Summary
Transforming growth factor beta 1 (TGF-β1) is a pro-fibrotic cytokine with a key role in wound repair and regeneration, including induction of fibroblast-to-myofibroblast transition. Genistein is a naturally occurring selective estrogen receptor modulator with promising anti-fibrotic properties. In the present study we aimed to investigate whether genistein modulates TGF-β1 (canonical and non-canonical) signaling in normal dermal fibroblasts at the protein level (Western blot and immunofluorescence). We demonstrated that TGF-β1 induces the myofibroblast-like phenotype in the studied fibroblast signaling via canonical (SMAD) and non-canonical (AKT, ERK1/2, ROCK) pathways. Genistein induced only ERK1/2 expression, whereas the combination of TGF-β1 and genistein attenuated the ERK1/2 and ROCK signaling. Of note, the other studied pathways remained almost unaffected. From this point of view, genistein does not impair conversion of normal fibroblasts to myofibroblast-like cells.
Keywords: Wound healing, Selective estrogen receptor modulator, Phytoestrogen
Ageing is associated with deterioration in physical condition (Hill et al. 2020) also related to estrogen deprivation during menopause. Estrogens have been shown to modulate a variety of biological processes (Herichova et al. 2019, Herichova et al. 2020) including wound healing (Ashcroft et al. 1997). In particular the age-related reduced rate of wound healing is associated with improved quality of scarring and reduced levels of transforming growth factor beta 1 (TGF-β1). TGF-β1 is a pro-fibrotic cytokine with a key role in wound repair and regeneration (Klass et al. 2009). TGF-β1 is transiently up-regulated in normal skin wounds, whereas its ability to induce fibroblast-to-myofibroblast conversion is crucial for the wound closure (Lichtman et al. 2016), even though myofibroblasts never represent the dominating cell population in contracting human wounds (Berry et al. 1998). Characteristically, these cells express α-smooth muscle actin (α-SMA) and secrete a variety of regulatory/signaling molecules, and they also actively participate in production and organization of the extracellular matrix (ECM) (Pakshir et al. 2020). TGF-β1 signaling occurs either via canonical (SMAD) or non-canonical (phosphoinositide-3-kinase (PI3K/AKT), mitogen activated protein kinases (MAPK/ERK1/2), Rho GTPase (ROCK) and p53) pathways (Shi et al. 2020) with the presence of further possible cross-talks (Derynck et al. 2003, Zhang 2003).
It has been shown that genistein (GEN), a naturally occurring isoflavone and selective estrogen receptor modulator (Diel et al. 2001), may act as an anti-fibrotic agent (Andugulapati et al. 2020, Ning et al. 2020) and effective skin wound healing modulator (Emmerson et al. 2010, Marini et al. 2010). Estrogen receptor (ER) signaling attenuates TGF-β1-induced activation of Sma and MAD-related protein 3 (SMAD3), whereas TGF-β1 signaling increases ER-mediated transcription activity (Matsuda et al. 2001). Since myofibroblasts persist in chronic inflammatory and fibroproliferative diseases, they contribute to the disease progress (Cannito et al. 2017). Thus, we were interested to answer the question whether genistein as a potential anti-fibrotic molecule also modulates the TGF-β1-induced fibroblast-to-myofibroblast conversion in cells isolated from the normal healthy skin.
HDFs were isolated (Dvorankova et al. 2019) from two healthy donors with the informed consent of the patient and Ethical Committee of the Third Faculty of Medicine, Charles University in Prague approval following the Helsinki declaration. Briefly, small pieces of residual skin specimens were enzymatically treated with 0.25 % trypsin (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C for 30 min. Epidermis was peeled off and dermis specimens were cut into small pieces and seeded on cultivation dishes containing Dulbecco’s medium (DMEM) with 10 % fetal bovine serum (FBS) and antibiotics (all from Biochrom, Berlin, Germany) at 37 °C and 5 % CO2/95 % air atmosphere. After 18/15 (donor 1/2) days, migrating cells were collected and further expanded by culturing. For the experiment passage 10 was used.
HDFs were seeded on Petri dishes/cover slips at the density of 3,000 cells/cm2 in standard cultivation medium. Next day, the medium was changed and cells were cultivated for nine days (medium was changed on day 3 and 7) in the presence (10, 100 and 1000 nM/ml) or absence (control) of genistein (Tocris Bioscience, Abingdon, UK). TGF-β1 (PeproTech, London, UK) at a final concentration of 30 ng/ml was used as positive control (Brenmoehl et al. 2009). To assess the genistein effect on TGF-β1-mediated myofibroblast differentiation, cells were treated with medium containing a combination of TGF-β1 and genistein.
Cells cultivated on Petri dishes were scratched and collected in Laemmli lysis buffer (0.1 M Tris/HCl (pH~6.8), 20 % glycerol, 10 % SDS (sodium dodecyl sulfate)) containing protease and phosphatase inhibitors (Sigma-Aldrich). Afterwards, samples were sonicated (QSonica, Newtown, CT, USA, 40 % amplitude, 15 s) to ensure complete cell lysis. Before loading into SDS-PAGE gel (10 % Bis-Tris), samples were shortly boiled (95 °C, 5 min). Following separation, proteins were dry blotted to PVDF membrane using the iBlot 2 (Thermo Fisher Scientific) system and blocked in 5 % NFDM/BSA (non-fat dry milk/bovine serum albumin) dissolved in TBS (tris-buffered saline) with 0.1 % Tween 20 (Sigma-Aldrich) at room temperature. After overnight incubation at 4 °C with primary antibody, membranes were incubated with appropriate HRP-conjugated secondary antibodies for 1 h at room temperature. Protein bands were detected as chemiluminescent signal from ECL (SuperSignal West Pico PLUS chemiluminescent Substrate, Thermo Fisher Scientific) acquired at MF-ChemiBis 2.0 (DNR Bio Imaging Systems, Israel). β-Actin was used to verify equal sample loading. The list of antibodies applied in Western blotting is shown in Table 1.
Table 1.
Antibodies used for Western blot and immunofluorescence.
| Antibodies used for Western blot | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Primary Antibody | Abbreviation | Host | Isotype | Clonality | Produced by | |
| α-Smooth muscle actin | α-SMA | Rabbit | IgG | Monoclonal | CST, Danvers, MA, USA | |
| Fibronectin | Fibr | Rabbit | IgG | Monoclonal | Abcam, Cambridge, UK | |
| Phospho-ERK1/2 | pERK1/2 | Rabbit | Polyclonal | CST, Danvers, MA, USA | ||
| Phospho-AKT | pAKT | Rabbit | IgG | Monoclonal | CST, Danvers, MA, USA | |
| Phospho-SMAD3 | pSMAD3 | Rabbit | IgG | Monoclonal | Abcam, Cambridge, UK | |
| ERK1/2 | ERK1/2 | Rabbit | IgG | Monoclonal | CST, Danvers, MA, USA | |
| SMAD3 | SMAD3 | Rabbit | IgG | Monoclonal | CST, Danvers, MA, USA | |
| AKT | AKT | Rabbit | Polyclonal | CST, Danvers, MA, USA | ||
| β-Actin | β-actin | Rabbit | IgG | Monoclonal | CST, Danvers, MA, USA | |
| D-Glyceraldehyde 3-phosphate | GAPDH | Rabbit | IgG | Monoclonal | CST, Danvers, MA, USA | |
|
| ||||||
| Secondary Antibody | Host | Isotype | Produced by | |||
|
| ||||||
| Anti-rabbit, HRP-linked | goat | IgG | CST, Danvers, MA, USA | |||
|
| ||||||
| Antibodies used for immunofluorescence | ||||||
|
| ||||||
| Primary Antibody | Abbreviation | Host | Produced by | Secondary Antibody | Produced by | Channel |
|
| ||||||
| α-Smooth muscle actin | α-SMA | Mouse monoclonal | DakoCytomation, Denmark | Goat anti-mouse | Sigma-Aldrich, St. Louis, MO, USA | TRITC-red |
| Fibronectin | Fibr | Rabbit polyclonal | DakoCytomation, Denmark | Goat anti-rabbit | Sigma-Aldrich, St. Louis, MO, USA | FITC-green |
Cells cultivated on cover slips were shortly fixed (2 % buffered paraformaldehyde, pH~7.2, 5 min) and washed with phosphate-buffered saline (PBS). Afterwards, cell membranes were permeabilized by Triton X-100 (Sigma-Aldrich) and blocked by porcine serum albumin (DAKO, Glostrup, Denmark). Following 1 h incubation at room temperature with primary antibody, samples were washed and incubated with appropriate secondary antibody. Cell nuclei were stained by 4′, 6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich). All coverslips were mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA) and investigated by a fluorescence microscope (Eclipse Ni-E, Nikon, Tokyo, Japan) equipped with filter cubes for fluorescein isothiocyanate (FITC), tetramethylrhodamine isothiocyanate (TRITC), DAPI, digital camera C11440 ORCA-flash 4.0 (Hamamatsu, Hamamatsu City, Japan), and NIS-Elements software (Nikon). The list of antibodies used for the immunofluorescent analysis is shown in Table 1.
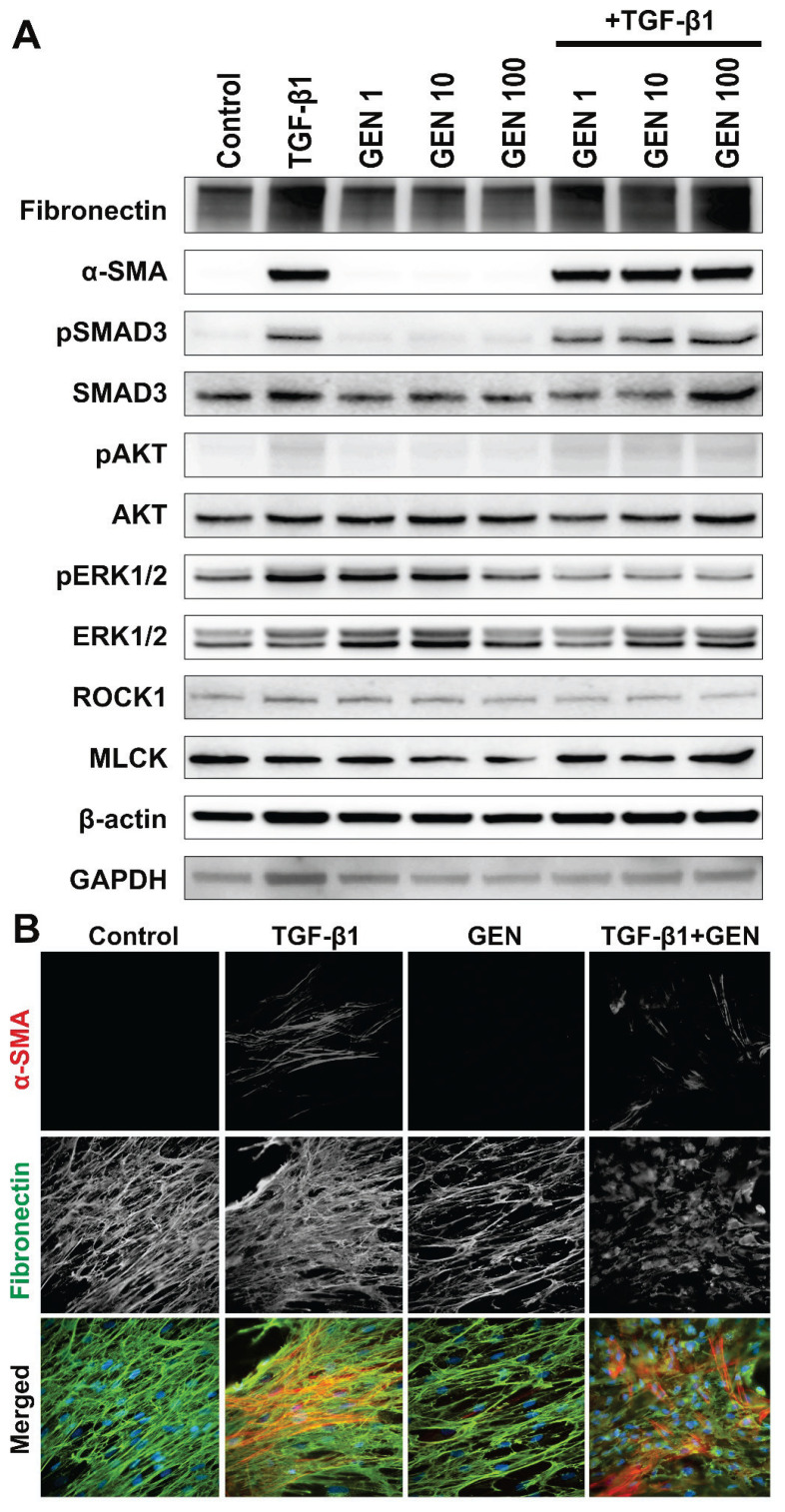
The capability of HDFs to differentiate into myofibroblasts following TGF-β1 exposure was affirmed by increased α-SMA and fibronectin expressions (Fig. 1A) and further confirmed by immunofluorescence (Fig. 1B).
Fig. 1.
(A) Western blot analysis of human dermal fibroblasts exposed to genistein (GEN – 1, 10 and 100 nM) and/or TGF-β1 (30 ng/m); (B) Immunofluorescence analysis of human dermal fibroblasts (HDF) exposed to genistein (GEN – 100 nM) and/or TGF-β1 (30 ng/ml); α-SMA – alpha-smooth muscle actin (red signal); Fibr – fibronectin (green signal); nuclei were labeled with DAPI (blue signal) (magnification 600×).
In detail, clearly visible SMA-positive stress fibers were present in TGF-β1 treated groups. Moreover, TGF-β1 treatment induced deposition of a more prominent fibronectin-rich ECM scaffold. In contrast, WB showed that GEN affects neither α-SMA nor fibronectin expressions; thus, it did not induce fibroblast-to-myofibroblast differentiation. Instead ECM deposition was rather inhibited by GEN. Interestingly, this effect persisted also in the presence of TGF-β1.
WB analysis (Fig. 1A) further revealed that TGF-β1 activated each studied signaling pathway to a different level. In particular, pSMAD and pERK1/2 were affected the most. On the other hand, GEN induced only pERK1/2 expression. Interestingly, the combination of GEN and TGF-β1 attenuated the TGF-β1-induced pERK1/2 and ROCK1 protein levels. Of note, GEN slightly decreased MLCK expression, but the effect was abolished in the presence of TGF-β1.
In the present study, we demonstrated that TGF-β1 induces the myofibroblast-like phenotype in the studied primary culture of HDFs. It has been previously shown that GEN inhibits secretion of ECM proteins and expression of TGF-β1 at both protein and mRNA levels in renal mesangial cells (Yuan et al. 2009) and keloid fibroblasts (Jurzak et al. 2014). However, the GEN ability to decrease TGF-β1-induced α-SMA expression was not observed in the present study. In contrast to a previously shown inhibitory effect of GEN on MAPK signaling in keloid fibroblasts (Cao et al. 2008), we observed induction of the MAPK signaling pathway (phosphorylation of ERK1/2) in HDFs. Although we showed that TGF-β1 induced both canonical (SMAD) and non-canonical (MAPK, AKT, ROCK) signaling, a combination of GEN and TGF-β1 attenuated the ERK1/2 and ROCK signaling in HDFs, whereas the other studied pathways remained almost unaffected. From this point of view, GEN should not impair the wound contraction induced by TGF-β1 in normal/healthy subjects.
Even though the exact mechanism is still not fully understood, several cytokines have been identified (IL-6, IL-8, IL-10, and TGF-β) to play major regulatory roles in the pathophysiology of keloids and hypertrophic scar development (Berman et al. 2017). In particular, genistein at high concentration (100 μM) arrested hypertrophic fibroblasts proliferation and decreased collagen deposition, whereas normal skin fibroblasts remained rather unaffected (Cao et al. 2009). Our IF analysis of normal HDFs revealed decreased fibronectin ECM deposition indicating certain level of GEN action specificity.
In conclusion, our results indicate that genistein does not impair conversion of normal dermal fibroblasts to myofibroblast-like cells. Accordingly, further research using an experimental animal as in vivo model is warranted by present set of in vitro data.
Acknowledgements
This study was supported in part by project „Centre for Tumour Ecology – Research of the Cancer Microenvironment Supporting Cancer Growth and Spread“ (No. CZ.02.1.01/0.0/0.0/16_019/0000785) supported by the Operational Programme “Research, Development and Education”, by Charles University in Prague (PROGRES Q28 and Q37), by the Slovak Research and Development Agency under the contracts No. APVV-16-0207, and Medical University Science Park in Košice (MediPark, Košice – Phase II) ITMS2014+ 313011D103 supported by the Operational Programme “Research and Innovations”, funded by the ERDF. Authors are grateful to Šárka Takáčová for the revision of English.
Abbreviations
- ER
estrogen receptor
- GEN
genistein
- HDFs
human dermal fibroblasts
- IL
interleukin
- MAPK
mitogen activated protein kinases
- MLCK
myosin light-chain kinase
- ROCK
Rho GTPase
- α-SMA
alpha smooth muscle actin
- TGF-β1
transforming growth factor beta 1
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- ANDUGULAPATI SB, GOURISHETTI K, TIRUNAVALLI SK, SHAIKH TB, SISTLA R. Biochanin-A ameliorates pulmonary fibrosis by suppressing the TGF-beta mediated EMT, myofibroblasts differentiation and collagen deposition in in vitro and in vivo systems. Phytomedicine. 2020;78:153298. doi: 10.1016/j.phymed.2020.153298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHCROFT GS, DODSWORTH J, Van BOXTEL E, TARNUZZER RW, HORAN MA, SCHULTZ GS, FERGUSON MW. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat Med. 1997;3:1209–1215. doi: 10.1038/nm1197-1209. [DOI] [PubMed] [Google Scholar]
- BERMAN B, MADERAL A, RAPHAEL B. Keloids and hypertrophic scars: Pathophysiology, classification, and treatment. Dermatol Surg. 2017;43(Suppl 1):S3–S18. doi: 10.1097/DSS.0000000000000819. [DOI] [PubMed] [Google Scholar]
- BERRY DP, HARDING KG, STANTON MR, JASANI B, EHRLICH HP. Human wound contraction: collagen organization, fibroblasts, and myofibroblasts. Plast Reconstr Surg. 1998;102:124–131. doi: 10.1097/00006534-199807000-00019. [DOI] [PubMed] [Google Scholar]
- BRENMOEHL J, MILLER SN, HOFMANN C, VOGL D, FALK W, SCHÖLMERICH J, ROGLER G. Transforming growth factor-beta 1 induces intestinal myofibroblast differentiation and modulates their migration. World J Gastroenterol. 2009;15:1431–1442. doi: 10.3748/wjg.15.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAO C, LI S, DAI X, CHEN Y, FENG Z, ZHAO Y, WU J. Genistein inhibits proliferation and functions of hypertrophic scar fibroblasts. Burns. 2009;35:89–97. doi: 10.1016/j.burns.2008.03.011. [DOI] [PubMed] [Google Scholar]
- CANNITO S, NOVO E, PAROLA M. Therapeutic pro-fibrogenic signaling pathways in fibroblasts. Adv Drug Deliv Rev. 2017;121:57–84. doi: 10.1016/j.addr.2017.05.017. [DOI] [PubMed] [Google Scholar]
- CAO C, LI SR, DAI X, CHEN YQ, FENG Z, QIN X, ZHAO Y, WU J. The effects of genistein on tyrosine protein kinase-mitogen activated protein kinase signal transduction pathway in hypertrophic scar fibroblasts. (Article in Chinese) Zhonghua Shao Shang Za Zhi. 2008;24:118–121. [PubMed] [Google Scholar]
- DERYNCK R, ZHANG YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- DIEL P, OLFF S, SCHMIDT S, MICHNA H. Molecular identification of potential selective estrogen receptor modulator (SERM) like properties of phytoestrogens in the human breast cancer cell line MCF-7. Planta Medica. 2001;67:510–514. doi: 10.1055/s-2001-16474. [DOI] [PubMed] [Google Scholar]
- DVORANKOVA B, LACINA L, SMETANA K., JR Isolation of normal fibroblasts and their cancer-associated counterparts (CAFs) for biomedical research. Methods Mol Biol. 2019;1879:393–406. doi: 10.1007/7651_2018_137. [DOI] [PubMed] [Google Scholar]
- EMMERSON E, CAMPBELL L, ASHCROFT GS, HARDMAN MJ. The phytoestrogen genistein promotes wound healing by multiple independent mechanisms. Mol Cell Endocrinol. 2010;321:184–193. doi: 10.1016/j.mce.2010.02.026. [DOI] [PubMed] [Google Scholar]
- HERICHOVA I, REIS R, HASAKOVA K, VICIAN M, ZEMAN M. Sex-dependent regulation of estrogen receptor beta in human colorectal cancer tissue and its relationship with clock genes and VEGF-A expression. Physiol Res. 2019;68(Suppl 3):S297–S305. doi: 10.33549/physiolres.934352. [DOI] [PubMed] [Google Scholar]
- HERICHOVA I, REIS R, HASAKOVA K, VICIAN M. Downregulation of miR-30c-5p expression in colorectal cancer tissue is sex-dependent. Physiol Res. 2020;69(Suppl 3):S479–S487. doi: 10.33549/physiolres.934598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL M, TŘÍSKALA Z, HONCŮ P, KREJČÍ M, KAJZAR J, BIČÍKOVÁ M, ONDŘEJÍKOVÁ L, JANDOVÁ D, STERZL I. Aging, hormones and receptors. Physiol Res. 2020;69(Suppl 2):S255–S272. doi: 10.33549/physiolres.934523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JURZAK M, ADAMCZYK K, ANTONCZAK P, GARNCARCZYK A, KUŚMIERZ D, LATOCHA M. Evaluation of genistein ability to modulate CTGF mRNA/protein expression, genes expression of TGFbeta isoforms and expression of selected genes regulating cell cycle in keloid fibroblasts in vitro. Acta Pol Pharm. 2014;71:972–986. [PubMed] [Google Scholar]
- KLASS BR, GROBBELAAR AO, ROLFE KJ. Transforming growth factor beta1 signalling, wound healing and repair: a multifunctional cytokine with clinical implications for wound repair, a delicate balance. Postgrad Med J. 2009;85:9–14. doi: 10.1136/pgmj.2008.069831. [DOI] [PubMed] [Google Scholar]
- LICHTMAN MK, OTERO-VINAS M, FALANGA V. Transforming growth factor beta (TGF-beta) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016;24:215–222. doi: 10.1111/wrr.12398. [DOI] [PubMed] [Google Scholar]
- MARINI H, POLITO F, ALTAVILLA D, IRRERA N, MINUTOLI L, CALÒ M, ADAMO EB, VACCARO M, SQUADRITO F, BITTO A. Genistein aglycone improves skin repair in an incisional model of wound healing: a comparison with raloxifene and oestradiol in ovariectomized rats. Br J Pharmacol. 2010;160:1185–1194. doi: 10.1111/j.1476-5381.2010.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUDA T, YAMAMOTO T, MURAGUCHI A, SAATCIOGLU F. Cross-talk between transforming growth factor-beta and estrogen receptor signaling through Smad3. J Biol Chem. 2001;276:42908–42914. doi: 10.1074/jbc.M105316200. [DOI] [PubMed] [Google Scholar]
- NING Y, CHEN J, SHI Y, SONG N, YU X, FANG Y, DING X. Genistein ameliorates renal fibrosis through regulation snail via m6A RNA demethylase ALKBH5. Front Pharmacol. 2020;11:579265. doi: 10.3389/fphar.2020.579265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAKSHIR P, NOSKOVICOVA N, LODYGA M, SON DO, SCHUSTER R, GOODWIN A, KARVONEN H, HINZ B. The myofibroblast at a glance. J Cell Sci. 2020;133:jcs227900. doi: 10.1242/jcs.227900. [DOI] [PubMed] [Google Scholar]
- SHI X, YOUNG CD, ZHOU H, WANG X. Transforming growth factor-beta signaling in fibrotic diseases and cancer-associated fibroblasts. Biomolecules. 2020;10:1666. doi: 10.3390/biom10121666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUAN WJ, JIA FY, MENG JZ. Effects of genistein on secretion of extracellular matrix components and transforming growth factor beta in high-glucose-cultured rat mesangial cells. J Artif Organs. 2009;12:242–246. doi: 10.1007/s10047-009-0479-y. [DOI] [PubMed] [Google Scholar]