] [Google Scholar]
- CRANE FL, NAVAS P. The diversity of coenzyme Q function. Mol Aspects Med. 1997;18:1–6. doi: 10.1016/S0098-2997(97)00016-2. [DOI] [PubMed] [Google Scholar]
- De PALMA L, CHILLEMI C, ALBANELLI S, RAPALI S, BERTONI-FREDDARI C. Muscle involvement in rheumatoid arthritis: an ultrastructural study. Ultrastruct Pathol. 2000;24:151–156. doi: 10.1080/01913120050132886. [DOI] [PubMed] [Google Scholar]
- ESTABROOK RW. Mitochondrial respiratory control and the polarographic measurement of ADP:O ratios. In: ESTABROOK RW, PULLMAN ME, editors. Methods in Enzymology. Academic Press; New York and London: 1967. pp. 41–47. [DOI] [Google Scholar]
- GIORDANO E, VISIOLI F. Long-chain omega 3 fatty acids: Molecular bases of potential antioxidant actions. Prostaglandins Leukot Essent Fatty Acids. 2014;90:1–4. doi: 10.1016/j.plefa.2013.11.002. [DOI] [PubMed] [Google Scholar]
- GIOXARI A, KALIORA AC, MARANTIDOU F, PANAGIOTAKOS DP. Intake of ω-3 polyunsaturated fatty acids in patient with rheumatoid arthritis: A systemic review and meta-analysis. Nutrition. 2018;45:114–124. doi: 10.1016/j.nut.2017.06.023. [DOI] [PubMed] [Google Scholar]
- GVOZDJAKOVA A. Mitochondrial physiology. In: GVOZDJAKOVA A, editor. Mitochondrial Medicine. Springer Science + Business Media; Germany: 2008. pp. 1–16. [DOI] [Google Scholar]
- KHANNA S, TRIPATHY A, PADHAN P, GUPTA B. Unravelling mitochondrial dysfunction in rheumatoid arthritis patients. Can J Biotech. 2017;1:95. doi: 10.24870/cjb.2017-a82. [DOI] [Google Scholar]
- KOHUTIAR M, ECKHARDT A, MIKŠÍK I, ŠANTOROVÁ P, WILHELM J. Proteomic analysis of peroxynitrite-induced protein nitration in isolated beef heart mitochondria. Physiol Res. 2018;67:239–250. doi: 10.33549/10.33549/physiolres.933608. [DOI] [PubMed] [Google Scholar]
- KOVES TR, NOLAND RC, BATES AL, HENES ST, MUOIO DM, CORTRIGHT RN. Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am J Physiol Cell Physiol. 2005;288:C1074–C1082. doi: 10.1152/ajpcell.00391.2004. [DOI] [PubMed] [Google Scholar]
- KUCHARSKA J, BRAUNOVA Z, ULICNA O, ZLATOS L, GVOZDJAKOVA A. Deficit of coenzyme Q in heart and liver mitochondria of rats with streptozotocin-induced diabetes. Physiol Res. 2000;49:411–418. [PubMed] [Google Scholar]
- LALIA AZ, DASARI S, ROBINSON MM, ABID H, MORSE DM, KLAUS KA, LANZA IR. Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging (Albany NY) 2017;9:1096–1129. doi: 10.18632/aging.101210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANG JK, GOHIL K, PACKER L. Simultaneous determination of tocopherols, ubiquinols, and ubiquinones in blood, plasma, tissue homogenates, and subcellular fractions. Anal Biochem. 1986;157:106–116. doi: 10.1016/0003-2697(86)90203-4. [DOI] [PubMed] [Google Scholar]
- LEE J, HONG YS, JEONG JH, YANG EJ, JHUN JY, PARK MK, JUNG YO, MIN JK, KIM HY, PARK SH, CHO ML. Coenzyme Q10 ameliorates pain and cartilage degradation in a rat model of osteoarthritis by regulating nitric oxide and inflammatory cytokines. PLoS One. 2013;8:e69362. doi: 10.1371/journal.pone.0069362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI X, GUO Y, HUANG S, HE M, LIU Q, CHEN W, LIU M, XU D, HE P. Coenzyme Q10 prevents the interleukin-1 beta induced inflammatory response via inhibition of MAPK signaling pathways in rat articular chondrocytes. Drug Dev Res. 2017;78:403–410. doi: 10.1002/ddr.21412. [DOI] [PubMed] [Google Scholar]
- LOPEZ-ARMADA MJ, RIVEIRO-NAVEIRA RR, VAAMONDE-GARCIA C, VALCARCEL-ARES MN. Mitochondrial dysfunction and the inflammatory response. Mitochondrion. 2013;13:106–118. doi: 10.1016/j.mito.2013.01.003. [DOI] [PubMed] [Google Scholar]
- LORENTE-CEBRIAN S, COSTA AG, NAVAS-CARRETERO S, ZABALA M, LAIGLESIA LM, MARTINEZ JA, MORENO-ALIAGA MJ. An update on the role of omega-3 fatty acids on inflammatory and degenerative diseases. J Physiol Biochem. 2015;71:341–349. doi: 10.1007/s13105-015-0395-y. [DOI] [PubMed] [Google Scholar]
- LOWRY DH, ROSENBROUGH NY, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–276. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- MIRO O, PEDROL E, CASADEMONT J, GARCIA-CARRASCO M, SANMARTI R, CEBRIAN M, GRAN JM. Muscle involvement in rheumatoid arthritis: clinicopathological study of 21 symptomatic cases. Semin Arthritis Rheum. 1996;25:421–428. doi: 10.1016/S0049-0172(96)80007-2. [DOI] [PubMed] [Google Scholar]
- MITCHEL P. The vital protonmotive role of coenzyme Q. In: FOLKERS K, LITTARRU GP, YAMAGAMI T, editors. Biomedical and Clinical Aspects of Coenzyme Q. Vol. 6. Amsterdam: Elsevier Science Publishers BV; 1991. pp. 3–10. [Google Scholar]
- MOSCA F, FATTORINI D, BOMPADRE S, LITTARRU GP. Assay of coenzyme Q10 in plasma by a single dilution step. Anal Biochem. 2002;305:49–54. doi: 10.1006/abio.2002.5653. [DOI] [PubMed] [Google Scholar]
- NAVARINI L, AFELTRA A, GALLO AFFLITO G, MARGIOTTA DPE. Polyunsaturated fatty acids: any role in rheumatoid arthritis. Lipids Health Dis. 2017;16:197. doi: 10.1186/s12944-017-0586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORDSTROM DC, HONKANEN VE, NASU Y, ANTILA E, FRIMAN C, KONTTINEN YT. Alpha-linolenic acid in the treatment of rheumatoid arthritis. A double-blind, placebo-controlled and randomized study: flaxseed vs. safflower seed. Rheumatol Int. 1995;14:231–234. doi: 10.1007/BF00262088. [DOI] [PubMed] [Google Scholar]
- PALMER JW, TANDLER B, HOPPEL CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–8739. doi: 10.1016/S0021-9258(19)75283-1. [DOI] [PubMed] [Google Scholar]
- PHULL AR, NASIR B, HAQ IU, KIM SJ. Oxidative stress, consequences and ROS mediated cellular signalling in rheumatoid arthritis. Chem Biol Interactions. 2018;281:121–136. doi: 10.1016/j.cbi.2017.12.024. [DOI] [PubMed] [Google Scholar]
- REMANS PH, SONT JK, WAGENAAR LW, WOUTERS-WESSELING W, ZUIJDERDUIN WM, JONGMA A, BREEDVELD FC, Van LAAR JM. Nutrient supplementation with polyunsaturated fatty acids and micronutrients in rheumatoid arthritis: clinical and biochemical effects. Eur J Clin Nutr. 2004;58:839–845. doi: 10.1038/sj.ejcn.1601883. [DOI] [PubMed] [Google Scholar]
- ROUSLIN W, MILLARD RW. Canine myocardial ischemia: Defect in mitochondrial electron transfer complex I. J Mol Cell Cardiol. 1980;12:639–645. doi: 10.1016/0022-2828(80)90021-8. [DOI] [PubMed] [Google Scholar]
- SLOVAK L, SVIK K, MIHALOVA D, TOTH J, CZIGLE S, PASKOVA L, BILKA F, BAUEROVA K. Ferulaldehyde improves the effect of methotrexate in experimental arthritis. Molecules. 2017;22:1911. doi: 10.3390/molecules22111911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULLIVAN EM, PENNINGTON ER, GREEN WD, BECK MA, BROWN DA, SHAIKH SR. Mechanisms by which dietary fatty acids regulate mitochondrial structure-function in health and disease. Adv Nutr. 2018;9:247–262. doi: 10.1093/advances/nmy007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUMBALOVA Z, KUCHARSKA J, KASPAROVA S, MLYNARIK V, BYSTRICKY P, BOZEK P, ULICNA O, VANCOVA O, SINGH RB, GVOZDJAKOVA A. Brain energy metabolism in experimental chronic diabetes: effect of long-term administration of coenzyme Q10 and ω-3 polyunsaturated fatty acids. Biologia. 2005;60:105–108. [Google Scholar]
- TAWFIK MK. Combination of coenzyme Q10 with methotrexate suppresses Freund’s complete adjuvant-induced synovial inflammation with reduced hepatotoxicity in rats: Effect on oxidative stress and inflammation. Int Immunopharmacol. 2015;24:80–87. doi: 10.1016/j.intimp.2014.11.018. [DOI] [PubMed] [Google Scholar]
- TSIKLAURI L, DRÁFI F, PONIŠT S, SLOVÁK L, CHRASTINA M, ŠVÍK K, KEMOKLIDZE Z, KEMERTELIDZE E, BAUEROVÁ K. Study of anti-inflammatory activity of Fatsiphloginum™ (Fatsia japonica) and a new purified triterpene-rich extract of saponins (PS-551) in experimental model of arthritis. Physiol Res. 2019;68(Suppl 1):S75–S85. doi: 10.33549/physiolres.934328. [DOI] [PubMed] [Google Scholar]
- UDHAYA LAVINYA B, BARDHAN I, EVAN PRINCE S. Efficacy of coenzyme Q10 in inhibiting monosodium urate crystal-induced inflammation in rats. Eur J Pharmacol. 2016;791:589–594. doi: 10.1016/j.ejphar.2016.09.036. [DOI] [PubMed] [Google Scholar]
- Van der WOUDE D, Van der HELM-Van MIL AHM. Update of the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2018;32:174–187. doi: 10.1016/j.berh.2018.10.005. [DOI] [PubMed] [Google Scholar]
- ZHANG J, SONG S, CAO W, LU J, WANG X, WANG G, WANG Z, CHEN X. Autophagy and mitochondrial dysfunction in adjuvant-arthritis rats’ treatment with resveratrol. Sci Rep. 2016;6:32928. doi: 10.1038/srep32928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOFKOVA I, NEMCIKOVA P. Osteoporosis complicating some inborn or acquired diseases. Physiol Res. 2018;67(Suppl 3):S441–S454. doi: 10.33549/physiolres.934027. [DOI] [PubMed] [Google Scholar]